Abstract
Cancer immunotherapy is a rapidly evolving concept that has been given the tag “fifth pillar” of cancer therapy while radiation therapy, chemotherapy, surgery and targeted therapy remain the other four pillars. This involves the stimulation of the immune system to control tumor growth and it specifically targets the neoplastic cells rather than the normal cells. Conventional chemotherapy has many limitations which include drug resistance, recurrence of cancer and severe adverse effects. Immunology has made major treatment breakthroughs for several cancers such as colorectal cancer, prostate cancer, breast cancer, lung cancer, liver cancer, kidney cancer, stomach cancer, acute lymphoblastic leukaemia etc. Currently, therapeutic strategies harnessing the immune system involve Checkpoint inhibitors, Chimeric antigen receptor T cells (CAR T cells), Monoclonal antibodies, Cancer vaccines, Cytokines, Radio-immunotherapy and Oncolytic virus therapy. The molecular characterization of several tumor antigens (TA) indicates that these TA can be utilized as promising candidates in cancer immunotherapy strategies. Here in this review, we highlight and summarize the different categories of emerging cancer immunotherapies along with the immunologically recognized tumor antigens involved in the tumor microenvironment.
Keywords: Cancer immunotherapy, Checkpoint inhibitor, PD-1, PD-L1, CTLA-4, CAR T cells, Oncolytic viruses, Monoclonal antibodies, Cancer vaccines, Cytokines
Graphical Abstract
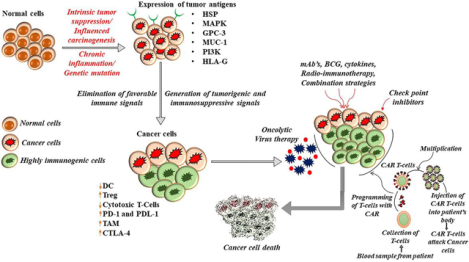
Introduction
Cancer has emerged as a significant health concern that affects more than 18.1 million people with more than 9.6 million deaths worldwide in 2018 [1]. Existing cancer therapies including chemotherapy, radiation therapy and hormone therapy are considered to be effective, but side effects and development of resistance during treatment significantly hamper their efficacy. This, in turn, increases the need for an alternative approach in combination with the conventional therapies to cure the patients [2]. Chemotherapy can be beneficial and continues to be the current standard therapy for cancer treatment. But it produces adverse effects as it destroys non-malignant cells and is also most likely to weaken the immune system and sometimes even cause drug-induced secondary carcinogenesis and recurrence following post-cancer chemotherapy [3]. In the case of radiation therapy, a broad spectrum of radiation is used for cancer treatment. However, these radiations cannot reach all parts of the body and cannot be effectively used for the course of action. The normal cells that are affected during radiation therapy usually recover within a few months after the therapy and some side effects (late effects) may show up in months or years after radiation [4]. Surgery also reflects a comparatively low survival rate due to the severe metastatic spread of cancer cells [5]. In this context, the scientific network actively pays more attention to immunotherapy and a copious number of developing immunotherapy approaches have introduced to treat cancer with maximum therapeutic effect with minimal adverse effects [6]. Furthermore, many studies revealed that a combinatorial approach with immunotherapy and conventional chemo-radio therapeutic agents could improve overall survival and reduce cancer recurrence more significantly than monotherapy [7]. Combining immunotherapy with conventional treatment might also enhance the quality of life of the patients and a collection of studies showed positive outcomes. In 2018, Nobel prize for physiology or medicine was awarded to James P. Allison, University of California, Berkeley and Tasuku Honjo, Kyoto University for the discovery of inhibitors of Cytotoxic T-lymphocyte -associated protein 4 (CTLA-4) and programmed cell death protein 1/programmed cell death protein ligand 1 (PD-1/PD-L1) for cancer therapy [7]. This significant recognition has accelerated immunotherapy-based research globally. The current review discusses the role of various TA in immune evasion as well as in anticancer immunity in tumor microenvironment. In addition to that, insights into various immunotherapy approaches targeting the aberrantly expressed TA in tumor microenvironment (TME) are also described briefly.
Harnessing the immune system against cancer
Oncogenesis is a complex process with an accumulation of an abundance of genetic as well as cellular alterations within the cells [8]. The malignant transformation of cells activates the immune system which mounts an anti-tumor response against the tumor cells. Two different immune arms, the innate immune system and the adaptive immune system are activated during the immune response with their corresponding activating molecules.
The innate immune system constitutes the primary host defence in our body that mounts an immune response without prior activation by antigens [9]. Innate immunity is encompassed of different components such as several physical barriers (tight junctions in the mucus, epithelial, skin, and mucous membrane surfaces), anatomical barriers, epithelial and phagocytic cell enzymes such as lysozyme, phagocytes (monocytes, neutrophils, macrophages), inflammation-mediated serum proteins (complement, C-reactive protein, lectin and ficolins), surface and phagocyte granule antimicrobial peptides (defensins and cathelicidin), cell receptors that sense microorganisms and signal a defensive response (Toll-like receptors) [10]. The adaptive immune system is comprised of CD4+ and CD8 + T lymphocytes, which get activated upon antigen presentation by antigen-presenting cells (APCs) followed by the production of specific antibodies against the antigen. Each individual is having a unique immune system component with varying ability to mount an immune response [7] due to the presence of several tumor associated antigen on the cancer cell surface characterized by the rapid cell proliferation and immune evasion in the tumor microenvironment (TME) [11, 12]. Turning these tumor cells into the APC is considered the next step to improve cancer immunotherapeutic approaches. Imperfectness in antigen presentation on cancer cells symbolizes a major immune escape mechanism in cancer survival [13]. Restoring/forcing antigen presentation by the cancer cells may augment the production of cytotoxic T cells and also escalate the capacity of T cells to recognise and destroy cancer cells. This may be accomplished by a variety of anticancer treatment strategies. These approaches may sensitise patients to T cell-based immunotherapies, including checkpoint inhibitors [13, 14].
When the immune system is activated, certain proteins are down-regulated via multiple immune signalling cascades and restrict the cancer cell survival. These tumor antigens can be either tumor-specific antigens (TSA) or tumor associated antigens (TAA). Tumor antigens are either Tumor-specific antigens (TSA), (found on cancer cells only, not on healthy cells) [15] or tumor-associated antigens (TAA), which have elevated levels on tumor cells, but are also expressed at lower levels on healthy cells [16] Several TAA and TSA are contributing immune evasion in TME. TAA may significantly contribute to immune evasion and tumor aggressiveness in the body. Many TAAs have been recognized and molecularly characterized [17–19]. Nevertheless, only a limited number of TAAs mostly recognized by CD8 + T cells in tumor patients have been clinically tested [20, 21]. The presence of a large variety of TAA in tumors provides opportunities for therapy via passively transferred antibodies or immune lymphocytes, as well as by immunization with tumor vaccines, and several tumor antigens are being routinely used as diagnostic markers [22, 23]. Similarly, TSAs are also causing immune evasion in the tumor milieu, which can evade cytotoxic T cells from TME lead to the generation of tumor-suppressive microenvironment and augment the cancer cell growth [24]. Moreover, the abnormal expression of TSA on neoplastic cells may lead to facilitating the escape of cancer cells from immune surveillance. They can increase the level of immune checkpoints in the tumor milieu and cause partial or complete loss of MHC I expression and also initiate the epigenetic silencing of neoantigens on the cancer cells and restrict the identification of neoantigens by the immune system [25, 26].
Immune evasion in cancer progression
Cancers can escape from immune surveillance by crippling cytotoxic T cells functionality through the generation of several immune-suppressive cytokines, either by the tumor cells or by the non-tumor cells present in the TME, particularly including several immune cells and epithelial cells [27]. The cluster of differentiation 8 + (CD8 +) and cytotoxic T cells (CTLs) are fundamental critical players involved in anticancer immunity. The molecules on the APC that present the antigen are called major histocompatibility complexes (MHC). There are two types of MHCs, MHC class I and MHC class II. MHC I present to cytotoxic T cells and MHC II presents to helper T cells. T cell receptors recognize MHC I/II bound antigenic peptides present in the cancer cells which lead to the priming and activation of T cells to cytotoxic phenotype. Naïve T cells get activated upon recognition of antigen-MHC complex presented by dendritic cells, typical antigen-presenting cells, which provides co-stimulatory molecules for T cell activation [28]. T cells also bind with the major histocompatibility complex II (MHC II) present in the APC. Immune recognition of tumor-associated antigens by cytotoxic T lymphocytes is largely triggered via MHC class I (MHC-I) molecules on the surface of cancer cells and MHC-I expression is also a negative regulator of natural killer (NK) cells. Tumor-specific MHC-II expression (tsMHC-II) may augment the recognition of cancer cells by the immune system and therefore may play a significant role in immunotherapy [29]. A few cancer cells can down-regulate the expression of MHC 1 and escapes from the immune reaction. Another mechanism is due to the mutation that causes the genetic alterations in MHC, which results in cancer cell proliferation, which in turn decreases the sensitivity towards the cytokines [27–29].
Natural Killer Cells (NK cells) or large granular lymphocytes are a type of cytotoxic lymphocyte, which can produce a cytotoxic effect in the way of the host rejection process. The early stimulation of NK cells is connected with the killing of allogeneic target cells and the release of immunomodulatory chemokines and cytokines, which can contribute to either rejection or tolerance. NK cells can distinguish between self and foreign tissues and play a key role in the initiation and regulation of adaptive immune responses after solid organ transplantation. Depending upon the types of NK cell receptors engaged and the nature of cytokines released, early NK cell activation can promote either acute rejection or tolerance. NK cells also serve as an allogeneic effector in adoptive tumor immunotherapy. They were named “natural killers” because of the concept that they do not necessitate activation to kill cells that are missing “self” markers of MHC I. This role is essential as the harmful cells that are missing MHC I markers cannot be detected and destroyed by other immune cells, such as T lymphocyte cells [29]. Various viral infections and abnormal cell growth have evolved to restricts the NK cells mediated immune responses illustrating the evolutionary struggles between viruses/cells and NK cells. Ligands for NK cell receptors are primary targets for immune evasion in the TME. On the other hand, cancer cells adopt a different mechanism to diverge from the immune clearance effect by reducing the level of co-stimulation by essential molecules such as CD28, CD86 and increasing the expression of Cytotoxic T lymphocyte-associated protein-4 (CTLA-4) and other immune checkpoint molecules, thus down-regulate the activation of cytotoxic T cells. The interaction of CTLA-4 with the B7 molecule delivers an inhibitory signal, effectively checked by CTLA-4 inhibitors [29]. The formation and development of regulatory T (Tregs) cells or suppressor T cells also consign a major role in the regulation of immune reaction because they down-regulate or inactivate the effector T cells and inhibit the interaction of T cells with the MHC I. Tregs from an immunosuppressive TME in cancer that helps in the rapid proliferation of cancer cells in the body [30].
Other well-established checkpoints in cancer immunotherapy are found to be PD 1 and PD L1. Programmed cell death protein 1, also known as PD-1 or CD279 (cluster of differentiation 279), is a protein situated on the surface of cells that belongs to the immunoglobulin superfamily and is expressed on T cells and pro-B cells that has a function in control the immune system response to the cells of the human body via down-regulating the immune system and upgrade self-tolerance by inhibiting cytotoxic T cell activity [31]. The protein restricts autoimmune diseases, but it can also prevent the immune system from killing cancer cells. PD 1 is an immune checkpoint and acts as a protector against autoimmunity via two mechanisms. First, it upgrades apoptosis (programmed cell death) of antigen-specific T-cells present in the lymph nodes. Second, it brings down apoptosis in regulatory T cells (anti-inflammatory or suppressive T cells) [32, 33]. PD-1 inhibitors, a new therapeutic approach that inhibits PD 1 and initiates the immune system to fight against cancer cells and is approved by the FDA to tackle several types of malignancies [34].
The PD 1 protein in humans is encoded by the PDCD1 gene [35]. PD 1 generally binds two ligands such as PD-L1 and PD-L2. Programmed death ligand 1 (PD-L1) also known as cluster of differentiation 274 (CD274) or B7 homolog 1 (B7–H1) is a protein that is encoded by the gene CD274 [36]. This protein plays a crucial role in inhibiting the adaptive signalings of the immune system during critical events such as pregnancy, tissue allografts, autoimmune disease and other disease states such as hepatitis etc. Remarkably the adaptive immune system reacts to antigens that are associated with immune system activation by exogenous or endogenous danger signals. The interactions of PD-L1 to the inhibitory checkpoint molecule PD1 exhibit a restrictive signal based on interaction with phosphatases such as SHP-1 or SHP-2 through immunoproteins such as Tyrosine-Based Switch Motif (ITSM) [37]. This restricts the proliferation of antigen-specific cytotoxic T-cells in lymph nodes, while at the same time it inhibits the apoptosis in regulatory T cells [38]. PD-1 ligand 2 (PD-L2) act as a second ligand for PD-1 also known as PD-L2, B7-DC, CD 273 is a protein that in humans is encoded by the gene PDCD1LG2 [39]. Binding to PD-1 with PD L2 can trigger the signalings that inhibiting TCR/BCR associated immune cell responses [40]. But, the prognostic value of PD-L2 in solid tumor patients after surgery remains controversial. The immune-modulatory role and expression of PD-L2 remain lesser unclear as compared to PD-L1, so its make as it a less obvious target for cancer immunotherapy [41]. Nevertheless, studies were demonstrated that PD-L2 plays a crucial role in the modulation of Th2 responses in cancer milieu, whereas in the background of anti-cancer immunity Th1 signalings are the most important, it does not seem good to choose PD-L2 as a target for tumor [42]. Even though, it is significant to notice that in the majority of research studies demonstrated that PD-L2 was expressed in only a minority of cancer patients. In addition, it is not implausible that PD-L2 expression is more important on the TME than PD-L1 [42].
