Abstract
Background and Objectives
To quantify interictal photophobia in migraine with and without aura using reflexive eye closure as an implicit measure of light sensitivity and to assess the contribution of melanopsin and cone signals to these responses.
Methods
Participants were screened to meet criteria for 1 of 3 groups: headache-free (HF) controls, migraine without aura (MO), and migraine with visual aura (MA). MO and MA participants were included if they endorsed ictal and interictal photophobia. Exclusion criteria included impaired vision, inability to collect usable pupillometry, and history of either head trauma or seizure. Participants viewed light pulses that selectively targeted melanopsin, the cones, or their combination during recording of orbicularis oculi EMG (OO-EMG) and blinking activity.
Results
We studied 20 participants in each group. MA and MO groups reported increased visual discomfort to light stimuli (discomfort rating, 400% contrast, MA: 4.84 [95% confidence interval 0.33, 9.35]; MO: 5.23 [0.96, 9.50]) as compared to HF controls (2.71 [0, 6.47]). Time course analysis of OO-EMG and blinking activity demonstrated that reflexive eye closure was tightly coupled to the light pulses. The MA group had greater OO-EMG and blinking activity in response to these stimuli (EMG activity, 400% contrast: 42.9%Δ [28.4, 57.4]; blink activity, 400% contrast: 11.2% [8.8, 13.6]) as compared to the MO (EMG activity, 400% contrast: 9.9%Δ [5.8, 14.0]; blink activity, 400% contrast: 4.7% [3.5, 5.9]) and HF control (EMG activity, 400% contrast: 13.2%Δ [7.1, 19.3]; blink activity, 400% contrast: 4.5% [3.1, 5.9]) groups.
Discussion
Our findings suggest that the intrinsically photosensitive retinal ganglion cells (ipRGCs), which integrate melanopsin and cone signals, provide the afferent input for light-induced reflexive eye closure in a photophobic state. Moreover, we find a dissociation between implicit and explicit measures of interictal photophobia depending on a history of visual aura in migraine. This implies distinct pathophysiology in forms of migraine, interacting with separate neural pathways by which the amplification of ipRGC signals elicits implicit and explicit signs of visual discomfort.
People with migraine experience discomfort from light both during1-3 and between headaches (i.e., interictally).4-8 Discomfort from light is a feature of daily life for people with migraine,8-11 and interictal photophobia is similar in people who have migraine with visual aura (MA) and in those without aura (MO).4,5,9 It is possible that the similarity of light sensitivity symptoms in MA and MO masks a difference in underlying physiology in these conditions.3
Photophobia is often measured by asking patients to report the light intensity threshold at which they begin to experience discomfort or pain. As an alternative to explicit self-report, an implicit sign of visual discomfort may be measured from the muscles of the eyelid.12,13 Reflexive blinking and squinting to bright light (i.e., “dazzle” or “photic blink”) is implemented by a subcortical (pretectal) reflex arc that does not require the visual cortex.14 There has been limited study of light-induced eyelid closure in people with migraine.13
In a recent study, we asked participants with migraine and headache-free (HF) controls to rate the discomfort produced by pulses of light.15 We found that explicit reports of discomfort were increased equally in MA and MO as compared to controls, and that this effect was driven by both types of daylight-sensitive photoreceptors in the retina (i.e., the cones and melanopsin). Using the same participants and stimuli, we now ask whether this pattern of results is present for an implicit sign of light sensitivity as measured by orbicularis oculi EMG (OO-EMG) and video-oculography of blinking.
Methods
In a preregistered study, we used silent substitution stimulation to selectively target melanopsin, the cones, or both. We presented 4-second pulses of these stimuli while we recorded OO-EMG and blinking in the contralateral eye via an infrared camera. The participants and stimuli presented here have been the subject of a previous article on pupil responses and self-reported visual discomfort15; the current data were collected in the same experimental sessions.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the University of Pennsylvania Institutional Review Board. This study was the subject of an initial preregistration document (osf.io/5ry67/) and subsequent addenda (project summary page: osf.io/qjxdf/). We have previously summarized our deviations from these protocols.15 There are 3 additional deviations pertaining to this study: (1) switching from root mean square to SD to calculate EMG activity; (2) using a shorter (3,400-ms) window for the quantification of responses; and (3) adding the quantification of the blink frames as a secondary measure. Written informed consent was obtained from all participants in this study.
Participants
A total of 60 participants (ages 25–40) were recruited from Philadelphia and the University of Pennsylvania. All recruited participants underwent screening using the Penn Online Evaluation of Migraine (POEM),16 an automated headache classification using the International Classification of Headache Disorders (ICHD)–3-beta criteria. The POEM also incorporates published questions regarding ictal and interictal photophobia that were scored with a point for each “yes” response (referred as the Choi score).17 Recruited participants also completed the Visual Discomfort Score (VDS) survey,18 which was scored as described in our previous report.15 Candidate participants were required to meet all of the following inclusion criteria:
MA: (a) classification of migraine with visual aura by the POEM, (b) Choi score of 6 or 7, (c) a response of “yes” to the Choi query regarding the presence of photophobia during headache-free periods, (d) one or more headaches within the prior month
MO: (a) classification of migraine without aura by the POEM, (b) Choi score of 6 or 7, (c) a response of “yes” to the Choi query regarding the presence of photophobia during headache-free periods, (d) one or more headaches within the prior month
Control: (a) classification of mild nonmigrainous headache or HF by the POEM, (b) a response of “No” or “I don’t know” to a question regarding a family history of migraine, (c) a response of “No” to a question regarding a history of childhood motion sickness, (d) VDS score of 7 or lower
Exclusion criteria related to impaired vision, inability to collect usable pupillometry, head trauma, and seizure history were as described previously.15 Participants were not excluded based on medication use and were allowed to continue to take their current medications during data collection.
