Abstract
Immune homeostasis in peripheral tissues is to a large degree maintained by the differentiation and action of regulatory T cells (Treg) specific for tissue antigens. Using a novel mouse model, we have studied the differentiation of naïve CD4+ T cells into Foxp3+ Treg in response to a cutaneous antigen (OVA). We found that expression of OVA resulted in fatal autoimmunity and in prevention of peripheral Treg generation. Inhibiting mTOR activity with rapamycin rescued the generation of Foxp3+ T cells. When we varied the level of antigen expression to modulate TCR signaling, we found that low antigen concentrations promoted the generation of Foxp3+ T cells whereas high levels expanded effector T cells and caused severe autoimmunity. Our findings indicate that the expression level of tissue antigen is a key determinant of the balance between tissue-reactive effector and peripheral Foxp3+ T cells, that determines the choice between tolerance and autoimmunity.
Introduction
Regulatory T cells (Treg) control potentially destructive responses of self-reactive effector T cells (Teff) and thus prevent autoimmunity (1, 2). Treg are classically divided into thymic Treg (tTreg), generated in the thymus, and peripheral Treg (pTreg) that arise from naïve T cells activated in the periphery (3–6). The latter constitute a mechanism to regulate immune responses towards antigens that are not present in the thymus, such as self and microbial antigens (7–9). Both tTreg and pTreg are characterized by the expression of the transcription factor Foxp3, which drives Treg identity (10). Mutations in the FOXP3 gene in mice and humans lead to loss of Treg function, causing a severe autoimmune phenotype called scurfy (in mice) and IPEX syndrome (in humans) (1). Foxp3 induces the expression of molecules related to Treg function, such as CTLA-4, while suppressing expression of inflammatory cytokines (11, 12).
T cell fate, including Treg differentiation, is defined by the local conditions naïve T cells are exposed to during their activation (13–15). Costimulatory signals and the local cytokine milieu regulate Foxp3 expression in activated T cells and their differentiation to pTreg (5, 16, 17). Additionally, the amount of antigen presented to naïve T cells during activation modulates the strength of TCR signaling and plays a major role in T cell differentiation (18, 19). Specifically, low antigen doses favor induction of Foxp3 in vitro, and pTreg generation and/or expansion in vivo in response to immunization (20, 21). Interestingly, a high affinity peptide administered at a low concentration induced Foxp3 expression in a naïve monoclonal population whereas a low-affinity agonist showed a diminished ability to induce a persistent Foxp3+ population in vivo (21). These studies were performed with administered foreign peptides and it is therefore unknown whether similar mechanisms govern the response to tissue antigen.
In the present study, we utilized a unique mouse model to study pTreg induction, function and stability in vivo. We investigated the impact of different expression levels of a transgene-encoded tissue antigen on the activation and differentiation of adoptively transferred antigen-specific naïve T cells. Understanding the mechanisms that regulate pTreg differentiation in response to tissue antigens could promote the development of better clinical interventions in inflammatory and autoimmune settings where induction of tolerance against tissue antigens is desirable.
Materials and Methods
Mice
All animal studies were performed in compliance with institutional guidelines in a specific pathogen-free facility and were approved by the Federal Ministry of Science, Research and Economy of Austria.
K5/rTA (22) or INV/rTA (23) and TGO (Tg(TetO-Tfrc/EGFP/OVA)#Sfz (MGI:5503055), TRE-OVA) mice were crossed and designated K5/TGO or INV/TGO mice. The double-transgenic K5/TGO or INV/TGO mouse lines were crossed onto TCRα−/− mice on BALB/c background. DO11.10 TCR-transgenic mice were crossed with Rag2−/−/CD90.1+/+ or Rag2−/−/CD45.1+/+ BALB/c mice as previously described (24).
Adoptive transfer of T cells and doxycycline administration
Single-cell suspensions from skin-draining (sdLNs: auricular, brachial, axillary, inguinal, and popliteal lymph nodes) and mesenteric lymph nodes of DO11.10/Rag2−/−/CD90.1+/+ or DO11.10/Rag2−/−/CD45.1+/+ mice were prepared by mechanical disruption of LNs. 0.5 – 2 × 106 LN cells were adoptively transferred by i.v. injection into K5/TGO/TCRα−/− or INV/TGO/TCRα−/− recipient mice. Cells were stained with 10 μM CFSE where indicated. Doxycycline (dox) (Sigma-Aldrich) was administered in the drinking water in concentrations ranging from 1200 – 1.2 μg/mL in a sterile 5% sucrose solution. Dox treatment started a day before the transfer of DO11.10/Rag2−/− cells and mice were maintained on dox for the duration of the experiments.
