Abstract
Background
Prognostic nutritional index (PNI), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII) are the most common factors to estimate nutritional and inflammatory status. The aim of this study is to systematically evaluate the prognostic significance of above nutritional and inflammatory indexes for overall survival (OS) and surgical complications in esophageal cancer patients.
Methods
Esophageal cancer patients who underwent esophagectomy were retrospectively collected. PNI, NLR, PLR, and SII were introduced to evaluate the baseline nutritional and inflammatory status.
Results
A total of 407 patients were included in the present study. Kaplan–Meier survival analysis revealed that PNI-low group, NLR-high group and PLR-high group, all showed a significantly shorter OS (34.38% vs 49.46%, P < 0.001; 36.13% vs 48.26%, P = 0.026 and 33.33% vs 48.52%, P = 0.001 respectively), while no significant difference was found in SII groups (42.33% vs 46.31%, P = 0.067). Multivariable analyses identified PNI (P = 0.002) was an independent prognostic factor for OS, but NLR (P = 0.672) and PLR (P = 0.186) were not. Postoperative complications occurred significantly more frequently in the low-PNI group (29.69% vs 13.26%, P < 0.001). However, no significant differences were found in the postoperative complication rates between different NLR (16.67% vs 22.69%, P = 0.124), PLR (18.03% vs 19.61%, P = 0.867) and SII (15.34% vs 20.49%, P = 0.326) groups. Multivariate logistic regression analysis showed only PNI (P = 0.008) was an independent prognostic factor for postoperative complications.
Conclusion
Preoperative low PNI was not only an independent prognostic factor for worse survival in esophageal cancer patients but also associated with high incidence of postoperative complications.
Keywords: esophageal cancer, prognostic nutritional index, prognosis, postoperative complications
Introduction
Esophageal cancer is one of the most prevalent malignant carcinomas with high mortality. Despite the development of multiple therapeutic regimens, the prognosis of esophageal cancer remains poor.1,2
In recent years, emerging studies focus on the relationship between tumor and malnutrition.3,4 Malignant patients tend to be malnutrition due to poor appetite, tumor consumption, treatment adverse effects, and so on.5,6 Malnutrition results in further reductions in tolerance to treatment modalities, and ultimately affects recurrence and survival.7,8 Besides, malnutrition can affect the quality of malignant patients’ lives.9 Thus, it is urgent to improve the nutrition status among malignant patients. Accumulating evidences suggest that the crosstalk between cancer cells and immune system and inflammation plays a crucial role in tumorigenesis and progression.10 With the application of immune therapy targeting programmed death 1 (PD1) and programmed death-ligand 1(PD-L1), tumor immunotherapy is extensively clarified as an important part of combined therapy of tumor in recent years.11 The correlation between systemic inflammation and the local immune response was recognized as one of the greatest milestones of cancer research. Cancer-related inflammation is determined by the levels of serum leukocytes, neutrophils, lymphocytes, platelets, and acute-phase proteins, such as C-reactive protein. The neutrophil–lymphocyte ratio (NLR) and platelet–lymphocyte ratio (PLR) are representative blood markers of the systemic inflammatory response. Recently, several investigators have demonstrated a close relationship between the systemic inflammatory response and tumor progression in various malignancies, including gastric cancer and colorectal cancer.12,13 So the relationships between NLR, PLR, SII and clinical outcomes in esophageal cancer are valuable to explore.
Prognostic nutritional index (PNI) is calculated by the serum albumin level and peripheral blood lymphocyte concentration. Hence, it integrates information on albumin and absolute lymphocyte count and simultaneously reflects the nutritional and inflammatory status of a patient. It was first used to indicate preoperative nutritional status and postoperative complications in patients with gastrointestinal cancers.14 Recent studies have revealed its predictive value in tumor progression and prognosis.15 A few studies reported the correlation between PNI and clinical outcomes in esophageal cancer, but with controversial results and there is room for improvement.16–18 Some studies suggested that PNI can predict survival,16,17 but they did not discuss the relationship between PNI and short-term outcomes.16 Some previous studies were hampered by small sample size.17 Other study indicated that PNI was not associated with prognosis.18 Few reports have described the relationship between PNI and the clinical outcomes of esophageal cancer systematically and comprehensively in large-scale patients.
The aim of this study is to systematically evaluate the association between PNI and OS and surgical complications, which, respectively, reflect the long-term and short-term clinical outcomes. Moreover, the correlation between NLR, PLR, SII and OS and postoperative complications are explored.
Patients and Methods
Patient Section
We collected patients who underwent curative resection at the Department of Thoracic Surgery, Qilu hospital of Shandong University from January 2010 to December 2014. The study participants were included according to the following criteria: (a) histologically confirmed esophageal cancer, (b) curative esophagectomy as the first therapeutic strategy, (c) patients with detailed clinicopathological and preoperative serum laboratory data.
