Abstract
A recent paper by Naderi et al. published in eLife shows that pair bonding in monogamous mice is protective against tumor growth, likely via changes in serum factors and cancer cell transcription. We propose that studying the pathway linking social stimuli to cancer cell gene regulation offers a means to identify novel pharmacological targets.
‘Love cures people - both the ones who give it and the ones who receive it’, claimed psychiatrist Karl Menninger. Were he alive today, Dr Menninger might support his claim by referencing a recent report by Naderi et al. from the group of Hippokratis Kiaris at the University of South Carolina, which provides evidence that pair bonding in monogamous mice is protective against tumor growth [1].
The salubrious effects of marriage and the deleterious effects of widowhood are well documented. Married men and women over 65 have life expectancies ~2 years longer than their unmarried counterparts, and the risk of mortality nearly doubles during the first 3 months after the loss of a spouse [2]. Married cancer patients are less likely to die from the disease than unmarried cancer patients, although this disparity may be due to differences in treatment regimens and diagnosis delays [3]. However, the mortality risk of bereaved partners is increased for almost all causes of death including cancer, infections, and cardiovascular disease [4]. The improved health outcomes of married people are often attributed to extrinsic factors such as emotional and financial support, as well as spousal motivation of healthy behaviors, but the causes of the detrimental ‘widowhood effect’ are largely unknown owing to the difficulty in discriminating between the behavioral and biological consequences of bereavement in humans.
The recent paper by Naderi et al. probes the biological basis of these phenomena using monogamous Peromyscus mice as a model. They show in vitro that human lung cancer grows larger when exposed to sera from mice with disrupted pair bonds compared to sera from mice with intact pair bonds. Transcriptomic analyses reveal that tumor cells grown with sera from bonded animals, compared to those grown with sera from either virgin or bond-disrupted animals, exhibit differential expression of several cancer-related genes involved in cell migration and tissue morphogenesis. In addition, tumors transplanted in vivo from bonded mice into virgin immuno-compromised mice show decreased tumor-igenicity compared to those transplanted from bond-disrupted mice, indicating that the effects of the pair-bond status of the initial animal persist even in the absence of any continuing influence from the pair bond itself.
Collectively, these findings indicate that the social relationships of an individual may influence cancer progression through intrinsic biophysiological mechanisms rather than through behavior and extrinsic lifestyle factors alone. Although the influence of the immune system on the brain and psychiatric conditions is clear [5], the possibility of reciprocal, psychosomatic influences is often regarded as dubious. Nevertheless, the idea that melancholy can promote the pathogenesis of cancer, which dates back to Galen in the second century AD, has persisted through the centuries [6]. As the authors note, studies in mice show that stimulation of a dopaminergic brain reward area (ventral tegmental area) can suppress tumor growth [7], and that stress can induce tumor metastasis via sympathetic nervous system activity [8]. The data of Naderi et al. lend additional support to the possible significance of psychosomatic influences on cancer. Specifically, they indicate a biochemical difference between the sera of bonded and bond-disrupted animals which is capable of altering gene expression in, and thus the growth of, tumors.
The crucial question is - how are extrinsic social stimuli transduced into intrinsic biological effects that influence health outcomes? Elucidating this process may reveal opportunities for pharmacological interventions to recapitulate the health benefits of social bonds. Given the results of Naderi et al., the process most likely involves three steps: (i) social information is encoded in a neural signal, (ii) the neural signal directly or indirectly induces the release of some humoral factor, (iii) the humoral factor binds to a receptor on cancer cells that induces changes in gene expression (Figure 1). Fortunately, the way in which the brain detects and processes social information is already well understood: it relies on specific neural networks and signaling molecules, most notably oxytocin, vasopressin (also known as antidiuretic hormone), dopamine, and corticotropin-releasing factor, which directly regulate pituitary gland activity [9]. In addition to mediating pair-bond formation and disruption in the brain, oxytocin, vasopressin, and stress hormones are also secreted by the pituitary into the peripheral circulation where they influence physiological processes throughout the body [10]. Triggered by social information processed in the brain, pituitary endocrine secretions may affect cancer cells directly, or they may work indirectly as secretagogues that affect an intermediary endocrine or immune tissue. Alternatively, social signals in the brain could influence peripheral endocrine or immune tissues via neural innervation. Irrespective of the origin, the data of Naderi et al. strongly suggest that some humoral factor is released into the bloodstream and subsequently alters transcription in cancer cells, most likely through receptor binding. We propose that subsequent studies should investigate the biological pathway leading from the perception of social stimuli to the regulation of transcription in cancer cells because each underlying mechanism is a potential entry point for identifying molecular targets for novel drug interventions.
