Abstract
RNA interference (RNAi) for spinocerebellar ataxia type 1 can prevent and reverse behavioral deficits and neuropathological readouts in mouse models, with safety and benefit lasting over many months. The RNAi trigger, expressed from adeno-associated virus vectors (AAV.miS1), also corrected misregulated microRNAs (miRNA) such as miR150. Subsequently, we showed that the delivery method was scalable, and that AAV.miS1 was safe in short-term pilot nonhuman primate (NHP) studies. To advance the technology to patients, investigational new drug (IND)-enabling studies in NHPs were initiated. After AAV.miS1 delivery to deep cerebellar nuclei, we unexpectedly observed cerebellar toxicity. Both small-RNA-seq and studies using AAVs devoid of miRNAs showed that this was not a result of saturation of the endogenous miRNA processing machinery. RNA-seq together with sequencing of the AAV product showed that, despite limited amounts of cross-packaged material, there was substantial inverted terminal repeat (ITR) promoter activity that correlated with neuropathologies. ITR promoter activity was reduced by altering the miS1 expression context. The surprising contrast between our rodent and NHP findings highlight the need for extended safety studies in multiple species when assessing new therapeutics for human application.
Polyglutamine (PolyQ) diseases are caused by abnormal expansion of CAG repeats in the coding region of different genes. A shared therapeutic strategy against the gain-of-function toxicity of PolyQ diseases is repressing or silencing expression of the mutant gene or gene product. One approach is to use adeno-associated virus (AAV) to deliver sequences that target the mutant gene via RNAi. This approach is already undergoing an early phase clinical trial for Huntington’s disease (NCT04120493), and is approaching the clinic1 for other polyQ diseases, such as spinocerebellar ataxia type 1 (SCA1; ref.1).
SCA1 is an adult-onset neurodegenerative disease caused by a CAG expansion in the coding region of the Ataxin-1 (ATXN1) gene. In SCA1 patients, a pure CAG expansion of greater than 40 repeats causes disease through a protein gain-of-function mechanism triggering protein aggregation, transcriptional dysregulation and cellular toxicity. Although ATXN1 is expressed ubiquitously, neurodegeneration occurs primarily in cerebellar Purkinje cells (PCs) and brainstem nuclei. Clinical symptoms of SCA1 include gait and limb ataxia, nystagmus, difficulty swallowing and abnormal speech, among others.
Disease reversal in SCA1 was established using a conditional transgenic mouse model wherein disease phenotypes normalized following reduction of the transgene expressing mutant ATXN1 (refs.2,3). This finding, together with the lack of ataxia or neurodegeneration in ataxin-1 null mice4, led us and others to pursue nonallele-specific gene silencing approaches as potential therapeutics. Promising strategies have used miRNAs (synthetic5–8 or endogenous9) or antisense oligonucleotides (ASOs10,11) to safely reduce ATXN1 levels in SCA1 rodent models. Previously, miRNAs were delivered using AAV and driven by the strong, ubiquitous RNA Pol III promoter U6. Delivery of these viruses to both knock-in5 and transgenic6,7 SCA1 rodents showed benefit extending from many months to well over a year, and were effective at both preventing and reversing disease phenotypes.
We previously performed studies in NHPs using a single AAV to deliver miS1, an miRNA targeting a conserved human/primate sequence in ataxin-1, along with an enhanced green fluorescent protein (eGFP) reporter driven by an RNA Pol II CAG promoter to assess our delivery method, effective dosage range and biodistribution. At 8 weeks postinjection (p.i.) of AAV.miS1.eGFP into the dentate, interposed and fastigial nuclei, we achieved targeted knockdown of ATXN1 (ref.12). No animals in the study showed behavioral abnormalities and there were no notable neuropathological findings12.
To advance miS1 towards clinical application, the miS1 expression cassette was modified to no longer express eGFP. The newly modified rAAV2/1.miS1 (hereafter referred to as AAV.miS1) was injected at escalating doses into pre- or postsymptomatic SCA1 mice. Similar to the viruses coexpressing eGFP, multiple doses improved and reversed behavioral, molecular and histological phenotypes, supporting the hypothesis that AAV.miS1 can improve pathology after SCA1 symptom onset7.
In support of an IND application to test AAV.miS1 in SCA1 patients, further NHP studies were done. This work showed findings unexpected from our earlier long-term rodent and short-term NHP work and has relevance to the community of investigators advancing AAV-based RNAi therapies to treat human diseases.
Results
Subacute cerebellar syndrome after AAV.miS1 delivery.
We previously reported that AAV-mediated expression of miS1, an artificial miRNA designed to target human and rhesus macaque ATXN1, could prevent or reverse disease with no toxicity over many months in a transgenic mouse model of SCA1 (ref.6). We reported that a similar artificial miRNA targeting rodent ataxin-1 in SCA1 knock-in mice was safe for over a year5. In all previous work, expression of miS1 was driven by the ubiquitous U6 promoter. In constructs lacking the eGFP reporter, the packaging genome size was adjusted to roughly 4.3 kb by inclusion of a noncoding stuffer derived from human intronic sequences6,13.
To assess the long-term safety and tolerability of miS1 in NHPs, AAV.miS1 (Fig. 1a) was generated using established GLP manufacturing processes and animals infused using ClearPoint—a real-time, magnetic resonance imaging (MRI)-guided neurosurgical platform at three escalating doses. Animals were randomized to experimental groups, with surgical team and veterinary staff completing postinfusion assessments blinded to the treatment group. All dosed animals had low to undetectable amounts of AAV neutralizing antibodies at baseline. Vector or vehicle was administered bilaterally in three, 50-μl infusions to target the dentate nuclei and midline structures as indicated (Fig. 1b), with planned euthanasia at 1, 3 and 6 months for molecular and tissue analyses along with standard in-life monitoring for neurological signs (Extended Data Table 1).
Fig. 1 |. AAV.miS1 delivery to NHP cerebella causes neurological deficits.
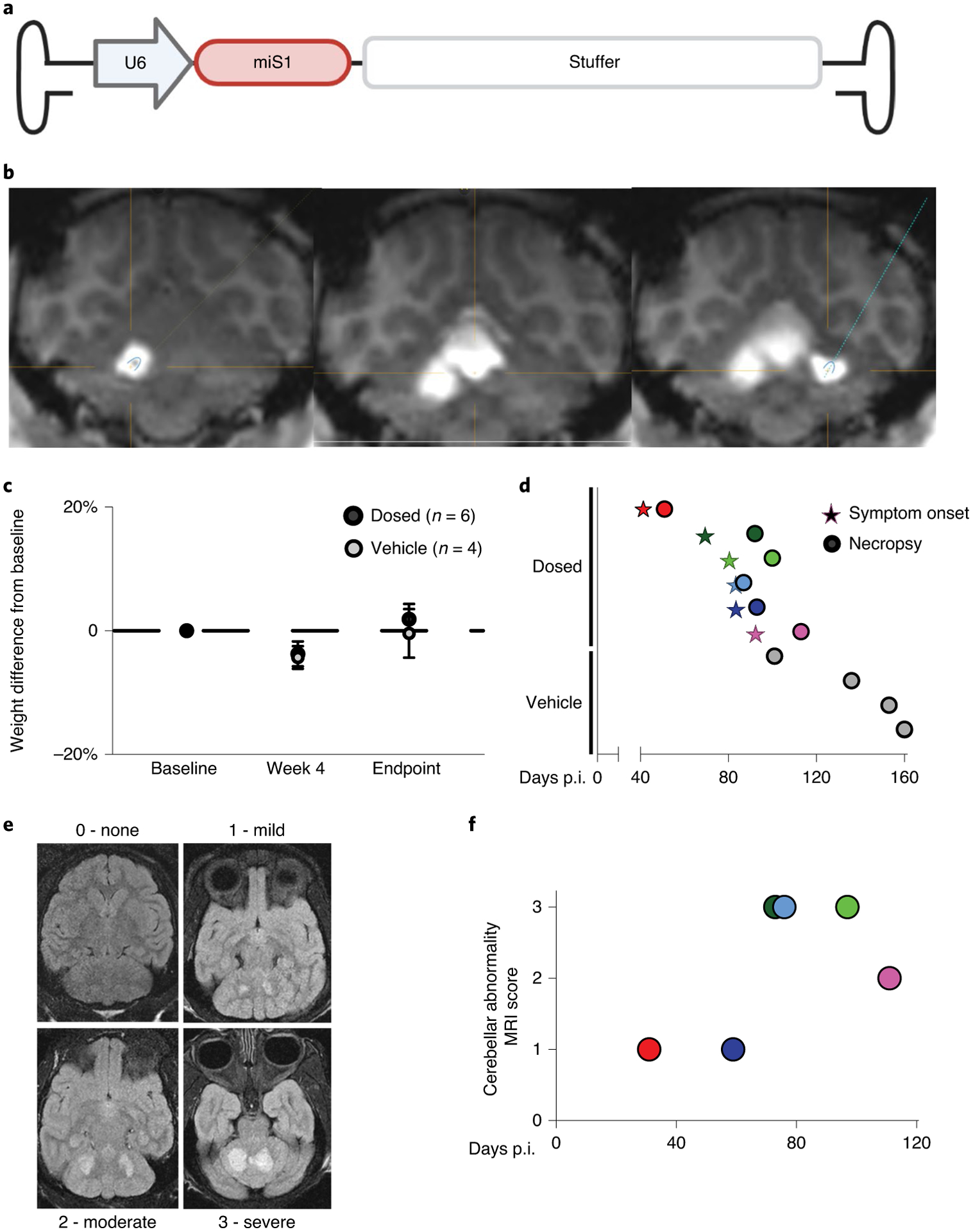
a, Schematic of viral construct. The murine U6 promoter drives expression of the artificial miRNA, miS1. A human intronic stuffer sequence normalizes AAV genome size. b, Magnetic resonance (MR) image from ClearPoint software after injection to the left dentate nuclei (left), medial (middle) cerebellar nuclei and right (right) dentate nuclei. c, Average percent change in weight from baseline. Data are presented as mean ± s.e.m.; no significant difference was determined by one-way ANOVA followed by Holm–Šídák’s multiple comparisons post hoc. d, Symptom onset (stars) and necropsy (circles) time in days p.i. for study animals (Extended Data Table 1). Each line on the y axis represents an individual subject. Dosed animal color designations are maintained throughout for all subsequent figures. e, Representative images of cerebellar abnormality score rankings. f, MR scores for each dosed animal (n = 6). Scan times are plotted relative to the time injected (days p.i.).
Around 3 months p.i., a group of animals developed ataxia, tremor, head-tilt and dysmetria. The study was terminated pre-maturely and unblinded to explore the underlying etiology of this subacute cerebellar syndrome. As a result, some planned treatment groups were incomplete and with variable times of vector exposure (Extended Data Table 1). There were no changes in weight among groups (Fig. 1c) and, at unblinding, it was noted that all symptomatic animals had received AAV.miS1 (Fig. 1d). Given the short in-life time frame for some animals, the presentation of symptoms is potentially underreported among groups as onset was at around 3 months p.i. and some animals were euthanized earlier (Fig. 1d and Extended Data Table 1).
Animals that showed cerebellar signs were evaluated by MRI before necropsy (Fig. 1e). Radiological assessment of scans from animals that received the planned target dose indicated toxicity in deep cerebellar nuclei (DCN) (Fig. 1f).
Neuropathology of AAV.miS1 in NHP cerebella.
The cerebellum and brainstem were processed into sagittal sections with alternating slabs dedicated to histopathology (immunohistochemistry (IHC)) or molecular analyses (Fig. 2a,b). Paired slices from left intermediate, right medial and right lateral slabs were stained with hematoxylin and eosin stain (H&E) or immunostained with anti-ionized calcium binding adapter molecule 1 (IBA1), a marker of microglial activation (Fig. 2c–h), or anti-glial fibrillary acidic protein (GFAP), a marker for astrocytosis (Extended Data Fig. 1a,b). In vehicle-treated animals, there were minor lesions consisting of cellular/macrophage infiltrates and gliosis consistent with the surgical intervention (Fig. 2c–e). Animals treated with vector at the middle (target) dose, however, showed necrosis, demyelination and perivascular/leptomeningeal lymphoid infiltrates (Fig. 2f–h), with a 2.4- and 9.3-fold increase in lesion severity in medial and intermediate cerebellar cortices, respectively, as compared with vehicle controls (Fig. 2i). Scores for lateral cerebellar cortex sections were unremarkable when compared with those from vehicle-treated animals. Purkinje cell (PC) quantification by stereology showed PC loss in the medial cerebellar cortex near the injection site, as compared with vehicle controls, with no significant changes in samples from the intermediate and lateral regions (Fig. 2j). Lesion severity for all animals at all doses was scored, with most toxicity noted in the medial and intermediate regions (Extended Data Fig. 1c,d and Extended Data Table 2). Representative images after anti-calbindin staining of vehicle and treated cerebellar sections show cell loss in all dosed animals (Extended Data Fig. 1e, f), with no correlation between viral dose, probably due to the variable time of vector expression in vivo.
Fig. 2 |. Molecular and histological readouts after AAV.miS1 delivery.