Various research studies such as several case studies, epidemiological analysis, clinical studies and tumor immunology revealed the prophylactical and therapeutical significance of pathogen associated molecular patterns (PAMP) in neoplastic diseases. Pathogen associated molecular patterns (PAMPs) are also plays a key role in cancer immunotherapy [43], which are small molecular motifs preserved within a class of microbes. They are established by toll-like receptors (TLRs) and other pattern recognition receptors (PRRs). Upon PAMP interaction, pattern recognition receptors (PRRs) initiate intracellular signaling cascades and finally terminate the expression of a variety of pro-inflammatory a protein, which together organize the early host response to infection, and also plays a role in the activation and shaping of innate immunity. TLRs are membrane-bound proteins localized at the cellular or endosomal membranes, recognizing PAMPs through the LRR domain and transducing signals to the intracellular environment through the TIR domain. TLRs via PAMP-TLR interaction stimulates receptor oligomerization, which afterwards induces intracellular signal transduction.
At present, 10 TLRs have been known in humans, and they each recognize distinct PAMPs derived from various microbial pathogens, including viruses, bacteria, fungi, and protozoa [44]. Initiation of TLR activation during vaccination, either as part of the immunogen or as an adjuvant, is the most explored application of TLR agonists [45]. Direct TLR stimulation has been demonstrated in the case of the bacillus Calmette-Guérin (BCG) vaccine for the prevention of tuberculosis and some types of cancers, in which cell wall components of BCG, including arabinogalactomannan and peptidoglycan, activate TLR2 and TLR4 in a MyD88-dependent manner [46]. An immunotherapy with a ragweed-TLR9 agonist vaccine against allergic rhinitis was found to be a successful example with an observed shift in the Th2/Th1 ratio with significant clinical outcomes [47]. In the treatment of infectious diseases, agonists of TLR3, TLR7, TLR8, and TLR9 have shown some prospect, particularly against chronic viral infections, where the mechanism seems to involve the production of type I IFN and generation of IFN-dependent antiviral responses. Eventually, a very important use of TLR agonists is in the treatment of different malignancies due to its immunomodulatory action [45, 48].
PAMPs also can recognise nod like receptor (NLR). Studies discovered that TLR-independent recognition of pathogens is completed by a large group of cytosolic PRRs, which can be broadly classified into retinoid acid-inducible gene I (RIG-I)-like receptors (RLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) [49]. Whereas NOD1 and NOD2 activation results primarily in the stimulation of proinflammatory gene expression, other NLR proteins are involved in the activation of caspases. Recently, augmentative evidence has extended the idea that chronic inflammation caused by aberrant NLR signaling is an omnipotent driver of carcinogenesis, where it abets genetic mutations, tumor growth, and progression [50].
Cancer immunotherapy is available in many ways, which include targeted monoclonal antibodies, adoptive T cell transfer, tumor vaccines, tumor-infecting viruses’ therapy, checkpoint inhibitors, cytokines, and adjuvants [51]. Immunotherapies are a form of biological therapy (Biological Response Modifier (BRM) therapy) as they contain materials from residing organisms to combat opposition to most cancer cells. Some immunotherapies make use of genetic engineering techniques to enhance immune surveillance against neoplastic cells in the body. Immunotherapy treatments for preventing, handling or treating specific cancers cells can also be utilized in combination with chemotherapeutic drugs, surgical treatments, radiation therapy or targeted treatments to enhance the effectiveness of the treatment methods. Gene therapy is also used in cancer to improve the selectivity of immune-checkpoint directed treatments and facilitate their entry to the tumor tissue and restore unbalanced mechanisms [51, 52].
Tumor immune evasion is one of the main obstacles in designing successful anticancer therapeutic approaches. Several factors produce cancer persistence even though having a normal host immune system. Tumor immune evasion is one of the significant characteristics with which cancers are escaping from the normal immune reaction. Abnormal presentation of tumor-associated antigens (TAA) on the cancer cells is normally hindered the anti-tumor immune response and elicit cancer cell invasion. Cancer immunotherapy is found to be very effective in many types of cancer. It is found to have the ability to reinforce the immune system to fight against tumors and demolish cancer. Some patients have shown a dramatic and lasting response to this new treatment method, which include Chimeric Antigen Receptor T cells and immune checkpoint inhibitors strategies [53]. In certain rare patients with malignant tumors, they had their tumors disappear absolutely following treatment with immunotherapy [54].
Tumor antigens—strategies in immune evasion and cancer progression
Tumor antigens are the antigenic substance that are present in cancer cells. The abnormal expression of these tumor antigens may lead to the development of cancer progression by suppressing the immune signals in the TME [55]. Tumor antigens serve as potential candidates in cancer therapy. When normal cells change into malignant cells, antigens on their surface may change. If the immune system observed that a foreign substance is present, the system will launch the body’s defence system such as cytotoxic T cells, NK cells, macrophages and other cytokines [56]. But, most of the time, the immune system fails to stop the malignant transformation of cells. Cancer develops in the background of a weak immune and scientists are now focusing on various aspects to shape immune cells into an inventive new anti-cancer weapon. They are named as immunologically relevant substances termed as biological response modifiers with the ability to mimic immune proteins to down-regulate the overexpressed immune oncoproteins [57]. Here we are choosing some potential tumor antigens that have relevance on cancer cell progression due to their abnormal signaling in the TME and also emphasize the role of these antigens in immune evasion.
Heat shock protein (HSP)
Heat shock proteins (HSPs) are generally expressed in cells under stressful environments, thus recognizing the highly conserved stress response chaperone proteins. HSPs are highly expressed in various types of tumors. Circulating HSPs are associated with cancer cell proliferation, differentiation, invasion and metastasis [58]. The level of circulating HSPs and their corresponding antibodies are considered as a biomarker for analysing the early stage and progression of cancers. Moreover, the overexpressed HSPs in tumor cells decrease the sensitivity of chemotherapeutic drugs. Protective and destructive immunological responses around the TME are also balanced by some of the HSPs that present in the cancer cells [59].
Several stress conditions in the TME lead to the generation of more numbers of tumor cells in the body. These kinds of induced stress factors can activate heat shock transcription factors (HSFs) present in the tissue and will favour their dissociation from heat shock proteins. The phosphorylation proceeds gradually after the dissociation. The dissociated heat shock transcription factors translocate into the nucleus. They bind with heat shock elements (HSE) induce the transcription of heat shock proteins such as HSP70, HSP90 and HSP27. This will help to maintain the up-regulated level of HSP 70. Recently, studies showed that HSPs are a potent multifaceted transformer of carcinogenesis. These are mostly distributed into the TME altering the immune response against malignant cells. In immunosuppressive circumstances, the HSPs increase the survival, proliferation and metastasis of tumor cells by activating their cellular safeguard machinery pathways [59, 60]. Sometimes the cancer cell death and the survivals are controlled in the optimal level by the immune response that is aroused by HSPs [61].
HSPs elicit tumor progression under stressful conditions such as hypoxia, radiation, and the presence of drugs [62]. HSPs also show critical roles in inhibiting apoptogenic molecules via modulation of several signaling cascades such as JNK, AKT, and NF-κB [63]. There are different types of HSPs present in the body. They produce cancer proliferation and immune evasion in different ways. HSP27 alter the WNT catenin and hippo pathways and consequently activate TGF- beta and SMAD and trigger metastatic and oncogenic pathways [64]. HSP40 activate the expression of IQGAP1 in various types of cancers and is more aggressive in colorectal and ovarian cancers; IQGAP1 is now established as a protein scaffold that integrates various signals that controls cell adhesion, actin cytoskeleton, the cell cycle and other cellular functions. IQGAP1 is particularly interesting as a therapeutic target since it plays significant role in many signaling pathways implicated in cancer progression such as Ras → Raf → MEK → ERK and MAPK signaling pathways [65]. HSP70 elicit tumor progression via the activation of different intracellular pathways such as P13K, AKT, EMT, JAK-STAT, FAK, Src and Pyk2 and extracellular pathways and proteins such as IFN-ƴ, STAT3 and MMPs [66]. HSP90 attenuate oncogenic signaling pathways through p53, ErbB, PI3K/EKT, JAK2/STAT3, ERK, JNK, HIF and EMT [67].
Studies indicate that Heat shock protein-peptide complex 96 (HSPPC 96), Glycoprotein 96 (GP 96) [68], HSP 70 based vaccine [69, 70] are under clinical trial for the treatment of adult high-grade glioma, colorectal cancer and cervical cancer respectively. Several significant advances have been made in the field of HSP-based onco-immunology, including the usage of anticancer vaccines [71–74]. Studies revealed that a combination of HSP vaccines with adjuvants and anti-CTLA antibodies will help to increase immunity and also produce anti-tumor immune responses in the TME [75].
Mitogen-activated protein kinase (MAPK or MAP kinase)
It is a type of protein kinase, which specifically contains amino acids such as serine and threonine. MAPKs can elicit the cellular response to diverse stimuli such as proinflammatory cytokines, mitogens, osmotic stress, heat shock etc. These proteins regulate cellular functions such as differentiation, mitosis, proliferation, gene expression and apoptosis. MAPKs signals are normally hyperactivated due to mutations that occur in the proto-oncogenes (B-Raf proto-oncogene, serine/threonine kinase) [76]. A variety of oncogenic stimulation can also cause hyperactivation of MAPKs pathways and these may lead to abnormal cancer cell progression in the body. Preclinical reports suggest that inhibition of MAPK-pathway can effectively increase the potency of cancer immunotherapy. Based on the current evidence, several components/proteins in the MAPKs network can be considered as a potential target for cancer immunotherapy.
Numerous pathogens target host intracellular signaling cascades including MAPK pathways that inhibit immune responses against cancer proliferation [77, 78]. MAPK signaling network can be exerted via three primary cross-signaling pathways such as ERK, p38, and JNK, each comprising several levels of kinases. MAPK signaling can be activated by internal and external stimuli, which results in either a tumor-suppressive or oncogenic event. ERK (extracellular signal-regulated kinase) is a protein that belongs to the category of MAPK. ERK signalling is prominent in a variety of cancers by controlling benign and malignant cellular functions during cancer development such as cancer cell proliferation, differentiation, motility and survival. On the other hand, ERK is also essential in the regulation of T-cell biology. In mature cytotoxic T-cells, ERK is generally activated upon the interaction between the T-cell receptor (TCR) and major histocompatibility complex (MHC) on APC [79, 80]. JNK (c-Jun N-terminal kinases), is another protein that belongs to the family of MAPK. JNK2 can target jun proto-oncogene to promote apoptosis, during cell proliferation. JNK2 specifically targets nuclear factor receptors present in the activated cytotoxic T-cells (NFAT) to facilitate DNA binding. After that, JNKs cooperates with MEKK2 protein signalling to enhance the IL-2 biosynthesis, playing crucial roles in TCR/CD3-mediated cytotoxic T-cell signalling [80]. RAS is a kind of proto-oncogene, which can elicit the activity of p38, another class of MAPKs. The activation of P38, which in turn activates the transcription of NF-κB. These transcription factors can regulate the intracellular pathways and integrate the signals from the surrounding tissue of cancer cells and the immune system. These signals maintain a balance between tumor cell survival and cell death. Upregulation of NF-κB activity can increase the activity of several genes that cause cancer cell survival, cell metastasis and progression [81].
The immune response is one of the respective key functions modulated by MAPKs, with the secretion of various immunomodulatory cytokines, such as tumor necrosis factor (TNFα), interleukin (IL)-1, IL-10, and IL-12 stimulated via the activation of p38 MAPK, JNK, and ERK pathways [82]. The restrictive cytokines, IL-12 and IL-10, synthesized by specialized dendritic cells (DCs) and macrophage cells, play an essential role in the coordination of the immune response since IL-12 inflect the development of a Th1 response [83]. MAPK inhibitors such as Trametinib, Selumetinib, G-38963, BVD 523 are under clinical trial with a combination of immunotherapy for the treatment of breast cancer, lung adenocarcinoma, colon carcinoma [79].
Glypican-3 (GPC- 3)
GPC3 is an immune protein consisting of 580 amino acids. The major constituent that presents in the GPC3 is heparan sulfate proteoglycan which is closely bound with the cell membrane by glycosylphosphatidylinositol linkage. This protein is expressed specifically in the liver of a healthy foetus but, not expressed in the adult liver. The protein core of GPC3 possesses two subunits named as the N-terminal subunit (~ 40 kDa) and the C-terminal subunit (~ 30 kDa). Cell-surface possessing heparan sulphate proteoglycan is composed of membrane-linked proteins attached with a variable number of heparan sulphate units present in the membrane. These proteins play a role in the metastasis and growth regulation of cancer cells [84].