Stimuli
We designed stimuli that target specific photoreceptor classes through silent substitution.19 In this approach, sets of light spectra are created that have the nominal property of producing equal excitation of one or more “silenced” classes of photoreceptors, and varying excitation on one or more “targeted” photoreceptors. We targeted 3 main photoreceptor mechanisms: melanopsin, the cones, or their combination (referred to as light flux).
Stimuli were generated as described in prior reports.15,20,21 Briefly, we used a digital light synthesis engine (OneLight Spectra) that produces stimulus spectra under digital control at 256 Hz. We created separate background and stimulation spectra that provided (1) a nominal 400% unipolar Weber contrast on melanopsin while silencing the cones (melanopsin-directed background/stimulus pair), (2) 400% contrast on each L-, M-, and S-cone classes while silencing melanopsin (cone-directed background/stimulus pair), and (3) 400% contrast each on melanopsin and each L-, M-, and S-cone classes (light flux background/stimulus pair). Background spectra for each stimulus type differed in luminance but had similar chromaticity.15 We also produced contrast levels of 100% and 200% for each stimulus direction by scaling the relevant stimulus spectra. We did not explicitly silence rods or penumbral cones,20 although the properties of our stimuli minimize the contribution of these photoreceptors.
Stimuli were presented through a custom-made eyepiece with a circular, spatially uniform field of 27.5° diameter. The central 5° (diameter) of the field was obscured to minimize effects of macular pigment. Apparatus calibration and stimulus validation have been described.15
Experiment Structure
Participants were studied during multiple sessions, usually held on different days. To reduce variation in circadian cycle across sessions, subsequent sessions for each participant were initiated within 3 hours of the time of day when that participant started the first session.
Acclimation to the testing room and apparatus, and pharmacologic dilation of the right eye, was as previously described.15 The participant remained in constant environmental light conditions during data collection. Participants viewed the stimuli through their pharmacologically dilated right eye and a 6-mm-diameter artificial pupil to control retinal irradiance.
On each trial, the participant viewed a pulsed spectral modulation designed to target melanopsin, the cones, or both. The transition from the background to the stimulation spectrum (melanopsin, cones, or light flux), and the subsequent return to the background, were windowed with a 500-ms half-cosine. This minimized the entopic percept of a Purkinje tree in the melanopsin-directed stimulus.20 The duration of the pulse was 4 seconds, after which the stimulus field returned to the background spectrum (Figure 1B). EMG and infrared camera recordings continued for another 12 seconds after the pulse ended. Twelve seconds after the pulse ended, participants were prompted by an auditory cue to verbally rate their visual discomfort on a 0–10 scale. After each trial, there was a variable intertrial interval of 1.5–2.5 seconds (uniformly distributed) that reduced the predictability of the onset of the next trial.
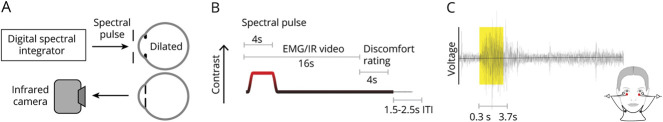
Figure 1. Experimental Overview.
(A) The light from a digital spectral integrator was presented to the pharmacologically dilated right eye of the participant through an artificial pupil. Blinking of the left eye was recorded with an infrared (IR) camera. (B) Each trial featured a 4-second spectral pulse. EMG and IR camera recordings started prior to the onset of the pulse and continued for 16 seconds. Then participants were prompted to verbally rate their visual discomfort on a 0–10 scale. There was an intertrial interval (ITI) that varied between 1.5 and 2.5 seconds. (C) To record orbicular oculi EMG (OO-EMG), electrodes were placed inferior to both eyes. OO-EMG activity was calculated from the percent change in the SD of the voltage from baseline over a 3,400 ms window starting 300 ms after pulse onset and ending 300 ms before pulse offset.
Ten consecutive trials that targeted the same photoreceptor direction but varied in contrast were grouped together during an acquisition. The ordering of the contrast levels (100%, 200%, 400%) was counterbalanced,22 and the first trial was discarded so that all retained trials had controlled first-order stimulus history. A total of 6 acquisitions, 2 of each photoreceptor direction, comprised a single session. Acquisitions were ordered such that consecutive acquisitions were not of the same photoreceptor direction. We attempted to gather 4 sessions of data for each participant but retained all participants who completed 2 sessions that contained at least 6 acceptable trials—as judged by pupillometry—for each stimulus type (photoreceptor direction and contrast level). Participants did not complete all 4 sessions for a variety of reasons.15
Electromyography
We recorded OO-EMG starting prior to pulse onset and ending 12 seconds after pulse offset (Figure 1, B and C). This measure is sensitive to tonic (squinting) and phasic (blinking) eye closure. There was a 1.1-s delay between the start of the trial and the initiation of EMG recording due to a slow computational operation; thus, the period of EMG recording prior to pulse onset was approximately 400 ms. Prior to electrode placement, the skin surface was wiped with an alcohol pad. Two small reference electrode pads were placed inferior to each eye, and a ground lead was placed on the neck. EMG was recorded with the BioNomadix 2-Channel EMG equipment (Biopac Systems, Inc). Participants were informed that the EMG electrodes measure an “eye response.” This wording was designed to avoid cueing the participant to the use of the EMG as an index of discomfort; we did not assess what assumptions participants had about our measurement.