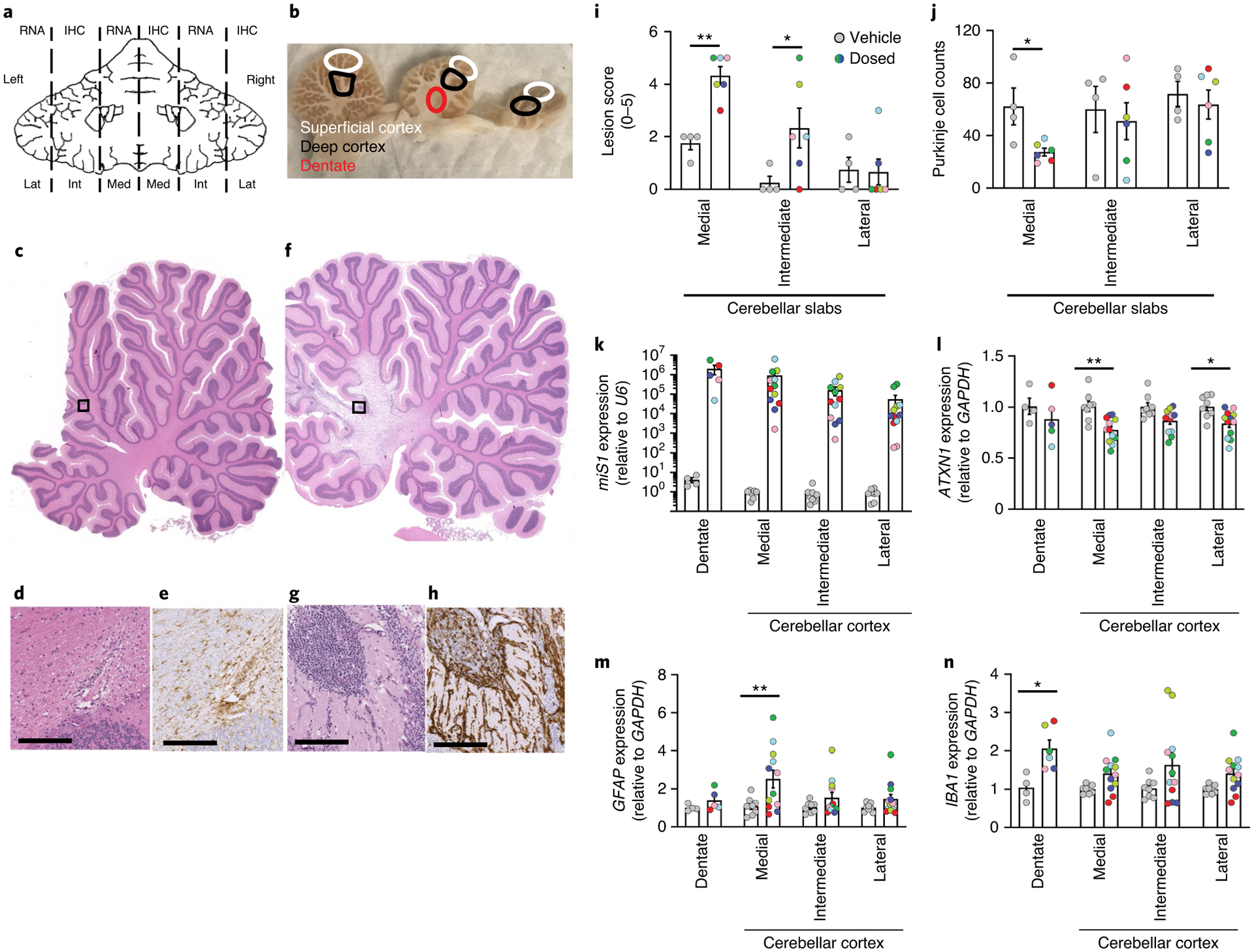
a, Schematic of tissue collection for molecular and histological (IHC) studies. b, Locations sampled from left lateral (Lat), right intermediate (Int) and left medial (Med) slabs. Superficial and deep cortex and dentate are indicated by white, black and red circles, respectively. c–e, Vehicle-treated NHPs (n = 4). f–h, AAV.miS1-treated NHPs (n = 6). Low- (c,f) and high- (d,g) magnification photomicrographs of H&E-stained medial sagittal sections. e,h, Photomicrographs of anti-IBA1 IHC sections from vehicle- (e) and vector- (h) treated animals. Scale bars, 300 μm. i, Lesion score quantitation (Extended Data Table 2; **P = 0.0062; *P = 0.0205 relative to vehicle). j, Average Purkinje cell counts. Each dot represents the average counts (n = 10 independent ×20 objective fields) per animal per location. Significance was determined by unpaired two-tailed t test for each location (*P = 0.0176 relative to vehicle). k, miS1 normalized to endogenous U6 from AAV.miS1- versus vehicle-treated control samples. l–n, Amounts of endogenous Rhesus ataxin-1 (ATXN1; **P = 0.0014; *P = 0.0335), glial fibrillary acidic protein (GFAP; **P = 0.0045) and ionized calcium binding adapter molecule 1 (IBA1; *P = 0.0239) mRNAs as measured by RT–qPCR. Data are represented as mean ± s.e.m. and significance determined by two-way ANOVA followed by a Holm–Šídák’s multiple comparisons post hoc (i, k–n). For k–n, each dot represents data derived from a single punch (DCN: n = 1 per animal, cerebellar cortex: n = 6 per animal).
Molecular analyses of NHP cerebella.
Quantitative PCR with reverse transcription (RT–qPCR) of cerebellar transcripts was consistent with neuropathological findings. Quantification of samples taken from superficial and deep cortices in medial, intermediate and lateral areas or the DCN (Fig. 2a,b) showed miS1 expression in all regions of the cerebellum (Fig. 2k), and significant reduction of ATXN1 in medial- (23% knockdown) and lateral- (17% knockdown) cerebellar cortex (Fig. 2l). As expected, animals with high miS1 showed stronger knockdown of target (Fig. 2k,l).
Changes in GFAP mRNA levels were 2.3-fold higher in medial cerebellar cortex samples when compared with vehicle-injected controls (Fig. 2m). Amounts of IBA1 mRNA also showed a 1.97-fold increase in the DCN, with slight increase of expression in cerebellar cortex locations, from AAV.miS1-treated samples relative to vehicle controls (Fig. 2n).
No deleterious effects on endogenous miRNA levels.
Although we showed earlier that doses of AAV.miS1 that improved the behavior and histological readouts of SCA1 mice rescued amounts of known dys-regulated miRNAs14, toxicity due to abnormal processing of endogenous miRNAs has been associated with high expression of exogenous miRNAs in brain15,16. To determine whether saturation of the endogenous miRNA processing machinery was a cause for our findings, we performed small RNA-seq. There was no evidence of perturbation of small RNAs and the miS1 transgene was the only annotated feature found to be significantly differentially expressed (Fig. 3a). Heatmaps comparing the expression of the 30 most abundant miRNAs and 11 neuronal miRNAs in samples from AAV.miS1- and vehicle-treated animals show limited and insignificant variation in miRNA expression among the groups (Fig. 3b,c and Extended Data Table 3).
Fig. 3 |. AAV.miS1 does not cause small RNA dysregulation.

a, Differential expression analysis on small RNAs in DCN tissue punches from AAV.miS1 and vehicle-treated animals. b,c, Heatmap representations of the top 30 most highly detected (b) or 11 neuronal specific (c) miRNAs.
Sequencing shows limited cross-packaging and transgene independent transcription.
We performed RNA sequencing on DCN tissue samples isolated from four vehicle- and six vector-dosed animals from our study including the pilot animal that received AAV.miS1 and was euthanized at day 31. Principal component analysis (PCA) showed that vehicle and AAV.miS1 samples segregated on the basis of expression of PC1 genes (Fig. 4a). AAV.miS1-treated animals showed a strong upregulation of genes by differential expression analysis (Fig. 4b). Gene ontology (GO) analysis showed that five of the top ten most enriched GO terms are related to immune response (indicated in red; Fig. 4c). Heatmaps generated from genes in GO:0002376 (immune system process) and GO:0006955 (immune response) demonstrate the striking upregulation of immune response genes including toll-like-receptors, interleukins, chemokines and cytokines in all AAV.miS1-treated animals (Fig. 4d and Extended Data Table 4).
Fig. 4 |. RNA-seq shows robust immune response in AAV.miS1 dosed animals.
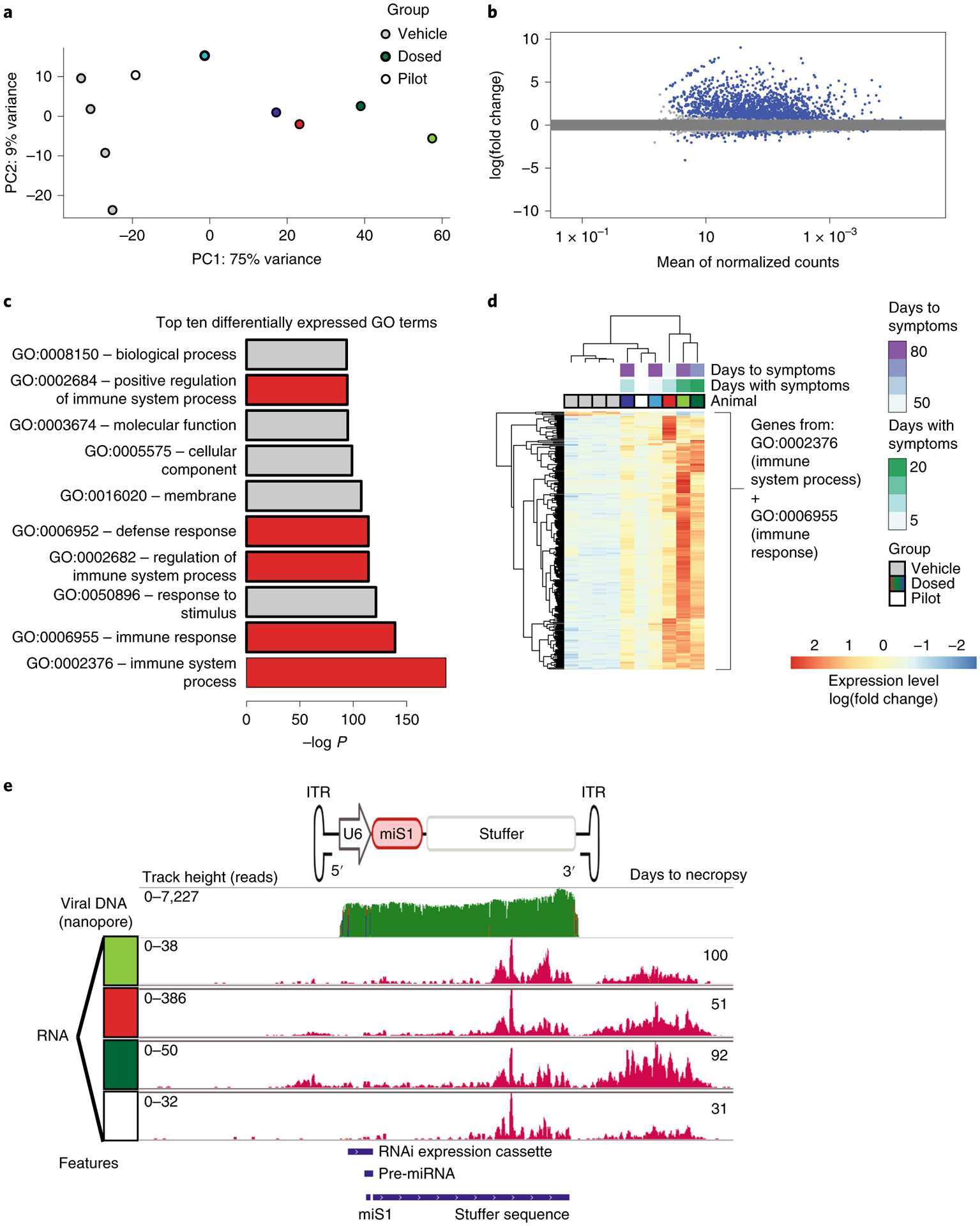
a–d, RNA sequencing of DCN-derived RNA in four vehicle-injected controls (n = 4) compared with dosed animals, including a pilot animal (n = 6) that received AAV.miS1 and was euthanized at day 31. a, PCA segregates vehicle and dosed groups on the basis of principal component 1 (PC1). b, Differential expression analysis between vehicle and AAV.miS1-treated animals. P values were determined by two-tailed Wald test. Adjusted P values (Padj) determined by Benjamini–Hochberg multiple testing correction. Genes reaching the threshold for significance (Padj < 0.1) are shown in blue; 2,086 (8.6%) of genes were significantly upregulated in samples from dosed NHPs and 313 (1.3%) genes were significantly upregulated in vehicle-treated animals. c, Top ten GO terms associated with differentially expressed genes when comparing dosed and vehicle-treated animals, half of which indicate an immune response (red). d, Heatmap of genes in GO:0002376 (immune system process) and GO:0006955 (immune response). e, Top, cartoon of the AAV.miS1 expression vector and alignment of Nanopore-sequenced vector DNA; bottom, RNA-seq reads align to the proviral vector used to generate AAV.miS1. Despite low detection of cross-packaged backbone by Nanopore sequencing (top), transcripts flanking the 3′ ITR map were detected in dosed samples, including in the pilot animal harvested at 31 days p.i. (white box). Transcripts mapping downstream of the 3′ ITR map to production plasmid DNA.
To quantify reads mapping to any part of the AAV.miS1 DNA or the proviral plasmid used in the AAV manufacturing process, we aligned RNA-seq reads to a version of the Mmul10 genome modified to include the entire miS1 plasmid used for viral production. As expected, we observed that only animals treated with AAV.miS1 had substantial reads mapping to the AAV.miS1 transgene. (Note that the total RNA method of library preparation would not be expected to effectively detect mature miS1.) Unexpectedly, there were a substantial number of reads mapping to regions upstream and downstream of the 3′ ITR, and some upstream of the 5′ ITR; reads were detected in four DCN samples from miS1-treated animals, including a pilot animal euthanized before development of clinical cerebellar signs (Fig. 4e). The transcribed region upstream of the 3′ ITR consists of a human derived stuffer sequence to right-size the AAV genome for packaging and has no intrinsic transcriptional activity (Extended Data Fig. 2).
To directly assess cross-packaging, which is inclusion of sequences from the packaging plasmid or host DNA from the packaging cells, we performed Nanopore long-read sequencing on AAV.miS1 (ref.17). Read alignments were made to the human reference genome (GRCh38) and the plasmids used for viral production. A total of 29,710 reads were base-called and MiniMap2 aligned 29,593 sequences. The desired cargo sequence consisting of the miS1 expression cassette, noncoding stuffer sequence and ITRs is 4,598 bp. Most sequence data (98.17%) aligned to AAV.miS1 cargo. Cross-packaging accounted for 1.83% of sequence coverage, with only 0.87% of sequencing depth attributed to the proviral backbone. This is in line with previous reports17. In addition, we assessed vector genomes from tissues harvested from AAV.miS1-injected animals, as well as transcription from cross-packaged DNA by digital droplet PCR (ddPCR; Extended Data Fig. 3a). Tissues had dose-dependent increases in correctly packaged cargo (stuffer region) and the 3′ cross-packaged plasmid backbone. There were low amounts of backbone DNA (Extended Data Fig. 3b). ddPCR, however, confirmed transcripts arising from the 3′ ITR (Extended Data Fig. 3c, d).
Toxicity after AAV.miS1 delivery is miRNA expression independent.
We next tested if the root cause of toxicity was due to the AAV capsid alone, off-targeting by miS1, or the noted 3′ ITR activity. In addition, we tested a new proviral construct that eliminated the stuffer and replaced it with a minigene encoding the human ATXN1 homolog, ataxin-1-like (ATXN1L). This sequence maintains the optimal cassette size. This new active ‘stuffer’ sequence was used because overexpression of ATXN1L alone is therapeutic in SCA1 mice6,18. Also, as Pol II promoters have been shown to impede ITR-driven transcription19,20, we replaced U6 with an EF1α promoter and placed the artificial miRNA encoding miS1 in an intron. When tested in the B05 SCA1 mouse model21, the resultant rAAV2/1.hATXN1L.miS1 (hereafter referred to as AAV.IntmiS1; Fig. 5a) showed correct splicing and dose-dependent expression of miS1 and hATXN1L that correspondingly reduced ATXN1 levels (Extended Data Fig. 4a–d). Relative to AAV.miS1, the AAV.IntmiS1 results in lower amounts of mature miS1 when assessed by RT–qPCR (Extended Data Fig. 4e). Nonetheless, the 2E9 vg dose induces the required 30% target silencing to prevent onset of disease in SCA1 mice7.