GPC3 can interact with both Wnt and frizzled (FZD) proteins to form a reaction complex and initiates a down-stream signaling process. The Wnt signalling process is normally activated by the core protein present in the GPC3. The hydrophobic core that is in the GPC3 is the N subunit, which is a cysteine-rich domain with the ability to interact with Wnt3a [85]. This particular interaction of GPC3 with the N terminal domains obstructs the Wnt activation. Studies demonstrated that overexpression of GPC3 in the cancer cell surface elicits rapid cell proliferation and metastasis. Studies revealed that GPC3 is overexpressed in liver cancer and it is crucial to understand the modulatory mechanisms of GPC3 expression during liver carcinogenesis [86]. Trinh et al. demonstrated that epigenetic alternations such as both DNA methylation and histone regulations were significant for the transcriptional modulation of GPC3 in various cancers [87]. Li et al. shows that GPC3 is a direct transcriptional target of c-Myc and that c-Myc overexpression augmented GPC3 levels in neoplastic cells [88]. c-Myc is a master regulator oncogene, GPC3 dependent over expression of c-Myc will cause metabolic changes in the cancer cells and produce cancer cell survival [89].
GPC3 is a promising therapeutic target for treating liver cancer. Several therapeutic anti-GPC3 antibodies such as GC33 and YP7have been introduced to improve the immune reactions in the body to fight against the cancer. YP7 and other murine monoclonal antibodies that recognize the C lobe of GPC3 and they were developed by the laboratory of Dr Mitchell Ho at the National Cancer Institute by hybridoma technology. Clinical application of these antibodies was further confirmed after being humanized. Human single-domain antibodies such as HN3 targeting the N-lobe of GPC3 and the human monoclonal antibody HS20 targeting the heparan sulfate moiety of GPC3 by phage display technology are identified at the same institution [86]. HN3 and HS20 antibodies can constrain the Wnt signaling in liver cancer cells.
Other GPC3 based therapies against hepatocellular carcinoma include immunotoxins based on HN3, GC33. The chimeric antigen receptor (CAR) T cell immunotherapies based on GC33, hYP7, the antibody–drug conjugates based on hYP7 and the T-cell engaging bispecific antibodies derived from YP7, and HN3. In mice with xenograft or orthotopic liver tumors, CAR (hYP7) T cells can eliminate GPC3-positive cancer cells, possibly by inducing perforin- and granzyme-mediated apoptosis or reducing Wnt signaling in tumor cells [86, 90].
Mucin 1 (MUC 1)
Mucins are specifically arranged in the apical surface of epithelial cells in the lungs, liver, intestines, stomach, eyes and several other organs [91]. The prime role of Mucins is to protect the body from infection caused by pathogen and further binding to oligosaccharides arranged in the extracellular space of the cells, anticipating the pathogen to reaching the cell surface. But, overexpression of MUC1 is associated with colon, liver, breast, ovarian, lung, and pancreatic cancers [92, 93]. Mucin 1 is a cell surface-associated TAA (MUC1) named polymorphic epithelial mucin (PEM) or epithelial membrane antigen (EMA) which consist of mucin protein encoded by the MUC1 gene in it [93]. MUC1 is a type of glycoprotein with extensive O-linked glycosylation to its extracellular protein domain or towards the cell surface.
MUC1 protein associated antibody-mediated cellular immune reactions have been shown to exert a positive effect on cancer patient’s therapeutic outcomes. The over expression of aberrantly glycosylated MUC 1 is prominently associated with various types of cancer. Aberrantly glycosylated MUC 1 always possesses a tumorigenic role in the cancer progression and thereby aberrant expression and cellular localization of MUC1 proteins in different cancers could act as prognostic marker. MUC 1 is more importantly act as a negative regulator of toll-like receptors (TLRs). TLRs play vital roles in the innate immune system through identifying pathogen-triggered molecular signals resulting from various microbes. They are single-pass membrane-spanning receptors frequently expressed on various immune cells such as macrophages and dendritic cells, thereby they increase the maturation of T cells and upregulate the level of co-stimulatory molecules such as CD80 and CD86 essential for the stimulation of naive CD4 + T-cells and suppression of regulatory T-cells (Treg) via IL-6 production [94, 95].
MUC 1 is a highly immunogenic TAA, where its hypo-glycosylated, it develops an immunosuppressive TME and is considered a hallmark for tumor progression. Aberrantly glycosylated MUC 1 may decide the effect of MUC 1 on the immune system and produce tumorigenesis [96]. As mentioned earlier intracellular and extracellular glycosylation of MUC 1 is mainly mediated by two carbohydrates groups, O-glycan and N-glycan and thereby, changes in these carbohydrates also indirectly affect the cancer-associated immune proteins and modulate the differentiation, proliferation, progression and development of tumor immunosurveillance. In addition to that, glycosylation of MUC 1 can affect the kinetic & thermal charges and thermostability of cancer-associated proteins in folded or unfolded state [97].
Improving MUC1-associated immunity through the use of MUC 1 based vaccines, MUC1-specific antibodies, and MUC 1 based T cell transfers represent a vital strategy in the fight against malignant cells [91]. Different types of MUC1-based vaccines that have been developed for cancer therapies are in preclinical and clinical trials. These vaccines include the vaccinia virus vaccine, peptide/protein vaccines, DNA vaccine and dendritic cell-based vaccine. To boost the immune responses of vaccines; vaccine carriers such as mannan, keyhole limpet hemocyanin, and as an adjuvant including Bacillus Calmette-Guerin, incomplete Freund’s adjuvants, and saponin, were all tried along with the vaccines with varied successes [98].
New evidence and hypotheses are evolving on the efficiency of MUC1 vaccines in treating the cancer patient population. The evidence suggests that the innate immune status of individual patients determine the success rate of MUC1-based immunotherapy. Conventional treatments in combination with MUC1 immunotherapy benefits oestrogen receptor-positive breast cancer patients. Promising results from the hypotheses leads to several active clinical trials, which confers a ray of hope into the field of MUC1 cancer immunotherapy. The future success of MUC1 Immunotherapy may also reside in the combination strategies of its use with chemotherapy, targeted therapy, and hormone therapy [99].
Phosphoinositide 3-kinases (PI3Ks)
P13Ks are a family of enzymes and different types of PI3K isomers plays a key role in cell proliferation, metastasis, cell differentiation, cell motility, cell survival and intracellular trafficking in the tumor. The PI3Ks increase the incidence of cancer cell survival by acting on the transformed cells directly, interfering with the supportive stroma and nutrient supply to the cells, or by enhancing more significant immune responses against the cancer cells [100]. PI3K has a crucial role in cancer immune evasion triggered by establishment of an inhibitory myeloid microenvironment in addition to its well-established, cell-intrinsic oncogenic role. Activating mutations in PI3K may be useful as a biomarker of poor response to immunotherapy. [101]. The PI3K signaling can be responsible to a certain extent, for transformed cells escaping from immunity. The over expressed P13K in the tumor mileu decreases the activity of NKG2D and down-regulate the activity of NK cells. A reduced NKG2D expression and function in NK cells following chronic exposure to NKG2D ligands and/or soluble forms of MIC (sMIC) leads to a immune surveillance failure [102].
Malignant cells can drive immune suppression via multiple mechanisms, such as the secretion of immune-suppressive cytokines and chemokines including TGFβ and IL-10 [103]. PI3K dependent, tumor cells can also elicit their malignancy via emulating some immune cell chemotactic responses in the TME. The chemokine CCL5 (RANTES), previously known as a motility factor for some leukocytes during inflammation, can generate migration and metastasis of neoplastic cells. This may further cause the de novo expression of CCL5 receptor (CCR5) at cancer cell surface, which is not present in non-cancerous cells [104]. Tang et al. have documented that chondrosarcoma cells express CCR5 and can sense CCL5 resulting in increased cell migration and metalloproteinases-3 secretion. The PI3K and NF-κB pathways have been shown to play an essential role in this scenario for immune evasion in cancer cells [105]. PI3Kδ inhibitors can enhance CD8 + T-cell-mediated cytotoxic anti-tumor response by activating dendritic cells to increase the production of more IL-12 and by inhibiting regulatory T cells and myeloid-derived suppressor cells, which can antagonize cell- cell-mediated immunity in cancers [106].
Human leukocyte antigen G (HLA G)
HLA-G (histocompatibility antigen, class I, G), is over expressed in cancer cells to suppress the cancer-induced immune response. Hence HLA G act as an immune checkpoint in cancer to defend against an immune response. This may result in the proliferation of tumor cells that do express HLA-G. Many studies demonstrated that, HLA-G as a new immune checkpoint in tumor [107]. Thus, HLA-G and its receptors might be targets for immune checkpoint blockade in cancer immunotherapy. The positive organization between the expression of pro-inflammatory transcripts and HLA-G could show that HLA-G is a counter-regulatory mechanism that follows the intra-tumoral infiltration of activated lymphocytes. In fact, the expression of classic immune checkpoints like programmed death ligand-1 (PD-L1), programmed cell death protein 1 (PD1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and indoleamine 2,3-dioxygenase dioxygenase 1 (IDO1), also has a high correlation with pro-inflammatory transcripts. Various in vitro studies demonstrated that HLA-G-negative leukaemia, glioma, ovarian carcinoma, and hepatocellular carcinoma cell lines are more expeditiously killed by NK cells compared to their HLA-G present cells [108, 109]. Interestingly, inhibition with HLA-G antibodies restored the NK cells triggered lysis of the targeted tumor cell lines, suggesting a role for HLA-G in tumor immune evasion that can be modulated. Additionally, the in vivo role of HLA-G as a tumor immune escape mechanism has been demonstrated in mouse models [110, 111]. It is important to consider that a murine counterpart of HLA-G does not exist and, therefore, it is debatable whether results from such murine models would translate to human physiology. In humans, the frequency of HLA-G expression has been widely studied using IHS and analyzed in relation to clinical outcome. Most studies show that HLA-G expression in cancer is associated with a poor clinical outcome in patients [112, 113], suggesting that HLA-G plays a role in immune evasion and disease progression.
Metadherin (MTDH)
Metadherin (MTDH) is a protein also known as LYRIC or astrocyte elevated gene-1 protein in humans (AEG-1), which is encoded by the gene MTDH [114]. Overexpression of MTDH was detected in large number of cancers. Notably, several immunogenic studies confirmed the presence of auto-antibodies against the MTDH in cancer patients [115]. MTDH is a multifaceted; pro- oncogenic factor which plays a central role in the cancer progression. Studies have demonstrated that MTDH can interfere the major cellular pathways such as MAPK, wnt/β-catenin, PI3K/AkT and NF-κβ, and promote tumor metastasis [116]. MTDH/AEG-1 stimulates cancer cell survival by obstructing apoptosis, and promote cancer progression via multiple PI3K/Akt signalling [117]. On the other hand, MTDH/AEG-1 mediated PI3K-Akt pathway elicits an invasive phenotype and angiogenesis in tumor cells [118]. MTDH mediated angiogenesis is mainly interconnected with the functions of vascular endothelial growth factor (VEGF), epithelial mesenchymal transition (EMT) and IGFBP7. Most of the research works clearly demonstrated that MTDH raises the expression of VEGF via the PI3K/Akt pathway in head and neck squamous cell carcinoma [119]. MTDH is also related with expression of N-cadherin markers in hepatocellular carcinoma (HCC) such as β-catenin, E-cadherin, snail, and N-cadherin. Evidences suggests that the association between MTDH with the pro-survival pathways promotes Wnt/β-catenin signalling by increasing the expression of the lymphoid enhancer binding factor 1 (LEF-1) and GSK3β in HCC and chronic lymphocytic leukemia [120, 121]. Overexpression of MTDH in HCC augments the up-regulation of multiple genes and signaling pathways including the stimulation of ERK 42/44, Mitogen Activated Protein Kinase (MAPK) signaling pathway and p38. Stimulation of ERK 42/44 and p38 MAPK enhanced GSK3β phosphorylation which in turn drives β-catenin nuclear translocation and thereby activate Wnt signalling [122]. Pro-inflammatory endotoxin Lipopolysaccharide (LPS) stimulate the expression of MTDH in triple negative breast cancer and produce cancer proliferation [123]. Studies show that Serum MTDH mRNA expression was reported to produce cancer proliferation in colorectal cancer. The increased expression of MTDH mRNA is connected with advanced tumor characteristics and lower disease-free survival rate. Therefore, it can be considered as a useful non-invasive biomarker for screening, early diagnosis, and prognosis of cancer patients [124].
Recently identified three T-cell epitopes within the MTDH protein supports its potential value as a cancer vaccine target. Studies have examined that MTDH based DNA vaccines have significant effect on cancer as they boost the anticancer immune responses in the TME [125, 126]. Several research works proved that the combination of MTDH based gene vaccine with granulocyte–macrophage colony-stimulating factor and paclitaxel may produce better therapeutic effect against prostate cancer [125] Table 1.
Table 1.
