Abstract
Exposures to endocrine disrupting chemicals (EDCs) perturb hormonal systems. EDCs are particularly problematic when exposure happens in the fetus and infant due to the high sensitivity of developing organisms to hormone actions. Previous work has shown that prenatal polychlorinated biphenyl (PCB) exposure disrupts hypothalamic development, reproductive physiology, mate preference behavior, and social behaviors in a sexually dimorphic manner. Based on evidence that EDCs perturb social behaviors in rodents, we examined effects of PCBs on the neuropeptides oxytocin (OXT) and vasopressin (AVP) systems that are involved in regulating these behaviors. Rats were exposed prenatally (gestational days 16 and 18) to the weakly estrogenic PCB mixture Aroclor 1221 (0.5 or 1 mg/kg), to estradiol benzoate (EB, a positive control), or to the vehicle (3% DMSO). In adult (~P90) brains, we counted immunolabeled oxytocin and vasopressin cell numbers in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus. EDCs did not change absolute numbers of oxytocin or vasopressin cells in either region, although there were some modest shifts in the rostral-caudal distribution. Second, expression of genes for these nonapeptides (Oxt, Avp), their receptors (Oxtr, Avpr1a), and the estrogen receptor beta (Esr2), was determined by qPCR. In the PVN, there were dose-dependent effects of PCBs in males (Oxt, Oxtr), and effects of EB in females (Avp, Esr2). In the SON, Oxt and Esr2 were affected by treatments in males. These changes to protein and gene expression caused by prenatal treatments suggest that transcriptional and post-transcriptional mechanisms play roles in mediating how EDCs reprogram hypothalamic development.
Keywords: Endocrine disrupting chemicals (EDCs), Polychlorinated biphenyls (PCBs), Aroclor 1221, oxytocin, vasopressin, paraventricular nucleus, supraoptic nucleus
Graphical Abstract
The distribution of oxytocin neurons in the PVN and SON was affected by prenatal PCB exposure. Shown is a series of immunolabeled sections from rostral (left) to caudal (right), relative to Bregma.
Introduction
Endocrine disrupting chemicals (EDCs) are a diverse group of chemicals that perturb hormones and their actions (Gore et al., 2015; Zoeller et al., 2012). Because of the high sensitivity of the developing brain to endogenous hormones, exposures to EDCs during these critical life periods have the potential to change the development of sexually-dimorphic neural circuits, leading to structural and functional neurobiological alterations. Among EDCs, polychlorinated biphenyls (PCBs) are associated with a wide variety of effects on brain development, including cognitive and neurobehavioral problems in humans (Braun et al., 2014; Jacobson and Jacobson, 1997; Winneke et al., 1998), and impairments of behaviors associated with anxiety, reproduction, and social interactions (Bell et al., 2016a; Bell et al., 2016b; Gillette et al., 2017; Reilly et al., 2018; Reilly et al., 2015). However, there are gaps in knowledge in the neural circuits underlying these behaviors and how they may be changed by early life EDC exposures.
Here, we focused on hypothalamic regions involved in the regulation of social behavior. A complex network of interconnected brain regions with a diverse complement of cell types controls these behaviors, and instrumental to these circuits are the nonapeptides oxytocin and vasopressin, synthesized in the hypothalamic paraventricular nucleus (PVN) and supraoptic nucleus (SON). Oxytocin and vasopressin play roles in sociality, including monogamy, pair bonding and affiliation, and social recognition and discrimination (Bielsky et al., 2005; Choleris et al., 2006; Lee et al., 2008; Ross and Young, 2009; Takayanagi et al., 2005). These behaviors are modulated by gonadal steroid hormones (Choleris et al., 2003). Both oxytocin and vasopressin neurons co-express estrogen receptor (ER) β (Kanaya et al., 2019; Oyola et al., 2017), and vasopressin (but not oxytocin) neurons co-express androgen receptor (Zhou et al., 1994) and progesterone receptor (Auger and De Vries, 2002; Bethea et al., 1994; Francis et al., 2002). Thus, the oxytocin and vasopressin systems are sites where converging actions of steroid hormones can affect social behaviors.
There is evidence that prenatal EDCs disrupt social and sociosexual behaviors in rodents (Colciago et al., 2009; Cummings et al., 2008; Jones et al., 2011; Lin et al., 2015; Monje et al., 2009; Porrini et al., 2005; Wang et al., 2016). Specific to the PCB mixture Aroclor 1221 (A1221), we reported that prenatal exposure led to deficits in tests sociability, social novelty, and sociosexual preference (Bell et al., 2016b; Hernandez Scudder et al., 2020b; Reilly et al., 2018; Reilly et al., 2015; Topper et al., 2019). However, evidence for whether behavioral effects of PCBs are due to changes in oxytocin or vasopressin is extremely limited. To our knowledge, PCB effects on oxytocin neurons have not been reported, and the few studies on the vasopressin system of the PVN and SON have been conducted in the context of the role of PCBs in water and electrolyte balance (Coburn et al., 2007; Coburn et al., 2005) but not social behavior.
The current study’s goal was to determine effects of PCB exposures on protein (immunohistochemistry) and gene expression of the oxytocin and vasopressin systems of the PVN and SON in order to provide a better understanding of molecular and cellular changes to these neuropeptide systems controlling social behaviors.
Methods
Animals and Husbandry.
Adult male and female Sprague-Dawley rats, aged approximately 3 months, were purchased from Harlan (Indianapolis, IN). All animals were housed in same-sex groups of 2–3 in polycarbonate cages with ad-libitum access to water and low-phytoestrogen chow (Harlan-Teklad Extruded 2019 Global Rodent diet). The colony was maintained at room temperature (21–22 C) on a partially reversed 12:12 light cycle (lights on at 12:00 am). Animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas at Austin, and followed NIH guidelines. After a one-week acclimation period, females were subjected to daily vaginal smears to monitor estrous cyclicity. On days of proestrus, the virgin female was placed in a cage with a sexually experienced male to confirm receptivity. Then, the pair was left together overnight and a vaginal smear containing sperm the next morning served as the marker for embryonic day 1 (E1). Males were removed and the females were left singly housed for the duration of their pregnancy; nestlets were provided several days prior to parturition.
Gestational Exposure to Endocrine-Disrupting Chemicals.
All protocols were conducted to exactly match prior work in the laboratory vis-à-vis the rat model, and the mode, route, and dose of agents (Dickerson et al., 2011a; Dickerson et al., 2011b; Gillette et al., 2017; Reilly et al., 2018; Reilly et al., 2015; Steinberg et al., 2007). In brief, pregnant dams were injected intraperitoneally twice, once on E16 and again with the same treatment on E18 with one of four experimental treatments: (a) vehicle (3% dimethylsulfoxide (DMSO) in sesame oil; negative control), (b) estradiol benzoate (EB 50 μg/kg; positive control for the estrogenic effects of PCBs), and the PCB mixture Aroclor 1221 at one of two concentrations: (c) (A1221, 0.5 mg/kg) or (d) (A1221, 1.0 mg/kg). Because of relatively short half-lives of EB and lightly chlorinated PCBs such as those in A1221 (Driowo et al., 1980; Sundstrom, Hutzinger, and Safe, 1976), exposure to the offspring likely is limited to placental transfer. Litters were weaned to 4 males and 4 females (Gillette et al., 2017; Reilly et al., 2015). The total number of animals used to generate tissues for IHC was: Vehicle – 11 females, 10 males; EB – 9 females, 10 males; A1221 (0.5) - 9 females, 9 males; A1221 (1.0) - 8 females, 9 males. For molecular work, siblings from the same litters were used, and n’s were: Vehicle – 11 females, 9 males; EB – 9 females, 10 males; A1221 (0.5) - 9 females, 8 males; A1221 (1.0) - 8 females, 9 males. For both immunohistochemistry and molecular work, within each sex, all individuals within a group came from different litters.
Euthanasia, Tissue Collection, and Processing.
All animals were euthanized at ~P90; to control for cycle status, females were euthanized on proestrus. For immunohistochemistry, rats were anesthetized with ketamine (150 mg/kg) and xylazine (30 mg/kg) and perfused with 4% paraformaldehyde (PFA) in PBS following lab protocols (Dickerson et al., 2011b; Kermath et al., 2014). The brains were postfixed overnight in 4% PFA at 4C and then stored in a sucrose cryoprotectant buffer at −20 for long-term storage. Tissues were blocked coronally and the region containing the hypothalamus was glued to a stage and sectioned on a Leica VT1000 vibrating microtome from Bregma −0.6 through Bregma 2.0 (Paxinos, 1986). For molecular work, rats were rapidly decapitated, brains removed and chilled on ice, and cut into 1 mm coronal slices onto slides that were frozen on dry ice and stored at −80C until use. Further tissue processing is described below.
