Abstract
Severe clinical diseases associated to αCoronavirus (αCoV) infections were recently demonstrated for the first time in humans and a closely related but distinct canine CoV (CCoV) variant was identified in the nasopharyngeal swabs of children with pneumonia hospitalized in Malaysia, in 2017–2018. The complete genome sequence analysis demonstrated that the isolated strain, CCoV-HuPn-2018, was a novel canine-feline-like recombinant virus with a unique nucleoprotein. The occurrence of three human epidemics/pandemic caused by CoVs in the recent years and the detection of CCoV-HuPn-2018, raises questions about the ability of these viruses to overcome species barriers from their reservoirs jumping to humans. Interestingly, in this perspective, it is interesting to consider the report concerning new CCoV strains with a potential dual recombinant origin through partial S-gene exchange with porcine transmissible gastroenteritis virus (TGEV) identified in pups died with acute gastroenteritis in 2009. The significance of the ability of CCoVs to evolve is still unclear, but several questions arisen on the biology of these viruses, focusing important epidemiological outcomes in the field, in terms of both virus evolution and prophylaxis. The new CCoV-Hupn-2018 should lead researchers to pay more attention to the mechanisms of recombination among CoVs, rather than to the onset of variants as a result of mutations, suggesting a continuous monitoring of these viruses and in particular of SARS-CoV-2.
Keywords: Canine coronavirus, Recombination, One health
1. Introduction
Coronaviruses (CoVs) are enveloped viruses with large and complex RNA genome up to 32 kb that encodes for 16 non-structural proteins regulating RNA synthesis and modification. The subfamily Orthocoronaviridae in the Coronaviridae family includes four genera, Alphacoronavirus (αCoV), Betacoronavirus (βCoV), Gammacoronavirus (γCoV) and Deltacoronavirus (δCoV) characterized by a variable tissue tropism and by the ability to easily cross interspecies barriers causing diseases with remarkably differences (Pratelli, 2011). These skills are the expression of the peculiar genome organization (large single-stranded RNA), of the low fidelity of the viral RNA polymerase, of the proofreading activity, of the high frequency of recombination and mutations events during RNA replication, and of the selection pressure during adaptation of the virus to the new host. All events that allow to escape lethal error and to generate quasispecies pools (Domańska-Blicharz et al., 2020; Pratelli, 2011).
αCoV and βCoV infect mammals, while γCoV and δCoV infect primarily birds with some mammalian spillover, as observed with the beluga whale and the pig (Domańska-Blicharz et al., 2020). Anyhow, it is known that CoVs are multitude and many of these have not yet been identified and classified and in the future could be found in other species. Human CoVs (HCoVs) are often of animal origin and most of them originated from bats and then adapted to humans by direct jumping or by jumping into an intermediate species. Before the emergence in 2003 of Severe Acute Respiratory Syndrome (SARS)-CoV, the first highly pathogenic HCoV, information was very scarce about these viruses, whereas there was extensive knowledge in veterinary medicine about animal CoVs, their evolution and their pathobiology. The known HCoVs were generally associated with common cold and acute gastroenteritis in immunocompetent patients and among them, HCoV-OC43 and HECoV-4408 originated by spillover from livestock (Vlasova and Saif, 2021). Before the first SARS epidemic, bats were not known to be hosts for CoVs, and only later, through in-deph investigations on various animal species, over 500 αCoV and βCoV were identified in bats all over the world (Drexler et al., 2014). The subsequent emergence of Middle East Respiratory Syndrome (MERS)-CoV in 2012 has confirmed that CoVs can cause severe-to-fatal disease and that bats are realistically the original source of both MERS- and SARS-CoVs (Vlasova and Saif, 2021). In the late 2019, a novel CoV, SARS-CoV-2, emerged among humans in Wuhan City, Hubei Province, China, likely, as universally narrated, via a spillover event originated at the largest Chinese wet market of the region, causing the current pandemic disease (Zhou et al., 2020) and pointing out the role of animals as reservoirs of new viruses with increased virulence that can adapt to humans. As a consequence, considering the latest CoV infections in humans and the main focus on the bats (Li et al., 2019), it is possible to assume that different animals may act as natural reservoirs/intermediate hosts for CoVs transmission to humans and among these, the potential threat of dogs and cats was poorly analyzed.