Clinical trials of drugs targeting tumor antigens
| Sl. no. | Tumor antigens | Types of cancer | Clinical trial details |
|---|---|---|---|
| 1 | Heat Shock Protein (HSP) | Colorectal cancer | NCT02324114 |
| Breast cancer | NCT02650193 | ||
| Squamous cell carcinoma | NCT04628806 | ||
| Leukaemia | NCT04437420 | ||
| 2 | Mitogen-activated protein kinase (MAPK) | Neoplasms | NCT01392521 |
| NSCLC | NCT01229150 | ||
| HCC | NCT01668017 | ||
| Ovarian cancer/NSCLC | NCT02607813 | ||
| 3 | Glypican-3(GPC3) | HCC | NCT04715191 |
| HCC | NCT03884751 | ||
| HCC | NCT03198546 | ||
| Advanced HCC | NCT03980288 | ||
| HCC | NCT02905188 | ||
| Lung Squamous Cell Carcinoma | NCT02876978 | ||
| HCC | NCT04121273 | ||
| 4 | Mucin 1 (MUC 1) | Advanced Esophageal Cancer | NCT03706326 |
| Metastatic Breast Cancer | NCT04020575 | ||
| Advanced Solid Tumor | NCT03179007 | ||
| Lung Neoplasm Malignant/Non-small Cell Lung Cancer | NCT03525782 | ||
| Gastric Cancer | NCT02602249 | ||
| 5 | Phosphoinositide-3- kinase (P13K) | Colorectal Cancer | NCT02945033 |
| Colon cancer | NCT03711058 | ||
| NSCLC | NCT01723800 | ||
| NSCLC | NCT02194049 | ||
| 6 | Human leukocyte antigen- G(HLA-G) | Leukaemia | NCT00075816 |
| Haematological Malignancies | NCT01246206 | ||
| Blood Cancer/ Multiple Myeloma | NCT00185614 | ||
| 7 | METADHERIN (MTDH) | Bladder cancer | NCT04286672 |
Cancer immunotherapy—different treatment approaches
The immunotherapy approaches combat against most cancers cells by way of: “revved up” (Boosting the immunity) immune device with “Marking” cancer cells so that the immune system can discover and destroy the malignant tumor cells [127]. This continues the cancer cells from being capable to hide, beyond that, helping the immune system of the body to discover most cancer cells and provides therapy like radiation, chemotherapy, or even T cells at once to the cancer cells. There are several types of immunotherapeutic approaches in cancer therapy.
Checkpoint inhibitors
Cancer immunity cycle is strictly regulated to prevent excessive immune activation and thereby the occurrence of uncontrolled inflammatory reactions in employing some co-inhibitory molecules. A co-inhibitory receptor with its corresponding ligand is called a checkpoint. Inhibition of these checkpoints helps in immune regulation and can be used as a novel therapeutic intervention for cancer treatment. Immune system checkpoints abrupt immune activation and thereby avoid the occurrence of autoimmune responses. Tumor cells can escape from the immune system attack by over-expressing checkpoints blockade molecules. These novel approaches are collectively termed checkpoint inhibitors. They slow down or prevent an immune cell reaction when healthy tissue is threatened by immune activity.
Some cancers will set off or down-regulate these checkpoints to avoid being attacked by the activated immune cells. New drugs, known as checkpoint inhibitors are planned to turn off these checkpoints and help the body to locate and fight against rapidly proliferating cells [128]. Most patients who manage immunotherapy are of PD-1 (Programmed death receptor-1) /PD-L1 (Programmed death receptor ligand-1) inhibitors or CTLA-4 (Cytotoxic T-lymphocyte-associated antigen 4, additionally referred to as CD152 (Cluster of differentiation 152)) inhibitors. Checkpoint inhibitors have been authorized for the following cancers include Merkel cell carcinoma, liver cancer, melanoma, non-small cell lung cancer, head and neck cancer, cutaneous squamous cell carcinoma, small cell lung cancer, kidney cancer, lymphoma, bladder cancer, breast cancer and cervical cancer [129]. In the case of checkpoint inhibitors, some clinical trials are available for checkpoint inhibitors in combination with chemotherapy or other immunotherapy methods.
Overexpression of PD-1 and PD-L1 leads to immune suppression in most of the cancers and inflammation processes. The binding of specific PD-1 ligand (PD-L1) on the tumor cell surface to the PD-1 receptors present on T cells leads to inactivation of cytotoxic T cells and they send an off signal to the immune cells which in turn leads to inactivation of T-cells. Activated PD-1 receptors bind with the corresponding ligand (PD-L1) present on the surface of cancer cells and the immune tolerance is developed. This tolerance may prevent the interaction of T cells with the MHC and shut down the immune reaction [130]. CTLA-4 is another checkpoint that is prominently overexpressed in most of the fatalities and is mainly involved in immune evasion of malignantly transformed cells. CTLA-4 is a homolog of CD28 and co-stimulatory molecules such as CD 28 acts as the substrate for CTLA-4 on T cells. The binding of CD28 to the overexpressed CTLA-4 hinders its co-stimulatory action and T cell priming [131, 132]. Anti-CTLA-4 antibodies can block the interplay of CTLA-4 with their corresponding ligand and facilitate the interaction of T cells with most cancer cells. Preclinical studies on PD-1, PD-L1, and CTLA-4 inhibitors indicate the decreased tumor shot and the survival improvement in most cancers. Monoclonal antibodies that block CTLA-4 (Ipilimumab) or PD-1 (Nivolumab, pembrolizumab)/ PDL1 (Avelumab, Durvalumab) have recently got approval for treating different cancers and clinical trials are ongoing for the treatment of several cancers [133] (Fig. 1).
Fig. 1.
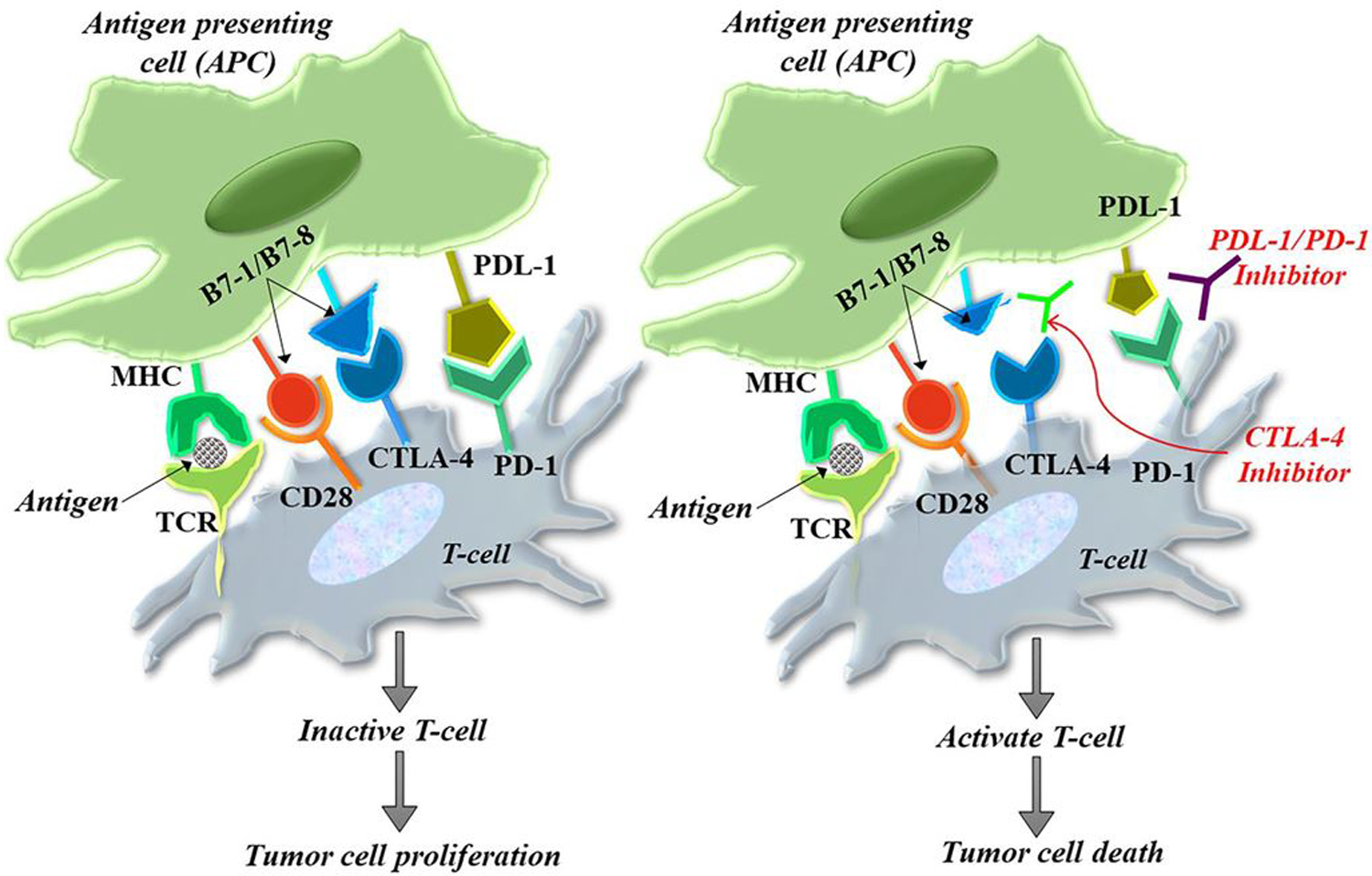
Working mechanism of CTLA-4 and PD-1 on cancer cells. The activation of T cells is mediated by the interaction of the T cell receptor and the CD28 receptor with class II major histocompatibility complex and B7 co-stimulatory molecule located on the antigen-presenting cells. The interaction of CTLA-4 with the B7 molecule delivers an inhibitory signal, effectively checked by CTLA-4 inhibitors. On the other hand, the negative regulation of T cells resulting from PD-1/PD-L1 interaction between T cells and tumor cells is prevented by PD-1/PD-L1 inhibitors
Other monoclonal antibodies
Checkpoint inhibitors are considered as a form of monoclonal antibody (mAb), but all mAbs are not having the same actions in cancer sites like check point inhibitors.
Monoclonal antibodies have the capability to recognize the tumor antigens present on the cancer cells and and attack most of the cancerous cells. Each mAbs are made to locate, connect, and communicate with a particular protein that occurs in the malignant cells [134]. There are several monoclonal antibodies, targeting the different tumor antigens present on the cancer cells such as EGFR, CD38, GD2, SLAMF7, CCR4, CD20, PDGFR, HER2, VEGFR2, Nectin 4, CD79B, TROP2, CD19, CD3 etc. [135]. mAbs work by recognising specific tumor proteins on cells in altered ways depending on the tumor antigen they are targeting. The prime mechanism of many antibodies that elicit cancer cell death is by the inhibition of growth factor receptor signaling. Pro-survival signaling is distressed when mAbs bind their corresponding target growth factor receptors and manipulate its signalings via the inhibition of ligand binding [136].
Epidermal growth factor receptor (EGFR) is overexpressed in many cancers and signaling via EGFR leads to cancer cell proliferation, migration, and cell invasion. Cetuximab, which is an anti-EGFR mAb, persuades apoptosis in neoplastic cells via inhibiting the ligand binding and receptor dimerization in the cancer cells [137]. Human epidermal growth factor receptor 2 (HER2) is a tyrosine kinase protein that is aberrantly expressed in several cancers, especially in breast carcinomas [138]. It is different from EGFR, that it has no known ligand for their activation and in its place hetero-dimerizes with other growth factor receptors to augment their activation [139]. Therefore, antibodies targeting HER2 achieve signaling perturbation by inhibiting heterodimerization and internalization. Trastuzumab was the first FDA approved anti-HER2 mAb and remains a vital component of therapy for HER2-amplified breast cancer [140].
CD20 was found to be abundantly expressed on the surface of cancerous B cells in non-Hodgkin’s lymphoma, but not found on healthy immature B cells. Therefore, a mAb treatment that targeted CD20 could eliminate the cancerous cells, but immature B cells would remain to replenish the supply of healthy cells. Thus, CD20 became the first target for mAb therapy and the anti-CD20 mAb rituximab was the first mAb to be approved for the treatment of cancer [141]. Most mAb remedies, which does not belong to category of checkpoint inhibitors such as Elotuzumab (multiple myeloma), Mogamulizumab (Cutaneous T-cell lymphoma), Olaratumab (sarcoma), Ramucirumab (gastric cancer), Pertuzumab (breast cancer), Enfortumab vedotin (bladder cancer), Sacituzumab govitecan (triple negative breast cancer) are used for the treatment of several cancers [142].
In immunotherapeutic aspect, several clinical studies are ongoing to establish the effects of mAbs in different malignancies. Combination of mFOLFOX6 + Bevacizumab + PD-1, phase II clinical trial is pursuing to appraise the efficacy and safety of this mAb combination in patients with local advanced colorectal cancer (NCT04895137). A phase II clinical trial in progress to assess the effect of ixazomib and rituximab in Bruton Tyrosine Kinase Inhibitor Resistant Mantle Cell Lymphoma. Immunotherapy with rituximab may induce changes in body’s immune system and may interfere with the ability of cancer cells, thereby inhibit its progression. Giving ixazomib and rituximab may be effective in treating patients with mantle cell lymphoma compared to rituximab alone (NCT04047797). More than a few numbers of clinical trials establish the effectiveness of different mAbs and its combination in different cancers (NCT01637532 (overian cancer), NCT04744649 (gastric cancer), NCT03950154 (colorectal cancer), NCT01922921 (breast cancer), NCT03579927 (mantle cell lymphoma) etc.).
Adoptive (or cart) cell therapies
CAR- T cell therapy is considered as a type of adaptive immune cell therapy where T cells are collected from a patient’s tumor sample, taken to a laboratory where the T-cell receptor is incorporated with tumor antigen-specific antibody receptor to get chimeric antigen T (CAR- T) cells. After expansion, once back to the patients, those changed T cells can locate or target and destroy most of the cancer cells by T cell-mediated immune reaction. This method is being tested in numerous types of cancers. The CAR- T cells usually consist of an Antigen recognition area, a hinge area, a transmembrane area and an intracellular T cell signalling area [143].
