Abstract
This study purposed to discover the connection between the central glutamatergic and histaminergic systems on feeding behavior in layer chickens. In the first experiment, chicks obtained intracerebroventricular (ICV) injections of saline (control solution), α-FMH (250 nmol), glutamate (300 nmol), and α-FMH + glutamate. Experiments 2-6 were comparable to the first experiment, apart from the birds being injected with chlorpheniramine (histamine H1 receptor antagonist, 300 nmol), famotidine (histamine H2 receptor antagonist, 82 nmol), and thioperamide (histamine H3 receptor antagonist, 300 nmol) instead of α-FMH. In Experiment five, experimental groups were divided into (A) control solution, (B) MK-801 (N-methyl-D-aspartate receptor antagonist, 15 nmol), (C) histamine (300 nmol) and (D) MK-801 + histamine. Experiments 6-10 and Experiment five were similar apart from the ICV injections of CNQX (AMPA receptor antagonist, 360 nm), UBP-302 (Kainate receptor antagonist, 390 nm), AIDA (mGluR1 antagonist, 2 nmol), LY341495 (mGluR2 antagonist, 150 nmol), and UBP1112 (mGluR3 antagonist, 2 nmol) given instead of MK-801. Afterward, cumulative food intake was recorded at30, 60, and 120 minutes after the injection process. According to the results, ICV injection of glutamate considerably reduced food intake (p<0.05). Co-injection of α-FMH + glutamate and/or chlorpheniramine + glutamate reduced the hypophagic influence of glutamate (p<0.05), whereas thioperamide + glutamate augmented glutamate-induced hypophagia in neonatal chicks (p<0.05). Co-injection of MK-801 + histamine or UBP-302 + histamine reduced the hypophagic influence of the histamine (p<0.05), whereas LY341495 + histamine augmented the hypophagic influence of the histamine (p<0.05). Given the results, it is suggested that the effect of the connection between these systems on the process of food intake regulation is mediated by H1 and H3 histamines as well as NMDA, Kainate, and mGluR2 glutamate receptors in neonatal layer chickens.
Keywords: central glutamate, histamine, food intake, layer chicken
1. Introduction
According to Yousefvand, Hamidi ( 1 ) and Hassanpour, Zendehdel ( 2 ), eating behavior can be considered as a complicated network between the brain and the gastrointestinal tract. According to Parker, Johns ( 3 ), the central nervous system (CNS) is capable of controlling various neurotransmitters through complicated neurological paths. Blandina, Provensi ( 4 ) suggested that histamine, a monoamine, contributes to various physiological roles in the brain. It is obvious that not only the paraventricular nucleus (PVN) but also the ventromedial hypothalamus (VMH) obtain afferent probable axons of histaminergic (HA-ergic) neurons from the tuberomammillary nucleus (TMN) ( 5 ). Schneider, Neumann ( 6 ) suggested that histamine receptors (H1, H2, H3, and H4) are dispersed in various divisions in the CNS. The central histaminergic (HAergic) system has an important function in activating locomotors, thermoregulation, and appetite ( 7 , 8 ).
ICV administration of histamine reduces appetite, whereas chlorpheniramine (histamine H1 receptor antagonist) or alpha-fluoromethylhistidine (α-FMH, selective inhibitor of histidine decarboxylase) provides the base to intake food in rats ( 9 ) and chicken ( 7 , 10 ). It has been reported that a single neuropeptide does not control food intake, but various neurotransmitters interact to control appetite ( 11 ). Glutamate can be considered an excitatory neurotransmitter, which provides various rewards ( 12 , 13 ). Rats could be fed properly by stimulating the lateral hypothalamic glutamate AMPA receptors. The injection of both NMDA and AMPA-kainite receptor opponents into the ventral striatal and ventral pallidal parts of the pigeon persuaded food intake ( 14 ). In addition, Taati, Nayebzadeh ( 15 ) suggested that the ICV injection of NMDA receptor antagonists amplifies food ingestion in three-hour food-deprived (FD3) broiler cockerels ( 15 ). Histamine H3 receptors provide a base for adjusting the release of dopamine, noradrenaline, glutamic acid, serotonin, and gamma-aminobutyric acid (GABA) ( 16 ). Apart from this, when H3 receptors in the mouse cerebral cortex are activated, the expressive part of anti-apoptotic proteins is boosted, and NMDA receptors are activated. According to Brown, Stevens ( 17 ), histamine is capable of altering and blocking the iconic recent arbitrated by NMDA receptors. Motor behavior can be regulated by restraining glutamate release from both thalamocortical axons; moreover, thalamostriatal terminals and their involvement in the expression of three factors, including histamine in the thalamus and the cerebellum, may prepare a base for such a factor ( 18 , 19 ). Zendehdel and Hassanpour ( 20 ) believed that ghrelin has an orexigenic function in rats, whereas it is an anorexigenic neurotransmitter in birds. The current study aimed to determine how central glutamatergic and HAergig systems interact on food intake regulation in chickens.
2. Material and Methods
2.1. Animals
In this research, 440 one-day-old layer chickens (Hy-Line) were bought from a hatchery (Mahan Co., Iran). Birds were kept as a group for two days and then arbitrarily moved into separate cages. At this stage, a temperature of 30±1 ºC with 50±2% humidity were used. ( 21 ). During the study, a profitable diet containing 21% simple protein plus 2850 kcal/kg of metabolizable energy was prepared (Chineh Co., Iran) (Table1).
Table 1.
