Abstract
Introduction:
Successful strategies are needed to address parental vaccine hesitancy, a significant public health issue. The study objective was to assess whether an Internet-based platform with vaccine information and interactive social media components improved parents’ vaccine-related attitudes.
Study design:
A three-arm RCT.
Setting/participants:
The study was conducted in a large Colorado integrated healthcare organization. Parents were enrolled during September 2013 through October 2015 and followed through November 2016; data were analyzed in 2017. Parents, recruited during pregnancy, were given a survey about vaccine-related attitudes at enrollment (i.e., baseline) and when their child was aged 3–5 months and 12–15 months (Timepoints 1 and 2, respectively). Parental vaccine hesitancy was assessed at baseline.
Intervention:
Study participants were randomized to the following: a study website with vaccine information and social media components (VSM arm); a website with vaccine information only (VI); or usual care.
Main outcome measures:
Change in parental vaccine attitudes over time by baseline degree of vaccine hesitancy.
Results:
Among 1,093 study participants, 945 (86.5%) completed all three surveys. Comparing baseline with Timepoint 1 among vaccine-hesitant parents, the VSM and VI arms were associated with significant improvements in attitudes regarding vaccination benefits compared to usual care (VSM mean change 0.23 on a 5-point scale, 95% CI=0.05, 0.40, VI mean change 0.22, 95% CI=0.04, 0.40). Comparing baseline with Timepoint 2 among hesitant parents, the VSM and VI arms were also associated with significant reductions in parental concerns about vaccination risks compared to usual care (VSM mean change −0.37, 95% CI= −0.60, −0.14, VI mean change −0.31, 95% CI= −0.55, −0.07). Self-efficacy around vaccine decision making also improved among vaccine-hesitant parents. No intervention effect was observed among parents not vaccine-hesitant at baseline.
Conclusions:
Among vaccine-hesitant parents, an Internet-based intervention improved parents’ attitudes about vaccines.
INTRODUCTION
Although immunization coverage remains high nationally,1,2 parental vaccine hesitancy3 is a significant public health concern in the U.S.4 and globally.5 An estimated 0.8% of children aged 19–35 months in the U.S. are completely unimmunized,1 10% to 20% of parents report having refused or delayed one or more vaccines,4,6,7 and even higher proportions of parents report concerns about vaccines.7,8 Children whose parents have refused vaccines are at increased risk of vaccine-preventable diseases9–11 and contribute to community disease outbreaks.12
Developing strategies to reduce parental vaccine hesitancy is a challenging task, because the phenomenon is complex and evolving.13,14 Seemingly sensible approaches may even have a negative effect: Nyhan and colleagues15 found that presenting a dramatic narrative about measles or showing pictures of children with vaccine-preventable diseases increased parental misperceptions about the measles, mumps, rubella (MMR) vaccine. Several systematic reviews of interventions targeting vaccine hesitancy were recently published. The review authors concluded that although parent-focused educational interventions may improve parents’ attitudes toward vaccination, the strength of evidence was relatively poor, and additional well-designed studies of novel interventions were needed.14,16,17
Using Internet-based interventions to address parents’ vaccine concerns may be a constructive strategy. Parents often seek vaccine information on the Internet.18,19 Additionally, Internet-based interventions can be delivered outside the confines of routine well-child visits. This may be important because some parents have begun considering vaccine-related decisions well before the 2-month well-child visit,20,21 and providers report insufficient time during well-child visits to adequately address parents’ vaccine concerns.22 Internet-based decisions aids were shown to improve attitudes about MMR vaccination in England, Australia, and New Zealand,23,24 but little is known about Internet-based interventions in the U.S., particularly the use of social media to engage with parents regarding early childhood vaccination. The objective of the current study is to assess whether an Internet-based platform with vaccine information and interactive social media components improved parents’ vaccine-related attitudes.
METHODS
During September 2013 through November 2016, an Internet-based platform of vaccine-related content was developed and tested in a single-site RCT. Results for the primary study outcome of vaccination timeliness are presented elsewhere.25 The intervention was delivered during pregnancy and early childhood, and the study assessed whether parents exposed to the intervention had a greater change in their vaccine-related attitudes and beliefs compared to parents receiving usual care. Parental attitudes and beliefs were assessed at three timepoints: when recruited during pregnancy, when their child was aged 3–5 months, and when their child was aged 12–15 months. The human subjects research review board at Kaiser Permanente Colorado (KPCO) reviewed and approved the study.
Study Population
The study was conducted at KPCO, a large integrated healthcare organization with ≅628,000 members. Each year, KPCO provides care to roughly 5,000 pregnant women and 130,000 children aged <18 years. Pediatricians, family physicians, and physician assistants provide routine pediatric care, with most children seen by pediatricians.
Study participants were recruited during September 2013 through October 2015 and followed through November 2016. To begin the recruitment process, electronic health record data were used to identify pregnant women in the third trimester of pregnancy. Women were considered study-eligible if they were aged ≥18 years, spoke English, had Internet access, and had health insurance through KPCO. Women were ineligible if they had a diagnosis of fetal demise, miscarriage, or congenital anomaly. Study-eligible women were recruited using a combination of letters, postcards, e-mails, and telephone calls. Informed consent was obtained online using a secure encrypted program.
Participants were randomized to one of three study arms: a vaccine social media (VSM) arm that received access to a website with vaccine information as well as interactive social media components, a vaccine information (VI) arm that had access to a website with vaccine information but without social media components, and a usual care arm. Participants were given a baseline survey at the time of study enrollment. As part of the baseline survey, participants were administered the Parent Attitudes and Childhood Vaccines (PACV) screener, a validated 15-item questionnaire assessing vaccine hesitancy on a scale from 0 to 100.26,27 Consistent with prior studies, participants with a PACV score ≥50 were classified as vaccine hesitant, whereas those with a score <50 were considered non-hesitant.26,27 To ensure a balance of vaccine hesitancy across study arms, randomization was conducted independently among hesitant and non-hesitant parents. A randomization allocation ratio of 3:2:1 was used across the VSM:VI:usual care study arms, respectively. Although an unequal randomization allocation ratio reduces statistical power,28,29 this allocation approach was taken to ensure that the VSM arm was of sufficient size to generate social media interactions among participants. SAS/STAT Proc Plan, version 9.2, was used to generate random allocation sequence lists, with randomization performed by an unblinded statistician. Given the nature of the intervention, the participants and study team were not blinded to study arm assignment.