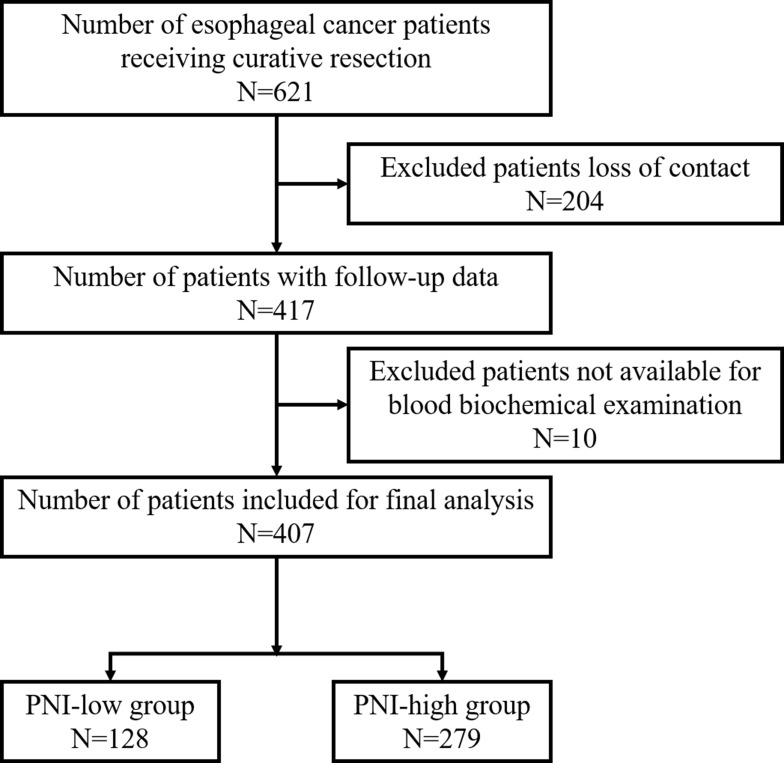
A total of 621 esophageal cancer patients underwent curative operation, including 204 patients who could not be contacted. The remaining 417 cases could be followed up, of which 10 cases without available data from blood biochemical examination. Consequently, 407 patients were retrospectively collected for the present study. Among them, 225 patients died during follow-up. (Figure 1)
Figure 1.
Flow chart showing patient recruitment.
Data Collection
The demographic and clinicopathological data were obtained from the patients’ medical records, including gender, age, smoking and alcohol history, past history, tumor location, T stage, N stage, M stage, TNM stage, and preoperative routine laboratory data. Past history included the history and clinical data of high blood pressure, diabetes, coronary heart disease. All of these data were recorded in the patients’ medical records during their first admission in the hospital, and we collected them from the patients’ medical records. All tumors were staged in accordance with the 7th edition American Joint Committee on Cancer TNM staging system.
The neutrophil, lymphocyte, and platelet counts were reviewed from the routine laboratory blood test, and the hemoglobin (Hb), total protein (TP), and albumin (Alb) level were also obtained using the blood test within 2 weeks before surgery. The prognostic nutritional index (PNI) is calculated as follows: PNI = serum albumin (g/L) +5*total lymphocyte count (109/L). The NLR, PLR, and SII were calculated as follows: NLR = neutrophil counts/lymphocyte counts; PLR = platelet counts/lymphocyte counts; SII = platelet counts*neutrophil counts/lymphocyte counts.
Surgical Treatment
Types of esophagectomy included thoracoscopic and transthoracic esophagectomy. Single thoracic incision, Ivor-Lewis esophagectomy, and McKeown esophagectomy (right thoracotomy, midline laparotomy and lateral cervical incisions) were common transthoracic esophagectomy.
Postoperative Complications
The postoperative complications were classified by the Clavien-Dindo classification.19 All of the parameters for the Clavien-Dindo classification rating could be found in the medical records during postoperative course, and we retrospectively collected the data from patients’ medical records.
Perioperative Parameters
We reviewed perioperative parameters including operation duration and blood loss. The American Society of Anesthesiologists (ASA) score was gained from the anesthetic risk assessment scale by an anesthesiologist according to the ASA classification.
Follow-Up
All patients were regularly followed up after discharge. January 2017 was the last censoring date for the evaluation of survival time. We assessed survival time from the date of surgery to date of the event or the last follow-up.
Statistical Analysis
Statistical analyses were performed using SPSS statistical package version 22 (SPSS, Inc., Chicago, IL, USA). The time‐dependent receiver operating characteristic (ROC) curve analysis was used to calculate the optimal cutoff values for PNI, NLR, PLR, and SII. Statistical analysis for comparing the parametric variables was performed using Chi-square test or Fisher’s exact test for categorical variables and Student’s t-test for continuous variables between the groups. The survival time distribution was evaluated by the Kaplan–Meier method and compared with the Log rank test. Univariate and multivariate analyses were calculated using the Cox proportional hazards regression model to determine prognostic factors for postoperative overall survival time (OS). Univariate and multivariate logistic regression analyses were employed to identify the potential risk factors associated with postoperative complications. In order to eliminate the risk of multicollinearity, we used stepwise multivariate analysis. A P value of less than 0.05 was considered to be statistically significant.
Results
Patients’ Characteristic
A total of 407 cases were included in the present study. The median age of the participants was 65. Among them, 324 (79.61%) were male and 83 (20.39%) were female. Table 1 shows the clinicopathological features of the recruited cases.
Table 1.
