Abstract
Aldose reductase (ALR2) is thought to be involved in the pathogenesis of various diseases associated with diabetes mellitus, such as cataract, retinopathy, neuropathy, and nephropathy. However, its physiological functions are not well understood. We developed mice deficient in this enzyme and found that they had no apparent developmental or reproductive abnormality except that they drank and urinated significantly more than their wild-type littermates. These ALR2-deficient mice exhibited a partially defective urine-concentrating ability, having a phenotype resembling that of nephrogenic diabetes insipidus.
Aldose reductase (ALR2) is the first enzyme in the polyol pathway. It was first described by Hers in 1956 (13). Using NADPH as a cofactor, it reduces glucose to sorbitol in addition to reducing other sugars to their respective polyols. The activation of the sorbitol pathway under hyperglycemic conditions is thought to be the cause of diabetic lesions in tissues where the import of glucose is independent of insulin, such as the lens, vascular cells, and nervous tissues (18, 28). Although ALR2 has been thoroughly studied for its role in the etiology of diabetic complications, its physiological functions are not well understood. ALR2 is present in most tissues surveyed and has been implicated in a wide variety of physiological functions. It is thought to be responsible for synthesizing fructose in the seminal vesicle to be used as the main energy source for sperm motility, because fructose is converted from sorbitol by the enzyme sorbitol dehydrogenase (SORD) (13). ALR2 can efficiently reduce methyglyoxal (35), 4-hydroxynonenal (34), and 3-deoxyglucosone (29), suggesting that it may be responsible for detoxification of these and other harmful metabolites. Another postulated function of ALR2 is osmoprotection in the kidney (1). This is based on the facts that sorbitol is an inert compound ideally suited as an osmolyte and that its intracellular concentration in certain tissues can reach a high enough level to affect osmotic pressure. Further, elevated extracellular NaCl was shown to elicit a marked increase in ALR2 expression and the accumulation of intracellular sorbitol in the cell line cultured from rabbit renal medullae (1), suggesting that kidney cells respond to an increase in extracellular osmotic pressure by producing more sorbitol. In support of this notion, osmotic response elements have been identified in the promoter regions of the rabbit (10) and human (20) ALR2 genes.
To test these proposed functions of ALR2, we developed two mouse lines deficient in this enzyme. ALR2 knockout mice (Aldor1−/−) appeared to grow normally and did not show any obvious abnormalities in their reproductive function. Upon closer examination, they were found to have developed polyuria and polydipsia. These Aldor1−/− mice exhibit a partially defective urine-concentrating ability, leading to a phenotype resembling that of diabetes insipidus (DI).
There are several known causes of DI. Deficiency in the synthesis or secretion of the antidiuretic hormone arginine vasopressin (AVP) leads to so-called hereditary hypothalamic DI (17, 32). This hormone signals the translocation of aquaporin 2 from the cytoplasm to the cell surface to facilitate the uptake of water (23, 26). Failure to respond to the AVP signal or defective aquaporins lead to DI of the nephrogenic type (6, 36). In this report we show that ALR2 deficiency also leads to nephrogenic DI. The impairment of water reabsorption in the kidneys of these mice is not due to a deficiency in AVP, AVP receptor, or aquaporin 2. These mice may provide further insights into the urine-concentrating mechanism in the kidney.
MATERIALS AND METHODS
Creation of Aldor1−/− mice.
A 2.7-kb fragment of the mouse Aldor1 gene containing part of exon 5 and all of exons 6, 7, and 8 was replaced with a phosphoglycerate kinase (PGK)-neor cassette. This targeting vector in pPNT, which also contains a hsv-tk gene, was linearized at the NotI site and electroporated into the AB2.2 embryonic stem (ES) cell line. Cells resistant to G418 and fialuridine were selected, and two ALR2 knockout clones (AR8 and AR51) from two independent transfections were identified by Southern blot analysis, using an EcoRI-digested probe from the Aldor1 gene. Cells from these two clones were injected into C57BL/6 embryos at the blastocyst stage. Chimeric offspring were mated with C57BL/6 mice, and offspring containing the Aldor1 null allele were identified by Southern analysis using DNA extracted from segments of the mouse tail. Northern blot analysis was done by agarose-formaldehyde gel electrophoresis of total RNA. The RNA in the gel was then transferred to a Hybond-N+ nylon membrane and hybridized with a 32P-radiolabeled Aldor1 cDNA probe containing exons 5 to 8. Western blot analysis was done by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of total protein, which was blotted onto nitrocellulose membrane, probed with primary rabbit anti-mouse ALR2 antibody and secondary antibody of donkey anti-rabbit immunoglobulin (Ig) linked to horseradish peroxidase (Amersham Life Science Ltd., Little Chalfont, Buckinghamshire, England), and detected by the enhanced chemiluminescence method.
Metabolic experiment and assays for serum and urine solutes.
Mice were maintained in a daily cycle of 12-h light and 12-h darkness and were allowed free access to standard mouse chow and water. Individual mice were put into metabolic cages to measure water consumption and urine output and for urine sample collection. Serum samples were collected from tail arteries. Na, K, Ca, Cl, urea, creatinine, and albumin levels in sera were measured with the Hitachi-747 autoanalyzer (Boehringer-Mannheim, Mannheim, Germany). Na, K, Ca, Cl, urea, and creatinine levels in urine were measured with the Synchron CX5 analyzer (Beckman Instruments, Inc., Fullerton, Calif.). Urine and serum osmolality were measured by the vapor pressure method, using the Vapro vapor pressure osmometer (Wescor Inc., Logan, Utah). Blood glucose was measured using a Glucometer Elite (Bayer Australia Ltd., Pymble, New South Wales, Australia). The urine AVP level was measured by a competitive enzyme assay method with an AVP enzyme immunoassay kit (Assay Designs Inc., Ann Arbor, Mich.), and each sample was run in duplicate with spiked standards as controls.