Measures
The multidirectional communication model30 served as the theoretic basis for the Internet-based social media intervention,25,31 and consisted of three components. Component one was a top-down process in which the study team developed and presented content to users on the study website. Component two was a bottom-up process that allowed website users to create content and interact with the study team. Component three was a side-to-side process in which website users could interact with each other and share information. This model is designed to empower users by allowing them to become active, engaged participants in the communication process, a process thought to promote positive changes in health behaviors.32 While the VSM study group received all three components, the VI group received only component one.
The vaccine content presented on the study website was developed using an adapted mental models approach.33 As described previously,31 the vaccine content and website design were extensively pilot-tested and revised using focus groups and individual interviews with pregnant women and parents of young children. The factual vaccine content was guided by the Health Belief Model34 and the Theory of Planned Behavior,35 and focused on encouraging parents to receive recommended vaccines on time. Seeking to accurately represent vaccination risks and benefits, the study website included content on the immune system, vaccine development, vaccine ingredients, vaccine safety, and vaccine-preventable diseases. Guided by best practices in website design and risk communication, content was clearly labeled and presented in short, easy-to-read sections.36,37 To convey credibility and transparency, the information sources were explicitly referenced and accompanied by hyperlinks that allowed users to review referenced studies for themselves.38 The vaccine content on the study website was identical for the VSM and VI groups.
Via the study website, the VSM group also had access to several social media formats including a blog, discussion forum, and chat room. Each month, the research team created one to two blog posts covering timely or controversial issues such as new vaccine safety research, recent vaccine-preventable disease outbreaks, or changes in state or national immunization policies. An “Ask a Question” portal was available for the VSM group, through which participants could direct questions to vaccine subject matter experts, who included a vaccine safety researcher, a pediatric infectious diseases specialist, a general pediatrician, and a risk communication specialist. For any questions submitted privately through the portal or by e-mail, personalized responses were provided within 2 business days. Each month, online chat sessions were held during which VSM participants could engage in real-time conversations with a team of vaccine experts. Finally, the VSM group was e-mailed monthly newsletters encouraging website use, highlighting website updates, and providing additional vaccine content. All interactive website and social media components were moderated to prevent bullying, abusive language, and disclosure of personal health information.
To limit access to study participants and prevent contamination across study arms, participants in the VSM and VI groups were required to create a personal login and password for the website. To reflect how a web-based tool might be used in routine clinical practice, individuals in the VSM and VI groups were given access to the website but were not required to visit it.
Routine pediatric preventive care was available to all participants in the three study arms. Structured well-child visits are typically scheduled at age 2 weeks, 2 months, 4 months, 6 months, and 12 months, and recommended vaccines are given at these visits according to national guidelines.39,40 It is standard practice at KPCO to provide parents with pre-visit information regarding vaccines due, as well as the applicable Vaccine Information Statements.41
The Health Belief Model and the Theory of Planned Behavior formed the conceptual framework for the survey instrument.34,35 Based on previously published work, survey items included questions about parents’ attitudes and beliefs regarding vaccine-preventable diseases, perceived risks of vaccination, and confidence in making vaccination decisions for their child.27,42–46 Survey items regarding attitudes and beliefs were asked on a 5-point Likert scale, with response options corresponding to a scale of strongly disagree to strongly agree or not at all confident to absolutely confident. After development, the survey instrument was tested in a group of 320 pregnant women and parents of young children, and was revised accordingly. When the survey instrument was administered at baseline, parents were also given the PACV vaccine-hesitancy screener, and asked standard demographic questions.
The survey was administered to study participants at three timepoints: at study enrollment (i.e., at baseline), when their child was aged 3–5 months (Timepoint 1), and when their child was aged 12–15 months (Timepoint 2). Surveys were administered online using a secure platform (SurveyGizmo). At Timepoints 1 and 2, participants received staggered e-mail reminders, up to a maximum of eight e-mails, if they had not yet completed their survey. Most participants completed their surveys within a short time frame, and consequently received fewer reminders. Participants also received a reminder if they coincidentally visited the study website when a survey was due. Participants were given compensation of $20 for each completed survey.
A confirmatory factor analysis was performed on baseline survey results examining all vaccine-related attitudes and beliefs. Based upon the factor analysis and resulting Cronbach’s α, all original survey items were retained and grouped into three vaccine-related constructs: (1) attitudes regarding the benefits of vaccination; (2) attitudes regarding the risks of vaccination; and (3) self-efficacy regarding vaccination decision making. The individual survey items that comprised each construct are shown in Table 1. For each survey respondent, scores on individual items within each construct were added, then divided by the number of contributing items, so that each individual had a mean score for each construct on a 5-point Likert-type scale ranging from 1 to 5. On the baseline survey, the Cronbach’s α for these constructs were 0.84 for the benefits of vaccination, 0.91 for the risks of vaccination, and 0.85 for perceived self-efficacy, indicating good internal consistency.47
Table 1.
Survey Items Assessing Parents’ Attitudes, Beliefs, and Perceived Self-Efficacy, Grouped by Vaccine-Related Construct
| Survey items |
|---|
|
|
| Attitudes and beliefs regarding benefits of vaccinationa |
| I believe it is better for my child to get the natural disease than to get a vaccineb |
| I believe that vaccines strengthen the immune system |
| Getting vaccines is a good way to protect my child from infectious diseases |
| Many of the illnesses vaccines prevent are serious |
| My child does not need vaccines for diseases that are not common anymore, like poliob |
| My child could get a serious disease if he or she were not vaccinated |
| I can protect my child from some types of infectious disease by vaccinating him or her |
| Attitudes and beliefs regarding risks of vaccinationc |
| I believe there has not been enough research on the safety of vaccines |
| My child’s immune system could be weakened by too many vaccines |
| Vaccines are safeb |
| Children get more vaccines than they need |
| I am concerned that vaccines have serious side effects |
| I am concerned that some vaccines cause autism in healthy children |
| I am concerned that the ingredients in vaccines are unsafe |
| Perceived self-efficacy regarding vaccination decision makingd |
| I am confident that I have the necessary information to make decisions about vaccination for my child |
| I am confident about my knowledge about how vaccines work |
| I am confident about my knowledge about infectious diseases |
| I am confident that I can express my views about vaccines to my obstetrician/pediatrician |
A higher score represents greater parental confidence regarding the benefits of vaccination.
Responses for this survey item were reverse-coded, so that a higher score had the same meaning across all survey items within the same construct.