Blink Quantification via Infrared Videography
We recorded blinks from the left eye of the participant (contralateral to the eye receiving stimuli) using an infrared camera (Pupil Labs GmbH) mounted on a post ∼25 mm from the eye. The camera has 2 infrared LEDs mounted adjacent to the lens, providing illumination of the eye. A 60-Hz video clip was recorded for each trial, starting 1.5 seconds prior to pulse onset and ending 12 seconds after pulse offset. These videos were processed using custom software (github.com/gkaguirrelab/transparentTrack).23 Blinking was quantified as the number of video frames in which the glints (first Purkinje images) from the active infrared light sources were absent.
Analysis
Data analysis was performed using custom MATLAB code (MathWorks). EMG activity was quantified by calculating the SD of the recorded voltage within a sliding 500-ms window. The activity time series were normalized as percentage change relative to activity occurring prior to pulse onset.
First, we examined the time course of EMG responses for each group of participants, averaged across the 3 stimulus types (cone, melanopsin, and light-flux). The mean OO-EMG response across trials and stimuli at each contrast level was calculated and then averaged across participants within each group.
Second, we examined the time course of blink activity for each group of participants, averaged across the 3 stimulus types (cone, melanopsin, and light-flux). Blink activity was defined as a binary vector (i.e., blink or no blink). Then, across trials for a participant, the average of such vectors was calculated, and a sliding window was applied to yield the percentage of trials in which a blink occurred. The mean percent trials with blinks at each contrast level were calculated and then averaged across participants within each group.
We quantified the effect of our stimuli upon the OO-EMG and blink measures by calculating the average response over a 3,400-ms window starting 300 ms after pulse onset and ending 300 ms prior to pulse offset. This window was selected to avoid the influence of blinking at stimulus onset or offset in the measured response.24 For both OO-EMG activity and percent of blink frames, we took the mean response across trials within a participant, and the mean across participants within a group.
Data Availability
Analysis results using the preregistered measures, analysis code, and data are available online (github.com/gkaguirrelab/melSquintAnalysis).
Results
Participant Characteristics
We studied 20 individuals in each of 3 groups: MA, MO, and HF controls. The groups were well matched for age (Table 1). The nonphotophobic HF group contained fewer female participants than the photophobic migraine groups, reflecting the higher female to male ratio observed in migraine.25 Participants in both migraine groups reported similar headache disability and frequency (∼4 headache days per month) from migraine, indicating that both migraine groups contained individuals with episodic migraine and moderate disability. Medication use among the 2 migraine groups was similar, although more MA participants reported aspirin/acetaminophen/caffeine (Excedrin) and triptan use.
Table 1.
Demographic and Clinical Characteristics
Patients With MA and Patients With MO Report Enhanced, Explicit Discomfort to Light
Patients were asked to verbally rate, on a scale of 0 to 10, the visual discomfort produced by each presented stimulus. In a previous presentation of these data,15 we found that stimulation of either the cones or melanopsin evoked a report of visual discomfort. Here, we collapsed the data across photoreceptor target and examined the effect of stimulus contrast and headache diagnosis. Figure 2 presents the mean reported discomfort level for each group as a function of the contrast of the stimulus. For all 3 groups, increased stimulus contrast evoked reports of greater discomfort. For both of the migraine populations, discomfort levels were increased overall. This was especially evident at the 400% contrast level (MA: 4.84 ± 2.30; MO: 5.23 ± 2.18; HF controls: 2.71 ± 1.92). Our previous analysis of these data showed that this effect of migraine was present for both cone and melanopsin stimulation.
Figure 2. Discomfort Rating in Response to Spectral Pulses.
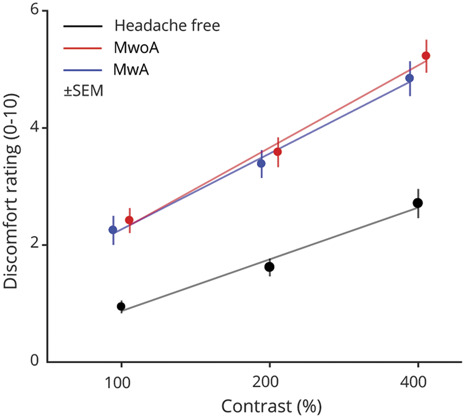
The average verbal discomfort rating on a scale of 0–10 within each group (n = 20 participants per group) is shown at each contrast level. The stimuli were presented at 3 different contrast levels (100%, 200%, and 400%), and these (log-spaced) values define the x-axis. The discomfort ratings for the 3 stimulus types were averaged across stimulus types within each group and shown as a filled circle. Error bars represent ±SEM. The best-fit line to the mean discomfort rating across participants as a function of log contrast is shown as a solid line for each group.
Only Patients With MA Demonstrate Enhanced OO-EMG Responses to Light
We examined the effect of the light stimulus pulse upon OO-EMG activity as a function of time and stimulus contrast across the 16-second recording interval (collapsed across photoreceptor target) for each of the studied groups. For the 100% contrast stimuli, the temporal evolution of EMG activity is indistinguishable between the studied groups (Figure 3A, left). There is a small increase in the EMG signal during the stimulus presentation, followed by an additional, transient increase at stimulus offset. Based on inspection of video recording of the eye contralateral to the stimulus, we interpret the offset response as participants suppressing blinks during the stimulus, and then engaging in increased blinking following stimulus offset. The response to the 200% contrast stimulus is similar (Figure 3A, center), except for an increased EMG response during the light pulse in only the MA group. This effect becomes pronounced in response to the maximal, 400% contrast pulse, for which only the MA group demonstrates a large change in EMG activity during the stimulus (Figure 3A, right).