Response to water deprivation and dDAVP injection in Aldor1+/+ and Aldor1−/− mice.
Mice were put into metabolic cages individually for 1 day with free access to standard mouse chow and water. Water bottles were then removed for a period of 24 h. Urine samples were collected by spontaneous voiding before and after the 24-h water deprivation period for the determination of osmolality by the vapor pressure method. Body weights were taken before and after the deprivation experiment. In some experiments, urine samples were collected by spontaneous voiding before and after the intraperitoneal injection of dDAVP (0.4 μg/kg of body weight) to measure osmolality.
Preparation of probes for Northern blot analysis of aquaporin 2, aquaporin 3, and V2R.
Northern blotting was performed as described above. Probes for detecting aquaporin 2, aquaporin 3, and V2R transcripts were PCR amplified from Marathon-Ready cDNA (Clontech, Palo Alto, Calif.) with primers designed from the published Mus musculus aquaporin 2 mRNA sequence (GenBank accession no. AF 020519, forward primer bp 1 to 24, reverse primer bp 793 to 816), M. musculus aquaporin 3 mRNA sequence (accession no. AF 104416, forward primer bp 70 to 93, reverse primer bp 917 to 940), and M. musculus V2R gene sequence (accession no. AJ 006691, forward primer bp 477 to 500, reverse primer bp 2,084 to 2,107), respectively.
Immunoblotting analysis.
Affinity-purified, peptide-derived rabbit polyclonal antibodies to aquaporin 1 (31) and to aquaporin 2 (7) and to the collecting duct urea transporter UTA-1 (27) were used for immunoblotting. Mice were sacrificed by decapitation and kidneys were excised. Inner medullae were isolated and homogenized in isolation buffer (10 mM triethanolamine and 250 mM sucrose [pH 7.6]) with protease inhibitors (Complete protease inhibitor cocktail tablets; Boehringer-Mannheim). Aliquots of homogenates were assayed for protein concentration by using Bradford's method (3) and brought to a final concentration in Laemmli sample buffer containing 7.5% SDS and 0.2 M dithiothrietol at −20°C till used.
SDS-PAGE was done using 10 or 12% polyacrylamide minigels. In all cases, to confirm equality of loading among lanes, electrophoresis was initially run for the entire set of samples in a given experiment on a single SDS–12% PAGE gel, which was then stained with Coomassie blue. Selected bands from these gels were analyzed by densitometry (Molecular Dynamics, San Jose, Calif.) to provide quantitative assessment of loading. These loading gels established that subsequent immunoblots (loaded identically) were uniformly loaded. Proteins were transferred electrophoretically from gels to nitrocellulose membranes. After blocking with 5 g of nonfat dry milk/dl, proteins were probed overnight at 4°C with the desired antibody at the following IgG concentrations (in micrograms per milliliter): 0.23 for aquaporin 1, 0.12 for aquaporin 2, and 0.09 for UTA-1. The antibodies were prepared in an antibody diluent containing 150 mM NaCl, 50 mM sodium phosphate, 10 mg of sodium azide/dl, 50 mg of Tween 20/dl, and 1 g of bovine serum albumin/dl (pH 7.5). The secondary antibody was goat anti-rabbit IgG conjugated to horseradish peroxidase (no. 31463, Pierce) and was used at a concentration of 0.16 μg/ml. Sites of antibody-antigen reaction were visualized using luminol-based enhanced chemiluminescence (LumiGLO; Kirkegaard and Perry Laboratories, Gaithersburg, Md.) before exposure to X-ray film (Kodak 3165-1579 scientific imaging film).
Determination of osmolyte content in mouse kidneys.
Kidneys were excised from sacrificed mice, quickly frozen in liquid N2, and stored at −80°C until processing. Cortex and medullary regions were excised by using razor-sharp blades while the kidneys were thawing on ice. Tissue from both kidneys was pooled for preparation. Sample preparation and high-pressure liquid chromatography conditions were as described previously (37). Briefly, samples were homogenized in 6% perchloric acid and centrifuged at ∼1,700 × g for 5 min. Pellets were later used for protein assays, and the supernatant was neutralized to pH ∼7 with 5 M KOH. The supernatant was then passed through a Sep-Pak C18 cartridge (Waters Corporation, Milford, Mass.) to remove fats and through a 0.45-μm-pore-size filter to remove particulate matter. Fifty microliters of each sample or standard was injected by an autosampler (Waters 717 Plus) to the high-pressure liquid chromatography column. Separation was achieved by a Sugar-Pak I column (Waters) isocratically perfused at 0.6 ml/min and 74°C using 50 mg of Ca-EDTA/liter of water as the mobile phase. Peaks were detected by a differential refractometer (Waters 410). Standards containing 5 to 200 nmol of various organic osmolytes were run to create the calibration curve. The amount of organic osmolytes in a specific kidney sample was calculated by comparing the peak area of the respective substance with the corresponding standard curve.