Evaluation of skin disease
A 15-point clinical scoring scale was used to quantify skin disease. The clinical parameters of scaling, alopecia, erythema, level of activity, and periocular inflammation were each given a score of 0–3. Scores for individual clinical parameters were summed for each mouse.
Rapamycin treatment
Mice received daily i.p. injections of 1 μg/g of body weight of rapamycin (Selleckchem; 2 mM stock solution stored in ethanol) dissolved in PBS starting one day before adoptive transfer of DO11.10/Rag2−/− cells.
Cell isolation from the skin
Skin from the tail, ears and shaved trunk was minced and digested for 45 min in 4 mL C10 medium with an enzyme mix containing collagenase XI, hyaluronidase, and DNase in supplemented RPMI medium as previously described (7). The cell suspension was filtered, washed, and stained for flow cytometry.
Restimulation for intracellular cytokine staining
Single-cell suspensions from sdLNs were stimulated with 70 ng/ml phorbol-12-myristate-13-acetate and 700 ng/ml ionomycin for 3 hours in C10 medium, with 20 μg/mL brefeldin A added for the last 2 h. Cells were washed with PBS before staining for flow cytometry.
Flow cytometry staining and evaluation
For flow cytometry staining 2×106 freshly isolated or in vitro restimulated single-cell suspensions from LNs or skin were stained using the Foxp3 staining kit (eBioscience) according to the manufacturer’s instructions. For the detection of phosphorylated S6 sdLNs were mechanically disrupted in PBS containing 1.6% paraformaldehyde and fixed for 10 mins at room temperature. The cells were permeabilized by adding 4 volumes of ice-cold methanol and stored at −80 °C. Cells were stained in PBS with 0.5% BSA. Phosphorylated molecules were stained in a two-step staining (using biotin and streptavidin).
Data were acquired on FACSCanto (BD Biosciences) flow cytometer and analyzed using FlowJo software (Tree Star, Inc.). The following gating strategies were applied: cells were gated on live, singlets, and DO11.10 T cells were defined by gating on CD4, KJ1–26, CD90.1 or CD45.1 (as stated in the Figure legends) and Foxp3- or Foxp3+, for Teff and Treg cells, respectively. When data was combined from measurements on different days it was normalized to naïve Foxp3+ tTreg isolated from DO11.10+ Rag2-sufficient mice and stained alongside pTreg according to the resolution metric:
RNA isolation and RT-PCR
Total RNA was isolated from the trunk skin using RNeasy plus mini kit (Qiagen, 74134) combined with a gDNA eliminator column and an on-column DNAse treatment. RNA (0.3–1 μg) was used for reverse transcription with the high capacity iScript™ reverse transcription super mix for RT qPCR (Biorad). qPCR assay was performed on a Rotor Gene using the GoTaq qPCR master mix 2X (Promega). HPRT expression was used as internal housekeeping control.
Primer sequences were: Mouse HPRT1 (forward: GTCCCAGCGTCGTGATTAGC reverse: GAGCAAGTCTTTCAGTCCTGTCC); TGO (forward: TGAAAAACTGACTGAATGGACCA, TGO reverse: TGCTGACCCTACCACCTCTC).
Statistical analysis
Statistical analysis of results was performed using GraphPad Prism Version 8.0. For comparison of two groups two-sample t or Mann Whitney test was used and for more than two groups, one-way ANOVA with Tukey’s multiple comparisons test or Kruskal-Wallis test with Dunn’s multiple comparisons test was used. Error bars indicate means ± SD. Denotation of asterisks is the following: * for p ≤ 0.05, ** for p ≤ 0.01, *** for p ≤ 0.001 and **** for p ≤ 0.0001. For Fig. 4C we applied a linear regression model to the curves after the dose shift and asked whether the slopes were different from each other using an ANCOVA equivalent test using GraphPad Prism.