Figure 3. Orbicularis Oculi EMG (OO-EMG) Activity in Response to Spectral Pulses.
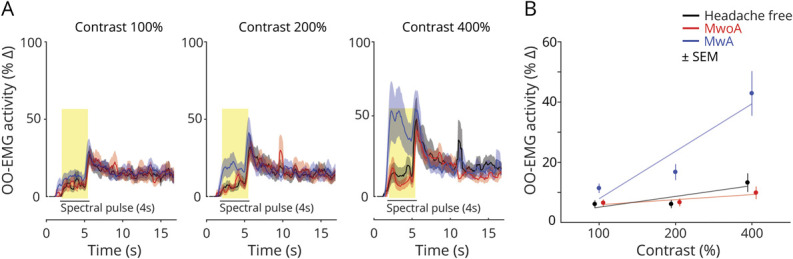
(A) Time course of OO-EMG activity. The average OO-EMG across participants within each group (n = 20 participants per group) is shown at each contrast level (columns: 100%, 200%, and 400%). For each contrast level, responses from the 3 groups are superimposed and shown as a function of time over the 16-second recording interval. The 4-second stimulus pulse is indicated by a black bar and the middle 3,400 ms of this period is highlighted in yellow. The shaded area is the ±SEM across participants within a group. OO-EMG is expressed as percentage change relative to activity occurring prior to pulse onset. (B) The across-subject, average OO-EMG activity during the middle 3,400 ms of the stimulus pulse is shown for each group (n = 20 participants per group) at each contrast level. The stimuli were presented at 3 different contrast levels (100%, 200%, and 400%), and these (log-spaced) values define the x-axis. Error bars represents ±SEM across participants.
We quantified the change in OO-EMG activity during the stimulus pulse for each group and contrast level (Figure 3B). We compared OO-EMG activity from the prestimulus baseline to activity during the middle 3,400 ms of the stimulus pulse. In MA participants, light pulses elicited elevated OO-EMG activity with increasing levels of contrast. This was particularly evident in response to 400% contrast, which evoked an increase in mean (±SEM) EMG activity of 42.9 ± 7.4%. Light pulses evoked smaller elevations in OO-EMG activity compared to baseline in controls or MO participants at 400% contrast (mean responses: HF controls: 13.2 ± 3.1%, MO: 9.9 ± 2.1%).
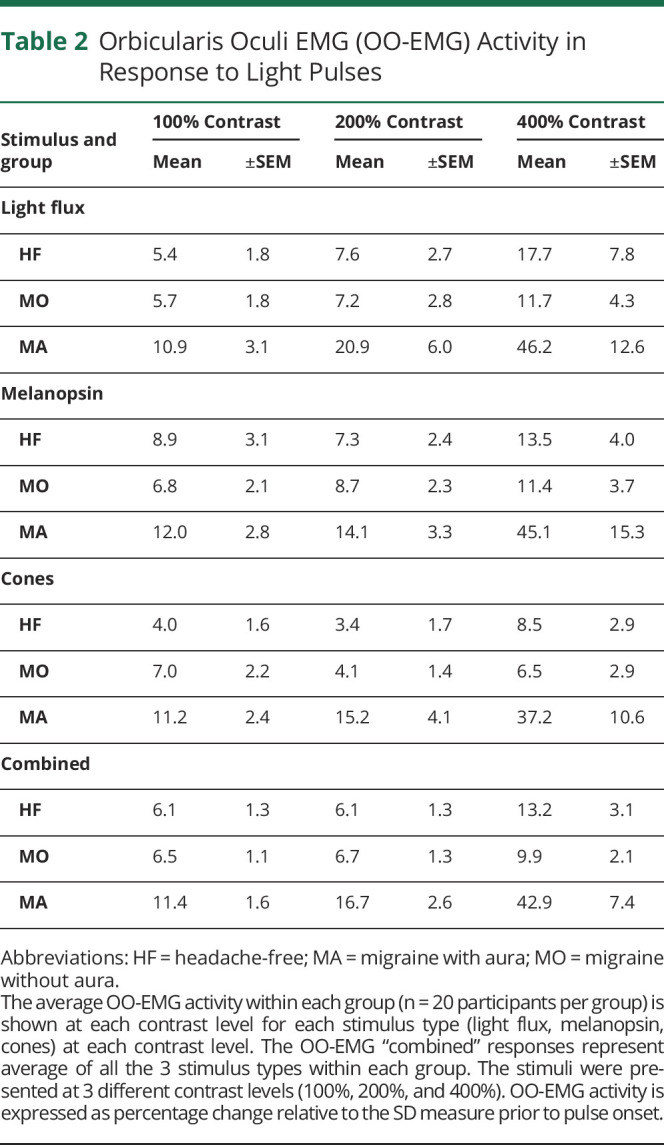
We examined the separate effect of stimuli that targeted the cones and melanopsin upon the OO-EMG response. Overall, we found that signals from both of these photoreceptor classes produced larger OO-EMG response in the MA group as compared to the MO and HF groups. Table 2 provides the mean OO-EMG response across participants as a function of group, contrast level, and photoreceptor target.
Table 2.
Orbicularis Oculi EMG (OO-EMG) Activity in Response to Light Pulses
Only Patients With MA Demonstrate Enhanced Blinking in Response to Light
People engage in both blinking and squinting in response to a bright light. The OO-EMG signal reflects both types of muscle activity. We sought to confirm our OO-EMG findings by examining a second measure of reflexive eyelid closure. Analyzing infrared video frames of the contralateral eye, we obtained an estimate of the percent of time participants spent blinking in the period following pulses of light.
