Abstract
Obstructive sleep apnea syndrome (OSAS) and obesity are frequently associated with hypertension (HTN), dyslipidemia (DLP), and insulin resistance (IR). In patients with obesity and OSAS scheduled for bariatric surgery (BS), guidelines recommend at least 4 weeks of preoperative continuous positive airway pressure (CPAP). Low-calorie ketogenic diets (LCKDs) promote pre-BS weight loss (WL) and improve HTN, DLP, and IR. However, it is unclear whether pre-BS LCKD with CPAP improves OSAS more than CPAP alone. We assessed the clinical advantage of pre-BS CPAP and LCKD in patients with obesity and OSAS. Seventy patients with obesity and OSAS were randomly assigned to CPAP or CPAP+LCKD groups for 4 weeks. The effect of each intervention on the apnea–hypopnea index (AHI) was the primary endpoint. WL, C-reactive protein (CRP) levels, HTN, DLP, and IR were secondary endpoints. AHI scores improved significantly in both groups (CPAP, p=0.0231; CPAP+LCKD, p=0.0272). However, combining CPAP and LCKD registered no advantage on the AHI score (p=0.863). Furthermore, body weight, CRP levels, and systolic/diastolic blood pressure were significantly reduced in the CPAP+LCKD group after 4 weeks (p=0.0052, p=0.0161, p=0.0008, and p=0.0007 vs baseline, respectively), and CPAP+LCKD had a greater impact on CRP levels than CPAP alone (p=0.0329). The CPAP+LCKD group also registered a significant reduction in serum cholesterol, LDL, and triglyceride levels (p=0.0183, p=0.0198, and p<0.001, respectively). Combined with CPAP, LCKD-induced WL seems to not have a significant incremental effect on AHI, HTN, DLP, and IR but lower CRP levels demonstrated a positive impact on chronic inflammatory status.
Keywords: Obstructive sleep apnea syndrome, Obesity, Weight loss, Ketogenic diet, Apnea– hypopnea index, C-reactive protein, Bariatric surgery
Introduction
Obstructive sleep apnea syndrome (OSAS) has a prevalence in the bariatric surgical population of up to 50–70% [1]. Clinical data associate OSAS with hypertension (HTN), dyslipidemia (DLP), insulin resistance (IR), and inflammation [2–4]. Polysomnography is the gold standard for the diagnosis of OSAS [5], and the apnea–hypopnea index (AHI) is used to quantify severity based on an international score [6].
Bariatric surgery (BS) is associated with a marked long-term weight loss (WL) and the resolution or improvement of co-morbid diseases [7]. To improve the AHI score in obese patients with severe OSAS (>30) scheduled for BS, preoperative continuous positive airway pressure (CPAP) is recommended for at least 4 weeks to reduce anesthesia risks [8]. Furthermore, body weight (BW) reduction is associated with improvements in OSAS, with a 10% reduction in BW predicting an approximate 26–32% change in the AHI score [9]. An accurate preoperative multidisciplinary assessment of bariatric patients plays an important role in improving performance status, surgical outcome, and WL [10–17]. Previous studies have reported that a low-calorie ketogenic diet (LCKD) is safe and effective in reducing BW and liver volume in patients scheduled for BS, resulting in a reduction of intraoperative complications and operating time [18–22]. To date, no prospective multicenter randomized trial has evaluated whether preoperative LCKD combined with CPAP improves OSAS versus treatment with CPAP alone. Therefore, the present pilot trial aims to assess the clinical advantage in combining two preoperative strategies (CPAP and LCKD) compared to CPAP alone, to improve AHI score, HTN, DLP, IR, and CRP levels in patients with severe obesity and OSAS scheduled for BS.
Patients and Methods
Study Design and Characteristics of the Patients at Baseline
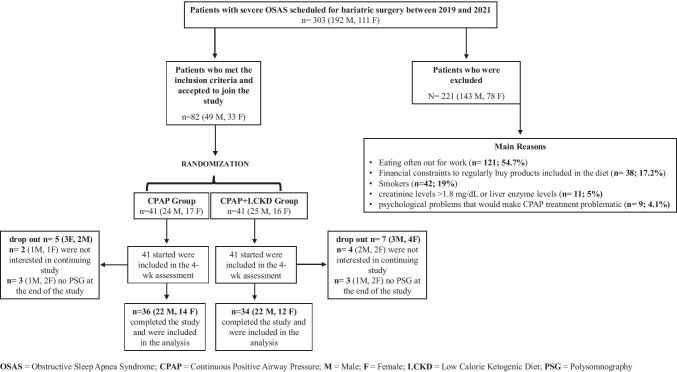
Between January 2019 and April 2021, we conducted a pilot, prospective, randomized, multicenter study involving a cohort of patients with severe OSAS (AHI ≥30) scheduled for BS. A total of 303 patients were selected, 82 underwent randomization, and 70 completed the study (Fig. 1). All patients fulfilled the criteria established by the International Federation for Surgery of Obesity and the Italian Society for Obesity Surgery and Related Diseases for the surgical treatment of morbid obesity [23]. Inclusion criteria included patients of both sexes with body mass index (BMI) ≥ 35 associated with severe OSAS (AHI ≥ 30); aged 18–65 years; and non-smokers or those who have quit smoking for at least 3 months. Exclusion criteria included kidney and/or liver conditions that would make LCKD unsuitable (creatinine levels >1.8 mg/dL or liver enzyme levels [glutamic pyruvic transaminase or glutamic oxaloacetic transaminase] less than three times over the upper normal threshold); psychological problems that would make CPAP treatment problematic; and patients with obesity >60 BMI. The study protocol was approved by the University Ethical Committee (0199138/2018) and was registered on ClinicalTrials.gov (NCT03791242). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments. Informed written consent was obtained from each participant after being informed about the purpose and nature of the study.
