Abstract
Various categories of coronavirus disease 19 (COVID-19) patients have exhibited major mortality rate differences and symptoms. Some papers have recently explained these differences in mortality rates and symptoms as a consequence of this virus infection acting in synergy with one or more latent pathogen infections in some patients. A latent pathogen infection likely to be involved in millions of these patients is the protozoan parasite Toxoplasma gondii, which infects approximately one third of the global human population. However, other papers have concluded that latent protozoan parasite infections can reduce the severity of viral infections. The aims and purposes of this paper include providing explanations for the contradictions between these studies and introducing a significant new category of T-cell exhaustion. Latent pathogens can have different genetic strains with great differences in their effects on a second pathogen infection. Furthermore, depending on the timing and effectiveness of drug treatments, pathogen infections that become latent may or may not later induce immune cell dysfunctions, including T-cell exhaustion. Concurrent multiple pathogen T-cell exhaustion is herein called "polyspecific T-cell exhaustion."
Keywords: Viral infections, Latent infections, Protozoa, Protozoan infections, Coronavirus, SARS-CoV-2 mortality
1. Introduction
Various categories of coronavirus disease 19 (COVID-19) patients have exhibited various mortality rate differences and symptoms [[1], [2], [3], [4], [5]]. A percentage of COVID-19 infected individuals in some categories have been virtually asymptomatic, or have exhibited several unusual symptoms, or have suffered fatal outcomes [[1], [2], [3], [4], [5]].
It has been proposed that these mortality rate differences and symptoms could result from a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus infection acting in synergy with one or more latent pathogen infections, through mutually beneficial induced immune cell dysfunctions, including T-cell exhaustion [6]. Two papers have also suggested COVID-19 can reactivate latent pathogens, such as Toxoplasma gondii [6,7]. However, other papers claim that latent pathogen infections, including protozoan parasite infections such as T. gondii, can exert antiviral effects by increasing the production of type I or type II interferons and can reduce the severity of COVID-19 and other viral infections [[8], [9], [10]].
Due to the potential impact of widespread latent pathogens on COVID-19 mortality world-wide, these apparent contradictions need to be resolved. The aims and purposes of this paper include providing explanations for these contradictions and providing an introduction to a new category of T-cell exhaustion.
The new category of T-cell exhaustion will be discussed in detail later and it is herein called "polyspecific T-cell exhaustion." Polyspecific T-cell exhaustion has significant implications and consequences for both primary and secondary pathogen infections, and these implications and consequences are not limited only to virulent viral pathogen infections.
2. Discussion
This discussion will focus on latent pathogen infections in several COVID-19 patients, including the protozoan parasite Toxoplasma gondii, which infects approximately one third of the global human population [6]. T. gondii protozoan parasites typically infect people after food or water ingestion [11]. T. gondii and other protozoan parasites can create latent infections of the muscles, brain and central nervous system (CNS), through intracellular cysts in hosts and cause immune system dysfunctions [12]. The fundamental observations below regarding major genetic variations in virulence and early drug treatment effects could also be relevant to several other bacterial, viral and fungal pathogens, and protozoan parasites including Cryptosporidium parvum, C. hominis, Blastocyst spp., Giardia lamblia (i.e., Giardia duodenalis), and Entamoeba histolytica, etc. [8].
It is known that long duration latent T. gondii infections can induce cluster of differentiation 8 (CD8) T-cell exhaustion, which can eventually result in T. gondii reactivations and tissue inflammations [12]. T-cell exhaustion, including CD8 T-cell exhaustion, and a weaker antiviral response have also been observed in COVID-19 patients exhibiting more severe infections and outcomes [4,5]. T-cell exhaustion will be discussed in more detail later.
2.1. Multiple genetic types of T. gondii exist globally with major differences
Globally, there are over ten different genetic types (haplogroups) of T. gondii, with major differences in their characteristics, and a factor of at least 100,000X in their differences in virulence and effects on a host's immune system [13]. For example, North America and Europe have the Type I, Type II, and Type III haplogroups of T. gondii, but the Type II T. gondii haplogroup predominates in North America and Europe. Furthermore, Type II and Type III T. gondii are considerably less virulent than the extremely virulent Type I T. gondii haplogroup that predominates in South America [13]. The other haplogroups in various countries could each have a virulence similar to either Type II or Type I, or have a milder or more severe virulence. Therefore, any immunological conclusions drawn from studies involving one T. gondii haplogroup may not apply to another T. gondii haplogroup. In particular, if a study includes individuals infected with a very mild genetic type of T. gondii haplogroup, there may be very little if any induced T cell exhaustion, and this could enable these infected individuals to have a strengthened immune response to a second pathogen infection [6,8]. Thus, it is essential that the T. gondii haplogroups being studied in a specific country be identified because of their greatly diverse consequences. Furthermore, in addition to the proven existence of over ten T. gondii haplogroups, there is a second critically important variable, the drug treatment history of each patient infected with T. gondii.