Patients’ Characteristic
| PNI-Low Group (≤48.33, n=128) | PNI-High Group (>48.33, n=279) | P value | NLR-Low Group (≤2.84, n=288) | NLR-High Group (>2.84, n=119) | P value | PLR-Low Group (≤170.61, n=305) | PLR-High Group (>170.61, n=102) | P value | SII-Low Group (≤433.25, n=163) | SII-High Group (>433.25, n=244) | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | 0.003 | 0.248 | 0.609 | 0.148 | ||||||||
| Female | 15 | 68 | 63 | 20 | 64 | 19 | 39 | 44 | ||||
| Male | 113 | 211 | 225 | 99 | 241 | 83 | 124 | 200 | ||||
| Age (mean±SD) | 62.73±7.84 | 59.96±7.63 | 0.001 | 60.50±7.51 | 61.63±8.41 | 0.183 | 60.82±7.59 | 60.85±8.40 | 0.973 | 61.31±7.40 | 60.51±8.04 | 0.314 |
| Alcohol use | 74 | 155 | 0.670 | 161 | 68 | 0.891 | 177 | 52 | 0.214 | 83 | 146 | 0.076 |
| Tobacco use | 71 | 160 | 0.722 | 163 | 68 | 0.919 | 179 | 52 | 0.174 | 85 | 146 | 0.125 |
| Past history | ||||||||||||
| High blood pressure | 27 | 54 | 0.683 | 54 | 27 | 0.345 | 60 | 21 | 0.841 | 28 | 53 | 0.261 |
| Diabetes | 8 | 11 | 0.306 | 14 | 5 | 0.774 | 14 | 5 | 0.897 | 6 | 13 | 0.440 |
| Coronary heart disease | 6 | 15 | 0.771 | 13 | 8 | 0.360 | 17 | 4 | 0.514 | 8 | 13 | 0.851 |
| Comorbidity | 30 | 54 | 0.345 | 59 | 25 | 0.906 | 62 | 22 | 0.789 | 36 | 48 | 0.555 |
| Cancer thrombus | 5 | 10 | 0.873 | 12 | 3 | 0.423 | 13 | 2 | 0.286 | 8 | 7 | 0.285 |
| Tumor location | 0.610 | 0.670 | 0.687 | 0.490 | ||||||||
| Cervical | 4 | 4 | 5 | 3 | 7 | 1 | 2 | 6 | ||||
| Upper | 6 | 13 | 15 | 4 | 15 | 4 | 5 | 14 | ||||
| Middle | 88 | 186 | 190 | 84 | 207 | 67 | 112 | 162 | ||||
| Lower | 30 | 76 | 78 | 28 | 76 | 30 | 44 | 62 | ||||
| T stage | 0.092 | 0.023 | 0.028 | 0.049 | ||||||||
| Tis | 1 | 10 | 11 | 0 | 10 | 1 | 6 | 5 | ||||
| T1 | 9 | 33 | 31 | 11 | 33 | 9 | 19 | 23 | ||||
| T2 | 34 | 77 | 87 | 24 | 93 | 18 | 55 | 56 | ||||
| T3 | 57 | 122 | 116 | 63 | 127 | 52 | 59 | 120 | ||||
| T4 | 27 | 37 | 43 | 21 | 42 | 22 | 24 | 40 | ||||
| N stage | 0.972 | 0.835 | 0.706 | 0.432 | ||||||||
| N0 | 70 | 155 | 161 | 64 | 173 | 52 | 97 | 128 | ||||
| N1 | 35 | 71 | 74 | 32 | 77 | 29 | 37 | 69 | ||||
| N2 | 15 | 36 | 34 | 17 | 38 | 13 | 21 | 30 | ||||
| N3 | 8 | 17 | 19 | 6 | 17 | 8 | 8 | 17 | ||||
| M stage | 0.139 | 0.119 | 0.083 | 0.413 | ||||||||
| M0 | 127 | 279 | 288 | 118 | 305 | 101 | 163 | 243 | ||||
| M1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | ||||
| TNM | 0.076 | 0.089 | 0.100 | 0.540 | ||||||||
| 0 | 1 | 9 | 10 | 0 | 9 | 1 | 5 | 5 | ||||
| I | 8 | 34 | 31 | 11 | 33 | 9 | 20 | 22 | ||||
| II | 58 | 126 | 133 | 51 | 144 | 40 | 76 | 108 | ||||
| III | 60 | 110 | 114 | 56 | 119 | 51 | 62 | 108 | ||||
| IV | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | ||||
| Hb (mean±SD) | 135.66±13.99 | 143.26±13.20 | <0.001 | 141.52±14.60 | 139.31±11.95 | 0.146 | 141.89±13.83 | 137.83±13.71 | 0.010 | 141.53±14.27 | 140.43±13.65 | 0.435 |
| TP (mean±SD) | 590.96±176.89 | 629.56 ±204.88 | 0.067 | 612.45±206.73 | 629.45 ±171.80 | 0.429 | 613.33±206.82 | 629.66 ±165.01 | 0.469 | 617.74±196.32 | 617.21 ±198.03 | 0.979 |
| Alb (mean±SD) | 38.53±2.93 | 43.63±3.05 | <0.001 | 42.42±3.89 | 41.06±3.51 | 0.001 | 42.29±3.75 | 41.23±3.99 | 0.016 | 42.32±3.43 | 41.83±4.08 | 0.209 |
| Lymphocyte (mean±SD) | 130.55±45.93 | 196.61±83.16 | <0.001 | 198.43±80.87 | 121.14±40.48 | <0.001 | 195.33±79.94 | 117.52±40.75 | <0.001 | 204.65±100.01 | 156.58±54.71 | <0.001 |
Abbreviations: Hb, hemoglobin; Alb, albumin; TP, total protein; PNI, prognostic nutritional index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
We determine the optimal cut‐off values of these biomarkers for predicting OS through an ROC analysis method. The optimal cut-off values were 48.33 for PNI, 2.84 for NLR, 170.61 for PLR, and 433.25 for SII, respectively, corresponding to the maximum Youden index.
According to the cut-off value, 128 (31.45%) patients were in PNI-low group and 279 (68.55%) were in PNI-high group. PNI was significantly associated with age (P = 0.001), sex (P = 0.003), hemoglobin (Hb) (P < 0.001), albumin (Alb) (P < 0.001), and lymphocyte counts (P < 0.001). There were no significant differences in alcohol use (P = 0.670), tobacco use (P = 0.722), history of high blood pressure (P = 0.683), diabetes (P = 0.306), coronary heart disease (P = 0.771), comorbidity (P = 0.345), cancer thrombus (P = 0.873), tumor location (P = 0.610), TNM stage (P = 0.076), or total protein (TP) (P = 0.067) between PNI-low and PNI-high groups.