RESULTS
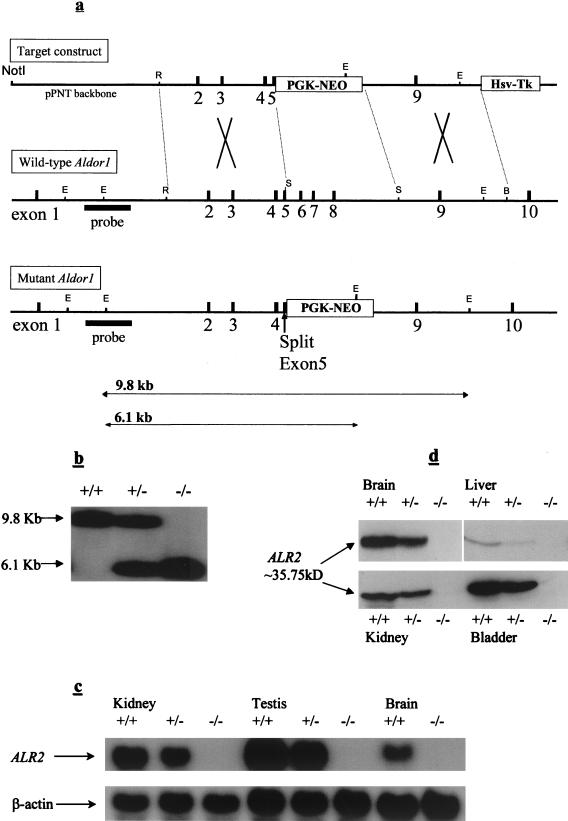
The Aldor1 gene has been previously cloned and characterized (14). The Aldor1 gene was disrupted in ES cells by homologous recombination with the transforming DNA containing the Aldor1 gene where exons 5, 6, 7, and 8 were replaced by the neomycin-resistant gene (Fig. 1a). Two independent Aldor1−/− ES cell lines were established from which two ALR2-deficient mouse lines (ARD1 and ARD2) were generated. Breeding of heterozygous founder mice produced wild-type heterozygous and homozygous progeny, as determined by Southern blot analysis (Fig. 1b), at a ratio consistent with the 1:2:1 Mendelian inheritance. Heterozygous (Aldor1+/−) and homozygous (Aldor1−/−) ALR2-deficient mice were normal in appearance, and their body weights were comparable to those of their wild-type littermates. Northern and Western blot analysis showed, respectively, that ALR2 mRNA and protein are absent in Aldor1−/− mice and reduced in Aldor1+/− mice to about half of that found in the wild type (Fig. 1c and d). Aldor1−/− mice have no sorbitol in their kidneys (data shown below), the only tissue where sorbitol is detectable in normal mice. These results indicate that Aldor1−/− mice are indeed deficient in ALR2 and that ALR2 is the major enzyme responsible for the synthesis of sorbitol in the kidney.
FIG. 1.
Generation of ALR2-deficient mice. (a) The gene targeting construct, containing the herpes simplex virus tk gene (tk) and the neomycin resistance gene (neo). The restriction map of the wild-type and mutant ALR2 genes are shown. Relative positions of all exons are shown in filled boxes. The NotI site used to linearize the construct and the outside probe (filled bar) and the expected sizes of EcoRV fragments used for analysis of genomic DNA are indicated. E, EcoRV; R, EcoRI; S, SpeI; B, BglII. (b) Genotyping the ALR2 allele by Southern blot analysis. The expected bands of ∼9.8 kb for the wild-type ALR2 allele and ∼6.1 kb for the mutant ALR2 allele were observed. (c) Northern analysis of total RNA (10 μg/lane) from kidney, testis, and brain tissue of F2 mice. The blot was analyzed with an ALR2 cDNA probe containing exons 5 to 8 and normalized with β-actin. An ∼1.3-kb band was detected representing the ALR2 transcript. (d) Western analysis of total protein isolated from brain, liver, bladder, and kidney extracts of F2 mice. ALR2 was detected using a polyclonal rabbit antibody specific for it.
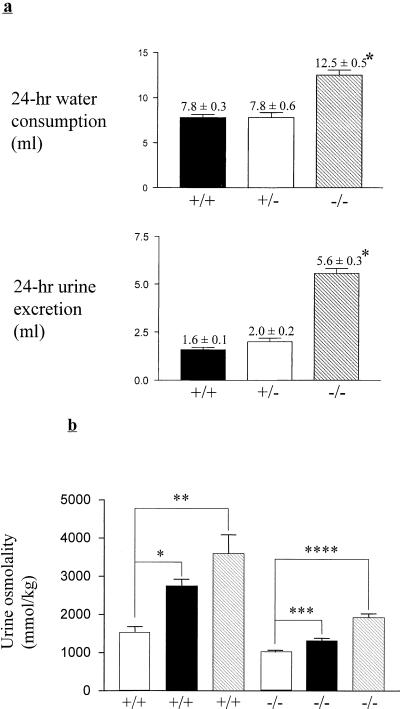
We soon noticed that Aldor1−/− mice exhibited polydipsia and polyuria (Fig. 2a). Since both independently derived mouse lines exhibit the same behavior, these traits are most likely the consequence of ALR2 deficiency, not the result of inactivation or activation of some genes in the process of engineering these mice. All subsequent data were derived from ARD1 mice. Polydipsia and polyuria are symptoms of diabetes mellitus as well as DI. However, ALR2-deficient mice have a normal blood glucose level (5.8 ± 0.23 and 6.1 ± 0.23 mmol/liter for Aldor1+/+ and Aldor1−/−, respectively). Therefore, it is unlikely that the mice's excessive drinking is due to diabetes mellitus. The urine osmolality of ALR2-deficient mice is about one-third of that of normal mice (Table 1). Urinary concentrations of sodium, potassium, calcium, and chloride ions as well as the concentrations of urea and creatinine were all lower in Aldor1−/− mice. However, the total amount of these urinary solutes excreted within a 24-h period was the same for ALR2 knockout and wild-type mice. This is also reflected by the similar levels of these solutes in the sera of ALR2 knockout and wild-type mice (Table 1). These results show that other than water absorption, the other functions of the kidney are not impaired and it is unlikely that polydipsia and polyuria in these mice are due to hypokalemia (15, 22) or hypercalcemia (8, 12).
