Abstract
Objective
To assess the relation of symptomatic knee osteoarthritis (OA), knee pain, and radiographic knee OA to all-cause mortality and identify mediators in the causal pathway.
Methods
Participants from the Osteoarthritis Initiative were divided into four groups: (1) symptomatic knee OA (i.e., both radiographic knee OA [Kellgren and Lawrence grade ≥2] and knee pain); (2) knee pain only; (3) radiographic knee OA only; and (4) neither radiographic knee OA nor knee pain. We examined the relation of knee OA status to all-cause mortality using a multivariable Cox-proportional model and assessed the extent to which the association was mediated by disability, physical (PCS) and mental component summary scores (MCS) of quality of life (QoL), and oral pain-relief medications (i.e. nonsteroidal anti-inflammatory drugs and opioids) use.
Results
Among 4,796 participants, 282 died over the 96-month follow-up period. Compared with those with neither radiographic knee OA nor knee pain, multivariable-adjusted hazard ratios (HRs) of mortality were 2.2 (95% confidence interval [CI]: 1.6–3.1) for symptomatic knee OA, 0.9 (95%CI: 0.6–1.4) for knee pain only, and 2.0 (95%CI: 1.4–2.9) for radiographic knee OA only, respectively. Indirect effects (HRs) of symptomatic knee OA on mortality via disability and PCS of QoL were 1.1 (95%CI: 1.0–1.4) and 1.2 (95%CI: 1.0–1.4), respectively. No apparent mediation effect was observed through either MCS of QoL or oral pain-relief medications use.
Conclusion
Participants with either symptomatic or radiographic knee OA were at an increased risk of all-cause mortality. Increased risk of mortality from symptomatic knee OA was partially mediated through its effect on disability and PCS of QoL.
Osteoarthritis (OA) is the most common joint disorder (1, 2), affecting approximately 10% of men and 18% of women aged 60 years or older around the world (3, 4). Besides its tremendous impact on morbidity and disability (5, 6), several studies also found that knee or hip OA were associated with an increased risk of all-cause mortality (7-14).
The mechanisms linking OA to mortality were not clearly understood. Such knowledge, however, has an important clinical implication because it would guide us to develop a more efficient intervention program that specifically targets a particular mechanism for the outcome. To our knowledge, only two studies explored the potential mechanisms linking OA to all-cause mortality (11, 12). The studies showed that an increased risk of all-cause mortality from OA was partly mediated through its effect on either walking disability (11) or physical function impairment (12), but none of them found that an increased all-cause mortality was mediated through nonsteroidal anti-inflammatory drugs (NSAIDs) use, a potential risk factor for an increased risk of either cardiovascular or gastrointestinal diseases mortality (15, 16). In both studies, however, potential mediators were either assessed at baseline or at the end of follow-up; thus, the time sequence from knee OA status to mediators to death was not clearly delineated.
To fill this knowledge gap, we examined the relation of knee OA status (i.e., symptomatic knee OA, knee pain only, radiographic knee OA only, and knee with neither radiographic OA nor pain) to the risk of all-cause mortality among participants in the Osteoarthritis Initiative (OAI). In addition, we assessed the extent to which three putative mediators, i.e., disability, quality of life (QoL), and oral pain-relief medications (i.e., NSAIDs and opioids) may impact the effect of OA on the risk of all-cause mortality.
PATIENTS AND METHODS
Study Design and Subjects
OAI is a multi-center observational study of natural history of OA and its risk factors. Individuals aged between 45 to 79 years were recruited from four clinical sites: Baltimore MD, Pittsburgh PA, Pawtucket RI, and Columbus OH. Data from each participant were collected at baseline and at each annual follow-up visit. A detailed description regarding the rationale and approach of OAI can be found at https://nda.nih.gov/oai/about-oai.
Assessment of Knee OA and Pain
Weight-bearing semi-fixed posteroanterior knee radiographs were obtained using a standard protocol. All radiographs were read by both a musculoskeletal radiologist and a rheumatologist. If there was a disagreement as to whether the knee had radiographic OA, the reading was adjudicated by a panel of three experienced readers including the two who originally read the radiographs and another rheumatologist. A consensus reading was reached when at least two of the three readers agreed. A knee was defined as having radiographic OA if its Kellgren and Lawrence grade ≥2. Knee pain (absent/present) was based on a self-report of whether participant had pain, aching, or stiffness in their right or left knee on most days for at least one month within the past year. This definition of knee pain has been employed previously using data from OAI (17) and in combination with radiographic knee OA to define symptomatic knee OA (18).
Participants were categorized into four groups: (1) symptomatic knee OA, i.e., at least one knee having both radiographic OA and pain; (2) knee pain only, i.e., at least one knee having pain but neither knee having both pain and radiographic OA; (3) radiographic knee OA only, i.e., at least one knee having radiographic OA but neither knee having pain; or (4) knee with neither radiographic OA nor pain. We assigned a participant into one of the four categories based on the following order: symptomatic knee OA, knee pain only, radiographic knee OA only, and knee with neither radiographic OA nor pain. For example, a participant was assigned to “knee pain only” category if s/he had radiographic OA on one knee, had pain on the contralateral knee, but had no knee with both radiographic OA and pain.