A higher score represents greater parental concern about the risks of vaccination.
A higher score represents higher perceived self-efficacy regarding vaccination decision making.
The primary study outcomes were changes in the three vaccine-related construct scores over time across the three study arms by baseline degree of vaccine hesitancy. It was hypothesized that vaccine-hesitant parents exposed to the VSM and VI interventions would have a greater increase in pro-vaccination attitudes and beliefs than parents exposed to usual care. It was also hypothesized that no intervention effect would be seen in parents who were not vaccine hesitant at baseline.
Statistical Analysis
The study sample size was determined based on the primary outcome of vaccination status, as described elsewhere.25 Frequencies and descriptive statistics were used to characterize study participants at baseline. For each of the three vaccine-related constructs (i.e., vaccination benefits, vaccination risks, perceived self-efficacy), mean scores were calculated for each participant at three timepoints. Linear mixed models for repeated measures48,49 were used to assess the change in mean scores for the constructs over time across the three study arms. The use of mixed models accounted for the correlation between observations made by the same parent across time, and allowed for missing data.48,49 A separate model was built for each of the three constructs. The models included the following variables: baseline hesitancy (hesitant or non-hesitant) assessed by the PACV screening tool26; study arm assignment (VSM, VI, or usual care); timepoint (baseline, Timepoint 1, Timepoint 2); and the two- and three-way interactions between these three variables. For hypothesis testing within each baseline hesitancy group, model contrasts estimated mean differences and 95% CIs comparing baseline to Timepoint 1, and baseline to Timepoint 2, across study arms. Several within-parent correlation structures were explored for each model. Akaike’s information criterion was used to compare the goodness of fit among models with different covariance structures, and the covariance structure with the smallest Akaike’s information criterion values was selected as the final model. All analyses were intention to treat because linear mixed models account for missing data48,49; participants who only completed the baseline survey nonetheless contributed to the baseline mean vaccine construct scores. Data were analyzed in 2017; analyses were performed using SAS, version 9.4.
RESULTS
As illustrated in Figure 1, a total of 5,302 parents were assessed for study eligibility, among whom 1,093 were eligible and enrolled in the study. After randomization, 41 parents (3.8%) were lost to follow-up. Reasons for loss to follow-up included the following: noncompletion of both Timepoint 1 and 2 surveys (n=30); disenrollment from the KPCO health plan prior to the Timepoint 1 survey (n=4); fetal demise (n=4); request to drop out of the study (n=2); and noncompletion of the baseline survey (n=1).
Figure 1.
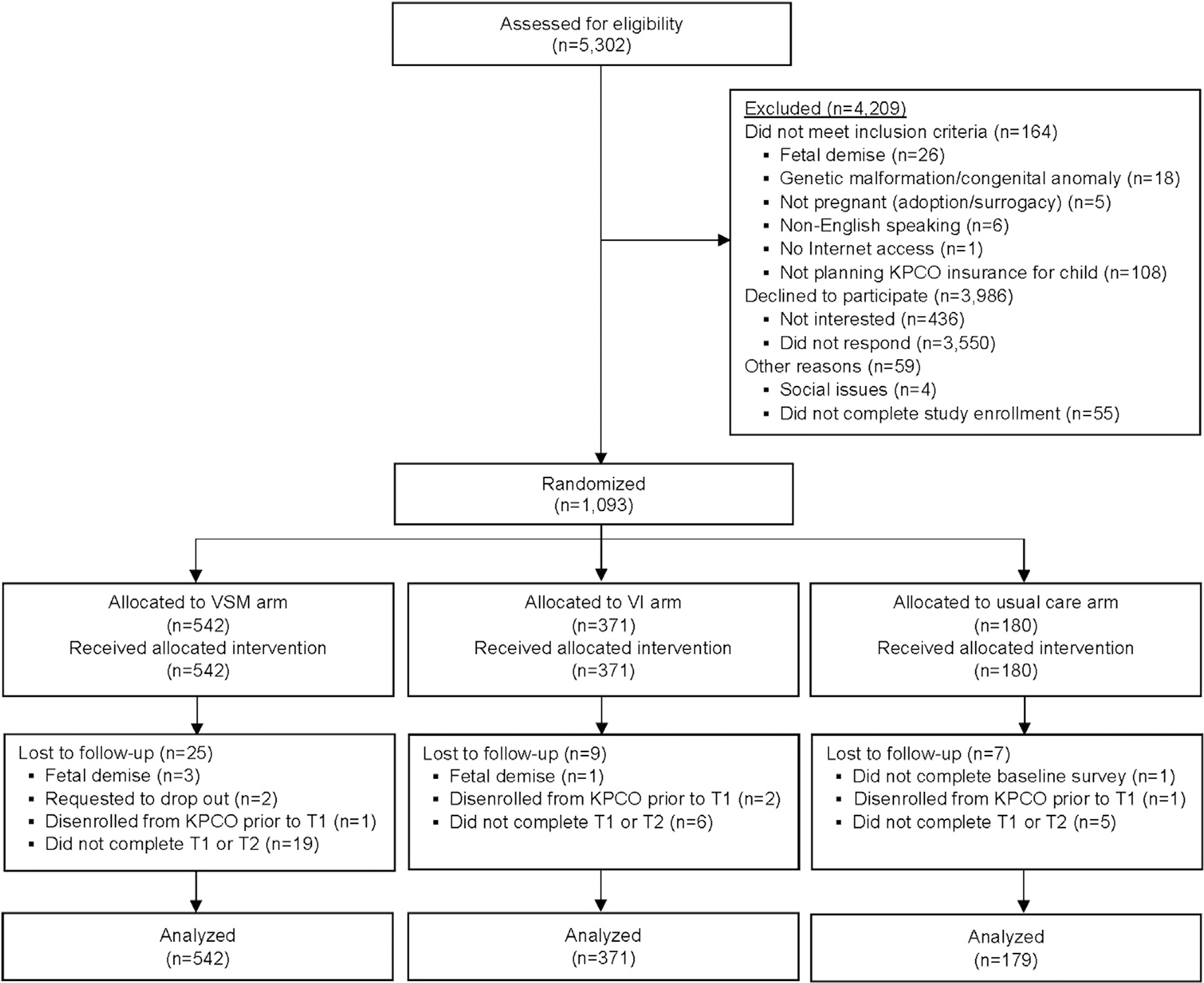
CONSORT study flow diagram.