Immunohistochemistry, microscopy, and cell counting.
Immunohistochemistry experiments were conducted with DAB to quantify the number of oxytocin and vasopressin neurons. Five sections per rat collected in a 1:6 tissue series through the hypothalamus from rostral to caudal were used, with the first section selected at Bregma −0.8. All tissues were processed in a single run each for oxytocin and vasopressin to avoid any inter-assay variability. Free floating sections were washed in PBS and subsequently quenched of any endogenous peroxidase in a 3:1 solution of methanol and 3% hydrogen peroxide in PBS for 20 minutes. Tissues were incubated for 1 hour in a blocking solution containing 10% normal goal serum (NGS; Vector Labs) and 0.5% Triton X (Vector Labs) in PBS. Incubation in primary antibody was carried out for oxytocin (Millipore MAB5296, mouse monoclonal, 1:20,000, RRID AB_2157626) or vasopressin (Immunostar 20069, rabbit polyclonal, 1:40,000, RRID AB_572219) in 2% NGS at 4C for 48 hours. Tissues were washed in PBS and then incubated for 2 hours in a solution containing 2% NGS and secondary antibody (oxytocin: Vector Laboratories Biotinylated Goat Anti-Mouse IgG #BA-9200; vasopressin: Vector Laboratories Biotinylated Goat Anti-Rabbit IgG Cat#BA-1000) at 1:400 in PBS. Tissues were then placed in an avidin-biotin solution (ABC kit, Vector) for 1 hour, followed by 3,3′-Diaminobenzidine reaction on ice for 2 minutes (oxytocin) or 4 minutes (vasopressin). Between every step, tissues were washed with PBS; all reactions occurred at room temperature unless otherwise noted. Tissues were mounted, dehydrated, counterstained with a cresyl violet solution, and coverslipped with DPX mountant (Sigma, Aldrich). Control tissues incubated without primary or secondary antibodies showed no specific labeling.
In order to assess the effects of treatment on the number of oxytocin and vasopressin neurons, all mounted sections were visualized on an Olympus BX61 microscope. Using Stereoinvestigator (MBF Bioscience, v10.0) the bilateral PVN and SON regional borders, as detected by cresyl violet, were outlined and the area determined. All immunopositive neurons within the borders were counted. Analyses were done separately for the 5 sections collected from rostral to caudal. For each section, based on the 40 μm tissue thickness, the sample density was estimated by the formula:
. Results were weighted if there were missing sections in a series so as not to bias results due to a missing data point.
RNA extraction, qPCR, and analysis
The 1 mm coronal sections containing the PVN and SON were used to punch out these regions using a Palkovits punch (0.75 mm). RNA was extracted and purified from frozen PVN and SON punches using a newly developed in-house protocol that has been optimized to yield high-quality RNA from small tissue punches. In brief, tissue was homogenized in 350 μl of a lysis buffer, an equal volume of 70% ethanol was added, and the sample transferred to a spin column [Epoch Life Science DNA/RNA Spin column (Cat. 1920250)] that was centrifuged for 30 seconds at 13,000 x g. The spin column containing the RNA was subsequently run through further buffer washes, given DNase I treatment and incubation, washed again, and finally eluted from the spin column. The full step-by-step protocol is in the Supplement. RNA quantity was determined using a nanodrop and diluted for the cDNA step. Samples from individual rats were converted to cDNA using High Capacity cDNA Reverse Transcription Kit (Cat. 4374967, ThermoFisher Scientific, Carlsbad, CA) according to the manufacturer’s protocol. Real-time PCR was run using TaqMan Gene Expression Master Mix, (4374967, ThermoFisher Scientific, Carlsbad, CA) on the ViiA7 Real-Time PCR System (4453552, Applied Biosystems, Foster City, CA) with the following parameters: 50°C for 2 minutes, 95°C for 10 minutes, and 45 cycles of 95°C for 15 seconds and 60°C for 1 minute. Primers and probes were purchased from ThermoFisher (Table 1). Relative expression was determined for each sample using the comparative CT method (Pfaffl, 2001; Schmittgen and Livak, 2008). Samples were normalized to Gapdh and calibrated to the median δ-cycle threshold of the DMSO females to determine the relative change in expression for each gene with treatment and by sex.
Table 1.
qPCR reagents.
| Gene | Primer/probe set | Lot # |
|---|---|---|
| Avp | Fam Rn00566449_m1 | 1504512 |
| Oxt | Fam Rn00563503_m1 | 1874285 |
| Avpr1a | Fam Rn00583910_m1 | 1713849 |
| Oxtr | Fam Rn00563503_m1 | 1874285 |
| Esr2 | Fam Rn00562610_m1 | 1863678 |
| Gapdh | Vic Rn01775763_g1 | P200716–007 H02 |
Statistical Analyses
For qPCR results and immunohistochemistry of total cell counts, data were analyzed using SPSS (v. 24). For each gene within each region, a two-way ANOVA (factors: sex, treatment) was run followed by Tukey’s post-hoc test if a significant main effect was found. In those cases where data were non-normally distributed, the non-parametric Kruskal-Wallis test was used followed by a Bonferroni post-hoc test if merited. An effect was considered significant at p < 0.05.
Immunohistochemical results across the rostral-caudal spectrum were analyzed to determine whether prenatal exposures to A1221 or EB changed the distribution of oxytocin- or vasopressin-immunoreactive cells. For oxytocin, the entire rostral-caudal profile was used (5 levels from Bregma −0.8 to −2.0). For vasopressin, the same five levels were used in the SON but in the PVN, only the three most caudal levels (Bregma −1.4, −1.7, −2.0) were used due to a near-absence of labeled cells in the two most rostral levels (−0.8, −1.1). We first evaluated whether there were any treatment effects at each level using the non-parametric Kruskal-Wallis test, finding no significant differences. Then, we used bootstrapping (10,000 bootstrap replicates per analysis) to evaluate differences in mean weighted cell count and cell density across treatment groups. For each sex, brain region, and nonapeptide, comparisons among the replicates were made for the three treatment groups [EB, A1221 (0.5), A1221 (1.0)] compared to DMSO. Male datasets were replicated to n = 10 per group and that of females to n = 11 per group to match the intended sampling design. Any missing values in the dataset due to tissue processing were also included in replicates. Replicates were discarded and redrawn if only missing values were contained in at least one brain section.
The mean weighted cell counts or cell density values were calculated for each replicate across sections. With the exception of oxytocin in the PVN, these means were then fitted to a quadratic function (y = ax2 + bx + c), where y is the mean weighted cell count or cell density and x is the brain section (distance from bregma). Coefficient a relates to concavity, b relates to horizontal shift, and c relates to vertical shift. The fitted values for coefficients a and b and constant c of the quadratic function were stored for every replicate. In other words, by comparing the fitted values to these coefficients and constants, it is possible to evaluate differences in the “shape” of cell distribution. We compared the “a” coefficient values of EB, low PCB, and high PCB treatment groups to those of DMSO by calculating the probability of their values being contained between the lowest and highest 2.5% of the DMSO coefficient values. In other words, we used the coefficients of each DMSO replicate to create a 95% confidence interval and counted how many times the “a” coefficients of other treatment groups fell within that range out of their 10,000 bootstrapped replications. In addition, histograms were generated for each of the four coefficients. The stored coefficient values represent sampling distributions from which we made group comparisons and calculated confidence intervals and p-values.
For oxytocin in the PVN, the quadratic function did not model the data, therefore, a cubic function was applied with the formula ax3 + bx2 + cx + d. In these cases, coefficient a relates to width and concavity, coefficients b and c relate to the x-axis intercepts, and constant d describes the vertical shift.
