Abstract
Optimal glycemic control in kidney transplant recipients with diabetes is associated with improved morbidity, mortality and allograft survival. Transplant options for patients with diabetes requiring insulin therapy and chronic kidney disease who are suitable candidates for kidney transplantation should include consideration of β-cell replacement therapy: pancreas or islet transplantation. International variation, related to national regulatory policies, exists in offering one or both of these options to suitable candidates, and is further affected by pancreas/islet allocation policies and waiting list dynamics. Selection of appropriate candidates depends on patient age, co-existent morbidities, timing of referral to the transplant center (pre-vs. on dialysis) and availability of living kidney donors. Early referral is therefore of the utmost importance (ideally when eGFR is <30 ml/min/1.73 m2), to ensure adequate time for informed decision making and thorough pre-transplant evaluation. Obesity, CVD, peripheral vascular disease, smoking, and frailty are some of the conditions that need to be considered prior to acceptance on the transplant list, and ideally prior to dialysis becoming imminent. This review offers insights into selection of pancreas/islet transplant candidates by transplant centers and an update on post-transplant outcomes, which may have practice implications for referring nephrologists.
Introduction
Patients with diabetes mellitus and advanced kidney disease require special consideration for possible β-cell replacement at the time of or following kidney transplantation. Two established forms of β-cell replacement therapy are whole pancreas1 and isolated islet cell2 transplantation. Either pancreas or islet transplantation may be performed simultaneously with or after kidney transplantation for improving glycemic control, eliminating problematic hypoglycemia, improving quality of life, and/or ameliorating the course of diabetes related complications including kidney graft damage. This review aims to provide an international perspective to these strategies with consideration for patient selection and anticipated outcomes and proposes an algorithm for identifying individuals appropriate for consideration of β-cell replacement therapy in conjunction with kidney transplantion. Pancreas and islet transplantation in non-uremic patients with type 1 diabetes (T1D) complicated by hypoglycemia unawareness has been the topic of a prior review.3
Pancreas or Islet Transplantation
The last decade has seen a significant decline in the numbers of pancreas transplants performed, especially pancreas after kidney transplantation (PAK) in the US and Europe.4–6 Possible reasons include decreased referrals due to technological advances in T1D therapy, specifically automated insulin delivery systems, and changing demographics of potential recipients (older age, higher BMI, more advanced cardiovascular disease). There is a higher prevalence of obese donors which influences pancreas utilisation due to increased surgical risks. Ironically, the increasing number of pancreata from high BMI donors may be better suited for islet isolation and transplantation,7 however, this option is not uniformly available around the world.
At the present time pancreas transplantation provides the best long-term outcomes with regard to insulin-independence, metabolic control, and stabilization or improvement of secondary complications. While both pancreas and islet transplantation may be options with T1D, whole organ pancreas transplantation is only being performed in selected individuals with insulin-dependent type 2 diabetes (T2D). Optimal β-cell graft function is defined as near-normal glycemic control (HbA1c≤6.5%) without severe hypoglycemia or requirement for insulin or other antihyperglycemic therapy, and with an increase in C-peptide from pre-trasplantation, while absence of clinically significant C-peptide production (<0.6 ng/mL [0.200 pmol/mL] stimulated) may indicate a failed β-cell graft.8Additionally, OPTN/UNOS defines pancreas graft failure as pancreas transplant removal, subsequent registration for pancreas or islet transplant or recipient death, or as insulin requirements equal to or greater than 0.5 units/kg/day for 90 consecutive days.9
Pancreas transplantation is typically performed intraperitoneally with arterial inflow from the right iliac artery and the venous drainage systemically into the inferior vena cava or the portal venous system.1 The pancreatic exocrine secretions are drained enterically, and occasionally by bladder drainage (Figure 1). Complications may include graft vascular thrombosis (approximately 5%), reperfusion pancreatitis, hemorrhage, anastomotic leaks, fluid collections, small bowel obstruction, and wound complications; up to 40% may need re-operation.10 This potential surgical morbidity precludes offering pancreas transplantation to significant numbers of patients with diabetes and advanced kidney disease. Morbidity is greater in older patients and those who have advanced cardiovascular disease (CVD) or peripheral vascular disease (PVD).10 Since the pancreas is preferably placed in the right lower abdomen, the kidney should be placed contralaterally, an important consideration for those receiving a kidney transplant alone (KTA) that may be followed by a PAK in the future.
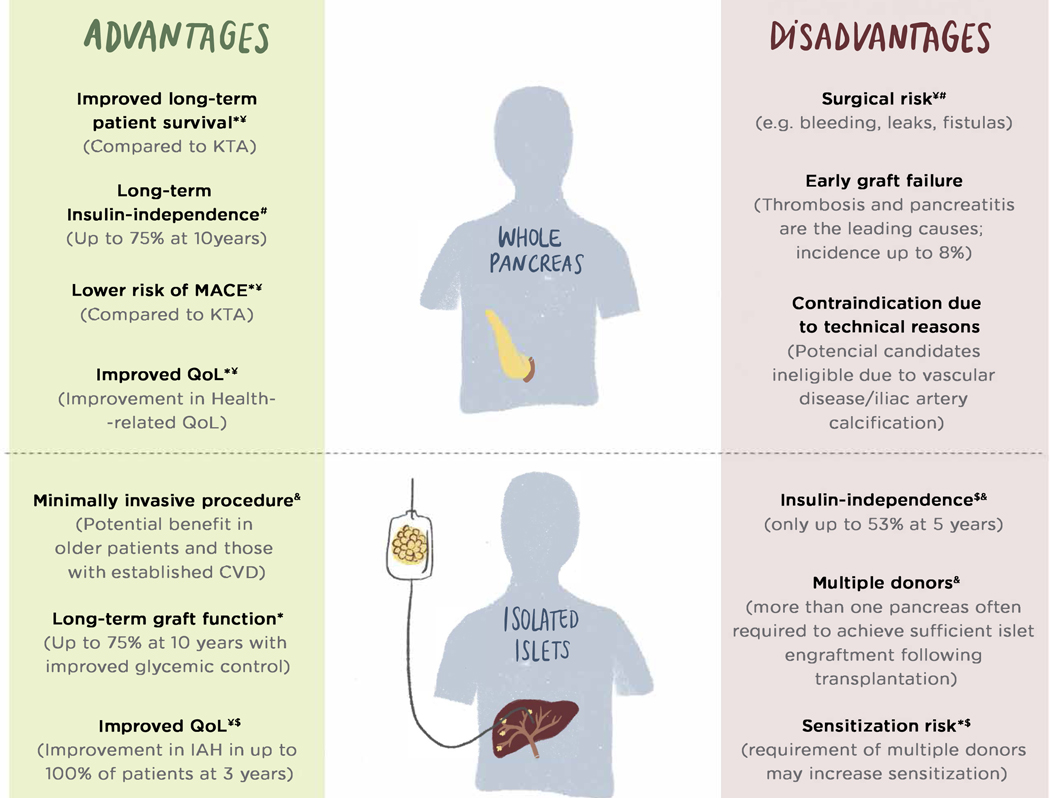
Figure 1 –
Diagram of clinical β-cell replacement treatment alternatives, highlighting major advantages and disadvantages of each procedure.
* Data from observational retrospective single center non-randomized or registries studies.