Fig. 1.
Numbers of patients who were included, randomly assigned to a study group, and included in the analysis
Polysomnography and Randomization
A 12-channel diagnostic polysomnography (in a sleep laboratory for a full night) was used to assess the AHI score in all patients. Patients with a polysomnogram that showed an AHI ≥30 were randomly assigned to a study group. In consideration of the small sample size, block randomization was used to achieve a balance in the allocation of patients to treatment arms and to increase the probability that each arm contained an equal number of patients [24]. The 70 patients who met the inclusion criteria were randomized into two groups: the CPAP group and the CPAP+LCKD group. The CPAP group comprised 36 patients with obesity (14 Females [F], 22 Males [M]) and severe OSAS suitable for BS who underwent 4 weeks of CPAP (these patients were not prescribed a change in eating habits) according to the standard. The CPAP+LCKD group comprised 34 patients with morbid obesity (12 F, 22 M) and severe OSAS suitable for BS who underwent 4 weeks of CPAP and an LCKD.
Interventions
In the CPAP and CPAP+LKCD groups, participants underwent an overnight in-laboratory sleep study to allow for the individual calibration of the CPAP therapy. Nightly CPAP therapy was provided thereafter through a fixed-pressure or auto-adjusting CPAP device. Adherence to CPAP therapy was monitored weekly by means of a wireless router attached to the CPAP device. Participants in the CPAP and CPAP+LCKD groups had individual weekly counseling sessions. In the CPAP+LCKD group, the goal for caloric intake was set at 1200 kcal/day.
Development of the LCKD
Before starting the LCKD, candidates were counseled individually about the diet that they would be expected to follow for 4 weeks. To ensure that all 34 patients that were included consumed a similar diet, we developed two LCKD meal plans, plan 1 (days 1–14) and plan 2 (days 15–28), and assigned individual foods a specific quantity using a free online keto diet application (https://www.eatthismuch.com). Each ketogenic food plan (from 1150 to 1250 kcal/day) consisted of 4% carbohydrates, 71% fats, and 25% proteins. Supplement composition (Ketocompleat, MVMedical Solutions, Serravalle, Repubblica San Marino) is reported in Table 1. Ketocompleat is included on the register of food supplements of the Italian Minister of Health (code number 94721) and, due to its carbohydrate-free formulation, may be associated with a low-carbohydrate ketogenic diet [19]. The diet plans and the Ketocompleat supplement were kindly provided free of charge to all trial participants by MVMedical Solutions, which had no role in designing trial and patient enrollment in the different participating sites or in processing any trial-related data.
Table 1.
Composition of the supplement administered during the course of the study (Ketocompleat, MVMedical Solution, Serravalle, San Marino)
| Element | Daily value (40 g) | RDA* |
|---|---|---|
| Soy Protein | 33.31 gr | - |
| Vitamin A | 0.483 mg | 60,38% |
| Vitamin B1 | 0.496 mg | 45,09% |
| Vitamin B2 | 0.833 mg | 59,50% |
| Vitamin B3 | 8.33 mg | 52,06% |
| Vitamin B5 | 1.28 mg | 21,33% |
| Vitamin B6 | 0.92 mg | 65,71% |
| Biotin | 0.028 mg | 56% |
| Vitamin B12 | 0.007 mg | 280% |
| Folic acid | 0.222 mg | 111% |
| Vitamin C | 83.28 mg | 104,10% |
| Vitamin D3 | 0.011 mg | 222% |
| Vitamin E | 12.65 mg | 105,42% |
| Vitamin K1 | 11.1 mg | 14,80% |
| Iron | 5.5 mg | 39,29% |
| Copper | 0.08 mg | 8% |
| Magnesium | 39.9 mg | 10,64% |
| Selenium | 0.015 mg | 27,27% |
| Manganese | 0.19 mg | 9,50% |
| Chromium | 0.003 mg | 7,50% |
| Calcium | 116.76 mg | 14,60% |
| Zinc | 8.74 mg | 87,40% |
| Inulin | 1.38 g | - |
| Phaseolamin | 1.11 g | - |
| Fructo-oligo-saccharides | 1.11 g | - |
| Lactobacillus plantarum | 2.24 mld | - |
| Lactobacillus acidophilus | 2.2 mld | - |
| Lactobacillus rhamnosus | 2.2 mld | - |
| Bifidobacterium longum | 2.24 mld | - |
| Saccharomyces boulardi | 4.48 mld | - |
Recommended Dietary Allowance
Study Assessment and Endpoints
An assessment was performed at baseline and 4 weeks after the initiation of the therapy in both study groups. The primary endpoint was to assess the clinical advantage in combining two preoperative strategies (CPAP+LCKD) vs CPAP alone, for the reduction of the AHI score. Secondary endpoints included changes in HTN, DLP, IR, and CRP levels in patients with obesity and severe OSAS who are scheduled for BS.