Results
Localization and numbers of oxytocin- and vasopressin-immunoreactive neurons
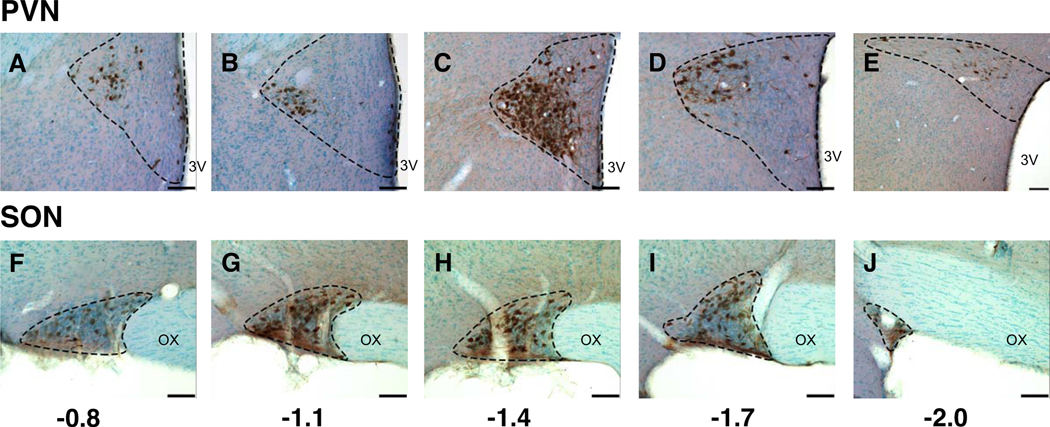
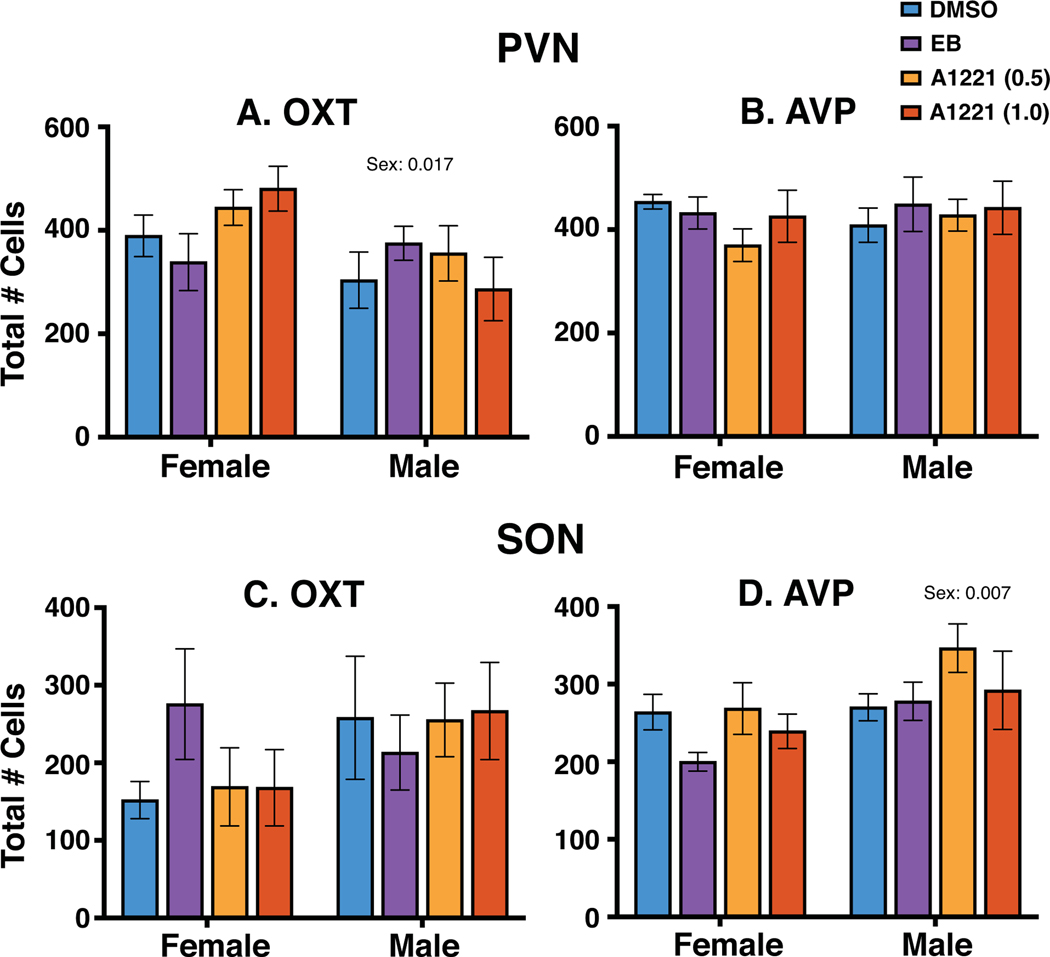
Oxytocin and vasopressin immunoreactive perikarya and fibers were detectable in the PVN and SON (Figure 1). A representative series of oxytocin-immunolabeled cells is shown from rostral to caudal for the PVN (Figure 2A–E) and SON (Figure 2F–J). A similar rostral-caudal series is shown for vasopressin-immunoreactive cells in the PVN (Figure 3A–E) and the SON (Figure 3F–J). Total numbers of oxytocin and vasopressin labeled cells for each region were counted within each rat. Results for all five sections combined are shown in Figure 4. In the PVN, there was a sex difference in oxytocin cell numbers (female > male, p = 0.017, Figure 4A), but no treatment effect or interactions. There were no significant sex or treatment effects for vasopressin cells in the PVN (Figure 4B).
Figure 1.
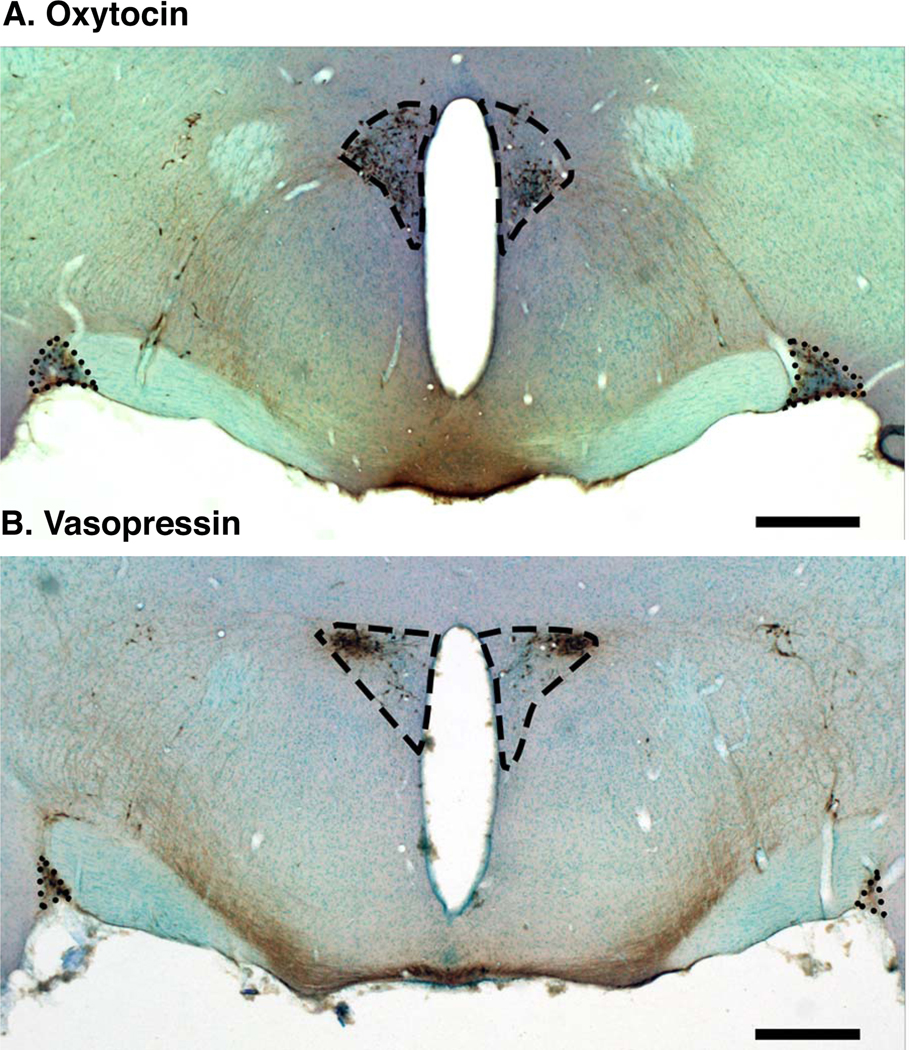
Low-magnification photomicrographs of coronal sections from a representative vehicle-treated male, showing oxytocin (A) and vasopressin (B) immunohistochemistry in the paraventricular nucleus (dashed outline) and the supraoptic nucleus (dotted outline). Scale bar = 500 μm.
Figure 2.
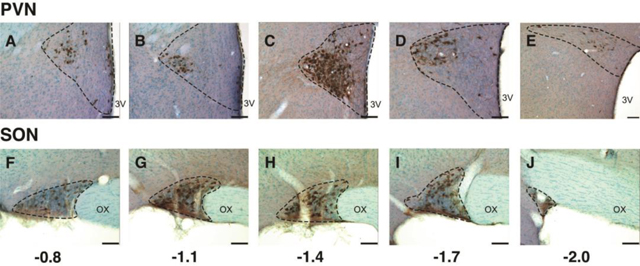
Representative photomicrographs showing oxytocin immunolabeling in a vehicle-treated female rat from rostral to caudal in the PVN (A through E) and SON (F through J). Rostral-caudal levels are shown relative to Bregma. Abbreviations: 3V, third ventricle; OX, optic chiasm. Scale bar = 100 μm.