Recently, clinical diseases associated to αCoV infections were demonstrated for the first time in humans and two αCoVs, a feline CoV (FCoV)-like (Silva et al., 2014), and a closely related but distinct canine CoV (CCoV) variants (Xiu et al., 2020), were identified. FCoVs-like were detected in the nasal swabs from patients with acute respiratory symptoms during a molecular epidemiologic investigation of HCoV strains circulating in Arkansas. The novel CCoV was conversely detected in the nasopharyngeal swabs from eight children with pneumonia hospitalized in Sarawak, Malaysia, in 2017–2018, during the validation of a sensitive pan-species semi-nested RT-PCR assay for the detection of CoVs. This was the first report suggesting that a CCoV, without major genomic re-arrangements or adaptive modifications in the spike (S) protein may replicate in association with pneumonia in human host (Vlasova et al., 2021). The young patients were from rural areas and the exposure or cohabitation with domestic and wildlife animals were frequent. Among these specimens, CCoV was confirmed in two samples with different, less sensitive, one-step RT-PCR assays. The virus from one specimen was cultivated in the canine A72 cell line, and the complete genome sequence analysis demonstrated that the isolated strain, CCoV-HuPn-2018, was a novel canine-feline-like recombinant virus with a unique nucleoprotein (N) (Vlasova et al., 2021). Interestingly, through the analysis of the virus's genes sequences emerged that the new CoV could have infected cats and pigs at one point, but it likely jumped directly from dogs to humans, as the majority of the genome was similar to canine strains (CCoV TN-449 and CCoV HLJ-073). The highest nucleotide (nt) identity of S1 and S2 domains with the canine strain UCD-1 and the feline strain WSU 79–1683, respectively, the lack of the ORF3 and of the furin-clevage site between S1 and S2 domains, and the ability to replicate in A72 cells, highlighted the recombinant nature of CCoV-HuPn-2018 (feature already observed in other CCoVs), and suggested that it belongs to CCoV genotype II (Vlasova et al., 2021). Furthermore, a disturbing clue about the virus's future was observed, as a very unique deletion in the middle portion of the N protein, was discovered. This 12-aa insertion/deletion within the middle region of SARS-CoVs N protein is not present in any other known αCoV 1 and CoVs, but it is very similar to one previously found in SARS-CoV and SARS-CoV-2 very soon after its introduction in human population (He et al., 2004; Vlasova and Saif, 2021). This feature resulted in dramatic changes in its cellular localization soon after its zoonotic transmission and, similarly, CCoV-HuPn-2018 possesses this unique genetic characteristic suggestive of zoonotic origin. Likely, this deletion may have helped the virus to infect or to persist in humans from dog, or it could be a key step required for CoVs jumping into people. This intriguing but dangerous observation still remains a hypothesis, and it's too early to call this new canine CoV a human pathogen because the virus has only been associated with children pneumonia, but no one has shown that it causes pneumonia. Therefore, genetic evidence suggests that CCoV-HuPn-2018 was likely intercepted in the early stages of human jump, while it's still trying to figure out how to infect human efficiently for spreading from man to man (Vlasova et al., 2021).
2. Evolution and recombination events in CCoV strains
Regardless of what emerges from these data and from their possible future evolution, attention should be paid to canine CoVs and to what these viruses have taught us over time. Since the first report in 1971 (Binn et al., 1974), CCoV has a preponderant role as a canine enteropathogen and serological and virological investigations have demonstrated that dogs of all age and breed are susceptible to infection and that the virus is widely spread in dog population, mainly in kennels and animal shelters (Bandai et al., 1999; Naylor et al., 2001b; Pratelli, 2011; Rimmelzwaan et al., 1991; Schulz et al., 2008; Tennant et al., 1993; Yeșilbaǧ et al., 2004). As demonstrated for other CoVs, CCoV underwent mutations/recombination over time and new genetically divergent strains were detected, some of them with more pronounced pathogenic potential. In 2001, the genetic analysis of several CCoV detected in fecal samples from pups with diarrhea in the South of Italy and later in the feces of two naturally infected pups during the latter stages of long-term viral shedding, revealed multiple point mutations accumulating over a fragment of the M gene (Pratelli et al., 2001, Pratelli et al., 2002). These CCoVs showed a genetic drift to FCoV type II and subsequent sequence analysis carried out on multiple regions of the genome of CCoV positive samples, demonstrated the existence of two different genetic clusters of CCoV: the first included strains intermingled with reference CCoV strains, such as Insavc-1 and K378, and the second, referred to as FCoV-like CCoV, segregated separately from classic CCoVs, presumably represented a genetic outlier (Pratelli et al., 2001, Pratelli et al., 2003b). Several hypotheses were advanced to explain this different segregation: i) in natural conditions homologous recombination between highly homologous CoVs may occur frequently, and even if where the recombination takes place is unknown, CCoV can use the feline aminopeptidase (fAPN) glycoprotein as a cellular receptor (Rossen et al., 2001) and under experimental conditions, cats can be infected with CCoVs (Barlough et al., 1984); ii) recombination events have taken place in a different host (i.e. wild carnivore), or a wild carnivore might have harbored the ancestor of CCoV, and CoV RNAs analysis from wild carnivore isolates could shed light on these hypotheses (Pratelli et al., 2001). These preliminary observations on the genetic drift of the M gene toward FCoV, gave a meaningful impulse to study the genetic evolution of CCoVs. The phylogenetic analysis on the inferred amino acid (aa) sequence of a region encompassing about 80% of the S gene of one of these FCoV-like CCoVs, strain Elmo/02, clearly showed that the virus segregates with FCoVs type I (about 81% identity) rather than reference CCoVs and FCoVs type II (about 54% identity), and that this novel CCoV circulates among dogs (Pratelli et al., 2003a). On the basis of the significant genetic similarity between Elmo/02 and FCoVs type I, this strain was designated as the prototype of the newly recognized CCoV type I, whereas reference CCoVs were designed as CCoV type II (Fig. 1 ) (Pratelli et al., 2003a). Interestingly, unlike αCoVs, CCoV type I shares a potential cleavage site in the S protein with members of βCoVs and γCoVs, and the high divergence in the aa composition compared to the most closely related CoVs (FCoV type I, FCoV type II, and typical CCoV), strongly suggests that Elmo/02 strain is antigenically poorly correlated to other CoVs of carnivores. Moreover, the presence of the stretch of basic residues RRXRR, present in all βCoVs and γCoVs, is indicative of a potential cleavage of the S protein (Pratelli et al., 2003a). Lastly, the genome of Elmo/02 contains an additional ORF, 624 nt in length and referred as ORF3, which has not been detected in CCoVs type II and in other αCoVs (Lorusso et al., 2007). This ORF encodes for a putative protein 207 aa long (molecular weight of about 24 kDa), and the analysis of hydrophobic profile showed a neutral median hydrophaty pattern with a highly hydrophobic region localized at the N-terminus. This region also contains a signal peptide with the aa cleavage site at position 15 (12VAAKD16), and the observation that no transmembrane region has been detected, suggests that the protein is secreted from the infected cells (Lorusso et al., 2008).