FIG. 2.
(a) Water intake and urine output in Aldor1+/+, Aldor1+/−, and Aldor1−/− mice. Water consumption and urine excretion by wild-type (n = 20), heterozygous (n = 15), and knockout (n = 20) male mice during a 24-h period were determined. Mice were housed individually in metabolic cages, and values were determined over a 2-day period. Data are means ± standard errors of the means. ∗, P < 0.0001 compared with Aldor1+/+ and Aldor1+/− mice. Statistical significance was determined by the unpaired Student t test. (b) Urine-concentrating ability before and after a 24-h water deprivation period or AVP injection in Aldor1+/+ and Aldor1−/− mice, measured as urine osmolality. White bars, before deprivation or AVP injection; dark bars, after AVP injection; striped bars, after deprivation. Data are means ± standard errors of the means (n = 4). ∗, P = 0.0005; ∗∗, P = 0.0024; ∗∗∗, P = 0.0014; ∗∗∗∗, P < 0.0001. Statistical significance was determined by the unpaired Student t test.
TABLE 1.
Urine and serum solutes of euhydrated Aldor1+/+ and Aldor1−/− micea
| Sample and substance | Aldor1+/+ | Aldor1−/− |
|---|---|---|
| Urine | Concn | |
| Osm (mmol/kg) | 3,173 ± 194 | 1,128 ± 54* |
| Na (mmol/liter) | 138.7 ± 8.3 | 59.5 ± 3.2* |
| K (mmol/liter) | 376.6 ± 22.0 | 147.2 ± 4.6* |
| Ca (mmol/liter) | 3.5 ± 0.3 | 1.4 ± 0.1* |
| Cl (mmol/liter) | 182.6 ± 12.0 | 70.43 ± 2.0* |
| Creatinine (μmol/liter) | 5,493 ± 306 | 2,257 ± 88* |
| Urea (mmol/liter) | 1,367 ± 68 | 569.7 ± 21* |
| AVP (ng/ml) | 0.80 ± 0.17 | 0.56 ± 0.11** |
| Serum | ||
| Osm (mmol/kg) | 316.9 ± 1.0 | 317.3 ± 1.4 |
| Na (mmol/liter) | 151.5 ± 0.5 | 153.6 ± 0.6 |
| K (mmol/liter) | 4.4 ± 0.08 | 4.4 ± 0.12 |
| Ca (mmol/liter) | 2.2 ± 0.02 | 2.3 ± 0.02 |
| Cl (mmol/liter) | 114.1 ± 0.5 | 115.5 ± 0.8 |
| Urea (mmol/liter) | 11.0 ± 0.6 | 10.6 ± 0.5 |
| Creatinine (μmol/liter) | 40.6 ± 1.2 | 44.3 ± 1.6 |
| Albumin (g/liter) | 25.6 ± 0.5 | 25.4 ± 1.1 |
| Urine | 24-h excretion | |
| Na (mmol) | 0.27 ± 0.02 | 0.31 ± 0.02 |
| K (mmol) | 0.68 ± 0.05 | 0.76 ± 0.02 |
| Ca (mmol) | 0.0059 ± 0.0005 | 0.0067 ± 0.0004 |
| Cl (mmol) | 0.32 ± 0.03 | 0.36 ± 0.02 |
| Creatinine (μmol) | 10.0 ± 0.8 | 11.9 ± 0.6 |
| Urea (mmol) | 2.5 ± 0.18 | 3.0 ± 0.11 |
| AVP (ng) | 1.6 ± 0.3 | 3.6 ± 0.7*** |
Values are means ± standard errors of the means (n = 10). *, P < 0.0001; **, P = 0.2551; ***, P = 0.0215 compared with Aldor1+/+ mice. Osm, osmolality.
In order to determine if the impairment of urine-concentrating ability in these mice is due to a defect in the secretion of AVP, the level of this hormone in urine was measured. Urinary AVP concentration was comparable for Aldor1+/+ and Aldor1−/− mice, while the total amount of AVP excreted in a 24-h period was higher for Aldor1−/− mice (Table 1), indicating that their AVP synthesis and secretion were not impaired. Furthermore, when these mice were intraperitoneally injected with V2R agonist dDAVP (0.4 μg/kg), their urine osmolality was increased by only about 28%, while similarly treated wild-type mice's urine osmolality was increased by 85% (Fig. 2b), suggesting that the defect in water reabsorption is not due to a deficiency in AVP secretion. Similar results were obtained when these mice were deprived of drinking water for 24 h (Fig. 2b). ALR2-deficient mice were not as efficient as their wild-type littermates in reabsorbing water from the urine and consequently lost 20% of their body weights in that period, compared to 11% for the wild type. Taken together, these results indicate that the defect in the urine-concentrating ability of ALR2-deficient mice is within the kidney.