NLR was significantly associated with T stage (P = 0.023), albumin (Alb) (P = 0.001), and lymphocyte counts (P < 0.001). PLR was significantly associated with T stage (P = 0.028), albumin (Alb) (P = 0.016), and lymphocyte counts (P < 0.001). SII was significantly associated with alcohol use (P = 0.076) and lymphocyte counts (P < 0.001).
Correlation of PNI, NLR, PLR, and SII with Survival and Prognosis Assessment
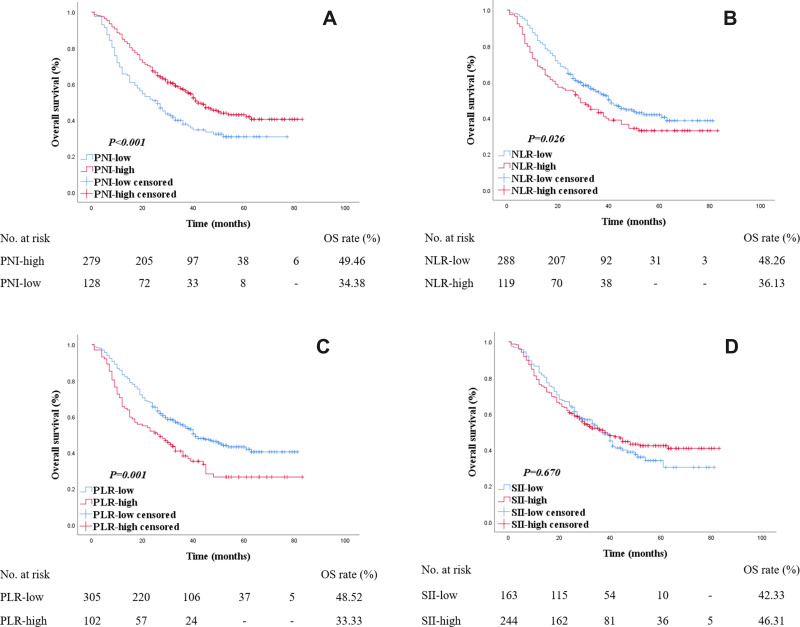
The median follow-up time was 29 months. The median follow-up time for survivor was 38 months. Overall, the 1-, 2-, 3-, and 5-year OS rates were 78.87%, 62.16%, 38.82%, and 11.30%, respectively. For the patients in PNI-low group, the 1-, 2-, 3-, and 5-year OS rates were 65.63%, 50.78%, 28.91%, and 6.25%. And the 1-, 2-, 3-, and 5-year OS rates were 84.95%, 67.38%, 43.37%, and 13.62% for the PNI-high group. The OS rate was high in PNI-high group (49.46%) than the PNI-low group (34.38%). Kaplan–Meier survival analysis revealed a correlation between PNI and overall survival times. The PNI-low group showed a significantly shorter OS (log rank P < 0.001) (Figure 2A).
Figure 2.
Kaplan–Meier survival curves for OS according to PNI, NLR, PLR and SII in esophageal cancer patients. The number at risk was shown below. (A) The PNI-low group showed a significantly shorter OS compared with the PNI-high group (34.38% VS 49.46%, log rank P < 0.001). (B) The NLR-high group showed a significantly shorter OS compared with the NLR-low group (36.13% VS 48.26%, log rank P = 0.026). (C) The PLR-high group showed a significantly shorter OS compared with the PLR-low group (33.33% VS 48.52%, log rank P = 0.001). (D) No significantly difference were found between OS in SII groups (46.31% VS 42.33%, log rank P = 0.067).
The OS rate was high in NLR-low group (48.26%) than the NLR-high group (36.13%). NLR-high group showed a significantly shorter OS (log rank P = 0.026) compared with the NLR-low group. PLR-high group showed a significantly shorter OS (33.33% VS 48.52%, log rank P = 0.001). No significant difference was found between OS in SII groups (42.33% VS 46.31%, log rank P = 0.067) (Figure 2B–D).
Univariate and Multivariate Survival Analyses
In the univariate Cox regression analyses, postoperative complications (P = 0.001), T stage (P < 0.001), N stage (P < 0.001), TNM stage (P < 0.001), Alb level (P = 0.002), PNI (P = 0.001), NLR (P = 0.028), and PLR (P = 0.001) were potentially prognostic factors for OS. Controlled variables included in the multivariable analysis were gender, age, T stage, N stage, TNM stage, Alb, PNI, NLR, PLR and postoperative complications. In our multivariable Cox model, postoperative complications (P = 0.028), T stage (P < 0.001), N stage (P = 0.014), TNM stage (P = 0.034), and PNI (P = 0.002) were independent prognostic factors in the study patients. Both NLR (P = 0.672) and PLR (P = 0.186) were not independent prognostic factors in the study patients (Table 2).
Table 2.