2.2. Timely and effective drug treatment for a first pathogen can prevent T-cell exhaustion to enable T-cell functionality against a second pathogen infection
A variety of drug treatments for T. gondii infections are available, using pyrimethamine and sulfadiazine, or using clindamycin or atovaquone as a substitute for sulfadiazine, or using sulfamethoxazole and trimethoprim or equivalents [13,14]. It has also been shown in murine experiments that using drug treatments, such as sulfamethoxazole and trimethoprim, in the early stages of active T. gondii infections can minimize T-cell exhaustion and other dysfunctions, in their later stage latent infections [14]. CD8 T-cells release interferon-γ and cytotoxic proteins perforin and serine protease granzymes, including granzyme B, to control toxoplasmosis and prevent reactivation of T. gondii cysts, but such releases are also utilized against viral infections of cells [13,14]. Thus, if T-cell exhaustion and other immune dysfunctions can be prevented or minimized by timely drug treatment of active protozoan parasite infections, then effective CD8 T-cell responses to secondary pathogen infections, including the virus SARS-CoV-2, are still possible [14]. In summary, prompt drug treatments of active protozoan parasite infections can avoid immune dysfunctions and later moderate the symptoms of COVID-19 patients.
2.3. Latent pathogen infections without T-cell exhaustion in some cases can strengthen the response to later pathogen infections
T-cell exhaustion is induced by the combination of chronic pathogen infections that cause a continuous and sustained inflammation and antigen stimulation of T-cells with significant antigen titers [6]. If all these requirements are not met, a relatively recent pathogen infection, or a latent pathogen infection that results in little inflammation or a low antigen titer, can avoid causing T-cell exhaustion [6]. In addition, if some variation in the genetic type of the pathogen or a timely and effective drug treatment can prevent T-cell exhaustion that reduces T-cell functions, some latent pathogen infections, such as protozoan parasite infections, can stimulate CD4 T-cells and CD8 T-cells to produce sufficient antiviral interferon-γ to effectively respond to a later viral infection [8,14].
One study of 375 COVID-19 Egyptian patients reported their outcomes where a majority of the patients had various types of protozoan parasite infections, such as T. gondii [8]. But the study did not report if any of these patients had received prompt drug treatments during their active protozoan parasite infections which would have prevented T-cell exhaustion [8]. In other words, if these very fortunate patients were able to receive early and comprehensive hospital treatment for COVID-19, were they also fortunate enough to have previously received early drug treatments for their protozoan parasite infections? This study reported that in most patients protozoan parasite stimulation of T-cells resulted in higher secretions of interferon-γ, and higher levels of interferon-γ were associated with less severe COVID-19 symptoms [8]. The open questions include: (1) were these recent protozoan parasite infections or did these patients previously receive anti-protozoan parasite drug treatments during their active infections which would have prevented T-cell exhaustion, and thus improve their later T-cell responses to COVID-19, and (2) which specific genetic types of T. gondii and the other protozoan parasites infected these patients? [13,14].
One method to detect latent pathogen infections, including T. gondii, is to measure the immunoglobulin G (IgG) antibodies created by past pathogen infections in each patient's blood [6,8]. However, the detection of such IgG antibodies for a latent pathogen will not necessarily indicate whether or not the patient received timely drug treatments for the pathogen infection that would prevent or minimize later induced T-cell exhaustion [6].
Therefore, various studies can reach different conclusions depending on a pathogen infection's time duration or genetic type, and each individual patient's history of drug treatment for the pathogen. In consequence, a pathogen's induced immune cell dysfunctions may or may not outweigh it's potential antiviral effects, such as increased releases of type I or type II interferons [8]; or in the case of active T. gondii infections, the secretion of immunostimulatory proteins, such as dense granule protein-7, that can induce higher releases of type I interferons and pro-inflammatory cytokines [10].