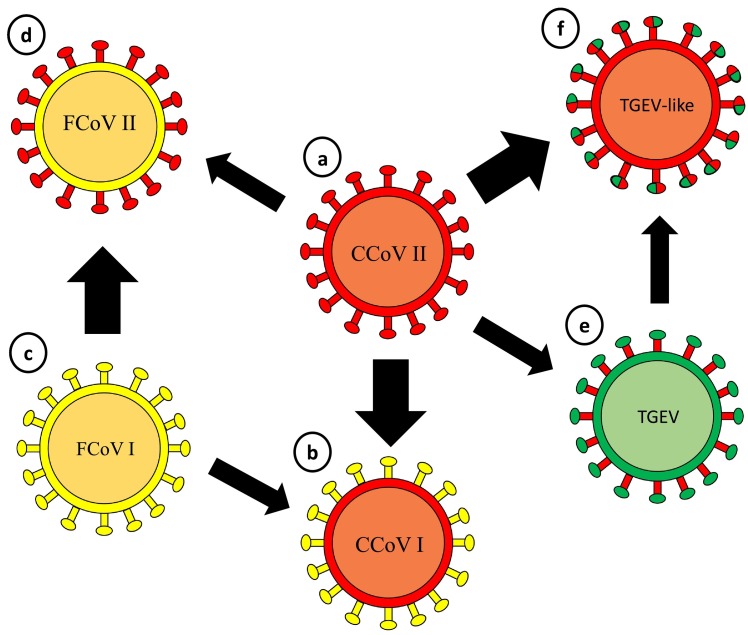
Fig. 1.
The evolution of CCoVs. The probable origin of FCoV type II, CCoV type I and TGEV-like CCoV as a result of recombination events.
a) CCoV II: “classical” canine coronavirus type II; b) CCoV I: canine coronavirus type I (in yellow: the spike protein originated from feline coronavirus type I); c) FCoV I: feline coronavirus type I; d) FCoV type II: feline coronavirus type II (in red: the spike protein originated from CCoV II; e) TGEV: transmissible gastroenteritis virus of swine (in red: the spike protein originated from CCoV II; f) TGEV-like: TGEV-like canine coronavirus (in green: the spike protein originated from CCoV II). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Following these preliminary observations, new strains were continuously reported, proving CCoVs skill to readily mutate and generate new potentially virulent or genetically divergent strains. By sequence analysis of a fragments of S and polymerase genes from an outbreak of fatal gastroenteritis in a breeding colony in Australia, Naylor et al. (2001a) identified the presence of a virulent strain (UWSMN-1) that appeared to be divergent from CCoVs type II circulating in other countries. Comparing the 751 nt in the 3’region of the S gene, UWSMN-1 had 21 unique sites and there were 112 sites, randomly interspersed, where the strain differs from other strains analyzed. These differences demonstrated that, probably, UWSMN-1 is not the result of recombination events between FCoVs and CCoVs, as would be indicated if the S gene shared blocks of homology with either FCoV or CCoV S genes, but rather it is a divergent strain originated after gradual accumulation of mutations throughout its genome, which may be reflective of its isolated evolution in Australia (Naylor et al., 2002).
In 2005, a highly virulent variant of CCoV type II (strain CB/05) which caused a systemic disease followed by fatal outcome in pups, was detected in Italy (Buonavoglia et al., 2006). CCoVs type I and type II were identified in the intestinal content of all infected pups and unexpectedly, CCoV type II RNA was also detected in lungs, spleen, liver, kidney and brain, and the virus was isolated on A-72 cells from all the examined tissues but brain. Sequence analysis of the 3’end of the genome, including ORFs 3a, 3b, 3c, 4 (E gene) 5 (M gene), 6 (N gene), 7a and 7b of this “pantropic” CCoV strain, showed high degree of aa identity to CCoV type II, but the S protein displayed the highest identity to FCoV type II strain 79–1683. Interestingly, the genetic marker of CB/05 genome consisted of a 38-nt deletion in the ORF3b which was responsible for a predicted truncated nonstructural protein 3b (Decaro et al., 2007). Experimental infection of seronegative pups confirmed the pantropism of the virus and its ability to induce severe clinical signs, lymphopenia and infection of the lymphoid tissue, strongly suggesting the ability of the virus to spread from the enteric tract to the internal organs (Decaro et al., 2010).