Univariate and Multivariate Analyses of Prognostic Factors for OS
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender | 0.337 | 0.285 | ||
| Female | 1 | |||
| Male | 1.180(0.841–1.656) | _ | ||
| Age | 0.268 | 0.067 | ||
| >65 | 1 | |||
| ≤65 | 0.857(0.652–1.126) | _ | ||
| Alcohol use | 0.895 | |||
| Absence | 1 | |||
| Presence | 1.018(0.782–1.325) | |||
| Tobacco use | 0.696 | |||
| Absence | 1 | |||
| Presence | 1.054(0.809–1.375) | |||
| High blood pressure | 0.256 | |||
| Absence | 1 | |||
| Presence | 1.201(0.875–1.649) | |||
| Diabetes | 0.574 | |||
| Absence | 1 | |||
| Presence | 0.834(0.442–1.572) | |||
| Coronary heart disease | 0.864 | |||
| Absence | 1 | |||
| Presence | 0.948(0.517–1.738) | |||
| T stage | <0.0001 | <0.0001 | ||
| T0+T1+T2 | 1 | 1 | ||
| T3+T4 | 2.706(2.007–3.648) | 1.989(1.400–2.826) | ||
| N stage | <0.0001 | |||
| N0+N1 | 1 | 1 | 0.014 | |
| N2+N3 | 2.293(1.699–3.094) | 1.557(1.094–2.217) | ||
| M stage | 0.861 | |||
| M0 | 1 | |||
| M1 | 1.191(0.167–8.502) | |||
| TNM | <0.0001 | 0.034 | ||
| 0+I+II | 1 | 1 | ||
| III+IV | 2.595(1.987–3.390) | 1.475(1.029–2.114) | ||
| Cancer thrombus | 0.165 | |||
| Absence | 1 | |||
| Presence | 1.569(0.831–2.961) | |||
| Hb | 0.997(0.988–1.006) | 0.539 | ||
| TP | 1.000(0.999–1.001) | 0.749 | ||
| Alb | 0.947(0.915–0.986) | 0.002 | _ | 0.684 |
| PNI | 0.001 | 0.002 | ||
| >48.33 | 1 | 1 | ||
| ≤48.33 | 1.604(1.224–2.103) | 1.529(1.163–2.011) | ||
| NLR | 0.028 | 0.672 | ||
| ≤2.84 | 1 | |||
| >2.84 | 1.363(1.033–1.797) | _ | ||
| PLR | 0.001 | 0.186 | ||
| ≤170.61 | 1 | |||
| >170.61 | 1.605(1.207–2.134) | _ | ||
| SII | 0.673 | |||
| ≤433.25 | 1 | |||
| >433.25 | 0.945(0.724–1.232) | |||
| Postoperative complications | 0.001 | 0.028 | ||
| 0+1+2 | 1 | 1 | ||
| 3+4 | 2.084(1.328–3.269) | 1.666(1.057–2.627) | ||
Abbreviations: Hb, hemoglobin; Alb, albumin; TP, total protein; PNI, prognostic nutritional index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
Correlation Between PNI NLR PLR SII and Postoperative Complications
The correlation of PNI with postoperative complications is presented in Table 3. In total 38 (29.69%) patients suffered at least one complication (Clavien-Dindo classification) in the PNI-low group and 37 (13.26%) in the PNI-high group. Postoperative complications occurred significantly more frequently in the low-PNI group (P < 0.001). The postoperative complication rates were 0.78% (n = 1) for classification I, 14.84% (n = 19) for classification II, 8.59% (n = 11) for classification III, 3.91% (n = 5) for classification IV and 1.56% (n = 2) for classification V in PNI-low group. The incidence of postoperative complications was 6.81% (n = 19) for classification I, 1.08% (n = 3) for classification II, 3.23% (n = 9) for classification III, 1.43% (n = 4) for classification IV, and 0.71% (n = 2) for classification V in PNI-high group. NLR (P = 0.124), PLR (P = 0.867) and SII (P = 0.326) were found not to be associated with postoperative complications.
Table 3.
Correlation of PNI NLR PLR SII with Postoperative Complications
| Clavien-Dindo Classification | PNI | NLR | PLR | SII | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (128) | High (278) | P | Low (288) | High (119) | P | Low (305) | High (102) | P | Low (163) | High (244) | P | |
| 1 | 1 | 19 | 17 | 3 | 16 | 4 | 7 | 13 | ||||
| 2 | 19 | 3 | 14 | 8 | 16 | 6 | 6 | 16 | ||||
| 3 | 11 | 9 | 10 | 10 | 13 | 7 | 5 | 15 | ||||
| 4 | 5 | 4 | 4 | 5 | 7 | 2 | 4 | 5 | ||||
| 5 | 2 | 2 | 3 | 1 | 3 | 1 | 3 | 1 | ||||
| Total (%) | 38 (29.69) | 37 (13.26) | <0.001 | 48 (16.67) | 27 (22.69) | 0.124 | 55 (8.03) | 20 (19.61) | 0.867 | 25 (15.34) | 50 (20.49) | 0.326 |
Note: Clavien-Dindo classification was used to evaluate the postoperative complications.
Abbreviations: PNI, prognostic nutritional index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
Univariate and Multivariate Regression Analysis on the Occurrence of Complications
The outcomes of univariate and multivariate logistic regression are shown in Table 4. In the univariate regression analysis, type of surgery (P = 0.018), blood loss (P = 0.008), age (P = 0.012), Alb (P = 0.002), TP (P < 0.001), and PNI (P < 0.001) were identified as significant risk factors for postoperative complications. In the multivariate model, age (P = 0.025), blood loss (P = 0.009), TP (P = 0.038), and PNI (P = 0.008) were independent risk factors for postoperative complications.
Table 4.