Anthropometric Evaluation of the Study Population and Blood Tests
In all patients, BW (kg) and height (cm) were determined under standard conditions (fasting state in light street clothes with shoes and any other heavy items removed). Height was measured using a mechanical measuring tape. BW was assessed by using medical weighing scales (capacity 250 Kg). BW evaluation was done at baseline and repeated weekly during the 4-week follow-up. Blood tests included glycemic profile parameters (glucose, insulin, and HOMA index), lipid profile (total cholesterol, high-density lipoprotein [HDL], low-density lipoprotein [LDL], and triglycerides), and ketonemia. All blood analyses were performed in an approved laboratory with internal and external quality controls using the reagents provided bythe manufacturer and following the manufacturer’s instructions. Data were compared with accepted clinical cutoff values.
Statistical Analysis
The possible clinical advantage of the combination of the two preoperative strategies (CPAP+LCKD) compared to preoperative CPAP treatment in reducing the surgical risk in morbidly obese OSAS patients were directly compared by using the paired sample t-test for continuous variables for comparison within groups and the Mann–Whitney test for comparison between CPAP and CPAP+LCKD groups. Block randomization was performed using free online GraphPad Quick Calcs Software (https://www.graphpad.com/quickcalcs/randomize1/). Data are reported as mean ± standard deviation (SD). A p < 0.05 was considered statistically significant. Furthermore, any p value less than 0.0001 was conventionally stated as p < 0.001. GraphPad Prism for Windows (Version 9.1.2p) was used for statistics.
Results
Impact of CPAP or CPAP and LCKD on AHI
The study included 70 subjects (16 females and 44 males) with a mean age of 42 (±13.7) years. Mean initial BW and BMI of participants are reported in Table 2.
Table 2.
Patient’s clinical parameters at baseline and after treatments (CPAP or CPAP+LCKD, 4 weeks). Data are reported as mean ± standard deviation. *Any p<0.05 was considered statistically significant
| Clinical parameters | Groups | Baseline | Follow-up (4 weeks) |
p |
|---|---|---|---|---|
| Body weight | CPAP | 132.7 ± 23 | 131.6 ± 22.3 | 0.816 |
| CPAP+LCKD | 143.6 ± 23.6 | 129.7 ± 23.7 | 0.0052* | |
| BMI | CPAP | 47.6 ± 5.9 | 47.2 ± 5.7 | 0.756 |
| CPAP+LCKD | 50.1 ± 5.9 | 45.3 ± 6.5 | <0.001* | |
| AHI score | CPAP | 63.3 ± 21 | 47.9 ± 20 | 0.0023 |
| CPAP+LCKD | 62.7 ± 22.4 | 50.4 ± 22.7 | 0.0272* | |
| CRP (mg/L) | CPAP | 5.95 ± 5.9 | 6.36 ± 6.0 | 0.855 |
| CPAP+LCKD | 6.12 ± 5.96 | 2.66 ± 2.57 | 0.0161* | |
| Blood pressure (systolic, mmHg) | CPAP | 134.2 ± 10.4 | 130 ± 9.7 | 0.0721 |
| CPAP+LCKD | 142.8 ± 13.3 | 133 ± 11.9 | 0.0008* | |
| Blood pressure (diastolic, mmHg) | CPAP | 87 ± 11.6 | 82 ± 9.5 | 0.0623 |
| CPAP+LCKD | 85.4 ± 8.38 | 78.7 ± 6.43 | 0.0007* | |
| Insulin (mcU/mL) | CPAP | 11 ± 7.04 | 10.4 ± 6.9 | 0.696 |
| CPAP+LCKD | 11.8 ± 6.3 | 10.6 ± 5.6 | 0.422 | |
| HOMA Index | CPAP | 2.67 ± 1.71 | 2.46 ± 1.66 | 0.430 |
| CPAP+LCKD | 3.46 ± 2.66 | 2.76 ± 2.14 | 0.181 | |
| Cholesterol (mg/dL) | CPAP | 196.1 ± 32.9 | 180.8 ± 33.0 | 0.153 |
| CPAP+LCKD | 200.1 ± 30.1 | 180.4 ± 35.2 | 0.0183* | |
| HDL (mg/dL) | CPAP | 46.4 ± 10.3 | 47.3 ± 9.8 | 0.612 |
| CPAP+LCKD | 48.3 ± 9.41 | 48.8 ± 10.4 | 0.910 | |
| LDL (mg/dL) | CPAP | 128 ± 30.2 | 112.9 ± 34.9 | 0.139 |
| CPAP+LCKD | 127.4 ± 26.8 | 107.1 ± 37.1 | 0.0198* | |