Ingredient and nutrient analysis of experimental diet
| Ingredient | (%) | Nutrient analysis | |
|---|---|---|---|
| Corn | 52.85 | ME, kcal/g | 2850 |
| Soybean meal, 48% CP | 31.57 | Crude protein (%) | 21 |
| Wheat | 5 | Linoleic acid (%) | 1.69 |
| Gluten meal, 61% CP | 2.50 | Crude fiber (%) | 3.55 |
| Wheat bran | 2.47 | Calcium (%) | 1 |
| Di-calcium phosphate | 1.92 | Available phosphorus (%) | 0. 5 |
| Oyster shell | 1.23 | Sodium (%) | 0.15 |
| Soybean oil | 1.00 | Potassium (%) | 0.96 |
| Mineral premix | 0.25 | Chlorine (%) | 0.17 |
| Vitamin premix | 0.25 | Choline (%) | 1.30 |
| Sodium bicarbonate | 0.21 | Arginine (%) | 1.14 |
| Sodium chloride | 0.20 | Isoleucine (%) | 0.73 |
| Acidifier | 0.15 | Lysine (%) | 1.21 |
| DL-Methionine | 0.10 | Methionine (%) | 0.49 |
| Toxin binder | 0.10 | Methionine + cystine (%) | 0.83 |
| L-Lysine HCl | 0.05 | Threonine (%) | 0.70 |
| Vitamin D3 | 0.1 | Tryptophan (%) | 0.20 |
| Multi enzyme | 0.05 | Valine (%) | 0.78 |
ME: metabolizable energy, CP: crude protein per kg of diet, the mineral supplement contains 35.2 g manganese from MnSO4∙H2O; 22 g iron from FeSO4∙H2O; 35.2 g zinc from ZnO; 4.4 g copper from CuSO4∙5H2O; 0.68 g iodine from ethylene diamine dihydroiodide; 0.12 g selenium from Na2SeO3. The vitamin supplement contains 1.188 g of retinyl acetate, 0.033 g of dl-α-tocopheryl acetate, 8.84 g of tocopherol, 1.32 g of menadione, 0.88 g of thiamine, 2.64 g of riboflavin, 13.2 g of nicotinic acid, 4.4 g of pantothenic acid, 1.76 g of pyridoxin, 0.022 g of biotin, 0.36 g of folic acid, 1500 mg of choline chloride.
Ad libitum food and freshwater were provided the birds during the research. The birds’ access to food was restricted for only 3 hours before the ICV injections, but the chickens could receive water. All five-day-old chickens received injections.
2.2. Experimental Drugs
Glutamate, MK-801 (NMDA receptor antagonist), AIDA (mGluR1 antagonist), histamine, chlorpheniramine (histamine H1 receptor antagonist), UBP1112 (mGluR3 antagonist), UBP-302 (Kainate receptor antagonist), α-FMH (histidine decarboxylase inhibitor), thioperamide (histamine H3 receptor antagonist), famotidine (histamine H2 receptor antagonist), CNQX (AMPA receptor antagonist), LY341495 (mGluR2 antagonist), and Evans blue were acquired from Sigma Co. and Tocris Co. (UK). In the first step, the drugs were completely dissolved in dimethyl sulfoxide (DMSO) and then diluted with 0.85% saline containing Evans blue rating 1.250. According to Blevins, Stanley ( 22 ) and Qi, Ding ( 23 ), there is no cytotoxic effect on DMSO at this percentage. The saline and DMSO combination was used as a solution in the process of control throughout the experiments.
2.3. ICV Injection Procedures
Ten different experiments were planned to examine the effects of the connection between the histaminergic and glutamatergic systems on the ingestion of cumulative food in neonatal chickens. According to the previous experiment and based on each chick's body weight, the birds were divided into experimental groups, and the weights between treatment groups were standardized. ICV injection was applied by microsyringe (Hamilton, Switzerland) without anesthesia according to the method explained by Davis, Masuoka ( 24 ) and Furuse, Matsumoto ( 25 ). The birds were grasped by the head using an acrylic tool whereas the bill container was an angle of about 45º and in the same direction as the exterior part of the table 1. A hole was drilled in the plate that was directly covered by the skull on top of the right lateral ventricle. A microsyringe was placed in the right ventricle through the hole with the tip of the needle entering 4 mm below the skin. According to Saito, Kaiya ( 26 ), this technique used in neonatal chicks causes no injection-induced physiological stress. Furuse, Ando ( 27 ) injected 10 μL of drug solution ICV into each chick. They further suggested that the control solution be saline with Evan’s blue, 10 μL. FD3 birds were returned to their cages immediately after the process of injection and received food plus fresh water (pre-weighed), and their cumulative food intake (g) was measured after 30, 60, and 120 minutes.
2.4. Feeding Experiments
In this research, ten experiments each with four treatment groups were designed (n=44 in each). In the first experiment, 4 groups of FD3 chicks received an ICV injection of (I) saline (control solution), (II) α-FMH (250 nmol), (III) glutamate (300 nmol), and (IV) α-FMH + glutamate. In the second experiment, injections of (I) control solution, (II) chlorpheniramine (300 nmol), (III) glutamate (300 nmol), and (IV) chlorpheniramine + glutamate were given. In the third experiment, birds were injected ICV with (I) control solution, (II) famotidine (82 nmol), (III) glutamate (300 nmol), and (IV) famotidine + glutamate. In the fourth experiment, chickens were injected ICV with (I) control solution, (II) thioperamide (300 nmol), (III) glutamate (300 nmol), and (IV) thioperamide + glutamate. In Experiment five, the experimental groups included (I) control solution, (II) MK-801 (15 nmol), (III) histamine (300 nmol), and (IV) MK- 801 + histamine. Injections in Experiment 6 included (I) control solution, (II) CNQX (390 nmol), (III) histamine (300 nmol), and (IV) MK- 801 + histamine. In Experiment seven, chickens received ICV injections of (I) control solution, (II) UBP1112 (2 nmol), (III) histamine (300 nmol), and (IV) UBP1112 + histamine. In the eighth experiment, chickens were injected ICV with (I) control solution, (II) AIDA (2 nmol), (III) histamine (300 nmol), and (IV) AIDA + histamine. In Experiment nine birds were treated with (I) control solution, (II) LY341495 (150 nmol), (III) histamine (300 nmol), and (IV) LY341495 + histamine. In the tenth experiment, (I) control solution, (II) UBP1112 (2 nmol), (III) histamine (300 nmol), and (IV) UBP1112 + histamine was injected. One injection was performed for each bird. Drug doses used in the experiments were calculated according to previous studies ( 15 , 28 - 30 ) as well as unpublished data from our experimental studies. The chickens were returned to their cages directly after the injection process and received ad libitum food plus freshwater (pre-weighed). Cumulative food intake was recorded as a percentage of body weight at30, 60, and 120 minutes after the injections.