Univariate and Multivariate Logistic Analyses on the Occurrence of Complications
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender | 0.050 | 0.957 | ||
| Female | 1 | |||
| Male | 2.103(1.001–4.421) | _ | ||
| Age | 0.012 | 0.025 | ||
| >65 | 1 | 1 | ||
| ≤65 | 0.520(0.312–0.867) | 0.426(0.202–0.898) | ||
| Operation duration | 0.616 | |||
| >3 hours | 1 | |||
| ≤3 hours | 0.880(0.533–1.452) | |||
| Type of surgery | 0.018 | 0.688 | ||
| Transthoracic | 1 | |||
| Thoracoscopic | 2.310(1.157–4.613) | _ | ||
| Blood loss | 0.008 | 0.009 | ||
| <100 | 1 | 1 | ||
| 100–200 | 1.914(0.931–3.934) | 2.050(0.760–5.531) | ||
| >200 | 6.739(1.663–24.474) | 9.209(2.051–41.342) | ||
| ASA score | 0.861 | |||
| 1+2 | 1 | |||
| 3+4 | 1.046(0.630–1.737) | |||
| Alb | 0.900(0.841–0.962) | 0.002 | _ | 0.627 |
| TP | 0.997(0.996–0.998) | <0.0001 | 0.998(0.997–1.000) | 0.038 |
| PNI | <0.0001 | 0.008 | ||
| >48.33 | 1 | 1 | ||
| ≤48.33 | 2.762(1.653–4.614) | 2.784(1.312–5.909) | ||
| NLR | 0.156 | 0.101 | ||
| ≤2.84 | 1 | |||
| >2.84 | 1.467(0.864–2.491) | _ | ||
| PLR | 0.723 | |||
| ≤170.61 | 1 | |||
| >170.61 | 1.109(0.627–1.959) | |||
| SII | 0.190 | 0.883 | ||
| ≤433.25 | 1 | |||
| >433.25 | 1.423(0.840–2.411) | _ | ||
Abbreviations: Alb, albumin; TP, total protein; PNI, prognostic nutritional index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index; ASA, American Society of Anesthesiologists.
Discussion
In the present study, we detected the relationship between PNI, NLR, PLR, SII and clinical outcomes of esophageal cancer who received curative operation. The optimal cut-off value was 48.33 for PNI corresponding to the maximum Youden index. According to the cut-off value, patients were divided into two groups separately for further analysis. The PNI cut-off value detected 128 patients (31.45%) with a risk of malnutrition. Patients in the cohort with PNI ≤48.33 need diet counseling and support to help maintain nutritional status, including oral nutritional supplements, enteral nutrition (EN), and/or parenteral nutrition.
We found that only PNI was significantly associated with both the long-term and short-term clinical outcomes. The PNI was identified as an independent prognostic factor for OS of esophageal cancer, which suggested that PNI could be used as a marker to identify patients who were likely to experience an unfavorable clinical outcome. Therefore, it is beneficial to carry out individualized treatment. Besides, the present study also concluded that low PNI was associated with the high incidence of postoperative complications.
PNI was initially calculated to estimate preoperative nutritional status and surgical complications in patients with gastrointestinal cancers, which reflected the nutritional and immunological status now.14 Malignant patients often develop malnutrition during cancer treatment. It is reported that malnutrition occurs in up to 80% of cancer patients at some point during cancer care.20 Malnutrition results in increased morbidity and mortality, increased risk of treatment delays and complications, decreased function and quality of life.21
Malnutrition also has been found associated with survivorship in various malignancies, including gastric cancer,15 hepatocellular cancer,22 and prostate cancer,23 and so on. Recently, the correlation between malnutrition and esophageal cancer has been found.16,17,24
Our study compared the prognostic values of various nutritional and inflammatory indicators and found that PNI was an independent predictive factor for OS of esophageal cancer, which was in consistent with many previous literatures.16,24 Zhang et al24 reported that the preoperative high SII and low PNI were powerful indicators of aggressive biology and poor prognosis for patients with esophageal squamous cell carcinoma (ESCC). Another study including 337 curatively resected esophageal cancers found the PNI-low cases showed significantly worse overall survival. Meanwhile, they confirmed that PNI affected their prognosis through local tumor immunity, which depended on their systemic nutritional and immunological status.16 However, these studies were aimed at long-term results. There was no analysis of short-term postoperative complications in the above studies. Postoperative complications, which can affect postoperative outcome, are a critical short-term outcome index. Our research has made up for the above deficiencies and made a comprehensive exposition from these two aspects. Moreover, there were several studies indicated that PNI was not an independent factor for prognosis in esophageal cancer.18,25 Sun et al18 conducted a retrospective study including 502 esophageal cancer patients and found low PNI was associated with poor OS, but it did not display reliably as an independent predictor. Pan et al25 estimated the relationship between various varied malnutrition criteria and survival in cell ESCC patients, and concluded that low BMI but not PNI at diagnosis was an independent prognostic factor for worse survival of ESCC patients.
As the PNI represents nutrition and immune status, but Alb and Hb did not show significant prognostic effect. PNI is not only a nutritional index but also an immune index. It is affected by ALB and lymphocytes and reflects the comprehensive level of different factors together. Combining the two indicators can reveal a patient’s inflammatory status and nutritional status, which can effectively predict prognosis. ALB is significant in univariate analysis in our study, but not in multivariate analysis, suggesting that it is related to prognosis, but not an independent predictor. Although Hb also reflects the nutritional status, its main function is related to the body’s ability to transport oxygen and energy. It reflects the nutritional supply capacity, which cannot reflect the nutritional status exactly. Therefore, it reflects the predictive value different from PNI.