CAR- T cell method is the best available choice for the treatment of cancer-based on immunotherapy. It is an accepted treatment procedure for both lymphomas and leukaemias [144]. CAR- T cells are artificially built that can target antigen-presenting cells (APC). They can bind with the target antigen via single-chain variable fragments and produce immune regulation to destroy the tumor cells [145]. A chimeric antigen receptor (CAR) is composed of several components, each of which contributes in the direction of the right activation of T Cells, capability, and endurance of CAR- T cells. The combinational strategies of chemotherapeutic drugs with CAR- T-cell therapy and checkpoint inhibitor such as antagonistic antibodies against CTLA-4 and PD1/PD1-L have a great potential in cancer therapy [146]. CAR-T cell strategies are an effective novel therapeutic with outstanding success in several solid tumors and emerge a new era for cancer immunotherapy (Fig. 2).
Fig. 2.
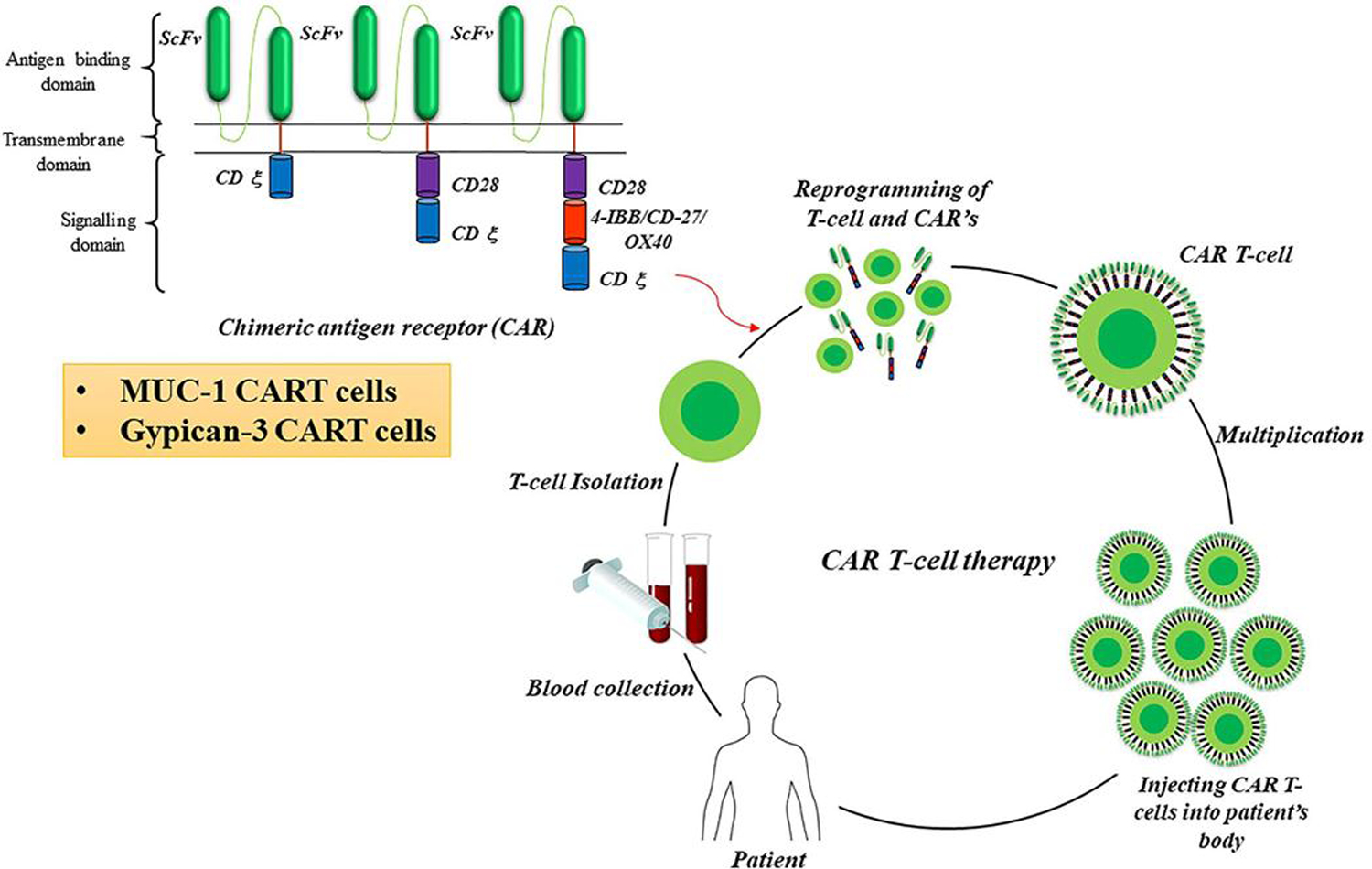
Generation of CAR-T Cells for immunotherapy. One form of adoptive cell therapy is CAR (Chimeric antigen receptors) T cell therapy. It is an accepted treatment procedure for both lymphomas and leukaemias. CAR T cell therapy is considered as a type of adoptive immune cell therapy where T cells are collected from a patient’s tumor sample, taken to a laboratory where the T-cell receptor is incorporated with tumor antigen-specific antibody receptor to get chimeric antigen receptor T (CAR T) cells. Nowadays MUC 1 and Glypican 3 targeting CAR-T cells are under clinical trial for different cancers
Cancer vaccines
Cancer vaccines are designed to elicit an immune response against TSP and TAA, encouraging the immune system to attack cancer cells bearing these antigens [147]. Different types of therapeutic vaccines have been developed including whole tumor cell, tumor-cell lysates (gene-modified tumor cells, protein- or peptide-based vaccines), RNA and DNA vaccines, viral vector engineered vaccines to express tumor antigen and DC-based vaccines loaded with DNA, RNA or peptides for cancer therapy [148–153]. Presently, Sipuleucel-T (Provenge®) is a well-established cell based cancer immunotherapy used as a vaccine to treat prostate cancer. Provenge® is made from the patient’s white blood cells. These cells are dispatched to the laboratory and they have the capacity to attack prostate tumor cells and they are then re-infused into the patient [154]. Many preclinical and clinical trials have been performed to evaluate the safety and efficacy of different vaccine approaches. A phase II/III, randomized clinical study is currently ongoing on glioblastoma patients to test the efficacy of DC-based vaccine (NCT03548571), Similarly, MUC-1 based and immunostimulatory cytokine IL-2 (TG4010) vaccines were tested in a phase II clinical trial in patients with metastatic renal cell carcinoma. ROSTVAC (PSA-TRICOM), a poxviral-based prostate-specific antigen vaccine has been assessed in a phase II clinical trial in patients with metastatic prostate cancer [155]. The combination of antiangiogenic treatments with different vaccines has also been emerged as an innovative strategy against cancer (NCT01582672) [156].
Bacillus Calmette-Guerin (BCG)
Bacillus Calmette-Guerin (BCG) is intravesical immunotherapy for primary-stage bladder cancer. It’s prepared from the weakened stress of Mycobacterium Bovis, generally used as a vaccine for tuberculosis. BCG is used for both non-invasive or stage zero and minimally invasive or stage one bladder cancers. It typically follows a method referred to as trans-urethral resection of bladder tumor (TURBT). BCG vaccine can increase the level of CD4 + T- cells inside the tumor site, which mediates the immune reaction towards the cancer cells inside the body. BCG vaccine therapy is injected into the bladder of the patients to drive an immune response towards bladder cancer cells. It prevents the recurrence of cancer in approximately 70 per cent of sufferers with early-stage bladder cancer [157].
Cytokines
Cytokines have the capacity to boosts T cell proliferation and activation and also have the ability to activate other immune proteins involved in the immune reaction. Interleukins and other interferon are the best-utilized cytokine class in immunotherapy, which have shown a significant role in immunotherapy. Cytokines are usually taken into consideration as an immune messenger, which can help the immune cells to communicate with each other. The interaction with these cells helps the rapid propagation of immune signalling within the body. Cytokines also stimulate the immune effector cells and cytotoxic effector cells at once to induce the immune reaction. A high dose of interleukin 2 (IL2) has substantially helped a small percentage of people with superior melanomas and kidney cancers. FDA has authorized two cytokines as single dealers for cancer treatment. High-dose of bolus interleukin-2 used for the treatment of metastatic melanoma and renal cell carcinoma, the other one is, IFN-α (Interferon-α) for the adjuvant therapy of these cancers [158].
The membrane-bound proteins generally secrete the cytokines, which act as an intracellular signalling molecule. They are secreted generally at the time for the development of response against the microbes or towards the tumor antigen. Cytokines can produce a significant degree of pleiotropism. They also target bone marrow, dendritic cells, T cells, macrophages, NKT cells, endothelial cells, B cells, monocytes, hypothalamus etc. The main pathogenic role of cytokines in the body related to the immune response is the development of co-stimulation, cell activation, homeostasis, inflammation, enhances MHC expression etc. Another way to increase the immunogenicity of the antigen-specific vaccine is recombinant DNA technology [159]. Recombinant virus cytokines (intra-tumor vaccine cytokine gene) are produced by recombinant technology which gives promising results on the in vivo murine tumor model. Nowadays, a variety of strategies are used to deliver the cytokines to treat malignancy such as cytokine antibody fusion molecule, recombinant virus as the delivery system for tumor immunotherapy, cell engineering approaches, cytokine pegylation (enhance the pharmacokinetic properties of the drugs) [160].
Radio-immunotherapy
Radio-immunotherapy (RIT) is a compilation of radiation therapy and immunotherapy. In immunotherapy, a laboratory-produced biological material known as a monoclonal antibody is engineered to target and bind to the surface of malignant cells. Monoclonal antibodies mimic the antibodies that are naturally produced by way of the body’s immune proteins, which can fight against foreign substances present in the body, including viruses and bacteria. In RIT, Mab is paired with radioactive material or radiotracer. When RIT is injected into the patient’s bloodstream, the radiation-related monoclonal antibody travels to detect and binds with the cancer cells, permitting an excessive dose of radiation to be introduced without delay to the tumor cells [161]. It is presently used to treat non-Hodgkin B-cell lymphoma (NHL), for newly recognized patients and also for victims who have not responded to chemotherapy or remedy with the monoclonal antibody.
Several new RIT approaches are under clinical trials. Potential RITs are used to treat various types of tumors such as ovarian cancers, leukaemia, high-grade brain glioma, prostate cancer, melanoma, and colorectal most cancers [162]. This treatment strategy uses an antibody with a radioactive core to straightforward the radiation to cancer cells for the destruction or alters the viability. Ibritumomab tiuxetan (Zevalin®) is the only radioimmunotherapy authorized by the US FDA. This cancer therapy allows physicians to target the cancer cells with higher doses of radiation safely [163]. Various clinical trials suggest the synergistic effect of combining radiotherapy with checkpoint inhibitors in several solid cancers [164, 165]. Recently, some of the preclinical studies demonstrated that the therapeutic efficiency of immune checkpoint inhibitors can be improved by the use of radiotherapy in a combination manner to treat head and neck cancer [166]. In this line, a few clinical trials are ongoing to establish the effect of a combination of radiotherapy with immune checkpoint inhibition in rectal cancer (NCT02298946, NCT02948348, NCT04124601, NCT04262687, NCT04558684) [167].
Oncolytic virus therapy
Oncolytic virus treatment is a well-established treatment approach for haematological malignancy and is also considered as an investigational form of immunotherapy for many other cancers [168]. The oncolytic virus infects both cancer cells and the normal cells during the therapy, but they have a mild effect on normal cells. After the administration of these kinds of viruses, they multiply inside tumor cells and induce apoptosis. Several viruses are currently in ongoing clinical trials. The only oncolytic virus therapy approved in the U.S [169]. T-VEC (Imlygic®) is a genetically engineered HSV-1 (herpes simplex virus-1) with GM- CSF (granulocyte–macrophage colony-stimulating factor). This is delivered locally which induce T cell-mediated cell death. Generally, they possess multimodal mechanisms such as cell autolysis, cell honing, vascular supply destruction, and potentiate other adjuvant therapies [170].
Combination strategies
Combination strategies using immunotherapy and chemotherapy showed prominent clinical outcomes in a series of clinical studies. Checkpoint blockade is the emerging and leading treatment method in immunotherapy. There are mainly two different types of checkpoint inhibitor molecules used as recent targets in cancer therapy, which include CTLA-4 and PD1/PDL1. Several antibodies in the particular category are approved namely Ipilimumab (an anti- CTLA-4 antibody) Pembrolizumab and Nivolumab (anti-PD-1 antibodies) for advanced melanoma treatment. The use of a single agent as a checkpoint inhibitor possesses only 20–30% of objective response. But the combination of both CTLA-4 and the PD1 antibodies increase the durable response rate as compared to the monotherapy [171]. Ipilimumab and Nivolumab combinations were found to be very effective for unresectable melanoma [172].
The combination of immunotherapy with radiation therapy is also considered an emerging field of cancer therapy. Antibodies against two immune checkpoints blockade molecules such as programmed death receptor-1 and cytotoxic T-lymphocyte antigen 4 in combination with radiation therapy are under evaluation in phase 1 and 2 clinical trials. Combinations of transforming growth factor-beta (TGF-β) with radiation therapy are presently being examined in phase 1/2 trials [173]. Clinical data supports the combination of radiation therapy with the cytokines such as interferon-α interleukins-2, granulocyte–macrophage, colony-stimulating, and tumor necrosis factor-α [174]. On the other hand, two different DNA methyltransferases inhibitors (DNMTi) approved by FDA for cancer immunotherapy, are azacitidine and decitabine. The combination of DNMTi with the other immunotherapies are also giving promising results against different types of cancer [175, 176]. Various immunotherapies for cancer treatment are summarized in Table 2.
Table 2.