2.5. Statistical Analysis
In this research, ten experiments with four treatment groups each were designed (I-IV). Single injections were applied for all groups, and the outcome of each experiment was kept separate from the other experiments. Each experiment was examined by the repeated measurement of two-way analysis of variance (ANOVA) based on the cumulative food intake considered as a percentage of body weight according to the model below:
Yijk= μ + αj + βk + (αβ)jk + εijk, with εijk ~ N (0, σ2).
where Yijk is the value of its personal examination for valuables, μ is considered as the grand mean, αj is the treatment that influences the time, βk is the treatment affecting the drugs, (αβ)jk is the interface affecting the time as well as drugs, and εijk is error. All examinations were carried out using SPSS 16.0 for Windows (SPSS, Inc., Chicago, IL, USA).
For treatments, the differences presented by ANOVA as means were compared using the Tukey test and shown as mean ± SEM (standard error of the mean). A value of p <0.05 was considered a significant difference.
3. Results
During the first experiment, no influence on food intake was observed (p>0.05) after ICV injection of α-FMH (250 nmol), despite the fact that glutamate (300 nmol) considerably reduces food intake compared to the control group (p <0.05). Neonatal chickens appeared to be less influenced by glutamate, when co-injection of α-FMH reduced the hypophagic consequences of it at30, 60, and 120 min compare to the control group (p <0.05) (Figure 1). Administration of the drug chlorpheniramine (300 nmol) in the second experiment showed no effect on food intake (p <0.05), whereas glutamate (300 nmol) considerably reduced food intake compared to the control group (p <0.05). Neonatal chickens appeared to be less influenced by the glutamate hypophagic effect after co-injection of chlorpheniramine + glutamate at 30, 60, and 120 min compared to the control group (p <0.05) (Figure 2). In the third experiment, ICV injection of famotidine (82 nmol) did not significantly influence food intake. ICV injection of glutamate (300 nmol) showed a considerable decrease in food intake compared to the control group (p <0.05). In Figure 3, co-injection of famotidine plus glutamate showed no effect on the hypophagic influence of glutamate compared to the control group (p >0.05).
Figure 1.
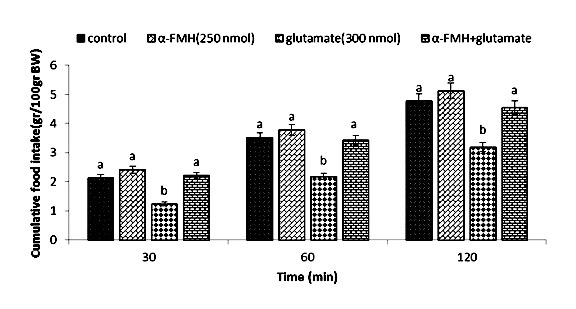
Effect of ICV injection of α-FMH (250 nmol), glutamate (300 nmol), and their combination on cumulative food intake (% BW) in neonatal layer chickens. α-FMH: alpha fluoromethyl histidine (inhibitor of histidine decarboxylase). Data expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments (p < 0.05).
Figure 2.
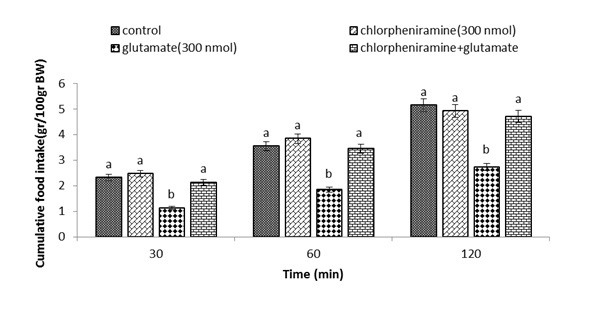
Effect of ICV injection of chlorpheniramine (300 nmol), glutamate (300 nmol), and their combination on cumulative food intake (%BW) in neonatal layer chickens. Chlorpheniramine: histamine H1 receptors antagonist. Data expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments (p < 0.05).
Figure 3.
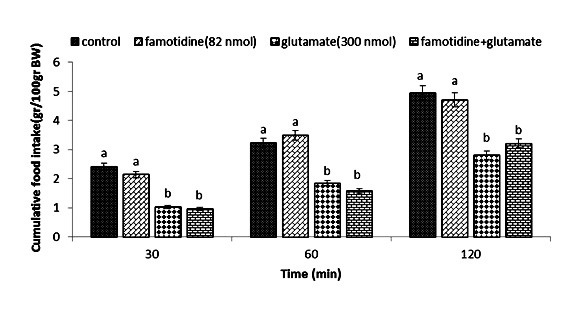
Effect of ICV injection of famotidine (82 nmol), glutamate (300 nmol), and their combination on cumulative food intake (% BW) in neonatal layer chickens. Famotidine: histamine H2 receptor antagonist. Different letters (a and b) indicate significant differences between treatments (p < 0.05).