Figure 1. Proposed model for the transduction of social stimuli into a physiological signal that influences cancer progression.
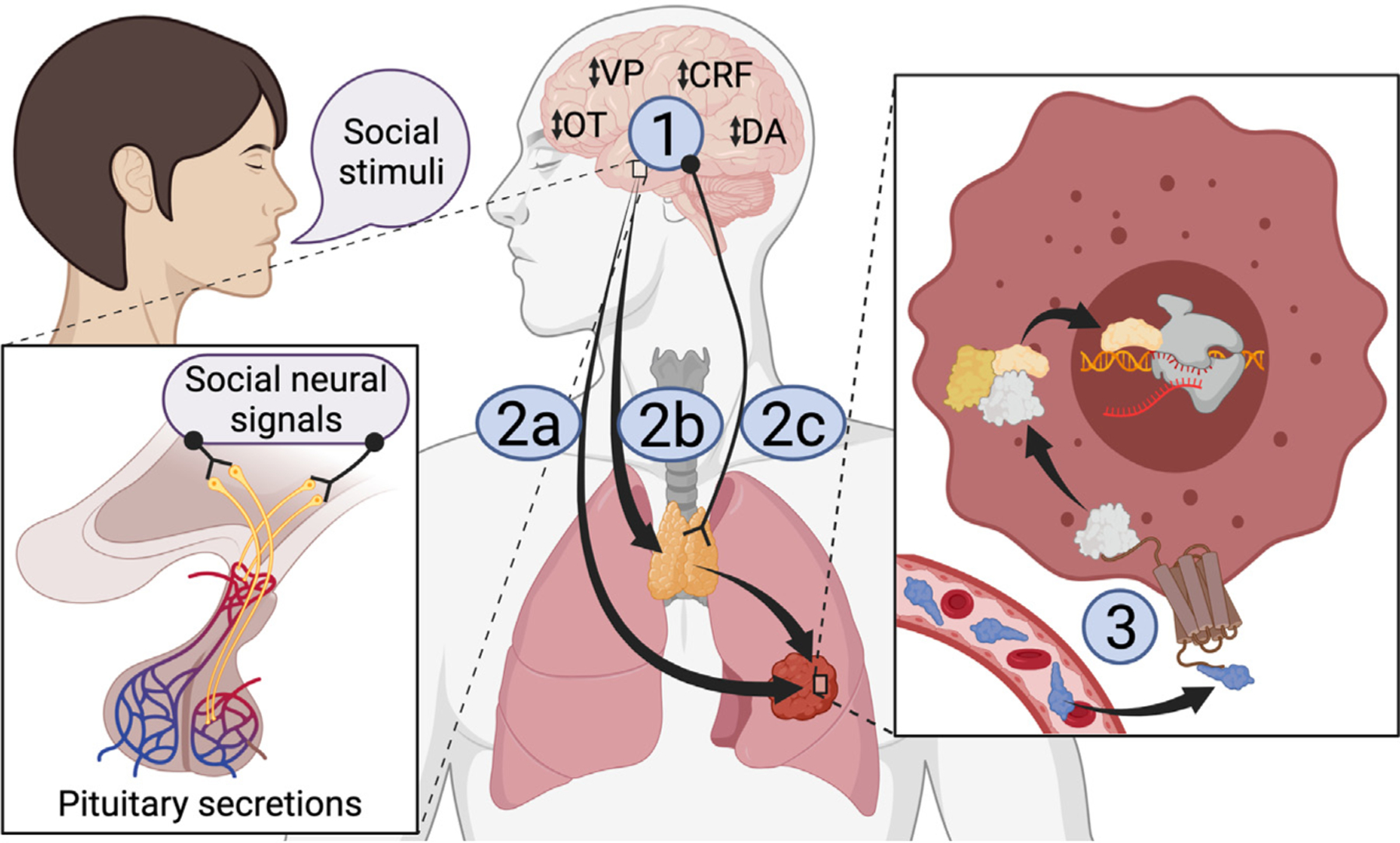
Social stimuli are processed by neural networks utilizing oxytocin (OT), vasopressin (VP), corticotropin-releasing factor (CRF), and dopamine (DA), which also regulate pituitary secretions into the circulation (1). These secretions may act as humoral factors that directly impact on the tumor (2a) or as secretagogues that trigger the release of a humoral factor from other tissues (2b). Alternatively, neural innervation of intermediary tissues could trigger the release of a humoral factor (2c). The humoral factor binds to receptors on tumor cells, thereby altering tumor physiology and progression (3).