| Triglycerides (mg/dL) | CPAP | 151.6 ± 62.5 | 129.7 ± 62.2 | 0.0985 |
| CPAP+LCKD | 191 ± 41.7 | 130 ± 79 | <0.001* | |
| Ketonemia (mmol/L) | CPAP | 0.246 ± 0.32 | 0.240 ± 0.20 | 0.410 |
| CPAP+LCKD | 0.299 ± 0.41 | 0.893 ± 1.22 | 0.0002* |
CPAP continuous positive airway pressure, CPAP+LCKD continuous positive airway pressure + low calories ketogenic diet, AHI apnea–hypopnea index, CRP C-reactive protein, HOMA Index homeostasis model assessment, HDL high-density lipoprotein, LDL low-density lipoprotein
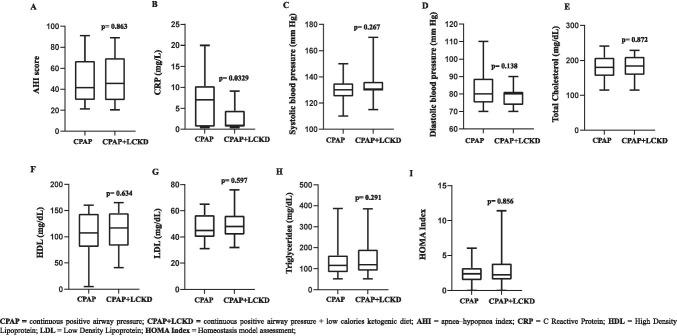
Concerning the primary endpoint, we observed a significant improvement in the AHI score in both groups (CPAP, p=0.0023; CPAP+LCKD, p=0.0272, Table 2). However, we did not observe any advantage in combining CPAP+LCKD compared with CPAP alone (p=0.863, Fig. 2A).
Fig. 2.
CPAP vs CPAP+LCKD on the AHI score (a), CRP levels (b), systolic and diastolic blood pressure (c and d, respectively), total cholesterol (e), HDL (f), LDL (g), triglycerides (h), and HOMA index (i)
Impact of CPAP or CPAP+LCKD on CRP Levels and Blood Pressure
As shown in Table 1, patients who received CPAP alone did not show any significant improvements in CRP levels (p=0.855) or systolic and diastolic blood pressure (p=0.0721 and 0.0623, respectively). On the contrary, patients who followed the CPAP+LCKD had significantly lower CRP levels and a significant improvement in systolic and diastolic blood pressure after 4 weeks (p=0.0161 vs baseline; p= 0.0008 vs baseline; p=0.0007 vs baseline, 155 respectively). We observed a further advantage in combining CPAP+LCKD compared with CPAP alone on CRP levels (p=0.0329, Fig. 2b), whereas we did not observe any further advantage on systolic and diastolic blood pressure (p=0.267 and p=0.138, respectively, Fig. 2c and d).
Impact of CPAP and CPAP+LCKD on BW, BMI, Lipid Profile, and IR
As expected, we observed a significant improvement in BW and BMI only in the CPAP+LCKD group (CPAP: BW and BMI p=0.816 and p=0.756, respectively; CPAP+LCKD: BW and BMI, p=0.0052 and p<0.001, respectively) (Table 2).
In the CPAP group, we did not observe any significant improvement in lipid profile and IR (Total cholesterol, p=0.153; HDL, p=0612; LDL, p=0.139; Triglycerides, p=0.0985; HOMA Index, p=0.430, respectively, Table 2) at the end of the treatment, whereas, in the CPAP+LCKD group, we observed a significant reduction in serum cholesterol, LDL, and triglyceride levels (p=0.0183, p=0.0198, and p<0.001, respectively), whereas no significant difference was found in HDL levels (p=0.910) (Table 2). Furthermore, in CPAP+LCKD vs CPAP alone, we observed a greater but not significant decrease in HOMA index (−20% vs −7.4%, p=0.181). Moreover, no significant incremental effects were detected in combining CPAP+LCKD compared with CPAP alone on lipid profile and IR (Fig. 2E–I).
Adherence in Following the LCKD and Side Effects
Ketone analysis was performed to assess the adherence of the CPAP+LCKD group in following the prescribed diet. Measurements were done at baseline and repeated at 4-week follow-up. As shown in Table 2, in the CPAP group, we did not observe any significant change in ketonemia levels (p=0.410), whereas in the CPAP+LCKD group, we observed a significant increase of ketonemia at the end of the treatment (p=0.0002, Table 2). Furthermore, in the CPAP+LCKD group, ketonemia was highly correlated with total percentage weight reduction at diet completion (r=0.61, p< 0.001). No side effects were reported.