$ Data from randomized clinical trials
¥ Compared to Kidney Transplant Alone
# Compared to islet transplantation
& Compared to Pancreas Transplantation
BMI – Body Mass Index; CVD – Cardiovascular disease; IAH – Impaired hypoglycemia awareness; KTA – Kidney Transplant Alone ; MACE – Major Adverse Cardiovascular Events; QoL – Quality of Life
In contrast, islet transplantation is a relatively low risk procedure, during which purified islet cells are infused into the portal vein either through a percutaneous transhepatic catheter or mini-laparatomy and then engraft in the liver (Figure 1).2, 11 Complications of islet transplantation are infrequent and include portal branch vein thrombosis in <5% and bleeding in <10% of infusion procedures if the percutaneous route is used.2 In simultaneous islet-kidney transplantation (SIK), islets are usually transplanted within 72 hours after the kidney graft, to allow for the separation of islet infusion from induction with high-dose glucocorticoids and/or T-cell depletion with resulting cytokine release, both potentially detrimental to islet survival.2
Limitations to islet transplantation include the need for more than one islet infusion from sequential donors (2–3) to achieve an adequate engrafted islet mass for insulin-independence and maintenance of long-term metabolic control. From CITR registry data, 71% of all recipients of islet transplantation required 2 or more islet infusions.12 Important predictors of insulin-independence following single-donor islet transplantation are pre-transplant insulin requirements, peri-transplant use of heparin and insulin,13 and the number of infused islets.14–16 Post-infusion hypoxia and the inflammatory response affect islet cell survival prior to revascularization by the hepatic arterial system. The use of multiple donors may increase the risk for sensitization against HLA. In a phase 3 single cohort study of IAK transplantation involving 24 subjects, the rate of de novo sensitization was up to 22% (5/23) over three years,17 similar to that reported in simultaneous pancreas-kidney transplantation (SPK) (21.3%).18
Outcomes between receiving a pancreas or islets with kidney transplantation in T1D were compared in a non-randomized single center retrospective analysis.19 The 5-year insulin independence rate was higher with SPK/PAK (73.6 vs 9.3% with SIK/IAK) and post-transplant HbA1c levels were lower (7.8 to 5.9% vs 8.0 to 6.5% with SIK/IAK).19 Although insulin dosage could only be decreased in <20% of SIK/IAK recipients, there was a significant improvement in HbA1c and severe hypoglycemic events were significantly reduced to rates similar to that observed with SPK/PAK.19 Importantly, procedure related complications were significantly less with islet compared to pancreas transplantation (re-laparotomy 10.5% vs 41.5%, respectively), and kidney allograft function shared similar low rates of eGFR decline in both pancreas and islet groups.19
Regulations governing cellular product manufacturing currently limit access to islet transplantation to clinical trials in the US. In contrast islet transplantation is performed and reimbursed in many other countries such as Australia, Belgium, Canada, Czech Republic, France, Italy, Japan, Netherlands, Norway, Poland, Switzerland, Sweden, and the United Kingdom.20
Patient Selection and Assessment
In general, pancreas transplantation is considered primarily in younger patients with insulin-dependent diabetes without major cardiovascular or surgical risks who require a kidney transplant, while islet transplantation may be an alternative in older patients with co-existing comorbidities. Carefully selected older recipients, however, may be considered for pancreas transplantation, especially in countries where islet transplantation is not available.21, 22 Older individuals may increasingly present as transplant candidates as modern approaches to diabetes treatment and more comprehensive management of risk factors including hypertension, hyperlipidemia and proteinuria have delayed progression to end-stage kidney disease (ESKD) in T1D by at least 10 years compared to previously reported cohorts. Several important factors determine patient selection, including the type of diabetes, degree of kidney dysfunction, degree of glycemic instability and hypoglycemia, disease and treatment burden, magnitude of obesity and insulin requirements, and the presence of comorbidities.
Diabetes Type
Insulin-dependent diabetes resulting from β-cell failure that may be addressed by β-cell replacement is typically T1D, but may also include selected cases of T2D and/or other types of diabetes characterized by decreased β-cell secretory capacity including diabetes associated with chronic pancreatitis or pancreatectomy, cystic fibrosis, some genetic forms of diabetes (especially type 3 Maturity Onset Diabetes of the Young: MODY3), mitochondrial cytopathy, etc.23 Differentiation of T2D from T1D is based on assessment of T1D-associated autoantibodies (against glutamic acid decarboxylase [GAD], islet-associated antigen-2 [IA-2], and zinc transporter-8 [ZnT8]) and C-peptide level. While an undetectable or very low C-peptide (<0.3 ng/ml [0.1 nmol/l]) is consistent with T1D, residual C-peptide production may be observed in cases of long-standing T1D, and the presence of uremia (C-peptide undergoes renal clearance) may allow for detection of higher than expected levels. Usually in T1D with advanced kidney disease, C-peptide levels are <2.0 ng/ml (0.7 nmol/l), and so the presence of C-peptide >2.0 ng/ml (0.7 nmol/l) in the absence of T1D-associated autoantibodies may be used to confirm T2D.24 While there are no uniformly accepted guidelines for pancreas transplantation in patients with T2D, in general patients without significant obesity (BMI <30–32 kg/m2) or insulin resistance (insulin requirements <1 units/kg/day) and low CVD risk are considered.25 More studies are needed to determine which patients with C-peptide positive diabetes benefit from pancreas transplantation.
The number of patients with T2D listed for kidney pancreas transplantation has been steadily growing in the US, reaching 17.7% for SPK and 10% for PAK.26 In selected T2D patients overall patient and graft survival are similar to T1D SPK recipients.27 Surgical and infectious complications and readmissions are similar to T1D recipients, even though BMI is typically higher in T2D recipients.28–31 Glycemic control up to 2-years following transplantation is comparable to T1D; however, more post-transplant weight gain is experienced in T2D recipients.30
Kidney Function
Patients with advanced kidney disease CKD stage 4 and 5 (eGFR 15–30 and <15 ml/min/1.73 m2 or on dialysis, respectively), should be evaluated for SPK or SIK as they can accrue waiting time once the eGFR is ≤20 ml/min/1.73 m2. Patients with CKD stage 3 (eGFR between 30–60 ml/min/1.73 m2), who have very labile glycemia and/or debilitating hypoglycemia unawareness and/or rapidly progressive diabetic complications remain the most challenging group to manage, as they are currently not offered a pancreas or islet transplant alone (PTA or ITA) due to the risk of accelerated kidney function decline associated with calcineurin-inhibitor based immunosuppression after transplantation.32, 33 Patients with eGFR >45–60 ml/min/1.73 m2 may still benefit from a transplant center evaluation, as under exceptional circumstances a PTA or ITA may be considered, granted the risks for kidney function decline requiring imminent dialysis are understood, with plans to follow with a living or deceased donor kidney transplant in that event.
Problematic Hypoglycemia/Hyperglycemia
Problematic hypoglycemia, defined as two or more episodes per year of severe hypoglycemia or as one episode associated with impaired awareness of hypoglycemia, extreme glycemic lability, or major fear and maladaptive behavior is the classical indication for β-cell replacement therapy in patients with preserved kidney function.8 Patients who have experienced a severe episode of hypoglycemia and also have impaired awareness of hypoglycemia and/or marked glycemic lability are at significantly increased risk for experiencing future severe hypoglycemia and mortality.34 Problematic hyperglycemia is defined by the presence of recurrent episodes of diabetic ketoacidosis or severe, rapidly progressing secondary complications of diabetes.8 Problematic hypo- or hyperglycemia is not a pre-requisite for kidney transplant candidates to be considered for simultaneous pancreas or islet transplantation, but may inform the decision to proceed with β-cell replacement therapy in KTA recipients, although it is not generally required.20
Quality of Life
Quality of life (QoL) may be severely affected in patients with diabetes due to the disease and treatment burden. Studies directly comparing QoL of SPK and KTA recipients are sparse and outdated and therefore may not be applicable to the current era with improved surgical techniques and pancreas allograft survival. Regardless, diabetes related QoL was shown to be consistently better in SPK versus KTA,35, 36 while general improvement in health was overall better post-transplant. Functioning pancreas allograft was found to be an important pre-requisite to improved QoL.37 More recent studies compared QoL pre- and post-SPK and confirmed an improvement post transplant.38–41 Improved metabolic control was also associated with better health-related and diabetes-related QoL in both, islet transplant alone and islet-after-kidney transplantation.17, 42
More studies are needed in the current era of modern insulin delivery technology to compare patient reported outcomes in pancreas versus islet transplant recipients transplanted simultaneously with or after the kidney, as well as KTA using standardized and validated surveys.