Approved immunotherapies for cancer treatment
| Approved immunotherapy | Targeted proteins | Approved cancers |
|---|---|---|
| Checkpoint inhibitors | ||
| Durvalumab | PD-L1 | Urothelial cancer, Non- small-cell lung cancer [177, 178] |
| Avelumab | PD-L1 | Advanced Merkel cell carcinoma, urothelial cancer [179] |
| Atezolizumab | PD-L1 | Urothelial cancer, Non- small-cell lung cancer [180] |
| Nivolumab | PD-1 | Melanoma, Hodgkin lymphoma, Colorectal cancer, Hepatocellular cancer, Non- small-cell lung cancer, Kidney cancer, Squamous cell carcinoma of the head and neck and Urothelial cancer, Bladder cancer [181] |
| Pembrolizumab | PD- 1 | Gastric cancer, Head and neck cancer, Urothelial bladder cancer [182] |
| Ipilimumab | CTL A4 | Melanoma [183] |
| Relatlimab | LAG-3 | Recurrent glioblastoma [184] |
| Urelumab | CD137 | Non-Hodgkin’s lymphoma [185] |
| T cell therapies | ||
| Axicabtagene ciloleucel | CD19-specific CAR T cells | B cell lymphoma [186] |
| Tisagenlecleucel | CD19-specific CAR T cells | B cell lymphocytic leukaemia, non- Hodgkin lymphoma [187] |
| Cancer vaccines | ||
| Bacillus Calmette-Guérin | The stain of Mycobacterium tuberculosis variant Bovis | Bladder cancer [188] |
| Sipuleucel- T | PBMCs activated with recombinant human PAP-GM- CSF | Prostate cancer [147] |
| Cytokines | ||
| Intron A | Recombinant IFNα2b | Leukaemia, Melanoma, Follicular lymphoma, AIDS-related Kaposi sarcomt [189] |
| Aldesleukin | Recombinant IL-2 | Melanoma, kidney cancer [190] |
| Roferon- A | Recombinant IFNα2a | Cell leukaemia, Chronic myelogenous leukaemia, AIDS-related Kaposi sarcoma [191] |
| Imiquimod | Stimulates TNF, IL-12, IFNγ | Basal cell carcinoma [192] |
| Radioimmunotherapy | ||
| Ibritumomabtiuxetan | CD20 | Non-Hodgkin’s lymphoma [193] |
| Tositumomab | CD20 | Non-Hodgkin’s lymphoma [194] |
| Oncolytic virus therapy | ||
| Talimogene laherparepvec | Genetically modified HSV type 1 | Melonoma [195] |
| Bispecefic antibodies | ||
| Blinatumomab | CD19, CD3 | B cell lymphocytic leukaemia [196] |
Conclusion and future perspective
Immunotherapy is a new strategy that helps the immune system fight against cancer. Cancer immunotherapy approaches should have prompt and efficient T cell responses against tumor antigens present on cancer cells but not on healthy cells. Various methods are employed to improve the activity of T cells in the body against cancer cells. The development of novel immunotherapies against cancer requires a meticulous study into the immune evasion in the TME, together with identifying specifically overexpressed tumor antigens in each type of cancer. The molecular characterization of numerous tumor antigens(TAA/TSA) recognized suitable candidates for the development of cancer immunotherapy.
There are several immunotherapy approaches available against various cancers. Checkpoint inhibitors, adoptive T cell therapies, cancer vaccines, and several combination therapies are currently used to control the rapid proliferation of cancer cells. But many factors still hamper the success rate of cancer immunotherapy. The critical hurdle of cancer immune therapies involves the emergence of unpredictable adverse effects on cancer patients, problems in characterizing clinically significant biomarkers for immunotherapy and tumor heterogeneity. The coming years will likely bring new insights to decrease the immune-associated side effects caused by immunotherapy with maximal therapeutic effects. Finally, the economic burden of immunotherapies on health care systems is turning unsustainable. Overall, for both medical and economic reasons, there is an urge to establish personalized immunotherapeutic strategies, which could provide an ideal remedy as per the unique tumor milieu of the patient. The success of clinical translation demonstrates the power of modulating the immune system to treat cancer. Many studies are continuing to resolve the issues for the development of new sophisticated immunotherapies against cancer.
Acknowledgements
We acknowledge the support from Amrita Vishwa Vidyapeetham. We express our sincere gratitude to Dr Shanthikumar V Nair, Dean of research, AIMS and Dr Sabitha M, Principal, Amrita School of Pharmacy for all the facilities provided.
Funding
We acknowledge the support of the Amrita Vishwa Vidyapeetham SEED grant (Project ID: K-PHAR-20-627) to LRN. This review work was supported by a research grant from Amrita Vishwa Vidyapeetham under the PhD Student Research University Grant Scheme to ARK.
Footnotes
Conflict of interest The authors declare that they have no competing interests.
Data availability
The authors confirm that the data supporting the findings of this research are available within the manuscript.
References
- 1.Freddie B, Ferlay J (2018) Global cancer statistics 2018:GLO-BOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CAAC 2018(68):394–424 [DOI] [PubMed] [Google Scholar]
- 2.Qiao J, Liu Z, Fu YX (2016) Adapting conventional cancer treatment for immunotherapy. J Mol Med 94(5):489–495 [DOI] [PubMed] [Google Scholar]
- 3.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G (2008) Immunological aspects of cancer chemotherapy. Nat Rev Immunol 8(1):59–73 [DOI] [PubMed] [Google Scholar]
- 4.Berkey FJ (2010) Managing the adverse effects of radiation therapy. Am Fam Phys 82(4):381–388 [PubMed] [Google Scholar]
- 5.Baskar R, Lee KA, Yeo R, Yeoh KW (2012) Cancer and radiation therapy: current advances and future directions. Int J Med Sci 9(3):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephan K, Matthias I, Sebastian K (2019) Advances in cancer immunotherapy 2019 – latest trends. Exp Clin Cancer Res 38(268):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato H, Okonogi N, Nakano T (2020) Rationale of combination of anti-PD-1/PD-L1 antibody therapy and radiotherapy for cancer treatment. Int J Clin Oncol 25(5):801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermeulen K, Van Bockstaele DR, Berneman ZN (2003) The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif 36(3):131–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demaria O, Cornen S, Daëron M, Morel Y, Medzhitov R, Vivier E (2019) Harnessing innate immunity in cancer therapy. Nature 574(7776):45–56 [DOI] [PubMed] [Google Scholar]
- 10.Mesri EA, Feitelson MA, Munger K (2014) Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe 15(3):266–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kambayashi T, Laufer TM (2014) Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol 14(11):719–730 [DOI] [PubMed] [Google Scholar]
- 12.Wang S, He Z, Wang X, Li H, Liu XS (2019) Antigen presentation and tumor immunogenicity in cancer immunotherapy response prediction. Elife 8:e49020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Charette M, Marabelle A, Houot R (2016) Turning tumor cells into antigen presenting cells: the next step to improve cancer immunotherapy? Eur J Cancer 68:134–147 [DOI] [PubMed] [Google Scholar]
- 14.Eggermont LJ, Paulis LE, Tel J, Figdor CG (2014) Towards efficient cancer immunotherapy: advances in developing artificial antigen-presenting cells. Trends Biotechnol 32(9):456–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith CC, Selitsky SR, Chai S, Armistead PM, Vincent BG, Serody JS (2019) Alternative tumor-specific antigens. Nat Rev Cancer 19(8):465–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang JY (2004) Tumor-associated antigen arrays to enhance antibody detection for cancer diagnosis. Cancer Detect Prev 28(2):114–118 [DOI] [PubMed] [Google Scholar]
- 17.Fortner RT, Damms-Machado A, Kaaks R (2017) Systematic review: tumor-associated antigen autoantibodies and ovarian cancer early detection. Gynecol Oncol 147(2):465–480 [DOI] [PubMed] [Google Scholar]
- 18.Akashi T, Oimomi H, Nishiyama KI, Nakashima M, Arita Y, Sumii T, Kimura T, Ito T, Nawata H, Watanabe T (2003) Expression and diagnostic evaluation of the human tumor–associated antigen RCAS1 in pancreatic cancer. Pancreas 26(1):49–55 [DOI] [PubMed] [Google Scholar]
- 19.Casiano CA, Mediavilla-Varela M, Tan EM (2006) Tumor-associated antigen arrays for the serological diagnosis of cancer. Mol Cell Proteom 5(10):1745–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribas A, Hanson DC, Noe DA, Millham R, Guyot DJ, Bernstein SH, Canniff PC, Sharma A, Gomez-Navarro J (2007) Tremelimumab (CP-675,206), a cytotoxic T lymphocyte–associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncologist 12(7):873–883 [DOI] [PubMed] [Google Scholar]
- 21.Degroote H, Van Dierendonck A, Geerts A, Van Vlierberghe H, Devisscher L (2018) Preclinical and clinical therapeutic strategies affecting tumor-associated macrophages in hepatocellular carcinoma. J Immunol Res 2018:7819520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandya PH, Murray ME (2016) The immune system in cancer pathogenesis: potential therapeutic approaches. J Immunol Res 2016:4273943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNamara MA, Nair SK, Holl EK (2015) RNA-based vaccines in cancer immunotherapy. J Immunol Res 2015:794528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minati R, Perreault C, Thibault P (2020) A roadmap toward the definition of actionable tumor-specific antigens. Front Immunol 11:583287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castle JC, Uduman M, Pabla S, Stein RB, Buell JS (2019) Mutation-derived neoantigens for cancer immunotherapy. Front Immunol 10:1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei J, Ren S, Zhou C (2019) Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol 12(1):1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Margolin K (2011) Cytokines in cancer immunotherapy. Cancers 3(4):3856–3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshal HT, Djamgoz MBA (2018) Immuno-oncology: emerging targets and combination therapies. Front Oncol 8:1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parayath N, Padmakumar S, Nair SV, Menon D, Amiji MM (2020) Strategies for targeting cancer immunotherapy through modulation of the tumor microenvironment. Regener Eng Transl Med 6(1):29–49 [Google Scholar]
- 30.Albini A, Bruno A, Noonan DM, Mortara L (2018) Contribution to tumor angiogenesis from innate immune cells within the tumor microenvironment: implications for immunotherapy. Front Immunol 9:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syn NL, Teng MW, Mok TS, Soo RA (2017) De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol 18(12):e731–e741 [DOI] [PubMed] [Google Scholar]
- 32.Francisco LM, Sage PT, Sharpe AH (2010) The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 236:219–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fife BT, Pauken KE (2011) The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann NY Acad Sci 1217(1):45–59 [DOI] [PubMed] [Google Scholar]
- 34.Zitvogel L, Kroemer G (2012) Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 1(8):1223–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braun-Prado K, Petzl-Erler ML (2007) Programmed cell death 1 gene (PDCD1) polymorphism and pemphigus foliaceus (fogo selvagem) disease susceptibility. Genet Mol Biol 30(2):314–321 [Google Scholar]
- 36.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F (2005) Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 11(8):2947–2953 [DOI] [PubMed] [Google Scholar]
- 37.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL (2004) SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 173(2):945–954 [DOI] [PubMed] [Google Scholar]
- 38.Jiao P, Geng Q, Jin P, Su G, Teng H, Dong J, Yan B (2018) Small molecules as PD-1/PD-L1 pathway modulators for cancer immunotherapy. Curr Pharm Des 24(41):4911–4920 [DOI] [PubMed] [Google Scholar]
- 39.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2(3):261–268 [DOI] [PubMed] [Google Scholar]
- 40.Chen L (2004) Co-inhibitory molecules of the B7–CD28 family in the control of T-cell immunity. Nat Rev Immunol 4(5):336–347 [DOI] [PubMed] [Google Scholar]
- 41.Yang H, Zhou X, Sun L, Mao Y (2019) Correlation between PD-L2 expression and clinical outcome in solid cancer patients: a meta-analysis. Front Oncol 9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rozali EN, Hato SV, Robinson BW, Lake RA, Lesterhuis WJ (2012) Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol 2012:656340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maverakis E, Kim K, Shimoda M, Gershwin ME, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB (2015) Glycans in the immune system and the altered glycan theory of autoimmunity: a critical review. J Autoimmunity 57:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124(4):783–801 [DOI] [PubMed] [Google Scholar]
- 45.Van Duin D, Medzhitov R, Shaw AC (2006) Triggering TLR signaling in vaccination. Trends Immunol 27(1):49–55 [DOI] [PubMed] [Google Scholar]
- 46.Tsuji S, Matsumoto M, Takeuchi O, Akira S, Azuma I, Hayashi A, Toyoshima K, Seya T (2000) Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect Immunity 68(12):6883–6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, Li H, Coffman R, Seyfert V, Eiden JJ, Broide D (2006) Immunotherapy with a ragweed–Toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med 355(14):1445–1455 [DOI] [PubMed] [Google Scholar]
- 48.Kanzler H, Barrat FJ, Hessel EM, Coffman RL (2007) Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med 13(5):552–559 [DOI] [PubMed] [Google Scholar]
- 49.Kanneganti TD, Lamkanfi M, Núñez G (2007) Intracellular NOD-like receptors in host defense and disease. Immunity 27(4):549–559 [DOI] [PubMed] [Google Scholar]
- 50.Saxena M, Yeretssian G (2014) NOD-like receptors: master regulators of inflammation and cancer. Front Immunol 5:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kondelkova K, Vokurková D, Krejsek J, Borská L, Fiala Z, Ctirad A (2010) Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica 53:73–77 [DOI] [PubMed] [Google Scholar]