In Experiment four, food intake appeared not to be affected by the ICV injection of thioperamide (300 nmol) (p >0.05). A considerable reduction in food intake was observed after ICV injection of glutamate (300 nmol) compared to the control group (p <0.05). In the Figure 4, the co-administration of thioperamide plus glutamate improved glutamate-induced hypophagia compared to the control group (p <0.05). ICV injection of MK-801 in Experiment 5 showed no significant effect on food intake (p >0.05), but at the same time, the chicks injected with histamine (300 nmol) showed a considerable reduction in food intake compared to the control group (p <0.05). Figure 5 shows that the co-injection of MK- 801 + histamine reduced the hypophagic influence of histamine compared to the control group (p <0.05). In Experiment 6, there was no major influence on cumulative food intake examined in birds receiving ICV injections of CNQX (390 nmol) (p >0.05). However, ICV injection of histamine (300 nmol) considerably reduced cumulative food intake compared to the control group (p <0.05). In addition, the co-injection of CNQX plus histamine showed no effect on the hypophagic influence of histamine compared to the control group (p >0.05) (Figure 6). In Experiment seven, ICV injection of UBP-302 (390 nmol) had no influence on food intake in neonatal broilers (p >0.05). However, the ICV injection of 300 nmol of histamine considerably reduced cumulative food intake (p <0.05). In Figure 7, the co-injection of UBP-302 and histamine reduced the hypophagic influence of histamine compared to the control group (p <0.05). In Experiment eight, no vital effect was observed on the cumulative food intake of birds receiving ICV injections of AIDA (2 nmol) (p >0.05). However, ICV injection of histamine (300 nmol) considerably reduced cumulative food intake compared to the control group (p <0.05). Moreover, in Figure 8, the co-injection of AIDA plus histamine showed no effect on histamine compared to the control group (p >0.05). In Experiment nine, ICV injection of LY341495 (150 nmol) had no significant influence on cumulative food intake (p >0.05), however, histamine (300 nmol) considerably reduced food intake (p <0.05). In Figure 9, the co-injection of LY341495 plus histamine augmented histamine-induced hypophagia in comparison with the control group (p <0.05). In Experiment three, no vital influence on cumulative food intake was observed in birds receiving ICV injections of UBP1112 (2 nmol) (p >0.05); However, ICV injection of histamine (300 nmol) considerably reduced food intake (p <0.05). In Figure 10, the co-injection of UBP1112 + histamine had no significant influence on the hypophagic consequences of histamine (p >0.05).
Figure 4.
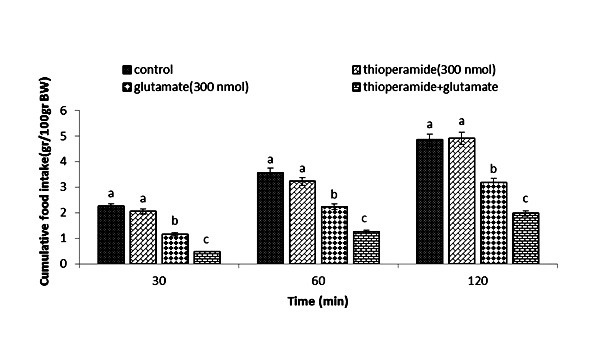
Effect of ICV injection of thioperamide (300 nmol), glutamate (300 nmol), and their combination on cumulative food intake (% BW) in neonatal layer chickens. Thioperamide: histamine H3 receptor antagonist. Data expressed as mean ± SEM. Different letters (a, b, and c) indicate significant differences between treatments (p < 0.05).
Figure 5.
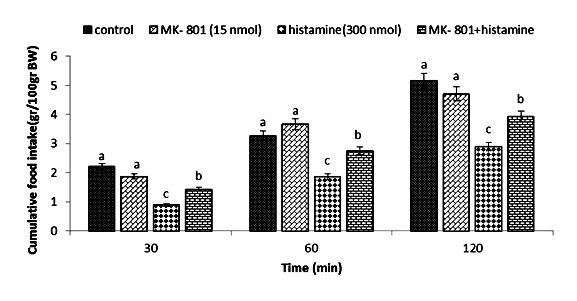
Effect of ICV injection of MK-801 (15 nmol), histamine (300 nmol). And their combination on cumulative food intake (% BW) in neonatal layer chickens. MK-801: NMDA receptor antagonist. Data expressed as mean ± SEM. Different letters (a, b, and c) indicate significant differences between treatments (p < 0.05).
Figure 6.
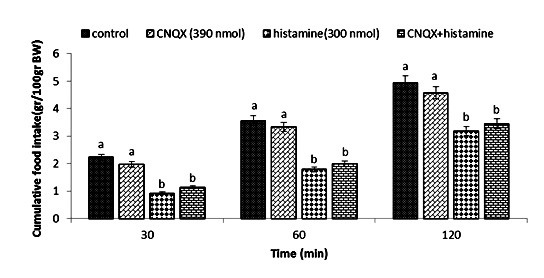
Effect of ICV injection of CNQX (390 nmol), histamine (300 nmol), and their combination on cumulative food intake (% BW) in neonatal layer chickens. CNQX: AMPA receptor antagonist. Data expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments (p < 0.05).
Figure 7.
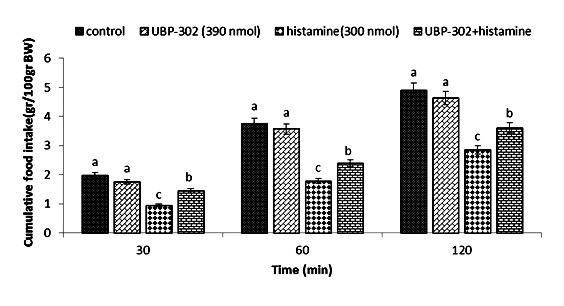
Effect of ICV injection of UBP-302 (390 nmol), histamine (300 nmol), and their combination on cumulative food intake (% BW) in neonatal layer chickens. UBP-302: Kainate receptor antagonist. Data expressed as mean ± SEM. Different letters (a, b, and c) indicate significant differences between treatments (p < 0.05).