Obesity
Increasing BMI in transplant candidates reflects the obesity trends in the general population as well as in T1D,43 with approximately 20% of wait-listed pancreas transplant candidates having BMI ≥30 kg/m2.26, 44 Obesity is associated with a higher risk of early graft loss due to graft thrombosis and technical failure as well as compromised long-term pancreas allograft survival and increased risk of mortality.45 Weight loss may improve transplant eligibility in candidates with both T1D and T2D and maximize the benefit-to-risk ratio of pancreas transplantation. Bariatric surgery could be considered in individuals unable to lose weight with diet and exercise prior to transplant listing.46 Sleeve gastrectomy is preferred over Roux-en-Y gastric bypass due to the lower risk of kidney allograft complications, no significant effect on the absorption of immunosuppression, and less risk of alimentary hypoglycemia, which may be exacerbated after pancreas transplantation.47, 48
Vascular Disease
CVD and PVD are common in patients with diabetes and advanced kidney disease. Additional risks factors include dyslipidemia and smoking,49as well asabnormal calcium and phosphate homeostasis, oxidative stress, and inflammation present in patients with renal failure and associated vascular calcification.50 The presence of arterial calcification increases the intraoperative technical difficulty for re-vascularization and correlates closely with CVD events, mortality and graft loss in pancreas (and kidney) transplant recipients.51 Thorough CVD disease workup prior to transplantation is required to mitigate unexpected postoperative cardiovascular events, but local policies vary.
Frailty
Frailty is a well-recognized risk factor leading to adverse outcomes in patients on dialysis and is associated with poor outcomes after kidney transplantation, including impaired physical and cognitive functioning and causing higher mortality. Transplant centers use various tools to assess for frailty and acceptance for transplantation.52 Frail patients are less likely to be considered for SPK due to the longer post-operative recovery and higher risk of complications. Research is urgently needed to identify interventions that could improve physical and cognitive functioning among frail patients that that they could be considered for transplantation. Whether patients with frailty benefit from SIK/IAK over SPK remains to be determined. Poor vision and severe, frequently debilitating diabetic polyneuropathy, including autonomic instability, often present in patients with long-standing diabetes, and add to the complexity of perioperative and post-transplant care.
Transplant Options
Patients with insulin-dependent diabetes and advanced kidney disease should preferably be referred for evaluation for a β-cell transplant as soon as eGFR reaches <30 ml/min/1.73 m2, especially in those with rapid kidney function decline (defined as GFR loss > 5 ml/min/1.73 m2 per year) (Figure 2). Early referral is advised to ensure adequate time for informed decision making and thorough pre-transplant evaluation, allowing for early identification and management of mental and physical health related barriers to transplantation. Early referrals can facilitate preemptive transplantation and therefore avoidance of debilitating dialysis related comorbidities and increased mortality, as well as improve patient and graft survival.53, 54
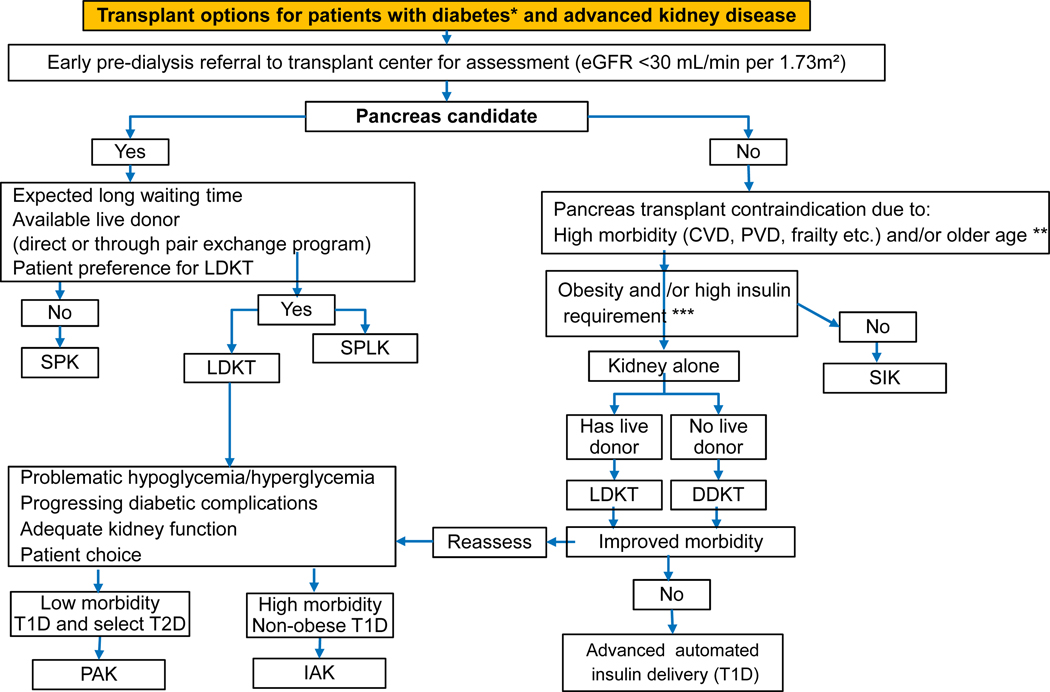
Figure 2 –
Practical decision making algorithm for β-cell replacement in patients with insulin-dependent diabetes and advanced kidney disease.
CVD – Cardiovascular Disease; DDKT – Deceased Donor Kidney Transplant; eGFR – estimated Glomerular Filtration Rate; IAK – Islet-After-Kidney; LDKT – Living Donor Kidney Transplant; PAK – Pancreas-After-Kidney; SIK – Simultaneous Islet-Kidney; SPK – Simultaneous Pancreas-Kidney; PVD – Peripheral Vascular Disease.