Like the OO-EMG activity, we examined the effect of the light stimulus pulse upon blinking as a function of time and stimulus contrast across the 16-second recording interval. For the 100% contrast stimuli, the temporal evolution of blinking is similar between the studied groups (Figure 4A, left). Participants attempt to keep their eye open during the stimulus, and then engage in increased blinking following the offset of the light pulse. At 200% contrast (Figure 4A, center), a slight increase in blinking during the spectral pulse is seen in the MA group as compared to the MO and HF control groups. This effect becomes prominent in the 400% contrast data, in which an increase in blinking with stimulus onset can be seen for the MA group (Figure 4A, right).
Figure 4. Blink Activity in Response to Spectral Pulses.
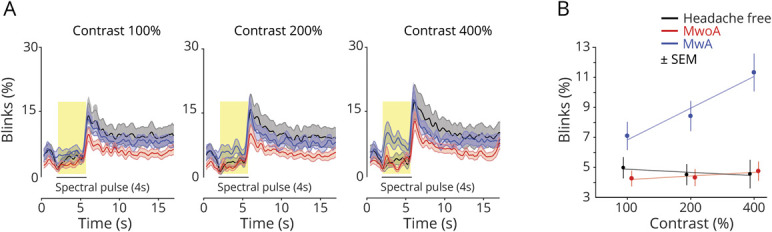
(A) Time course of blinking activity. The average blink activity across participants within each group (n = 20 participants per group) is shown at each contrast level (columns: 100%, 200%, and 400%). For each contrast level, responses from the 3 groups are superimposed and shown as a function of time over the 16-second recording interval. The 4-second stimulus pulse is indicated by a black bar, and the middle 3,400 ms of this period is highlighted in yellow. The shaded area is the ±SEM across participants within a group. Blink activity was quantified as the percentage of video frames classified as blinks. (B). The across-subject, average blink activity during the middle 3,400 ms of the stimulus pulse is shown for each group (n = 20 participants per group) at each contrast level. The stimuli were presented at 3 different contrast levels (100%, 200%, and 400%), and these (log-spaced) values define the x-axis. Error bars represents ±SEM across participants.
We quantified the percent of time spent blinking during the middle 3,400 ms of the stimulus pulses for each group and contrast level (Figure 4B). In MA participants, spectral pulses increased the percent of blink frames with increasing levels of contrast, particularly at 400% contrast (mean ± SEM blink frames: 11.2 ± 1.2%). Lower rates of blinking were seen in the MO (4.7 ± 0.6%) and HF controls (4.5 ± 0.7%). These findings parallel our measurements of OO-EMG activity.
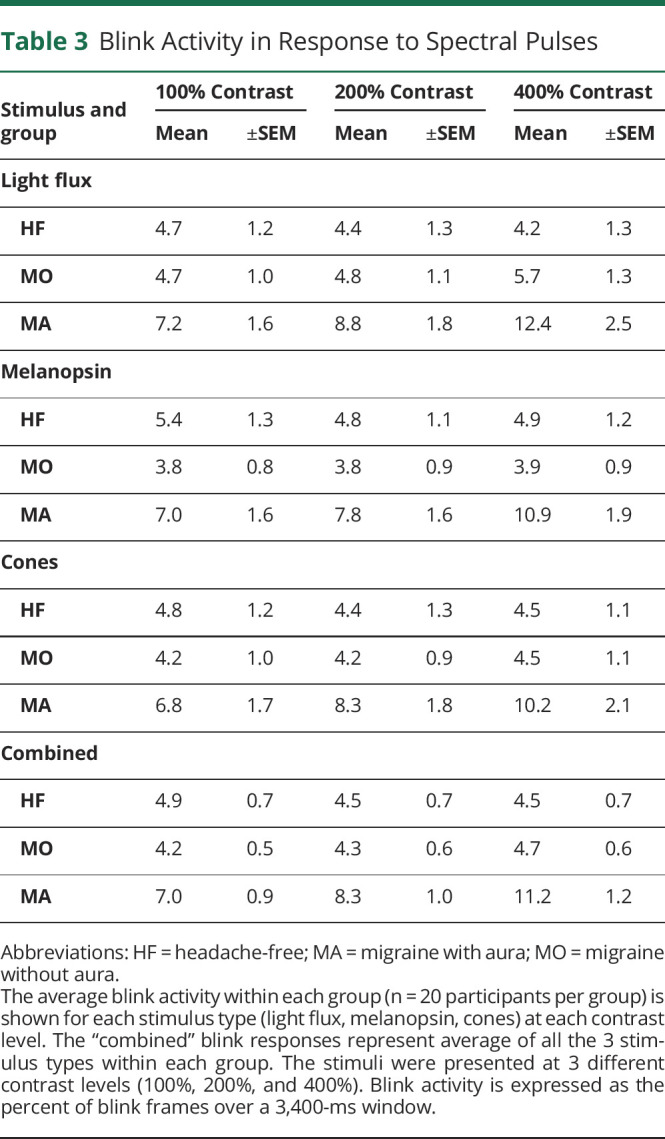
We examined the separate effect of stimuli that targeted the cones and melanopsin upon the blink response. Again, we found that stimulation of either of these photoreceptor classes produced a larger blink response in the MA group as compared to the MO or HF groups (Table 3).
Table 3.