Fig. 5 |. Transcripts derived from stuffer and/or packaged backbone are sufficient to induce toxicity.

a, Cartoon diagrams of AAV.IntmiS1, AAV.miSCA7 and AAV.Stuffer. b, Experimental timeline for each individual animal injected with indicated vectors or purified empty capsids. Symptom onset denoted by asterisks; necropsy denoted by filled shapes. c, MR cerebellar abnormality score from Study 2: 4-month animals at both 2- and 4-month timepoints. Rankings were scored as in Fig. 1e. Each dot represents a single animal (****P < 0.0001; ***P = 0.0003 relative to empty capsids; NS, not significant). Data are represented as mean ± s.e.m. (n = 2 or 3 animals per group) with significance determined by two-way ANOVA followed by Holm–Šídák’s multiple comparisons post hoc. d,e, Total read counts mapping to the AAV.miS1 (d) and AAV.IntmiS1 (e) vectors. f, Differential expression analysis between the AAV.Stuffer and empty capsid-injected DCNs. P values and Padj determined as in Fig. 4b. Genes reaching the threshold for significance (Padj < 0.1) are shown in blue; 3,292 (11%) were significantly upregulated and 2,272 (7.4%) were significantly downregulated. g, Differential expression analysis between the AAV.IntmiS1- and empty capsid-injected DCN tissues. Genes reaching the threshold for significance (Padj < 0.1) 50 (0.16 %) were significantly upregulated and 167 (0.54%) were significantly downregulated. h, Heatmap representation of the top 100 most differentially expressed genes by adjusted P value with hierarchical clustering. AAV.IntmiS1 (pink square) clusters with vehicle-injected samples. i, Targeted heatmap of genes comprising the ‘immune system process’ GO term. Asterisks indicate samples from an animal euthanized 28 days postadministration.
To assess how miS1 expression from the EF1α promoter affected 3′ ITR-driven transcription, we performed ddPCR on tissues from mice injected with AAV.miS1 or AAV.IntmiS1. Despite identical 3′ ITRs, there was greater 3′ ITR-driven transcription of cross-packaged product in AAV.miS1 compared with AAV.IntmiS1 (Extended Data Fig. 4f,g).
Next, groups of three NHPs each were treated with AAV.IntmiS1, empty AAV1 capsids, a vector generated using a proviral plasmid with the stuffer sequence only (AAV.Stuffer; devoid of any expression sequence, Extended Data Fig. 2) and a vector containing a miRNA targeting an alternative gene, ATXN7 (AAV.miSCA7, Fig. 5a). Viruses were delivered at 3E12 vg and animals aged 4 months following injection except one AAV.IntmiS1-treated NHP, which was euthanized at day 28 for interim pathology and transcript analysis (Study 2, Extended Data Table 1). Two of three NHPs receiving AAV.Stuffer or AAV.miSCA7 developed a cerebellar syndrome similar to AAV.miS1, with onset between 53 and 119 days postinfusion (Fig. 5b and Extended Data Table 1). However, none of the animals injected with AAV.IntmiS1 developed neurological symptoms or showed MRI abnormalities (Fig. 5b,c). Thus, toxicity arose from some, but not all, miRNA-containing expression cassettes (AAV.miS1, AAV.miSCA7 but not AAV.IntmiS1) and from cassettes lacking miRNA expression (AAV.Stuffer). This suggests that miS1 concentrations, although more robust in AAV.miS1 versus AAV.IntmiS1, were not the main contributor to the toxicity noted in the first study.
Tissues were harvested for pathology and molecular readouts as before. First, RNA-seq reads were aligned to the proviral plasmids. As with AAV.miS1, RNA reads from samples treated with AAV.miSCA7 and AAV.Stuffer mapped to regions adjacent to the 3′ ITR in both directions, covering sequences from the expected packaged genome and proviral plasmid backbone (Fig. 5d). Unlike AAV.miS1, RNA-seq analyses of cerebellar tissue samples from AAV.IntmiS1-treated animals showed reads aligning to the proviral plasmid only in the ITRs (Fig. 5e; pink). The observation of similar neurological onset and gene expression changes in animals treated with AAV.Stuffer and AAV.miSCA7 suggests that RNAs expressed from the 3′ ITR were sufficient to induce toxicity rather than expression of a miRNA per se.
RNA-seq differential expression analysis on DCN and cerebellar cortex samples identified 3292 (11%) upregulated and 2272 (7.4%) downregulated genes passing the threshold for significance (multiple testing adjusted P value <0.1, shown in blue) between the AAV.Stuffer and empty capsid conditions (Fig. 5f). In contrast, the number of differentially expressed genes between IntmiS1 and empty capsid is 50 (0.16 %) upregulated and 167 (0.54%) downregulated (Fig. 5g). To visualize the variability in highly differentially expressed genes across all four conditions (AAV.miSCA7, AAV.Stuffer, AAV.IntmiS1 and empty capsid) a heatmap depicting the top 100 most differentially expressed genes was generated (Fig. 5h). Similar to our earlier RNA-seq findings (Fig. 4b–d), differential expression analysis again identified immune-response-associated genes as the primary differentially expressed genes between vehicle-, empty capsid- or AAV.IntmiS1-treated animals and the animals that received AAV.Stuffer or AAV.miSCA7 (Fig. 5h). The top five biological GO terms of differentially expressed genes were ‘immune system process’ (P = 3.01 × 10−68), ‘biological process’ (P = 2.29 × 10−56), ‘response to stimulus’ (P = 4.64 × 10−56), ‘immune response’ (P = 7.64 × 10−50) and ‘cellular process’ (P = 2.35 × 10−48). A focused heatmap representation of the ‘immune system process’ GO term genes showed distinct groups of samples segregated by the inclusion of the stuffer sequence (Fig. 5i and Extended Data Table 4).
Next, cerebellar cortex samples were subjected to small RNA-seq analysis. Limited differential expression was observed between AAV.IntmiS1 and empty capsid groups with only seven upregulated (0.054%) and three downregulated (0.023%) genes meeting the threshold for significance (multiple testing adjusted P value <0.1, shown in blue; Extended Data Fig. 5a). A focused analysis of the top 30 most highly detected miRNAs and 11 neuronal miRNAs showed no significant differences between AAV.IntmiS1 and empty capsid-treated samples (Extended Data Table 3 and Extended Data Fig. 5b,c).
Neuropathological assessment of brain tissue from AAV.IntmiS1-treated NHPs showed mild-to-moderate effects following treatment, similar to empty capsid-treated animals (Extended Data Fig. 6a). Quantitation of transcripts show ATXN1L and miS1 expression (Extended Data Fig. 6b–d). There was no significant elevation in either GFAP or IBA1 concentrations between AAV.IntmiS1-treated animals and animals that received empty capsids (Extended Data Fig. 6e,f). These data indicate that moving the miS1 sequence into the intron retains its expression, albeit at lower amounts relative to AAV.miS1, yet 3′ ITR transcription and toxicity in NHP brain is reduced.
Discussion
Our data show that, despite long-term safety of AAVs expressing therapeutic RNAi in rodent models at doses that could prevent or reverse disease readouts5–7, similar in-life exposure in NHPs showed ITR-driven transcription that correlated with a toxic neurological response. The findings were unexpected and required next generation sequencing of the input vector, testing further AAVs expressing no miRNAs or miRNAs targeting other genes, and assessing the transcriptional products arising from AAV.miS1 to dissect the root cause. Cumulatively, this work highlights a need for safety testing of AAVs intended for human therapies in NHPs and contributes to the growing body of knowledge about AAV vectors for RNAi therapy for central nervous system disorders.
While there was variability in symptom onset and the extent of neuropathology in NHPs receiving AAV.miS1, AAV.miSCA7 and AAV.Stuffer, a consistent finding was that neurological symptoms correlated with 3′ ITR transcription from low amounts of cross-packaged plasmid backbone. This occurred in AAVs expressing miRNAs from the U6 promoter as well as AAVs devoid of any expression construct. Others have noted expression arising from ITRs after transduction of airway epithelia22, or in liver22,23 but, to our knowledge, this has not been noted previously in primate brain after AAV delivery, nor have transcripts been mapped to cross-packaged material. Whether the RNA or protein products arising from these transcripts are responsible remains unknown at this time. While we did not have the ability to test for cell-mediated responses, there was elevated expression of genes implicated in cell-mediated responses in AAV.Stuffer-, AAV.miS1- and AAV.miSCA7-treated animals. Indeed, there was strong concordance in immune-response genes overall between these three groups, indicating that the miRNA expression from a U6 promoter per se was not the main factor inducing the symptomatology, neuropathology and notable MRI findings.
AAV.miS1, a construct previously shown to be well tolerated and effective in reversing or protecting from disease phenotypes in SCA1 mice6,7, showed similar 3′ ITR activity when reassessed by RNA-seq after delivery to mice. Again, transcripts mapped to the low amounts of cross-packaged product. Why these products are tolerated in mice but not NHPs is intriguing and warrants further study as cross-packaged material, albeit at low concentrations, is a common feature in products manufactured for preclinical and clinical use. In our work, expression of transcripts from the 3′ ITR into cross-packaged DNA was dependent on the lack of a Pol II promoter.
When the pre-miRNA encoding miS1 was moved into an intron downstream of the EF1α promoter the 3′ ITR activity after AAV delivery to NHPs was mitigated; no RNA-seq transcripts mapped to any cross-packaged DNA. Results from ddPCR-based 3′ ITR transcription assays after injections into mice were consistent with the NHP data where AAV.miS1 showed 3′ ITR activity and AAV. IntmiS1 did not.
Cumulatively, our data show that 3′ ITR promoter activity can be neurotoxic in NHPs and show that assays in mice may be useful in predicting this activity. Taken together with previous work in knock-in and transgenic preclinical models, the presence or absence of 3′ ITR transcription does not affect therapeutic outcomes when testing in mice, and highlights the utility of examining vectors intended for clinical testing in NHPs.
Methods
Virus production.
AAV-based viral vectors have been described previously24,25. GLP grade AAVs were manufactured by the Children’s Hospital of Philadelphia (CHOP) Clinical Vector Core (CVC) by transient triple transfection of adherent human embryonic kidney epithelial cells (HEK293) from a certified Working Cell Bank (WCB). Cells were expanded in tissue culture flasks and roller bottles before transfection. We tested GLP product in-house at the CVC QC laboratory and by contract testing laboratories qualified by CVC. Test methods, procedures and results are reported on a certificate of analysis for each lot.
Humane care guidelines.
This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council) and the Animal Welfare Act of 1966 (P.L. 89–544), as amended. Animals were housed at the CHOP Research Institute (RI). All procedures were approved by the CHOP RI Institutional Animal Care and Use Committee. The CHOP RI is fully accredited by AAALAC International.
Animals.
A total of 18 female and 11 male Rhesus macaques (Macaca mulatta) were used in this study. All NHPs were screened for AAV1 neutralizing antibodies before enrollment and tested negative. Physical exams were performed by a veterinarian trained in NHP medicine. Comprehensive blood chemistry analysis and complete blood counts were performed before study enrollment to ensure animal health before surgery. Female NHPs were 6.3 ± 2.0 years of age and weighed 5.01 kg ± 0.90 kg at the time of surgery. Male NHPs were 4.6 ± 0.5 years of age and weighed 4.62 ± 0.44 kg at the time of surgery. All NHPs were captive-born and socially housed while on study. NHPs were exposed to a 12-h light:dark cycle, offered ad libitum access to reverse osmosis purified water and fed twice daily with portions of Purina LabDiet Certified Primate Diet (5048) supplemented with fresh fruits and vegetables as part of their behavioral enrichment program.
Nine male transgenic B05 SCA1 mice bred on a FVB background and six wildtype FVB male mice were used for experiments. Mice were housed in a controlled temperature environment on a 12-h light/dark cycle. Temperature was maintained between 20 °C and 26 °C, humidity was maintained between 30% and 70% according to the Guide for the Care and Use of Laboratories animal. Food and water were provided ad libitum.
Blood collection and processing.
Blood was collected from NHPs to run prestudy and endpoint comprehensive blood chemistry analysis and complete blood counts (Antech Diagnostics), and neutralizing antibody screening. NHPs were fasted overnight, anesthetized and blood collected by percutaneous venipuncture at a femoral vein. Blood was collected in K2EDTA and serum clot activator Vacuette Blood Collection Tubes (Greiner Bio-One). Animals recovered in home cages. Blood was stored on ice until processing. Within 2 h of collection, blood was centrifuged at 2,500g for 10 min to separate serum and aliquots were frozen on dry ice and stored at −80 °C until processing.
Neutralizing antibody assay.
Serum samples were heat inactivated at 56 °C for 30 min. rAAV1.CAGeGFP (multiplicity of infection: 5 × 103) was diluted in serum-free Dulbecco’s modified Eagle’s medium and incubated with twofold serial dilutions (initial dilution, 1:10) of heat-inactivated serum samples on Dulbecco’s modified Eagle’s medium for 1 h at 37 °C. Subsequently, the serum-vector mixture was added to 96-well plates seeded with 4 × 104 HEK293 cells per well. After 48 h, the intensity of enhanced GFP was measured with Spectra Max i3x Multi-Mode detection platform and processed using SoftMax Pro v.7.0.2; build number 235200. The neutralizing antibody titer was reported as the highest serum dilution that inhibited rAAV.CAGeGFP transduction (eGFP intensity) by 50% compared with the no-serum control.
Anesthesia and surgery.
NHPs were fasted overnight, sedated, intubated and given isoflurane. The head and chest were shaved, and ECG electrodes were attached to the ventral thorax. Following surgery and before recovery, a dose of buprenorphine sustained release (SR; 0.2 mg kg−1 subcutaneously (SC)) was administered. During surgery, ECG, SpO2, ETCO2, NIBP, rectal temperature, heart rate and respiration rate were all monitored continuously. Thermal support was provided via a warmwater recirculating blanket and several layers of insulating material wrapped around the animal and centered on the thoracic region.
We performed NHP brain imaging on a 3 T research MRI unit (Siemens Trio; SYNGO Vb17) using a T1-weighted 3D MPRAGE sequence to obtain high resolution (0.35 × 0.35 × 1 mm3) volumetric images of the cerebellum before and after injection of the treatment. Preinjection T2-weighted 3D SPACE (0.7 × 0.7 × 1 mm3) volumetric images were also obtained. Trajectories were determined using ClearPoint v.1.6 and v.2.0 software.
