Abstract
Background:
Magnetic resonance spectroscopy (MRS) methods have quantified changes in levels of neurotransmitters and other neurometabolites in patients with major depression across the lifespan. 7T field strengths and greater have not been a major focus of study in patients with late-life depression (LLD).
Methods:
Nine LLD patients who met DSM-IV criteria for a current major depressive episode and nine non-depressed, healthy, age-matched controls underwent clinical and neuropsychological assessment and single-voxel 7T 1H-MRS at baseline and after 10–12 weeks of antidepressant treatment (Citalopram; patients only). Spectra were acquired from two brain regions implicated in both depressive symptoms and neuropsychological deficits in LLD, the anterior (ACC) and posterior cingulate (PCC). Levels of γ-aminobutyric acid (GABA), glutamate (Glu), glutathione (GSH), N-acetylaspartylglutamate (NAAG), N-acetylaspartate (NAA), and myo-inositol (mI) were quantified relative to total creatine (tCr) using linear-combination modeling.
Results:
Baseline Glu/tCr levels were not significantly different between groups. Decreased Glu/tCr levels levels after Citalopram treatment was observed in a subset of LLD patients, although the group difference was not statistically significant. Exploratory analyses showed that LLD patients had lower NAA levels in the PCC relative to controls. Higher levels of ml in the LLD patients relative to the controls and decreases after Citalopram treatment had large effect sizes but were not statistically significant. Further, decreases in PCC Glu/tCr and increases in ACC GSH/tCr were associated with improvement in depressive symptoms.
Limitations:
Sample size.
Conclusions:
These preliminary results suggest a role of neurochemicals and neurometabolites in the neurobiology of LLD and antidepressant treatment response.
Keywords: magnetic resonance spectroscopy, depression, late-life, Citalopram
Introduction
Depression in late life is associated with greater variability in antidepressant treatment response and greater rate of relapse compared to younger depressed patients (Dew et al., 1997). Further, depression in late life is associated with an increase in the rate of all-cause dementia (Alzheimer’s and vascular dementia; (Diniz et al., 2013). The neurobiological mechanisms underlying heterogeneity in treatment response and increased dementia risk are not well understood. Positron Emission Tomography (PET) studies have shown increased cerebral glucose metabolism in LLD patients relative to controls, which correlated with greater depressive symptom severity. The regional extent of hypermetabolism in LLD is much greater than that observed in mid-life depressed patients who show decreases in metabolism or no change in these regions and overlaps with areas of hypometabolism in individuals at genetic risk for Alzheimer’s disease (AD), as well as AD patients (Reiman et al., 1996; Smith et al., 2009, 1992). Antidepressant treatment with Citalopram, a selective serotonin reuptake inhibitor (SSRI), decreased metabolism in neural networks that were hypermetabolic prior to treatment that were correlated with improvement in (depression and anxiety) and cognition (Smith et al., 2002, 2009, 2011; Diaconescu et al., 2011). Understanding the mechanisms that underlie these metabolic alterations may inform the development of more effective treatments for depressive symptoms and neuropsychological deficits. Cerebral glucose metabolism is a final common pathway of synaptic activity. Astrocytes and glutamate signaling play an important role in the coupling between neuronal function and glucose metabolism (Magistretti, 2006). MRS and preclinical studies suggest that higher glutamate concentrations and decreases with antidepressant treatment may be a neurobiological mechanism that might contribute to the glucose metabolism changes (Binesh et al., 2004; Golembiowska K. Dziubina, 2000).
Magnetic resonance spectroscopy (MRS) is a non-invasive method for quantifying the levels of endogenous metabolites. MRS-detectable molecules that have been implicated in neuropsychiatric and neurodegenerative disease include the major excitatory and inhibitory neurotransmitters in the brain, glutamate (Glu) and γ-aminobutyric acid (GABA), N-acetylaspartylglutamate (NAAG; a modulator of glutamatergic neurotransmission), N-acetylaspartate (NAA; a measure of neuronal integrity), the primary antioxidant glutathione (GSH), and myo-inositol (ml), which is a potential marker of phospholipid metabolism, gliosis, or neuroinflammation (Rae, 2014). MRS methods are used to detect a broad variety of low-molecular-weight compounds, including neurotransmitters, antioxidants, and compounds reflecting neuronal loss or inflammation; they therefore provide complementary information to that provided by target-specific PET molecular imaging methods. Field strengths of 7T and greater have the advantage of increased spectral resolution and signal-to-noise ratio (SNR) compared to commonly used clinical MRI scanners, resulting in greater sensitivity for the quantification of low-concentration compounds, and improved separation of overlapping resonances (e.g. glutamate and glutamine, or NAA and NAAG).
MRS has been applied to the study of depression across the lifespan, as reviewed previously (Moriguchi et al., 2019; Maddock and Buonocore, 2012; Mathias et al., 2017; Reddy-Thootkur et al., 2020; Yksel and Öngür, 2010). The majority of studies were performed at 3T field strength. The most consistent findings in mid-life depression patients relative to controls are lower levels of Glx (glutamate + glutamine; reported as a composite due to low spectral resolution) and of GABA in frontal, cingulate and occipital cortices (Reddy-Thootkur et al., 2020; Arnone et al., 2015; Godfrey et al., 2018; Luykx et al., 2012; Moriguchi et al., 2019; Yksel and Öngür, 2010, Bhagwagar et al., 2007; Sanacora et al., 1999). Studies of glutamate and glutamine at higher field strength (7T) have shown, similarly, mixed results, including an increase, decrease or no change in frontal cortex and anterior cingulate (Godlewska et al., 2018). MRS studies of the effects of antidepressant treatment (selective serotonin reuptake inhibitors; SSRIs) have shown either decreases or no change in GABA and Glx (Brian P. Brennan et al., 2017; Sanacora et al., 2002; Taylor et al., 2012).
Prior studies in LLD patients relative to controls have revealed higher Glx, lower NAA in frontal cortex, increased GSH in the anterior cingulate and increased myo-inositol in basal ganglia and medial temporal lobe (Binesh, et al., 2004, Chen et al., 2009; Duffy et al., 2015; Venkatraman et al., 2009). Lower hippocampal Glx levels were associated with poorer working memory performance and higher hippocampal Glx levels with more severe depressive symptoms and earlier age at onset (Jayaweera et al., 2015). These studies were performed at field strengths of 3T or lower. The application of MRS to evaluate the effects of antidepressant treatment in LLD patients have not been a focus of study.
The purpose of the present study was to measure neurotransmitter and neurometabolite levels in the anterior (ACC) and posterior cingulate (PCC) of patients with LLD prior to and after 10–12 weeks of treatment with the SSRI, Citalopram, using 7T 1H-MRS. These two regions were chosen for study, rather than the dorsal-lateral prefrontal cortex, for example, because prior MRS and PET glucose metabolism studies have shown disease- and treatment-related changes in the ACC and PCC (A.O. et al., 2011; Arnone et al., 2015; Luykx et al., 2012; Maddock and Buonocore, 2012; Smith et al., 2009; Yksel and Öngür, 2010). Three primary hypotheses were tested: 1) prior to antidepressant treatment, LLD patients would have higher Glu levels relative to controls; 2) Glu levels in LLD patients would decrease with antidepressant treatment and 3) decreases in Glu would be associated with improved depressive symptoms and cognition (executive function and memory). Exploratory analyses characterized baseline diagnostic and prospective treatment-related alterations in levels of GABA, GSH, NAAG, NAA, and mI, as well as their associations with changes in depressive symptoms and cognition.