Blink Activity in Response to Spectral Pulses
Discussion
We find a clear dissociation between explicit and implicit measures of visual discomfort in participants who have migraine with interictal light sensitivity. While migraine headache generally is associated with a verbal report of increased discomfort from pulses of light, only those participants with migraine with visual aura had changes in implicit, reflexive measures of visual discomfort (blinking and squinting). Our study adds to a relatively limited set of findings of measurable differences in migraine between people with and without aura. Similar to our prior study with these stimuli,15 we find that both cone and melanopsin stimulation elicit reflexive eye closure. This finding implicates the intrinsically photosensitive retinal ganglion cells (ipRGCs), which contain melanopsin and receive extrinsic input from the cones.
The 2 migraine study populations were well matched in demographic properties and headache frequency and disability, in their reports of visual disability in daily life, and in measures of circadian and seasonal sensitivity (Table 1).15 The MA and MO groups also reported nearly identical levels of visual discomfort in response to the light pulse stimuli (Figure 2). Despite these manifest similarities, the 2 groups had markedly different responses in measures of reflexive eyelid closure (Figures 3B and 4B). It is possible that there are further, individual differences related to aura or headache frequency. In post hoc tests within the MA group, we did not find a significant relationship between headache recency and either EMG (Spearman ρ = −0.29, p = 0.12) or blinking response (ρ = −0.07, p = 0.72). A larger study focused upon individual differences may reveal that reflexive eyelid closure is a postdrome phenomenon, similar to observations of altered pupillary responses following a migraine event26 or an effect of more frequent aura as greater light sensitivity has been observed in chronic compared to episodic migraine.27 Interestingly, the stimuli in our protocol induced aura in the midst of an experimental session in one instance, and we are aware of at least 8 instances of participants experiencing migraine within 6 hours following an experimental session.
Whether migraine with and without aura reflect distinct entities has been the subject of some debate. Despite clinical and demographic differences,3,28 genetic differences, for example, have proven elusive.29,30 There are several reports of altered neural or vascular physiology specific to MA.10,28 A consistent finding has been a reduction in neural habituation in MA,31 although similar findings have also been observed in MO.32
We found a difference between migraine with and without aura in reflexive eyelid closure in response to light. The photic blink reflex relies upon brainstem mechanisms,14 which are intimately related with pathways for the acoustic and tactile (i.e., trigeminal) blink reflex.33 A decrease in habituation of the trigeminally mediated blink reflex has been shown in migraine generally,31,32 and a recent study found that people with migraine respond with a threshold amount of OO-EMG activity at lower levels of light compared to healthy controls (although that study did not distinguish between migraine participants with and without visual aura).13
Trigeminal and retinal signals appear to interact and potentiate each other. In migraine, noxious trigeminal stimulation decreases visual discomfort thresholds,4 increases light-induced pain,34 and potentiates visual cortex activity in response to light.35 The reverse association is also found, as light decreases pain thresholds for trigeminal stimulation.36 Trigeminal sensitization also appears to facilitate photophobia in blepharospasm, which is a dystonia of repetitive eye closure.37 In an fMRI study of a patient with transient photophobia from corneal irritation, visual stimulation was found to activate the trigeminal ganglion and trigeminal nucleus caudalis,38 in agreement with similar measurements in rodents.39-41 The ability of corneal irritation to induce light aversion is attenuated in mice lacking ipRGCs, suggesting a link between ipRGC signals and trigeminal nociception.42 Furthermore, the somatic discomfort from bright light may in part derive from the convergence of ipRGC and dura-sensitive trigeminal afferents upon the posterior thalamus.43
An intriguing possibility is that melanopsin expression in trigeminally innervated tissue could itself contribute to visual discomfort. Melanopsin has been found in the trigeminal ganglia of mice and humans44,45 and the cornea45 and iris of mice.46 In rodents with optic nerve lesions, bright light has been reported to potentiate the trigeminal blink reflex47 and induce light aversion in a migraine-like state.44 In our study, however, extraretinal melanopsin signaling seems unlikely to have played a substantial role, as the stimulus was transmitted through an artificial pupil into the pharmacologically dilated eye, minimizing the area of stimulated cornea and iris.
Given the dissociation of explicit and implicit discomfort measures in the presence and absence of visual aura, it seems likely that the physiologic mechanism of enhanced reflexive eye closure differs at some point from that of an enhanced conscious report of visual discomfort. Evidence from animal studies,41,48-50 and from our recent15 and current measures in humans, suggest that the ipRGCs are a source of signals for light aversion. There are several classes of these melanopsin-containing cells, and they have diverse and widespread projections both to cortical pathways (including the visual and somatosensory thalamic nuclei) and to subcortical, brainstem sites. While we suspect that there exists a brainstem site that receives converging trigeminal and ipRGC input, and which mediates reflexive eye closure, we are unaware of the demonstration of a neuroanatomic site with these properties. We interpret our findings as suggesting that people with MA and MO have an amplification of ipRGC-based signals for the conscious report of visual discomfort (perhaps via a cortical route), but that only in MA is there an alteration of brainstem mechanisms for light-induced, reflexive eyelid closure.
Glossary
- HF
headache-free
- ipRGC
intrinsically photosensitive retinal ganglion cell
- MA
migraine with aura
- MO
migraine without aura
- OO-EMG
orbicularis oculi EMG
- POEM
Penn Online Evaluation of Migraine
- VDS
Visual Discomfort Score
Appendix. Authors
Footnotes
Podcast: NPub.org/rc7ke4
Study Funding
This work was supported by grants from the National Eye Institute (R01EY024681 to G.K.A. and D.H.B.; Core Grant for Vision Research P30 EY001583), National Institute of Neurological Disorders and Stroke (R25 NS065745), National Institute on Aging (5T32AG000255-13), and the Department of Defense (W81XWH-151-0447 to G.K.A.).