Note: All analyses were intention-to-treat; because linear mixed models were used, participants who did not complete the T1 or T2 surveys nonetheless contributed to analyses of vaccine-related constructs.
VSM, vaccine social media; VI, vaccine information; KPCO, Kaiser Permanente Colorado; T1, Timepoint 1 survey; T2, Timepoint 2 survey.
The baseline characteristics of study participants are presented in Table 2. The sociodemographic characteristics of participants did not differ significantly across study arms. As shown, between 45.4% and 50.9% of participants across study arms were pregnant with their first child, and a majority reported using the Internet for health-related information at least weekly. On a scale of 0–100, median vaccine hesitancy scores on the PACV screener26,27 were 13, 17, and 15 for the VSM, VI, and usual care study groups, respectively (p=0.44). For the study population overall, 14.1% were classified as vaccine hesitant at baseline (PACV score ≥50).26,27
Table 2.
Baseline Characteristics of Study Participants by Study Arm (N=1,093 total)
| Characteristica | Vaccine social media arm (n=542) | Vaccine information arm (n=371) | Usual care arm (n=180) |
|---|---|---|---|
|
| |||
| Mother’s age at enrollment, years, M (SD) | 31.6 (4.4) | 31.5 (4.3) | 31.4 (4.1) |
| Mother’s race, n (%)b | |||
| White | 476 (87.8) | 315 (84.9) | 159 (88.3) |
| Black | 12 (2.2) | 8 (2.2) | 4 (2.2) |
| Asian/Pacific Islander | 21 (3.9) | 19 (5.1) | 4 (2.2) |
| Multi-racial/other | 31 (5.7) | 27 (7.3) | 12 (6.7) |
| Missing | 2 (0.4) | 2 (0.5) | 1 (0.6) |
| Mother’s ethnicity, n (%)b | |||
| Hispanic | 47 (8.7) | 48 (12.9) | 22 (12.2) |
| Non-Hispanic | 489 (90.2) | 321 (86.5) | 155 (86.1) |
| Missing | 6 (1.1) | 2 (0.5) | 3 (1.7) |
| Number of children, n (%)b | |||
| Pregnant with first child | 246 (45.4) | 189 (50.9) | 83 (46.1) |
| Have other child/children | 296 (54.6) | 181 (48.8) | 96 (53.3) |
| Missing | 0 (0) | 1 (0.3) | 1 (0.6) |
| Annual household income, n (%)b | |||
| <$40,000 | 45 (8.3) | 35 (9.4) | 15 (8.3) |
| $40,000–$80,000 | 163 (30.1) | 122 (32.9) | 60 (33.3) |
| $81,000–$120,000 | 188 (34.7) | 103 (27.8) | 57 (31.7) |
| >$120,000 | 118 (21.8) | 91 (24.5) | 40 (22.2) |
| Decline to answer/missing | 28 (5.2) | 20 (5.4) | 8 (4.4) |
| Mother’s education, n (%)b | |||
| High school or less | 21 (3.9) | 14 (3.8) | 2 (1.1) |
| Some college | 72 (13.3) | 49 (13.2) | 28 (15.6) |
| College | 222 (41.0) | 136 (36.7) | 72 (40.0) |
| Graduate school | 227 (41.9) | 171 (46.1) | 77 (42.8) |
| Missing | 0 (0) | 1 (0.3) | 1 (0.6) |
| Use of Internet for health, n (%)b | |||
| Not at all | 11 (2.0) | 5 (1.3) | 3 (1.7) |
| Once a month or less | 199 (36.7) | 135 (36.4) | 57 (31.7) |
| Every week | 272 (50.2) | 199 (53.6) | 100 (55.6) |
| Every day | 60 (11.1) | 31 (8.4) | 19 (10.6) |
| Missing | 0 (0) | 1 (0.3) | 1 (0.6) |
| Vaccine hesitancy by PACV,c median (IQR) | 13.0 (26.0) | 17.0 (34.0) | 15.0 (31.5) |
No significant differences in characteristics across study arms (all p-values ≥0.15).
Percentages represent column percentages.
PACV, a validated screening questionnaire assessing vaccine hesitancy on a scale from 0 to 100; higher scores indicate a higher degree of hesitancy.
IQR, interquartile range; PACV, Parent Attitudes and Childhood Vaccines.
Among 1,093 study participants, 945 (86.5%) completed all three surveys, 107 (9.8%) were missing either the Timepoint 1 or 2 surveys, and 41 (3.8%) were lost to follow-up. The percentage who completed all three surveys was lower in the VSM study arm (82.7% completion) than in the VI (90.0%) or usual care (90.6%) arms, p=0.001. Also, the percentage who completed all three surveys was lower among parents who were vaccine hesitant compared with non-hesitant at baseline (81.2% vs 87.3% completion, respectively, p=0.04).
Among 542 participants in the VSM study arm, 189 (34.9%) visited the study website at least once, with a mean of 1.9 visits (SD = 1.8) and a range of one to 15 visits. A greater proportion of vaccine-hesitant participants (41.2%) visited the website compared with non-hesitant participants (34.0%), although the difference was not statistically significant (p=0.24). Over the study period, the VSM component of the website offered 59 blog entries and 31 chat sessions, and VSM participants contributed 90 comments and questions. Website social media interactions were monitored for abusive or otherwise inappropriate behavior; no interactions required moderation, and no VSM participants were issued warnings or restricted from website access.
For participants in the VSM arm, topics raised during chat sessions and through the Ask a Question portal varied widely. For example, several participants expressed concern regarding the number of vaccines recommended at the 12-month well-child visit and asked why “so many” vaccines were needed. Others asked why an “alternative” or delayed schedule wasn’t a reasonable approach to vaccination. Finally, some participants asked detailed safety-related questions, such as about vaccine ingredients, or whether certain children were particularly vulnerable to experiencing vaccine adverse events.
Among 371 participants in the VI study arm, 122 (32.9%) visited the study website at least once, with a mean of 1.7 visits (SD=1.5) and a range of one to ten visits. A greater proportion of vaccine-hesitant participants (43.4%) visited the website compared with non-hesitant participants (31.1%, p=0.08 for the comparison).
Mean scores on 5-point Likert-type scales for each of the three vaccine constructs, stratified by baseline level of vaccine hesitancy, are presented in Table 3. As shown, parents who were vaccine hesitant at baseline had significantly less confidence in the benefits of vaccination (baseline scores across study arms ranged from 3.33 to 3.61 among hesitant, compared with 4.22 to 4.28 among non-hesitant). Vaccine-hesitant parents also had greater concerns about the risks of vaccination and lower perceived self-efficacy at baseline than non-hesitant parents.