A fiducial grid that was visible using MRI was placed on the head and gadoteridol administered intravenously (i.v.) to visualize the cerebral vasculature and enable cannula trajectory planning. A Clearpoint workstation was used to triangulate between the fiducial grid and the neuroanatomical target to optimize the entry point on the fiducial grid. Next, the Clearpoint scalp-mount was attached over the desired entry point and the targeting tower attached for guide tube alignment. A 2-mm diameter entry point through the skull was created percutaneously using the Clearpoint handtwist drill, the drug infusion catheter attached and vector or vehicle (50 μl per target) mixed with gadoteridol infused with a Harvard Apparatus infusion pump programmed at 1.0–5.0 μl min−1. The skin was sutured and animals recovered.
NHP necropsy.
NHPs were fasted overnight, sedated, intubated and given isoflurane. Upon confirmation of deep anesthesia (stage III, plane 3), we performed a thoracotomy before cardiac perfusion of ice-cold PBS. Brains were removed and chilled before grossly removing the hindbrain from the cerebrum at the level of the midbrain for sagittal slabbing. All brain tissue was cut into 4-mm thick slabs. Tissues were microdissected from alternating left and right cerebellar sagittal slabs and snap frozen in liquid nitrogen and stored at ≤ −60 °C until further processing. Punches were taken from the right dentate nucleus using a 3-mm Disposable Biopsy Punch with Plunger (Integra). Cassetted tissue slabs were postfixed in 10% neutral buffered formalin for ≥2 weeks at 4 °C before paraffin embedding by the CHOP Pathology Core.
Radiology analyses and scan grading.
More MRIs from Study 1 symptomatic animals were obtained on a 3 T research MRI unit (Siemens Trio; SYNGO Vb17). Scans were reviewed for toxicity, focusing primarily on T1-weighted 3D MPRAGE (TR/TE 2,300/3.59 ms, resolution 0.5 × 0.5 × 1 mm3) without and with i.v. contrast and FLAIR (TR/TE/TI 9,000/85/2,500 ms, resolution 0.5 × 0.5 × 2 mm3) without i.v. contrast. Diffusion and susceptibility weighted data were acquired for later cases as well but not included in this analysis. Contrast enhancement was not a prominent feature in these cases, evaluation thus focused primarily on FLAIR images, which were graded blinded to animal data including symptoms by a board-certified neuroradiologist on a four-point scale targeting extent of signal abnormalities in the cerebellum near injection sites. Scoring as follows: 3, severe; 2, moderate; 1, mild; 0, no abnormalities present.
Reverse transcription and quantitative PCR of RNA samples.
Total RNA was extracted from tissues using TRIzol (Ambion by Life Technologies) as per the manufacturer’s protocol. RNA (1 μg) was treated with DNase I, RNase-free (ThermoScientific) as per the manufacturer’s protocol. miRNA-specific cDNA was generated using miRNA stem-loop-specific primers (miS1 and U6) and the High Capacity cDNA Reverse Transcription Kit (Life Technologies). Samples were run on the Bio-Rad CFX384 Real Time System C1000 Touch using Bio-Rad CFX Manager v.3.1 software. Exogenous miS1 was quantified by designed primer/probes to be used with TaqMan Master Mix (Applied Biosystems). Endogenous Rhesus U6 was used to normalize expression across samples; primer/probes for miS1 and U6 were ordered from Integrated DNA Technologies. In addition, complementary DNA libraries were also generated. Endogenous mRNA of ATXN1 (Hs00165656_m1), GFAP (Rh00909240_m1) and IBA1(AIF1) (Rh00894882_m1) and transgene expression of ATXN1L (Hs04964302_s1) were quantified by (Rh02621745_g1) commercial TaqMan primer/probe sets (Applied Biosystems). Endogenous Rhesus GAPDH was used to normalize expression across samples.
RNA sequencing.
Total RNA (1 μg) was extracted from tissue using TRIzol (Ambion by Life Technologies) and treated to remove contaminating DNA using RNAeasy Plus Miniprep kit (Qiagen). RNA integrity number values were acquired using RNA Nano Chips (Agilent Technologies) in an Agilent 2100 BioAnalyzer as per the manufacturer’s protocol. Sequencing libraries were prepared using NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (New England Biolabs). We performed rRNA depletion using combined human-, mouse- and rat-species specific QIAseq FastSelect RNA Removal Kit (Qiagen). SPRIselect Beads (Beckman Coulter) were used for purification. cDNA libraries were indexed using the NEBNext Dual Index Kit (New England Biolabs) and samples analyzed on High Sensitivity DNA Chips (Agilent Technologies) with an Agilent 2100 BioAnalyzer as per the manufacturer’s protocol to determine library size, concentration and purity. Libraries were indexed and pooled at concentrations of 1 nM and run on a NovaSeq 6000 S1 flow cell (Illumina) using NovaSeq Control Software v.1.5. The resulting sequencing reads, in FASTQ format, were aligned to the Rhesus macaque genome (Mmul10) obtained from https://www.ensembl.org. We performed alignment with the STAR (STAR_2.6.0c) aligner26. Read counts-per-gene values generated by STAR were used as the basis for differential expression analysis performed using DESeq2 (ref.27) v.1.24.0. Further analyses done by R (v.3.6.1) and associated modalities: Readr (v.1.3.1), Dpylr (v.0.8.3), MASS (v.7.4–54), Reshape (v.3.6.1), Pheatmap (v.1.0.12) and RColorBrewer (v.1.1–2). All data is accessible through https://github.com/DavidsonLabCHOP/Keiser_NatMed_2021.
Small RNA sequencing.
Small RNA was extracted from tissue using the miRNeasy Kit (Qiagen). RNA integrity number values were acquired as above. Sequencing libraries were prepared using TruSeq Small RNA Library Prep Kit (Illumina) with the QIAquick Gel Extraction Kit DNA extraction supplement (Qiagen). SPRIselect Beads (Beckman Coulter) were used for the purification steps. cDNA libraries were indexed using the TruSeq Small RNA Library Prep Kit-Set A Indices (Illumina). Samples were analyzed on High Sensitivity DNA Chips (Agilent Technologies) with an Agilent 2100 BioAnalyzer and libraries indexed, pooled to 1 nM and run on a NovaSeq 6000 S1 flow cell (Illumina). The resulting sequencing reads, in FASTQ format, were aligned to the Rhesus macaque genome (Mmul10) obtained from https://www.ensembl.org. We performed alignment with the STAR aligner26. Feature count values generated by STAR were used as the basis for differential expression analysis performed using DESeq2 (ref.27) as described above.
Nanopore sequencing.
Around 5 × 1011 viral genomes of AAV.miS1 were treated with DNAse for 1 h, and the DNAse and viral particles denatured. Single-stranded DNA (ssDNA) was purified using Agencourt XP beads and libraries prepared using the Nanopore Rapid Sequencing Kit (catalog no. SQK-RAD004) according to the manufacturer’s recommendations.
Around 150 fmol of ssDNA was used for library preparation and subsequently loaded onto a Nanopore Flongle (Oxford Nanopore Technologies) flow cell and run on a GridION using MinKNOW core (v.3.4.8). Raw FAST5 sequencing files were base-called with Guppy (v.3.0.6), processed through Porechop (v.0.2.4) to remove leading adapter sequences and aligned using MiniMap2 (v.2.17)28. Alignments were made to provided reference genome that included human reference chromosomes and the three plasmids used for viral production. A total of 29,710 reads were base-called; 26,783 reads had the sequencing adapter removed from the beginning of the sequence and 147 reads were split upon removal of the adapter from the middle of the sequence. MiniMap2 aligned 29,593 sequences using the noisy reads presets and excluding secondary alignment output.
Histopathology.
Formalin-fixed paraffin-embedded (FFPE) NHP cerebellar tissue blocks were sectioned and stained at the CHOP Pathology Core using Leica Bond Max autostainer. We performed immunostaining with IBA1 (rabbit α Iba1; 1:1,000; catalog no. 019–19741; Wako Chemicals), GFAP (rabbit α Gfap; 1:1,000; catalog no. z033401-2; Dako), or Calbindin (rabbit α Calbindin D-28K; 1:1,000; catalog no. CB-38a; Swant). Images were acquired using Aperio AT2 Digital Whole Slide Scanner (ImageScope v.12.3.3.5048) or Leica Application Suite X (v.3.7.1.21655). The pathological analyses were carried out blindly by a board-certified neuropathologist at the University of Pennsylvania School of Veterinary Medicine.
Generation of intronic miS1 vectors.
The intronic miS1 transgene was modified from our hATXN1L transgene described previously6. The 5′ UTR of our IntmiS1 construct was derived from the ATXN1L gene. Two genomic segments (hg38, chr16:71848010–71848392 and chr16:71849295–71850155) were amplified from HEK293 DNA using NEB HiFi DNA Assembly compatible primers: Segment 1, Fwd: 5′ TTC AGG TGT CGT GAA CAC GTG GCT CCC GAG CCA GCC GG and Rev: 5′ ACG CGT TGA AGC TAG CTG AAA TTT CGA AGT CTG CTC CAG GCA CCA CTC C; Segment 2, Fwd: 5′ TTC GAA ATT TCA GCT AGC TTC AAC GCG TGG CAG AAA AGA GAC AAG GTG and Rev: 5′ ACT GCA GCG AGG TGG ATG. Fragment assembly introduced an intronic cloning site linker BstBI–NheI–BmtI–MluI and was incorporated into our previously described hATXN1L transgene at PmlI and BlpI sites. miS1 was ligated at NheI and MluI sites. Bold sequences denote hybridizing regions of primer probe.
Recombinant AAV serotype AAV2/1 vector was prepared in the Davidson laboratory according to previously published protocols29. Vector titer was determined by quantitative PCR using primers and probe targeting the hATXN1L cDNA (ThermoFisher, catalog no. Hs04964302_s1).
Mouse AAV injection and tissue isolation.
At 6 weeks of age, B05 mice were injected bilaterally into the DCN (coordinates: −6.0 mm caudal to bregma, ±2 mm from the midline and −2.2 mm deep from the cerebellar surface30) with AAV. IntmiS1. Virus was delivered in 4 μl volumes per hemisphere at concentrations of 2.7 × 107, 2.7 × 108 or 2.7 × 109 vector genomes per microliter.
At 10 weeks of age, wildtype FVB/NJ mice were injected bilaterally into the striatum (coordinates: +0.86 mm rostral to bregma, ±1.8 mm from the midline and −3.5 mm deep from the skull with AAV.IntmiS1or AAV.miS1. Virus was delivered in 5 μl volumes per hemisphere at concentrations of 5 ×109 vector genomes per microliter.
All animals were euthanized 3 weeks p.i. and cerebellar hemispheres were transferred to TRIzol and snap frozen in liquid nitrogen followed by storage at −80 °C.
Mouse RNA isolation and quantification.
RNA was isolated using TRIzol according to manufacturer’s instructions (Invitrogen, catalog no. 15596018) and quantified using the Qubit RNA BR Assay (Invitrogen, catalog no. Q10210). RNA samples were DNase-treated (DNA-free DNA Removal Kit, Invitrogen catalog no. AM1906) and reverse transcribed using MultiScribe RT (Invitrogen, catalog no. 4304134). RNA samples were reverse transcribed using Random Hexamers for mRNA quantification, stem-loop primers for miRNA quantification and a transgene-specific primer localized to the BGH polyA region to assess transgene splicing (5′ ACA GTG GGA GTG GCA CCT TC). Quantitative RT–PCR measurements were made using TaqMan primer/probe sets directed against hATXN1 (ThermoFisher, catalog no. Hs00165656_m1), hATXN1L (ThermoFisher, catalog no. Hs04964302_s1) and mouse β-actin (ThermoFisher, catalog no. 4352341E). miS1 was quantified as described above and normalized to endogenous mouse U6.
Digital droplet PCR assays.
ddPCR were performed on the QX100 (Bio-Rad) according to manufacturer’s recommendations for probe-based assays, with 0.1–5 ng of genomic DNA used to measure vector copies. To measure expression of the vector components, we performed reverse transcription of 800 ng RNA using the High Capacity Reverse Transcription Kit (ThermoFisher) in 20 μl total volume. cDNA was diluted 1:10 with nuclease free water and 10 μl was used for ddPCR assays. All ddPCR assays were multiplexed and performed in 20 μl reactions. One assay (Mm-Tfrc) was purchased from ThermoFisher, the remaining assays were synthesized as follows. Assay ‘miS1–3’bb’; forward primer 5′ ACGCCGCTGGAGAAATATAC, reverse primer 5′ GCCATCAGCGTGTTGTAATC, probe /56-FAM/ TGAACAAGG /ZEN/CACTGAAAGACGGGA/3IABkFQ/. Assay ‘mis1atxn1L-3ITR’; forward primer 5′ GCCGTGCGGTTGATATTG, reverse primer 5′ CTTGCTGGCATCCTTGAATAG, probe /56-FAM/ TCGAGAAAG/ZEN/AGTGCGGAAGATGCA/3IABkFQ/. Assay ‘mis1atxn1L-5ITR’; forward primer 5′ CTACAGCGTGAGCTATGAGAAA, reverse primer 5′ CGAAACCCGACAGGACTATAAA, probe /56-FAM/ AAGGGAGAA/ZEN/AGGCGGACAGGTATC/3IABkFQ/. Assay ‘miS1-stuffer’; forward primer 5′ GGTCAGATTGCTGTGCTTATTG, reverse primer 5′ CACTGAGGGTCCAAGGAATTAG, probe /56-FAM/ AACCCTTTC/ZEN/TTCCCTGGGCTCTTC /3IABkFQ/. Assay ‘Mm-GusB Mm.PT.39a .22214848’; forward primer 5′ ACCACACCCAGCCAATAAAG, reverse primer 5′ AGCAATGGTACCGGCAG, probe /5HEX/ ACATCACCC/ZEN /AAGAAGCAGCCCT/3IABkFQ/. Assay ‘Rh-GusB1’; forward primer 5′ GCTTTGAGGAGC AGTGGTA, reverse primer 5′ CCTGGCTGATGTCGTTGAA, probe /5HEX/CTCTGCGGG/ZEN/ AGTCAGGCCC/3IABkFQ/. Assay ‘Rh-ref-rpp30’; forward primer 5′ GGTGTTTGCAGACTTGGAC, reverse primer 5′ CCGCTGTCTCCACAAGT, probe /5HEX/ TTCTGACCT/ZEN/ GAAGGCTCTGCGC/3IABkFQ/.
Stuffer expression studies.