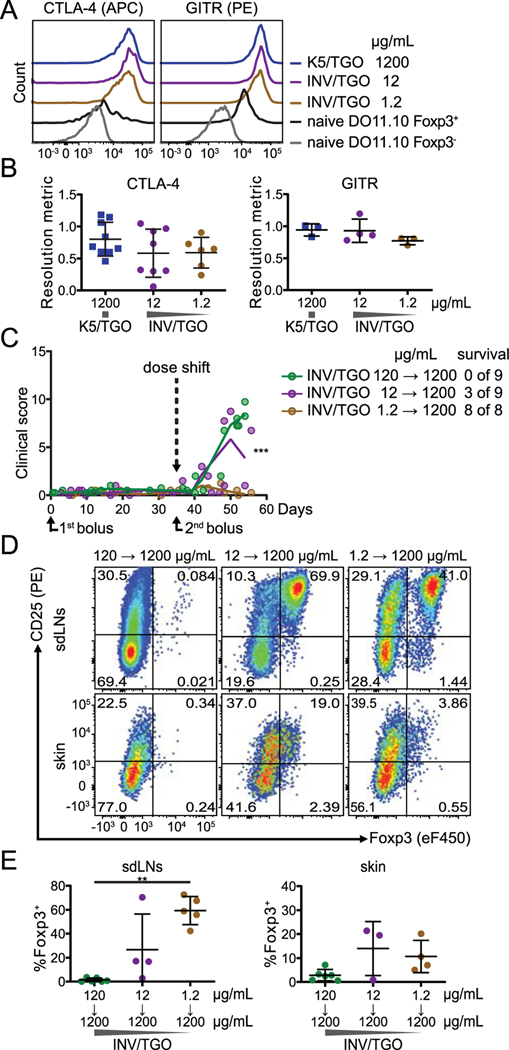
Figure 4: Low dose of tissue antigen favors the generation of stable Foxp3+ cells.
(A and B) OVA expression was induced by the indicated doses of dox (1200, 12, 1.2 μg/mL) and 0.875–1×106 LN cells from DO11.10 Rag2−/− mice were transferred to K5/TGO or INV/TGO mice. Cells were isolated from sdLNs between 22–57 days after transfer. (A, B) Representative overlays (on day 50) and summary graphs displaying the intracellular expression of CTLA-4 (left) or the surface expression of GITR (right) by Foxp3+ DO11.10 cells compared to naïve Foxp3+ tTreg and Foxp3- cells isolated from DO11.10+ Rag2-sufficient mice (all populations gated on live CD4+ DO11.10+). Cumulative data from 3 experiments, N ≥ 3/group. (C, D and E) OVA expression in the skin was induced by different doses of dox (120 – 1.2 μg/mL) and 0.5–0.9×106 LN cells from DO11.10 Rag2−/−CD90.1+/+ mice were transferred into INV/TGO mice. On day 35, 0.9 ×106 LN cells from DO11.10 Rag2−/−CD45.1+/+ mice were transferred into the mice as a second bolus and the dose of dox was shifted to 1200 μg/mL for all groups. (C) Clinical disease course over the duration of the experiment (N ≥ 2/group). The slopes of the curves after the dose shift are significantly different (p= 0.0004). Survival was determined by day 54 after transfer. (D) Representative flow plots of the CD90.1+/+ DO11.10 population (i.e. first bolus) isolated from sdLNs or skin 56–90 days after transfer (from left to right). (E) Percentages of Foxp3+ cells of CD90.1+/+ DO11.10 cells isolated from the sdLNs (left) and the skin (right) 56–90 days after transfer. Cumulative data from 3 experiments, N ≥ 3/group. Mean and SD is shown.
In all plots individual animals are represented by individual symbols.
A detailed list of antibodies and reagents can be found in supplemental table S1.
Results and Discussion
Absence of pTreg generation in INV/TGO mice correlates with lethal autoimmunity
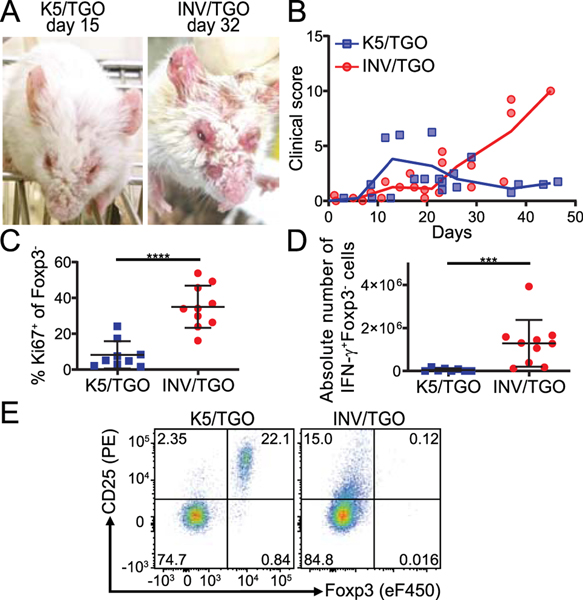
We previously demonstrated that an endogenous skin antigen can promote pTreg generation and protect from autoimmune skin disease. For these studies we utilized a tetracycline-inducible system for the expression of a membrane bound GFP-OVA fusion protein, designated TGO, under the control of the keratin 5 promoter (K5) expressed in the basal cell layer of the epidermis of transgenic mice (K5/TGO) and we followed adoptively transferred naïve OVA-specific CD4+ T cells (DO11.10) in vivo (scheme Supplemental Fig. 1A) (7, 24). As previously described, adoptive transfer recipients developed cutaneous inflammation that quickly resolved, and resolution was associated with the development of OVA-specific Foxp3+ pTreg (7, 24). However, we observed a lethal autoimmune phenotype when we used the same adoptive transfer model but expressed OVA under the control of the involucrin promoter (INV) in the upper layers of the epidermis (INV/TGO). These animals developed progressive inflammation and had to be euthanized within 22–50 days after adoptive transfer, due to disease progression without resolution (Fig. 1A, B). A significantly higher fraction of DO11.10 cells isolated from the sdLNs of INV/TGO mice expressed the proliferation marker Ki67 compared to K5/TGO recipients and significantly more T cells produced the effector cytokine IFN-γ in INV/TGO mice (Fig. 1C, D). In addition, no Foxp3+CD25+ pTreg were generated in INV/TGO recipients whereas a sizeable fraction of DO11.10 cells within K5/TGO mice developed into pTreg (Fig. 1E, and (7)).