Table 3.
Parents’ Vaccine-Related Attitudes, Beliefs, and Perceived Self-Efficacy, Measured Over Time on a 5-Point Scale
| Vaccine-hesitant at baseline |
Not vaccine-hesitant at baseline |
|||||
|---|---|---|---|---|---|---|
| Vaccine-related construct | Baseline | Timepoint 1 | Timepoint 2 | Baseline | Timepoint 1 | Timepoint 2 |
|
| ||||||
| Attitudes regarding benefits of vaccinationa | ||||||
| Vaccine social media | 3.33 (0.65) | 3.74 (0.64) | 3.74 (0.75) | 4.28 (0.44) | 4.48 (0.38) | 4.52 (0.38) |
| Vaccine information | 3.48 (0.48) | 3.88 (0.48) | 3.87 (0.49) | 4.28 (0.46) | 4.47 (0.41) | 4.44 (0.42) |
| Usual care | 3.61 (0.55) | 3.79 (0.60) | 3.88 (0.65) | 4.22 (0.47) | 4.43 (0.50) | 4.46 (0.40) |
| Attitudes regarding risks of vaccinationb | ||||||
| Vaccine social media | 3.74 (0.57) | 3.39 (0.66) | 3.26 (0.79) | 2.25 (0.62) | 2.13 (0.60) | 2.08 (0.60) |
| Vaccine information | 3.65 (0.54) | 3.29 (0.58) | 3.22 (0.76) | 2.24 (0.66) | 2.16 (0.71) | 2.15 (0.71) |
| Usual care | 3.52 (0.67) | 3.37 (0.72) | 3.42 (0.74) | 2.29 (0.64) | 2.20 (0.69) | 2.12 (0.66) |
| Perceived self-efficacy regarding vaccination decision makingc | ||||||
| Vaccine social media | 2.87 (0.87) | 3.22 (0.84) | 3.63 (0.69) | 3.54 (0.75) | 3.87 (0.64) | 3.96 (0.66) |
| Vaccine information | 2.55 (0.92) | 3.05 (0.91) | 3.32 (0.79) | 3.51 (0.78) | 3.79 (0.73) | 3.83 (0.73) |
| Usual care | 2.66 (0.71) | 3.15 (0.69) | 3.06 (0.71) | 3.48 (0.77) | 3.83 (0.70) | 3.91 (0.64) |
Note: Values represent the unadjusted mean scores (SD) on a 5-point Likert-type scale for each vaccine-related construct, stratified by vaccine hesitancy at baseline.
A higher score represents greater parental confidence regarding the benefits of vaccination.
A higher score represents greater parental concern about the risks of vaccination.
A higher score represents higher perceived self-efficacy regarding vaccination decision making.
Also shown in Table 3, mean scores changed over time in the usual care group among hesitant and non-hesitant parents. For example, vaccination benefits scores in the usual care group rose from baseline to Timepoint 2 among vaccine-hesitant parents (from 3.61 to 3.88, respectively, p < 0.001) and among non-hesitant parents (4.22 to 4.46, respectively, p < 0.001).
The intervention effect on the change in parental vaccine-related attitudes over time is presented in Table 4. There was a significant interaction between study arm, baseline vaccine hesitancy, and timepoint, and the effect of the intervention was assessed for the two categories of baseline hesitancy. Comparing baseline with Timepoint 1, the VSM and VI interventions were associated with significant improvements in attitudes regarding vaccination benefits compared to usual care among vaccine-hesitant parents. Comparing baseline with Timepoint 2, the VSM and VI study arms were also associated with significant reductions in parental concerns about vaccination risks compared to usual care among hesitant parents. Perceived self-efficacy also improved, although a significant change was only observed when comparing VI with usual care. No significant differences were observed when comparing the VSM versus VI study arms.
Table 4.
Adjusted Mean Change in Parents’ Vaccine-Related Attitudes, Beliefs, and Perceived Self-Efficacy, From Baseline to Follow-up
| Vaccine-hesitant at baseline |
Not vaccine-hesitant at baseline |
|||
|---|---|---|---|---|
| Study arm comparisons | Mean change from baseline to Timepoint 1 | Mean change from baseline to Timepoint 2 | Mean change from baseline to Timepoint 1 | Mean change from baseline to Timepoint 2 |
|
| ||||
| Attitudes regarding benefits of vaccinationa | ||||
| Vaccine social media vs usual care | 0.23 (0.05, 0.40) | 0.12 (−0.07, 0.31) | −0.02 (−0.10, 0.06) | −0.01 (−0.09, 0.07) |
| Vaccine information vs usual care | 0.22 (0.04, 0.40) | 0.09 (−0.11, 0.29) | −0.03 (−0.11, 0.05) | −0.08 (−0.17, 0.01) |
| Vaccine social media vs vaccine information | 0.01 (−0.14, 0.16) | 0.03 (−0.13, 0.19) | 0.01 (−0.05, 0.06) | 0.07 (0.01, 0.13) |
| Attitudes regarding risks of vaccinationb | ||||
| Vaccine social media vs usual care | −0.19 (−0.39, 0.002) | −0.37 (−0.60, −0.14) | −0.02 (−0.10, 0.07) | 0.01 (−0.09, 0.11) |
| Vaccine information vs usual care | −0.18 (−0.39, 0.02) | −0.31 (−0.55, −0.07) | 0.03 (−0.07, 0.12) | 0.08 (−0.03, 0.18) |
| Vaccine social media vs vaccine information | −0.01 (−0.18, 0.16) | −0.06 (−0.26, 0.14) | −0.04 (−0.11, 0.03) | −0.06 (−0.14, 0.01) |
| Perceived self-efficacy regarding vaccination decision makingc | ||||
| Vaccine social media vs usual care | −0.12 (−0.40, 0.16) | 0.29 (−0.03, 0.60) | −0.02 (−0.14, 0.11) | −0.02 (−0.15, 0.12) |
| Vaccine information vs usual care | 0.01 (−0.28, 0.30) | 0.37 (0.04, 0.69) | −0.06 (−0.19, 0.07) | −0.09 (−0.24, 0.05) |
| Vaccine social media vs vaccine information | −0.13 (−0.38, 0.12) | −0.08 (−0.35, 0.19) | 0.05 (−0.05, 0.14) | 0.08 (−0.03, 0.18) |
Note: Values represent adjusted mean change (95% CI) in scores on a 5-point Likert-type scale for each vaccine-related construct, assessed for the two categories of vaccine hesitancy measured at baseline. Boldface indicates statistical significance (p<0.05).