Figure 3.
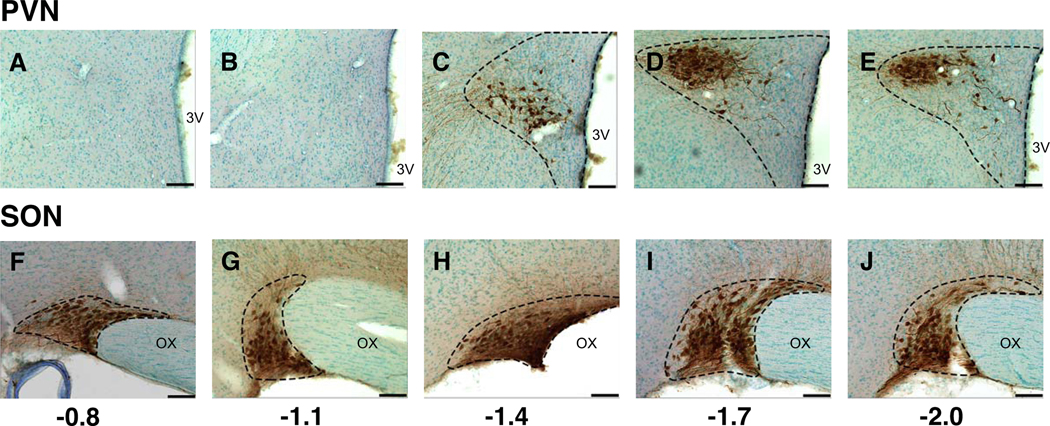
Representative photomicrographs showing vasopressin immunolabeling in a representative vehicle-treated female rat from rostral to caudal in the PVN (A through E) and SON (F through J). Rostral-caudal levels are shown relative to Bregma. Abbreviations: 3V, third ventricle; OX, optic chiasm. Scale bar = 100 μm.
Figure 4.
The total number of oxytocin- and vasopressin-immunoreactive cells in the PVN (top) and SON (bottom) were determined for all sections combined. There were no significant treatment effects on oxytocin or vasopressin cell numbers in either region. Sex differences were found for oxytocin in the PVN (female > male) and for vasopressin in the SON (male > female). Data shown are mean ± SEM.
In the SON, there were no significant effects of sex or treatment on oxytocin cell numbers, although it should be noted that this endpoint had the most variability within groups (Figure 4C). For vasopressin in the SON, while there was no treatment effect, there was a significant sex difference (male > female, p = 0.007, Figure 4D).
Rostral-caudal distribution of oxytocin-immunoreactive neurons
Oxytocin-immunoreactive cells were detectable across the full rostral-caudal extent of the series in both sexes (Figure 2). Because the distribution of oxytocin neurons in the PVN and SON is heterogeneous (Kania et al., 2020) we analyzed the rostral to caudal dataset using bootstrap statistics fitted to a quadratic (SON) or a cubic function (PVN) to determine whether prenatal treatment altered oxytocin distribution (see Methods for details). Data for the total number of cells counted are presented below; similar results were obtained for cell density (not shown).
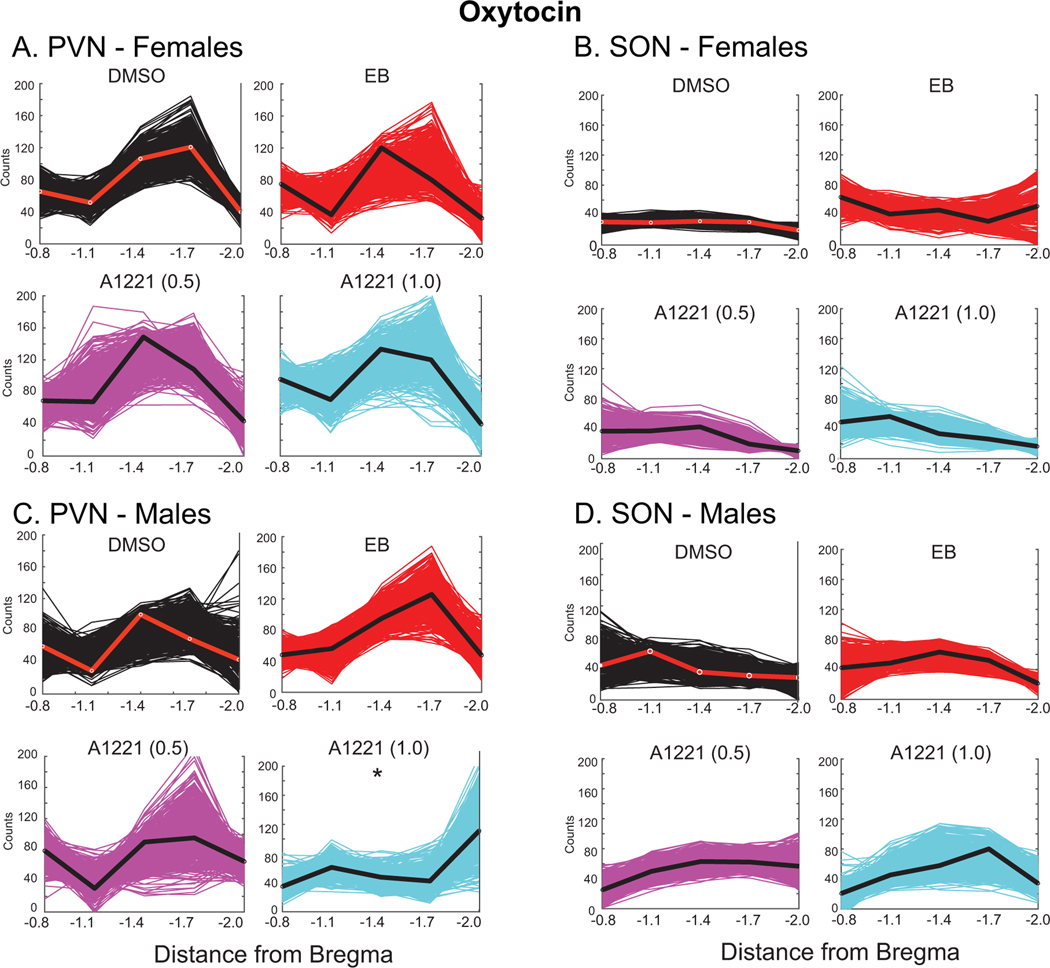
In the female PVN, as analyzed by bootstrap statistics, the oxytocin distribution was not significantly affected by the three treatments compared to DMSO (Figure 5A). The peak of the curve was at Bregma −1.7 in the DMSO group, and Bregma −1.4 for the three treatment groups, suggestive of some qualitative differences in distribution. In the female SON, the distributions of oxytocin did not differ, and there was greater variability in the replicates of the three treatment groups compared to DMSO (Figure 5B; note difference in width of the plots).
Figure 5.
Numbers of oxytocin-immunoreactive cells were counted from rostral to caudal in the PVN (A, C) and SON (B, D). We fit curves to 500 bootstrapped replicates (fits to the original datasets are indicated by red line for DMSO, and black line for EB, A1221 (0.5) and A1221 (1.0)). For oxytocin in the PVN, we fit a cubic function, and in the SON, we fit a quadratic. Statistical comparisons between the control DMSO curve with the other curves were conducted. The curve whose parameter was significantly different from the DMSO curve is designated with *.
In the male PVN, the PCB (1.0) group was significantly different in oxytocin distribution compared to the DMSO group (p = 0.024; Figure 5C). Although the shapes of other curves were qualitatively different, we did not reach a quantitative threshold of significance. In the male SON, comparisons with DMSO curves revealed no significant effect of treatment (Figure 5D).