Recipients with Living Kidney Donors
Successful kidney transplantation is a major determinant of improved survival in kidney and pancreas transplant candidates, mainly due to the reduction of cardiovascular disease (CVD) mortality, compared to remaining on dialysis.55, 56 Gruessner and colleagues demonstrated 4-year patient survival on the waiting lists of 81.7% for kidney transplant recipients waiting for pancreas transplant, compared to 58.7% for patients with renal failure waiting for a SPK transplant.56 Hence, patients on or close to dialysis who have a suitable living donor may benefit from proceeding with KTA. Kidney donors and recipients who are blood type incompatible, cross-match positive or with high donor specific antibody profiles may be transplanted through paired exchange programs, which are being increasingly utilized worldwide.57
KTA has a short-term advantage over SPK transplants in terms of lower surgical morbidity and mortality.55, 58–60 In the long term, SPK recipients have been shown to accrue a survival benefit compared to living donor KTA recipients,59–62 with a reduction up to 37% of CVD-related mortality (HR 0.63, 95% CI [0.40, 0.99]).62 However in the absence of randomized controlled trials, these outcomes have to be interpreted cautiously, due to potential selection bias where healthier patients receive a SPK, as well as a shorter dialysis duration due to donor allocation policies.59, 62
Simultaneous Pancreas-Kidney Transplant and Simultaneous Islet-Kidney Transplant
SPK transplant offers the benefits of a single surgical procedure and induction immunosuppression with superior pancreas allograft outcomes, compared to a pancreas-after-kidney (PAK) transplant.63–65 In some countries the pancreas is often allocated with a better quality kidney allograft, due to stricter pancreas acceptance criteria, and typically with a shorter waiting time, compared to a deceased donor KTA.66 Improvement in surgical techniques and immunosuppression have greatly prolonged pancreas allograft survival,67 particularly with the use of T-cell depleting antibodies as induction therapy.55, 68–70 The survival benefit in SPK is observed as early as 170 days following transplantation compared to remaining on the wait list.71
SPK recipients have long-term survival benefits over KTA which may be explained by a long-term reduced risk of CVD events62 and increased kidney allograft survival at 10 years.61 Pancreas allograft survival in SPK recipients at 1-year and 10-years is 89% and 75%, respectively,61, 67, 72 and at 25 years up to 13%.73 In addition, insulin-dependent KTA recipients have inferior kidney allograft survival at 10 years (50% vs 61% for SPK or 66% for PAK),61 and are at increased risk of recurrent diabetic nephropathy,74, 75 demonstrated histologically by the presence of mesangial matrix expansion in up to 64.9% of patients at 5 years post-transplant (compared to 27.1% in non-diabetic and 27.7% in non-insulin-treated patients with diabetes).74–76
Simultaneous deceased donor pancreas with living donor kidney (SPLK) transplant is a potential alternative to a SPK when waiting times for a deceased donor is prolonged and a living kidney donor is available.77, 78 SPLK transplantation has universal and patient specific benefits, including expanding the pool of available kidneys and potential shorter waiting time for a deceased donor pancreas alone.77, 78 However, the logistics of coordinating a living donor kidney operation with the simultaneous implant of a deceased donor pancreas limits wider acceptance in countries where wait times for SPK are relatively short.79 Less commonly, a simultaneous islet-kidney (SIK) may be offered from a deceased donor, particularly for recipients with T1D awaiting deceased donor kidney transplant who are not candidates for, or willing to accept the risks of pancreas transplantation.20
Pancreas-after-Kidney and Islet-after-Kidney
For patients with T1D who have undergone successful KTA from either a living or deceased donor, a subsequent pancreas-after-kidney (PAK) or islet-after-kidney (IAK) transplant may be an option. PAK may also be considered for selected individuals with insulin-dependent T2D.20 PAK is usually done in recipients who have difficulty with achieving target glycemic control and/or management of diabetes related complications, and who are willing to accept the potential morbidity of additional surgery. Adequate kidney allograft function, ideally eGFR at least 40–45 ml/min/1.73 m2 is needed to buffer the impact of potential perioperative pancreas transplant complications and intensified immunosuppression on long-term kidney allograft function.80 PAK has been reported to improve kidney graft survival at 10 years when compared to KTA (66% vs 50%, respectively)61 but results may be biased by retrospective analysis, patient selection, and lack of a standardized insulin therapy approach in KTA recipients. As for the pancreas allograft, survival has been reported to be inferior in PAK recipients, compared to SPK (at 10 years 45% vs 58%, respectively),61, 63 although center variations may exist, with some reporting similar allograft survival between both techniques.81 Biopsying the kidney as a surrogate marker of pancreas graft rejection in SPK has been considered a key reason for these differences in graft survival,4 though in concurrent graft biopsies in SPK only 40% of patients presented simultaneous rejection, with 34% and 27% showing discordant kidney and pancreas rejection.82
IAK is an alternative β-cell replacement strategy for patients with T1D and a functioning kidney transplant – including those with early technical pancreas allograft failure after an SPK or PAK. While insulin-independence may be inferior with islet compared to pancreas transplantation (at 10 years 28% vs 45% in PAK),61, 83 insulin independence is observed in 38–62% at 1-year,17, 84 and islet graft survival up to 78% at 10-years.83 IAK is associated with a significant improvement in HbA1c to ≤6.0% at 1-year17, 83 that is maintained at 6.3% at 3-years17 and 6.7% at 10-years,83 and further restores awareness of hypoglycemia with a significant improvement in quality-of-life.17 While limited to 6 months follow-up, the TRIMECO study randomized participants to ITA or IAK vs intensive insulin therapy and confirmed HbA1c was significantly lower with islet transplantation (5.6 vs. 8.2%) with 23 of 25 subjects protected from severe hypoglycemic events compared to only 8 of 22 subjects receiving optimized medical management. Importantly, a significant improvement in health-related quality-of-life was also confirmed in the transplant compared to insulin group.85 Following islet transplantation, kidney allograft function remains stable,17, 83 without evidence of sensitization against the transplanted kidney despite multiple islets infusion. Longer-term studies are needed to define the impact of islet transplant associated sensitization on long term kidney allograft outcomes and retransplantation for allograft failure. Whether KTA recipients with acceptable glucose control and no hypoglycemia unawareness benefit from PAK/IAK over state-of-the-art individualized intensive insulin therapy in terms of overall survival and prevention/decreased progression of micro- and macrovascular complications remains to be determined.
Level of Evidence
The algorithm proposed here is based primarily on retrospective cohort studies and registry analysis data. Studies of IAK have been conducted prospectively,17, 83, 84 including under phase 3 registration with the US Food and Drug Administration.17 One randomized clinical trial comparing ITA and IAK to intensive insulin therapy has been carried out to date.86 Table 1 summarizes the most relevant studies and outcomes for each treatment alternative. Further studies are required to evaluate differences among treatment alternatives (Box 1).