Figure 8.
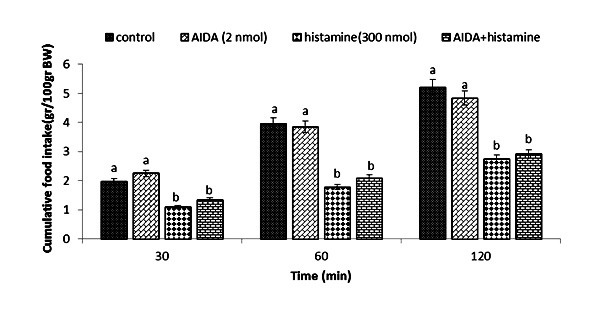
Effect of ICV injection of AIDA (2 nmol), histamine (300 nmol), and their combination on cumulative food intake (% BW) in neonatal layer chickens. AIDA: Effect of ICV injection of AIDA (2 nmol), histamine (300 nmol), and their combination on cumulative food intake (% BW) in neonatal layer chickens. AIDA: mGluR1 antagonist. Data expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments (p < 0.05).
Figure 9.
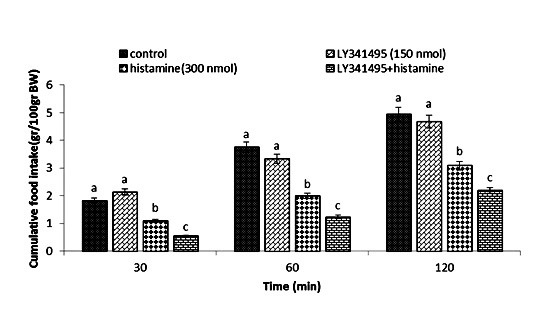
Effect of ICV injection of LY341495 (150 nmol), histamine (300 nmol), and their combination on cumulative food intake (% BW) in neonatal layer chickens. LY341495: mGluR2 antagonist. Data expressed as mean ± SEM. Different letters (a, b, and c) indicate significant differences between treatments (p < 0.05)
Figure 10.
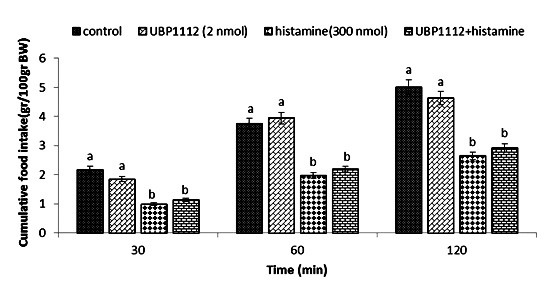
Effect of ICV injection of UBP1112 (2 nmol), histamine (300 nmol), and their combination on cumulative food intake (% BW) in neonatal layer chickens. UBP1112: mGluR3 antagonist. Data expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments (p < 0.05)
4. Discussion
This report is the most comprehensive on the interaction between both HAergic and glutamatergic systems in the reward instruction in FD3 neonatal layer chickens. Based on the findings, food intake can be reduced by ICV injection of histamine. According to ( 9 , 15 ), the ypophagic function in rats and broilers is related to H1 receptors. Broilers may be affected by H2 receptors that show an anorexic influence ( 31 ). Moreover, thioperamide reduces feeding behavior in broilers ( 15 ). Zendehdel, Hamidi ( 30 ) suggested that in poultry, the mediation of histamine effects will be done through H1 receptors; however, contentious reports exist for H3 receptors. Taati, Babapour ( 10 ) suggested that ICV injection of thioperamide could reduce food intake in broilers. According to Zendehdel, Hamidi ( 30 ), in poultry brains, limited information is recognized for H4 receptors. Passani, Blandina ( 32 ) believed that in lighting period, deprived or fasted rates, ICV injection of thioperamide showed no effect on the feeding behavior of chickens. They also suggested that feeding might be affected when the activity in the histaminergic system is not high. According to Hancock and Brune ( 33 ), the barrier of the H3 receptors decreases food intake in rats, and H1 receptor antagonists reduces these effects.
Clearly, the amount of food taken in by FD3 neonatal layer chickens is reduced by ICV injection of glutamate. According to Baghbanzadeh and Babapour ( 28 ), because of the significant function of glutamate in controlling food intake, manipulation of vesicular concentration can influence food intake in broilers. Glutamate has the capability of reducing food intake in broiler chickens, and this outcome is moderated by not only ionotropic, but also metabotropic receptors ( 28 , 29 ). According to Duva, Siu ( 34 ), NMDA receptors may possibly mediate some features of eating as well as satiety. Ciranna ( 35 ) suggested that neurons use glutamate and consider it a co-transmitter that functions through AMPA/Kainate-mediated excitatory post-synaptic potentials (EPSPs). Baghbanzadeh and Babapour ( 28 ) suggested that ionotropic glutamate receptor antagonists boost the process of feeding behavior and reduce birds’ latency to begin feeding, whereas metabotropic glutamate receptor antagonists reduce food intake.
The co-injection of α-FMH reduces hypophagic influence of glutamate. In addition, the co-injection of the H1 receptor antagonist (chlorpheniramine) + glutamate reduces the hypophagic outcome of the glutamate. Co-injection of the H3 receptor antagonist (thioperamide) + glutamate increased glutamate-induced hypophagia. The hypophagic influence of histamine could be reduced by the co-injection of the NMDA receptor antagonist (MK-801) + histamine. Apart from this, the hypophagic influence of histamine could be reduced by the co-injection of the Kainate receptor antagonist (UBP-302) + histamine. Histamine-induced hypophagia was improved by the co-injection of the mGluR2 antagonist (LY341495) + histamine. Release of glutamate could be modulated by histamine H3 receptors ( 16 ). The expression of anti-apoptotic proteins is boosted when H3 receptors in the mouse cerebral cortex were activated, and at this level, the activation of the NMDA will be started ( 16 ).