Materials and Methods
Participant Screening and Selection
Participants were recruited with advertisements in the community and from the Johns Hopkins University Alzheimer’s Disease Research Center (2P50AG005146). All potential participants underwent psychiatric and cognitive screening, including the Structured Clinical Interview for DSM-IV by a clinical psychologist (NG), clinical dementia rating scale (CDR), Mini Mental State Examination (MMSE; First et al., 1995; Morris, 1993; Folstein et al., 1975). Potential participants also underwent a physical and neurological examination, had laboratory testing performed (including complete blood count and blood chemistries) and had toxicology screening performed (psychotropic drugs and drugs of abuse).
Inclusion criteria for the controls was no DSM-IV diagnosis of a psychiatric disorder and a CDR score of 0 (normal control). Inclusion criteria for the LLD patients was DSM-IV diagnosis of major depressive disorder (non-bipolar, non-psychotic), age at onset of first depressive episode after age 55, and no antidepressant treatment in the past year. Chronic health conditions such as hypertension and diabetes had to be well-controlled in all participants. Exclusionary criteria were contraindications for MR imaging (pacemakers, aneurism clamps and metal), a history of or active neurological or Axis I psychiatric disorders (except for a diagnosis of current major depressive episode, non-bipolar, non-psychotic, in the LLD patients), and not medically stable. Participants were excluded from participating with a positive toxicology screen for psychotropic drugs or medications with central nervous system effects (e.g. antihistamines, cold medications) or if they used such medications within two weeks before enrollment. The Institutional Review Board and the Radiation Research Committee of the Johns Hopkins University School of Medicine approved the study protocol and consent forms (Protocol Number NA_00021615). Participants received transcribed and verbal descriptions of the research study and written informed consent was obtained.
Citalopram Treatment
After the baseline study procedures were completed, LLD patients began treatment with Citalopram (Celexa; 10mg oral dose per day) for one week to minimize side effects. Then, the dose was increased to 20 mg. If significant clinical improvement was not observed at the 20mg dosage after three weeks of treatment based on an improvement rating of 3 or greater on the Clinical Global Impression Scale (CGI; National Institute (National Institute of Mental Health, 1976), the dose was increased to 30 mg and then if necessary, to 40 mg. All patients were followed clinically on a weekly basis. Clinical ratings for depressive symptoms included the (Hamilton Depression Rating Scale-24 item [HDRS]; (Hamilton, 1960) and Beck Depression Inventory [BDI]; (Beck et al., 1996).
Neuropsychological Testing
Neuropsychological assessments were performed at baseline and repeated after 10–12 weeks of treatment in patients and after an equivalent period in controls. The neuropsychological test battery included measures of executive function, attention, auditory-verbal and visual-spatial memory and decision making (data not shown). Four measures were chosen a priori to represent two domains affected in LLD patients (executive function and memory) using tests that are sensitive to detecting deficits in LLD patients and that show improvement with antidepressant treatment. The executive function tests were the Delis-Kaplan Executive Function System [DKEFS] letter and category fluency (Delis, Kaplan, Kramer, 2001). The memory tests included a test of auditory-verbal memory (California Verbal Learning Test; CVLT) and a test of visual-spatial memory (Brief Visual Memory Test-Revised; BVMT-R; (Benedict, 1997; Delis et al., 1987). Alternate forms of the tests were used. The CVLT variable analyzed was the total number of words recalled without perseverations and intrusions over the first five trials. Similarly, the BVMT-R variable analyzed was the number of shapes and their location recalled over three learning trials.
MRS Acquisition
MRS acquisition was performed within one week of the neuropsychological assessment, prior to and after 10–12 weeks of Citalopram treatment. The MRS acquisition and analysis methods have been described previously (Oeltzschner et al., 2019) and are briefly summarized. All data were acquired on a 7T Philips Achieva scanner (Philips Healthcare, Best, The Netherlands) with a 32-channel receive and quadrature transmit head coil (Nova Medical, Wilmington, MA). After a high-resolution (0.96 mm isotropic) anatomical T1-weighted magnetization prepared gradient echo (MP-RAGE) scan, MRS voxels were placed in two brain regions: the ACC and PCC (Figure 1). For both regions, the MRS voxels had dimensions of 28 mm (anterior-posterior) × 20 mm (left-right) × 16 mm (caudal-cranial). Voxels were centered at the midline with the anteriorposterior edge tangential to the corpus callosum. The ACC voxel was placed in the dorsal ACC with the caudal-cranial edge perpendicular to the genu of the corpus callosum, and the PCC voxel was placed in PCC with the caudal-cranial edge perpendicular to the splenium of the corpus callosum. A researcher who was present at all acquisitions monitored reproducibility of voxel placement between pre- and post-treatment scans using anatomical landmarks.
Fig. 1:
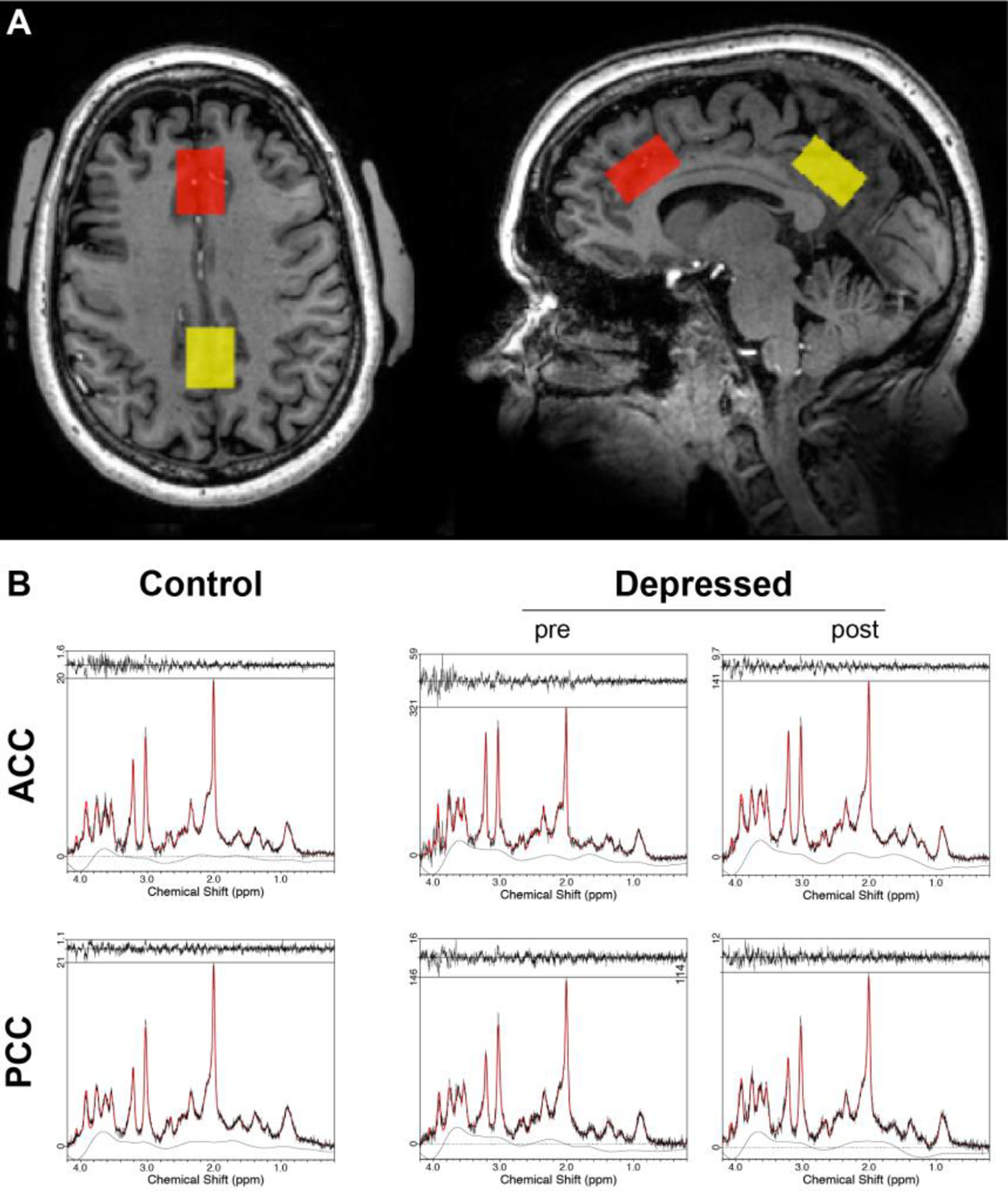
A. Example MRS voxel placement in the anterior cingulate (red) and posterior cingulate (yellow) cortices. Voxel dimensions were 28 mm (anterior-posterior) × 20 mm (left-right) × 16 mm (caudal-cranial). The axial image shows signals outside the head originating from padding used to stabilize the subject’s head position.