Table 1 –
Outcomes among transplant options for patients with insulin-dependent diabetes and advanced kidney disease
| Study population | Study design | Follow-up | Time on waiting list and/or dialysis | Patient survival | Pancreas/islet graft survival | Kidney graft survival | |
|---|---|---|---|---|---|---|---|
| SPK vs KTA | |||||||
| Lindahl JP (2016)62 | SPK (n=256) vs LDKT (n=230) | Retrospective single center | 7.9 years | Waiting list Not mentioned Dialysis SPK 0.9 years LDKT 0.6 years | Survival on follow-up - SPK 61% - LDKT 44% Mortality risk for SPK (LDKT as reference) - CVD-related (HR 0.63, 95% CI 0.40, 0.99; p= 0.047) - All-cause (HR 0.81, 95% CI 0.57, 1.16; p = 0.25) - CAD-related (HR 0.63, 95% CI 0.36, 1.12; p =0.12) |
- | - |
| Sollinger HW (2009)55 | SPK (n=1000) vs LDKT (n=403) Vs DDKT (n=697) | Retrospective single center | 20 years | Waiting list Not mentioned Dialysis Not mentioned LDKT Not mentioned DDKT Not mentioned | At 10 years SPK : 80% LDKT: 50–60% DDKT: 40–50% Dialysis: follow up only up to 5years | - | At 10 years SPK: 38% LDKT: not mentioned DDKT: not mentioned |
| Barlow A (2017)60 | SPK (n=1739) vs LDKT (n=370) | Retrospective registry analysis | 13 years | Waiting list SPK 0.87 years KTA 0.90 years Dialysis Not mentioned | Better in SPK (with functioning pancreas at 90days) vs LDKT (p=0.042) | - | Delayed graft function (p<0.001) SPK 15.5% LDKT 7.3% Graft survival at 10years (p=0.25) SPK 77% LDKT 80% |
| Fridell JA (2018)61 | SPK (n=19725) vs PAK (n=5636) | Retrospective registry analysis | 10 years | Waiting list SPK 1.2 years KTA No reference Dialysis Not mentioned | At 10 years SPK : 70.3% KTA†: 86.3% | - | PALK vs LDKT 69.8 vs 61.0% PADK vs DDKT 66.0 vs 50.4% |
| SPK vs PAK | |||||||
| Fridell JA (2018)61 | SPK (n=19725) vs PAK (n=5636) | Retrospective registry analysis | 10 years | Waiting list SPK 1.2years PAK 1.3years Dialysis Not mentioned | SPK : 70.3% PAK : 63.2% (P < .001) | SPK 58.7% PALK: 44.4% PADK 41.7% (P < .001) | SPK 61% PALK: 69.8% PADK 66–0% (P < .001) |
| Ventura-Aguiar P (2018)63 | SPK (n=139) vs PALK (n=18) vs PADK (n=28) | Retrospective single center | 10 years | Waiting list SPK 1.6 years PALK 0.5 years PADK 0.3 years Dialysis SPK 2.9 years PALK 1.0 years PADK 2.8 years | SPK vs PALK vs PADK (p>0.05) | PALK and PADK inferior to SPK (p<0.05) | SPK vs PALK vs PADK (p>0.05) |
| Parajuli S (2019)81 | SPK (n=611) vs PALK (n=12) vs PADK (n=12) | Retrospective Single center | 15 years | Waiting list SPK 0.5years PAK 1.2years Dialysis Not mentioned | SPK 68% PAK 71% (p=0.79) | SPK 62% PAK 71% (p=0.38) SPK vs PALK vs PADK (p=0.68) | SPK 66% PAK 50% (p=0.11) SPK vs PALK vs PADK (p=0.59) |
| SIK/IAK vs SPK/PAK | |||||||
| Frank A (2004) 87 | IAK (n=4) vs SPK/ PAK (n=30) | Retrospective single center | IAK (1,4years) SPK/PAK (1,2 years) | Not mentioned | At Fup SPK/PAK 96.6% vs IAK 100% | Graft function (as per C-Peptide secretion) No difference Insulin independence (Fup) Superior for SPK/PAK (p<.04) | No reference |
| Lehmann R (2015) 19 | SIK/IAK (n=38) vs SPK/PAK (n=94) | Retrospective single center | SPK/PAK (5.6 years) SIK/IAK (6.4 years) | Waiting list SPK/PAK 0.9 years SIK/IAK 1.4 years | At 10y SPK/PAK 88.5% vs SIK/IAK 65.4% | Insulin independence (5y) SPK/PAK 73.6% vs SIK/IAK 9.3% | ΔeGFR at 13years SPK/PAK −9.5±23ml/min vs SIK/IAK −13.3±13.8ml/min |
for only those awaiting a PAK
DGF – delayed graft function; eGFR – estimated glomerular filtration rate; IAK – islet-after-kidney; ITA - islet transplant alone; KTA – kidney transplant alone; PADK – pancreas-after-deceased-donor-kidney; PAK – pancreas-after-kidney; PALK – pancreas-after-living-donor-kidney; PTA - pancreas transplant alone; SIK - simultaneous islet-kidney; SPK - simultaneous pancreas-kidney.
Conclusions
For patients with insulin-dependent diabetes and advanced kidney disease requiring kidney transplantation, β-cell replacement should be considered to provide a complete spectrum of cure for β-cell deficiency. Advances in β-cell replacement allow individualized therapy options depending on patient priority, co-existent morbidity, and the availability of a living kidney donor. Ideally, both pancreas and islet transplantation should be offered according to medical condition and patient preference20 rather than dictated by regional availability for a particular patient with diabetes and advanced kidney disease.
Box 1.
Future Research
| • Benefits of early (eGFR >20 ml/min/1.73 m2) SPK transplant in patients with impaired kidney function and rapidly progressive diabetic complications and/or problematic hypo- and hyperglycemia despite advanced automated insulin delivery system. |
| • Patient survival and micro- and macro-vascular diabetic complications in PAK recipients as compared to KTA on an advanced automated insulin delivery systems. |
| • Kidney allograft survival in PAK recipients as compared to KTA on advanced automated insulin delivery systems. |
| • Outcomes of pancreas versus islet transplantation in patients with C-peptide positive diabetes; appropriate selection of candidates for degrees of insulin resistance. |
| • Assessment of pancreas insulin secretory reserve in patients with type 2 diabetes and advanced chronic kidney disease. |
| • Preservation of renal function in PTA/ITA recipients with diabetic kidney disease. |
| • Surgical pancreas transplant related morbidity and long-term outcomes in high risk recipients-e.g. age >55 years, frailty or significant physical impairment, advanced cardiovascular and peripheral vascular disease, etc. |
| • Pancreas allograft outcomes in SPK recipients as compared to the solitary pancreas transplant in terms of alloimmune rejection, autoimmune recurrence, and graft survival. |
| • Long-term risk of sensitization with islet transplantation in IAK recipients. |
| • The impact of CMV disease and its prevention on pancreas and islet transplant outcomes. |
| • Organ donor allocation for pancreas versus islet transplantation. |
| • Randomized studies evaluating efficacy of less nephrotoxic and diabetogenic immunosuppression. |
| • The impact of recipient prehabilitation strategies on long term outcomes. |
| • Organ preservation strategies to reduce ischemia reperfusion injury and optimize isolated islet yields and function. |
| • Comparison of patients reported outcomes between SPK, SIK and KTA recipients, as well as between PAK, IAK and KTA recipeints. |
eGFR – estimated Glomerular Filtration Rate; IAK- islet after kidney; ITA – Islet Transplant Alone; KTA-kidney transplant alone, PAK – Pancreas after kidney; PTA – Pancreas Transplant Alone; SPK – Simultaneous Pancreas-Kidney.
Acknowledgments:
The authors thank the Council of the International Pancreas & Islet Transplant Association, a section of The Transplantation Society, for providing advice during concept development and the formation of the writing committee, and Aristea Slikas of the University of Pennsylvania Institute for Diabetes, Obesity & Metabolism for providing editorial assistance.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Support: Prof. Saudek was supported by a research grant from the Ministry of Education Youth and Sports of the Czech Republic No. LTAUSA19073. Prof. Vantyghem was supported by the French Ministry of Health, PHRC (Programme hospitalier de recherche clinique) 2001, the European Community (Fond Européen de Développement Régional), Conseil Régional du Nord-Pas-de-Calais (IFR 114, Institut de Médecine Prédictive et de Recherche Thérapeutique), Programme d’investissements d’avenir Labex European Genomic Institute for Diabetes ANR-10-LABX-46). Prof. Ventura-Aguiar was supported by a research grant from the Spanish Ministry of Science and Innovation (grant PI16-00167 of Instituto Salud Carlos III); Prof. Rickels was supported by Public Health Services Research Grant R01 DK091331 and the Institute for Diabetes, Obesity &Metabolism at the University of Pennsylvania Perelman School of Medicine. None of the funders had a role in the design, content, interpretation, reporting, or decision to submit for publication.
Acronym list
- BMI
Body Mass Index
- CITR
Collaborative Islet Transplant Registry
- CVD
Cardiovascular disease
- CAD
Coronary Artery Disease
- DDKT
Deceased Donor Kidney Transplant
- DGF
Delayed Graft Function
- eGFR
estimated Glomerular Filtration Rate
- ESKD
End-Stage Kidney Disease
- GAD
Glutamic Acid Decarboxylase
- HLA
Human Leukocyte Antigens
- IAK
Islet-After-Kidney
- IA-2
Islet-associated Antigen-2
- ITA
Islet Transplant Alone
- KTA
Kidney Transplant Alone
- LDKT
Living Donor Kidney Transplant
- MACE
Major Adverse Cardiovascular Events
- MODY
Maturity Onset Diabetes of the Young
- OPTN
Organ Procurement and Transplantation Network
- PADK
Pancreas after Deceased-donor Kidney
- PAK
Pancreas-After-Kidney
- PALK
Pancreas After Living-donor Kidney
- PTA
Pancreas Transplant Alone
- PVD
Peripheral Vascular Disease
- QoL
Quality of Life
- SIK
Simultaneous Islet-Kidney
- SPK
Simultaneous Pancreas-Kidney
- SPLK
Simultanous deceased donor Pancreas with Living donor Kidney
- SRTR
Scientific Registry of Transplant Recipients
- T1D
Type 1 Diabetes
- T2D
Type 2 Diabetes
- US
United States
- ZnT8
Zinc Transporter-8
Footnotes
Peer Review: Received November 15, 2020. Evaluated by 3 external peer reviewers, with direct editorial input from an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form February 15, 2021. The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Aleksandra Kukla, Division of Nephrology and Hypertension, Mayo Clinic, Rochester, Minnesota USA..