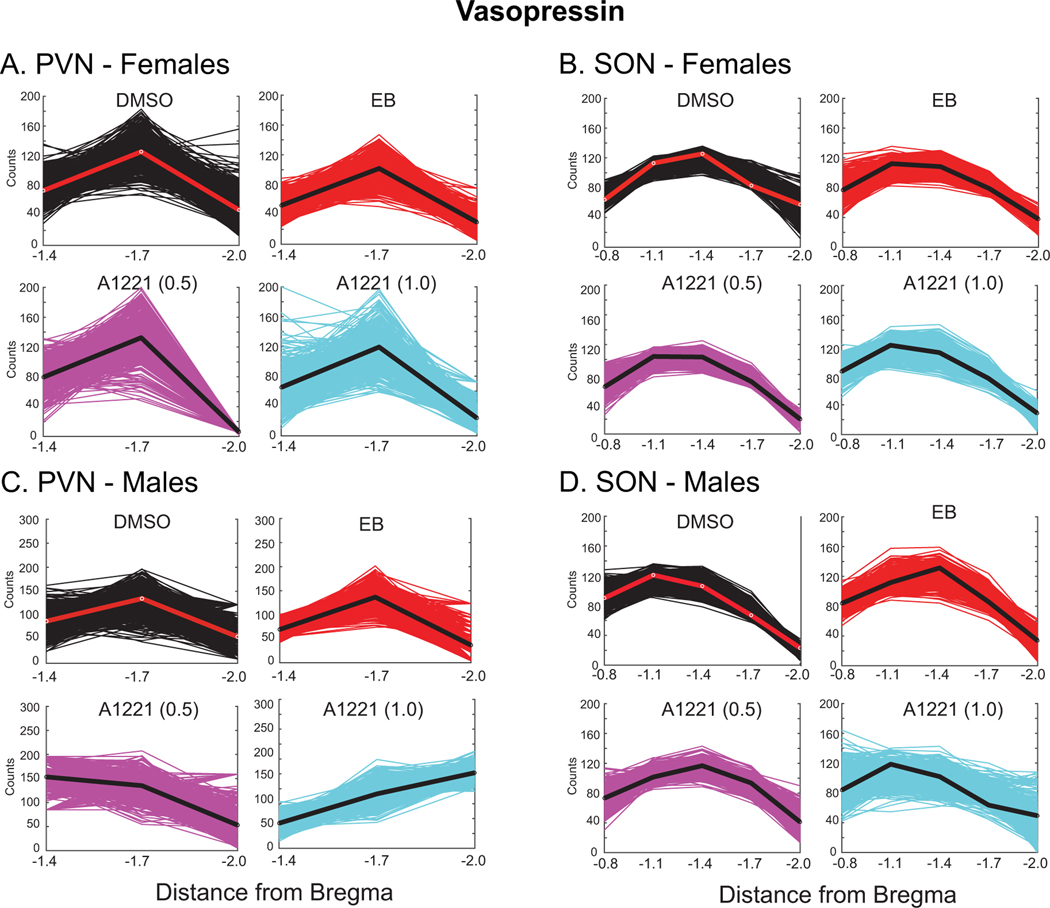
Rostral-caudal distribution of vasopressin-immunoreactive neurons
Vasopressin-immunoreactive cells in the PVN were more narrowly distributed in the PVN, with few cells in the two most rostral sections (Bregma levels −0.8 and −1.1; see Figure 3). Therefore, analyses were limited to the 3 caudal-most sections selected at Bregma = −1.4, −1.7, and −2.0. The vasopressin curve was modeled by a quadratic function in bootstrapping analyses. Mean vasopressin cell counts peaked at −1.7 for all treatment groups except for A1221 (1.0) in the male PVN, which peaked at Bregma −2.0 (Figure 6A, 6C). In the SON of males, vasopressin-immunoreactive cells were detectable across the entire rostral-caudal extent (see Figure 3). No significant treatment effects were found for either sex (Figure 6B; Figure 6D).
Figure 6.
Numbers of vasopressin-immunoreactive cells were counted from rostral to caudal in the PVN (A, C) and SON (B, D). We fit curves to 500 bootstrapped replicates (fits to the original datasets are indicated by red line for DMSO, and black line for EB, A1221 (0.5) and A1221 (1.0)). All curves in the PVN and SON were fit with a quadratic function. No significant differences between treatments and the DMSO groups were found.
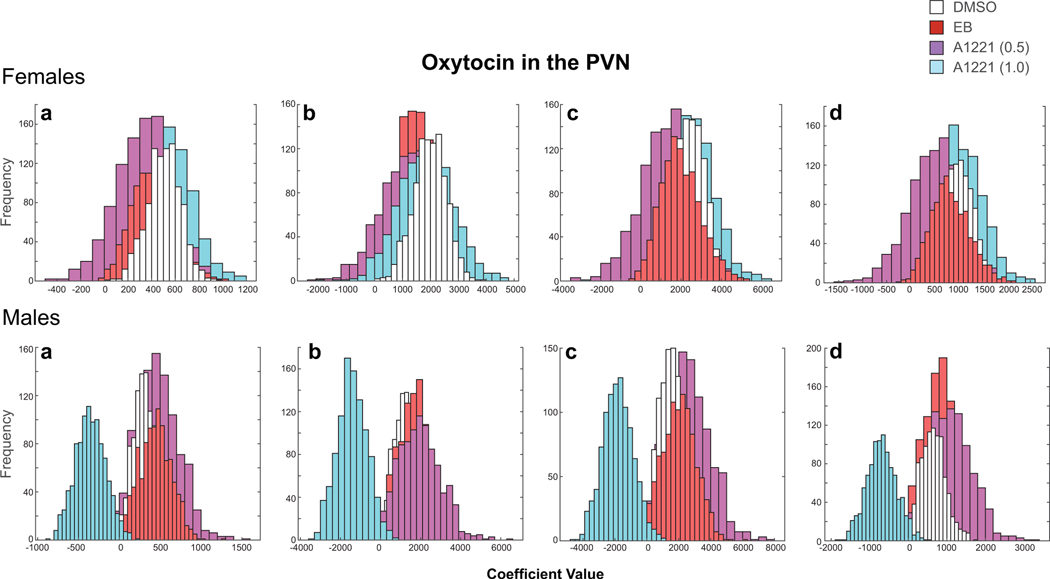
Figure 7 shows histograms of the four fitted coefficients (a, b, c) and constant (d) from the cubic formula for oxytocin in the PVN of each replicate. In females, all of the histograms showed a high degree of overlap for fitted values. In males, the A1221 (1.0) group is offset from the DMSO group. We utilized a 95% confidence interval created by eliminating the tail ends of the DMSO sampling distribution (each tail containing 2.5% of the fitted coefficient a values for that group). We then counted the number of times the A1221 (1.0) group had a fitted coefficient a value that fell within that range (p < 0.05). Our findings suggest a significant difference in the distribution of weighted mean oxytocin cell counts in the PVN between DMSO and A1221 (1.0) treated animals (Figure 7).
Figure 7.
An example of how histograms of the four fitted coefficients (a, b, c, d) from the cubic fits were used for statistical analysis. This analysis was undertaken for all of the distributions, and illustrated here for oxytocin in the PVN. These histograms represent sampling distributions, from which we made group comparisons and calculated p-values. The x axis shows the range of coefficient values assigned to the fitted curves of each replicate. The y axis shows the frequency at which these values occurred over the 10,000 replications. In females, all of the histograms overlapped for the coefficients and there were no significant treatment effects. In males, the A1221 (1.0) histogram (blue) is offset from the others, indicating that this group is dissimilar from the other groups in its distribution of oxytocin.