It is especially noteworthy that active T. gondii infections induce increased releases of type I interferons and pro-inflammatory cytokines [10]. This is interesting, because sustained high levels of type I interferons and sustained inflammation are known factors for inducing cluster of differentiation 4 (CD4) and CD8 T-cell exhaustion [15,16]. In summary, the protozoan parasite T. gondii has some particularly effective characteristics for inducing extensive T-cell exhaustion in some cases.
2.4. T-cell exhaustion affects both CD4 T-cells and CD8 T-cells
T-cell exhaustion has far-reaching consequences for pathogen suppression by both CD4 T-cells and CD8 T-cells [15,16]. T-cell exhaustion can be reversed and/or prevented by blockades of inhibitory T-cell receptors, such as a blockade of the interleukin-10 receptor, a blockade of the programmed cell death protein 1 (PD-1) receptor on CD8 T-cells, or a blockade of type I interferon (interferon α and β) signaling to CD4 T-cells [15,16]. While the type I interferons α and β are critical antiviral cytokines in the early stages of infection for the activation and differentiation of CD8 T-cells, their continued presence can induce CD4 T-cell exhaustion [15]. CD4 T-cell exhaustion also increases CD8 T-cell exhaustion, because the absence of an interleukin-21 signal that is normally secreted by CD4 T-cells will directly increase CD8 T-cell exhaustion [15,16]. And the loss of CD4 T-cell functions, either from CD4 T-cell exhaustion, or caused by destructive attacks on CD4 T-cells by some pathogens, such as the human immunodeficiency virus (HIV), will reduce the levels of interferon-γ, which is an essential cytokine necessary to control both acute and chronic T. gondii infections [13].
2.5. Other pathogen infections can also cause T-cell exhaustion
T-cell exhaustion is caused by long duration antigen exposures and persistent inflammation, and these conditions can result from several long duration latent pathogen infections [15,17,18]. And the severity of T-cell exhaustion is determined by both the abundance (titer) of the antigens and by the time duration of the antigen stimulation [15,17,18]. Thus, T-cell exhaustion can also be induced by latent infections of various protozoan, fungal, viral, or bacterial pathogens, including the hepatitis B virus, hepatitis C virus, cytomegalovirus, etc. [[19], [20], [21], [22], [23], [24], [25]] In fact, almost all protozoan, fungal, viral and bacterial pathogen infections will induce various combinations of inflammatory cytokines [26].
2.6. Antigen-specific T-cell exhaustion can cause other T-cells' exhaustion
A question may be raised concerning how T-cell exhaustion in antigen-specific T-cells can inhibit or exhaust T-cells specific for other antigens. There are multiple direct and indirect pathways for concurrent pathogens to induce T-cell exhaustion or T-cell inhibition [15,[27], [28], [29], [30]]. T-cell exhaustion can be induced through inhibitory receptor expressions and co-stimulatory receptor desensitization on T-cells [15]. As a specific example, chronic T. gondii infections cause a higher T-cell expression of inhibitory PD-1 receptors and cause a higher programmed cell death protein ligand 1 (PD-L1) expression by T. gondii infected cells [14]. This could easily facilitate cross-activation of the inhibitory T-cell receptor PD-1 on T-cells by cells concurrently infected by both T. gondii and the SARS-CoV-2 virus, and this cross-activation of the inhibitory PD-1 receptors on T-cells can thereby result in CD4 and CD8 T-cell exhaustion for both pathogens.
Since T-cell receptors were orginally believed to be receptive to only one peptide antigen, the cross-activation of the inhibitory PD-1 receptors on a T-cell with antigenic activation of the T-cell receptor by antigens of two different pathogens may be considered unlikely [31]. However, the polyspecificity of a T-cell's receptors, i.e., the ability to recognize several distinct major histocompatibility complex (MHC) bound peptides, and a T-cell's cross-reactivity through its T-cell receptors, defined as the ability to be activated by multiple peptides different from the peptide that initially defined the T-cell, have been extensively reported and discussed [[31], [32], [33], [34]]. In summary, a T-cell's antigen receptors can potentially be activated by multiple peptide antigens of distinctly different pathogens, including protozoan parasites and viruses [[31], [32], [33], [34]]. Multiple pathogen T-cell inhibition or exhaustion will herein be called "polyspecific T-cell inhibition" or "polyspecific T-cell exhaustion."
It is important to note that there are actually two possible types of "polyspecific T-cell exhaustion." The first type would be a group of T-cells that were originally defined by one distinctive antigen, where each T-cell is exhausted with respect to two different pathogens using the polyspecificity of the T cell receptor. The second type would be simpler and involve two groups of T-cells, in which a first group of T-cells is exhausted with respect to a first pathogen, and the second group of T-cells is exhausted with respect to a second pathogen. The second type of polyspecific T-cell exhaustion is more significant and used herein.