Pedro Ventura-Aguiar, Division of Nephrology, Hospital Clinic of Barcelona, Barcelona, Spain..
Matthew Cooper, Medstar Georgetown Transplant Institute, Washington DC USA..
Eelco J.P. de Koning, Department of Medicine, Leiden University Medical Center, Leiden, Netherlands..
David J. Goodman, Department of Nephrology, St. Vincent’s Hospital, Melbourne, Australia..
Paul R. Johnson, Nuffield Department of Surgical Sciences, University of Oxford, Oxford, UK..
Duck J. Han, Division of Transplantation, Department of Surgery, Asan Medical Center, Seoul, South Korea..
Didier A. Mandelbrot, Division of Nephrology, Department of Medicine, University of Wisconsin, Madison, WI USA..
Martha Pavlakis, Division of Nephrology, Department of Medicine, Beth Isreal Deaconess Medical Center, Boston, MA USA..
Frantisek Saudek, Diabetes Center, Institute for Clinical and Experimental Medicine, Prague, Czech Republic..
Marie-Christine Vantyghem, CHU Lille, Department of Endocrinology, Diabetology and Metabolism, Inserm U1190, Translational Research for Diabetes, Univ Lille, European Genomic Institute for Diabetes, F-59000, Lille, France.
Titus Augustine, Division of Diabetes, Endocrinology and Gastroenterology, Faculty of Biology Medicine and Health, Manchester Academic Health Centre, University of Manchester, Manchester UK..
Michael R. Rickels, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA USA..
References
- 1.Larsen JL. Pancreas transplantation: Indications and consequences. Endocrine Reviews.2004;25(6): 919–946. [DOI] [PubMed] [Google Scholar]
- 2.Rickels MR, Robertson RP. Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions. Endocr Rev. 2019;40(2): 631–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhary P RM, Senior PA, Vantyghem MC, Maffi P, Kay TW, Keymeulen B, Inagaki N, Saudek F, Lehmann R, Hering BJ. Evidence-Informed clinical practice recommendations for treatment of type 1 diabetes complicated by problematic hypoglycemia. Diabetes Care. 2015;38(6): 1016–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fridell JA, Powelson JA. Pancreas after kidney transplantation: why is the most logical option the least popular? Curr Opin Organ Transplant. 2015;20(1): 108–114. [DOI] [PubMed] [Google Scholar]
- 5.Stratta RJ, Fridell JA, Gruessner AC, Odorico JS, Gruessner RW. Pancreas transplantation: a decade of decline. Curr Opin Organ Transplant. 2016;21(4): 386–392. [DOI] [PubMed] [Google Scholar]
- 6.Benjamens S, Leemkuil M, Margreiter C, Huurman VA, Leuvenink HG, Pol RA. A steady decline in pancreas transplantation rates. Pancreatology. 2019;19(1): 31–38. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto I, Sawada T, Nakano M, et al. Improvement in islet yield from obese donors for human islet transplants. Transplantation. 2004;78(6): 880–885. [DOI] [PubMed] [Google Scholar]
- 8.Rickels MR, Stock PG, de Koning EJP, et al. Defining Outcomes for beta-cell Replacement Therapy in the Treatment of Diabetes: A Consensus Report on the Igls Criteria From the IPITA/EPITA Opinion Leaders Workshop. Transplantation. 2018;102(9): 1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kandaswamy R, Stock PG, Gustafson SK, et al. OPTN/SRTR 2016 Annual Data Report: Pancreas. Am J Transplant. 2018;18 Suppl 1: 114–171. [DOI] [PubMed] [Google Scholar]
- 10.Goodman J, Becker YT. Pancreas surgical complications. Curr Opin Organ Transplant. 2009;14(1): 85–89. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017;13(5): 268–277. [DOI] [PubMed] [Google Scholar]
- 12.(CITR) CITR. Collaborative Islet Transplant Registry - Ninth Annual Report 2016. https://citregistry.org/content/citr-9th-annual-report.
- 13.Koh A, Senior P, Salam A, et al. Insulin-heparin infusions peritransplant substantially improve single-donor clinical islet transplant success. Transplantation. 2010;89(4): 465–471. [DOI] [PubMed] [Google Scholar]
- 14.Al-Adra DP, Gill RS, Imes S, et al. Single-donor islet transplantation and long-term insulin independence in select patients with type 1 diabetes mellitus. Transplantation. 2014;98(9): 1007–1012. [DOI] [PubMed] [Google Scholar]
- 15.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. Jama-Journal of the American Medical Association. 2005;293(7): 830–835. [DOI] [PubMed] [Google Scholar]
- 16.Rickels MR, Liu C, Shlansky-Goldberg RD, et al. Improvement in beta-cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes. 2013;62(8): 2890–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markmann JF, Rickels MR, Eggerman TL, et al. Phase 3 Trial of Human Islet-after-Kidney Transplantation in Type 1 Diabetes. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelletier RP, Rajab AA, Diez A, et al. Early immunosuppression treatment correlates with later de novo donor-specific antibody development after kidney and pancreas transplantation. Clin Transplant.2015;29(12): 1119–1127. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann R, Graziano J, Brockmann J, et al. Glycemic Control in Simultaneous Islet-Kidney Versus Pancreas-Kidney Transplantation in Type 1 Diabetes: A Prospective 13-Year Follow-up. Diabetes Care. 2015;38(5): 752–759. [DOI] [PubMed] [Google Scholar]
- 20.Flatt AJS, Bennett D, Counter C, Brown AL, White SA, Shaw JAM. beta-Cell and renal transplantation options for diabetes. Diabet Med. 2020;37(4): 580–592. [DOI] [PubMed] [Google Scholar]
- 21.Scalea JR, Redfield RR 3rd, Arpali E, et al. Pancreas transplantation in older patients is safe, but patient selection is paramount. Transpl Int. 2016;29(7): 810–818. [DOI] [PubMed] [Google Scholar]
- 22.Montagud-Marrahi E, Molina-Andújar A, Pané A, et al. Outcomes of pancreas transplantation in older diabetic patients. BMJ Open Diabetes Res Care. 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(WHO) WHO. Classification of Diabetes Mellitus 2019. https://apps.who.int/iris/rest/bitstreams/1233344/retrieve.