Figure 1: INV/TGO mice developed a lethal autoimmune disease in contrast to the self-resolving disease in the K5/TGO model.
OVA expression in the skin was induced by 1200 μg dox/mL and 0.8–1×106 LN cells from DO11.10 Rag2−/− animals were transferred to K5/TGO or INV/TGO mice. (A) Representative pictures of K5/TGO and INV/TGO mice at the height of disease (15 and 32 days after transfer, respectively). (B) Clinical disease course over the duration of the experiment (representative of 3 experiments, N ≥ 8/group in total). (C, D) sdLNs were analyzed by flow cytometry 22–57 days after transfer. Graphical summary of the Ki67 expression and IFN-γ production by live gated CD4+Foxp3-DO11.10-TCR+ Teff cells. Cumulative data from 4 experiments, N ≥ 8/group. (E) Representative flow plots of Foxp3 and CD25 expression by live gated CD4+DO11.10-TCR+ T cells in the sdLNs of recipient animals 22 days after transfer. Mean and SD is shown.
mTOR signaling regulates de novo expression of Foxp3 in peripheral T cells responding to tissue antigen
It is well known that the PI3K/Akt/mTOR network, activated downstream of the TCR, regulates many metabolic and cell differentiation processes and plays a pivotal role in Teff cell differentiation choices via metabolic adaptation (25–30). Additionally, rapamycin (rapa), an immunosuppressive drug that preferentially inhibits the mTORC1 complex (31, 32), has been utilized to generate Foxp3+ iTreg from naïve T cells in vitro (33) or expand tTreg in vivo. However, few studies investigated de novo expression of Foxp3 by naïve T cells in the periphery using rapa (34).
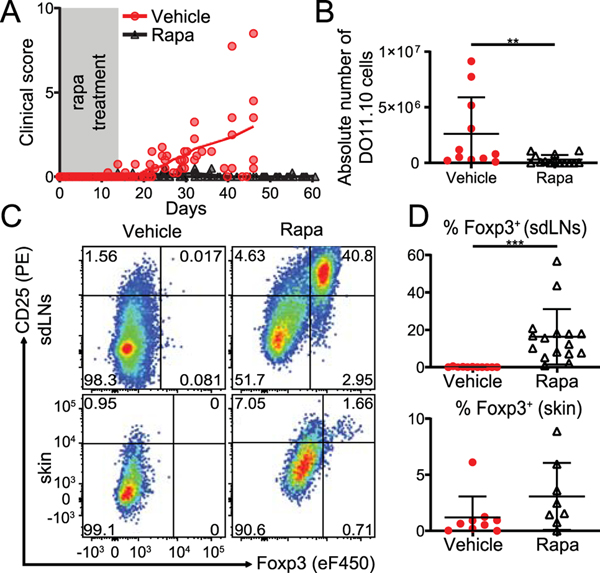
Strong T cell proliferation and lack of Foxp3 induction in INV/TGO mice (Fig. 1C, E) would be consistent with high mTOR activity. To test the role of mTOR, we administered rapa to the recipients of naïve DO11.10 cells (scheme Supplemental Fig. 1B). 18 of 23 (78%) rapa treated mice did not show signs of clinical disease and survived long-term beyond the duration of rapa treatment, while 13 of 16 (81%) of the vehicle treated mice succumbed to autoimmunity (Fig. 2A). Rapa treatment inhibited T cell expansion (Fig. 2B), an effect that has been observed before (35) and which may impact clinical disease. However, most notably in rapa treated mice, Foxp3+ T cells differentiated from naïve T cell precursors upon encountering cutaneous antigen. Foxp3+ T cells could be detected in the blood (Supplemental Fig. 1C, D), sdLNs, and the skin (Fig. 2C, D and Supplemental Fig. 1E). Importantly, once generated Foxp3+ T cells were maintained over time and could be readily detected after up to 10 weeks (i.e. 8 weeks after rapamycin was discontinued) in the face of continued exposure to the tissue antigen for the duration of the experiment (Fig 2C, D).