The correlation between PNI and postoperative complications was reported before. Hideo Matsumoto et al17 calculated PNIs before surgery, discharge, and 1, 2, and 6 months after discharge, and found that the mean PNI for patients with complications of more than Grade 2 by the C–D classification was 37.4, which was significantly lower than that for Grades 0 or 1. In the present study, we found that the incidence of grade 2, 3, and 4 were all significantly high in PNI-low group. After adjusted by many other clinicopathological factors, PNI was clarified as an independent predictor for complications. Thus, preoperative nutritional assessment and intervention are essential to reduce the postoperative complications. Reducing postoperative complications can thus potentially shorten the length of hospital stay, reduce healthcare costs, and improve patients’ quality of life. Therefore, elucidating the factors that affect postoperative complications is crucial for surgical patients.26 Patients with low PNI require dietician intervention, in conjunction with a nurse or physician in clinical work. Once a cancer-associated nutritional issue has been identified and triaged, nutrition counseling by a healthcare professional is regarded as the first line of nutrition therapy.27 The emphasis of nutrition support is needed following treatment and throughout survivorship.
The underlying mechanism of PNI and esophageal cancer clinical outcomes remains poorly understood. PNI is composed of both serum albumin concentration and lymphocyte count, which is used to assess immune‐nutritional status. As mentioned before, local tumor immunity may be one potential mechanism.16 Malnutrition has been clarified to correlate with the immune‐suppressed condition.28 Lymphocytes have been implicated in immunomodulation in the tumor microenvironment, which might establish the human immune response to tumor cells.29 Thus, low lymphocyte counts are associated with an immunosuppressed condition, which provides a favorable microenvironment for tumor proliferation and migration. This immunosuppressed condition in low‐PNI patients provides a favorable microenvironment for tumor progression and cause the unfavorable outcome.30 On the other hand, patients with low PNI may not be treated promptly due to a period of nutritional support treatment, and treatment delay also leads to poor outcomes. Patients with a superior nutritional status were likely to complete the whole cycles of chemotherapy and gain better clinical outcomes.8
Many nutritional and inflammatory measures have been reported to be associated with esophageal cancer prognosis, such as the modified GPS (mGPS), controlling nutritional status (CONUT) score, and Geriatric Nutritional Risk Index (GNRI). mGPS was calculated on the basis of C-reactive protein (CRP) levels plus Alb levels, which is considered as an independent marker of poor prognosis for patients with SCC and superior to NLR, PLR and PNI.31 CONUT score was reported as an independent prognostic factor for OS and relapse-free survival among thoracic esophageal squamous cell carcinoma patients,32 and was superior to PLR, NLR, and GPS. It is also identified as an independent prognostic factor in patients with stage II–III gastric cancer receiving curative resection and adjuvant chemotherapy.33 CONUT scores may be more useful in stratifying patients. Unlike CONUT score and mGPS, there were only two groups by PNI.34
GNRI was revealed to be an ideal predictor of baseline nutritional status in elderly. Nutritional status at diagnosis based on the evaluation of the GNRI criteria was associated with better clinical response and increased OS and PFS in ESCC patients aged over 70 years. Routine use of the GNRI criteria may help in the risk stratification of elderly patients undergoing combined treatment.35 However, it remains controversial as to which one of those measures is the most useful. Since these measures cannot be used to fully assess the nutritional status of patients, appropriate nutritional assessments should be performed in further study.
This study has several limitations. This is a retrospective study in a single institute, and selection bias may exist. Moreover, we just discuss the relationship and the underlying mechanism still remains unclear. Further well-designed prospective trials in multi-institute should be considered to verify our findings for esophageal cancer patients.
In summary, PNI is not only a valuable factor for predicting the long-term outcome in esophageal cancer but also can predict the short-term outcome. Preoperative nutritional support should be considered for low PNI patients to improve the outcome for esophageal cancer patients.
Funding Statement
The present work was supported by the National Natural Science Foundation of China (No.81700469).
Data Sharing Statement
All data are available in the manuscript or upon request to the author Wang Nana, Email: wangna03@163.com.
Ethical Statement
The research has been carried out in accordance with the World Medical Association Declaration of Helsinki. The study was approved by the Medical Ethics Committee of Shandong University Qilu hospital. Informed consent was obtained from all individual participants included in the study.
Consent for Publication
The authors agree for publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Wei W, Zeng H, Zheng R, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 2020;21:e342–e349. doi: 10.1016/S1470-2045(20)30073-5 [DOI] [PubMed] [Google Scholar]
- 3.Wiseman MJ. Nutrition and cancer: prevention and survival. Br J Nutr. 2019;122:481–487. doi: 10.1017/S0007114518002222 [DOI] [PubMed] [Google Scholar]
- 4.Tobberup R, Thoresen L, Falkmer UG, et al. Effects of current parenteral nutrition treatment on health-related quality of life, physical function, nutritional status, survival and adverse events exclusively in patients with advanced cancer: a systematic literature review. Crit Rev Oncol Hematol. 2019;139:96–107. doi: 10.1016/j.critrevonc.2019.04.014 [DOI] [PubMed] [Google Scholar]
- 5.Bouleuc C, Anota A, Cornet C, et al. Impact on health-related quality of life of parenteral nutrition for patients with advanced cancer cachexia: results from a randomized controlled trial. Oncologist. 2020;25:e843–e851. doi: 10.1634/theoncologist.2019-0856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uster A, Ruehlin M, Mey S, et al. Effects of nutrition and physical exercise intervention in palliative cancer patients: a randomized controlled trial. Clin Nutr. 2018;37:1202–1209. doi: 10.1016/j.clnu.2017.05.027 [DOI] [PubMed] [Google Scholar]