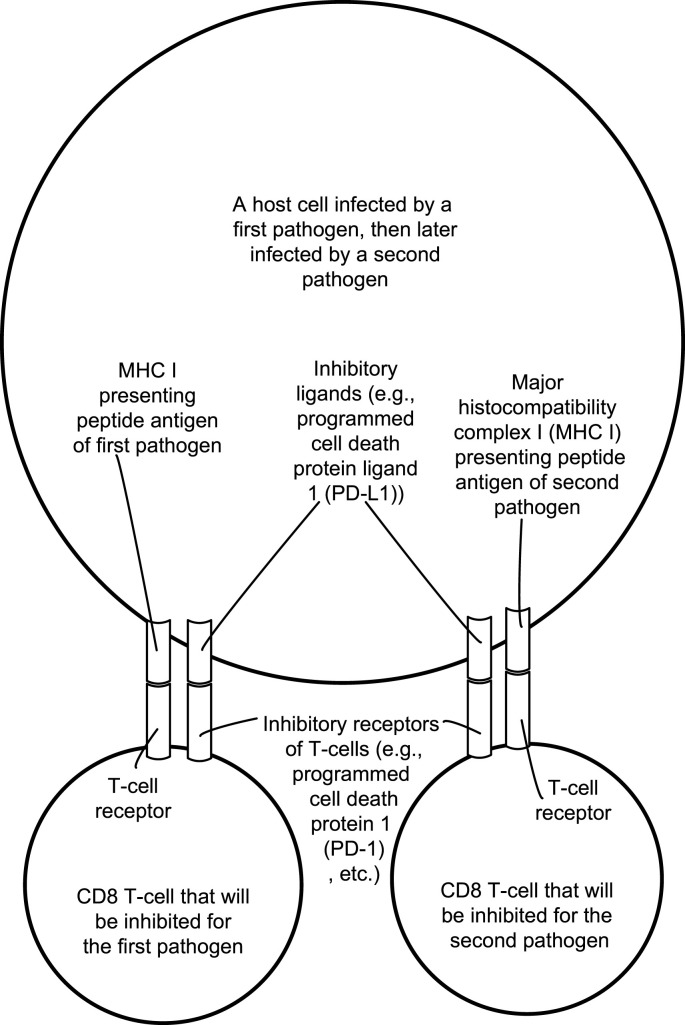
Fig. 1 illustrates the T-cell receptors and inhibitory receptors of T-cells being activated by a host cell infected by both Toxoplasma gondii and a virus, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as a simplified conceptual illustration of the second type of polyspecific T-cell exhaustion. Particularly shown are inhibitory T-cell receptors, such as the programmed cell death protein 1 (PD-1) receptor, programmed cell death protein ligands 1 (PD-L1) induced on the infected host cell and examples of a major histocompatibility complex I (MHC I) presenting peptide antigens on the infected host cell to the CD8 T-cells.
Fig. 1.
Illustrates the T-cell receptors and inhibitory receptors of T-cells being activated by a host cell infected by both the protozoan parasite Toxoplasma gondii and a virus such as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
2.7. T-cell exhaustion also has several far-reaching mediators
In addition, T-cell exhaustion can result from multiple mediators that will decrease T-cell functionality, such as cytokines interleukin-10 (IL-10) and transforming growth factor-β (TGF-β), and indoleamine 2,3 dioxygenase (IDO), and the type I interferons α and β that can eventually induce T-cell exhaustion [15]. T-cell exhaustion can also result from dendritic cells, macrophages and B cells that can become immunoregulatory antigen presenting cells that release IL-10, TGF-β and IDO; immunoregulatory T-cells (TREG) cells; and myeloid-derived suppressor cells capable of causing T-cell dysfunctionality and causing T-cell exhaustion [15,[27], [28], [29], [30]].
It has been long known that TREG cells can directly suppress a target T-cell in an antigen-specific manner [35]. However, TREG cells can also generally suppress T-cells using bystander suppression, in which a TREG cell specific for one antigen can suppress T-cell immune responses against other antigens because of their proximity to the TREG cell [35]. In summary, there are several direct and indirect pathways that enable antigen-specific T-cell exhaustion induced by one pathogen to also cause T-cell exhaustion in T-cells specific to other pathogens.