Another example of the evident evolution of dog CoVs was the identification of a canine respiratory coronavirus (CRCoV) in tissue samples from the respiratory tract of diseased dogs in United Kingdom (Erles et al., 2003). The virus resulted only distantly related to known CCoVs, displaying only a 21.2% aa identity in the S protein, and showed a close relationship to the βCoVs in the polymerase and S genes (Erles et al., 2003). In particular, S gene sequence analysis revealed a nt identity of 97.3% and 96.9% to bovine coronavirus (BCoV) and HCoV-OC43, respectively, suggesting a recent common ancestor, as well as the occurrence of repeated host-species shifts (Vijgen et al., 2005, Vijgen et al., 2006). The presence of the HE gene in the CRCoV genome, characteristic of the βCoVs, confirmed the hypothesis that CRCoV might have originated from BCoV (Erles et al., 2007).
Lastly, in 2009 CCoVs with a potential dual recombinant origin through partial S-gene exchange with TGEV were identified in the gastrointestinal tract and organs of pups imported from Hungary and died with acute gastroenteritis (Fig. 1) (Decaro et al., 2009). Recombination events involving partial S-gene sequences were previously described for FCoV (Herrewegh et al., 1998) and CCoV (Escutenaire et al., 2007; Wesley, 1999). In particular, Wesley (1999) characterized a TGEV-like CCoV strain (UCD1) in the feces of a dog with diarrhea, through sequence analysis of the N-terminal domain of the S protein, but the rest of the genome was strictly related to CCoV type II. Conversely, the Hungarian viruses with canine/porcine origin formed monophyletic group clustered with TGEV and porcine respiratory CoV and segregated separately from CCoVs type II in the 5’end of the S gene, but in the C terminus, the TGEV-like CCoVs clustered together with CCoV type II and separately from TGEV/porcine respiratory CoVs. Moreover, these viruses, later detected in many countries, were identified through the analysis of the 3’end of four strains and the nearly full-length genome of two of those strains, confirming the stability of the recombination events (Decaro et al., 2009). Subsequent experimental infections highlighted also an immunological impairment respect to classical CCoVs and consequently, because of these genetic and antigenic differences between original CCoVs type II and recombinant TGEV-like CCoVs, CCoV type II were further divided into two different subtypes, CCoV-IIa and CCoV-IIb, including reference and TGEV-like CCoV type II isolates, respectively (Decaro et al., 2009). It is interesting to note that these recombination events affecting canine and porcine CoVs represent a kind of “sliding door” where original CCoV gave rise to TGEV and then, TGEV participated to TGEV-like CCoV appearance (Fig. 1) (Pratelli et al., 2021).
The significance of all these data on the ability of CCoVs to evolve is still unclear, but several questions regarding the biology of these viruses arisen, focusing important epidemiological outcomes in the field, in terms of both virus evolution and prophylaxis.
3. Genetic plasticity of CoVs
One of RNA's most intriguing feature is their ability to carry genetic information despite its labile nature (Jarvis and Kirkegaard, 1991; Steinhauer and Holland, 1986). CoVs are unique among RNA viruses in many aspects of their biology, such as the extremely large genome, the nested set of subgenomic mRNAs, the discontinuous transcription mechanism, and the high frequency of RNA recombination events because of the high error frequencies of RNA polymerase that are predicted to accumulate several base substitutions per round of replication. Genetic recombination ensures the proliferation of new virus strains, serotypes and subtypes that may have selective advantages over parental genomes (Dolja and Carrington, 1992), and the consequent acquired characteristics, such as changes in virulence and tissue tropisms, and/or interspecies transmission, right occur through genetic variations in structural and/or non-structural proteins (Decaro and Buonavoglia, 2008; Guan et al., 2003; Laude et al., 1993; Rottier et al., 2005; Song et al., 2005; Vennema et al., 1998; Vijgen et al., 2005). The history of animal CoVs has demonstrated that genetic changes can be generated by mutations (i.e. deletions, insertions and substitutions), but gain/loss mechanisms mainly concerned accessory protein genes and mostly recombination could generate new strains with drastic changes, i.e. adaptation to different host, ability to avoid the immune response and variation in virulence (Domańska-Blicharz et al., 2020; Forni et al., 2017). Striking examples emerged from animal CoVs in which recombination have played a role in the evolution of different CoV species.