Gene expression of Oxt, Avp, Oxtr, Avpr1a, and Esr2
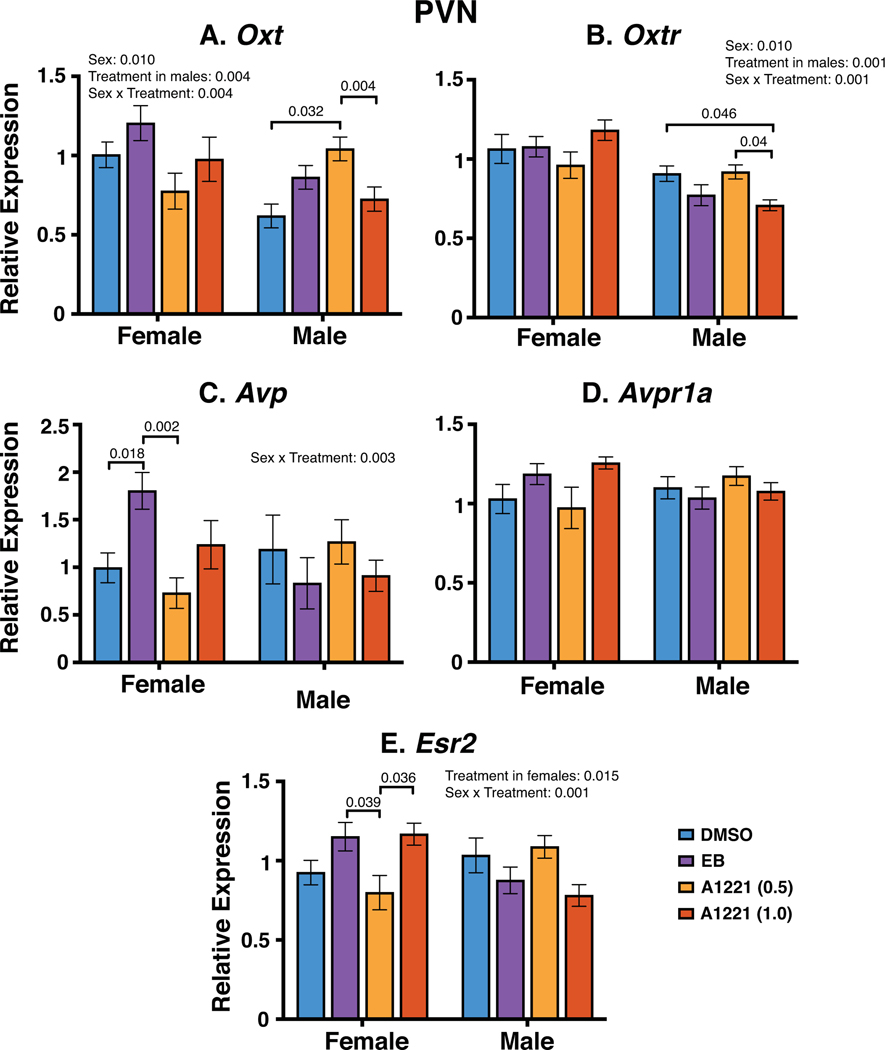
PVN:
Expression of five genes (Oxt, Oxtr, Avp, Avpr1a, Esr2) were quantified by qPCR (Figure 8A–E). For Oxt in the PVN, there was a significant sex dimorphism (female > male; p = 0.010), a significant treatment effect in males (A1221 (0.5) > vehicle, A1221 (1.0)), and a sex by treatment interaction (p = 0.004). For the latter, expression of Oxt in the A1221 (0.5) group was significantly higher than both vehicle and A1221 (1.0) in males. Oxtr was also sexually dimorphic (female > male, p = 0.010). There was a treatment effect in males (p = 0.001), and a sex by treatment interaction (p = 0.001). For the latter, the A1221 (1.0) group was lower than both vehicle and A1221 (0.5) in males. In the PVN, Avp had a significant sex by treatment interaction (p = 0.003), with the EB group higher than vehicle and A1221 (0.5) in females. No significant differences were detected for Avpr1a. The Esr2 gene was affected by treatment in females (p = 0.015) and there was a sex by treatment interaction (p = 0.001), with the A1221 (0.05) group lower than both EB and A1221 (1.0) of females.
Figure 8.
Gene expression of Oxt, Oxtr, Avp, Avpr1a and Esr2 was determined by qPCR in the PVN. Significant main effects and interactions are indicated, with post-hoc values shown with brackets. Data are shown as mean ± SEM.
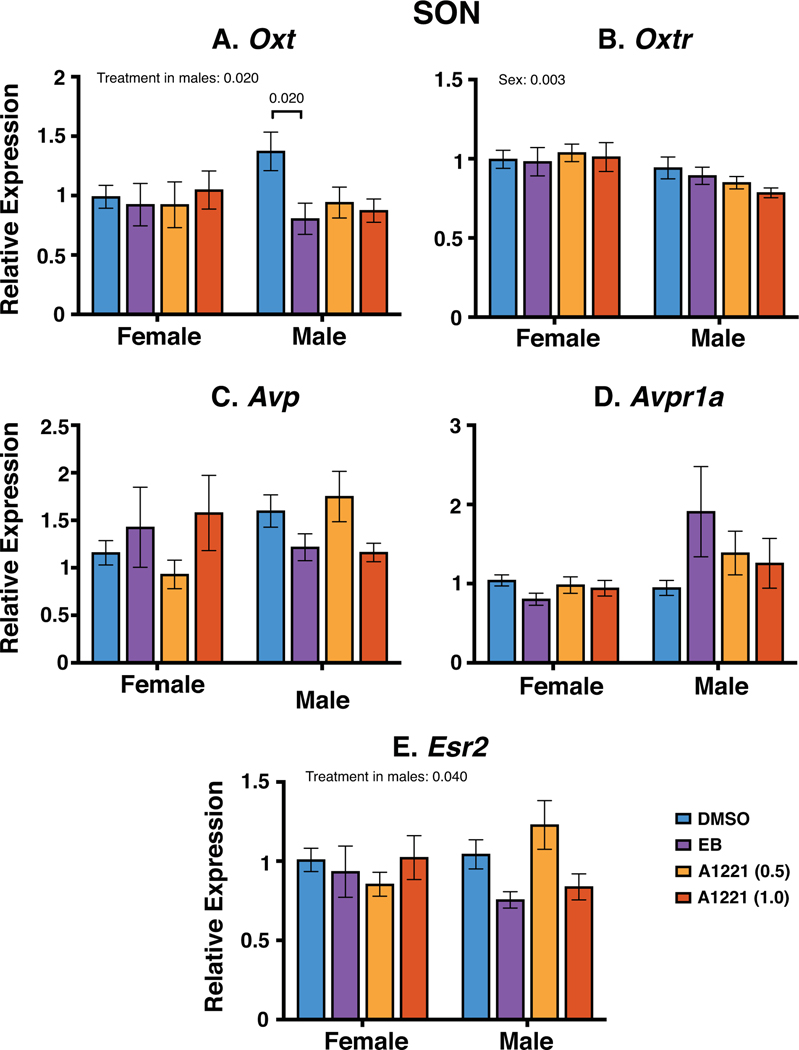
SON:
The same five genes were quantified in the SON (Figure 9). One gene (Oxtr) was sexually dimorphic (female > male; p = 0.003). Oxt was affected by treatment in males (p = 0.02) with the vehicle group significantly higher than the EB group. Esr2 had a significant treatment effect in males but post hoc analysis only revealed a marginal difference between groups (A1221 (0.5) > EB, p = 0.064). Avp and Avpr1a did not differ by sex, treatment, or their interactions.
Figure 9.
Gene expression of Oxt, Oxtr, Avp, Avpr1a and Esr2 as determined by qPCR in the SON. Significant main effects and interactions are indicated, with post-hoc values shown with brackets. Data are shown as mean ± SEM.
Discussion
Endocrine-disrupting chemicals, including PCBs, affect social and affective behaviors in rats (Bell et al., 2016a; Bell et al., 2016b; Gillette et al., 2017; Reilly et al., 2018; Reilly et al., 2015), but the neural circuits acted upon by EDCs to change behavior are not well-understood. Here, we evaluated whether and how prenatal exposures to EDCs affected protein and gene expression of oxytocin and vasopressin systems of the PVN and SON, with these populations selected because of both their importance in social behaviors and their steroid hormone sensitivity (Clipperton-Allen et al., 2012; Spiteri and Agmo, 2009). We found small but significant changes to the nonapeptide protein and genes, and in the case of gene expression, effects of treatment on expression of other genes involved in oxytocin and vasopressin signaling and regulation. These results add to knowledge about neurobiological substrates underlying behavioral changes reported previously (Bell et al., 2016a; Bell et al., 2016b; Gillette et al., 2017; Reilly et al., 2018; Reilly et al., 2015).
Few prior studies have been published on PCB effects on the oxytocin and vasopressin neuronal populations in the PVN and/or SON. Another PCB mixture, Aroclor 1254 (A1254), was studied for its effects on the central vasopressin system’s role in the control of osmoregulation in an adult male rat model of dehydration. A1254 abrogated vasopressin release in SONs ex vivo but enhanced plasma vasopressin (Coburn et al., 2005). Early postnatal chlorpyrifos, a pesticide, increased oxytocin and decreased vasopressin peptide in whole hypothalamus in a dose-dependent manner (Tait et al., 2009). In another study, neonatal bisphenol A increased oxytocin cell numbers in the PVN of female rats (Adewale et al., 2011). BPA had both direct (F1) and transgenerational effects on vasopressin populations, especially in the amygdala of mice (Goldsby et al., 2017). In the female prairie vole, postnatal exposure to BPA changed both vasopressin- and oxytocin-immunoreactive neurons in the PVN (Sullivan et al., 2014). As a whole, these reports suggest the vulnerability of the hypothalamic nonapeptide systems to EDCs.