A positive number represents an increase over time in parental confidence regarding the benefits of vaccination.
A negative number represents a decrease over time in parental concern about the risks of vaccination.
A positive number represents an increase over time in perceived self-efficacy regarding vaccination decision making.
Table 4 also presents the change in attitudes over time among parents who were not vaccine hesitant at baseline. The VSM and VI interventions were not associated with any significant changes in vaccine-related attitudes compared to usual care.
DISCUSSION
In an RCT conducted in a large integrated healthcare organization, an Internet-based intervention delivered during pregnancy and early childhood led to significant improvements in vaccine-related attitudes among vaccine-hesitant parents. The intervention was effective among hesitant parents whether interactive social media components were included or not. Positive attitudes about vaccination benefits and perceived self-efficacy increased, whereas concerns about vaccination risks decreased compared with parents who received usual care. No intervention effect was observed among parents who were not vaccine hesitant at baseline. Interestingly, among parents not exposed to any intervention (i.e., those in the usual care group), pro-vaccination attitudes increased over the first 15 months of their child’s life.
As was highlighted in recent reviews of parental vaccine hesitancy, interventions are needed that change not only vaccine attitudes but vaccination behaviors.14,16,17 Vaccination rates did improve in the current study: as described elsewhere, children whose parents received the vaccine social media intervention were more likely to be vaccinated on time compared with parents who received usual care.25 Models of health decision making, including the Health Belief Model and the Theory of Planned Behavior, postulate that changes in relevant attitudes and beliefs are often necessary to produce behavior change.34,35 The finding that the intervention improved vaccine-related attitudes as well as vaccination behavior may be viewed as strengthening the likelihood of causality of the observed effects. Additionally, improving parents’ attitudes about vaccines remains important independent of the direct effect on vaccination behavior. Through discussing the topic online, and in-person with family and friends, parents contribute to the broader public discourse and help define social norms regarding the importance of vaccination for disease prevention.50–52
Several aspects of the vaccine social media intervention were unique compared with other Internet-based interventions in this field.23,24,53,54 The intervention included interactive features, such as a discussion forum, chat room, and blog. Parents could ask detailed questions of the vaccine subject matter experts and receive tailored responses within a few days. Study website users were required to have a login and password, which may have created a more civil discourse by preventing staunchly anti-vaccination individuals from outside the KPCO community from posting highly negative comments. Finally, engagement with parents began during the third trimester of pregnancy, a time when parents may begin considering their upcoming vaccination decisions.20,21
Presenting vaccination information on the study website without social media components also led to significant improvements in parents’ vaccine-related attitudes. What distinguished the content and tone of the study website from other public websites (such as www.cdc.gov/vaccines, www.immunize.org, or www.chop.edu/centers-programs/vaccine-education-center)? First, the study website was explicitly linked to KPCO, the organization providing care for study participants’ children, which may have tacitly indicated an endorsement of the material by their children’s primary care providers. Second, the study website included relevant local information, such as regarding community disease outbreaks or changes to Colorado’s school immunization requirements. Third, the study website, developed in collaboration with a health communications expert, tried to adopt a collaborative and non-judgmental tone by acknowledging that parental vaccine concerns were understandable given the ubiquity of vaccine misinformation.
Although the intervention was successful at changing parents’ vaccine-related attitudes, potential challenges to dissemination exist. Because Internet and social media technology rapidly evolve, ongoing resources will be needed to update the study website to the newest operating systems and portable device platforms. Creating new content, moderating the chat room and discussion forum, and directly responding to participants’ questions was time intensive, and many medical practices may not have the resources to create and maintain a vaccine-related social media forum for their own patients. A formal cost-effectiveness analysis of the trial is under way and should provide additional insight into the resources required to introduce this intervention elsewhere. Finally, answering participants’ questions required substantial vaccine subject matter expertise, which may not be available in all communities. Although this represents an important barrier to widespread dissemination, it is possible that communities could collaborate with regional immunization coalitions to identify individuals with the knowledge and willingness to address challenging vaccine-related questions.
Limitations
These findings should be interpreted in the context of several study limitations. Although survey response rates were high, vaccine-hesitant parents were less likely than non-hesitant parents to complete all three surveys, and response bias could have affected results. Although the survey questions were based on established models of health decision making,34,35 it is possible that important vaccine-related attitudes were left unexamined. Additionally, the primary study outcomes of changes in scores of three vaccine-related constructs are not entirely intuitive, and the clinical meaningfulness of a small change in scores (i.e., a 0.2-point change on a 5-point scale) may be difficult to gauge, notwithstanding the finding that the study also demonstrated improvements in vaccination timeliness.25 Also, parents’ attitudes were not assessed after their child was aged 15 months, so it is not possible to determine how long-lasting the observed changes in attitudes will be. Parents were required to have a user name and password; although this created a secure online environment, doing so could have created a barrier to website usage, and only one third of subjects in the VSM and VI study arms accessed the website one or more times.
Only 20.6% of those assessed for eligibility enrolled in the study, which could affect study generalizability. Of the subjects successfully enrolled, 14.1% were vaccine hesitant at baseline. Although this degree of vaccine hesitancy is similar to rates reported in other studies,4,6,7 it suggests that substantial resources were needed to target a relatively small population. Also, sample size and power calculations were based on the primary study outcome,25 not the outcomes presented here. Finally, because this study was conducted in a single organization among an insured population, similar results may not be found in different settings or among different patient populations, such as the uninsured.
CONCLUSIONS
An Internet-based intervention improved parents’ attitudes about vaccines, among parents who were vaccine hesitant at baseline. Perceived self-efficacy around vaccination decision making increased, as did positive attitudes about vaccination benefits, whereas concerns about vaccination risks decreased. Although these results are promising, several challenges to widespread dissemination exist.
ACKNOWLEDGMENTS
We would like to acknowledge the contribution of Stanley Xu, PhD, who provided consultation on the statistical analyses. This study was supported by a research grant from the Agency for Healthcare Research and Quality (R01HS021492). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Agency for Healthcare Research and Quality.