In the kidney collecting duct cells, AVP binds to its receptor, V2R, which activates adenylyl cyclase and increases the production of cyclic AMP (cAMP). The cAMP in turn stimulates the translocation of the major water channel, aquaporin 2, from the cytoplasm to the apical membrane of the cell (23, 26). Together with other water channels, mainly aquaporin 3 and aquaporin 4, this allows water to permeate through the cell from the luminal to the basolateral compartments, enhancing water reabsorption in the collecting ducts (2, 19).
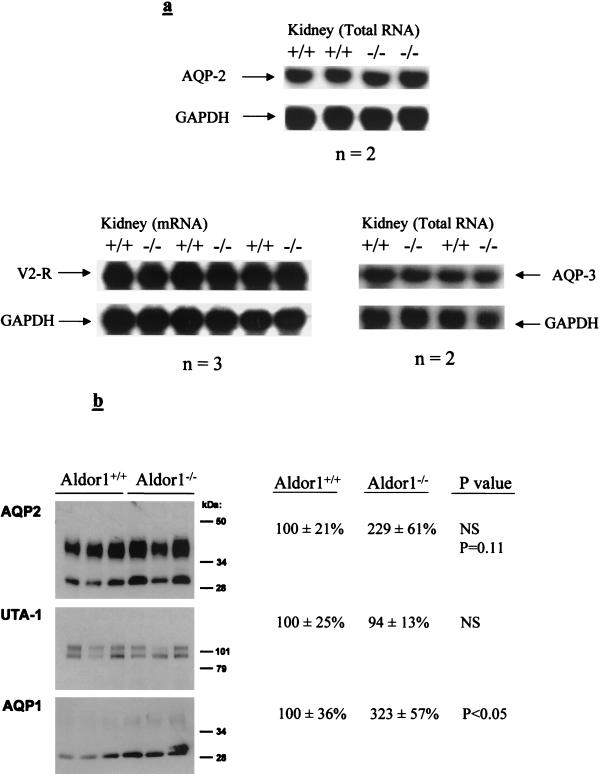
We therefore determined if the expression of V2R, aquaporin 2, and aquaporin 3 is affected by ALR2 deficiency, leading to the impairment of urine-concentrating ability. The mRNA levels of V2R, aquaporin 2, and aquaporin 3 were analyzed by using Northern blot hybridization. The expression levels of these three transcripts were not significantly different in ALR2-deficient and wild-type mice (Fig. 3a). To determine whether there was a change of aquaporin 2 protein levels in Aldor1−/− mice, immunoblots were performed using protein extracts from kidney inner medullae. In addition, the levels of aquaporin 1 and UTA-1 proteins were also determined. The result showed no significant change in aquaporin 2 and UTA-1 protein levels for Aldor1+/+ and Aldor1−/− mice, while the protein level of aquaporin 1 was increased in Aldor1−/− mice (Fig. 3b).
FIG. 3.
(a) Detection of aquaporin 2 (AQP-2), aquaporin 3 (AQP-3), and V2R mRNA levels. Results of Northern blot analysis of total RNA (10 μg/lane) and mRNA (3 μg/lane) from kidney extracts of Aldor1+/+ and Aldor1−/− mice are shown. The blots were analyzed with probes designed from the cDNA sequences of the three genes published in GenBank (discussed in Methods and Materials). The blots were normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (b) Immunoblots comparing AQP2, AQP1, and UTA-1 expression in kidney inner medullae from Aldor1+/+ and Aldor1−/− mice. Loading was 2 μg/lane for AQP1 and AQP2 and 10 μg/lane for UTA-1. Densitometry data are shown on the right. Statistical significance was determined by the unpaired Student t test.
One possible explanation for the reduced ability to concentrate urine in these mice is their inability to synthesize sorbitol, which led to reduced total osmolyte content in the epithelial cells of the collecting tubules. This may reduce the osmotic gradient such that water absorption is impaired. We measured the level of the major osmolytes in the renal medullae of normal and ALR2-deficient mice. The levels of myo-inositol, taurine, glycerophosphorylcholine, and betaine were comparable for these mice (Table 2). As expected, sorbitol was undetectable (<0.01 nM/mg of wet weight) in the kidney samples of ALR2-deficient mice, while the kidneys of their wild-type littermates contained ∼1.4 nM sorbitol/mg of wet weight. However, that amount of sorbitol constitutes less than 2% of the total osmolality in the wild-type mouse kidney. Therefore, it would be unlikely that a 2% drop in the osmolyte content in ALR2-deficient mice would cause a drastic impairment of water absorption.
TABLE 2.
Kidney organic osmolyte, glucose, and glycine content in Aldor1+/+ and Aldor1−/− mice
| Substance | Concn (nM/mg of wet wt)a
|
|||
|---|---|---|---|---|
|
Aldor1+/+
|
Aldor1−/−
|
|||
| Medulla | Cortex | Medulla | Cortex | |
| myo-Inositol | 9.54 ± 0.46 | 3.99 ± 0.16 | 9.20 ± 0.30 | 4.53 ± 0.31 |
| Taurine | 12.33 ± 0.25 | 13.69 ± 0.59 | 12.98 ± 0.30 | 13.55 ± 0.53 |
| GPC | 6.48 ± 0.40 | 1.42 ± 0.20 | 5.88 ± 0.35 | 1.69 ± 0.23 |
| Sorbitol | 1.42 ± 0.15 | 0.087 ± 0.011 | <0.01* | <0.01* |
| Betaine | 7.84 ± 0.76 | 9.68 ± 0.55 | 8.40 ± 0.97 | 9.70 ± 0.50 |
| Glucose | 6.92 ± 0.97 | 8.12 ± 0.67 | 6.80 ± 0.73 | 7.41 ± 0.56 |
| Glycine | 5.43 ± 0.38 | 6.53 ± 0.45 | 5.59 ± 0.22 | 6.29 ± 0.20 |
Data are means ± standard errors of the means (n = 10). GPC, glycerophosphorylcholine. No significant difference between the two genotypes in osmolyte levels was observed except for that of sorbitol (*, P < 0.0001). Statistical significance was determined by the unpaired Student t test.