Disclosure
E.A. Kaiser received royalties from patents in association with Alder Biopharmaceuticals related to anti-CGRP monoclonal antibodies for the treatment of migraine and photophobia and received investigator-driven grant funding from Amgen, which manufactures an anti-CGRP monoclonal antibody for the treatment of migraine and not used for this study. H. McAdams, A. Igdalova, E.B. Haggerty, and B.L. Cucchiara report no disclosures. D.H. Brainard has received grant funding and personal fees from Johnson & Johnson. G.K. Aguirre has received grant funding from Johnson & Johnson. Go to Neurology.org/N for full disclosures.
Publication History
This manuscript was pre-published in BioRxiv. doi: https://doi.org/10.1101/2020.12.12.422528. Received by Neurology March 24, 2021. Accepted in final form August 16, 2021.
References
- 1.Selby G, Lance JW. Observations on 500 cases of migraine and allied vascular headache. J Neurol Neurosurg Psychiatry. 1960;23(1):23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drummond PD. A quantitative assessment of photophobia in migraine and tension headache. Headache. 1986;26(9):465-469. [DOI] [PubMed] [Google Scholar]
- 3.Russell MB, Rasmussen BK, Fenger K, Olesen J. Migraine without aura and migraine with aura are distinct clinical entities: a study of four hundred and eighty-four male and female migraineurs from the general population. Cephalalgia. 1996;16(4):239-245. [DOI] [PubMed] [Google Scholar]
- 4.Drummond PD, Woodhouse A. Painful stimulation of the forehead increases photophobia in migraine sufferers. Cephalalgia. 1993;13(5):321-324. [DOI] [PubMed] [Google Scholar]
- 5.Vanagaite J, Pareja JA, Storen O, White LR, Sand T, Stovner LJ. Light-induced discomfort and pain in migraine. Cephalalgia. 1997;17(7):733-741. [DOI] [PubMed] [Google Scholar]
- 6.Main A, Dowson A, Gross M. Photophobia and phonophobia in migraineurs between attacks. Headache. 1997;37(8):492-495. [DOI] [PubMed] [Google Scholar]
- 7.Main A, Vlachonikolis I, Dowson A. The wavelength of light causing photophobia in migraine and tension-type headache between attacks. Headache. 2000;40(3):194-199. [DOI] [PubMed] [Google Scholar]
- 8.Perenboom MJL, Zamanipoor Najafabadi AH, Zielman R, Carpay JA, Ferrari MD. Quantifying visual allodynia across migraine subtypes: the Leiden visual sensitivity scale. Pain. 2018;159(11):2375-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulleners WM, Aurora SK, Chronicle EP, Stewart R, Gopal S, Koehler PJ. Self-reported photophobic symptoms in migraineurs and controls are reliable and predict diagnostic category accurately. Headache. 2001;41(1):31-39. [DOI] [PubMed] [Google Scholar]
- 10.Datta R, Aguirre GK, Hu S, Detre JA, Cucchiara B. Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia. 2013;33(6):365-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong CD, Starling AJ, Schwedt TJ. Interictal photosensitivity associates with altered brain structure in patients with episodic migraine. Cephalalgia. 2016;36(6):526-533. [DOI] [PubMed] [Google Scholar]
- 12.Berman SMB MA, Jacobs RJ, Bailey IL, Gandhi N. An objective measure of discomfort glare. J Illuminating Eng Soc J. 2016;23(2):40-49. [Google Scholar]
- 13.Zele AJ, Dey A, Adhikari P, Feigl B. Melanopsin hypersensitivity dominates interictal photophobia in migraine. Cephalalgia. 2020;41(2):217-220. [DOI] [PubMed] [Google Scholar]
- 14.Hackley SA, Johnson LN. Distinct early and late subcomponents of the photic blink reflex: response characteristics in patients with retrogeniculate lesions. Psychophysiology. 1996;33(3):239-251. [DOI] [PubMed] [Google Scholar]
- 15.McAdams H, Kaiser EA, Igdalova A, et al. Selective amplification of ipRGC signals accounts for interictal photophobia in migraine. Proc Natl Acad Sci USA. 2020;117(29):17320-17329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser EA, Igdalova A, Aguirre GK, Cucchiara B. A web-based, branching logic questionnaire for the automated classification of migraine. Cephalalgia. 2019;39(10):1257-1266. [DOI] [PubMed] [Google Scholar]
- 17.Choi JY, Oh K, Kim BJ, Chung CS, Koh SB, Park KW. Usefulness of a photophobia questionnaire in patients with migraine. Cephalalgia. 2009;29(9):953-959. [DOI] [PubMed] [Google Scholar]
- 18.Conlon EG, Lovegrove WJ, Chekaluk E, Pattison PE. Measuring visual discomfort. Vis Cogn. 1999;6(6):637-663. [Google Scholar]
- 19.Estevez O, Spekreijse H. The “silent substitution” method in visual research. Vis Res. 1982;22(6):681-691. [DOI] [PubMed] [Google Scholar]
- 20.Spitschan M, Aguirre GK, Brainard DH. Selective stimulation of penumbral cones reveals perception in the shadow of retinal blood vessels. PLoS One. 2015;10(4):e0124328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spitschan M, Bock AS, Ryan J, Frazzetta G, Brainard DH, Aguirre GK. The human visual cortex response to melanopsin-directed stimulation is accompanied by a distinct perceptual experience. Proc Natl Acad Sci USA. 2017;114(46):12291-12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguirre GK, Mattar MG, Magis-Weinberg L. de Bruijn cycles for neural decoding. Neuroimage. 2011;56(3):1293-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aguirre GK. A model of the entrance pupil of the human eye. Sci Rep. 2019;9(1):9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stringham JM, Fuld K, Wenzel AJ. Action spectrum for photophobia. J Opt Soc Am A Opt Image Sci Vis. 2003;20(10):1852-1858. [DOI] [PubMed] [Google Scholar]