B. Example spectra from the ACC (upper row) and PCC (bottom row) for healthy controls at baseline (left column), and LLD patients pre- (middle column) and post-treatment (right column).
Prior to data acquisition, shimming was performed up to 2nd order using a FASTMAP-based routine, and RF power was optimized on the localized volume. Data were acquired using the Stimulated Echo Acquisition Mode (STEAM) sequence with the following parameters: TR = 3000 ms; 96 averages; 2048 data points; 3 kHz spectral width; VAPOR (Tkac et al., 1999) water suppression. Four water-unsuppressed averages per voxel were recorded with the same settings. TE was set to the shortest possible value (14 ms in ACC, 15 ms in PCC).
MRS Data processing
Spectroscopic data were analyzed with LCModel v6.3–0D (Provencher, 2001, 1993), using TE-specific simulated basis sets including alanine (Ala), aspartate (Asp), creatine (Cr), GABA, glucose (Glc), glutamate (Glu), glutamine (Gln), GSH, glycerophosphocholine (GPC), glycine (Gly), lactate (Lac), mI, NAA, NAAG, phosphocholine (PCh), phosphocreatine (PCr), phosphoethanolamine (PE), serine (Ser), scyllo-inositol (sI), taurine (Tau), and resonances from lipids (Lip09, Lip13a-d, Lip20). Basis functions for macromolecules and lipids (MM09, MM12, MM14, MM17, MM20, Lip09, Lip13, Lip 20) were internally simulated by LCModel using the default settings. Baseline flexibility was likewise set to internal LCModel defaults (DKNTMN = 0.15). Levels of GABA, Glu, GSH, NAA, NAAG, and mI (estimated with respect to the total creatine signal tCr = Cr + PCr) were used for further analysis. Individual metabolite measures with %SD Cramér-Rao lower bounds (%SD, as determined by LCModel) higher than 15% were excluded. Detection of gross outliers was performed by calculating the mean Cook’s distance across both groups (HC, MCI) and regions (ACC, PCC) for each metabolite (Stevens, 1984). Individual data points with more than 5 times the mean Cook’s distance were regarded as gross outliers and discarded.
Statistical Analysis
Two sample t-tests were used to compare LLD patients to healthy controls on baseline depressive symptoms, neuropsychological measures and metabolite levels. Paired t-tests were used to assess within-subject change over time in the LLD patients in depressive symptoms, neuropsychological measures and metabolites. Effect sizes were calculated according to published methods (Cohen, 1988). Linear regression models were used to assess the relationship between change in depressive symptoms and neuropsychological measures with change in metabolite levels. The models were adjusted for the baseline depressive symptoms and neuropsychological measures, respectively. The coefficients were scaled so that they could be interpreted as the expected difference in the change outcome measure between two individuals who differed in their change in metabolite levels by 0.1 units.
Results
Nine LLD patients and nine healthy controls were enrolled in the study. The demographic and clinical characteristics for both groups are summarized in Table 1a. Three of the patients had been treated with SSRIs in the past (only one patient with an adequate course of treatment), although not within the past two years. One of the controls and none of the LLD patients were left-handed. All controls and LLD patients had a CDR score of 0 (normal).
Table 1a:
Demographic characteristics of patients with Late-Life Depression and Healthy Elderly Control Subjects (mean ± standard deviation)
| Elderly Control Subjects (n = 9) | Patients with Late-Life Depression (n = 9) Baseline | |
|---|---|---|
|
|
||
| Age | 67 ± 7 | 70 ± 7 |
|
|
||
| Sex (M/F) | 5/4 | 4/5 |
|
|
||
| Years of Education | 15 ± 2 | 16 ± 3 |
|
|
||
| Mini-Mental Status Examination | 29 ± 1 | 29 ± 1 |
|
|
||
| Hamilton Depression Rating Scale | 1 ± 2 | 17 ± 2a |
|
|
||
| Beck Depression Inventory | 1.4 ± 2 | 22 ± 8 b |
|
|
||
| Total California Verbal Learning Test (CVLT) Score (Sum of Trials 1–5) | 55 ± 7 | 54 ± 9 |
|
|
||
| Brief Visuospatial Memory Test, Revised (Sum of Trials 1–5) | 19 ± 3 | 18 ± 9 |
|
|
||
| DKEFS Letter Fluency* | 41 ± 13 | 42 ± 7 |
|
|
||
| DKEFS Category Fluency* | 40 ± 5 | 40 ± 8 |
D-KEFS™ = Delis-Kaplan Executive Function System™
Significant difference between healthy controls and LLD patients (t16=−16.9, p<0.001)
Significant difference between healthy controls and LLD patients (t16=−7.6, p<0.001)
The groups did not differ significantly in age or sex distribution or in baseline neuropsychological measures (MMSE, CVLT, BVMT, DKEFS-Category and Letter Fluency). As expected, the LLD group showed significantly higher HDRS t16=−16.9, p<0.001) and BDI scores (t16=−7.6, p<0.001) at baseline, corresponding to moderate and moderate-to-severe levels of depression, respectively. The mean Citalopram dose was 18 mg ± 7 (range 10–40 mg) at the time of the follow-up MRS scan. Medication side effects were not reported. HDRS (t8=7.7, p<0.001) and BDI (t8=4.5, p<0.001) scores showed similar improvement with Citalopram treatment (Table 1b). All of the LLD patients met criteria for treatment response (score of 10 or below on the HDRS for two weeks consistently; (Dew et al., 1997). There were no significant changes in the verbal fluency and verbal and visual-spatial memory measures with Citalopram treatment.