MFD helped conceptualize the study design, provided input for the statistical analyses, interpreted the results, and drafted the manuscript. KJN provided input on the study design, managed the data, conducted the statistical analysis, and helped draft the manuscript and protocol. JAS helped conceptualize the intervention, helped design the survey instrument, helped manage the data, and provided input on the manuscript. NMW provided input on the study design, helped draft the study protocol, and contributed to drafting the manuscript. JMG conceptualized the study design, obtained the funding, provided input for the statistical analysis, interpreted the results, and helped draft the manuscript. All authors reviewed and revised the manuscript and approved the current manuscript for publication.
This study was registered at www.clinicaltrials.gov, NCT01873040. The authors MFD, KJN, and JMG had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
No financial disclosures were reported by the authors of this paper.
Footnotes
Trial registration: This study was registered at www.clinicaltrials.gov NCT01873040.
REFERENCES
- 1.Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Dietz V. Vaccination coverage among children aged 19–35 months—United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(39):1065–1071. 10.15585/mmwr.mm6539a4. [DOI] [PubMed] [Google Scholar]
- 2.Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kolasa M. National, state, and selected local area vaccination coverage among children aged 19–35 months—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(33):889–896. 10.15585/mmwr.mm6433a1. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald NE, Sage Working Group on Vaccine Hesitancy. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33 (34):4161–4164. 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 4.Frew PM, Fisher AK, Basket MM, et al. Changes in childhood immunization decisions in the United States: results from 2012 & 2014 National Parental Surveys. Vaccine. 2016;34(46):5689–5696. 10.1016/j.vaccine.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. 2014;32(19):2150–2159. 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 6.Dempsey AF, Schaffer S, Singer D, Butchart A, Davis M, Freed GL. Alternative vaccination schedule preferences among parents of young children. Pediatrics. 2011;128(5):848–856. 10.1542/peds.2011-0400. [DOI] [PubMed] [Google Scholar]
- 7.McCauley MM, Kennedy A, Basket M, Sheedy K. Exploring the choice to refuse or delay vaccines: a national survey of parents of 6- through 23-month-olds. Acad Pediatr. 2012;12(5):375–383. 10.1016/j.acap.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics. 2008;122 (4):718–725. 10.1542/peds.2007-0538. [DOI] [PubMed] [Google Scholar]
- 9.Glanz JM, McClure DL, Magid DJ, et al. Parental refusal of pertussis vaccination is associated with an increased risk of pertussis infection in children. Pediatrics. 2009;123(6):1446–1451. 10.1542/peds.2008-2150. [DOI] [PubMed] [Google Scholar]
- 10.Glanz JM, McClure DL, Magid DJ, Daley MF, France EK, Hambidge SJ. Parental refusal of varicella vaccination is associated with an increased risk of varicella infection in children. Arch Pediatr Adolesc Med. 2010;164(1):66–70. 10.1001/archpediatrics.2009.244. [DOI] [PubMed] [Google Scholar]
- 11.Glanz JM, McClure DL, O’Leary ST, et al. Parental decline of pneumococcal vaccination and risk of pneumococcal related disease in children. Vaccine. 2011;29(5):994–999. 10.1016/j.vaccine.2010.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. JAMA. 2016;315(11):1149–1158. 10.1001/jama.2016.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams SE. What are the factors that contribute to parental vaccine-hesitancy and what can we do about it? Hum Vaccin Immunother. 2014;10(9):2584–2596. 10.4161/hv.28596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarrett C, Wilson R, O’Leary M, Eckersberger E, Larson HJ, Sage Working Group on Vaccine Hesitancy. Strategies for addressing vaccine hesitancy—a systematic review. Vaccine. 2015;33(34):4180–4190. 10.1016/j.vaccine.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 15.Nyhan B, Reifler J, Richey S, Freed GL. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133(4):e835–e842. 10.1542/peds.2013-2365. [DOI] [PubMed] [Google Scholar]
- 16.Sadaf A, Richards JL, Glanz J, Salmon DA, Omer SB. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31(40):4293–4304. 10.1016/j.vaccine.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Dube E, Gagnon D, MacDonald NE, Sage Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33(34):4191–4203. 10.1016/j.vaccine.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 18.Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Sources and perceived credibility of vaccine-safety information for parents. Pediatrics. 2011;127(suppl 1):S107–S112. 10.1542/peds.2010-1722P. [DOI] [PubMed] [Google Scholar]
- 19.Jones AM, Omer SB, Bednarczyk RA, Halsey NA, Moulton LH, Salmon DA. Parents’ source of vaccine information and impact on vaccine attitudes, beliefs, and nonmedical exemptions. Adv Prev Med. 2012;2012:932741. 10.1155/2012/932741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glanz JM, Wagner NM, Narwaney KJ, et al. A mixed methods study of parental vaccine decision making and parent-provider trust. Acad Pediatr. 2013;13(5):481–488. 10.1016/j.acap.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham RM, Minard CG, Guffey D, Swaim LS, Opel DJ, Boom JA. Prevalence of vaccine hesitancy among expectant mothers in Houston, Texas. Acad Pediatr. 2018;18(2):154–160. 10.1016/j.acap.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Kempe A, Daley MF, McCauley MM, et al. Prevalence of parental concerns about childhood vaccines: the experience of primary care physicians. Am J Prev Med. 2011;40(5):548–555. 10.1016/j.amepre.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Wallace C, Leask J, Trevena LJ. Effects of a web based decision aid on parental attitudes to MMR vaccination: a before and after study. BMJ. 2006;332(7534):146–149. 10.1136/bmj.38678.681840.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shourie S, Jackson C, Cheater FM, et al. A cluster randomised controlled trial of a web based decision aid to support parents’ decisions about their child’s measles mumps and rubella (MMR) vaccination. Vaccine. 2013;31(50):6003–6010. 10.1016/j.vaccine.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glanz JM, Wagner NM, Narwaney KJ, et al. Web-based social media intervention to increase vaccine acceptance: a randomized controlled trial. Pediatrics. 2017;140(6):e20171117. 10.1542/peds.2017-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Opel DJ, Taylor JA, Zhou C, Catz S, Myaing M, Mangione-Smith R. The relationship between parent attitudes about childhood vaccines survey scores and future child immunization status: a validation study. JAMA Pediatr. 2013;167(11):1065–1071. 10.1001/jamapediatrics.2013.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Opel DJ, Taylor JA, Mangione-Smith R, et al. Validity and reliability of a survey to identify vaccine-hesitant parents. Vaccine. 2011;29(38): 6598–6605. 10.1016/j.vaccine.2011.06.115. [DOI] [PubMed] [Google Scholar]