According to Garduño-Torres, Treviño ( 18 ), the expression of both receptors in the thalamus as well as the cerebellum evolves the instruction of motor behavior, and this happens by restraining glutamate release from thalamostriatal and thalamocortical terminals. According to Faucard, Armand ( 36 ), both NMDA receptors and neuronal glutamate transporters are demonstrated on the cell bodies in the hypothalamus, where histaminergic cell bodies are situated and obtain main excitatory glutamatergic inputs coming from the prefrontal cortex and other brain parts at the same time ( 37 ). According to Okakura-Mochizuki, Mochizuki ( 38 ), the NMDA receptor agonist boosts the histamine release when it is injected into the frontal hypothalamus, also signifying that glutamate has a stimulatory function on the release of histamine. To bind glutamate NMDA receptors in the brain, three histamine receptors are required. Haas, Sergeeva ( 39 ) suggested that both H1and H2 receptor-mediated actions are typically excitatory, whereas H3 receptors are inhibitory heteroreceptors. Pre-treating the animals with LY379268 (3-10 mg/kg) histamine efflux boosts the nucleus accumbens (NAcc) and reduces the histamine reaction to ketamine. According to Fell, Katner ( 37 ), when mGluR2 receptors are activated histamine release is reduced in the rat limbic area, and this influence is held by the presynaptic inhabitation of glutamate release. Receptors may show a new mechanism to reduce unnecessary histamine neurotransmission. According to Fell, Flik ( 40 ), histamine release, which is modulated by mGluR2 receptor agonists, can probably compensate for offered antipsychotic drugs which are potent antagonists of histamine H1 receptors. Fell, Katner ( 37 ) suggested that these receptors have a key function in numerous physiological roles, including wakefulness, learning, memory, and appetite regulation. According to Fell, Flik ( 40 ), the activation of mGluR2 receptors may possibly demonstrate a new mechanism for modulating improved histaminergic tone which has no acquired side effects related to the blockade of histamine H1 receptors. Faucard, Armand ( 36 ) believed that NMDA antagonists amplify the synthesis, provide a base for histamine to turnover, and indicate the inhibitory action of NMDA receptors on tuberomammillary nucleus (TMN) neurons.
Wake-active histaminergic neurons could create paracrine GABAergic signs, which serve to put a brake on histamine activation; however, it could boost the precision of neocortical dispensation ( 41 ). The HAergic neurons utilize vesicular GABA transporter for releasing GABA. According to Yu, Ye ( 41 ), when GABA is released from HAergic neurons, it is somewhat like histamine and functions in a paracrine mode that could make these neurons an unanticipated basis of GABAergic volume transmission in both the neocortex as well as the striatum. Light generates a broad increase in synaptic drive onto pyramidal neurons when the vesicular GABA transporter is absent; in this situation, pyramidal neurons are blocked by H1 and H2 receptor antagonists. According to Ellender, Huerta-Ocampo ( 42 ), the influence of histamine probably reflects the engagement of various modifications in the inputs impinging onto pyramidal neurons.
Prast, Tran ( 43 ) believed that the H3 receptor-mediated inhibition of GABA or dopamine release could consequently reduce the inhibitory control by these transmitters of acetylcholine release.
Although neither the cellular nor the molecular mechanism for the HAergic interaction and glutamatergic system are not entirely understood, but it is obvious that the second messenger-mediated modulation of ionotropic receptors could be mediated when NMDA is facilitated by NMDA receptors through PKC and a decrease in the Mg2+ block as a consequence of H1 receptor activation ( 39 ). According to Haas, Sergeeva ( 39 ), histamine directly assists NMDA receptors and boosts excitatory transmission by its polyamine modulator site. Osorio-Espinoza, Alatorre ( 44 ) believed that pre-synaptic H3 receptors reduce glutamatergic transmission in various parts including rat hippocampus, thalamus, striatum, and basolateral amygdale. According to Osorio-Espinoza, Alatorre ( 44 ), when the H3 receptor is activated in striatal and thalamic synaptosomes, glutamate release is reduced and intracellular Ca2+ is boosted and induced by depolarization. In conclusion, and according to the results, there is an interconnection between the mentioned systems on food intake regulation through H1, H3 histamine and NMDA, Kainate, and mGluR2 glutamate receptors in neonatal layer chickens. No previous research has investigated the function of central HAergic or glutamatergic systems on food intake in poultry. Therefore, based on our information, we were unable to compare our findings.
Authors' Contribution
Study concept and design: B. V.
Acquisition of data: M. M. F.
Analysis and interpretation of data: M. Z.
Drafting of the manuscript: B. V.
Critical revision of the manuscript for important intellectual content: B. V.
Statistical analysis: A. A.
Administrative, technical, and material support: M. Z.
Ethics
All procedures performed in this study involving animal participants were in accordance with the ethical standards of the Islamic Azad University of Tehran, Iran under the project number of 2019-4747456-2.
Grant Support
This study was supported by Islamic Azad University, Science and research Branch, Veterinary Faculty.