When the kidneys of 36-week-old mice were examined under a light microscope, the inner medulla of ALR2-deficient mice appeared to be abnormal. The lumens of the collecting tubules were extended (data not shown). However, this is likely to be the consequence rather than the cause of defective urine-concentrating ability, because the morphology of the kidneys of 4-week- and 12-week-old ALR2-deficient mice appeared to be quite normal, while impairment of their urine-concentrating ability was already evident at 4 weeks, the earliest time tested. Renal lesions similar to those seen in ALR2-deficient mice were also observed in Brattleboro rats (24) and in SWR/J mice (21). The defect in water reabsorption in Brattleboro rats is due to a lack of AVP, while the defect in SWR/J mice is caused by the relative inability of the kidneys to respond to AVP. Thus, the abnormal morphology of the renal collecting tubules is common to DI of different origins. It is most likely the consequence of chronic polydipsia and polyuria.
DISCUSSION
We generated ALR2-deficient mice to determine ALR2's physiological functions and role in the pathogenesis of various diabetic complications. These mice have no apparent growth or reproductive abnormality. Their general appearance, body weights, blood glucose levels, and litter sizes were no different from those of wild-type mice, indicating that ALR2 is not essential for the survival of mice. The only phenotypic trait that we were able to identify was the defect in their urine-concentrating ability, which resulted in polyuria and polydipsia.
Heterozygous mice having one normal and one mutant allele do not have the polyuric and polydipsic phenotypes, indicating that the abnormality stems from a complete lack of the enzyme. Although the data reported here were obtained exclusively from male mice, female ALR2-deficient mice also have the same phenotype (data not shown). Furthermore, this phenotype was not affected by genetic background (129/SV × C57BL/6N hybrid, or a six-generation backcross with C57BL/6N).
At this point we do not know why ALR2 deficiency leads to impairment of water reabsorption in the kidney. These mice can synthesize and secrete AVP. It appears that the kidney's response to AVP is impaired. The levels of V2R, aquaporin 2, and aquaporin 3 mRNAs are normal, and so are the levels of aquaporin 2 and UTA-1 proteins. We have not determined if V2R, aquaporin 2, and aquaporin 3 proteins are in their proper locations on the apical or basolateral membrane of the epithelial cells. However, it seems unlikely that ALR2 deficiency would interfere with the translocation of these proteins to their proper sites. The level of aquaporin 1 was increased in Aldor1−/− mice. This should facilitate rather than impede water reabsorption. Overexpression of this protein is probably the kidney's response to polyuria rather than the cause of it.
A strain of mice called DI+/+ also exhibit nephrogenic DI (9). These mice have high levels of rolipram-sensitive cAMP-phosphodiesterase type IV activity and low cytosolic cAMP levels and are unable to raise the cytosolic cAMP in response to vasopressin (33). They have extensive polyuria and low urine osmolality (4, 16). These mice were found to have greatly reduced expression of aquaporin 2 as well as impairment of aquaporin 2 trafficking (11). Since the expression of aquaporin 2 in our ALR2-deficient mice is normal, this mechanism cannot explain the defect.
It is interesting that an inhibitor of ALR2 (sorbinil) has a diuretic effect in rabbits and rats but only during antidiuresis (30). This drug also has a natriuretic effect in these animals. In ALR2-deficient mice, impairment of water reabsorption was evident whether water was freely available or withheld. Furthermore, there was no abnormality in sodium or potassium secretion. Therefore, diuresis induced by an ALR2 inhibitor most likely involves a different mechanism. The natriuretic effect of the drug may be the result of inhibition of enzymes other than ALR2.
ALR2 and SORD are two enzymes in the polyol pathway, which is thought to be responsible for the synthesis of fructose. Fructose is very abundant in the semen and is thought to be the major energy source for sperm. While we have not determined the fructose level in the semen of ALR2-deficient mice, we found that fructose is absent in the coagulating glands of SORD-deficient mice (unpublished result), indicating that the polyol pathway is indeed the major source of fructose in this tissue. However, both ALR2-deficient and SORD-deficient mice (25) are fertile, suggesting that fructose is dispensable for reproduction. ALR2 is also thought to be involved in the detoxification of harmful cellular metabolites such as methyglyoxal, 3-deoxyglucosone, and 4-hydroxynonenal. ALR2-deficient mice appeared to be healthy. It is likely that the detoxification function may be taken over by enzymes such as glyoxalase, aldehyde reductase, fibroblast growth factor regulated protein, or other enzymes in the absence of ALR2. It would be interesting to see if the levels of these toxic metabolites are increased in ALR2-deficient mice.
The involvement of ALR2 in the development of diabetic complications was suggested by the use of ALR2 inhibitors (5, 38). However, these drugs may interact with other enzymes. Furthermore, the role of ALR2 in the pathogenesis of these diseases is not clear. ALR2-deficient mice as well as SORD-deficient mice may provide better models for studying the role of the polyol pathway in the development of various diabetic complications.