- 25.Robbins MS, Lipton RB. The epidemiology of primary headache disorders. Semin Neurol. 2010;30(2):107-119. [DOI] [PubMed] [Google Scholar]
- 26.Ali EN, Carle CF, Lueck CJ, Kolic M, Maddess T. Assessing migraine patients with multifocal pupillographic objective perimetry. BMC Neurol. 2021;21(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cortez MM, Rea NA, Hunter LA, Digre KB, Brennan KC. Altered pupillary light response scales with disease severity in migrainous photophobia. Cephalalgia. 2017;37(8):801-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen JM, Charles A. Differences in treatment response between migraine with aura and migraine without aura: lessons from clinical practice and RCTs. J Headache Pain. 2019;20(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ligthart L, Boomsma DI, Martin NG, Stubbe JH, Nyholt DR. Migraine with aura and migraine without aura are not distinct entities: further evidence from a large Dutch population study. Twin Res Hum Genet. 2006;9(1):54-63. [DOI] [PubMed] [Google Scholar]
- 30.Chasman DI, Schurks M, Anttila V, et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet. 2011;43(7):695-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrotta A, Anastasio MG, De Icco R, et al. Frequency-dependent habituation deficit of the nociceptive blink reflex in aura with migraine headache: can migraine aura modulate trigeminal excitability? Headache. 2017;57(6):887-898. [DOI] [PubMed] [Google Scholar]
- 32.Di Clemente L, Coppola G, Magis D, Fumal A, De Pasqua V, Schoenen J. Nociceptive blink reflex and visual evoked potential habituations are correlated in migraine. Headache. 2005;45(10):1388-1393. [DOI] [PubMed] [Google Scholar]
- 33.Rimpel J, Geyer D, Hopf HC. Changes in the blink responses to combined trigeminal, acoustic and visual repetitive stimulation, studied in the human subject. Electroencephalogr Clin Neurophysiol. 1982;54(5):552-560. [DOI] [PubMed] [Google Scholar]
- 34.Drummond PD. Photophobia and autonomic responses to facial pain in migraine. Brain. 1997;120(pt 10):1857-1864. [DOI] [PubMed] [Google Scholar]
- 35.Boulloche N, Denuelle M, Payoux P, Fabre N, Trotter Y, Geraud G. Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry. 2010;81(9):978-984. [DOI] [PubMed] [Google Scholar]
- 36.Kowacs PA, Piovesan EJ, Werneck LC, et al. Influence of intense light stimulation on trigeminal and cervical pain perception thresholds. Cephalalgia. 2001;21(3):184-188. [DOI] [PubMed] [Google Scholar]
- 37.Hallett M, Evinger C, Jankovic J, Stacy M. Update on blepharospasm: report from the BEBRF international workshop. Neurology. 2008;71(16):1275-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moulton EA, Becerra L, Borsook D. An fMRI case report of photophobia: activation of the trigeminal nociceptive pathway. Pain. 2009;145(3):358-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okamoto K, Thompson R, Tashiro A, Chang Z, Bereiter DA. Bright light produces Fos-positive neurons in caudal trigeminal brainstem. Neuroscience. 2009;160(4):858-864. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. Pain. 2010;149(2):235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marek V, Reboussin E, Degardin-Chicaud J, et al. Implication of melanopsin and trigeminal neural pathways in blue light photosensitivity in vivo. Front Neurosci. 2019;13:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matynia A, Parikh S, Deot N, et al. Light aversion and corneal mechanical sensitivity are altered by intrinsically photosensitive retinal ganglion cells in a mouse model of corneal surface damage. Exp Eye Res. 2015;137:57-62. [DOI] [PubMed] [Google Scholar]
- 43.Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13(2):239-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matynia A, Nguyen E, Sun X, et al. Peripheral sensory neurons expressing melanopsin respond to light. Front Neural Circuits. 2016;10(10):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delwig A, Chaney SY, Bertke AS, et al. Melanopsin expression in the cornea. Vis Neurosci. 2018;35:E004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xue T, Do MT, Riccio A, et al. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479(7371):67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dolgonos S, Ayyala H, Evinger C. Light-induced trigeminal sensitization without central visual pathways: another mechanism for photophobia. Invest Ophthalmol Vis Sci. 2011;52(11):7852-7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delwig A, Logan AM, Copenhagen DR, Ahn AH. Light evokes melanopsin-dependent vocalization and neural activation associated with aversive experience in neonatal mice. PLoS One. 2012;7(9):e43787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matynia A, Parikh S, Chen B, et al. Intrinsically photosensitive retinal ganglion cells are the primary but not exclusive circuit for light aversion. Exp Eye Res. 2012;105:60-69. [DOI] [PubMed] [Google Scholar]
- 50.Semo M, Gias C, Ahmado A, et al. Dissecting a role for melanopsin in behavioural light aversion reveals a response independent of conventional photoreception. PLoS One. 2010;5(11):e15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Analysis results using the preregistered measures, analysis code, and data are available online (github.com/gkaguirrelab/melSquintAnalysis).