- 28.Peckham E, Brabyn S, Cook L, Devlin T, Dumville J, Torgerson DJ. The use of unequal randomisation in clinical trials—an update. Contemp Clin Trials. 2015;45(pt A):113–122. 10.1016/j.cct.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Vozdolska R, Sano M, Aisen P, Edland SD. The net effect of alternative allocation ratios on recruitment time and trial cost. Clin Trials. 2009;6 (2):126–132. 10.1177/1740774509103485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thackeray R, Neiger BL. A multidirectional communication model: implications for social marketing practice. Health Promot Pract. 2009;10(2):171–175. 10.1177/1524839908330729. [DOI] [PubMed] [Google Scholar]
- 31.Shoup JA, Wagner NM, Kraus CR, Narwaney KJ, Goddard KS, Glanz JM. Development of an interactive social media tool for parents with concerns about vaccines. Health Educ Behav. 2015;42(3):302–312. 10.1177/1090198114557129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Q. Are social networking sites making health behavior change interventions more effective? A meta-analytic review. J Health Commun. 2017;22(3):223–233. 10.1080/10810730.2016.1271065. [DOI] [PubMed] [Google Scholar]
- 33.Morgan MG. Risk Communication: A Mental Models Approach. New York, NY: Cambridge University Press, 2002. [Google Scholar]
- 34.Skinner CS, Tiro J, Champion VL. The health belief model. In: Glanz K, Rimer B, Viswanath K, eds. Health Behavior: Theory, Research, and Practice, 5th ed., San Francisco, CA: John Wiley & Sons, 2015:75–94. [Google Scholar]
- 35.Montano DE, Kasprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. In: Glanz K, Rimer B, Viswanath K, eds. Health Behavior: Theory, Research and Practice, 5th ed., San Francisco, CA: John Wiley & Sons, 2015:95–124. [Google Scholar]
- 36.Covello VT. Best practices in public health risk and crisis communication. J Health Commun. 2003;8(suppl 1):5–8. 10.1080/713851971. [DOI] [PubMed] [Google Scholar]
- 37.Fischhoff B, Brewer NT, Downs JS. Communicating Risks and Benefits: An Evidence Based User’s Guide. Silver Spring, MD: Food and Drug Administration, 2012. [Google Scholar]
- 38.Flanagin AJ, Metzger MJ. The role of site features, user attributes, and information verification behaviors on the perceived credibility of web-based information. New Media Soc. 2007;9(2):319–342. 10.1177/1461444807075015. [DOI] [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC). Recommended immunization schedules for persons aged 0 through 18 years-United States, 2015. www.cdc.gov/vaccines/schedules/downloads/past/2015-child.pdf. Accessed September 25, 2017. [PMC free article] [PubMed]
- 40.Centers for Disease Control and Prevention (CDC). Recommended immunization schedules for persons aged 0 through 18 years-United States, 2016. www.cdc.gov/vaccines/schedules/downloads/past/2016-child.pdf. Accessed September 25, 2017.
- 41.Frew PM, Chung Y, Fisher AK, Schamel J, Basket MM. Parental experiences with vaccine information statements: implications for timing, delivery, and parent-provider immunization communication. Vaccine. 2016;34(48):5840–5844. 10.1016/j.vaccine.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gellin BG, Maibach EW, Marcuse EK. Do parents understand immunizations? A national telephone survey. Pediatrics. 2000;106 (5):1097–1102. 10.1542/peds.106.5.1097. [DOI] [PubMed] [Google Scholar]
- 43.Salmon DA, Moulton LH, Omer SB, Patricia deHart M, Stokley S, Halsey NA. Factors associated with refusal of childhood vaccines among parents of school-aged children: a case-control study. Arch Pediatr Adolesc Med. 2005;159(5):470–476. 10.1001/archpedi.159.5.470. [DOI] [PubMed] [Google Scholar]
- 44.Gust DA, Kennedy A, Shui I, Smith PJ, Nowak G, Pickering LK. Parent attitudes toward immunizations and healthcare providers: the role of information. Am J Prev Med. 2005;29(2):105–112. 10.1016/j.amepre.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Kennedy A, LaVail K, Nowak G, Basket M, Landry S. Confidence about vaccines in the United States: understanding parents’ perceptions. Health Aff (Millwood). 2011;30(6):1151–1159. 10.1377/hlthaff.2011.0396. [DOI] [PubMed] [Google Scholar]
- 46.Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Parental vaccine safety concerns in 2009. Pediatrics. 2010;125 (4):654–659. 10.1542/peds.2009-1962. [DOI] [PubMed] [Google Scholar]
- 47.Nunnally JC. Assessment of reliability. Psychometric Theory, 2nd ed., New York, NY: McGraw-Hill Inc, 1978: 225–255. [Google Scholar]
- 48.Ibrahim JG, Molenberghs G. Missing data methods in longitudinal studies: a review. Test (Madr). 2009;18(1):1–43. 10.1007/s11749-009-0138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer, 2000. [Google Scholar]
- 50.Kang GJ, Ewing-Nelson SR, Mackey L, et al. Semantic network analysis of vaccine sentiment in online social media. Vaccine. 2017;35 (29):3621–3638. 10.1016/j.vaccine.2017.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oraby T, Thampi V, Bauch CT. The influence of social norms on the dynamics of vaccinating behaviour for paediatric infectious diseases. Proc Biol Sci. 2014;281(1780):20133172. 10.1098/rspb.2013.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larson HJ, Smith DM, Paterson P, et al. Measuring vaccine confidence: analysis of data obtained by a media surveillance system used to analyse public concerns about vaccines. Lancet Infect Dis. 2013;13 (7):606–613. 10.1016/S1473-3099(13)70108-7. [DOI] [PubMed] [Google Scholar]
- 53.Lau AY, Sintchenko V, Crimmins J, Magrabi F, Gallego B, Coiera E. Impact of a web-based personally controlled health management system on influenza vaccination and health services utilization rates: a randomized controlled trial. J Am Med Inform Assoc. 2012;19(5):719–727. 10.1136/amiajnl-2011-000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gowda C, Schaffer SE, Kopec K, Markel A, Dempsey AF. A pilot study on the effects of individually tailored education for MMR vaccine-hesitant parents on MMR vaccination intention. Hum Vaccin Immunother. 2013;9(2):437–445. 10.4161/hv.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]