The demonstration by Naderi et al. of biologically mediated effects of bond status on cancer progression has implications beyond its physiological mechanisms. As the authors mention, it illustrates the value of using animal models that better reflect human socioemotional dynamics than conventional mice and rats. It also highlights the importance of considering the social support and relationship health of patients as consequential lifestyle factors similar to diet, exercise, or substance use. More provocatively, the authors suggest ‘that cancers at widowhood represent a distinct pathological entity that may deserve targeted therapeutic strategies’. This idea is supported by evidence of bond-dependent alterations in tumor cell gene expression and the fact that cancers are often defined by, and their treatments tailored to, their gene expression profiles. Transcriptomic analyses of tumors from matched cohorts of married and recently widowed cancer patients could help to determine whether a similar phenomenon occurs in humans. The authors further suggest that ‘targeted therapeutic strategies… should take into consideration social interactions.’ Certainly, the therapeutic benefits of social interactions and bonds should be maximized. However, although powerful social bonds such as love may be easily prescribed, they are difficult to obtain. Therefore, the healing power of love may be more easily harnessed pharmacologically by targeting the biochemical processes that underlie the results of Naderi et al.
Acknowledgments
Preparation of this commentary was supported by National Institutes of Health grant P50MH100023. Figure created with BioRender.com.
Footnotes
Declaration of interests
The authors declare no conflicts of interest.
References
- 1.Naderi A et al. (2021) Persistent effects of pair bonding in lung cancer cell growth in monogamous Peromyscus californicus. Elite 10, e64711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jia H and Lubetkin EI (2020) Life expectancy and active life expectancy by marital status among older U.S. adults: results from the U.S. Medicare Health Outcome Survey (HOS). SSM Popul. Health 12, 100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen ZH et al. (2021) Assessment of modifiable factors for the association of marital status with cancer-specific survival. JAMA Netw. Open 4, e2111813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elwert F and Christakis NA (2008) The effect of widowhood on mortality by the causes of death of both spouses. Am. J. Public Health 98, 2092–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller AH et al. (2017) Therapeutic implications of brain-immune interactions: treatment in translation. Neuropsychopharmacology 42, 334–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karamanou M et al. (2016) Melancholy as a risk factor for cancer: a historical overview. J. BUON 21, 756–759 [PubMed] [Google Scholar]
- 7.Ben-Shaanan TL et al. (2016) Activation of the reward system boosts innate and adaptive immunity. Nat. Med 22, 940–944 [DOI] [PubMed] [Google Scholar]
- 8.Sloan EK et al. (2010) The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 70, 7042–7052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walum H and Young LJ (2018) The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci 19, 643–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pohl TT et al. (2019) Lost connections: oxytocin and the neural, physiological, and behavioral consequences of disrupted relationships. Int. J. Psychophysiol 136, 54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]