New strains of infectious bronchitis virus (IBV) in poultry flocks are the results of recombination between different field and/or vaccinal strains, and some IBV-like strains from turkey CoV (TCoV) (Bande et al., 2017; Brown et al., 2016). FCoVs type II arisen by recombination events between FCoV type I and CCoV (Fig. 1) (Herrewegh et al., 1998). The enteric biotype of FCoV in the persistent infected cats may undergo mutations in the S gene and/or deletions in the genes 3c, 7b or 7a, involving changes in the tropism of the virus causing the appearance of pathogenetic strain of feline peritonitis virus (FIPV) (Rottier et al., 2005; Vennema et al., 1998). Similar drastic shifts of tissue tropism have also been observed with murine coronaviruses mouse hepatitis virus (MHV) (Haspel et al., 1978). Swine CoVs are the expression of the ability of CoVs to cross species barriers infecting new hosts. The βCoV porcine haemagglutinating encephalomyelitis virus (PHEV) was a derivative of BCoV, which in turn is believed to have descended from a bat virus through adaptation in a rodent species (Decaro et al., 2020). Sequence alignments based on ORF1a and ORF1b of porcine epidemic diarrhea virus (PEDV) clearly shows that PEDV is most closely related to HCoV 229E and that HCoV 229E is more similar in sequence to PEDV than it is to TGEV (Kocherhans et al., 2001). TGEV likely originated from CCoV through cross-species transmission (adaptation of CCoV type II to swine apparently was accompanied by inactivation of ORF3b and loss of ORF7) (Lorusso et al., 2008) and gave rise to the less virulent porcine respiratory CoV (PRCoV) that shows a 200-aa deletion in the N-terminus with respect to TGEV (Decaro et al., 2007; Vaughn et al., 1994).
There is genetic evidence that several αCoVs, such as HCoV-OC43, HECV-4408 and porcine hemagglutinanting encephalomyelitis virus (PHEV), have arisen by host jumps from BCoV (Erles et al., 2007; Vijgen et al., 2005, Vijgen et al., 2006; Zhang et al., 1994), which likely derived from a bat virus through adaptation in a rodent species, as well as porcine deltacoronavirus (PDCoV) has emerged from avian δCoVs (Decaro et al., 2020; Wang et al., 2019). Recently, bovine-like CoVs were identified in wild or domesticated ruminants, even if the genetic events that have caused the interspecies transmission have not been identified so far. In addition, severe acute diarrhea syndrome CoV (SADS-CoV), a porcine αCoV recently emerged, derived from CoVs circulating in bats (Decaro et al., 2020).
4. Conclusion
The occurrence of three human epidemics/pandemic caused by CoVs in the recent years and the detection of CCoV-HuPn-2018 in the nasopharyngeal swabs from children with pneumonia in Malaysia, asks questions about the ability of these viruses to overcome species barriers from their reservoirs and to jump to humans. The viruses' animal-to-human transmission has already occurred in the past, but it seems that its frequency has increased in the last decades, involving in a short time not only CoVs, but also many genetically and biologically different viruses with zoonotic potential, such as Ebola virus, influenza viruses, flaviviruses, Hendra and Nipah viruses (McMahon et al., 2018).
CCoVs, and in general αCoVs, have the ability to infect different hosts inducing variable clinical disease and this emphasizes their complex evolution. Cui et al. (2019) stated that given the great diffusion and the genetic diversity of bat CoVs related to human SARS-CoVs, novel viruses will emerge in the future. Despite this dangerous hypothesis, no concrete actions have been performed to limit strict contact or cohabitation of animals (especially wildlife animals) with humans, and due to increasing urbanization, in the near future the world will deal with new severe health emergencies.
The discovery of the likely new human pathogen with CCoV characteristics, along with the report of a CoV that likely jumped from pigs to people in Haitian pediatric patients (Lednicky et al., 2021), pose another global threat, and attention should be focused on animals that could act as reservoirs or intermediate hosts to CoVs transmittable to humans (Vlasova et al., 2021). Considering that molecular analysis has confirmed the animal origin of the recent HCoVs epidemics/pandemic, important countermeasures should be taken to avoid next viral spillover from animals to humans, and continuous genomic surveillance in wild/domestic animals, ban of the wet markets and development of new antiviral drugs and vaccines should be carried out to prevent animal-to-human infections (Decaro and Lorusso, 2020). Considering that, in the past two decades, animal CoVs have jumped in humans at least three times, the veterinary medicine should play a more decisive role in promoting sustainable prevention measures and should support health policy makers in managing human health in order to advance the “One Global” Health Movement (Decaro et al., 2020).
Last but not least, the newly identified CCoV-Hupn-2018 should lead researchers to pay more attention to the mechanisms of recombination among CoVs, rather than to the onset of variants as a result of mutations, and continuous monitoring of these viruses are required because (without saying as Cassandra…!!!) recombination observed in CCoVs may represent a warning for SARS-CoV-2 (Pratelli et al., 2021).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None.