Acknowledgement
The authors express their appreciation to the central laboratory (Dr. Rastegar Lab.) of the Faculty of Veterinary Medicine, University of Tehran for their cooperation. This research was conducted as part of the PhD thesis of the first author.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.ReferencesYousefvand S, Hamidi F, Zendehdel M, Parham A. Interaction of neuropeptide Y receptors (NPY1, NPY2 and NPY5) with somatostatin on somatostatin-induced feeding behaviour in neonatal chicken. Br Poult Sci. 2019;60(1):71–8. doi: 10.1080/00071668.2018.1547359. [DOI] [PubMed] [Google Scholar]
- 2.Hassanpour S, Zendehdel M, Babapour V, Charkhkar S. Endocannabinoid and nitric oxide interaction mediates food intake in neonatal chicken. Br Poult Sci. 2015;56(4):443–51. doi: 10.1080/00071668.2015.1059407. [DOI] [PubMed] [Google Scholar]
- 3.Parker KE, Johns HW, Floros TG, Will MJ. Central amygdala opioid transmission is necessary for increased high-fat intake following 24-h food deprivation, but not following intra-accumbens opioid administration. Behav Brain Res. 2014;260:131–8. doi: 10.1016/j.bbr.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blandina P, Provensi G, Munari L, Passani MB. Histamine neurons in the tuberomamillary nucleus: a whole center or distinct subpopulations? . Front Syst Neurosci. 2012;6(33) doi: 10.3389/fnsys.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannoni P, Passani M-B, Nosi D, Chazot PL, Shenton FC, Medhurst AD, et al. Heterogeneity of histaminergic neurons in the tuberomammillary nucleus of the rat. Eur J Neurosci. 2009;29(12):2363–74. doi: 10.1111/j.1460-9568.2009.06765.x. [DOI] [PubMed] [Google Scholar]
- 6.Schneider EH, Neumann D, Seifert R. Modulation of behavior by the histaminergic system: Lessons from HDC-, H3R- and H4R-deficient mice. Neurosci Biobehav Rev. 2014;47:101–21. doi: 10.1016/j.neubiorev.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Rafiei M, Taati M, Alavi S, Nayebzadeh H, Zendehdel M. Effects of intracerebroventricular injection of histamine and H1, H2 receptor antagonists on electrocardiographic parameters in broiler chickens. Iranian J Vet Res. 2011;12 (3):192–8. [Google Scholar]
- 8.Rozov SV, Zant JC, Karlstedt K, Porkka-Heiskanen T, Panula P. Periodic properties of the histaminergic system of the mouse brain. Eur J Neurosci . 2014;39(2):218–28. doi: 10.1111/ejn.12397. [DOI] [PubMed] [Google Scholar]
- 9.Morimoto T, Yamamoto Y, Yamatodani A. Brain histamine and feeding behavior. Behavioural Brain Research. Behav Brain Res. 2001;124(2):145–50. doi: 10.1016/s0166-4328(01)00225-x. [DOI] [PubMed] [Google Scholar]
- 10.Taati M, Babapour V, Kheradmand A, Tarrahi MJ. The role of central endogenous histamine and H1, H2 and H3 receptors on food intake in broiler chickens. Iranian J Vet Res . 2009;10(1):54–60. [Google Scholar]
- 11.Irwin N, Hunter K, Frizzell N, Flatt PR. Antidiabetic effects of sub-chronic administration of the cannabinoid receptor (CB1) antagonist, AM251, in obese diabetic (ob/ob) mice. Eur J Pharmacol. 2008;581(1):226–33. doi: 10.1016/j.ejphar.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Charles JR, Duva MA, Ramirez GJ, Lara RL, Yang CR, Stanley BG. Activation of lateral hypothalamic mGlu1 and mGlu5 receptors elicits feeding in rats. Neuropharmacology. 2014;79:59–65. doi: 10.1016/j.neuropharm.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 13.McFadden KL, Cornier M-A, Tregellas JR. The role of alpha-7 nicotinic receptors in food intake behaviors. Front Psychol. 2014;5(553) doi: 10.3389/fpsyg.2014.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Da Silva AA, Marino-Neto J, Paschoalini MA. Feeding induced by microinjections of NMDA and AMPA–kainate receptor antagonists into ventral striatal and ventral pallidal areas of the pigeon. Brain Res . 2003;966(1):76–83. doi: 10.1016/s0006-8993(02)04196-3. [DOI] [PubMed] [Google Scholar]
- 15.Taati M, Nayebzadeh H, Zendehdel M. The effects of DL-AP5 and glutamate on ghrelin-induced feeding behavior in 3-h food-deprived broiler cockerels. J Physiol Biochem. 2011;67(2):217–23. doi: 10.1007/s13105-010-0066-y. [DOI] [PubMed] [Google Scholar]
- 16.García-Gálvez AM, Arias-Montaño JA. Isoforms of the human histamine H3 receptor: Generation, expression in the central nervous system and functional implications. Gaceta medica de Mexico. Gac Med Mex. 2016;152:82–90. [PubMed] [Google Scholar]
- 17.Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63(6):637–72. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 18.Garduño-Torres B, Treviño M, Gutiérrez R, Arias-Montaño J-A. Pre-synaptic histamine H3 receptors regulate glutamate, but not GABA release in rat thalamus. Neuropharmacology. 2007;52(2):527–35. doi: 10.1016/j.neuropharm.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Zendehdel M, Ebrahimi-Yeganeh A, Hassanpour S, Koohi MK. Interaction of the dopaminergic and Nociceptin/Orphanin FQ on central feed intake regulation in chicken. Br Poult Sci. 2019;60(3):317–22. doi: 10.1080/00071668.2019.1596225. [DOI] [PubMed] [Google Scholar]
- 20.Zendehdel M, Hassanpour S. Ghrelin-induced hypophagia is mediated by the β2 adrenergic receptor in chicken. J Physiol Sci. 2014;64(5):383–91. doi: 10.1007/s12576-014-0330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olanrewaju HA, Purswell J, Collier SD, Branton SL. Effects of light ingress through ventilation fan apertures on selected blood variables of male broilers. Int J Poult Sci . 2017;16:288–95. [Google Scholar]