- 52.Mary LD (2014) Mechanism of action of immunotherapy. Semin Oncol 41:3–13 [DOI] [PubMed] [Google Scholar]
- 53.Davis BP, Ballas ZK (2017) Biologic response modifiers: indications, implications, and insights. J Allergy Clin Immunol 139(5):1445–1456 [DOI] [PubMed] [Google Scholar]
- 54.Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674 [DOI] [PubMed] [Google Scholar]
- 55.Knutson KL, Disis ML (2005) Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother 54(8):721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Upadhyay S, Sharma N, Gupta KB, Dhiman M (2018) Role of immune system in tumor progression and carcinogenesis. J Cell Biochem 119(7):5028–5042 [DOI] [PubMed] [Google Scholar]
- 57.Weinberg RA, Hanahan D (2000) The hallmarks of cancer. Cell 100(1):57–70 [DOI] [PubMed] [Google Scholar]
- 58.Sauerborn M, Brinks V, Jiskoot W, Schellekens H (2010) Immunological mechanism underlying the immune response to recombinant human protein therapeutics. Trends Pharmacol Sci 31(2):53–59 [DOI] [PubMed] [Google Scholar]
- 59.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31(3):164–172 [DOI] [PubMed] [Google Scholar]
- 60.Wang XY, Kaneko Y, Repasky E, Subjeck JR (2000) Heat shock proteins and cancer immunotherapy. Immunol Investig 29(2):131–137 [DOI] [PubMed] [Google Scholar]
- 61.Chatterjee S, Burns TF (2017) Targeting heat shock proteins in cancer: a promising therapeutic approach. Int J Mol Sci 18(9):1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Das JK, Xiong X, Ren X, Yang JM, Song J (2019) Heat shock proteins in cancer immunotherapy. J Oncol. 10.1155/2019/3267207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C (2008) Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med 12(3):743–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vidyasagar A, Wilson NA, Djamali A (2012) Heat shock protein 27 (HSP27): biomarker of disease and therapeutic target. Fibrogenesis Tissue Repair 5(1):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eftekharzadeh B, Banduseela VC, Chiesa G, Martínez-Cristóbal P, Rauch JN, Nath SR, Schwarz DM, Shao H, Marin-Argany M, Di Sanza C, Giorgetti E (2019) Hsp70 and Hsp40 inhibit an inter-domain interaction necessary for transcriptional activity in the androgen receptor. Nat Commun 10(1):1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mayer MP, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62(6):670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moser C, Lang SA, Stoeltzing O (2009) Heat-shock protein 90 (Hsp90) as a molecular target for therapy of gastrointestinal cancer. Anticancer Res 29(6):2031–2042 [PubMed] [Google Scholar]
- 68.Caudill MM, Li Z (2001) HSPPC-96: a personalised cancer vaccine. Expert Opin Biol Ther 1(3):539–547 [DOI] [PubMed] [Google Scholar]
- 69.Geng H, Zhang GM, Xiao H, Yuan Y, Li D, Zhang H, Qiu H, He YF, Feng ZH (2006) HSP70 vaccine in combination with gene therapy with plasmid DNA encoding sPD-1 overcomes immune resistance and suppresses the progression of pulmonary metastatic melanoma. Int Jn Cancer 118(11):2657–2664 [DOI] [PubMed] [Google Scholar]
- 70.Gong J, Zhu B, Murshid A, Adachi H, Song B, Lee A, Liu C, Calderwood SK (2009) T cell activation by heat shock protein 70 vaccine requires TLR signaling and scavenger receptor expressed by endothelial cells-1. J Immunol 183(5):3092–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gong J, Zhang Y, Durfee J, Weng D, Liu C, Koido S, Song B, Apostolopoulos V, Calderwood SK (2010) A heat shock protein 70-based vaccine with enhanced immunogenicity for clinical use. J Immunol 184(1):488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zong J, Wang C, Liu B, Liu M, Cao Y, Sun X, Yao Y, Sun G (2013) Human hsp70 and HPV16 oE7 fusion protein vaccine induces an effective antitumor efficacy. Oncol Rep 30(1):407–412 [DOI] [PubMed] [Google Scholar]
- 73.Klimczak M, Biecek P, Zylicz A, Zylicz M (2019) Heat shock proteins create a signature to predict the clinical outcome in breast cancer. Sci Rep 9(1):1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar S, Principe DR, Singh SK, Viswakarma N, Sondarva G, Rana B, Rana A (2020) Mitogen-activated protein kinase inhibitors and t-cell-dependent immunotherapy in cancer. Pharmaceuticals 13(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murshid A, Gong J, Stevenson MA, Calderwood SK (2011) Heat shock proteins and cancer vaccines: developments in the past decade and chaperoning in the decade to come. Expert Rev Vaccines 10(11):1553–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiong D, Wang Y, Singavi AK, Mackinnon AC, George B, You M (2018) Immunogenomic landscape contributes to hyperprogressive disease after anti-PD-1 immunotherapy for cancer. iScience 9:258–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tournier C (2013) The 2 faces of JNK signaling in cancer. Genes Cancer 4(9–10):397–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roy CR, Mocarski ES (2007) Pathogen subversion of cell-intrinsic innate immunity. Nat Immunol 8(11):1179–1187 [DOI] [PubMed] [Google Scholar]
- 79.Arthur JS, Ley SC (2013) Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 13(9):679–692 [DOI] [PubMed] [Google Scholar]
- 80.Bhattacharyya ND, Feng CG (2020) Regulation of T helper cell fate by TCR signal strength. Front Immunol 11:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ohkusu-Tsukada K, Toda M, Udono H, Kawakami Y, Takahashi K (2010) Targeted inhibition of IL-10-secreting CD25–Treg via p38 MAPK suppression in cancer immunotherapy. Eur J Immunol 40(4):1011–1021 [DOI] [PubMed] [Google Scholar]
- 82.Romagnani S (2006) Regulation of the T cell response. Clin Exp Allergy 36(11):1357–1366 [DOI] [PubMed] [Google Scholar]
- 83.Boislève F, Kerdine-Römer S, Pallardy M (2005) Implication of the MAPK pathways in the maturation of human dendritic cells induced by nickel and TNF-α. Toxicology 206(2):233–244 [DOI] [PubMed] [Google Scholar]
- 84.Homet B, Ribas A (2014) New drug targets in metastatic melanoma. J Pathol 232(2):134–141 [DOI] [PubMed] [Google Scholar]
- 85.Shimizu Y, Suzuki T, Yoshikawa T, Endo I, Nakatsura T (2019) Next-generation cancer immunotherapy targeting glypican-3. Front Oncol 9:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ho M, Kim H (2011) Glypican-3: a new target for cancer immunotherapy. Eur J Cancer 47(3):333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trinh TL, Puszyk W, Liu C (2014) Epigenetic regulation of glypican-3 in hepatocellular carcinoma. Cancer Res 74(19):418 [Google Scholar]
- 88.Li L, Jin R, Zhang X, Lv F, Liu L, Liu D, Liu K, Li N, Chen D (2012) Oncogenic activation of glypican-3 by c-Myc in human hepatocellular carcinoma. Hepatology 56(4):1380–1390 [DOI] [PubMed] [Google Scholar]
- 89.Miller DM, Thomas SD, Islam A, Muench D, Sedoris K (2012) c-Myc and cancer metabolism. Clin Cancer Res 18(20):5546–5553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimizu Y, Suzuki T, Yoshikawa T, Tsuchiya N, Sawada Y, Endo I, Nakatsura T (2018) Cancer immunotherapy-targeted glypican-3 or neoantigens. Cancer Sci 109(3):531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ratan C, Cicily KD, Nair B, Nath LR (2020) MUC glycoproteins: potential biomarkers and molecular targets for cancer therapy. Curr Cancer Drug Targets 21:132. [DOI] [PubMed] [Google Scholar]
- 92.Hollingsworth MA, Swanson BJ (2004) Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 4(1):45–60 [DOI] [PubMed] [Google Scholar]
- 93.Moncada DM, Kammanadiminti SJ, Chadee K (2003) Mucin and Toll-like receptors in host defense against intestinal parasites. Trends Parasitol 19(7):305–311 [DOI] [PubMed] [Google Scholar]
- 94.Mahla RS, Reddy CM, Prasad D, Kumar H (2013) Sweeten PAMPs: role of sugar complexed PAMPs in innate immunity and vaccine biology. Front Immunol 4:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ueno K, Koga T, Kato K, Golenbock DT, Gendler SJ, Kai H, Kim KC (2008) MUC1 mucin is a negative regulator of toll-like receptor signaling. Am J Respir Cell Mol Biol 38(3):263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ (1994) Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res 54(11):2856–2860 [PubMed] [Google Scholar]
- 97.Shental-Bechor D, Levy Y (2008) Effect of glycosylation on protein folding: a close look at thermodynamic stabilization. Proc Natl Acad Sci 105(24):8256–8261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taylor-Papadimitriou J, Burchell JM, Graham R, Beatson R (2018) Latest developments in MUC1 immunotherapy. Biochem Soc Trans 46(3):659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roulois D, Grégoire M, Fonteneau JF (2013) MUC1-specific cytotoxic T lymphocytes in cancer therapy: induction and challenge. BioMed Res Int 2013:871936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Syrkina MS, Rubtsov MA (2019) MUC1 in cancer immunotherapy: new hope or phantom menace? Biochem Mosc 84(7):773–781 [DOI] [PubMed] [Google Scholar]
- 101.Collins NB, Al Abosy R, Miller B, Bi K, Manguso R, Yates K, Haining WN. PI3K activated tumors evade tumor immunity by promoting an inhibitory myeloid microenvironment
- 102.Groh V, Wu J, Yee C, Spies T (2002) Tumor-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419(6908):734–738 [DOI] [PubMed] [Google Scholar]
- 103.Nicolini A, Carpi A (2009) Immune manipulation of advanced breast cancer: an interpretative model of the relationship between immune system and tumor cell biology. Med Res Rev 29(3):436–471 [DOI] [PubMed] [Google Scholar]
- 104.Thomas MS, Mitchell JS, DeNucci CC, Martin AL, Shimizu Y (2008) The p110γ isoform of phosphatidylinositol 3-kinase regulates migration of effector CD4 T lymphocytes into peripheral inflammatory sites. J Leukoc Biol 84(3):814–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tang CH, Yamamoto A, Lin YT, Fong YC, Tan TW (2010) Involvement of matrix metalloproteinase-3 in CCL5/CCR5 pathway of chondrosarcomas metastasis. Biochem Pharmacol 79(2):209–217 [DOI] [PubMed] [Google Scholar]
- 106.O’Donnell JS, Massi D, Teng MW, Mandala M (2018) PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. In: Kim JB (ed) Seminars in cancer biology, vol 48. Academic Press, Cambridge, pp 91–103 [DOI] [PubMed] [Google Scholar]
- 107.Lin A, Yan WH (2015) Human leukocyte antigen-G (HLA-G) expression in cancers: roles in immune evasion, metastasis and target for therapy. Mol Med 21(1):782–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin A, Chen HX, Zhu CC, Zhang X, Xu HH, Zhang JG, Wang Q, Zhou WJ, Yan WH (2010) Aberrant human leucocyte antigen-G expression and its clinical relevance in hepatocellular carcinoma. J Cell Mol Med 14(8):2162–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wiendl H, Mitsdoerffer M, Hofmeister V, Wischhusen J, Bornemann A, Meyermann R, Weiss EH, Melms A, Weller M (2002) A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol 168(9):4772–4780 [DOI] [PubMed] [Google Scholar]
- 110.Agaugué S, Carosella ED, Rouas-Freiss N (2011) Role of HLA-G in tumor escape through expansion of myeloid-derived suppressor cells and cytokinic balance in favor of Th2 versus Th1/Th17. Blood 117(26):7021–7031 [DOI] [PubMed] [Google Scholar]
- 111.Lin A, Zhang X, Xu HH, Xu DP, Ruan YY, Yan WH (2012) HLA-G expression is associated with metastasis and poor survival in the Balb/c nu/nu murine tumor model with ovarian cancer. Int J Cancer 131(1):150–157 [DOI] [PubMed] [Google Scholar]
- 112.Zhang Y, Yu S, Han Y, Wang Y, Sun Y (2018) Human leukocyte antigen-G expression and polymorphisms promote cancer development and guide cancer diagnosis/treatment. Oncol Lett 15(1):699–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zeestraten EC, Reimers MS, Saadatmand S, Dekker JT, Liefers GJ, Van Den Elsen PJ, Van De Velde CJ, Kuppen PJ (2014) Combined analysis of HLA class I, HLA-E and HLA-G predicts prognosis in colon cancer patients. Br J Cancer 110(2):459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown DM, Ruoslahti E (2004) Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell 5(4):365–374 [DOI] [PubMed] [Google Scholar]
- 115.Chen X, Dong K, Long M, Lin F, Wang X, Wei J, Ren J, Zhang H (2012) Serum anti-AEG-1 auto-antibody is a potential novel biomarker for malignant tumors. Oncol Lett 4(2):319–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dhiman G, Srivastava N, Goyal M, Rakha E, Lothion-Roy J, Mongan NP, Miftakhova RR, Khaiboullina SF, Rizvanov AA, Baranwal M (2019) Metadherin: a therapeutic target in multiple cancers. Front Oncol 9:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB (2008) Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene 27(8):1114–1121 [DOI] [PubMed] [Google Scholar]
- 118.Emdad L, Lee SG, Su ZZ, Jeon HY, Boukerche H, Sarkar D, Fisher PB (2009) Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc Natl Acad Sci 106(50):21300–21305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu GC, Yu CY, She L, Tan HL, Li G, Ren SL, Su ZW, Wei M, Huang DH, Tian YQ, Su RN (2015) Metadherin regulation of vascular endothelial growth factor expression is dependent upon the PI3K/Akt pathway in squamous cell carcinoma of the head and neck. Medicine 94(6):e502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li PP, Feng LL, Chen N, Ge XL, Lv X, Lu K, Ding M, Yuan D, Wang X (2015) Metadherin contributes to the pathogenesis of chronic lymphocytic leukemia partially through Wnt/β-catenin pathway. Med Oncol 32(2):21. [DOI] [PubMed] [Google Scholar]