Figure 2: High mTOR activation inhibits pTreg generation in INV/TGO mice.
OVA expression in the skin was induced with 1200 μg dox/mL and 0.5–0.9×106 LN cells from DO11.10 Rag2−/− animals were transferred into INV/TGO mice. Starting a day before adoptive transfer, mice were treated with either rapamycin (1 μg/g of body weight) or vehicle i.p. injections daily for 16 days. (A) Clinical disease course over the duration of the experiment (representative of 4 experiments, N ≥ 16/group in total). (B) Absolute number of recovered DO11.10 cells from sdLNs between 41–89 days. (C) Representative flow plots of DO11.10 cells recovered from sdLNs or skin (67 and 89 days after transfer in vehicle and rapa treated groups, respectively). (D) Percentages of Foxp3+ DO11.10 cells recovered from the sdLNs (top) and the skin (bottom) between days 41–67 (vehicle) and 71–89 (rapa). B, D show Cumulative data from 4 experiments, N ≥ 8/group. Mean and SD is shown.
Concentration of tissue antigen affects T cell activation and differentiation in vivo
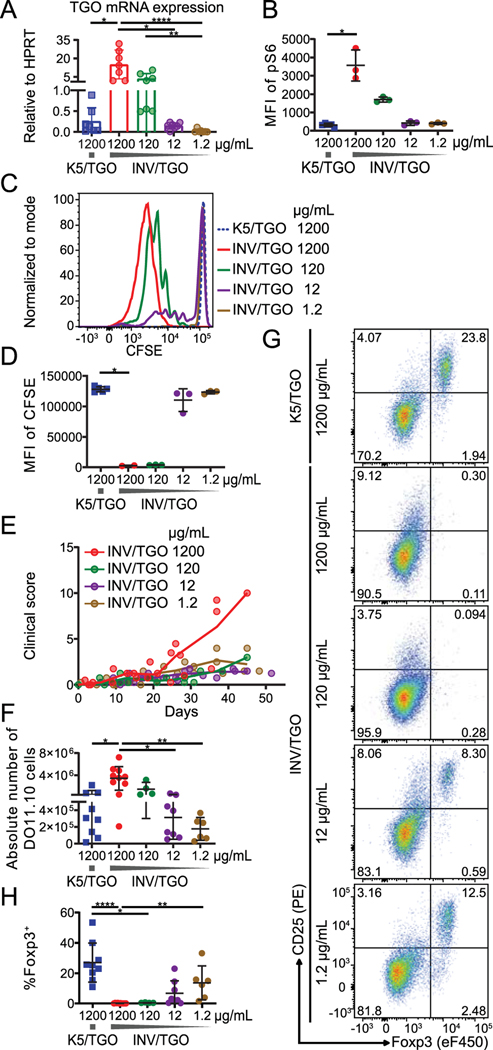
We next aimed to study the endogenous regulation of mTOR activity by the dose of cognate antigen. We hypothesized that high levels of antigen sensed by the TCR lead to high mTOR activity and lack of Treg generation in INV/TGO mice. To test this, we modulated the levels of tissue antigen expressed under the control of a tetracycline-responsive element by varying the dose of the tetracycline analog, doxycycline (dox). By RT-PCR analysis we found that INV/TGO mice displayed about 50-fold higher TGO mRNA expression in the skin than K5/TGO mice (Fig. 3A), and the amount of TGO mRNA expressed by the keratinocytes could be reduced in a dose dependent manner with doses of dox ranging from 1200 to 1.2 μg/mL in the drinking water. We further found that TGO mRNA levels correlated with the activity of mTOR pathway measured by phosphorylation of the ribosomal protein S6, a downstream target of mTORC1 (29, 31), in DO11.10 cells 48–96 hours after adoptive (Fig. 3B and Supplemental Fig. 2A).
Figure 3: T cell activation and differentiation depends on dose of tissue antigen in INV/TGO mice.