- 22.Blevins JE, Stanley BG, Reidelberger RD. DMSO as a vehicle for central injections: tests with feeding elicited by norepinephrine injected into the paraventricular nucleus. Pharmacol Biochem Behav. 2002;71(1):277–82. doi: 10.1016/s0091-3057(01)00659-1. [DOI] [PubMed] [Google Scholar]
- 23.Qi W, Ding D, Salvi RJ. Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res. 2008;236(1):52–60. doi: 10.1016/j.heares.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A. Autoradiographic distribution of L-proline in chicks after intracerebral injection. Physiol Behav. 1979;22(4):693–5. doi: 10.1016/0031-9384(79)90233-6. [DOI] [PubMed] [Google Scholar]
- 25.Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S. The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol. 1997;339(2):211–3. doi: 10.1016/s0014-2999(97)01391-5. [DOI] [PubMed] [Google Scholar]
- 26.Saito E-S, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, et al. Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept. 2005;125(1):201–8. doi: 10.1016/j.regpep.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Furuse M, Ando R, Bungo T, Shimojo M, Masuda Y. Intracerebroventricular injection of orexins does not stimulate food intake in neonatal chicks. Br Poult Sci. 1999;40(5):698–700. doi: 10.1080/00071669987115. [DOI] [PubMed] [Google Scholar]
- 28.Baghbanzadeh A, Babapour V. Glutamate ionotropic and metabotropic receptors affect feed intake in broiler cockerels. J Vet Res. 2007;62(4):125–9. [Google Scholar]
- 29.Zendehdel M, Baghbanzadeh A, Babapour V, Cheraghi J. The effects of bicuculline and muscimol on glutamate-induced feeding behavior in broiler cockerels. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195(8):715–20. doi: 10.1007/s00359-009-0446-3. [DOI] [PubMed] [Google Scholar]
- 30.Zendehdel M, Hamidi F, Hassanpour S. The Effect of Histaminergic System on Nociceptin/Orphanin FQ Induced Food Intake in Chicken. Int J Pept Res Ther . 2015;21(2):179–86. [Google Scholar]
- 31.Meade S, Denbow DM. Feeding, drinking, and temperature responses of chickens to intracerebroventricular histamine. Physiol Behav. 2001;73(1):65–73. doi: 10.1016/s0031-9384(01)00438-3. [DOI] [PubMed] [Google Scholar]
- 32.Passani MB, Blandina P, Torrealba F. The histamine H3 receptor and eating behavior. J Pharmacol Exp Ther . 2011;336(1):24–9. doi: 10.1124/jpet.110.171306. [DOI] [PubMed] [Google Scholar]
- 33.Hancock AA, Brune ME. Assessment of pharmacology and potential anti-obesity properties of H3 receptor antagonists/inverse agonists. Expert Opin Investig Drugs. 2005;14(3):223–41. doi: 10.1517/13543784.14.3.223. [DOI] [PubMed] [Google Scholar]
- 34.Duva MA, Siu A, Stanley BG. The NMDA receptor antagonist MK-801 alters lipoprivic eating elicited by 2-mercaptoacetate. Physiol Behav. 2005;83(5):787–91. doi: 10.1016/j.physbeh.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 35.Ciranna L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol. 2006;4(2):101–14. doi: 10.2174/157015906776359540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faucard R, Armand V, Héron A, Cochois V, Schwartz J-C, Arrang J-M. N-methyl-d-aspartate receptor antagonists enhance histamine neuron activity in rodent brain. J Neurochem. 2006;98(5):1487–96. doi: 10.1111/j.1471-4159.2006.04002.x. [DOI] [PubMed] [Google Scholar]
- 37.Fell MJ, Katner JS, Johnson BG, Khilevich A, Schkeryantz JM, Perry KW, et al. Activation of metabotropic glutamate (mGlu)2 receptors suppresses histamine release in limbic brain regions following acute ketamine challenge. Neuropharmacology. 2010;58(3):632–9. doi: 10.1016/j.neuropharm.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Okakura-Mochizuki K, Mochizuki T, Yamamoto Y, Horii A, Yamatodani A. Endogenous GABA Modulates Histamine Release from the Anterior Hypothalamus of the Rat. J Neurochem. 1996;67(1):171–6. doi: 10.1046/j.1471-4159.1996.67010171.x. [DOI] [PubMed] [Google Scholar]
- 39.Haas HL, Sergeeva OA, Selbach O. Histamine in the Nervous System. Physiol Rev. 2008;88(3):1183–241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 40.Fell MJ, Flik G, Dijkman U, Folgering JHA, Perry KW, Johnson BJ, et al. Glutamatergic regulation of brain histamine neurons: In vivo microdialysis and electrophysiology studies in the rat. Neuropharmacology. 2015;99:1–8. doi: 10.1016/j.neuropharm.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 41.Yu X, Ye Z, Houston Catriona M, Zecharia Anna Y, Ma Y, Zhang Z, et al. Wakefulness Is Governed by GABA and Histamine Cotransmission. Neuron. 2015;87(1):164–78. doi: 10.1016/j.neuron.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellender TJ, Huerta-Ocampo I, Deisseroth K, Capogna M, Bolam JP. Differential Modulation of Excitatory and Inhibitory Striatal Synaptic Transmission by Histamine. J Neurosci. 2011;31(43):15340–51. doi: 10.1523/JNEUROSCI.3144-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prast H, Tran MH, Fischer H, Kraus M, Lamberti C, Grass K, et al. Histaminergic neurons modulate acetylcholine release in the ventral striatum: role of H3 histamine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;360(5):558–64. doi: 10.1007/s002109900097. [DOI] [PubMed] [Google Scholar]
- 44.Osorio-Espinoza A, Alatorre A, Ramos-Jiménez J, Garduño-Torres B, García-Ramírez M, Querejeta E, et al. Pre-synaptic histamine H3 receptors modulate glutamatergic transmission in rat globus pallidus. Neuroscience. 2011;176:20–31. doi: 10.1016/j.neuroscience.2010.12.051. [DOI] [PubMed] [Google Scholar]