Table 1b:
The Effects of Citalopram on Depressive Symptoms and Neuropsychological Function in patients with Late-Life Depression (mean ± standard deviation)
| Patients with Late-Life Depression (n = 9) Baseline | Patients with Late-Life Depression (n = 9) During Citalopram Treatment | |
|---|---|---|
|
|
||
| Hamilton Depression Rating Scale | 17 ± 2a | 4 ± 2a |
|
|
||
| Beck Depression Inventory | 22 ± 8 b | 7 ± 3b |
|
|
||
| Mini-Mental Status Examination | 29 ± 1 | 30 ± 1 |
|
|
||
| Total California Verbal Learning Test (CVLT) Score (Sum of Trials 1–5) | 54 ± 9 | 53 ± 10 |
|
|
||
| Brief Visuospatial Memory Test, Re vis d (Sum of Trials 1–5) | 18 ± 9 | 21 ± 7 |
|
|
||
| DKEFS Letter Fluency* | 42 ± 7 | 42 ± 9 |
|
| ||
| DKEFS Category Fluency * | 40 ± 8 | 40 ± 6 |
D-KEFS™ = Delis-Kaplan Executive Function System™
Significant difference in the LLD patients before and during Citalopram treatment (t8=7.7, p<0.001)
Significant difference in the LLD patients before and during Citalopram treatment (t8=4.5, p<0.001)
There were no significant baseline between-group differences in Glu/tCr levels in the ACC or PCC between the LLD patients and controls (Table 2, Figure 2). PCC NAA/tCr levels were significantly lower in the LLD patients relative to the controls (t16=2.2, p=0.04). There were no statistically significant between-group differences in the other metabolite measures.
Table 2:
Metabolite levels in patients with Late-Life Depression (LLD) and Healthy Elderly Control (HC) subjects
| Metabolite [/tCr] | Anterior Cingulate | t | df | p | Effect Size1 | |
|---|---|---|---|---|---|---|
| HC Mean (SD) | LLD Mean (SD) | |||||
| GABA | 0.353 (0.080) | 0.334 (0.087) | 0.463 | 16 | 0.649 | 0.238 |
| Glu | 1.422 (0.163) | 1.405 (0.154) | 0.237 | 16 | 0.816 | 0.104 |
| GSH | 0.225 (0.049) | 0.226 (0.043) | −0.026 | 16 | 0.980 | −0.020 |
| NAA | 1.465 (0.128) | 1.411 (0.218) | 0.639 | 16 | 0.532 | 0.422 |
| NAAG | 0.166 (0.052) | 0.150 (0.097) | 0.423 | 16 | 0.678 | 0.308 |
| mI | 0.703 (0.142) | 0.785 (0.126) | −1.230 | 16 | 0.212 | −0.577 |
| Metabolite [/tCr] | Posterior Cingulate | t | df | p | Effect Size1 | |
|---|---|---|---|---|---|---|
| HC Mean (SD) | LLD Mean (SD) | |||||
| GABA | 0.337 (0.081) | 0.338 (0.061) | −0.023 | 16 | 0.982 | −0.012 |
| Glu | 1.323 (0.090) | 1.290 (0.140) | 0.585 | 16 | 0.568 | 0.366 |
| GSH | 0.217 (0.052) | 0.220 (0.045) | −0.102 | 16 | 0.920 | −0.058 |
| NAA | 1.510 (0.159) | 1.370 (0.102) | 2.209 | 16 | 0.042 | 0.881 |
| NAAG | 0.225 (0.098) | 0.209 (0.091) | 0.351 | 16 | 0.730 | 0.163 |
| mI | 0.670 (0.043) | 0.733 (0.088) | −1.024 | 16 | 0.321 | −1.465 |
Total creatine (tCr), γ-aminobutyric acid (GABA), glutamate (Glu), glutathione (GSH), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG) and myo-inositol (mI)
Effect size was calculated as the ratio of the between-group difference to the SD of the healthy older adult comparison group.
Statistically significant effects are indicated in boldface.
Fig. 2:
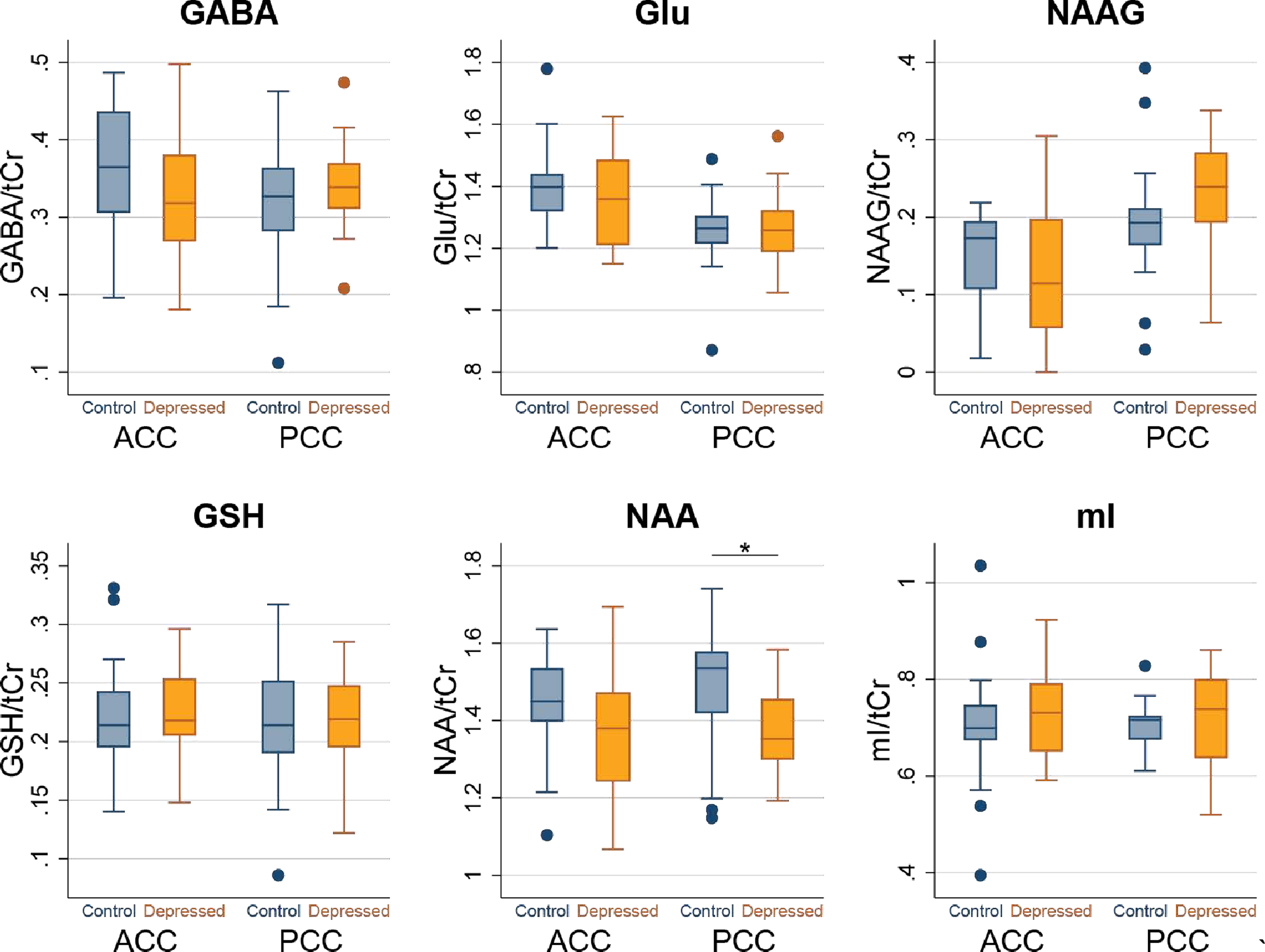
Box plots of metabolite levels at baseline from the ACC and PCC in LLD patients and Comparison Healthy Elderly Subjects. Dots represent outliers.
Comparing metabolites levels in the LLD group at baseline to post-Citalopram treatment revealed lower ACC and PCC Glu/tCr levels, although shy of achieving statistical significance. (Table 3, Figure 3). Likewise, ACC mI/tCr levels showed a notable decrease with treatment, which, while yielding a p value (0.058) slightly above the significance threshold, had a substantial effect size. The other metabolite measures were not significantly affected by treatment.