OVA expression was induced by the indicated doses of dox (1200 – 1.2 μg/mL) and 0.9–2×106 CFSE-labeled (A-D) or 0.8–1×106 unlabeled (E-H) LN cells from DO11.10 Rag2−/− mice were transferred to K5/TGO or INV/TGO mice. (A) TGO mRNA expression in the skin analyzed by RT-PCR 2–4 days after dox administration. (B) sdLNs were isolated after 2–4 days, immediately fixed ex vivo and pS6 stained for flow cytometry. The Median fluorescence intensity (MFI) of pS6 in transferred DO11.10 cells is plotted (representative of 2 experiments, N ≥ 4/group in total). (C, D) Representative overlay of the CFSE dilution and summary graph of the MFI of CFSE in live DO11.10 cells in the sdLNs after 2–4 days (representative of 2 experiments, N ≥ 4/group in total). (E) Clinical disease course over the duration of the experiment (representative of 2 experiments, N ≥ 5/group in total) (F) Absolute number of live CD4+ DO11.10 cells in sdLNs after 22–57 days. (G) Representative flow plots of the expression of Foxp3 and CD25 by live CD4+ DO11.10 cells from sdLNs 46 days after transfer. (H) Percentages of live CD4+ Foxp3+ DO11.10 cells in the sdLNs after 22–57 days. F-H show cumulative data from 4 experiments, N ≥ 4/group. Mean and SD is shown.
We found that DO11.10 T cell proliferation, measured by CFSE dilution 4 days after transfer, correlated with the titrated antigen levels (Fig. 3C, D). The expression of activation markers and IFN-γ was similarly dependent on the dose of dox (Supplemental Fig. 2B, C).
Mice that received reduced doses of dox developed little clinical disease (Fig. 3E) and in line with the dose-dependent activation of responding T cells, the absolute numbers of DO11.10 cells isolated from the sdLNs of INV/TGO recipients at low doses of dox were comparable to the K5/TGO model (Fig. 3F). Most importantly, at lower antigen doses in INV/TGO recipients, Foxp3+ T cells were detectable in the periphery (Fig. 3G, H) similar to animals treated with rapa, which permitted expression of Foxp3 even at high antigen concentration (Fig. 2D). Thus, de novo Foxp3 expression in peripheral T cells can be achieved through expression of endogenous tissue antigen at low levels.
Foxp3+ T cells induced by low dose of tissue antigen are stable
Treg generated from naïve T cells can be insufficiently functional or lose Foxp3 expression upon inflammatory challenge thus converting into Teff cells (36). To investigate whether Foxp3+ T cells generated at permissive doses of dox (12 and 1.2 μg/mL) in the INV/TGO model were functional and stable, we first interrogated their phenotype by flow cytometry. The in vivo generated Foxp3+ T cells expressed the canonical Treg markers GITR and CTLA-4 (Fig. 4A, B). The expression of these molecules was even higher than that of naïve thymic Treg (Fig. 4A).
To functionally test the suppressive activity and stability of Foxp3+ T cells generated at permissive doses, we challenged these cells by increasing the dose of dox to 1200 μg /mL after 35 days of low dose exposure. At the same time, we adoptively transferred a second bolus of congenically marked naïve DO11.10 cells (scheme Supplemental Fig. 1F) which cause lethal disease at the high dose of dox (Fig. 3E). Recipients without Foxp3+ T cells from the first bolus were not protected from autoimmunity (0% survival at initial dose of 120 μg dox/mL), while 33%, 3/9 animals, survived in hosts where the first bolus of DO11.10 cells was primed at 12 μg dox/mL and 100% survived after receiving 1.2 μg dox/mL in the initial dox treatment (Fig. 4C). In line with reduced or absent disease, Foxp3+ T cells generated from the first bolus of DO11.10 cells in response to low dose tissue antigen (12 and 1.2 μg dox/mL dox) retained Foxp3 expression in vivo and could be recovered from the sdLNs as well as from the skin up to 8 weeks after increasing the dose of dox and exposing T cells to high levels of antigen (Fig. 4D, E).
Taken together, using a system in which de novo expression of Foxp3 can be studied in vivo in the absence of any potentially confounding impact of expanding tTreg (37), we show that reducing antigen-induced signals in T cells is permissive for the development of Foxp3+ T cells in the periphery. Although similar conclusions have been reached using titrated administration of foreign antigens (with and without rapamycin) and by in vitro studies (20, 21, 33), we now show that Foxp3 expression is induced in response to low levels of endogenous tissue antigen in vivo. Our results also show that Foxp3+ T cells induced under conditions of limiting TCR signals are stable in vivo long-term. These findings suggest that this approach may be beneficial in protocols for therapeutic tolerance induction to treat autoimmunity.