3. Conclusion
Various categories of COVID-19 patients have exhibited major mortality rate differences and symptoms. Some papers have explained these differences in mortality rates and symptoms as a consequence of SARS-CoV-2 virus infections acting together with one or more latent pathogen infections in COVID-19 patients. However, other papers have contradicted this and have concluded that latent pathogen infections have antiviral effects that reduce the severity of COVID-19 infections. These contradictions can be explained. Latent pathogens can express different genetic strains that can have vast differences in their immunological effects that affect a second pathogen infection through induced immune cell dysfunctions, such as T-cell exhaustion. Multiple pathogen T-cell exhaustion is herein called "polyspecific T-cell exhaustion."
Ethics statement
No ethical approval was required as this is a review article with no original research data.
Author contribution
No other author contributed to this paper.
Funding declaration
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and material statement
Not applicable.
Declaration of competing interest
The author has no potential conflicts of interest.
Acknowledgments
There are no acknowledgments. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao- Tournois C., Laribi S., Flament T., et al. Follow-up of adults with non- critical COVID-19 two months after symptoms' onset. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendelson M., Nel J., Blumberg L., Madhi S.A., Dryden M., Stevens W., Venter F.W.D. Long-COVID: an evolving problem with an extensive impact. S. Afr. Med. J. 2020;111(1):10–12. doi: 10.7196/SAMJ.2020.v111i11.15433. [DOI] [PubMed] [Google Scholar]
- 3.Yelin D., Margalit I., Yahav D., Runold M., Bruchfeld J. Long COVID- 19–-it's not over until? Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng H.Y., Zhang M., Yang C.X., Zhang N., Wang X.C., Yang X.P., Dong X.Q., Zheng Y.T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17:541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roe K. A role for T-cell exhaustion in long COVID-19 and severe outcomes for several categories of COVID-19 patients. J. Neurosci. Res. 2021 doi: 10.1002/jnr.24917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proal A.D., VanElzakker M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 2021 doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdel-Hamed E.F., Ibrahim M.N., Mostafa N.E., Moawad H., Elgammal N.E., Darwiesh E.M., El-Rafey D.S., ElBadawy N.E., Al-Khoufi E.A., Hindawi S.I. Role of interferon gamma in SARS-CoV-2-positive patients with parasitic infections. Gut Pathog. 2021;(29) doi: 10.1186/s13099-021-00427-3. 13(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jankowiak L., Rozsa L., Tryjanowski P., Moller A.P. A negative covariation between toxoplasmosis and COVID-19 with alternative interpretations. Sci. Rep. 2020;27:12512. doi: 10.1038/s41598-020-69351-x. 10(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weeratunga P., Herath T.U.B., Kim T.-H., et al. Dense Granule Protein-7 (GRA-7) of Toxoplasma gondii inhibits viral replication in vitro and in vivo. J. Microbiol. 2017;55:909–917. doi: 10.1007/s12275-017-7392-5. [DOI] [PubMed] [Google Scholar]
- 11.Król-Turmińska K., Olender A. Human infections caused by free- living amoebae. Ann. Agric. Environ. Med. 2017;24(2):254–262. doi: 10.5604/12321966.1233568. [DOI] [PubMed] [Google Scholar]
- 12.Xiao J., Prandovszky E., Kannan G., Pletnikov M.V., Dickerson F., Severance E.G., Yolken R.H. Toxoplasma gondii: biological parameters of the connection to schizophrenia. Schizophr. Bull. 2018;44(5):983–992. doi: 10.1093/schbul/sby082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halonen S.K., Weiss L.M. Toxoplasmosis. Handb. Clin. Neurol. 2013;114:125–145. doi: 10.1016/B978-0-444-53490-3.00008-X. Elsevier B.V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhadra R., Gigley J.P., Weiss L.M., Khan I.A. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc. Natl. Acad. Sci. U.S.A. 2011;108(22):9196–9201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurachi M. CD8+ T cell exhaustion. Semin. Immunopathol. 2019;41:327–337. doi: 10.1007/s00281-019-00744-5. [DOI] [PubMed] [Google Scholar]