- 24.Katz LE, Jawad AF, Ganesh J, Abraham M, Murphy K, Lipman TH. Fasting c-peptide and insulin-like growth factor-binding protein-1 levels help to distinguish childhood type 1 and type 2 diabetes at diagnosis. Pediatr Diabetes. 2007;8(2): 53–59. [DOI] [PubMed] [Google Scholar]
- 25.Weems P, Cooper M. Pancreas transplantation in type II diabetes mellitus. World J Transplant. 2014;4(4): 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kandaswamy R, Stock PG, Gustafson SK, et al. OPTN/SRTR 2018 Annual Data Report: Pancreas. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2020;20 Suppl s1: 131–192. [DOI] [PubMed] [Google Scholar]
- 27.Gruessner AC, Gruessner RWG. Pancreas Transplantation for Patients with Type 1 and Type 2 Diabetes Mellitus in the United States: A Registry Report. Gastroenterol Clin North Am. 2018;47(2): 417–441. [DOI] [PubMed] [Google Scholar]
- 28.Al-Qaoud TM, Odorico JS, Redfield RR 3rd. Pancreas transplantation in type 2 diabetes: expanding the criteria. Curr Opin Organ Transplant. 2018;23(4): 454–460. [DOI] [PubMed] [Google Scholar]
- 29.Rohan V, Taber D, Palanisamy A, et al. Impact of Type 1 and Type 2 Diabetes Mellitus on Pancreas Transplant Outcomes. Exp Clin Transplant. 2019;17(6): 796–802. [DOI] [PubMed] [Google Scholar]
- 30.Andacoglu OM, Himmler A, Geng X, et al. Comparison of glycemic control after pancreas transplantation for Type 1 and Type 2 diabetic recipients at a high volume center. Clin Transplant. 2019;33(8): e13656. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki TM, Gray RS, Ratner RE, et al. Successful long-term kidney-pancreas transplants in diabetic patients with high C-peptide levels. Transplantation. 1998;65(11): 1510–1512. [DOI] [PubMed] [Google Scholar]
- 32.Smail N, Paraskevas S, Tan X, Metrakos P, Cantarovich M. Renal function in recipients of pancreas transplant alone. Current opinion in organ transplantation. 2012;17(1): 73–79. [DOI] [PubMed] [Google Scholar]
- 33.Odorico JS, Voss B, Munoz Del Rio A, et al. Kidney function after solitary pancreas transplantation. Transplant Proc. 2008;40(2): 513–515. [DOI] [PubMed] [Google Scholar]
- 34.Rickels MR. Hypoglycemia-associated autonomic failure, counterregulatory responses, and therapeutic options in type 1 diabetes. Ann N Y Acad Sci. 2019;1454(1): 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sureshkumar KK, Patel BM, Markatos A, Nghiem DD, Marcus RJ. Quality of life after organ transplantation in type 1 diabetics with end-stage renal disease. Clin Transplant. 2006;20(1): 19–25. [DOI] [PubMed] [Google Scholar]
- 36.Gross CR, Limwattananon C, Matthees B, Zehrer JL, Savik K. Impact of transplantation on quality of life in patients with diabetes and renal dysfunction. Transplantation. 2000;70(12): 1736–1746. [DOI] [PubMed] [Google Scholar]
- 37.Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233(4): 463–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheuermann U, Rademacher S, Jahn N, et al. Impact of pre-transplant dialysis modality on the outcome and health-related quality of life of patients after simultaneous pancreas-kidney transplantation. Health Qual Life Outcomes. 2020;18(1): 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajkumar T, Mazid S, Vucak-Dzumhur M, Sykes TM, Elder GJ. Health-related quality of life following kidney and simultaneous pancreas kidney transplantation. Nephrology (Carlton). 2019;24(9): 975–982. [DOI] [PubMed] [Google Scholar]
- 40.Gibbons A, Cinnirella M, Bayfield J, et al. Changes in quality of life, health status and other patient-reported outcomes following simultaneous pancreas and kidney transplantation (SPKT): a quantitative and qualitative analysis within a UK-wide programme. Transpl Int. 2020;33(10): 1230–1243. [DOI] [PubMed] [Google Scholar]
- 41.Nijhoff MF, Hovens J, Huisman SD, et al. Psychological Symptoms and Quality of Life After Simultaneous Kidney and Pancreas Transplantation. Transplant Direct. 2020;6(5): e552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foster ED, Bridges ND, Feurer ID, et al. Improved Health-Related Quality of Life in a Phase 3 Islet Transplantation Trial in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care. 2018;41(5): 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corbin KD, Driscoll KA, Pratley RE, et al. Obesity in Type 1 Diabetes: Pathophysiology, Clinical Impact, and Mechanisms. Endocr Rev. 2018;39(5): 629–663. [DOI] [PubMed] [Google Scholar]
- 44.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med. 2019;381(25): 2440–2450. [DOI] [PubMed] [Google Scholar]
- 45.Bedat B, Niclauss N, Jannot AS, et al. Impact of recipient body mass index on short-term and long-term survival of pancreatic grafts. Transplantation. 2015;99(1): 94–99. [DOI] [PubMed] [Google Scholar]
- 46.Diwan TS, Lee TC, Nagai S, et al. Obesity, transplantation, and bariatric surgery: An evolving solution for a growing epidemic. Am J Transplant. 2020. [DOI] [PubMed] [Google Scholar]
- 47.Dziodzio T, Biebl M, Ollinger R, Pratschke J, Denecke C. The Role of Bariatric Surgery in Abdominal Organ Transplantation-the Next Big Challenge? Obes Surg. 2017;27(10): 2696–2706. [DOI] [PubMed] [Google Scholar]
- 48.Aminian A. Bariatric procedure selection in patients with type 2 diabetes: choice between Roux-en-Y gastric bypass or sleeve gastrectomy. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2019. [DOI] [PubMed] [Google Scholar]
- 49.Mann DM, Fernandez S, Mondal Z, et al. Role of Coronary Angiography in the Assessment of Cardiovascular Risk in Kidney Transplant Candidates. The American journal of cardiology. 2016;118(5): 679–683. [DOI] [PubMed] [Google Scholar]
- 50.Wu M, Rementer C, Giachelli CM. Vascular calcification: an update on mechanisms and challenges in treatment. Calcified tissue international. 2013;93(4): 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis JR, Wong G, Taverniti A, Vucak-Dzumhur M, Elder GJ. Association between Aortic Calcification, Cardiovascular Events, and Mortality in Kidney and Pancreas-Kidney Transplant Recipients. American journal of nephrology. 2019;50(3): 177–186. [DOI] [PubMed] [Google Scholar]
- 52.McAdams-DeMarco MA, Van Pilsum Rasmussen SE, Chu NM, et al. Perceptions and Practices Regarding Frailty in Kidney Transplantation: Results of a National Survey. Transplantation. 2020;104(2): 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parajuli S, Swanson KJ, Patel R, et al. Outcomes of simultaneous pancreas and kidney transplants based on preemptive transplant compared to those who were on dialysis before transplant - a retrospective study. Transpl Int. 2020;33(9): 1106–1115. [DOI] [PubMed] [Google Scholar]
- 54.Kaku K, Kitada H, Noguchi H, et al. Living Donor Kidney Transplantation Preceding Pancreas Transplantation Reduces Mortality in Type 1 Diabetics With End-stage Renal Disease. Transplantation Proceedings. 2015;47(3): 733–737. [DOI] [PubMed] [Google Scholar]
- 55.Sollinger HW, Odorico JS, Becker YT, D’Alessandro AM, Pirsch JD. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Annals of surgery. 2009;250(4): 618–630. [DOI] [PubMed] [Google Scholar]
- 56.Gruessner RWG, Sutherland DER, Gruessner AC. Mortality Assessment for Pancreas Transplants. American Journal of Transplantation. 2004;4(12): 2018–2026. [DOI] [PubMed] [Google Scholar]