Our present study extends prior work in several ways. In contrast to other studies, our exposure period was limited to embryonic days 16 and 18, which is the beginning of the critical period of brain sexual differentiation of rats (Arnold and Gorski, 1984). This is also a time when oxytocin and vasopressin neurons are developing (Bloch et al., 1990; Jing et al., 1998; Lipari et al., 2001; Sinding et al., 1980). Buijs et al. reported that vasopressin content in the brain of developing rats increased beginning on E16, and that oxytocin content decreased from E16 to E18 but increased thereafter (Buijs et al., 1980). It is also notable that neurogenesis in the hypothalamus of rodents occurs from mid to late gestation (Cheung et al., 2013; Hernandez Scudder et al., 2020a; Shimada and Nakamura, 1973). Therefore, our exposure window overlaps several key neurodevelopmental events involved in the generation and organization of hypothalamic neural circuits, including those involved in the control of social behaviors.
Although transient prenatal exposure to A1221 or EB did not change the overall numbers of vasopressin or oxytocin neurons in either hypothalamic region, there was one example of a significant shift in profile (oxytocin in the PVN of male A1221 (1.0) rats). We also noticed that the location of the peak number of mean neurons often differed among groups, albeit non-significantly, for oxytocin in the PVN of females and males, vasopressin in the PVN of males, and vasopressin in the SON of males. When we first designed this study we had the a priori hypothesis that this might be the case based on evidence that in the PVN and SON, numbers and density of oxytocin and vasopressin neurons are not uniformly distributed and that there may be subpopulations of cells that receive different afferents or make different efferents depending upon localization [see Figures 1–3; (Kania et al., 2020; Rhodes et al., 1981; Swaab et al., 1975)]. We do not wish to over-interpret this type of qualitative observation, but we think that this finding suggests further research to extend this type of study, using larger sample sizes. To our knowledge, only one other study has addressed whether early life EDCs causes population changes to hypothalamic vasopressin and oxytocin populations. Using prairie voles as a model, Sullivan et al. evaluated vasopressin and oxytocin cell numbers in the rostral, medial, and caudal PVN (Sullivan et al., 2014). They showed that postnatal exposure to BPA increased vasopressin-immunoreactive neuron numbers in the rostral PVN, and decreased oxytocin neurons in the caudal PVN. Thus, BPA in the prairie vole affected a selected population of neurons in a sex-dependent manner.
This study also enabled us to compare expression of the oxytocin and vasopressin genes and proteins. Although there were no significant differences in total immunoreactive cell counts for oxytocin or vasopressin in either PVN or SON, gene expression of Oxt was affected by treatment in the PVN of males (highest in A1221 (0.5)), and Avp was affected in the PVN of females (highest in EB). In the SON, there was a significant treatment effect for Oxt in males (highest in DMSO) and no effect for Avp in either sex. The finding that gene expression changes are not reflected by total cell counts is not entirely surprising, as transcriptional, post-transcriptional, translational, and post-translational modifications all affect protein levels. Thus, the gene expression changes seen in the current study may be compensated for by any of these molecular processes. It is also notable that effects of EB did not mirror those of A1221. Although generally considered a weakly estrogenic class of EDCs (Frame, 1997), A1221 is not a pure estrogen receptor agonist/antagonist and signals through other pathways. Our previous work looking at other gene and protein endpoints demonstrate that some, but not all effects of A1221 are similar to those of EB as reported for gene expression in the preoptic area and the ventromedial nucleus, anxiety-like behaviors, and ultrasonic vocalizations, among others (Dickerson et al., 2011a; Gillette et al., 2017; Topper et al., 2019).
As a whole, these data suggest that early life EDC exposures influence the hypothalamic vasopressin and oxytocin systems in a sex, region, and treatment-specific manner. Although the observed effects were small, it is important to consider the current results in the greater context of other neuroendocrine effects of prenatal PCBs. The control of complex behaviors involves the coordination of multiple cell types within multiple brain regions, together with peripheral hormones and external influences (the social system, the nature of the environment, etc.). Small changes in many parts of the neural circuit instigated by environmental EDCs can lead to biologically-relevant behavioral and functional outcomes.
Supplementary Material
Acknowledgments
Grant Support: NIH RO1 ES023254 and RO1 ES029464
Footnotes
Data Availability: Data will be made available upon request.
Conflict of interest: Authors declare no competing interests.
REFERENCES
- Adewale HB, et al. (2011). The impact of neonatal bisphenol-A exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology, 32(1), 38–49. 10.1016/j.neuro.2010.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP and Gorski RA (1984). Gonadal steroid induction of structural sex differences in the central nervous system. Ann Rev Neurosci, 7, 413–442. [DOI] [PubMed] [Google Scholar]
- Auger CJ and De Vries GJ (2002). Progestin receptor immunoreactivity within steroid-responsive vasopressin-immunoreactive cells in the male and female rat brain. J Neuroendocrinol, 14(7), 561–567. 10.1046/j.1365-2826.2002.00809.x [DOI] [PubMed] [Google Scholar]
- Bell MR, et al. (2016a). Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 2. Sex-specific neuromolecular effects in the brain. Mol Cell Endocrinol, 420, 125–137. 10.1016/j.mce.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, et al. (2016b). Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 1. Sexually dimorphic effects on social and anxiety-like behaviors. Horm Behav, 78, 168–177. 10.1016/j.yhbeh.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, et al. (1994). Search for progestin receptors (PR) in prolactin-releasing peptidergic neurons: oxytocin neurons lack PR, but respond to gonadal steroids in monkeys. Endocrinology, 134(2), 945–953. 10.1210/endo.134.2.8299589 [DOI] [PubMed] [Google Scholar]
- Bielsky IF, et al. (2005). The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron, 47(4), 503–513. 10.1016/j.neuron.2005.06.031 [DOI] [PubMed] [Google Scholar]
- Bloch B, et al. (1990). Presence of neuropeptide messenger RNAs in neuronal processes. Neuroscience Letters, 109, 259–264. [DOI] [PubMed] [Google Scholar]
- Braun JM, et al. (2014). Gestational Exposure to Endocrine-Disrupting Chemicals and Reciprocal Social, Repetitive, and Stereotypic Behaviors in 4- and 5-Year-Old Children: The HOME Study. Environmental health perspectives. 10.1289/ehp.1307261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, et al. (1980). Ontogeny of vasopressin and oxytocin in the fetal rat: early vasopressinergic innervation of the fetal brain. Peptides, 1(4), 315–324. [DOI] [PubMed] [Google Scholar]
- Cheung CC, et al. (2013). Genetic labeling of steroidogenic factor-1 (SF-1) neurons in mice reveals ventromedial nucleus of the hypothalamus (VMH) circuitry beginning at neurogenesis and development of a separate non-SF-1 neuronal cluster in the ventrolateral VMH. Journal of Comparative Neurology, 521(6), 1268–1288. 10.1002/cne.23226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, et al. (2003). An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci U S A, 100(10), 6192–6197. 10.1073/pnas.0631699100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, et al. (2006). Involvement of estrogen receptor α, β and oxytocin in social discrimination: a detailed behavioral analysis with knockout female mice. Genes, Brain and Behavior, 5(7), 528–539. 10.1111/j.1601-183X.2006.00203.x [DOI] [PubMed] [Google Scholar]
- Clipperton-Allen AE, et al. (2012). Oxytocin, vasopressin and estrogen receptor gene expression in relation to social recognition in female mice. Physiol Behav, 105(4), 915–924. 10.1016/j.physbeh.2011.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn CG, et al. (2007). Polybrominated Diphenyl Ethers and ortho-Substituted Polychlorinated Biphenyls as Neuroendocrine Disruptors of Vasopressin Release: Effects during Physiological Activation In Vitro and Structure–Activity Relationships. Toxicological Sciences, 98(1), 178–186. 10.1093/toxsci/kfm086 [DOI] [PubMed] [Google Scholar]
- Coburn CG, et al. (2005). Dietary exposure to aroclor 1254 alters central and peripheral vasopressin release in response to dehydration in the rat. Toxicol Sci, 84(1), 149–156. [DOI] [PubMed] [Google Scholar]
- Colciago A, et al. (2009). Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat. Part 2: Effects on reproductive parameters, on sex behavior, on memory retention and on hypothalamic expression of aromatase and 5alpha-reductases in the offspring. Toxicol Appl Pharmacol, 239, 46–54. [DOI] [PubMed] [Google Scholar]
- Cummings JA, et al. (2008). Exposure to PCB 77 affects partner preference but not sexual behavior in the female rat. Physiology & behavior, 95(3), 471–475. 10.1016/j.physbeh.2008.07.016 [DOI] [PubMed] [Google Scholar]