Plasmids to test the promoter activity of noncoding stuffer sequence were generated using the psiCheck2 vector (Promega, catalog no. C8021). Stuffer sequence and ITR components were digested from proviral plasmids using appropriate enzymes, blunted and cloned into a blunted psiCheck2 backbone following digestion with KpnI and NheI to remove the SV40 promoter. Plasmids (200 ng per well) were transfected into HEK293 cells using Lipofectamine 3000 according to manufacturer’s protocol (Invitrogen, catalog no. L3000001). Cells were harvested 24 h post-transfection in Passive Lysis Buffer (Promega, catalog no. E1941). After 30 min on ice, lysates were spun at 18,000g for 30 s and luciferase activity measured using the Dual-Luciferase Reporter Assay (Promega, catalog no. E1910). Signal was normalized to Firefly activity encoded in the psiCheck2 plasmid.
Statistics.
Differences between lesion scores from vehicle and AAV.miS1 groups were compared using two-way analysis of variance (ANOVA) with Holm-Šídák’s post hoc. Differences between vehicle and treatment groups for individual cerebellar cortex locations were assessed using unpaired two-tailed t test for Purkinje cell counts. Differences in RT–qPCR amounts from Study 1 were assessed using a two-way ANOVA with Holm–Šídák’s Multiple Comparisons post hoc.
Differences between all treatment groups for Study 2 used a two-way ANOVA with Dunnett’s Multiple Comparisons post hoc. Differences between groups were considered to be significant at a P value of < 0.05. Statistical analyses were performed with GraphPad Prism v.9.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Extended Data
Extended Data Fig. 1 |. Immunohistochemistry and lesion scores in AAV.miS1 treated animals.
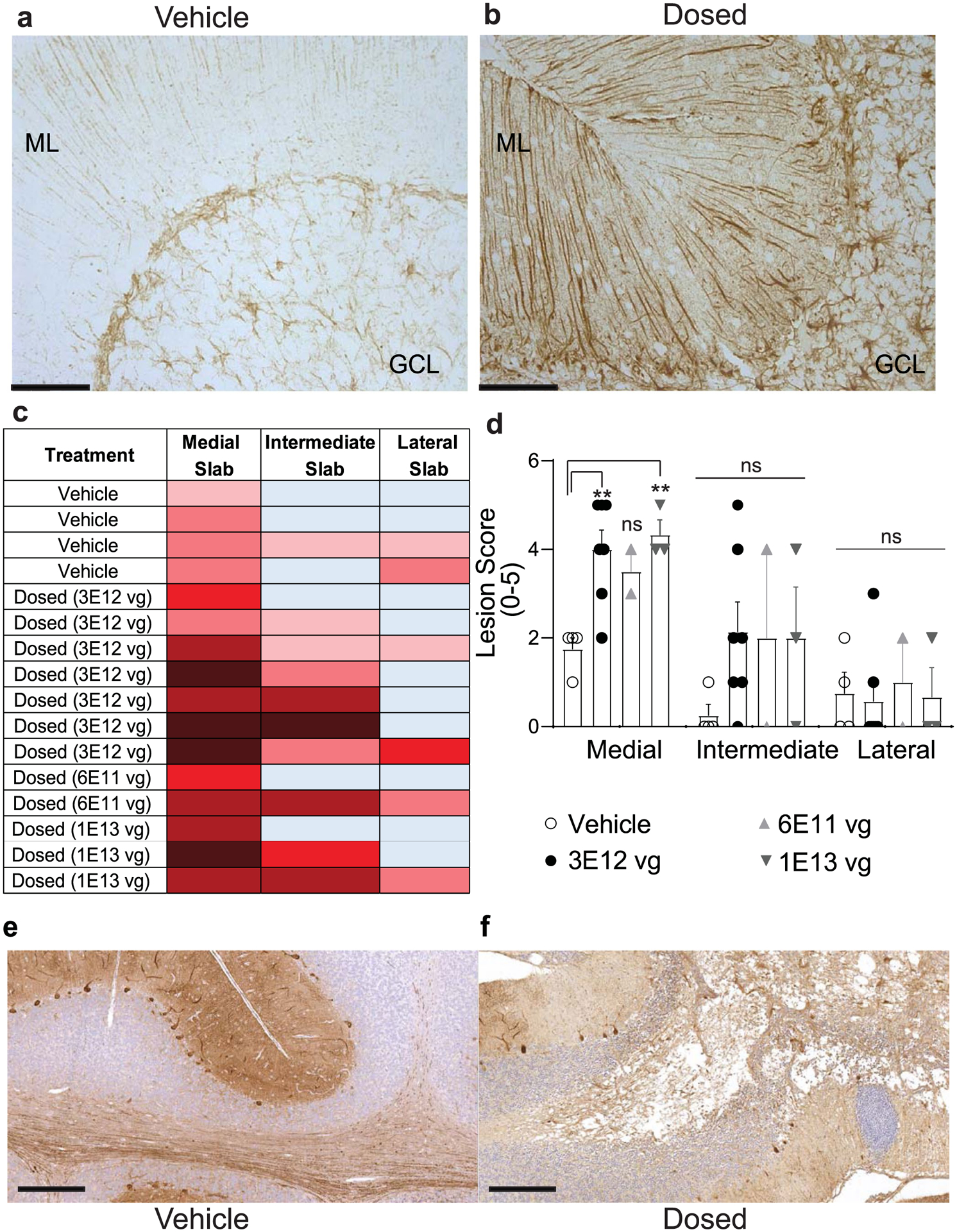
Representative images from vehicle- (a) and AAV.miS1-injected (b) sagittal cerebellar sections (10 μm thick) immunostained for glial fibrillary acidic protein (GFAP). N = 4 or 6 animals per group. Granule cell layer (GCL) and molecular layers (ML) are identified. Scale bar = 100 μm. c, Tissue lesion scoring parameters and associated heat chart (See Extended Data Table 2). d, Quantitation and statistics of lesion scores. Each dot represents a single animal. Data are represented as mean ± SEM (N = 2, 3, 4 or 7 animals per group), significance was determined by one-way ANOVA followed by Dunnett’s multiple comparison post hoc for each location (** P(3E12vg) = 0.0052; **P(1E13vg) = 0.0079 relative to vehicle). E–f, Representative images (N = 10 independent 20X objective fields) from cerebellar sections immunostained for Calbindin in vehicle- (e; N = 4 animals) and AAV.miS1-injected (f; N = 6 animals) used to quantify Purkinje cell counts throughout the cerebellum. Scale bar = 300 μm.
Extended Data Fig. 2 |. Non-coding stuffer sequence does not exhibit promoter activity in HEK293 cells.
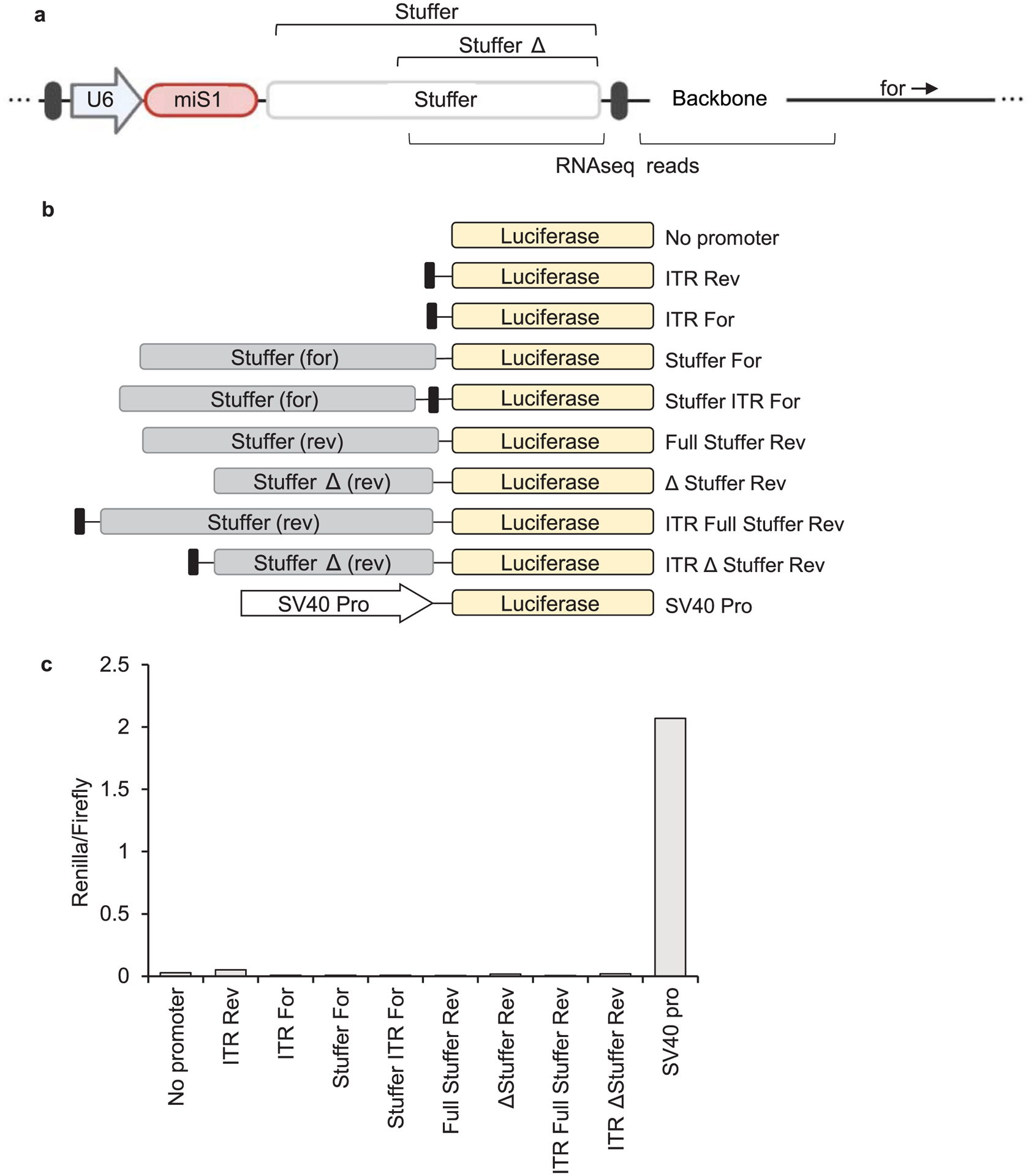
a, Cartoon map of AAV.miS1 proviral plasmid. Bracketed regions below indicate locations of unexpected RNA-seq reads. Bracketed regions above represent the full or partial stuffer regions tested for activity. b, Construct series designed to test promoter activity of sequences near the 3’ ITR. Proviral plasmid segments were cloned upstream Renilla luciferase coding sequence. c, Renilla luciferase activity in HEK293 cells at 24 hrs post-transfection. Activity was normalized to Firefly luciferase activity encoded within the same plasmid.
Extended Data Fig. 3 |. Probe based ddPCR assays.
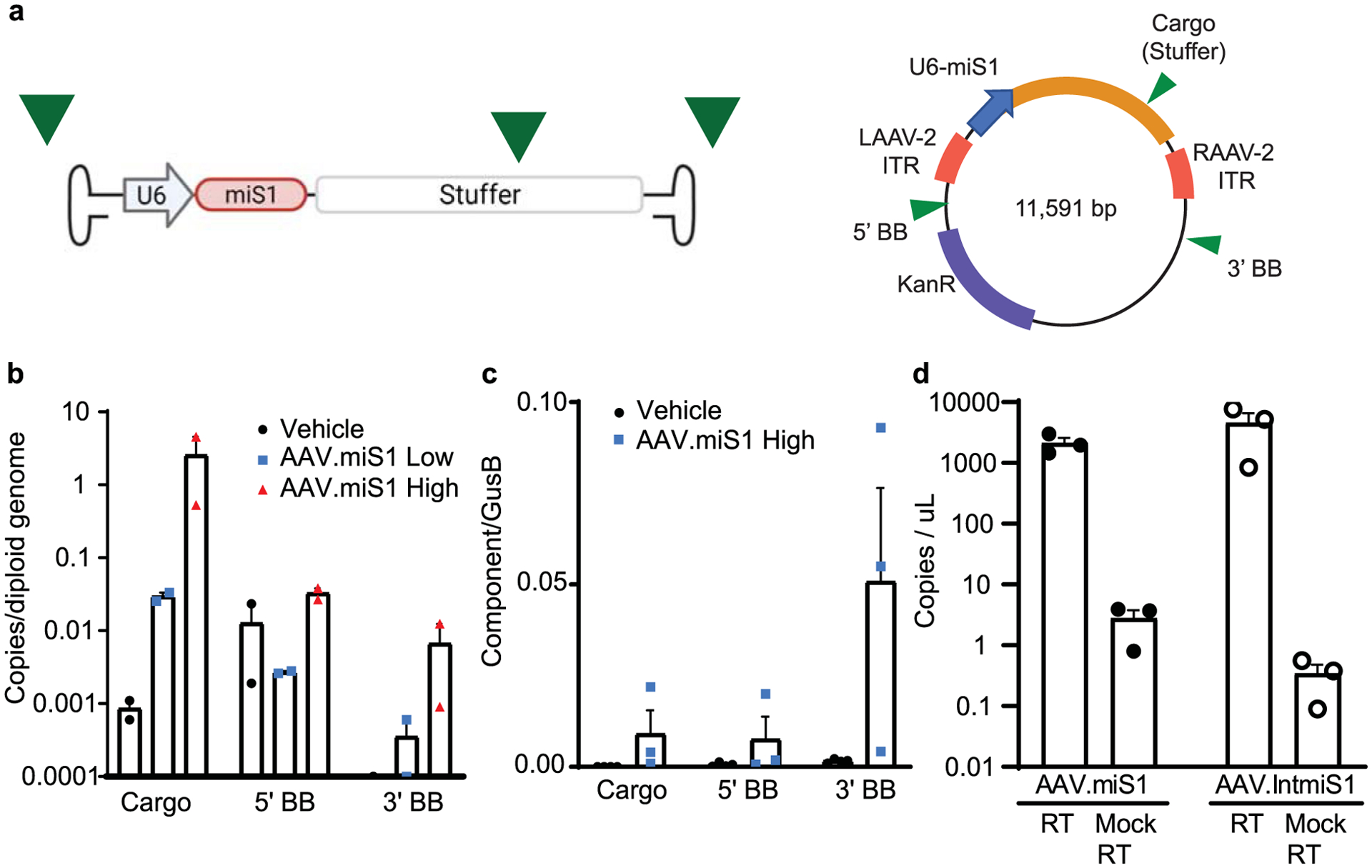
a, Assays were designed to quantify cross-packaged sequence upstream of the left ITR (5’ backbone (BB)), downstream of the right ITR (3’ BB), or within the cargo (stuffer sequence) of AAV.miS1, to reflect transcripts identified by RNA-seq (See Fig. 4). b, Copies of vector cargo (stuffer sequence) and cross-packaged sequence measured from punches taken from the intermediate lobule of NHPs. c, Cargo, 5’- and 3’- transcript levels isolated from the superficial medial punches of animals injected with high dose AAV.miS1. d, Mock RT cDNA for the cargo sequence (stuffer for AAV.miS1 and EF1α for AAV.IntmiS1) was quantified in parallel to confirm that expression was not arising from contaminating vector DNA. All data are represented as mean ± SEM (N = 3 animals per group).