Supplementary Material
Key points:
Expression level of tissue antigen determines the fate of responding T cells in vivo
Rapa rescues the generation of peripheral Foxp3+ T cells even at high antigen dose
Low level of endogenous tissue antigen differentiates stable Foxp3+ T cells in vivo
Acknowledgements
This work was supported by the Ph.D. program Immunity in Cancer and Allergy (Grant W1213 to I.K.G. and J.T.) and the Special Research Program (SFB F 70 to I.K.G.), both funded by the Austrian Science Fund. The work was also supported by National Institutes of Health Grant R03AR064554 (to I.K.G.), a grant from the Dystrophic Epidermolysis Bullosa Research Association Austria, and the Focus Program Allergy Cancer BioNano Research Centre of the University of Salzburg. M.M.K. was a recipient of a DOC fellowship awarded by the Austrian Academy of Sciences.
Abbreviations used in this article:
- dox
doxycycline
- Foxp3
forkhead boxP3
- GITR
glucocorticoid-induced TNFR-family-related gene
- INV
involucrin
- IPEX syndrome
Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome
- K5
keratin 5
- mTOR
mammalian target of rapamycin
- rapa
rapamycin
- (sd)LN
(skin-draining) lymph node
- Teff cells
effector T cell
- TGO
TGO construct encodes a fusion protein linking the transferrin receptor transmembrane domain, GFP, and aa 230–359 of chicken OVA
- tTreg or pTreg
thymic or peripheral regulatory T cell
References
- 1.Ramsdell F, and Ziegler SF. 2014. FOXP3 and scurfy: how it all began. Nat. Rev. Immunol. 14: 343–349. [DOI] [PubMed] [Google Scholar]
- 2.Gratz IK, and Campbell DJ. 2014. Organ-specific and memory treg cells: specificity, development, function, and maintenance. Front. Immunol. 5: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, and Konkel JE. 2015. The development of thymic Foxp3+ regulatory T cells: TGF-β matters. Eur. J. Immunol. 45: 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowan JE, Jenkinson WE, and Anderson G. 2015. Thymus medulla fosters generation of natural Treg cells, invariant γδ T cells, and invariant NKT cells: What we learn from intrathymic migration. Eur. J. Immunol. 45: 652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apostolou I, Verginis P, Kretschmer K, Polansky J, Hühn J, and von Boehmer H. 2008. Peripherally induced Treg: mode, stability, and role in specific tolerance. J. Clin. Immunol. 28: 619–624. [DOI] [PubMed] [Google Scholar]
- 6.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, Rudensky A, Sakaguchi S, Shevach EM, Vignali DAA, and Ziegler SF. 2013. Regulatory T cells: recommendations to simplify the nomenclature. Nat. Immunol. 14: 307–308. [DOI] [PubMed] [Google Scholar]
- 7.Gratz IK, Rosenblum MD, Maurano MM, Paw JS, Truong H-A, Marshak-Rothstein A, and Abbas AK. 2014. Cutting Edge: Self-antigen controls the balance between effector and regulatory T cells in peripheral tissues. J. Immunol. Baltim. Md 1950 192: 1351–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, and Rudensky AY. 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T cell generation. Nature 504: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio C-W, Santacruz N, Peterson DA, Stappenbeck TS, and Hsieh C-S. 2011. Peripheral education of the immune system by colonic commensal microbiota. Nature 478: 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontenot JD, Gavin MA, and Rudensky AY. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4: 330–336. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Rowell EA, Thomas RM, Hancock WW, and Wells AD. 2006. Transcriptional regulation by Foxp3 is associated with direct promoter occupancy and modulation of histone acetylation. J. Biol. Chem. 281: 36828–36834. [DOI] [PubMed] [Google Scholar]
- 12.Bettelli E, Dastrange M, and Oukka M. 2005. Foxp3 interacts with nuclear factor of activated T cells and NF-κB to repress cytokine gene expression and effector functions of T helper cells. Proc. Natl. Acad. Sci. U. S. A. 102: 5138–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verhagen J, Wegner A, and Wraith DC. 2015. Extra-thymically induced T regulatory cell subsets: the optimal target for antigen-specific immunotherapy. Immunology 145: 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O’Gorman WE, and Abbas AK. 2010. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J. Immunol. Baltim. Md 1950 185: 6426–6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, and Rudensky AY. 2010. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463: 808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, de Bovis B, Alexopoulou L, Dalod M, and Malissen B. 2010. Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood 115: 1958–1968. [DOI] [PubMed] [Google Scholar]