Discussion
The results of the present pilot study found that CPAP combined with LCKD-induced WL did not have a significant incremental effect on AHI score, HTN, DLP, and IR after 4 weeks, but there was a positive impact on chronic inflammatory condition in the population with obesity by lowering CRP levels. Concerning the primary endpoint of the study (AHI score), we found that an improvement in the AHI score of 24% and 24.4% occurred in the CPAP and CPAP+LCKD groups, respectively, after 4 weeks. Furthermore, while we did not observe an improvement in the BW of the CPAP group (these patients were not prescribed a change in eating habits) at the end of the treatment, a reduction of −10.7% was found in the BW of the CPAP+LCKD group. Peppard et al. report that even modest reductions in BW are associated with changes in OSAS, with a 10% reduction in BW predicting an approximative change of 26 to 32% in the AHI index [9].
Concerning CRP levels, Chrinos et al. found that in adults with obesity and OSAS, CPAP combined with a WL intervention did not reduce CRP levels more than intervention with CPAP alone [25]. Indeed, although we did not observe significant improvements in CRP levels in patients who received CPAP alone, patients who followed the CPAP and LCKD showed a significantly lower CRP level at 4 weeks. Possible explanations could be the different dietary compositions used and/or the WL levels. Chrinos et al. observed that in the CPAP combined with a WL intervention group, the dietary composition was aligned with recommendations from the National Cholesterol Education Program (NCEP), and the decline in BW was 7.0 kg in 24 weeks.
In the NCEP diet, the percentages of calories from fat, carbohydrate, and protein are 30%, 55%, and 15% [26], respectively, whereas the LCKD we used in this study consisted of 4% carbohydrates, 71% fats, and 25% proteins. Although it has been well-documented that WL improves inflammation [27, 28], there is little understanding or agreement on the role of carbohydrate restriction or macronutrient composition on inflammation. Some evidence indicates that higher-fat diets are associated with higher serum inflammatory markers [29] and higher protein diets may be beneficial to inflammation [30], although data in this area is very limited. In agreement with the present study, consuming a hypocaloric high fat low-carbohydrate diet for 12 weeks has been reported to lower CRP. On the other hand, other studies have reported that WL, but not macronutrient content, improves systemic inflammation [31–34]. Herein, we report a decrease in BW of 10.7% in 4 weeks of LCKD. Taken together, our data indicate that LCKD-induced WL improves CRP levels because of its composition and the capacity to determine an effective and rapid WL. Furthermore, in accordance with the Chrinos et al. study, we found no discernible decline in BW in the CPAP group.
In this study, and in previous studies, WL significantly reduced HTN and IR, whereas CPAP therapy did not have a significant effect [35, 36]. CPAP alone for 4 weeks did not improve DLP; this finding is consistent with the results of other studies, which showed no significant changes in total cholesterol levels after CPAP therapy [37, 38]. Systolic and diastolic blood pressure significantly decreased only in the CPAP+LCKD group. This was in contrast with the study by Chrinos et al., who reported decreased blood pressure in patients with obesity treated with CPAP [25]. However, data regarding the improvements in blood pressure in patients with obesity and OSAS and treated with CPAP therapy are limited [39–41].
Furthermore, our findings suggest that both OSAS and obesity have an independent causal relation to HTN. We also found that CPAP therapy combined with a WL intervention had an incremental effect on IR when compared with CPAP intervention alone (−20% vs−7.8%), but no significant incremental effects were detected for combination therapy in accordance with a previous study [25].
We acknowledge some methodological limitations in our study. First, we did not include a sham CPAP intervention. However, both sham CPAP and the absence of treatment for OSAS are considered to be adequate controls for an active CPAP intervention [25, 42]. Nevertheless, considering that control conditions are vital to the scientific integrity of clinical trials and may differ depending on the specific trial, due to the complexity of ethical issues, consultation with the institutional review board and an ethicist should be considered during the design phase of CPAP clinical trials. Second, in the CPAP-LCKD group, we were not able to directly measure adherence in following the prescribed diet by a validated questionnaire, such as the 3-day estimated food record and the 72-h recall [43–45]. However, the significant WL associated with the significant increase of blood ketonemia at the end of the treatment (p<0.001, Table 2) may be considered an indirect index of adherence. Furthermore, in both study groups, we were not able to directly measure the health-related quality of life. Finally, our findings cannot be extended to the population with mild and moderate OSAS because of our exclusion criteria. Thereafter, although the data on the reduction of CRP levels is a very interesting finding, our sample of patients is made up of almost 37% women and CRP is a marker of non-specific inflammation possibly linked with endogenous estradiol [46]. Therefore, the complex link between estradiol and inflammation may influence the result. However, at the beginning of the study, we did not consider in the exclusion criteria confounding factors such as sex, the presence of menstruation or menopause, and hormone replacement therapy as it was not among the end points of the research. Moreover, we retain that further researches may necessitate to understand the complex estradiol-inflammation link, as one may act as an important confounder, mediator, or moderator of the other. In any case, accordingly to our finding, a recent paper by Wu et al. has demonstrated that in their study sample, made up of 61% males and 39% females; similar in percentage to our group of patients, obesity is associated with the levels of CRP independently of OSAS severity and sex [47]. Finally, the long period of recruitment is a consequence of COVID-19, which determined a worldwide stop of bariatric surgical programs during the first wave (March–May 2020) and partially during the second wave (October 2020–January 2021). The initial study draft involved three more bariatric centers and a large cohort. However, national and regional restrictions during the pandemic in Italy limited the participating centers.