Table 3:
Metabolite levels in patients with Late-Life Depression at baseline and during Citalopram treatment
| Metabolite [/tCr] | Anterior Cingulate | t | df | p | Effect Size1 | |
|---|---|---|---|---|---|---|
| Time 1 Mean (SD) | Time 2 Mean (SD) | |||||
| GABA | 0.334 (0.087) | 0.319 (0.079) | 0.501 | 8 | 0.630 | 0.200 |
| Glu | 1.405 (0.154) | 1.318 (0.148) | 1.358 | 8 | 0.212 | 0.455 |
| GSH | 0.226 (0.043) | 0.224 (0.036) | 0.162 | 8 | 0.875 | 0.051 |
| NAA | 1.411 (0.218) | 1.369 (0.112) | 0.731 | 8 | 0.485 | 0.243 |
| NAAG | 0.150 (0.097) | 0.118 (0.097) | 0.725 | 8 | 0.489 | 0.244 |
| mI | 0.785 (0.126) | 0.702 (0.065) | 2.214 | 8 | 0.058 | 0.735 |
| Metabolite [/tCr] | Posterior Cingulate | t | df | p | Effect Size1 | |
|---|---|---|---|---|---|---|
| Time 1 Mean (SD) | Time 2 Mean (SD) | |||||
| GABA | 0.338 (0.061) | 0.337 (0.062) | 0.035 | 8 | 0.973 | 0.013 |
| Glu | 1.290 (0.140) | 1.244 (0.105) | 1.399 | 8 | 0.199 | 0.470 |
| GSH | 0.220 (0.047) | 0.221 (0.038) | −0.081 | 8 | 0.937 | −0.017 |
| NAA | 1.370 (0.102) | 1.388 (0.109) | −0.501 | 8 | 0.623 | 0.168 |
| NAAG | 0.209 (0.091) | 0.252 (0.039) | −1.072 | 8 | 0.315 | −0.361 |
| mI | 0.733 (0.088) | 0.703 (0.099) | 1.300 | 8 | 0.230 | 0.429 |
Total creatine (tCr), γ-aminobutyric acid (GABA), glutamate (Glu), glutathione (GSH), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG) and myo-inositol (mI)
Effect size was calculated as the ratio of the between-time-points difference to the SD of the between-time-points difference.
Fig. 3:
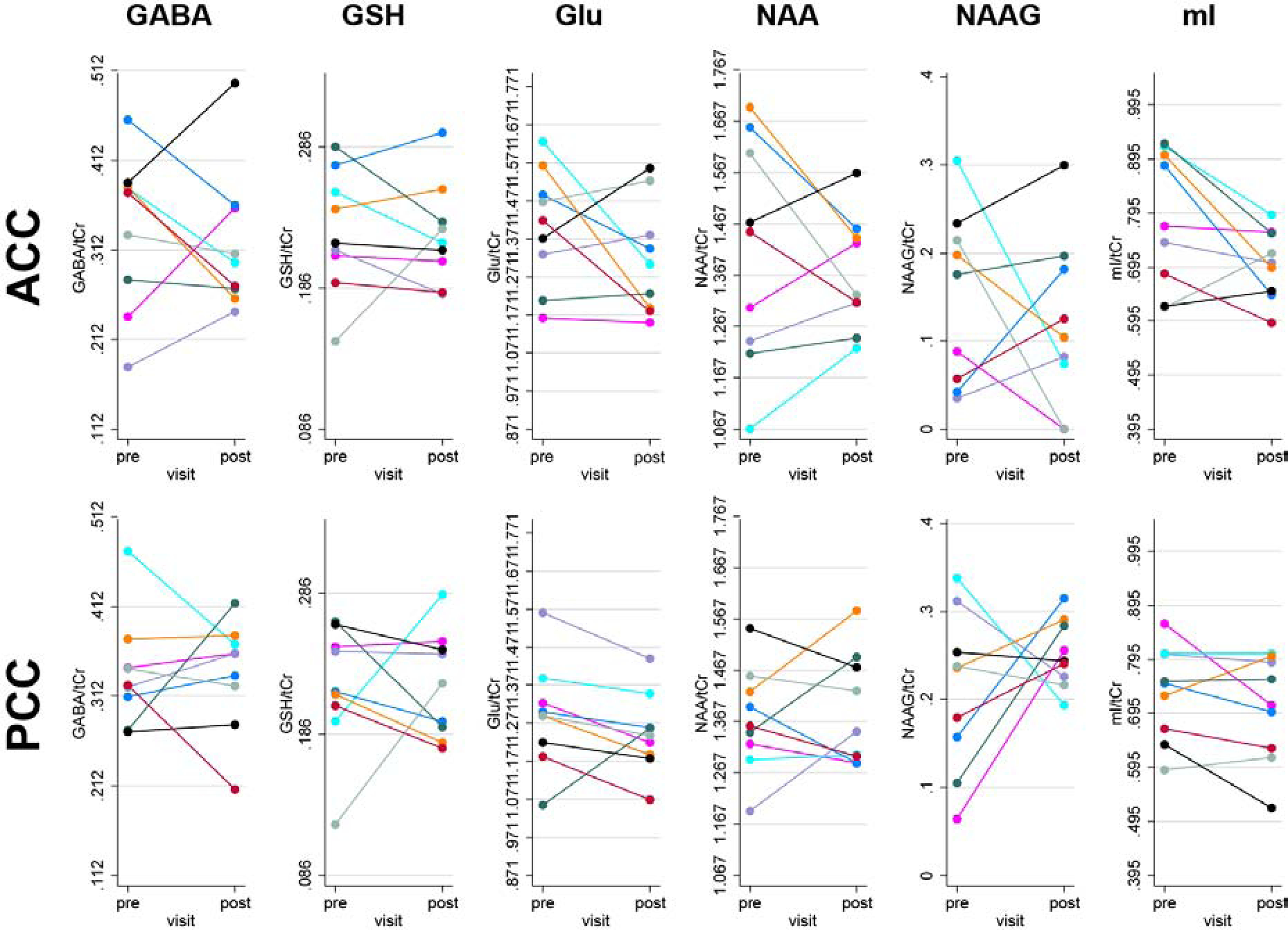
Metabolite levels from the ACC (upper row) and PCC (bottom row) in the LLD patients before (pre) and after Citalopram treatment (post). Individual subjects are color-coded.
Exploratory linear-regression modeling (Table 4) detected an association between decreased Glu/tCr in PCC with depressive symptom improvement (BDI score) during Citalopram treatment, as well as an association between increased GSH/tCr in ACC and depressive symptom improvement (BDI score). A 0.1 difference in Glu/tCr decrease was associated with 1.94 (SE: 0.75; p=0.042) points improvement (decrease) in depressive symptoms (BDI score). Conversely, a 0.1 increase in GSH/tCr in ACC was associated with a 5.9 (SE: 1.9; p=0.021) point improvement (decrease) in depressive symptoms (BDI score).