References
- Bandai C., Ishiguro S., Masuya N., Hohdatsu T., Mochizuki M. Canine coronavirus infections in Japan: virological and epidemiological aspects. J. Vet. Med. Sci. 1999;61:731–736. doi: 10.1292/jvms.61.731. [DOI] [PubMed] [Google Scholar]
- Bande F., Arshad S.S., Omar A.R., Hair-Bejo M., Mahmuda A., Nair V. Global distributions and strain diversity of avian infectious bronchitis virus: a review. Anim. Health Res. Rev. 2017;18:70–83. doi: 10.1017/S1466252317000044. [DOI] [PubMed] [Google Scholar]
- Barlough J.E., Stoddart C.A., Sorresso G.P., Jacobson R.H., Scott F.W. Experimental inoculation of cats with canine coronavirus and subsequent challenge with feline infectious peritonitis virus. Lab. Anim. Sci. 1984;34:592–597. [PubMed] [Google Scholar]
- Binn L.N., Lazar E.C., Keenan K.P., Huxsoll D.L., Marchwicki B.S., Strano A.J. Recovery and characterization of a coronavirus from military dogs with diarrhea. Proc. 78th Annu. Meet. U. S. Anim. Health Assoc. 1974;78:359–366. [PubMed] [Google Scholar]
- Brown P.A., Touzain F., Briand F.X., Gouilh A.M., Courtillon C., Allee C., Lemaitre E., De Boisseson C., Blanchard Y., Eterradossi N. First complete genome sequence of European Turkey coronavirus suggests complex recombination history related with US Turkey and guinea fowl coronaviruses. J. Gen. Virol. 2016;97:110–120. doi: 10.1099/jgv.0.000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonavoglia C., Decaro N., Martella V., Elia G., Campolo M., Desario C., Castagnaro M., Tempesta M. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 2006;12:492–494. doi: 10.3201/eid1203.050839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Buonavoglia C. An update on canine coronaviruses: viral evolution and pathobiology. Vet. Microbiol. 2008;132:221–234. doi: 10.1016/j.vetmic.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Lorusso A. Novel human coronavirus (SARS-CoV-2): a lesson from animal coronaviruses. Vet. Microbiol. 2020;244 doi: 10.1016/j.vetmic.2020.108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Martella V., Elia G., Campolo M., Desario C., Cirone F., Tempesta M., Buonavoglia C. Molecular characterization of the virulent canine coronavirus CB/05 strain. Virus Res. 2007;125:54–60. doi: 10.1016/j.virusres.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Mari V., Campolo M., Lorusso A., Camero M., Elia G., Martella V., Cordioli P., Enjuanes L., Buonavoglia C. Recombinant canine coronaviruses related to transmissible gastroenteritis virus of swine are circulating in dogs. J. Virol. 2009;83:1532–1537. doi: 10.1128/JVI.01937-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Elia G., Martella V., Campolo M., Mari V., Desario C., Lucente M.S., Lorusso E., Kanellos T., Gibbons R.H., Buonavoglia C. Immunity after natural exposure to enteric canine coronavirus does not provide complete protection against infection with the new pantropic CB/05 strain. Vaccine. 2010;28:724–729. doi: 10.1016/j.vaccine.2009.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Martella V., Saif L.J., Buonavoglia C. COVID-19 from veterinary medicine and one health perspectives: what animal coronaviruses have taught us. Res. Vet. Sci. 2020;131:21–23. doi: 10.1016/j.rvsc.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolja V.V., Carrington J.C. Evolution of positive-strand RNA viruses. Semin. Virol. 1992;3:315–326. [Google Scholar]
- Domańska-Blicharz K., Woźniakowski G., Konopka B., Niemczuk K., Welz M., Rola J., Socha W., Orłowska A., Antas M., Śmietanka K., Cuvelier-Mizak B. Animal coronaviruses in the light of COVID-19. J. Vet. Res. 2020;64:333–345. doi: 10.2478/jvetres-2020-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler J.F., Corman V.M., Drosten C. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antivir. Res. 2014;101:45–56. doi: 10.1016/j.antiviral.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Toomey C., Brooks H.W., Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Shiu K.-B., Brownlie J. Isolation and sequence analysis of canine respiratory coronavirus. Virus Res. 2007;124:78–87. doi: 10.1016/j.virusres.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escutenaire S., Isaksson M., Renstrom L.H., Klingeborn B., Buonavoglia C., Berg M., Belak S., Thoren P. Characterization of divergent and atypical canine coronaviruses from Sweden. Arch. Virol. 2007;152:1507–1514. doi: 10.1007/s00705-007-0986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni D., Cagliani R., Clerici M., Sironi M. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017;25:35–48. doi: 10.1016/j.tim.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., Luo S.W., Li P.H., Zhang L.J., Guan Y.J., Butt K.M., Wong K.L., Chan K.W., Lim W., Shortridge K.F., Yuen K.Y., Peiris J.S., Poon L.L. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Haspel M.V., Lampert P.V., Oldstone M.B. Temperature-sensitive mutants of mouse hepatitis virus produce a high incidence of demyelination. Proc. Natl. Acad. Sci. U. S. A. 1978;75:4033–4036. doi: 10.1073/pnas.75.8.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R., Dobie F., Ballantine M., Leeson A., Li Y., Bastien N., Cutts T., Andonov A., Cao J., Booth T.F., Plummer F.A., Tyler S., Baker L., Li X. Analysis of multimerization of the SARS coronavirus nucleocapsid protein. Biochem. Biophys. Res. Commun. 2004;316:476–483. doi: 10.1016/j.bbrc.2004.