Conclusions
The results of this pilot study may suggest that CPAP combined with LCKD-induced WL did not have a significant incremental effect on AHI score, HTN, DLP, and IR, whereas it has a positive impact in lowering CRP levels. However, our data show that an LCKD-induced WL intervention is effective in improving HTN, DLP, IR, and inflammation, in patients with severe obesity and OSAS scheduled for BS. Further and larger randomized clinical trials are needed to confirm these preliminary data.
Declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by the university ethical committee (0199138/2018). The study was registered on ClinicalTrials.gov with inscription number NCT03791242.
Conflict of Interest
Luigi Schiavo, Roberto Pierro, Carmela Asteria, Pietro Calabrese, Alberto Di Biasio, Ilenia Coluzzi, Lucia Severino, Alessandro Giovanelli, Vincenzo Pilone, and Gianfranco Silecchia declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Luigi Schiavo, Email: lschiavo@unisa.it.
Roberto Pierro, Email: robertopierro1985@libero.it.
Carmela Asteria, Email: carmela_asteria@yahoo.com.
Pietro Calabrese, Email: pietro.calabrese@gmail.com.
Alberto Di Biasio, Email: a.dibiasio8@gmail.com.
Ilenia Coluzzi, Email: ilenia.dietista@gmail.com.
Lucia Severino, Email: luciaseverino1994@libero.it.
Alessandro Giovanelli, Email: alessandro.giovanelli@grupposandonato.it.
Vincenzo Pilone, Email: vpilone@unisa.it.
Gianfranco Silecchia, Email: gianfranco.silecchia@uniroma1.it.
References
- 1.Frey WC, Pilcher J. Obstructive sleep-related breathing disorders in patients evaluated for bariatric surgery. Obes Surg. 2003;13(5):676–683. doi: 10.1381/096089203322509228. [DOI] [PubMed] [Google Scholar]
- 2.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 3.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165(5):670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 4.Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, Somers VK. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105(21):2462–2464. doi: 10.1161/01.CIR.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 5.Markun LC, Sampat A. Clinician-Focused Overview and Developments in Polysomnography. Curr Sleep Med Rep. 2020;23:1–13. doi: 10.1007/s40675-020-00197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonas DE, Amick HR, Feltner C, Weber RP, Arvanitis M, Stine A, Lux L, Harris RP. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US preventive services task force. JAMA. 2017;317(4):415–433. doi: 10.1001/jama.2016.19635. [DOI] [PubMed] [Google Scholar]
- 7.Thorell A. The 2020 ESPEN Arvid Wretlind lecture: Metabolic response in bariatric surgery - Mechanisms and clinical implications. Clin Nutr. 2021;40(5):2602–2608. doi: 10.1016/j.clnu.2021.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Tham KW, Lee PC, Lim CH. Weight Management in Obstructive Sleep Apnea: Medical and Surgical Options. Sleep Med Clin. 2019;14(1):143–153. doi: 10.1016/j.jsmc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 10.Schiavo L, Sans A, Scalera G, Barbarisi A, Iannelli A. Why preoperative weight loss in preparation for bariatric surgery is important. Obes Surg. 2016;26(11):2790–2792. doi: 10.1007/s11695-016-2381-z. [DOI] [PubMed] [Google Scholar]
- 11.Schiavo L, Pilone V, Rossetti G, Iannelli A. The Role of the Nutritionist in a Multidisciplinary Bariatric Surgery Team. Obes Surg. 2019;29(3):1028–1030. doi: 10.1007/s11695-019-03706-w. [DOI] [PubMed] [Google Scholar]
- 12.Schiavo L, Scalera G, Sergio R, De Sena G, Pilone V, Barbarisi A. Clinical impact of Mediterranean enriched-protein diet on liver size, visceral fat, fat mass, and fat-free mass in patients undergoing sleeve gastrectomy. Surg Obes Relat Dis. 2015;11(5):1164–1170. doi: 10.1016/j.soard.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Schiavo L, De Stefano G, Persico F, Gargiulo S, Di Spirito F, Griguolo G, Petrucciani N, Fontas E, Iannelli A, Pilone V. A randomized, controlled trial comparing the impact of a low-calorie ketogenic vs a standard low-calorie diet on fat-free mass in patients receiving an elipse™ intragastric balloon treatment. Obes Surg. 2021;31(4):1514–1523. doi: 10.1007/s11695-020-05133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilone V, Tramontano S, Renzulli M, Romano M, Cobellis L, Berselli T, Schiavo L. Metabolic effects, safety, and acceptability of very low-calorie ketogenic dietetic scheme on candidates for bariatric surgery. Surg Obes Relat Dis. 2018;14(7):1013–1019. doi: 10.1016/j.soard.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Schiavo L, Scalera G, Pilone V, De Sena G, Capuozzo V, Barbarisi A. Micronutrient Deficiencies in Patients Candidate for Bariatric Surgery: A Prospective, Preoperative Trial of Screening, Diagnosis, and Treatment. Int J Vitam Nutr Res. 2016;10:1–8. doi: 10.1024/0300-9831/a000282. [DOI] [PubMed] [Google Scholar]