- 16.Osokine I., Snell L.M., Cunningham C.R., Yamada D.H., Wilson E.B., Elsaesser H.J., de la Torre J.C., Brooks D. Type I interferon suppresses de novo virus-specific CD4 Th1 immunity during an established persistent viral infection. Proc. Natl. Acad. Sci. U.S.A. 2014;111(20):7409–7414. doi: 10.1073/pnas.1401662111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyck L., Mills K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017;47:765–779. doi: 10.1002/eji.201646875. [DOI] [PubMed] [Google Scholar]
- 18.Kong Y., Zhu L., Schell T.D., Zhang J., Claxton D.F., Ehmann W.C., Rybka W.B., et al. T-cell immunoglobulin and ITIM domain (TIGIT) associates with CD8+ T- cell exhaustion and poor clinical outcome in AML. Clin. Cancer Res. 2016;22(12):3057–3066. doi: 10.1158/1078-0432.CCR-15-2626. [DOI] [PubMed] [Google Scholar]
- 19.Dittfeld A., Gwizdek K., Michalski M., Wojnicz R. A possible link between the Epstein-Barr virus infection and autoimmune thyroid disorders. Cent. Eur. J. Immunol. 2016;41(3):297–301. doi: 10.5114/ceji.2016.63130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handous I., Achour B., Marzouk M., Rouis S., Hazgui O., Brini I., Khelif A., Hannachi N., Boukadida J. Co-infections of human herpesviruses (CMV, HHV-6, HHV-7 and EBV) in non-transplant acute leukemia patients undegoing chemotherapy. Virol. J. 2020;17(1):37. doi: 10.1186/s12985-020-01302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McHugh D., Caduff N., Murer A., Engelmann C., Deng Y., Zdimerova H., Zens K., et al. Infection and immune control of human oncogenic γ-herpeviruses in humanized mice. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019;374(1773):20180296. doi: 10.1098/rstb.2018.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pallett L.J., Schmidt N., Schurich A. T cell metabolism in chronic viral infection. Clin. Exp. Immunol. 2019;197(2):143–152. doi: 10.1111/cei.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schildermans J., De Vlieger G. Cytomegalovirus: a troll in the ICU? overview of the literature and perspectives for the future. Front. Med. 2020;7(188) doi: 10.3389/fmed.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virgin H.W., Wherry E.J., Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 25.Wherry E.J., Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roe K. An inflammation classification system using cytokine parameters. Scand. J. Immunol. 2021;93(2):e12970. doi: 10.1111/sji.12970. [DOI] [PubMed] [Google Scholar]
- 27.Goh C., Narayanan S., Hahn Y.S. Myeloid-derived suppressor cells: the dark knight or the joker in viral infections? Immunol. Rev. 2013;255(1):210–221. doi: 10.1111/imr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norris B.A., Uebelhoer L.S., Nakaya H.I., Price A.A., Grakoui A., Pulendran B. Chronic but not acute virus infection induces sustained expansion of myeloid suppressor cell numbers that inhibit viral-specific T cell immunity. Immunity. 2013;38(2):309–321. doi: 10.1016/j.immuni.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sevilla N., McGavern D.B., Teng C., Kunz S., Oldstone M.B.A. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J. Clin. Invest. 2004;113(5):7837–8745. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson E.B., Kidani Y., Elsaesser H., Barnard J., Raff L., Karp C.L., Bensinger S., Brooks D.G. Emergence of distinct multiarmed immunoregulatory antigen-presenting cells during persistent viral infection. Cell Host Microbe. 2021;11(5):481–491. doi: 10.1016/j.chom.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wucherpfennig K.W., Allen P.M., Celada F., Cohen I.R., De Boar R., Garcia K.C., Goldstein B., et al. Polyspecificity of T cell and B cell receptor recognition. Semin. Immunol. 2007;19(4):216–224. doi: 10.1016/j.smim.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrova G., Ferrante A., Gorski J. Cross-reactivity of T cells and its role in the immune system. Crit. Rev. Immunol. 2012;32(4):349–372. doi: 10.1615/critrevimmunol.v32.i4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frankild S., de Boer R.J., Lund O., Nielsen M., Kesmir C. Amino acid similarity accounts for T cell cross-reactivity and for "holes" in the T cell repetoire. PLoS One. 2008;3(3):e1831. doi: 10.1371/journal.pone.0001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar A., Delogu F. Dynamical footprint of cross-reactivity in a human autoimmune T-cell receptor. Sci. Rep. 2017;7:42496. doi: 10.1038/srep42496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eggenhuizen P.J., Ng B.H., Ooi J.D. Treg enhancing therapies to treat autoimmune diseases. Int. J. Mol. Sci. 2020;21(19):7015. doi: 10.3390/ijms21197015. [DOI] [PMC free article] [PubMed] [Google Scholar]