Extended Data Fig. 4 |. Testing of AAV.IntmiS1.
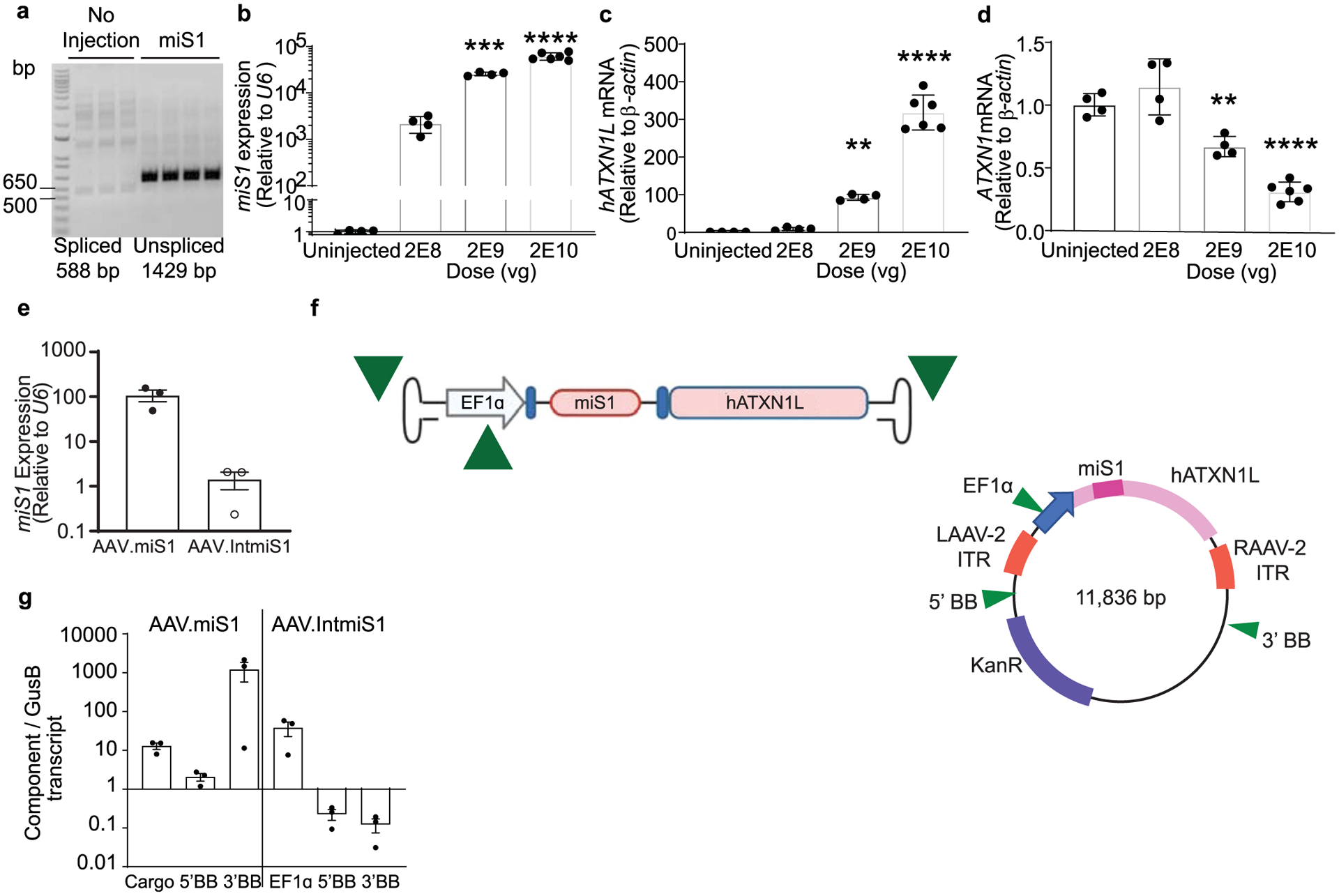
AAV1.IntmiS1 was injected bilaterally into B05 SCA1 mice cerebella (N = 4 or 6) and tissues collected 3 weeks later. a, Representative agarose gels of cDNAs following PCR using primers flanking the miRNA-containing intron (N = 3 biological replicates). b–d, mRNA levels of miS1 (***P = 0.0003; ****P < 0.0001), (b) hATXN1L (**P = 0.001; ****P < 0.0001), (c) and hATXN1 (**P = 0.0060; ****P, 0.0001), (d) in mice cerebella following AAV.IntmiS1 injection at the indicated doses. All data are represented as mean ± SD (N = 4 and 6 animals per treatment group), with significance determined by one-way ANOVA followed by Dunnett’s multiple comparisons post hoc. e) Relative expression of miS1 as quantified by stem-loop qPCR in WT mice injected bilaterally with AAV.miS1 or AAV.IntmiS1 into the striatum. Data are represented as mean ± SD, (N = 3 animals per group). f) Cartoons of the packaged AAV genome and plasmid sequences for assessing transcript levels by ddPCR. Green arrowheads depict the probe locations for assessing transcripts arising from the 5’ ITR (5’ backbone (BB)), the 3’ ITR (3’ BB), or the EF1α promoter of AAV.IntmiS1. g, ddPCR quantification of RNA isolated from striatum of mice injected with AAV.miS1 or AAV.IntmiS1. Data are represented as mean ± SEM (N = 3 animals per group).
Extended Data Fig. 5 |. AAV.IntmiS1 does not cause small RNA dysregulation.

a, Differential expression analysis on small RNASeq data obtained from medial deep cerebellar tissue punches from AAV.IntmiS1 and empty capsid treated NHPs. b,c, Heatmaps of the top 30 most highly detected (b) or 11 specific neuronal miRNAs (c). * denotes 28 day in-life animal.
Extended Data Fig. 6 |. Study 2 histological and molecular readouts.

a, Quantitation of lesion scores from cerebellar sections of NHPs injected with the indicated AAVs (Empty, miSCA7, Stuffer, IntmiS1). Each dot represents a single animal. b, Human ATXN1L levels normalized to endogenous GAPDH as assessed by RT-qPCR. c, Total read counts of human ATXN1L by RNA-seq. d, miS1 levels in AAV.IntmiS1 treated animals, normalized to endogenous U6 RNA and relative to empty capsid treated animals. e, Ionized calcium binding adapter molecule 1 (IBA1) mRNA levels. f, Glial fibrillary acidic protein (GFAP) mRNA levels. Data are represented as mean ± SEM (N = 3 animals per group). There was no significant difference as measured by two-way ANOVA followed by a Dunnett’s multiple comparisons post hoc.
Extended Data Table 1 |.
Study designs and demographics
| Treatment | Sex | Age (Years) | MRI Scan(s) | Days To Symptoms | Days With Symptoms | Days In Life | Planned Days in Life | Symptoms |
|---|---|---|---|---|---|---|---|---|
| Vehiclea | female | 8.40 | NA | NA | NA | 101 | 180 | None |
| Vehiclea | male | 4.62 | NA | NA | NA | 136 | 180 | None |
| Vehiclea | female | 7.35 | NA | NA | NA | 153 | 180 | None |
| Vehiclea | female | 7.28 | NA | NA | NA | 160 | 90 | None |
| AAV.miS1a (1E13vg) |
male | 4.59 | 29 | 24 | 6 | 30 | 90 | Dysmetria; ataxia; tremor |
| AAV.miS1a (1E13vg) |
female | 6.52 | 62 | 55 | 9 | 64 | 90 | Inducible horizontal nystagmus, ataxia |
| AAV.miS1a (1E13vg) |
female | 4.79 | 43 | NA | NA | 49 | 90 | None |
| AAV.miS1a (6E11vg) |
male | 4.73 | 64 | 60 | 5 | 65 | 90 | Head tilt |
| AAV.miS1a (6E11vg) |
female | 4.73 | 66 | NA | NA | 84 | 90 | None |
| AAV.miS1a (3E12vg) |
female | 4.81 | 31 | 41 | 10 | 51 | 90 | Ataxia |
| AAV.miS1a (3E12vg) |
female | 4.62 | 73 | 69 | 23 | 92 | 90 | Head tilt; tremor; dysmetria |
| AAV.miS1a (3E12vg) |
female | 7.53 | 76 | 83 | 4 | 87 | 90 | Tremor |
| AAV.miS1a (3E12vg) |
male | 4.44 | 59 | 83 | 10 | 93 | 180 | Ataxia, tremor |
| AAV.miS1a (3E12vg) |
female | 7.37 | 111 | 92 | 21 | 113 | 180 | Head tilt; ataxia |
| AAV.miS1a (3E12vg; pilot) |
female | 7.00 | NA | NA | NA | 31 | Pilot (30) | None |
| AAV.miS1a (3E12vg) |
male | 4.60 | 97 | 80 | 20 | 100 | 180 | Tremor; ataxia |
| Empty Capsidsb | female | 5.48 | 60,128 | NA | NA | 130 | 120 | None |
| Empty Capsidsb | male | 4.29 | 64,125 | NA | NA | 127 | 120 | None |
| Empty Capsidsb | male | 4.31 | 57,118 | NA | NA | 123 | 120 | None |
| AAV.miSCA7b | male | 4.23 | 59,123 | 119 | 6 | 125 | 120 | Dysmetria |
| AAV.miSCA7b | female | 5.63 | 57,121 | 53 | 63 | 116 | 120 | Head tilt, hindlimb ataxia |
| AAV.miSCA7b | female | 6.42 | 67,128 | NA | NA | 135 | 120 | None |
| AAV.Stufferb | male | 5.03 | 64,120 | NA | NA | 122 | 120 | None |
| AAV.Stufferb | female | 5.28 | 56,120 | 85 | 42 | 127 | 120 | Tremor, dysmetria; head tilt |
| AAV.Stufferb | female | 4.33 | 61,125 | 119 | 13 | 132 | 120 | Tremor, dysmetria, ataxia |
| AAV.IntmiS1b | male | 3.77 | NA | NA | NA | 28 | Pilot (30) | None |
| AAV.IntmiS1b | male | 4.62 | 58,121 | NA | NA | 132 | 120 | None |
| AAV.IntmiS1b | male | 4.69 | 55,115 | NA | NA | 126 | 120 | None |
denotes Study 1;
denotes Study 2
Extended Data Table 2 |.
Lesion scoring parameters
| Score | Color | Lesion Description |
|---|---|---|
| 0 | Unremarkable/background finding | |
| 1 | Minimal/mild gliosis affecting a focal confined region, usually within the deep cerebellar nuclei and the arbor vitae, with limited extension distally into the lobules. | |
| 2 | Mild parenchymal necrosis and/or accumulation of foamy macrophages/gitter cells with focal involvement of the arbor vitae (most commonly the base) and/or deep cerebellar nuclei. Extension of the process distally into the lobules is limited and the involvement of the overlying cortex is usually minimal. Perivascular and/or meningeal aggregates of lymphocytes and histiocytes are unfrequently observed. Accompanying gliosis and demyelination of the more distal white matter is overall modest. | |
| 3 | Moderate parenchymal necrosis and accumulation of foamy macrophages/gitter cells with regional involvement of the arbor vitae (most commonly the base) and/or deep cerebellar nuclei. Extension of the process distally into the lobules and the involvement of the overlying cortex is limited with occasional neuronal loss. Perivascular and/or meningeal aggregates of lymphocytes and histiocytes are usually present. Accompanying gliosis and demyelination of the more distal white matter is evident. | |
| 4 | Severe parenchymal necrosis and accumulation of foamy macrophages/gitter cells with extensive involvement of the arbor vitae (most commonly the base), deep cerebellar nuclei, and more distal lobular tracts of the cerebellar white matter. Extension of the process into the overlying cortex is prominent with remarkable neuronal loss. Perivascular and/or meningeal aggregates of lymphocytes and histiocytes are common. Gliosis and demyelination of the more distal white matter are extensive. | |
| 5 | Massive parenchymal necrosis with complete tissue destruction and loss characterized by cavitation. Extension of the process into the overlying cortex is prominent with extensive neuronal loss. Perivascular and/or meningeal aggregates of lymphocytes and histiocytes are present. Gliosis and demyelination of the more distal white matter are severe. |
Extended Data Table 3 |.
Study 1 and 2 top 30 most abundant miRNAs
| Stable ID | Mean Read Counts | Stable ID | Mean Read Counts |
|---|---|---|---|
| mml-mir-9-2 | 764531.33 | mml-mir-125a | 322948.67 |
| mml-mir-30d | 231572.50 | mml-mir-26a-1 | 94935.67 |
| mml-mir-30c-1 | 211687.00 | mml-mir-30c-1 | 19041.83 |
| mml-mir-125a | 206365.83 | mml-mir-181c | 13865.33 |
| mml-mir-103-2 | 148113.83 | mml-mir-30d | 8852.17 |
| mml-mir-181a-2 | 102387.33 | mml-mir-100 | 7099.83 |
| mml-mir-342 | 67410.00 | mml-mir-30a | 6127.33 |
| mml-mir-181a-1 | 27590.33 | mml-mir-30b | 5530.33 |
| mml-mir-301a | 26687.17 | mml-mir-342 | 5286.33 |
| mml-mir-320a | 25510.83 | mml-mir-181b-1 | 5062.17 |
| mml-mir-30a | 21784.67 | mml-mir-143 | 4750.17 |
| mml-mir-30e | 10017.17 | mml-mir-128b | 3813.33 |
| mml-mir-21 | 9518.83 | mml-mir-16-1 | 3560.50 |
| mml-mir-107 | 8752.33 | mml-mir-204 | 3252.17 |
| mml-mir-92a-1 | 7524.50 | mml-mir-340 | 3134.33 |
| mml-mir-339 | 7338.00 | mml-mir-153-2 | 3103.33 |
| mml-mir-425 | 7227.00 | mml-mir-186 | 2552.17 |
| mml-mir-361 | 7195.50 | mml-mir-30e | 2541.67 |
| mml-mir-9-3 | 6698.00 | mml-mir-9-3 | 2429.00 |
| mml-mir-30c-2 | 5986.83 | mml-mir-149 | 2230.00 |
| mml-mir-127 | 5392.50 | mml-mir-129-2 | 1576.83 |
| mml-mir-140 | 5332.83 | mml-mir-98 | 1518.17 |
| mml-mir-7-3 | 4387.83 | mml-mir-181a-1 | 1319.83 |
| mml-mir-26a-1 | 4038.67 | mml-mir-320a | 1289.50 |
| mml-mir-124a-2 | 3926.00 | mml-mir-148b | 1247.67 |
| mml-mir-124a-1 | 3819.33 | mml-mir-145 | 1237.17 |
| mml-mir-660 | 3636.17 | mml-mir-361 | 1203.67 |
| mml-mir-500a | 3427.00 | mml-mir-107 | 1031.50 |
| mml-mir-130a | 3367.33 | mml-mir-301a | 1029.00 |
| mml-mir-26a-2 | 2675.50 | mml-mir-195 | 885.50 |
Columns alternate ‘Stable ID’ and ‘Mean Read Counts.’ Blue cells denote the most abundant miRs from Study 1. Orange cells denote the most abundant miRs from Study 2.