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrewegh A.A., Smeenk I., Horzinek M.C., Rottier P.J., de Groot R.J. Feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J. Virol. 1998;72:4508–4514. doi: 10.1128/jvi.72.5.4508-4514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis T.C., Kirkegaard K. The polymerase in its labyrinth: mechanisms and implications of RNA recombination. Trends Genet. 1991;7:186–191. doi: 10.1016/0168-9525(91)90434-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocherhans R., Bridgen A., Ackermann M., Tobler K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes. 2001;23:137–144. doi: 10.1023/A:1011831902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laude H., Van Reeth K., Pensaert M. Porcine respiratory coronavirus: molecular features and virus-host interactions. Vet. Res. 1993;24:125–150. [PubMed] [Google Scholar]
- Lednicky J.A., Tagliamonte M.S., White S.K., Elbadry M.A., Alam M.M., Stephenson C.J., Bonny T.S., Loeb J.C., Telisma T., Chavannes S., Ostrov D.A., Mavian C., De Rochars V.M.B., Salemi M., Morris J.G., Jr. Emergence of porcine delta-coronavirus pathogenic infections among children in Haiti through independent zoonoses and convergent evolution. MedRxiv. 2021;2021 Mar 25:2021.03.19.21253391. [Google Scholar]
- Li H., Mendelsohn E., Zong C., Zhang W., Hagan E., Wang N., Li S., Yan H., Huang H., Zhu G., Ross N., Chmura A., Terry P., Fielder M., Miller M., Shi Z., Daszak P. Human-animal interactions and bat coronavirus spillover potential among rural residents in southern China. Biosaf. Health. 2019;1:84–90. doi: 10.1016/j.bsheal.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorusso A., Haijema B.J., Decaro N., Schellen P., Buonavoglia C., de Groot R.J. Proceedings of the Third European Congress of Virology “EuroVirology 2007”, Nuernberg, 1–5 September 2007. 2007. Identification and biochemical characterization of a novel protein unique to canine coronavirus type I. [Google Scholar]
- Lorusso A., Decaro N., Schellen P., Rottier P.J., Buonavoglia C., Haijema B.J., de Groot R.J. Gain, preservation and loss of a group 1a coronavirus accessory glycoprotein. J. Virol. 2008;82:10312–10317. doi: 10.1128/JVI.01031-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon B.J., Morand S., Gray J.S. Ecosystem change and zoonoses in the Anthropocene. Zoonoses Public Health. 2018;65:755–765. doi: 10.1111/zph.12489. [DOI] [PubMed] [Google Scholar]
- Naylor M.J., Harrison G.A., Monckton R.P., McOrist S., Lehrbach P.R., Deane E.M. Identification of canine coronavirus strains from faeces by S gene nested PCR and molecular characterization of a new Australian isolate. J. Clin. Microbiol. 2001;39:1036–1041. doi: 10.1128/JCM.39.3.1036-1041.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor M.J., Monckton R.P., Lehrbach P.R., Deane E.M. Canine coronavirus in Australian dogs. Aust. Vet. J. 2001;79:116–119. doi: 10.1111/j.1751-0813.2001.tb10718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor M.J., Walia C.S., McOrist S., Lehrbach P.R., Deane E.M., Harrison G.A. Molecular characterization confirms the presence of a divergent strain of canine coronavirus (UWSMN-1) in Australia. J. Clin. Microbiol. 2002;40:3518–3522. doi: 10.1128/JCM.40.9.3518-3522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A. The evolutionary processes of canine coronaviruses. Adv. Virol. 2011;2011 doi: 10.1155/2011/562831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Martella V., Elia G., Decaro N., Aliberti A., Buonavoglia D., Tempesta M., Buonavoglia C. Variation of the sequence in the gene encoding for transmembrane protein M of canine coronavirus (CCV) Mol. Cell. Probes. 2001;15:229–233. doi: 10.1006/mcpr.2001.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Elia G., Martella V., Tinelli A., Decaro N., Marsilio F., Buonavoglia D., Tempesta M., Buonavoglia C. M gene evolution of canine coronavirus in naturally infected dogs. Vet. Rec. 2002;151:758–761. [PubMed] [Google Scholar]
- Pratelli A., Martella V., Decaro N., Tinelli A., Camero M., Cirone F., Elia G., Cavalli A., Corrente M., Greco G., Buonavoglia D., Gentile M., Tempesta M., Buonavoglia C. Genetic diversity of a canine coronavirus detected in pups with diarrhoea in Italy. J. Virol. Methods. 2003;110:9–17. doi: 10.1016/S0166-0934(03)00081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Martella V., Pistello M., Elia G., Decaro N., Buonavoglia D., Camero M., Tempesta M., Buonavoglia C. Identification of coronaviruses in dogs that segregate separately from the canine coronavirus genotype. J. Virol. Methods. 2003;107:213–222. doi: 10.1016/S0166-0934(02)00246-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Buonavoglia A., Lanave G., Tempesta M., Camero M., Martella V., Decaro N. One world, one health, one virology of the mysterious labyrinth of coronaviruses: the canine coronavirus affair. Lancet Microbe. 2021 doi: 10.1016/S2666-5247(21)00282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelzwaan G.F., Groen J., Egberink H., Borst G.H., Uytde-Haag F.G., Osterhaus A.D. The use of enzyme-linked immunosorbent assay systems for serology and antigen detection in parvovirus, coronavirus and rotavirus infections in dogs in the Netherlands. Vet. Microbiol. 