Table 4a:
Relationships between change in metabolite levels in patients with late-life depression (LLD) and change in depressive symptoms (Beck Depression Inventory): results of linear regression analyses
| Anterior Cingulate Metabolite [/tCr] | β | SE | p | 95% CI |
|---|---|---|---|---|
| GABA | −0.320 | 1.128 | .786 | (−3.080, 2.439) |
| Glu | −0.282 | 0.550 | .626 | (−1.627, 1.063) |
| GSH | −5.886 | 1.889 | .021 | (−10.509, −1.264) |
| NAA | 0.704 | 0.589 | .277 | (−0.737, 2.146) |
| NAAG | 0.297 | 0.810 | .727 | (−1.685, 2.278) |
| mI | −1.364 | 0.769 | .127 | (−3.245, 0.518) |
| Posterior Cingulate Metabolite [/tCr] | β | SE | p | 95% CI |
| GABA | 1.163 | 1.337 | .418 | (−2.109, 4.344) |
| Glu | 1.944 | 0.753 | .042 | (0.103, 3.786) |
| GSH | −2.194 | 1.648 | .231 | (−6.225, 1.838) |
| NAA | 0.404 | 1.008 | .702 | (−2.063, 2.871) |
| NAAG | 1.096 | 0.842 | .241 | (−0.963, 3.155) |
| mI | −0.337 | 1.534 | .833 | (−4.091, 3.417) |
Total creatine (tCr), γ-aminobutyric acid (GABA), glutamate (Glu), glutathione (GSH), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG) and myo-inositol (mI) Statistically significant effects are indicated in boldface.
Discussion
This study is among the first to investigate the neurobiology of LLD using high-field MRS to measure levels of neurometabolites in LLD patients relative to healthy, age-matched, controls. In contrast to the study hypotheses, the LLD patients did not have higher Glu/tCr levels than the controls at baseline. While the lower Glu/tCr levels in the LLD patients after Citalopram treatment were not statistically significant for the group as a whole, the pre- compared to post-treatment plots (Figure 3) did reveal that a sub-group of LLD patients showed reductions of GABA/tCr and Glu/tCr levels with Citalopram treatment. This suggests that the small sample size may have reduced the statistical sensitivity for detecting effects of Citalopram treatment, which is a limitation of the present study. The main limitation of the study, the small sample size, was in part due to the “black box” warning for Citalopram that was issued during the conduct of the study (Vieweg et al., 2012), at which point, study recruitment ended. Statistical testing was performed using parametric testing, which may inflate Type I error rates, but was chosen since a non-parametric version of the regression analysis is not available. Another limitation is the lack of correction for multiple comparisons. Further, it is not possible to conclude whether the MRS results are attributable to the effect of reduced depressive symptoms or an effect of Citalopram and other neurobiological mechanisms may be involved in the response to Citalopram. The strengths of the study included enrolling a group of well-characterized, late-onset LLD patients who had limited or no previous exposure to antidepressant treatment and monitoring outcomes for both depressive symptoms and neuropsychological outcomes during a prospective treatment trial with the same SSRI. All of the LLD patients were considered treatment responders. Moreover, as a group, the LLD patients did not show evidence of significant neuropsychological impairment at baseline or significant change during Citalopram treatment, although some patients improved. If the patient cohort included LLD patients, who were treatment-resistant and/or who had greater neuropsychological deficits, larger between-group differences and stronger relations between the MRS measures and clinical and neuropsychological outcomes may have been observed.
Exploratory analyses revealed that at baseline, LLD patients had lower PCC NAA/tCr levels relative to the controls. This observation is consistent with previous reports in younger depressed patients (Tan et al., 2016; Li et al., 2016). Lower concentrations of NAA/tCr and higher levels of mI/tCr in the PCC have been observed also in mild cognitive impairment, where declines in PCC NAA/tCr and increases in mI/tCr are greater in individuals who are “amyloid-positive” (Voevodskaya et al., 2019). The PCC shows early hypometabolism and amyloid deposition in mild cognitive impairment. Decreased PCC NAA may reflect a loss of neuronal integrity that could be associated with cognitive decline in LLD (Klunk et al., 2004; Minoshima et al., 1997). The effect size for between-group differences in ml (higher levels were observed in LLD patents relative to controls) were large relative to the other metabolites, but the values did not reach statistical significance. Previous studies have shown increased mI/tCr in the basal ganglia and medial temporal lobe in LLD (Chen et al., 2009; Venkatraman et al., 2009).
Exploratory analyses did not show significant changes in the metabolites after antidepressant treatment in the LLD patients, but showed associations between improvement in depressive symptoms and decrease in Glu/tCr in the PCC and increase in GSH in the ACC. The association between depressive symptom improvement and Glu/tCr is consistent with the study hypotheses regarding the role of glutamate in treatment response in LLD patients.
MRS studies in mid-life depressed patients have shown that SSRIs variably affect GABA and Glx, in some studies, depending on treatment duration (B P Brennan et al., 2017; Sanacora et al., 2002). In mid-life depressed patients, baseline Glx levels were unchanged after one week of Citalopram treatment, but nevertheless correlated, inversely, with depressive symptoms (Taylor et al., 2012). Another study showed significantly reduced hippocampal Glx levels after 8 weeks of Citalopram treatment in mid-life depressed patients (Block et al., 2009). Two studies have shown decreased GABA concentrations in the occipital cortex after SSRI treatment (Bhagwagar et al., 2004; Sanacora et al., 2002).
Preclinical and human MRS studies have shown reductions in GSH with Citalopram treatment (Godlewska et al., 2015; Gupta et al., 2018). However, the role of GSH in depression is not well understood. While lower GSH levels in mid-life depressed patients have been interpreted as evidence for increased oxidative stress (Godlewska et al., 2015; Shungu et al., 2012), it is conceivable that recovery from depression with SSRI treatment may be associated with less oxidative stress as reflected by increased GSH (Behr et al., 2012).
Similar to the between-group analysis, the effect size for within-group differences in ml/tCr was large relative to the other measures, although with a p value slightly above the nominal significance threshold level. Notably, ml/tCr was decreased in the ACC and PCC with Citalopram treatment. There are limited data on the effects of SSRI’s on ml/tCr. One study in mid-life depressed patients showed lower mI/tCr levels compared to controls and an increase to normal levels after SSRI treatment (Chen et al., 2014). As increased mI/tCr may reflect abnormalities in metabolism and intracellular cell signaling (Stork and Renshaw, 2005), further investigation of the effects of SSRI’s on mI/tCr, particularly in relation to cognitive function and AD pathology may be informative.
Conclusions
In summary, these preliminary results indicate that 7T MRS may elucidate the role of neurotransmitters and neurometabolites in depressive symptoms and cognitive deficits LLD patients. Studies are in progress to further evaluate the relationship between the MRS metabolite measures and PET measures of Alzheimer’s disease pathology (beta-amyloid). Understanding the associations between MRS and PET measures may elucidate disease mechanisms and inform the development of biomarkers for diagnosis and prognostication.
Table 4b:
Relationships between change in metabolite levels in patients with LLD and change in episodic verbal memory (California Verbal Learning Test): results of linear regression analyses
| Anterior Cingulate Metabolite [/tCr] | β | SE | p | 95% CI |
|---|---|---|---|---|
| GABA | 4.924 | 2.403 | .086 | (−0.954, 10.803) |
| Glu | 2.570 | 1.156 | .068 | (−0.257, 5.398) |
| GSH | −4.833 | 7.340 | .535 | (−22.795, 13.128) |
| NAA | 2.396 | 1.407 | .139 | (−1.046, 5.838) |
| NAAG | −0.918 | 2.295 | .703 | (−6.534, 4.697) |
| mI | 3.583 | 2.328 | .175 | (−2.113, 9.280) |
| Posterior Cingulate Metabolite [/tCr] | β | SE | p | 95% CI |
| GABA | 3.285 | 3.613 | .398 | (−5.557, 12.126) |
| Glu | 4.431 | 2.404 | .115 | (−1.452, 10.314) |
| GSH | 1.063 | 5.288 | .847 | (−11.875, 14.001) |
| NAA | −1.174 | 3.478 | .747 | (−9.684, 7.336) |
| NAAG | 1.372 | 3.217 | .685 | (−6.450, 9.244) |
| mI | −7.187 | 4.340 | .153 | (−17.951, 3.578) |
Total creatine (tCr), γ-aminobutyric acid (GABA), glutamate (Glu), glutathione (GSH), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG) and myo-inositol (mI)
Highlights.