- 16.Schiavo L, Pilone V, Rossetti G, Romano M, Pieretti G, Schneck AS, Iannelli A. Correcting micronutrient deficiencies before sleeve gastrectomy may be useful in preventing early postoperative micronutrient deficiencies. Int J Vitam Nutr Res. 2019;89(1-2):22–28. doi: 10.1024/0300-9831/a000532. [DOI] [PubMed] [Google Scholar]
- 17.Pilone V, Tramontano S, Cutolo C, Marchese F, Pagano AM, Di Spirito F, Schiavo L. Clinical factors correlated with vitamin D deficiency in patients with obesity scheduled for bariatric surgery: A single center experience. Int J Vitam Nutr Res. 2020;90(3-4):346–352. doi: 10.1024/0300-9831/a000662. [DOI] [PubMed] [Google Scholar]
- 18.Leonetti F, Campanile FC, Coccia F, Capoccia D, Alessandroni L, Puzziello A, Coluzzi I, Silecchia G. Very low-carbohydrate ketogenic diet before bariatric surgery: prospective evaluation of a sequential diet. Obes Surg. 2015;25(1):64–71. doi: 10.1007/s11695-014-1348-1. [DOI] [PubMed] [Google Scholar]
- 19.Schiavo L, Pilone V, Rossetti G, Barbarisi A, Cesaretti M, Iannelli A. A 4-week preoperative ketogenic micronutrient-enriched diet is effective in reducing body weight, left hepatic lobe volume, and micronutrient deficiencies in patients undergoing bariatric surgery: a prospective pilot study. Obes Surg. 2018;28(8):2215–2224. doi: 10.1007/s11695-018-3145-8. [DOI] [PubMed] [Google Scholar]
- 20.Albanese A, Prevedello L, Markovich M, Busetto L, Vettor R, Foletto M. Pre-operative very low calorie ketogenic diet (VLCKD) vs. very low calorie diet (VLCD): surgical impact. Obes Surg. 2019;29(1):292–296. doi: 10.1007/s11695-018-3523-2. [DOI] [PubMed] [Google Scholar]
- 21.Bettini S, Belligoli A, Fabris R, Busetto L. Diet approach before and after bariatric surgery. Rev Endocr Metab Disord. 2020;21(3):297–306. doi: 10.1007/s11154-020-09571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muscogiuri G, El Ghoch M, Colao A, Hassapidou M, Yumuk V, Busetto L, Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO) European guidelines for obesity management in adults with a very low-calorie ketogenic diet: a systematic review and meta-analysis. Obes Facts. 2021;14(2):222–245. doi: 10.1159/000515381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fried M, Yumuk V, Oppert JM, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. 2014;24(1):42–55. doi: 10.1007/s11695-013-1079-8. [DOI] [PubMed] [Google Scholar]
- 24.Efird J. Blocked randomization with randomly selected block sizes. Int J Environ Res Public Health. 2011;8(1):15–20. doi: 10.3390/ijerph8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, Foster GD, Maislin G, Saif H, Broderick P, Chittams J, Hanlon AL, Pack AI. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370(24):2265–2275. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aude YW, Agatston AS, Lopez-Jimenez F, Lieberman EH, Almon M, Hansen M, Rojas G, Lamas GA, Hennekens CH. The national cholesterol education program diet vs a diet lower in carbohydrates and higher in protein and monounsaturated fat: a randomized trial. Arch Intern Med. 2004;164(19):2141–2146. doi: 10.1001/archinte.164.19.2141. [DOI] [PubMed] [Google Scholar]
- 27.Lira FS, Rosa JC, Dos Santos RV, Venancio DP, Carnier J, Sanches Pde L, do Nascimento CM, de Piano A, Tock L, Tufik S, de Mello MT, Dâmaso AR, Oyama LM. Visceral fat decreased by long-term interdisciplinary lifestyle therapy correlated positively with interleukin-6 and tumor necrosis factor-α and negatively with adiponectin levels in obese adolescents. Metabolism. 2011;60(3):359–365. doi: 10.1016/j.metabol.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Valsamakis G, McTernan PG, Chetty R, Al Daghri N, Field A, Hanif W, Barnett AH, Kumar S. Modest weight loss and reduction in waist circumference after medical treatment are associated with favorable changes in serum adipocytokines. Metabolism. 2004;53(4):430–434. doi: 10.1016/j.metabol.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, Esposito K, Jönsson LS, Kolb H, Lansink M, Marcos A, Margioris A, Matusheski N, Nordmann H, O'Brien J, Pugliese G, Rizkalla S, Schalkwijk C, Tuomilehto J, Wärnberg J, Watzl B, Winklhofer-Roob BM. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(Suppl 3):S5–78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 30.Basu A, Devaraj S, Jialal I. Dietary factors that promote or retard inflammation. Arterioscler Thromb Vasc Biol. 2006;26(5):995–1001. doi: 10.1161/01.ATV.0000214295.86079.d1. [DOI] [PubMed] [Google Scholar]