- 7.Muller-Richter U, Betz C, Hartmann S, et al. Nutrition management for head and neck cancer patients improves clinical outcome and survival. Nutr Res. 2017;48:1–8. doi: 10.1016/j.nutres.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Yu B, Ye Y, et al. Predictive value of nutritional risk screening 2002 and prognostic nutritional index for esophageal cancer patients undergoing definitive radiochemotherapy. Nutr Cancer. 2018;70:879–885. doi: 10.1080/01635581.2018.1470656 [DOI] [PubMed] [Google Scholar]
- 9.Furness K, Huggins CE, Hanna L, et al. A process and mechanism of action evaluation of the effect of early and intensive nutrition care, delivered via telephone or mobile application, on quality of life in people with upper gastrointestinal cancer: a study protocol. BMC Cancer. 2018;18:1181. doi: 10.1186/s12885-018-5089-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bader JE, Voss K, Rathmell JC. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell. 2020;78:1019–1033. doi: 10.1016/j.molcel.2020.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23:6261–6272. doi: 10.3748/wjg.v23.i34.6261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirahara T, Arigami T, Yanagita S, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. 2019;19:672. doi: 10.1186/s12885-019-5903-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85:1001–1005. Japanese. [PubMed] [Google Scholar]
- 15.Oh SE, Choi MG, Seo JM, et al. Prognostic significance of perioperative nutritional parameters in patients with gastric cancer. Clin Nutr. 2019;38:870–876. doi: 10.1016/j.clnu.2018.02.015 [DOI] [PubMed] [Google Scholar]
- 16.Okadome K, Baba Y, Yagi T, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271:693–700. doi: 10.1097/SLA.0000000000002985 [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto H, Okamoto Y, Kawai A, et al. Prognosis prediction for postoperative esophageal cancer patients using onodera’s prognostic nutritional index. Nutr Cancer. 2017;69:849–854. doi: 10.1080/01635581.2017.1339093 [DOI] [PubMed] [Google Scholar]
- 18.Sun P, Zhang F, Chen C, et al. Comparison of the prognostic values of various nutritional parameters in patients with esophageal squamous cell carcinoma from Southern China. J Thorac Dis. 2013;5:484–491. doi: 10.3978/j.issn.2072-1439.2013.08.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trujillo EB, Dixon SW, Claghorn K, et al. Closing the gap in nutrition care at outpatient cancer centers: ongoing initiatives of the oncology nutrition dietetic practice group. J Acad Nutr Diet. 2018;118:749–760. doi: 10.1016/j.jand.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 21.Jensen GL, Mirtallo J, Compher C, et al. Adult starvation and disease-related malnutrition: a proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. JPEN J Parenter Enteral Nutr. 2010;34:156–159. doi: 10.1177/0148607110361910 [DOI] [PubMed] [Google Scholar]
- 22.Man Z, Pang Q, Zhou L, et al. Prognostic significance of preoperative prognostic nutritional index in hepatocellular carcinoma: a meta-analysis. HPB (Oxford). 2018;20:888–895. doi: 10.1016/j.hpb.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 23.Fan L, Wang X, Chi C, et al. Prognostic nutritional index predicts initial response to treatment and prognosis in metastatic castration-resistant prostate cancer patients treated with Abiraterone. Prostate. 2017;77:1233–1241. doi: 10.1002/pros.23381 [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Shang X, Ren P, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. 2019;234:1794–1802. doi: 10.1002/jcp.27052 [DOI] [PubMed] [Google Scholar]
- 25.Pan YP, Hsu TY, Lin JY, et al. Prognostic significance of low body mass index and betel-quid use in the 5-year survival rates of esophageal squamous cell carcinoma patients. Nutr Cancer. 2018;70:1315–1321. doi: 10.1080/01635581.2019.1588983 [DOI] [PubMed] [Google Scholar]
- 26.Huang TH, Hsieh CC, Kuo LM, et al. Malnutrition associated with an increased risk of postoperative complications following hepatectomy in patients with hepatocellular carcinoma. HPB (Oxford). 2019;21:1150–1155. doi: 10.1016/j.hpb.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 27.Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48. doi: 10.1016/j.clnu.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 28.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Giorgi U, Mego M, Scarpi E, et al. Relationship between lymphocytopenia and circulating tumor cells as prognostic factors for overall survival in metastatic breast cancer. Clin Breast Cancer. 2012;12:264–269. doi: 10.1016/j.clbc.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 30.Shimizu K, Iyoda T, Okada M, et al. Immune suppression and reversal of the suppressive tumor microenvironment. Int Immunol. 2018;30:445–454. doi: 10.1093/intimm/dxy042 [DOI] [PubMed] [Google Scholar]
- 31.Fan H, Shao ZY, Xiao YY, et al. Comparison of the Glasgow Prognostic Score (GPS) and the modified Glasgow Prognostic Score (mGPS) in evaluating the prognosis of patients with operable and inoperable non-small cell lung cancer. J Cancer Res Clin Oncol. 2016;142:1285–1297. doi: 10.1007/s00432-015-2113-0 [DOI] [PubMed] [Google Scholar]
- 32.Toyokawa T, Kubo N, Tamura T, et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer. 2016;16:722. doi: 10.1186/s12885-016-2696-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Zhang D, Lin E, et al. Preoperative controlling nutritional status (CONUT) score as a predictor of long-term outcome after curative resection followed by adjuvant chemotherapy in stage II-III gastric Cancer. BMC Cancer. 2018;18:699. doi: 10.1186/s12885-018-4616-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takamizawa Y, Shida D, Boku N, et al. Nutritional and inflammatory measures predict survival of patients with stage IV colorectal cancer. BMC Cancer. 2020;20:1092. doi: 10.1186/s12885-020-07560-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Wang L, Fang M, et al. Prognostic value of the geriatric nutritional risk index in patients exceeding 70 years old with esophageal squamous cell carcinoma. Nutr Cancer. 2020;72:620–626. doi: 10.1080/01635581.2019.1650189 [DOI] [PubMed] [Google Scholar]