Extended Data Table 4 |.
Top differentially upregulated genes from Study 1 and 2 for GO terms ‘Immune System Process’ and ‘Immune Response’
| Gene ID | Fold Change | Gene ID | Fold Change | Gene ID | Fold Change | Gene ID | Fold Change | Gene ID | Fold Change | Gene ID | Fold Change | Gene ID | Fold Change | Gene ID | Fold Change |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GPR174 | 7.679 | ELF4 | 2.962 | ACTN1 | 1.993 | NFKB1 | 0.8157 | TNFSF15 | 3.5638 | GAT A3 | 2.2702 | NLRP3 | 1.39 | SOCS6 | 1.3653 |
| LCK | 7.4746 | BTK | 2.9403 | CEBPB | 1.9686 | CSK | 0.8066 | MAMU-DMA | 3.5289 | C5AR2 | 2.2028 | SOCS6 | 1.3653 | PSAP | 1.3406 |
| RAC2 | 7.1899 | C1QC | 2.9391 | NLRP1 | 1.9161 | TRIM11 | 0.7957 | ITGA4 | 3.5141 | CFD | 2.1979 | PSAP | 1.3406 | TRIB1 | 1.3015 |
| CXCL9 | 6.103 | MZB1 | 2.918 | CD276 | 1.8996 | ITGA6 | 0.7586 | CCL4L1 | 3.4887 | CCR4 | 2.1845 | TRIB1 | 1.3015 | FES | 1.3011 |
| SLAMF1 | 6.0418 | FCGR2B | 2.9119 | NRROS | 1.8522 | PELI1 | 0.7499 | C1QC | 3.4764 | MFNG | 2.1807 | FES | 1.3011 | LTBR | 1.2846 |
| CLEC6A | 5.9503 | NA | 2.8876 | WAS | 1.8483 | CD9 | 0.6992 | HLA-DRA | 3.4608 | IRF1 | 2.1733 | LTBR | 1.2846 | NA | 1.2717 |
| NA | 5.2527 | C5AR1 | 2.845 | GCNT1 | 1.8369 | ABI1 | 0.6929 | EGR1 | 3.4428 | AQP3 | 2.1523 | NA | 1.2717 | SLA2 | 1.2661 |
| MAMU-DOA | 5.144 | CCL8 | 2.8387 | IFI16 | 1.8293 | TXLNA | 0.6857 | COL3A1 | 3.4094 | CLEC7A | 2.1427 | SLA2 | 1.2661 | MYC | 1.2497 |
| CXCL10 | 5.1439 | EGR1 | 2.8158 | CD4 | 1.8076 | ACTG1 | 0.6691 | ICAM1 | 3.3926 | SPIB | 2.1332 | MYC | 1.2497 | PSME1 | 1.235 |
| CDKN1A | 5.1036 | CCRL2 | 2.7827 | NA | 1.8038 | FAU | 0.6606 | HCST | 3.3511 | HLA-F | 2.1023 | PSME1 | 1.235 | RFTN1 | 1.2317 |
| MAFB | 4.7929 | LGMN | 2.7738 | NLRP3 | 1.7979 | STAMBPL1 | 0.6443 | VSIG4 | 3.3368 | TGFBR2 | 2.0934 | RFTN1 | 1.2317 | ANO6 | 1.2058 |
| IKBKE | 4.7629 | CXCL8 | 2.7668 | IL1B | 1.7947 | SH3KBP1 | 0.5924 | CCRL2 | 3.2455 | EVI2B | 2.0786 | ANO6 | 1.2058 | SMAD3 | 1.1862 |
| NR1H3 | 4.651 | NA | 2.763 | RAB20 | 1.7808 | RB1 | 0.5829 | CCL2 | 3.2286 | TGFB1 | 2.0561 | SMAD3 | 1.1862 | RIPK2 | 1.1838 |
| IRF4 | 4.6452 | PYCARD | 2.761 | THBS1 | 1.7739 | FAM49B | 0.5195 | CNR2 | 3.2271 | RNASEL | 2.0449 | RIPK2 | 1.1838 | MYD88 | 1.1715 |
| CTLA4 | 4.6048 | OAS1 | 2.7605 | TAPBP | 1.758 | IL2RA | 0.1258 | Mamu-DPA1 | 3.1958 | NMI | 2.0393 | MYD88 | 1.1715 | PRKD2 | 1.1453 |
| CCL3 | 4.5644 | ICAM1 | 2.7412 | TLR6 | 1.7148 | CCL18 | 0.1011 | CTSS | 3.1813 | RARRES2 | 2.0107 | PRKD2 | 1.1453 | STK4 | 1.1382 |
| ECM1 | 4.5354 | PSMB9 | 2.7345 | PLA2G4A | 1.6682 | CXCL9 | 9.6973 | PLSCR1 | 3.1785 | CEBPB | 1.9991 | STK4 | 1.1382 | CBFB | 1.1378 |
| NA | 4.492 | NMI | 2.7318 | SH2B3 | 1.6592 | CCL18 | 9.4452 | KLHL6 | 3.1686 | IKBKE | 1.9836 | CBFB | 1.1378 | TLR10 | 1.0948 |
| PPARG | 4.4513 | CH25H | 2,7247 | TLR5 | 1.6587 | PLA2G2D | 9.0084 | CLEC6A | 3.1653 | C1R | 1.9796 | TLR10 | 1.0948 | PARP3 | 1.0932 |
| COL3A1 | 4.3786 | C1R | 2.703 | FES | 1.653 | CCR7 | 8.4027 | LY96 | 3.1371 | CCR1 | 1.976 | PARP3 | 1.0932 | PRKCB | 1.093 |
| NA | 4.3602 | HCLS1 | 2.7021 | CMTM3 | 1.6515 | ADGRE2 | 7.0749 | ISG20 | 3.1076 | PIK3CD | 1.9731 | PRKCB | 1.093 | MYH9 | 1.0844 |
| ITK | 4.2421 | HLA-E | 2.7005 | OAS3 | 1.6076 | RAC2 | 6.5359 | CFB | 3.1075 | CXCL16 | 1.9687 | MYH9 | 1.0844 | BCL2L11 | 1.0819 |
| CD36 | 4.238 | MDK | 2.6674 | PSME1 | 1.562 | KLRK1 | 6.4721 | LAT2 | 3.0985 | RAB20 | 1.9604 | BCL2L11 | 1.0819 | TLR4 | 1.0757 |
| TOP2A | 4.2293 | IL1A | 2.6574 | PARP3 | 1.5477 | HOXB6 | 6.4469 | B2M | 3.0955 | TNFSF8 | 1.9597 | TLR4 | 1.0757 | DDX58 | 1.0732 |
| SOCS1 | 4.2056 | CXCR5 | 2.6435 | ANO6 | 1.5218 | IL10 | 6.3916 | BST2 | 3.0754 | APOD | 1.954 | DDX58 | 1.0732 | TMOD3 | 1.0715 |
| Mamu-DPA1 | 4.1802 | CXCR3 | 2.6409 | NFATC2 | 1.4925 | MZB1 | 6.2091 | APOBEC3G | 3.0604 | TBC1D10C | 1.9505 | TMOD3 | 1.0715 | IRF9 | 1.0588 |
| NFAM1 | 4.1788 | IKZF3 | 2.568 | PSAP | 1.4778 | NA | 5.9505 | MX2 | 3.0472 | PIK3R6 | 1.9492 | IRF9 | 1.0588 | OAS3 | 1.0578 |
| CD79A | 4.1536 | RUNX1 | 2.5261 | MYC | 1.427 | LAX1 | 5.9152 | NA | 3.0096 | TNFSF13B | 1.9423 | OAS3 | 1.0578 | TLR6 | 1.0447 |
| VIM | 4.1342 | RNASE6 | 2.5244 | IL18 | 1.3986 | IL2RA | 5.8945 | CD74 | 3.0004 | CMTM3 | 1.9403 | TLR6 | 1.0447 | ZC3H12A | 1.0294 |
| CD7 | 4.0943 | HRH1 | 2.5102 | JUN | 1.3972 | LCK | 5.8512 | LGMN | 2.9879 | JUN | 1.9271 | ZC3H12A | 1.0294 | IRF3 | 1.0248 |
| CFP | 4.0631 | CCR2 | 2.5056 | TNIP2 | 1.3929 | GPR174 | 5.8395 | IKZF3 | 2.9874 | FYB1 | 1.9259 | IRF3 | 1.0248 | ACTR3 | 1.0214 |
| MMP14 | 3.9382 | CLEC7A | 2.489 | TLR1 | 1.388 | MAFB | 5.7716 | CD3E | 2.9619 | RNASE6 | 1.9097 | ACTR3 | 1.0214 | CD40 | 0.9926 |
| LY96 | 3.8811 | PTPN6 | 2.4852 | CCR1 | 1.3595 | IRF4 | 5.5766 | TLR8 | 2.9465 | TNFAIP8L2 | 1.9021 | CD40 | 0.9926 | TRIM11 | 0.9824 |
| HLA-DRA | 3.8315 | VAMP8 | 2.4679 | C3 | 1.3501 | SELL | 5.5644 | SPI1 | 2.9372 | AIF1 | 1.8956 | TRIM11 | 0.9824 | CD160 | 0.9758 |
| TNFSF15 | 3.8131 | KLF10 | 2.4587 | TNFAIP8L2 | 1.3245 | ZAP70 | 5.3923 | GPR183 | 2.9171 | NA | 1.8887 | CD160 | 0.9758 | THBS1 | 0.97 |
| ITGA4 | 3.7981 | AIF1 (IBA1 | 2.4476 | ZC3H12A | 1.3154 | CCL3 | 5.1926 | RAB7B | 2.9078 | IL18 | 1.8858 | THBS1 | 0.97 | HIST1H2BK | 0.9503 |
| ID01 | 3.7739 | SPIB | 2.4437 | DDX58 | 1.3071 | NR1H3 | 5.1662 | PTPN22 | 2.8406 | FOS | 1.8828 | HIST1H2BK | 0.9503 | TNIP2 | 0.9329 |
| LAX1 | 3.7669 | MILR1 | 2.4376 | TRIM21 | 1.2969 | CFP | 5.1197 | NCR3 | 2.8336 | ACTN1 | 1.8813 | TNIP2 | 0.9329 | TRIM14 | 0.901 |
| CLEC12B | 3.7154 | PLSCR1 | 2.4285 | CD46 | 1.2939 | CXCL8 | 5.0928 | HCLS1 | 2.821 | GAB3 | 1.8769 | TRIM14 | 0.901 | SWAP70 | 0.8682 |
| ICOS | 3.7145 | TNFSF13B | 2.4211 | SLC11A1 | 1.2761 | OSM | 5.0613 | PDCD1 | 2.8115 | SH2B3 | 1.8672 | SWAP70 | 0.8682 | BCL10 | 0.864 |
| IL1RN | 3.6396 | LCP2 | 2.4094 | GAB3 | 1.2487 | NFAM1 | 5.0384 | PTGER4 | 2.8036 | KLF10 | 1.8415 | BCL10 | 0.864 | ATP6AP1 | 0.8636 |
| RAB7B | 3.4948 | COCH | 2.3997 | TRIB1 | 1.2387 | ICOS | 4.9333 | HRH1 | 2.7982 | ZFP36 | 1.8076 | ATP6AP1 | 0.8636 | LBR | 0.8602 |
| CD1C | 3.4836 | IRF1 | 2.3722 | POLD1 | 1.2169 | ITK | 4.9017 | TMEM173 | 2.7946 | LOXL3 | 1.7906 | LBR | 0.8602 | DUSP1 | 0.8529 |
| BST2 | 3.4779 | IRF5 | 2.3701 | ALOX5 | 1.2105 | IL7 | 4.7247 | CCL19 | 2.7757 | PDCD1LG2 | 1.7727 | DUSP1 | 0.8529 | NFIL3 | 0.8355 |
| C1QB | 3.4652 | THEMIS2 | 2.3643 | IL7R | 1.2029 | CXCR3 | 4.6543 | CD14 | 2.747 | TWF2 | 1.755 | NFIL3 | 0.8355 | POLD1 | 0.8263 |
| CD74 | 3.458 | MX1 | 2.3613 | TRIM56 | 1.2007 | SCIN | 4.6471 | LPXN | 2.7469 | GCNT1 | 1.7497 | POLD1 | 0.8263 | MEF2C | 0.8115 |
| B2M | 3.4321 | TNF | 2.3517 | RIPK2 | 1.2002 | CXCL10 | 4.6425 | TMEM106A | 2.732 | B4GALT1 | 1.7114 | MEF2C | 0.8115 | LRCH1 | 0.7869 |
| TMEM173 | 3.4182 | CGAS | 2.3355 | MYH9 | 1.1974 | LY6D | 4.581 | CH25H | 2.7089 | IFI16 | 1.7095 | LRCH1 | 0.7869 | RPS3 | 0.7524 |
| SCIN | 3.3752 | PIK3CD | 2.3311 | SAMHD1 | 1.1949 | LTB | 4.5685 | RUNX1 | 2.6899 | ZNF335 | 1.709 | RPS3 | 0.7524 | ZBTB1 | 0.7124 |
| PTGER4 | 3.3069 | TRIM34 | 2.3094 | CBFB | 1.1798 | PPARG | 4.4799 | PSMB9 | 2.6847 | TRIM34 | 1.6743 | ZBTB1 | 0.7124 | CEBPG | 0.7038 |
| NA | 3.3069 | ZFP36 | 2.2775 | STK4 | 1.1572 | NA | 4.4725 | C5AR1 | 2.6779 | NRROS | 1.6706 | CEBPG | 0.7038 | SLC11A1 | 0.6844 |
| CXCR4 | 3.3068 | MFNG | 2.275 | HIST1H2BK | 1.1547 | FCGR2B | 4.4582 | PTPN6 | 2.6754 | TKFC | 1.6658 | SLC11A1 | 0.6844 | PRELID1 | 0.6835 |
| LPXN | 3.2965 | TGFBR2 | 2.251 | CD80 | 1.15 | CD7 | 4.4165 | ELF4 | 2.6665 | NA | 1.6657 | PRELID1 | 0.6835 | ZYX | 0.6782 |
| ADGRE2 | 3.2736 | CMKLR1 | 2.242 | RFTN1 | 1.1495 | MAMU-DOA | 4.349 | PYCARD | 2.6606 | TRIM21 | 1.6477 | ZYX | 0.6782 | EMILIN1 | 0.6426 |
| CCL2 | 3.2573 | IRF8 | 2.2336 | IRF9 | 1.1419 | MAMU-A | 4.2707 | NFKBID | 2.6457 | TRIM22 | 1.6261 | EMILIN1 | 0.6426 | RHOA | 0.641 |
| CTSS | 3.2373 | GPR183 | 2.2228 | CCR5 | 1.1417 | CST7 | 4.164 | TLR2 | 2.6243 | CD44 | 1.6249 | RHOA | 0.641 | NUP85 | 0.6317 |
| TLR8 | 3.2247 | FYB1 | 2.1992 | MEF2C | 1.1247 | ECM1 | 4.1615 | HLA-E | 2.6192 | TNFRSF17 | 1.6198 | NUP85 | 0.6317 | STAT2 | 0.6224 |
| MAMU-A | 3.224 | TRIM22 | 2.1676 | SWAP70 | 1.0997 | NA | 4.1407 | SOCS1 | 2.605 | CLEC4A | 1.6094 | STAT2 | 0.6224 | TFEB | 0.6103 |
| FCER1G | 3.2054 | FOS | 2.1672 | TWF2 | 1.0952 | VAMP8 | 4.1396 | CMKLR1 | 2.6038 | THEMIS2 | 1.5992 | TFEB | 0.6103 | PELI1 | 0.6061 |
| PDCD1 | 3.1867 | CD3E | 2.1585 | B4GALT1 | 1.0818 | VIM | 4.0994 | CCL5 | 2.5979 | CD28 | 1.5886 | PELI1 | 0.6061 | FLCN | 0.6054 |
| CFB | 3.185 | CD44 | 2.1431 | PRKD2 | 1.0488 | KLRD1 | 4.095 | BTK | 2.5628 | TAPBP | 1.5637 | FLCN | 0.6054 | MPP1 | 0.5434 |
| MAMU-DMA | 3.1385 | RNASEL | 2.1118 | ANXA1 | 1.0358 | CD79A | 4.043 | NA | 2.5571 | CD80 | 1.5573 | MPP1 | 0.5434 | SLC11A2 | 0.5385 |
| CD14 | 3.1384 | IL33 | 2.0977 | ZFP36L2 | 1.0221 | SH2D1A | 3.9068 | NA | 2.5322 | MDK | 1.5389 | SLC11A2 | 0.5385 | MAP3K14 | 0.4673 |
| SPI1 | 3.1334 | CCL4L1 | 2.0956 | MYD88 | 1.0134 | CD19 | 3.9051 | IRF8 | 2.5254 | NLRP1 | 1.5336 | MAP3K14 | 0.4673 | CXCL1 | 0.463 |
| KLRK1 | 3.1141 | ISG15 | 2.0953 | TRIM14 | 0.9962 | IL1RN | 3.8962 | WAS | 2.5157 | CCR2 | 1.5082 | CXCL1 | 0.463 | CLEC12B | 0.4183 |
| PTPN22 | 3.1106 | CFD | 2.0771 | MOV10 | 0.9419 | MMP14 | 3.8415 | PLA2G4A | 2.4558 | TLR1 | 1.4939 | CLEC12B | 0.4183 | TRAT1 | 0.3686 |
| ISG20 | 3.0677 | LYN | 2.0702 | ACTR3 | 0.9349 | CXCR4 | 3.8335 | MILR1 | 2.4327 | CD40LG | 1.4718 | TRAT1 | 0.3686 | CTLA4 | 0.3655 |
| LAT2 | 3.0326 | RHEX | 2.0546 | ZYX | 0.9233 | CDKN1A | 3.8184 | IRF7 | 2.4099 | IL1B | 1.4697 | CTLA4 | 0.3655 | TNFRSF13B | 0.324 |
| CCL5 | 3.0258 | TNFSF13 | 2.0536 | SMAD3 | 0.9227 | C1QB | 3.7108 | LYN | 2.3702 | F11R | 1.4529 | TNFRSF13B | 0.324 | IL36G | 0.3198 |
| MX2 | 3.0242 | EVI2B | 2.022 | SOCS6 | 0.9117 | NA | 3.6905 | IRF5 | 2.339 | RARA | 1.4117 | IL36G | 0.3198 | FASLG | 0.2825 |
| IRF7 | 2.9943 | CXCL16 | 2.017 | F11R | 0.9028 | IL1A | 3.6412 | CGAS | 2.3223 | IL7R | 1.4096 | FASLG | 0.2825 | SLAMF1 | 0.2424 |
| HLA-F | 2.9854 | TMEM106A | 2.0052 | STAT2 | 0.8967 | CD36 | 3.6216 | LCP2 | 2.3201 | NFATC2 | 1.4068 | SLAMF1 | 0.2424 | CD3D | 0.1604 |