- 31.Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr. 2009;90(1):23–32. doi: 10.3945/ajcn.2008.27326. [DOI] [PubMed] [Google Scholar]
- 32.Wycherley TP, Brinkworth GD, Keogh JB, Noakes M, Buckley JD, Clifton PM. Long-term effects of weight loss with a very low carbohydrate and low fat diet on vascular function in overweight and obese patients. J Intern Med. 2010;267(5):452–461. doi: 10.1111/j.1365-2796.2009.02174.x. [DOI] [PubMed] [Google Scholar]
- 33.Sharman MJ, Volek JS. Weight loss leads to reductions in inflammatory biomarkers after a verylow-carbohydrate diet and a low-fat diet in overweight men. Clin Sci (Lond). 2004;107(4):365–369. doi: 10.1042/CS20040111. [DOI] [PubMed] [Google Scholar]
- 34.Keogh JB, Brinkworth GD, Clifton PM. Effects of weight loss on a low-carbohydrate diet on flow-mediated dilatation, adhesion molecules and adiponectin. Br J Nutr. 2007;98(4):852–859. doi: 10.1017/S0007114507747815. [DOI] [PubMed] [Google Scholar]
- 35.Horvath K, Jeitler K, Siering U, Stich AK, Skipka G, Gratzer TW, Siebenhofer A. Long-term effects of weight-reducing interventions in hypertensive patients: systematic review and metaanalysis. Arch Intern Med. 2008;168(6):571–580. doi: 10.1001/archinte.168.6.571. [DOI] [PubMed] [Google Scholar]
- 36.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 37.Nicklas BJ, Ambrosius W, Messier SP, Miller GD, Penninx BW, Loeser RF, Palla S, Bleecker E, Pahor M. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79(4):544–551. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 38.Craig SE, Kohler M, Nicoll D, Bratton DJ, Nunn A, Davies R, Stradling J. Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised controlled trial. Thorax. 2012;67(12):1090–1096. doi: 10.1136/thoraxjnl-2012-202178. [DOI] [PubMed] [Google Scholar]
- 39.Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A, Velkeniers B. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167(8):757–764. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 40.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50(2):417–423. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 41.Barbé F, Durán-Cantolla J, Capote F, de la Peña M, Chiner E, Masa JF, Gonzalez M, Marín JM, Garcia-Rio F, de Atauri JD, Terán J, Mayos M, Monasterio C, del Campo F, Gomez S, de la Torre MS, Martinez M, Montserrat JM, Spanish Sleep and Breathing Group Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181(7):718–726. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 42.Brown DL, Anderson CS, Chervin RD, Kushida CA, Lewin DS, Malow BA, Redline S, Goldman EB. Ethical issues in the conduct of clinical trials in obstructive sleep apnea. J Clin Sleep Med. 2011;7(1):103–108. doi: 10.5664/jcsm.28049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiavo L, Favrè G, Pilone V, Rossetti G, De Sena G, Iannelli A, Barbarisi A. Low-purine diet is more effective than normal-purine diet in reducing the risk of gouty attacks after sleeve gastrectomy in patients suffering of gout before surgery: a retrospective study. Obes Surg. 2018;28(5):1263–1270. doi: 10.1007/s11695-017-2984-z. [DOI] [PubMed] [Google Scholar]
- 44.Schiavo L, Scalera G, Pilone V, De Sena G, Ciorra FR, Barbarisi A. Patient adherence in following a prescribed diet and micronutrient supplements after laparoscopic sleeve gastrectomy: our experience during 1 year of follow-up. J Hum Nutr Diet. 2017;30(1):98–104. doi: 10.1111/jhn.12427. [DOI] [PubMed] [Google Scholar]
- 45.Schiavo L, Scalera G, Pilone V, De Sena G, Quagliariello V, Iannelli A, Barbarisi A. A comparative study examining the impact of a protein-enriched vs normal protein postoperative diet on body composition and resting metabolic rate in obese patients after sleeve gastrectomy. Obes Surg. 2017;27(4):881–888. doi: 10.1007/s11695-016-2382-y. [DOI] [PubMed] [Google Scholar]
- 46.Eldridge RC, Wentzensen N, Pfeiffer RM, Brinton LA, Hartge P, Guillemette C, Kemp TJ, Pinto LA, Trabert B. Endogenous estradiol and inflammation biomarkers: potential interacting mechanisms of obesity-related disease. Cancer Causes Control. 2020;31(4):309–320. doi: 10.1007/s10552-020-01280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu MF, Chen YH, Chen HC, Huang WC. Interactions among obstructive sleep apnea syndrome severity, sex, and obesity on circulatory inflammatory biomarkers in patients with suspected obstructive sleep apnea syndrome: a retrospective, cross-sectional study. Int J Environ Res Public Health. 2020;17(13):4701. doi: 10.3390/ijerph17134701. [DOI] [PMC free article] [PubMed] [Google Scholar]