- 17.Semple K, Nguyen A, Yu Y, Wang H, Anasetti C, and Yu X-Z. 2011. Strong CD28 costimulation suppresses induction of regulatory T cells from naive precursors through Lck signaling. Blood 117: 3096–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purvis HA, Stoop JN, Mann J, Woods S, Kozijn AE, Hambleton S, Robinson JH, Isaacs JD, Anderson AE, and Hilkens CMU. 2010. Low-strength T-cell activation promotes Th17 responses. Blood 116: 4829–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner MS, Isse K, Fischer DK, Turnquist HR, and Morel PA. 2014. Low TCR signal strength induces combined expansion of Th2 and regulatory T cell populations that protect mice from the development of type 1 diabetes. Diabetologia 57: 1428–1436. [DOI] [PubMed] [Google Scholar]
- 20.van Panhuys N. 2016. TCR Signal Strength Alters T–DC Activation and Interaction Times and Directs the Outcome of Differentiation. Front. Immunol. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottschalk RA, Corse E, and Allison JP. 2010. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J. Exp. Med. 207: 1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diamond I, Owolabi T, Marco M, Lam C, and Glick A. 2000. Conditional Gene Expression in the Epidermis of Transgenic Mice Using the Tetracycline-Regulated Transactivators tTA and rTA Linked to the Keratin 5 Promoter. J. Invest. Dermatol. 115: 788–794. [DOI] [PubMed] [Google Scholar]
- 23.Jaubert J, Patel S, Cheng J, and Segre JA. 2004. Tetracycline-Regulated Transactivators Driven by the Involucrin Promoter to Achieve Epidermal Conditional Gene Expression. J. Invest. Dermatol. 123: 313–318. [DOI] [PubMed] [Google Scholar]
- 24.Gratz IK, Truong H-A, Yang SH-Y, Maurano MM, Lee K, Abbas AK, and Rosenblum MD. 2013. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J. Immunol. Baltim. Md 1950 190: 4483–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo Y-C, Lee C-F, and Powell JD. 2014. Insight into the role of mTOR and metabolism in T cells reveals new potential approaches to preventing graft rejection. Curr. Opin. Organ Transplant. 19: 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu JSL, and Cui W. 2016. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Dev. Camb. Engl. 143: 3050–3060. [DOI] [PubMed] [Google Scholar]
- 27.Salmond RJ, Emery J, Okkenhaug K, and Zamoyska R. 2009. MAPK, phosphatidylinositol 3-kinase, and mammalian target of rapamycin pathways converge at the level of ribosomal protein S6 phosphorylation to control metabolic signaling in CD8 T cells. J. Immunol. Baltim. Md 1950 183: 7388–7397. [DOI] [PubMed] [Google Scholar]
- 28.Apostolidis SA, Rodríguez-Rodríguez N, Suárez-Fueyo A, Dioufa N, Ozcan E, Crispín JC, Tsokos MG, and Tsokos GC. 2016. Phosphatase PP2A is requisite for the function of regulatory T cells. Nat. Immunol. 17: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman NM, and Chi H. 2014. mTOR Links Environmental Signals to T Cell Fate Decisions. Front. Immunol. 5: 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, and Merkenschlager M. 2008. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. U. S. A. 105: 7797–7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Lawrence JC, Sturgill TW, and Harris TE. 2009. Mammalian target of rapamycin complex 1 (mTORC1) activity is associated with phosphorylation of raptor by mTOR. J. Biol. Chem. 284: 14693–14697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballou LM, and Lin RZ. 2008. Rapamycin and mTOR kinase inhibitors. J. Chem. Biol. 1: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haxhinasto S, Mathis D, and Benoist C. 2008. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 205: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniel C, Wennhold K, Kim H-J, and von Boehmer H. 2010. Enhancement of antigen-specific Treg vaccination in vivo. Proc. Natl. Acad. Sci. U. S. A. 107: 16246–16251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sehgal SN, and Bansbach CC. 1993. Rapamycin: in vitro profile of a new immunosuppressive macrolide. Ann. N. Y. Acad. Sci. 685: 58–67. [DOI] [PubMed] [Google Scholar]
- 36.Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, Fehling HJ, and Bluestone JA. 2013. Self-antigen driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 39: 949–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Wang W, Xu J, and Le Q. 2013. Effect of rapamycin and interleukin-2 on regulatory CD4+CD25+Foxp3+ T cells in mice after allogenic corneal transplantation. Transplant. Proc. 45: 528–537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.