1991;26:25–40. doi: 10.1016/0378-1135(91)90039-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen J.W.A., Kouame J., Goedheer A.J.W., Vennema H., Rottier P.J.M. Feline and canine coronaviruses are released from the basolateral side of polarized epithelial LLCPK1 cells expressing the recombinant feline aminopeptidase-N cDNa. Arch. Virol. 2001;146:791–799. doi: 10.1007/s007050170147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P.J., Nakamura K., Schellen P., Volders H., Haijema B.J. Acquisition of macrophage tropism during the pathogenesis of feline infectious peritonitis is determined by mutations in the feline coronavirus spike protein. J. Virol. 2005;79:14122–14130. doi: 10.1128/JVI.79.22.14122-14130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz B.S., Strauch C., Mueller R.S., Eichhorn W., Hartmann K. Comparison of the prevalence of enteric viruses in healthy dogs and those with acute haemorrhagic diarrhoea by electron microscopy. J. Small Anim. Pract. 2008;49:84–88. doi: 10.1111/j.1748-5827.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C.S., Mullis L.B., Pereira O., Jr., Saif L.J., Vlasova A., Zhang X., Owens R.J., Paulson D., Yaylor D., Haynes L.M., Azevedo M.P. Human respiratory coronaviruses detected in patients with influenza-like illness in Arkansas, USA. Virol. Mycol. 2014;2014(Suppl. 2):004. doi: 10.4172/2161-0517.S2-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H.D., Tu C.C., Zhang G.W., Wang S.Y., Zheng K., Lei L.C., Chen Q.X., Gao Y.W., Zhou H.Q., Xiang H., Zheng H.J., Chern S.W., Cheng F., Pan C.M., Xuan H., Chen S.J., Luo H.M., Zhou D.H., Liu Y.F., He J.F., Qin P.Z., Li L.H., Ren Y.Q., Liang W.J., Yu Y.D., Anderson L., Wang M., Xu R.H., Wu X.W., Zheng H.Y., Chen J.D., Liang G., Gao Y., Liao M., Fang L., Jiang L.Y., Li H., Chen F., Di B., He L.J., Lin J.Y., Tong S., Kong X., Du L., Hao P., Tang H., Bernini A., Yu X.J., Spiga O., Guo Z.M., Pan H.Y., He W.Z., Manuguerra J.C., Fontanet A., Danchin A., Niccolai N., Li Y.X., Wu C.I., Zhao G.P. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc. Natl. Acad. Sci. 2005;102:2430–2435. doi: 10.1073/pnas.0409608102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer D.A., Holland J.J. Direct method for quantitation of extreme polymerase error frequencies at selected single base sites in viral RNA. J. Virol. 1986;57:219–228. doi: 10.1128/jvi.57.1.219-228.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant B.J., Gaskell R.M., Jones R.C., Gaskell C.J. Studies on the epizootiology of canine coronavirus. Vet. Rec. 1993;132:7–11. doi: 10.1136/vr.132.1.7. [DOI] [PubMed] [Google Scholar]
- Vaughn E.M., Halbur P.G., Paul P.S. Three new isolates of porcine respiratory coronavirus with various pathogenicities and spike (S) gene deletions. J. Clin. Microbiol. 1994;32:1809–1812. doi: 10.1128/jcm.32.7.1809-1812.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., Poland A., Foley J., Pedersen N.C. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology. 1998;243:150–157. doi: 10.1006/viro.1998.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgen L., Keyaerts E., Moes E., Thoelen I., Wollants E., Lemey P., Vandamme A.M., Van Ranst M. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 2005;79:1595–1604. doi: 10.1128/JVI.79.3.1595-1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgen L., Keyaerts E., Lemey P., Maes P., Van Reeth K., Nauwynck H., Pensaert M., Van Ranst M. Evolutionary history of the closely related group 2 coronaviruses: porcine hemagglutinating encephalomyelitis virus, bovine coronavirus, and human coronavirus OC43. J. Virol. 2006;80:7270–7274. doi: 10.1128/JVI.02675-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova A.N., Saif L.J. Bovine coronavirus and the associated diseases. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.643220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova A.N., Diaz A., Damtie D., Xiu L., Toh T.H., Lee J.S., Saif L.J., Gray G.C. Novel canine coronavirus isolated from a hospitalized pneumonia patient, East Malaysia. Clin. Infect. Dis. 2021;May 20 doi: 10.1093/cid/ciab456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Vlasova A.N., Kenney S.P., Saif L.J. Emerging and re-emerging coronaviruses in pigs. Curr. Opin. Virol. 2019;34:39–49. doi: 10.1016/j.coviro.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley R.D. The S gene of canine coronavirus, strain UCD-1, is more closely related to the S gene of transmissible gastroenteritis virus than to that of feline infectious peritonitis virus. Virus Res. 1999;61:145–152. doi: 10.1016/S0168-1702(99)00032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu L., Binder R.A., Alarja N.A., Kochek K., Coleman K.K., Than S.T., Bailey E.S., Bui V.N., Toh T.H., Erdman D.D., Gray G.C. RT-PCR assay for the detection of coronaviruses from four genera. J. Clin. Virol. 2020;128 doi: 10.1016/j.jcv.2020.104391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeșilbaǧ K., Yilmaz Z., Torun S., Pratelli A. Canine coronavirus infection in Turkish dog population. J. Vet. Med. B Infect. Dis Vet. Public Health. 2004;51:353–355. doi: 10.1111/j.1439-0450.2004.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.M., Herbst W., Kousoulas K.G., Storz J. Biological and genetic characterization of a hemagglutinating coronavirus isolated from a diarrhoeic child. J. Med. Virol. 1994;44:152–161. doi: 10.1002/jmv.1890440207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]