- 121.Yoo BK, Emdad L, Su ZZ, Villanueva A, Chiang DY, Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA, Llovet JM, Fisher PB (2009) Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J Clin Investig 119(3):465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shi X, Wang X (2015) The role of MTDH/AEG-1 in the progression of cancer. Int J Clin Exp Med 8(4):4795. [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao Y, Kong X, Li X, Yan S, Yuan C, Hu W, Yang Q (2011) Metadherin mediates lipopolysaccharide-induced migration and invasion of breast cancer cells. PLoS ONE 6(12):e29363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abdel Ghafar MT, Gharib F, Abdel-Salam S, Elkhouly RA, Elshora A, Shalaby KH, El-Guindy D, El-Rashidy MA, Soliman NA, Abu-Elenin MM, Allam AA (2020) Role of serum Metadherin mRNA expression in the diagnosis and prediction of survival in patients with colorectal cancer. Mol Biol Rep 47(4):2509. [DOI] [PubMed] [Google Scholar]
- 125.Wang Z, Wei YB, Gao YL, Yan B, Yang JR, Guo Q (2014) Metadherin in prostate, bladder, and kidney cancer: a systematic review. Mol Clin Oncol 2(6):1139–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dhiman G, Lohia N, Jain S, Baranwal M (2016) Metadherin peptides containing CD4+ and CD8+ T cell epitopes as a therapeutic vaccine candidate against cancer. Microbiol Immunol 60(9):646–652 [DOI] [PubMed] [Google Scholar]
- 127.Oh T, Ivan ME, Sun MZ, Safaee M, Fakurnejad S, Clark AJ, Sayegh ET, Bloch O, Parsa AT (2014) PI3K pathway inhibitors: potential prospects as adjuncts to vaccine immunotherapy for glioblastoma. Immunotherapy 6(6):737–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hongming Z, Jibei C (2018) Current status and future directions of cancer immunotherapy. JCA 9(10):1773–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bonavita E, Bromley CP, Jonsson G, Pelly VS, Sahoo S, Walwyn-Brown K, Mensurado S, Moeini A, Flanagan E, Bell CR, Chiang SC (2020) Antagonistic inflammatory phenotypes dictate tumor fate and response to immune checkpoint blockade. Immunity 53(6):1215–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martha MR, Estuardo AC, Augusto RM (2017) Immunotherapy and gene therapy as novel treatments for cancer. Colomb Med 48(3):138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Julian A, Marin A, Bhagirathbhai D, Aixa E (2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol 11(39):1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hashem O, Samaresh S, Rami A (2017) PD-1 and PD-L1 Checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol 8:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garber K (2018) Driving T-cell Immunotherapy to solid tumors. Nat Biotechnol 36:215–219 [DOI] [PubMed] [Google Scholar]
- 134.Elizabeth IB, Anupam D (2016) CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. Am J Clin Oncol 39(1):98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zahavi D, Weiner L (2020) Monoclonal antibodies in cancer therapy. Antibodies 9(3):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM (2005) Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7(4):301–311 [DOI] [PubMed] [Google Scholar]
- 137.Patel D, Bassi R, Hooper A, Prewett M, Hicklin DJ, Kang X (2009) Anti-epidermal growth factor receptor monoclonal antibody cetuximab inhibits EGFR/HER-2 heterodimerization and activation. Int J Oncol 34(1):25–32 [PubMed] [Google Scholar]
- 138.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244(4905):707–712 [DOI] [PubMed] [Google Scholar]
- 139.Chen JS, Lan K, Hung MC (2003) Strategies to target HER2/neu overexpression for cancer therapy. Drug Resist Updates 6(3):129–136 [DOI] [PubMed] [Google Scholar]
- 140.Wang J, Xu B (2019) Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct Target Ther 4(1):1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maloney DG, Grillo-López AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles TM, Dallaire BK, Wey K (1997) IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood 90(6):2188–2195 [PubMed] [Google Scholar]
- 142.Anand R (2019) Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res 38(255):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kimiz G, Gulce I, Biray AC (2018) Monoclonal antibodies in cancer immunotherapy. Mol Biol Rep 45(6):2935–2940 [DOI] [PubMed] [Google Scholar]
- 144.Miliotou AN, Papadopoulou LC (2018) CAR T-cell therapy: a new era in cancer immunotherapy. Curr Pharm Biotechnol 19(1):5–18 [DOI] [PubMed] [Google Scholar]
- 145.Darvin P, Toor SM, Nair S (2018) Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 50(165):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xiuyan W, Isabelle R (2016) Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncol 3:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hammerstrom AE, Cauley DH, Atkinson BJ, Sharma P (2011) Cancer immunotherapy: sipuleucel-T and beyond. Pharmacotherapy 31(8):813–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lollini PL, Cavallo F, Nanni P, Quaglino E (2015) The promise of preventive cancer vaccines. Vaccines 3(2):467–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hu Z, Ott PA, Wu CJ (2018) Towards personalized, tumor-specific, therapeutic vaccines for cancer. Nat Rev Immunol 18(3):168–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ (2016) Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer 16(4):219–233 [DOI] [PubMed] [Google Scholar]
- 151.Wong KK, Li WA, Mooney DJ, Dranoff G (2016) Advances in therapeutic cancer vaccines. Adv Immunol 130:191–249 [DOI] [PubMed] [Google Scholar]
- 152.Maeng H, Terabe M, Berzofsky JA (2018) Cancer vaccines: translation from mice to human clinical trials. Curr Opin Immunol 51:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Finn OJ (2018) The dawn of vaccines for cancer prevention. Nat Rev Immunol 18(3):183–194 [DOI] [PubMed] [Google Scholar]
- 154.Gardner T, Elzey B, Hahn NM (2012) Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum Vaccines Immunother 8(4):534–539 [DOI] [PubMed] [Google Scholar]
- 155.Mougel A, Terme M, Tanchot C (2019) Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front Immunol 10:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bose A, Taylor JL, Alber S, Watkins SC, Garcia JA, Rini BI, Ko JS, Cohen PA, Finke JH, Storkus WJ (2011) Sunitinib facilitates the activation and recruitment of therapeutic anti-tumor immunity in concert with specific vaccination. Int J Cancer 129(9):2158–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Oliver F, Nikhil V (2015) Immunotherapy for bladder cancer. Res Rep Urol 7:65–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alice M, Magali T, Corinne T (2019) Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front Immunol 10:1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Berraondo P, Sanmamed MF, Ochoa MC (2019) Cytokines in clinical cancer immunotherapy. BJC 120:6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sylvia L, Kim M (2011) Cytokines in cancer immunotherapy. Cancers 3(4):3856–3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zaheer J, Kim H, Lee YJ, Kim JS, Lim SM (2019) Combination radioimmunotherapy strategies for solid tumors. Int J Mol Sci 20(22):5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Larson SM, Carrasquillo JA (2015) Radioimmunotherapy of human tumors. Nat Rev Cancer 15(6):347–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Puvvada SD, Guillén-Rodríguez JM, Yan J, Inclán L, Heard K, Rivera XI, Anwer F, Mahadevan D, Schatz JH, Persky DO (2018) Yttrium-90-Ibritumomab tiuxetan (Zevalin®) radioimmunotherapy after cytoreduction with ESHAP chemotherapy in patients with relapsed follicular non-Hodgkin lymphoma: final results of a phase II study. Oncology 94(5):274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Di Maria Jiang AF, Nguyen LT, Neel BG, Sacher A, Rottapel R, Wang BX, Ohashi PS, Sridhar SS (2019) Phase I study of local radiation and tremelimumab in patients with inoperable locally recurrent or metastatic breast cancer. Oncotarget 10(31):2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bahig H, Aubin F, Stagg J, Gologan O, Ballivy O, Bissada E, Nguyen-Tan FP, Soulières D, Guertin L, Filion E, Christopoulos A (2019) Phase I/II trial of Durvalumab plus Tremelimumab and stereotactic body radiotherapy for metastatic head and neck carcinoma. BMC Cancer 19(1):1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Karam SD, Raben D (2019) Radioimmunotherapy for the treatment of head and neck cancer. Lancet Oncol 20(8):e404–e416 [DOI] [PubMed] [Google Scholar]
- 167.Corrò C, Dutoit V, Koessler T (2021) Emerging trends for radioimmunotherapy in rectal cancer. Cancers 13(6):1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bauerschmitz GJ, Kanerva A, Wang M, Herrmann I, Shaw DR, Strong TV, Desmond R, Rein DT, Dall P, Curiel DT, Hemminki A (2004) Evaluation of a selectively oncolytic adenovirus for local and systemic treatment of cervical cancer. Int J Cancer 111(2):303–309 [DOI] [PubMed] [Google Scholar]
- 169.Said D, Anne CA (2002) Cancer vaccines and immunotherapy. Br Med Bull 62:149–162 [DOI] [PubMed] [Google Scholar]
- 170.Junaid R, Johannes ML, Gettinger SN (2018) Oncolytic virus immunotherapy: future prospects for oncology. JITC 6(140):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kumar AR, Devan AR, Nair B, Nath LR (2021) Anti-VEGF mediated immunomodulatory role of phytochemicals: scientific exposition for plausible HCC treatment. Curr Drug Targets 22:1288. [DOI] [PubMed] [Google Scholar]
- 172.Emmett VS (2019) Developing combination strategies using PD-1 checkpoint inhibitors to treat cancer. Semin Immunopathol 41(1):21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Duaa OK, Heather JB, Silvia M (2019) Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol 10:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yifan W, Weiye D, Li N (2018) Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front Pharmacol 9:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Asna N, Livoff A, Batash R (2018) Radiation therapy and immunotherapy: a potential combination in cancer treatment. Curr Oncol 25(5):454–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ralph EV, Benjamin TC, Claire V (2014) Combinations of immunotherapy and radiation in cancer therapy. Front Oncol 4:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Powles T, Balar A, Gravis G, Jones R, Ravaud A, Florence J, Grivas P, Petrylak DP, Galsky M, Carles J, Sridhar S (2019) An adaptive, biomarker directed platform study in metastatic urothelial cancer (BISCAY) with durvalumab in combination with targeted therapies. Ann Oncol 30:v356–v357 [Google Scholar]
- 178.Shuji M (2019) Durvalumab for the treatment of non-small cell lung cancer. Expert Rev Anticancer Ther 19(12):1009–1016 [DOI] [PubMed] [Google Scholar]
- 179.Julie MC, James LG (2019) Product review: avelumab, an anti-PD-L1 antibody. Hum VaccinImmunother 15(4):891–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Akintunde A, Zoaib R (2019) Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol 12(92):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wu X, Gu Z, Chen Y (2019) Application of PD-1 Blockade in Cancer Immunotherapy. Comput Struct Biotechnol J 17:661–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rusquec P, Calbiac O, Robert M, Campone M, Frenel JS (2019) Clinical utility of pembrolizumab in the management of advanced solid tumors: an evidence-based review on the emerging new data. Cancer Manag Res 11:4297–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Verma V, Sprave T, Haque W (2018) A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer 6(128):1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Qin S, Xu L, Yi M, Yu S (2019) Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer 18(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL (2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol 11(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jain MD, Bachmeier CA, Phuoc VH, Chavez JC (2018) Axicabtageneciloleucel (KTE-C19), an anti-CD19 CAR T therapy for the treatment of relapsed/refractory aggressive B-cell non-Hodgkin’s lymphoma. Ther Clin Risk Manag 14:1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Boyiadzis MM, Dhodapkar MV, Brentjens RJ (2018) Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother Cancer 6(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kamat AM, Bellmunt J, Galsky MD (2017) Society for Immunotherapy of Cancer consensus statement on Immunotherapy for the treatment of bladder carcinoma. J Immunother Cancer 5(68):1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Riley RS, June CH, Langer R, Mitchell MJ (2019) Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov 18(3):175–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Amaria RN, Reuben A, Cooper ZA, Wargo JA (2015) Update on use of aldesleukin for treatment of high-risk metastatic melanoma. Immunotargets Ther 4:79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Asmana NR (2014) Human interferon alpha-2b: a therapeutic protein for cancer treatment. Scientifica (Cairo) 2014:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bubna AK (2015) Imiquimod - Its role in the treatment of cutaneous malignancies. Indian J Pharmacol 47(4):354–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ventola CL (2017) Cancer Immunotherapy, part 2: efficacy, safety, and other clinical considerations. Phar Ther 42(7):452–463 [PMC free article] [PubMed] [Google Scholar]
- 194.Coulson A, Levy A, Gossell WM (2014) Monoclonal antibodies in cancer therapy: mechanisms, successes and limitations. West Indian Med J 63(6):650–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kohlhapp FJ, Zloza A, Kaufman HL (2015) Talimogenela-herparepvec (T-VEC) as cancer immunotherapy. Drugs Today (Barc) 51(9):549–558 [DOI] [PubMed] [Google Scholar]
- 196.Newman MJ, Benani DJ (2016) A review of blinatumomab, a novel immunotherapy. J Oncol Pharm Pract 22(4):639–645 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this research are available within the manuscript.