Late-life depressed patients had lower posterior cingulate Nacetyl-aspartate levels
Improved depressive symptoms correlated with decreased posterior cingulate glutamate
Improved depressive symptoms correlated with increased anterior cingulate glutathione
Acknowledgements:
The authors gratefully acknowledge Terri Brawner, Ivana Kusevic, and Kathy Kahl for their invaluable contribution to the acquisition of the MRI data.
Funding/Support: This study was supported by: National Institute of Health: MH064823 (GSS), MH086881 (GSS), AG038893 (GSS), AG041633, AG059390 (GSS), K99AG062230 (GO), and UL1 TR 001079 (Daniel E. Ford, MD, MPH).
Footnotes
Conflict of Interest
The authors have no competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnone D, Mumuni AN, Jauhar S, Condon B, Cavanagh J, 2015. Indirect evidence of selective glial involvement in glutamate-based mechanisms of mood regulation in depression: Meta-analysis of absolute prefrontal neuro-metabolic concentrations. Eur. Neuropsychopharmacol 10.1016/j.euroneuro.2015.04.016 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Manual for the Beck Depression Inventory-II. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Behr GA, Moreira JCF, Frey BN, 2012. Preclinical and clinical evidence of antioxidant effects of antidepressant agents: Implications for the pathophysiology of major depressive disorder. Oxid. Med. Cell. Longev 10.1155/2012/609421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RHB, 1997. Brief Visuospatial Memory Test -Revised: Professional manual. Psychological Assessment Resources, Inc, Odessa, FL. [Google Scholar]
- Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ, 2004. Increased Brain GABA Concentrations Following Acute Administration of a Selective Serotonin Reuptake Inhibitor. Am. J. Psychiatry 10.1176/appi.ajp.161.2.368 [DOI] [PubMed] [Google Scholar]
- Binesh N, Kumar A, Hwang S, Mintz J, Thomas MA, 2004. Neurochemistry of late-life major depression: A pilot two-dimensional MR spectroscopic study. J. Magn. Reson. Imaging 10.1002/jmri.20214 [DOI] [PubMed] [Google Scholar]
- Block W, Träber F, Von Widdern O, Metten M, Schild H, Maier W, Zobel A, Jessen F, 2009. Proton MR spectroscopy of the hippocampus at 3 T in patients with unipolar major depressive disorder: Correlates and predictors of treatment response. Int. J. Neuropsychopharmacol 10.1017/S1461145708009516 [DOI] [PubMed] [Google Scholar]
- Brennan BP, Admon R, Perriello C, LaFlamme EM, Athey AJ, Pizzagalli DA, Hudson JI Jr, P.H.G., JE. J, 2017. Acute change in anterior cingulate cortex GABA, but not glutamine/glutamate, mediates antidepressant response to citalopram. Psychiatry Res Neuroimaging 269, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Chiang IC, Li CW, Lin WC, Lu CY, Hsieh TJ, Liu GC, Lin HF, Kuo YT, 2009. Proton magnetic resonance spectroscopy of late-life major depressive disorder. Psychiatry Res. - Neuroimaging 10.1016/j.pscychresns.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Chen LP, Dai HY, Dai ZZ, Xu CT, Wu RH, 2014. Anterior cingulate cortex and cerebellar hemisphere neurometabolite changes in depression treatment: A 1H magnetic resonance spectroscopy study. Psychiatry Clin. Neurosci 10.1111/pcn.12138 [DOI] [PubMed] [Google Scholar]
- Chiotis K, Saint-Aubert L, Savitcheva I, Jelic V, Andersen P, Jonasson M, Eriksson J, Lubberink M, Almkvist O, Wall A, Antoni G, Nordberg A, 2016. Imaging in-vivo tau pathology in Alzheimer’s disease with THK5317 PET in a multimodal paradigm. Eur. J. Nucl. Med. Mol. Imaging 10.1007/s00259-016-3363-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical Power for the Behavioral Sciences (2nd Edition), Hillsdale, NJ: Laurence Erlbaum and Associates. [Google Scholar]
- Delis DC, Kaplan E, Kramer J, 2001. Examiner’s Manual for the Delis-Kaplan Executive Function System, Child Neuropsychology. 10.1080/09297040490911140 [DOI] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B, 1987. California Verbal Learning Test (CVLT) Manual. Psychol. Corp 10.1207/s15328023top2503_18 [DOI] [Google Scholar]
- Dew MA, Reynolds CF, Houck PR, Hall M, Buysse DJ, Frank E, Kupfer DJ, 1997. Temporal profiles of the course of depression during treatment: Predictors of pathways toward recovery in the elderly. Arch. Gen. Psychiatry 10.1001/archpsyc.1997.01830230050007 [DOI] [PubMed] [Google Scholar]
- Diaconescu AO, Kramer E, Hermann C, Ma Y, Dhawan V, Chaly T, Eidelberg D, Mcintosh AR, Smith GS, 2011. Distinct functional networks associated with improvement of affective symptoms and cognitive function during citalopram treatment in geriatric depression. Hum. Brain Mapp 32, 1677–1691. 10.1002/hbm.21135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF, 2013. Late-life depression and risk of vascular dementia and Alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry 10.1192/bjp.bp.112.118307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SL, Lagopoulos J, Cockayne N, Hermens DF, Hickie IB, Naismith SL, 2015. Oxidative stress and depressive symptoms in older adults: A magnetic resonance spectroscopy study. J. Affect. Disord 10.1016/j.jad.2015.03.007 [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Rounsaville B, 1995. The Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition, Biometrics Research Department. 10.1521/pedi.1995.9.2.92 [DOI] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Godfrey KEM, Gardner AC, Kwon S, Chea W, Muthukumaraswamy SD, 2018. Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: A systematic review and meta-analysis. J. Psychiatr. Res 10.1016/j.jpsychires.2018.08.015 [DOI] [PubMed] [Google Scholar]
- Godlewska BR, Masaki C, Sharpley AL, Cowen PJ, UE E, 2018. Brain glutamate in medication-free depressed patients: a proton MRS study at 7 Tesla. Psychol MedJul; 48, 1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewska BR, Near J, Cowen PJ, 2015. Neurochemistry of major depression: A study using magnetic resonance spectroscopy. Psychopharmacology (Berl). 10.1007/s00213-014-3687-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golembiowska K. Dziubina A, 2000. Effect of acute and chronic administration of citalopram on glutamate and aspartate release in the rat prefrontal cortex. Polish J Pharm 52, 441–448. [PubMed] [Google Scholar]
- Gupta S, Upadhayay D, Sharma U, Jagannathan NR, 2018. Gupta YK Citalopram attenuated neurobehavioral, biochemical, and metabolic alterations in transient middle cerebral artery occlusion model of stroke in male Wistar rats. J Neurosci Res 96, 1277–1293. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaweera HK, Lagopoulos J, Duffy SL, Lewis SJG, Hermens DF, Norrie L, Hickie IB, Naismith SL, 2015. Spectroscopic markers of memory impairment, symptom severity and age of onset in older people with lifetime depression: Discrete roles of N-acetyl aspartate and glutamate. J. Affect. Disord 10.1016/j.jad.2015.04.023 [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B, 2004. Imaging Brain Amyloid in Alzheimer’s Disease with Pittsburgh Compound-B. Ann. Neurol 10.1002/ana.20009 [DOI] [PubMed] [Google Scholar]