| APOBEC3G | 2.9825 | TNFSF8 | 2.0004 | DUSP1 | 0.8937 | FCER1G | 3.6194 | CCR5 | 2.3112 | TLR5 | 1.4039 | CD30 | 0.1604 | ||
| TLR2 | 2.9621 | TGFB1 | 1.999 | ZFP36L1 | 0.8276 | CXCR5 | 3.6076 | TNFSF13 | 2.3049 | CD4 | 1.3975 | NLRP3 | 1.39 |
Columns alternate ‘Gene ID’ and Fold Change.’ Genes are ranked by Log2 Fold Change. Blue gene denote top differentially upregulated genes between AAV.miS1 and vehicle (Study 1). Orange genes denote top differentially upregulated genes between AAV.Stuffer and empty capsids (Study 2).
Acknowledgements
This work was funded by the NIH NS094355 (M.K., P.G.-A., T.J.L., B.L.D.), NIH T32 HG009495 (P.T.R.) and the Children’s Hospital of Philadelphia Research Institute.
Competing interests
B.L.D. is a founder of Spark Therapeutics and Spirovant Sciences. She serves an advisory role and/or receives sponsored research support for her laboratory from Roche, NBIR, Homology Medicines, Triplet Therapeutics, Resilience, Intellia Therapeutics, Spirovant Sciences, Panorama Medicines and Voyager Therapeutics. P.G.-A. has consulted for Eisai Therapeutics, Spark Therapeutics and NeuExcell Therapeutics. The remaining authors declare no competing interests.
Footnotes
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41591-021-01522-3.
Code availability
The software tools generated as a part of this study are archived at https://github.com/DavidsonLabCHOP/Keiser_NatMed_2021.
Extended data is available for this paper at https://doi.org/10.1038/s41591-021-01522-3.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41591-021-01522-3.
Data availability
Sequencing datasets generated as a part of this manuscript can be accessed using NCBI Gene Expression Omnibus (GEO) at accession number: GSE182666. The following individual files in GSE182666are associated with the indicated figure: Fig. 3, GSM5534318, GSM5534319, GSM5534320, GSM5534321, GSM5534322, GSM5534323; Fig. 4, GSM5534296, GSM5534297, GSM5534298, GSM5534299, GSM5534300, GSM5534301, GSM5534302, GSM5534303, GSM5534304, GSM5534305; Fig. 5, GSM5534306, GSM5534307, GSM5534308, GSM5534309, GSM5534310, GSM5534311, GSM5534312, GSM5534313, GSM5534314, GSM5534315, GSM5534316, GSM5534317; Extended Data Fig. 5, GSM5534324, GSM5534325, GSM5534326, GSM5534327, GSM5534328, GSM5534329. Raw data are available as Supplementary Data for graphs shown in Figs. 1c,f, 2i–n and 5c and Extended Data Figs. 2c, 3b–d, 4b–e,g and 6b,d–f. The original uncropped gel is given in the Supplementary Data for Extended Data Fig. 3a. The following public datasets used: Ensemble, Rhesus macaque genome (Mmul10), http://ftp.ensembl.org/pub/release-104/fasta/macaca_mulatta/dna/Macaca_mulatta.Mmul_10.dna.toplevel.fa.gz for Figs. 3–5 and Extended Data Fig. 5; Ensembl, (GRCh38), http://ftp.ensembl.org/pub/release-104/fasta/homo_sapiens/dna/Homo_sapiens.GRCh38.dna.toplevel.fa.gz for Fig. 4 and Extended Data Figs. 4 and 5. All vectors presented in this work are available on request with approval from the CHOP Office of Technology Transfer. Source data are provided with this paper.
References
- 1.Gonzalez-Alegre P Recent advances in molecular therapies for neurological disease: triplet repeat disorders. Hum. Mol. Genet 28, R80–R87 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oz G et al. In vivo monitoring of recovery from neurodegeneration in conditional transgenic SCA1 mice. Exp. Neurol 232, 290–298 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zu T et al. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J. Neurosci 24, 8853–8861 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matilla A et al. Mice lacking ataxin-1 display learning deficits and decreased hippocampal paired-pulse facilitation. J. Neurosci 18, 5508–5516 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keiser MS, Boudreau RL & Davidson BL Broad therapeutic benefit after RNAi expression vector delivery to deep cerebellar nuclei: implications for spinocerebellar ataxia type 1 therapy. Mol. Ther 22, 588–595 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keiser MS, Geoghegan JC, Boudreau RL, Lennox KA & Davidson BL RNAi or overexpression: alternative therapies for spinocerebellar ataxia type 1. Neurobiol. Dis 56, 6–13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keiser MS, Monteys AM, Corbau R, Gonzalez-Alegre P & Davidson BL RNAi prevents and reverses phenotypes induced by mutant human ataxin-1. Ann. Neurol 80, 754–765 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia H et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat. Med 10, 816–820 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Nitschke L et al. miR760 regulates ATXN1 levels via interaction with its 5′ untranslated region. Genes Dev. 34, 1147–1160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedrich J et al. Antisense oligonucleotide-mediated ataxin-1 reduction prolongs survival in SCA1 mice and reveals disease-associated transcriptome profiles.JCI Insight. 3, e123193 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Callaghan B et al. Antisense oligonucleotide therapeutic approach for suppression of ataxin-1 expression: a safety assessment. Mol. Ther. Nucleic Acids 21, 1006–1016 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keiser MS, Kordower JH, Gonzalez-Alegre P & Davidson BL Broad distribution of ataxin 1 silencing in rhesus cerebella for spinocerebellar ataxia type 1 therapy. Brain 138, 3555–3566 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monteys AM et al. Single nucleotide seed modification restores in vivo tolerability of a toxic artificial miRNA sequence in the mouse brain. Nucleic Acids Res. 42, 13315–13327 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Lebron E, Liu G, Keiser M, Behlke MA & Davidson BL Altered Purkinje cell miRNA expression and SCA1 pathogenesis. Neurobiol. Dis 54, 456–463 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boudreau RL, Martins I & Davidson BL Artificial microRNAs as siRNA shuttles: improved safety as compared to shRNAs in vitro and in vivo. Mol. Ther 17, 169–175 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Gestel MA et al. shRNA-induced saturation of the microRNA pathway in the rat brain. Gene Ther. 21, 205–211 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Radukic MT, Brandt D, Haak M, Muller KM & Kalinowski J Nanopore sequencing of native adeno-associated virus (AAV) single-stranded DNA using a transposase-based rapid protocol. NAR Genom. Bioinform. 2, lqaa074 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crespo-Barreto J, Fryer JD, Shaw CA, Orr HT & Zoghbi HY Partial loss of ataxin-1 function contributes to transcriptional dysregulation in spinocerebellar ataxia type 1 pathogenesis. PLoS Genet. 6, e1001021 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chadeuf G, Ciron C, Moullier P & Salvetti A Evidence for encapsidation of prokaryotic sequences during recombinant adeno-associated virus production and their in vivo persistence after vector delivery. Mol. Ther 12, 744–753 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Tai PWL et al. Adeno-associated virus genome population sequencing achieves full vector genome resolution and reveals human-vector chimeras. Mol. Ther. Methods Clin. Dev 9, 130–141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burright EN et al. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell 82, 937–948 (1995). [DOI] [PubMed] [Google Scholar]
- 22.Flotte TR et al. Gene expression from adeno-associated virus vectors in airway epithelial cells. Am. J. Respir. Cell Mol. Biol 7, 349–356 (1992). [DOI] [PubMed] [Google Scholar]
- 23.Earley LF et al. Adeno-associated virus serotype-specific inverted terminal repeat sequence role in vector transgene expression. Hum. Gene Ther 31, 151–162 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayuso E et al. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther. 17, 503–510 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Maguire AM et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet 374, 1597–1605 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Love MI, Huber W & Anders S Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobin A et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YH, Keiser MS & Davidson BL Adeno-associated virus production, purification, and titering. Curr. Protoc. Mouse Biol 8, e56 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Keiser MS, Chen YH & Davidson BL Techniques for intracranial stereotaxic injections of adeno-associated viral vectors in adult mice. Curr. Protoc. Mouse Biol 8, e57 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequencing datasets generated as a part of this manuscript can be accessed using NCBI Gene Expression Omnibus (GEO) at accession number: GSE182666. The following individual files in GSE182666are associated with the indicated figure: Fig. 3, GSM5534318, GSM5534319, GSM5534320, GSM5534321, GSM5534322, GSM5534323; Fig. 4, GSM5534296, GSM5534297, GSM5534298, GSM5534299, GSM5534300, GSM5534301, GSM5534302, GSM5534303, GSM5534304, GSM5534305; Fig. 5, GSM5534306, GSM5534307, GSM5534308, GSM5534309, GSM5534310, GSM5534311, GSM5534312, GSM5534313, GSM5534314, GSM5534315, GSM5534316, GSM5534317; Extended Data Fig. 5, GSM5534324, GSM5534325, GSM5534326, GSM5534327, GSM5534328, GSM5534329. Raw data are available as Supplementary Data for graphs shown in Figs. 1c,f, 2i–n and 5c and Extended Data Figs. 2c, 3b–d, 4b–e,g and 6b,d–f. The original uncropped gel is given in the Supplementary Data for Extended Data Fig. 3a. The following public datasets used: Ensemble, Rhesus macaque genome (Mmul10), http://ftp.ensembl.org/pub/release-104/fasta/macaca_mulatta/dna/Macaca_mulatta.Mmul_10.dna.toplevel.fa.gz for Figs. 3–5 and Extended Data Fig. 5; Ensembl, (GRCh38), http://ftp.ensembl.org/pub/release-104/fasta/homo_sapiens/dna/Homo_sapiens.GRCh38.dna.toplevel.fa.gz for Fig. 4 and Extended Data Figs. 4 and 5. All vectors presented in this work are available on request with approval from the CHOP Office of Technology Transfer. Source data are provided with this paper.