ACKNOWLEDGMENTS
We thank Martin Matzuk and Allan Bradley at Baylor College of Medicine, Houston, Tex., for providing ES cells and for their invaluable help and advice in gene targeting technique and K. H. Gabbay at Baylor College of Medicine for providing the rabbit anti-mouse ALR2 antibody. We also thank Wai Lap Wong for Western blot analysis and Kai Ming Chan, Fuk Ki Lee, and Karen W. Y. Lee for valuable advice and technical assistance.
This work was supported by grants from the Hong Kong Research Grant Council (7225/97M), Biotechnology Research Institute at the Hong Kong University of Science and Technology, and CRCG from the University of Hong Kong.
REFERENCES
- 1.Bagnasco S M, Uchida S, Balaban R S, Kador P F, Burg M B. Induction of aldose reductase and sorbitol in renal inner medullary cells by elevated extracellular NaCl. Proc Natl Acad Sci USA. 1987;84:1718–1720. doi: 10.1073/pnas.84.6.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bichet D G, Oksche A, Rosenthal W. Congenital nephrogenic diabetes insipidus. J Am Soc Nephrol. 1997;8:1951–1958. doi: 10.1681/ASN.V8121951. [DOI] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Brown D, Shields G I, Valtin H, Morris J F, Orci L. Lack of intramembranous particle clusters in collecting ducts of mice with nephrogenic diabetes insipidus. Am J Physiol. 1985;249:F582–F589. doi: 10.1152/ajprenal.1985.249.4.F582. [DOI] [PubMed] [Google Scholar]
- 5.Costantino L, Rastelli G, Vianello P, Cignarella G, Barlocco D. Diabetes complications and their potential prevention: aldose reductase inhibition and other approaches. Med Res Rev. 1999;19:3–23. doi: 10.1002/(sici)1098-1128(199901)19:1<3::aid-med2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Deen P M, Verdijk M A, Knoers N V, Wieringa B, Monnens L A, van Os C H, van Oost B A. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- 7.DiGiovanni S R, Nielsen S, Christensen E I, Knepper M A. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci USA. 1994;91:8984–8988. doi: 10.1073/pnas.91.19.8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein G H, Rivera M J, Carone F A. The effect of hypercalcemia induced by calciferol upon renal concentrating ability. J Clin Investig. 1958;37:1702–1709. doi: 10.1172/JCI103762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falconer D S, Latyszewski M, Isaacson J H. Diabetes insipidus associated with oligosyndactyly in the mouse. Genet Res. 1964;5:473–488. [Google Scholar]
- 10.Ferraris J D, Williams C K, Jung K Y, Bedford J J, Burg M B, Garcia-Perez A. ORE, a eukaryotic minimal essential osmotic response element. The aldose reductase gene in hyperosmotic stress. J Biol Chem. 1996;271:18318–18321. doi: 10.1074/jbc.271.31.18318. [DOI] [PubMed] [Google Scholar]
- 11.Frokiaer J, Marples D, Valtin H, Morris J F, Knepper M A, Nielsen S. Low aquaporin-2 levels in polyuric DI +/+ severe mice with constitutively high cAMP-phosphodiesterase activity. Am J Physiol. 1999;276:F179–F190. doi: 10.1152/ajprenal.1999.276.2.F179. [DOI] [PubMed] [Google Scholar]
- 12.Gill J R, Jr, Bartter F C. On the impairment of renal concentrating ability in prolonged hypercalcemia and hypercalciuria in man. J Clin Investig. 1961;40:716–722. doi: 10.1172/JCI104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hers H G. The mechanism of transformation of glucose to fructose by the seminal vesicles. Biochim Biophys Acta. 1956;22:202–203. doi: 10.1016/0006-3002(56)90247-5. [DOI] [PubMed] [Google Scholar]
- 14.Ho H T, Jenkins N A, Copeland N G, Gilbert D J, Winkles J A, Louie H W, Lee F K, Chung S S, Chung S K. Comparisons of genomic structures and chromosomal locations of the mouse aldose reductase and aldose reductase-like genes. Eur J Biochem. 1999;259:726–730. doi: 10.1046/j.1432-1327.1999.00110.x. [DOI] [PubMed] [Google Scholar]
- 15.Hollander W, Winter R W, Williams T F, Bradley J, Oliver J, Welt L G. Defects in the renal tubular reabsorption of water associated with potassium depletion in rats. Am J Physiol. 1957;189:557–563. doi: 10.1152/ajplegacy.1957.189.3.557. [DOI] [PubMed] [Google Scholar]
- 16.Homma S, Gapstur S M, Coffey A, Valtin H, Dousa T P. Role of cAMP-phosphodiesterase isozymes in pathogenesis of murine nephrogenic diabetes insipidus. Am J Physiol. 1991;261:F345–F353. doi: 10.1152/ajprenal.1991.261.2.F345. [DOI] [PubMed] [Google Scholar]
- 17.Ivell R, Burback P H, Van Leeuwen F W. The molecular biology of the Brattleboro rat. Front Neuroendocrinol. 1990;4:313–338. [Google Scholar]
- 18.Kinoshita J H, Nishimura C. The involvement of aldose reductase in diabetic complications. Diabetes Metab Rev. 1988;4:323–337. doi: 10.1002/dmr.5610040403. [DOI] [PubMed] [Google Scholar]