- Li H, Xu H, Zhang Y, Guan J, Zhang J, Xu C, Shen Z, Xiao B, Liang C, Chen K, Zhang J, Wu R, 2016. Differential neurometabolite alterations in brains of medication-free individuals with bipolar disorder and those with unipolar depression: a two-dimensional proton magnetic resonance spectroscopy study. Bipolar Disord; 18, 583–590. [DOI] [PubMed] [Google Scholar]
- Luykx JJ, Laban KG, van den Heuvel MP, Boks MPM, Mandl RCW, Kahn RS, Bakker SC, 2012. Region and state specific glutamate downregulation in major depressive disorder: A meta-analysis of 1H-MRS findings. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2011.05.014 [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH, 2012. MR Spectroscopic studies of the brain in psychiatric disorders. Curr. Top. Behav. Neurosci 10.1007/7854_2011_197 [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, 2006. Neuron-glia metabolic coupling and plasticity. J. Exp. Biol 10.1242/jeb.02208 [DOI] [PubMed] [Google Scholar]
- Mathias LK, Monette PJ, Harper DG, Forester BP, 2017. Application of magnetic resonance spectroscopy in geriatric mood disorders. Int. Rev. Psychiatry 10.1080/09540261.2017.1397608 [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE, 1997. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann. Neurol 10.1002/ana.410420114 [DOI] [PubMed] [Google Scholar]
- Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S, Plitman E, Sano Y, Tarumi R, ElSalhy M, Katayama N, Ogyu K, Miyazaki T, Kishimoto T, Graff-Guerrero A, Meyer JH, Blumberger DM, Daskalakis ZJ, Mimura M, Nakajima S, 2019. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol. Psychiatry 10.1038/s41380-018-0252-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, 1993. The clinical dementia rating (cdr): Current version and scoring rules. Neurology. 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Guy W. National Institute of Mental Health, 1976. Clinical global impressions, in: Bonato RR (Ed.), National Institute of Mental Health;, Manual for the ECDEU assessment battery. 2. Rockville, MD. [Google Scholar]
- Oeltzschner G, Wijtenburg SA, Mikkelsen M, Edden RAE, Barker PB, Joo JH, Leoutsakos JMS, Rowland LM, Workman CI, Smith GS, 2019. Neurometabolites and associations with cognitive deficits in mild cognitive impairment: a magnetic resonance spectroscopy study at 7 Tesla. Neurobiol. Aging 10.1016/j.neurobiolaging.2018.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW, 2001. Automatic quantitation of localized in vivo1H spectra with LCModel - Provencher - 2001 - NMR in Biomedicine - Wiley Online Library. NMR Biomed. [DOI] [PubMed] [Google Scholar]
- Provencher SW, 1993. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med 10.1002/mrm.1910300604 [DOI] [PubMed] [Google Scholar]
- Rae CD, 2014. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem. Res 10.1007/s11064-013-1199-5 [DOI] [PubMed] [Google Scholar]
- Reddy-Thootkur M, Kraguljac NV, Lahti AC, 2020. The role of glutamate and GABA in cognitive dysfunction in schizophrenia and mood disorders –A systematic review of magnetic resonance spectroscopy studies. Schizophr. Res 10.1016/j.schres.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D, 1996. Preclinical evidence of Alzheimer’s disease in persons homozygous for the ε4 allele for apolipoprotein E. N. Engl. J. Med 10.1056/NEJM199603213341202 [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Krystal JH, 2002. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am. J. Psychiatry 10.1176/appi.ajp.159.4.663 [DOI] [PubMed] [Google Scholar]
- Shungu DC, Weiduschat N, Murrough JW, others, 2012. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed 25, 1073–1087. 10.1002/nbm.2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GS, Kramer E, Ma Y, Kingsley P, Dhawan V, Chaly T, Eidelberg D, 2009. The functional neuroanatomy of geriatric depression. Int. J. Geriatr. Psychiatry 24. 10.1002/gps.2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GS, Leon MJ, George AE, Kluger A, Volkow ND, Mcrae T, Golomb J, Ferris SH, Reisberg B, Ciaravino J, Regina ME, 1992. Topography of cross-sectional and longitudinal glucose metabolic deficits in alzheimer’s disease: Pathophysiologic implications. Arch. Neurol 49. 10.1001/archneur.1992.00530350056020 [DOI] [PubMed] [Google Scholar]
- Smith GS, Reynolds CF, Houck PR, Dew MA, Ma Y, Mulsant BH, Pollock BG, 2002. Glucose metabolic response to total sleep deprivation, recovery sleep, and acute antidepressant treatment as functional neuroanatomic correlates of treatment outcome in geriatric depression. Am. J. Geriatr. Psychiatry 10. 10.1097/00019442200209000-00009 [DOI] [PubMed] [Google Scholar]
- Smith GS, Workman CI, Kramer E, Hermann CR, Ginsberg R, Ma Y, Dhawan V, Chaly T, Eidelberg D, 2011. The relationship between the acute cerebral metabolic response to citalopram and chronic citalopram treatment outcome. Am. J. Geriatr. Psychiatry 10.1097/JGP.0b013e3181eafde4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JP, 1984. Outliers and influential data points in regression analysis. Psychol. Bull 10.1037/0033-2909.95.2.334 [DOI] [Google Scholar]
- Stork C, Renshaw PF, 2005. Mitochondrial dysfunction in bipolar disorder: Evidence from magnetic resonance spectroscopy research. Mol. Psychiatry 10.1038/sj.mp.4001711 [DOI] [PubMed] [Google Scholar]
- Tan HZ, Li H, Liu CF, Guan JT, Guo XB, Wen CH, Ou SM, Zhang YN, Zhang J, Xu CT, Shen ZW, Wu RH, Wang XQ, 2016. Main Effects of Diagnoses, Brain Regions, and their Interaction Effects for Cerebral Metabolites in Bipolar and Unipolar Depressive Disorders. Sci. Rep 10.1038/srep37343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Godlewska BR, Norbury R, Selvaraj S, Near J, Cowen PJ, 2012. Early increase in marker of neuronal integrity with antidepressant treatment of major depression: 1H-magnetic resonance spectroscopy of N-acetyl-aspartate. Int. J. Neuropsychopharmacol 10.1017/S1461145712000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkac I, Starcuk Z, Choi IY, Gruetter R, 1999. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 41, 649. [DOI] [PubMed] [Google Scholar]
- Venkatraman TN, Krishnan RR, Steffens DC, Song AW, Taylor WD, 2009. Biochemical abnormalities of the medial temporal lobe and medial prefrontal cortex in late-life depression. Psychiatry Res. - Neuroimaging 10.1016/j.pscychresns.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieweg WVR, Hasnain M, Howland RH, Hettema JM, Kogut C, Wood MA, Pandurangi AK, 2012. Citalopram, QTc interval prolongation, and torsade de pointes. How should we apply the recent FDA ruling? Am. J. Med 10.1016/j.amjmed.2011.12.002 [DOI] [PubMed] [Google Scholar]
- Voevodskaya O, Poulakis K, Sundgren P, Westen D. Van, Palmqvist S, Wahlund LO, Stomrud E, Hansson O, Westman E, 2019. Brain myoinositol as a potential marker of amyloid-related pathology: A longitudinal study. Neurology. 10.1212/WNL.0000000000006852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yksel C, Öngür D, 2010. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol. Psychiatry 10.1016/j.biopsych.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]