- Dickerson SM, et al. (2011a). Prenatal PCBs disrupt early neuroendocrine development of the rat hypothalamus. Toxicol Appl Pharmacol, 252(1), 36–46. 10.1016/j.taap.2011.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, et al. (2011b). Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology, 152(2), 581–594. 10.1210/en.2010-1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM and Gore AC (2007). Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord, 8(2), 143–159. [DOI] [PubMed] [Google Scholar]
- Driowo MA, et al. (1980). A comparison of the pharmacokinetic properties of three estradiol esters. Contraception, 21, 415–424. [DOI] [PubMed] [Google Scholar]
- Frame G. (1997). A collaborative study of 209 PCB congeners and 6 Aroclors on 20 diffrent HRGC columns, 2. Semi-quantitative Aroclor congener distributions. Fresenius J Anal Chem, 357, 714–722. [Google Scholar]
- Francis K, et al. (2002). Progesterone receptor expression in the pregnant and parturient rat hypothalamus and brainstem. Brain Res, 927(1), 18–26. [DOI] [PubMed] [Google Scholar]
- Gillette R, et al. (2017). Anxiety-like behaviors in adulthood are altered in male but not female rats exposed to low dosages of polychlorinated biphenyls in utero. Horm Behav, 87, 8–15. 10.1016/j.yhbeh.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsby JA, et al. (2017). Multi- and Transgenerational Consequences of Bisphenol A on Sexually Dimorphic Cell Populations in Mouse Brain. Endocrinology, 158(1), 21–30. 10.1210/en.2016-1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, et al. (2015). EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev, 36(6), E1–e150. 10.1210/er.2015-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Scudder ME, et al. (2020a). Exposure to prenatal PCBs shifts the timing of neurogenesis in the hypothalamus of developing rats. J Exp Zool A Ecol Integr Physiol, 333(8), 550–560. 10.1002/jez.2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Scudder ME, et al. (2020b). Prenatal EDCs impair mate and odor preference and activation of the VMN in male and female rats. Endocrinology, 161(9). 10.1210/endocr/bqaa124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL and Jacobson SW (1997). Evidence for PCBs as neurodevelopmental toxicants in humans. Neurotoxicology, 18(2), 415–424. [PubMed] [Google Scholar]
- Jing X, et al. (1998). Ontogeny of the vasopressin and oxytocin RNAs in the mouse hypothalamus. Neurosci Res, 30(4), 343–349. 10.1016/s0168-0102(98)00017-0 [DOI] [PubMed] [Google Scholar]
- Jones BA, et al. (2011). Pre- and postnatal bisphenol A treatment results in persistent deficits in the sexual behavior of male rats, but not female rats, in adulthood. Hormones and behavior, 59(2), 246–251. 10.1016/j.yhbeh.2010.12.006 [DOI] [PubMed] [Google Scholar]
- Kanaya M, et al. (2019). Neurochemical Characterization of Neurons Expressing Estrogen Receptor β in the Hypothalamic Nuclei of Rats Using in Situ Hybridization and Immunofluorescence. Int J Mol Sci, 21(1). 10.3390/ijms21010115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania A, et al. (2020). Electrophysiology and distribution of oxytocin and vasopressin neurons in the hypothalamic paraventricular nucleus: a study in male and female rats. Brain Structure and Function, 225(1), 285–304. 10.1007/s00429-019-01989-4 [DOI] [PubMed] [Google Scholar]
- Kermath BA, et al. (2014). Hypothalamic molecular changes underlying natural reproductive senescence in the female rat. Endocrinology, 155(9), 3597–3609. 10.1210/en.2014-1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, et al. (2008). A conditional knockout mouse line of the oxytocin receptor. Endocrinology, 149(7), 3256–3263. 10.1210/en.2007-1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, et al. (2015). In Utero Exposure to Diethylhexyl Phthalate Affects Rat Brain Development: A Behavioral and Genomic Approach. Int J Environ Res Public Health, 12(11), 13696–13710. 10.3390/ijerph121113696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipari EF, et al. (2001). The hypothalamic magnocellular neurosecretory system in developing rats. Eur J Histochem, 45(2), 163–168. 10.4081/1626 [DOI] [PubMed] [Google Scholar]
- Monje L, et al. (2009). Neonatal exposure to bisphenol A alters estrogen-dependent mechanisms governing sexual behavior in the adult female rat. Reproductive toxicology, 28(4), 435–442. 10.1016/j.reprotox.2009.06.012 [DOI] [PubMed] [Google Scholar]
- Oyola MG, et al. (2017). Distribution and chemical composition of estrogen receptor β neurons in the paraventricular nucleus of the female and male mouse hypothalamus. J Comp Neurol, 525(17), 3666–3682. 10.1002/cne.24295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos (1986). The rat brain in stereotaxic coordinates. San Diego, CA, Academic Press. [Google Scholar]
- Pfaffl MW (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res, 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrini S, et al. (2005). Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Research Bulletin, 65(3), 261–266. 10.1016/j.brainresbull.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Reilly MP, et al. (2018). Application of a Novel Social Choice Paradigm to Assess Effects of Prenatal Endocrine-Disrupting Chemical Exposure in Rats (Rattus norvegicus). J Comp Psychol, 132, 253–267. 10.1037/com0000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly MP, et al. (2015). The effects of prenatal PCBs on adult social behavior in rats. Horm Behav, 73, 47–55. 10.1016/j.yhbeh.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CH, et al. (1981). Immunohistochemical analysis of magnocellular elements in rat hypothalamus: distribution and numbers of cells containing neurophysin, oxytocin, and vasopressin. J Comp Neurol, 198(1), 45–64. 10.1002/cne.901980106 [DOI] [PubMed] [Google Scholar]
- Ross HE and Young LJ (2009). Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol, 30(4), 534–547. 10.1016/j.yfrne.2009.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD and Livak KJ (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc, 3(6), 1101–1108. [DOI] [PubMed] [Google Scholar]
- Shah A, et al. (2011). Altered cardiovascular reactivity and osmoregulation during hyperosmotic stress in adult rats developmentally exposed to polybrominated diphenyl ethers (PBDEs). Toxicology and applied pharmacology, 256(2), 103–113. 10.1016/j.taap.2011.07.014 [DOI] [PubMed] [Google Scholar]
- Shimada M. and Nakamura T. (1973). Time of neuron origin in mouse hypothalamic nuclei. Experimental Neurology, 41(1), 163–173. 10.1016/0014-4886(73)90187-8 [DOI] [PubMed] [Google Scholar]
- Sinding C, et al. (1980). Levels of neurohypophyseal peptides in the rat during the first month of life. I. Basal levels in plasma, pituitary, and hypothalamus. Endocrinology, 107(3), 749–754. 10.1210/endo-107-3-749 [DOI] [PubMed] [Google Scholar]
- Spiteri T. and Agmo A. (2009). Ovarian hormones modulate social recognition in female rats. Physiol Behav, 98(1–2), 247–250. 10.1016/j.physbeh.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Steinberg RM, et al. (2007). The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm Behav, 51, 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AW, et al. (2014). A novel model for neuroendocrine toxicology: neurobehavioral effects of BPA exposure in a prosocial species, the prairie vole (Microtus ochrogaster). Endocrinology, 155(10), 3867–3881. 10.1210/en.2014-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom G, Hutzinger O, and Safe S. (1976). The metabolism of chlorobiphenyls: a review. Chemosphere, 5, 267–298. [Google Scholar]
- Swaab DF, et al. (1975). Immunofluorescence of vasopressin and oxytocin in the rat hypothalamo-neurohypophypopseal system. J Neural Transm, 36(3–4), 195–215. 10.1007/bf01253126 [DOI] [PubMed] [Google Scholar]
- Tait S, et al. (2009). Long-term effects on hypothalamic neuropeptides after developmental exposure to chlorpyrifos in mice. Environmental health perspectives, 117(1), 112–116. 10.1289/ehp.11696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, et al. (2005). Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A, 102(44), 16096–16101. 10.1073/pnas.0505312102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper VY, et al. (2019). Social and neuromolecular phenotypes are programmed by prenatal exposures to endocrine-disrupting chemicals. Mol Cell Endocrinol, 479, 133–146. 10.1016/j.mce.2018.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, et al. (2016). Pubertal exposure to di-(2-ethylhexyl) phthalate influences social behavior and dopamine receptor D2 of adult female mice. Chemosphere, 144, 1771–1779. 10.1016/j.chemosphere.2015.10.062 [DOI] [PubMed] [Google Scholar]
- Winneke G, et al. (1998). Developmental neurotoxicity of polychlorinated biophenyls (PCBS): cognitive and psychomotor functions in 7-month old children. Toxicology Letters, 102–103, 423–428. [DOI] [PubMed] [Google Scholar]
- Zhou L, et al. (1994). Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology, 134(6), 2622–2627. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, et al. (2012). Endocrine-Disrupting Chemicals and Public Health Protection: A Statement of Principles from The Endocrine Society. Endocrinology, 153, 4097–4110. 10.1210/en.2012-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.