- 19.Knepper M A. Molecular physiology of urinary concentrating mechanism: regulation of aquaporin water channels by vasopressin. Am J Physiol. 1997;272:F3–F12. doi: 10.1152/ajprenal.1997.272.1.F3. [DOI] [PubMed] [Google Scholar]
- 20.Ko B C B, Ruepp B, Bohren K M, Gabbay K H, Chung S S. Identification and characterization of multiple osmotic response sequences in the human aldose reductase gene. J Biol Chem. 1997;272:16431–16437. doi: 10.1074/jbc.272.26.16431. [DOI] [PubMed] [Google Scholar]
- 21.Kutscher C L, Miller M, Schmalbach N L. Renal deficiency associated with diabetes insipidus in the SWR/J mouse. Physiol Behav. 1975;14:815–818. doi: 10.1016/0031-9384(75)90075-x. [DOI] [PubMed] [Google Scholar]
- 22.Manitius A, Levitin H, Beck D, Epstein F H. On the mechanism of impairment of renal concentrating ability in potassium deficiency. J Clin Investig. 1960;39:684–692. doi: 10.1172/JCI104084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marples D, Knepper M A, Christensen E I, Nielsen S. Redistribution of aquaporin-2 water channels induced by vasopressin in rat kidney inner medullary collecting duct. Am J Physiol. 1995;269:C655–C664. doi: 10.1152/ajpcell.1995.269.3.C655. [DOI] [PubMed] [Google Scholar]
- 24.McAuliffe W G. Histochemistry and ultrastructure of the interstitium of the renal papilla in rats with hereditary diabetes insipidus (Brattleboro strain) Am J Anat. 1980;157:17–26. doi: 10.1002/aja.1001570103. [DOI] [PubMed] [Google Scholar]
- 25.Ng T F, Lee F K, Song Z T, Calcutt N A, Lee A Y, Chung S S, Chung S K. Effects of sorbitol dehydrogenase deficiency on nerve conduction in experimental diabetic mice. Diabetes. 1998;47:961–966. doi: 10.2337/diabetes.47.6.961. . (Erratum, 47:1374.) [DOI] [PubMed] [Google Scholar]
- 26.Nielsen S, Chou C L, Marples D, Christensen E I, Kishore B K, Knepper M A. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen S, Terris J, Smith C P, Hediger M A, Ecelbarger C A, Knepper M A. Cellular and subcellular localization of the vasopressin-regulated urea transporter in rat kidney. Proc Natl Acad Sci USA. 1996;93:5495–5500. doi: 10.1073/pnas.93.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pugliese G, Tilton R G, Williamson J R. Glucose-induced metabolic imbalances in the pathogenesis of diabetic vascular disease. Diabetes Metab Rev. 1991;7:35–59. doi: 10.1002/dmr.5610070106. [DOI] [PubMed] [Google Scholar]
- 29.Sato K, Inazu A, Yamaguchi S, Nakayama T, Deyashiki Y, Sawada H, Hara A. Monkey 3-deoxyglucosone reductase: tissue distribution and purification of three multiple forms of the kidney enzyme that are identical with dihydrodiol dehydrogenase, aldehyde reductase, and aldose reductase. Arch Biochem Biophys. 1993;307:286–294. doi: 10.1006/abbi.1993.1591. [DOI] [PubMed] [Google Scholar]
- 30.Springate J E, Feld L G, Van Liew J B, Fildes R D, Acara M A. Diuretic and natriuretic effects of sorbinil, an aldose reductase inhibitor. Pharmacol Res. 1991;23:279–283. doi: 10.1016/s1043-6618(05)80087-8. [DOI] [PubMed] [Google Scholar]
- 31.Terris J, Ecelbarger C A, Nielsen S, Knepper M A. Long-term regulation of four renal aquaporins in rats. Am J Physiol. 1996;271:F414–F422. doi: 10.1152/ajprenal.1996.271.2.F414. [DOI] [PubMed] [Google Scholar]
- 32.Valtin H. Hereditary hypothalamic diabetes insipidus in rats (Brattleboro strain). A useful experimental model. Am J Med. 1967;42:814–827. doi: 10.1016/0002-9343(67)90098-8. [DOI] [PubMed] [Google Scholar]
- 33.Valtin H. Genetic models of diabetes insipidus. In: Windhager E E, editor. Handbook of physiology. section 8: renal physiology. II. Bethesda, Md: American Physiology Society; 1992. pp. 1281–1315. [Google Scholar]
- 34.Vander Jagt D L, Kolb N S, Vander Jagt T J, Chino J, Martinez F J, Hunsaker L A, Royer R E. Substrate specificity of human aldose reductase: identification of 4-hydroxynonenal as an endogenous substrate. Biochim Biophys Acta. 1995;1249:117–126. doi: 10.1016/0167-4838(95)00021-l. [DOI] [PubMed] [Google Scholar]
- 35.Vander Jagt D L, Robinson B, Taylor K K, Hunsaker L A. Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic complications. J Biol Chem. 1992;267:4364–4369. [PubMed] [Google Scholar]
- 36.van Lieburg A F, Knoers N V, Deen P M. Discovery of aquaporins: a breakthrough in research on renal water transport. Pediatr Nephrol. 1995;9:228–234. doi: 10.1007/BF00860757. [DOI] [PubMed] [Google Scholar]
- 37.Wolff S D, Yancey P H, Stanton T S, Balaban R S. A simple HPLC method for quantitating major organic solutes of renal medulla. Am J Physiol. 1989;256:F954–F956. doi: 10.1152/ajprenal.1989.256.5.F954. [DOI] [PubMed] [Google Scholar]
- 38.Yabe-Nishimura C. Aldose reductase in glucose toxicity: a potential target for the prevention of diabetic complications. Pharmacol Rev. 1998;50:21–33. [PubMed] [Google Scholar]