- 57.Vivek B Kute NP, Pankaj R Shah, Pranjal R Modi. Kidney exchange transplantation current status, an update and future perspectives World J Transplant. 2018;June 28(8(3)): 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruiz S, Amor AJ, Pane A, et al. Cardiovascular risk factors and cardiovascular disease in patients with type 1 diabetes and end-stage renal disease candidates for kidney-pancreas transplantation: Trends from 1999 to 2017. Diabetes Research and Clinical Practice. 2020;163: 108135. [DOI] [PubMed] [Google Scholar]
- 59.Sung RS, Zhang M, Schaubel DE, Shu X, Magee JC. A Reassessment of the Survival Advantage of Simultaneous Kidney-Pancreas Versus Kidney-Alone Transplantation. Transplantation. 2015;99(9): 1900–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barlow AD, Saeb-Parsy K, Watson CJE. An analysis of the survival outcomes of simultaneous pancreas and kidney transplantation compared to live donor kidney transplantation in patients with type 1 diabetes: a UK Transplant Registry study. Transplant international : official journal of the European Society for Organ Transplantation. 2017;30(9): 884–892. [DOI] [PubMed] [Google Scholar]
- 61.Fridell JA, Niederhaus S, Curry M, Urban R, Fox A, Odorico J. The survival advantage of pancreas after kidney transplant. American journal of transplantation. 2019;19(3): 823–830. [DOI] [PubMed] [Google Scholar]
- 62.Lindahl JP, Hartmann A, Aakhus S, et al. Long-term cardiovascular outcomes in type 1 diabetic patients after simultaneous pancreas and kidney transplantation compared with living donor kidney transplantation. Diabetologia. 2016;59(4): 844–852. [DOI] [PubMed] [Google Scholar]
- 63.Ventura-Aguiar P, Ferrer J, Revuelta I, et al. Pancreas outcomes between living and deceased kidney donor in pancreas after kidney transplantation patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2018;33(11): 2052–2059. [DOI] [PubMed] [Google Scholar]
- 64.Niederhaus SV, Leverson GE, Lorentzen DF, et al. Acute cellular and antibody-mediated rejection of the pancreas allograft: incidence, risk factors and outcomes. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(11): 2945–2955. [DOI] [PubMed] [Google Scholar]
- 65.Dong M, Parsaik AK, Kremers W, et al. Acute pancreas allograft rejection is associated with increased risk of graft failure in pancreas transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(4): 1019–1025. [DOI] [PubMed] [Google Scholar]
- 66.Esmeijer K, Hoogeveen EK, van den Boog PJM, et al. Superior Long-Term Survival for Simultaneous Pancreas-Kidney Transplantation as Renal Replacement Therapy: 30-Year Follow-up of a Nationwide Cohort. Diabetes Care. 2019. [DOI] [PubMed] [Google Scholar]
- 67.Gruessner AC, Gruessner RWG. Pancreas Transplantation of US and Non-US Cases from 2005 to 2014 as Reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). The Review of Diabetic Studies. 2016;13(1): 35–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kandaswamy R, Stock PG, Gustafson SK, et al. OPTN/SRTR 2015 Annual Data Report: Pancreas. American Journal of Transplantation. 2017;17: 117–173. [DOI] [PubMed] [Google Scholar]
- 69.Kopp WH, Verhagen MJ, Blok JJ, et al. Thirty Years of Pancreas Transplantation at Leiden University Medical Center: Long-term Follow-up in a Large Eurotransplant Center. Transplantation. 2015;99(9): e145–151. [DOI] [PubMed] [Google Scholar]
- 70.Finger EB, Radosevich DM, Dunn TB, et al. A composite risk model for predicting technical failure in pancreas transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(7): 1840–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ojo AO, Meier-Kriesche HU, Hanson JA, et al. The impact of simultaneous pancreas-kidney transplantation on long-term patient survival. Transplantation. 2001;71(1): 82–90. [DOI] [PubMed] [Google Scholar]
- 72.Ventura-Aguiar P, Ferrer J, Paredes D, et al. Outcomes From Brain Death Donors With Previous Cardiac Arrest Accepted for Pancreas Transplantation. Annals of Surgery. 2019: 1-1. [DOI] [PubMed] [Google Scholar]
- 73.Parajuli S, Bath NM, Aziz F, et al. More than 25 years of pancreas graft survival after simultaneous pancreas and kidney transplantation. Transplantation. 2019: 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lindahl JP, Reinholt FP, Eide IA, et al. In patients with type 1 diabetes simultaneous pancreas and kidney transplantation preserves long-term kidney graft ultrastructure and function better than transplantation of kidney alone. Diabetologia. 2014;57(11): 2357–2365. [DOI] [PubMed] [Google Scholar]
- 75.Helantera I, Ortiz F, Raisanen-Sokolowski A, Koskinen P. Impact of glucose metabolism abnormalities on histopathological changes in kidney transplant protocol biopsies. Transplant international : official journal of the European Society for Organ Transplantation. 2010;23(4): 374–381. [DOI] [PubMed] [Google Scholar]
- 76.Coemans M, Van Loon E, Lerut E, et al. Occurrence of Diabetic Nephropathy After Renal Transplantation Despite Intensive Glycemic Control: An Observational Cohort Study. Diabetes Care. 2019;42(4): 625–634. [DOI] [PubMed] [Google Scholar]
- 77.Farney AC, Cho E, Schweitzer EJ, et al. Simultaneous cadaver pancreas living-donor kidney transplantation: a new approach for the type 1 diabetic uremic patient. Ann Surg. 2000;232(5): 696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choi JY, Jung JH, Shin S, Kim YH, Han DJ. Association between the pancreas transplantation and survival of patients with diabetes: A single center experience. PLoS One. 2017;12(11): e0186827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farney AC, Rogers J, Orlando G, Stratta RJ. Simultaneous transplantation of the living donor kidney and deceased donor pancreas and other transplant options for diabetic and uremic patients. Curr Opin Organ Transplant. 2015;20(1): 103–107. [DOI] [PubMed] [Google Scholar]
- 80.Pavlakis M, Khwaja K, Mandelbrot D, et al. Renal allograft failure predictors after PAK transplantation: results from the New England Collaborative Association of Pancreas Programs. Transplantation. 2010;89(11): 1347–1353. [DOI] [PubMed] [Google Scholar]
- 81.Parajuli S, Arunachalam A, Swanson KJ, et al. Outcomes after simultaneous kidney-pancreas versus pancreas after kidney transplantation in the current era. Clin Transplant. 2019;33(12): e13732. [DOI] [PubMed] [Google Scholar]
- 82.Uva PD, Papadimitriou JC, Drachenberg CB, et al. Graft dysfunction in simultaneous pancreas kidney transplantation (SPK): Results of concurrent kidney and pancreas allograft biopsies. Am J Transplant. 2019;19(2): 466–474. [DOI] [PubMed] [Google Scholar]
- 83.Vantyghem MC, Chetboun M, Gmyr V, et al. Ten-Year Outcome of Islet Alone or Islet After Kidney Transplantation in Type 1 Diabetes: A Prospective Parallel-Arm Cohort Study. Diabetes Care. 2019;42(11): 2042–2049. [DOI] [PubMed] [Google Scholar]
- 84.Nijhoff MF, Engelse MA, Dubbeld J, et al. Glycemic Stability Through Islet-After-Kidney Transplantation Using an Alemtuzumab-Based Induction Regimen and Long-Term Triple-Maintenance Immunosuppression. Am J Transplant. 2016;16(1): 246–253. [DOI] [PubMed] [Google Scholar]
- 85.Lablanche S, Vantyghem MC, Kessler L, et al. Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2018. [DOI] [PubMed] [Google Scholar]
- 86.Lablanche S, Vantyghem MC, Kessler L, et al. Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6(7): 527–537. [DOI] [PubMed] [Google Scholar]
- 87.Frank A, Deng S, Huang X, et al. Transplantation for type I diabetes: comparison of vascularized whole-organ pancreas with isolated pancreatic islets. Ann Surg. 2004;240(4): 631–640; discussion 640–633. [DOI] [PMC free article] [PubMed] [Google Scholar]