Assessment of All-cause Mortality
All-cause mortality, hereafter referred to as mortality, during the 96-month follow-up period was confirmed and adjudicated based on autopsy report, coroner’s report, death certificate, National Death Index, obituary, or Social Security Death Index (19).
Assessment of Potential Confounders
Information on age, sex, race, height, weight, education level, weekly alcohol consumption, smoking habits, history of knee injury, comorbidities, depressive symptoms and widespread pain were obtained at baseline. Body Mass Index (BMI) was computed as weight (kg)/ height (m2). The modified Charlson comorbidity index was computed based on self-reported comorbidities (20), and the presence of depressive symptoms was classified using a score ≥16 on the Center for Epidemiologic Studies Depression Scale (21). Widespread pain was defined as pain above and below the waist, pain on the right and left sides of the body, and axial pain based on a standard homunculus (22).
Assessment of Mediators
Three putative mediators were assessed at the closest follow-up visit prior to death, or prior to the last follow-up visit if participant was lost to follow-up, or prior to the end of follow-up (i.e., 96-month follow-up visit). These included: 1) disability, measured by the physical function subscale (range from 0 to 68) of Western Ontario and McMaster Universities Osteoarthritis Index (23); 2) physical and mental component summary scores of QoL (range from 0 to 100), assessed with the 12-item Short-Form Health Survey, i.e., PCS of QoL and MCS of QoL (24, 25); and 3) the use of oral pain-relief medications (i.e., NSAIDs and opioids) that was collected by questionnaires in the OAI database. Participants were asked whether they had used prescription or over-the-counter medications (acetaminophen, NSAIDs, coxibs and opioids) for joint pain or arthritis more than half of the days of the month, in the past 30 days (26).
Statistical Analysis
Person-years of follow-up for each participant were computed as the amount of time from the date of the baseline examination to the date of death, the last date of contact, or the end of follow up (i.e., 96-month follow-up visit). Mortality rates for each knee OA category were calculated by dividing the number of deaths by the number of person-years of follow-up. We fitted a Cox proportional-hazards model to determine the relation of knee OA status at baseline to the risk of mortality. In the multivariable Cox proportional-hazards model, we adjusted for baseline age, sex, race, BMI, education level, weekly alcohol consumption, smoking status, history of knee injury, modified Charlson comorbidities index, depressive symptoms and widespread pain. We tested the proportional hazards assumption using the Kolmogorov supremum test.
We used a marginal structural model to assess the extent to which the association between knee OA status and mortality (i.e., total effect) was mediated through each of the putative mediators (27). Specifically, we decomposed the total effect of knee OA status on mortality into two components: 1) the indirect effect (or mediated effect), i.e., the effect of knee OA status on mortality mediated through each of the three mediators, i.e., disability, PCS/MCS of QoL and oral use of pain-relief medications; and 2) the direct effect, i.e., the effect of knee OA status on mortality that was not through a specific aforementioned mediator. In addition, we estimated the percentage of the total effect that was mediated via each mediator using the following formula: [(indirect effect - 1)*direct effect]/(indirect effect*direct effect - 1)*100% (28).
We conducted two sensitivity analyses to assess the robustness of our study findings. First, we used a new algorithm to define knee OA status at baseline according to the following order: symptomatic knee OA, radiographic knee OA only, knee pain only, and knee with neither radiographic OA nor pain. For example, a participant was assigned to “radiographic knee OA only” category if s/he had radiographic OA on one knee, had pain on the contralateral knee, but had neither knee with both radiographic OA and pain; whereas a participant was assigned to “knee pain only” category if s/he had knee pain on at least one knee but had neither knee with radiographic OA. Participants who were assigned to “symptomatic knee OA” (i.e., at least one knee having both radiographic OA and pain) or to “knee with neither radiographic OA nor pain” categories based on the previous algorithm did not change their knee OA status. Second, considering that knee OA status of a participant may change during the follow-up period, we used the knee OA status right before the mediators were assessed. For example, if a participant with neither radiographic knee OA nor pain at baseline developed radiographic knee OA at 24-month follow-up visit, and this person did not develop knee pain since then, his/her knee OA status was assigned to “radiographic knee OA only” category, and his/her follow-up time would start from the date of 24-month follow-up visit. Data on confounders were also updated using the information collected at the closest follow-up visit prior to the assessment of knee OA status. We then took the same approach as described above to examine the relation of each of the OA status derived from these two new definitions to the risk of mortality and estimate its direct and indirect effects through each mediator.
All statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Of the 4,796 participants at baseline, 6.2% (n=297) did not have adequate data for assessment of either radiographic knee OA or knee pain; thus, they were excluded from the analyses. Of the remaining, 31% (n=1,387) participants had symptomatic knee OA, 19% (n=856) had knee pain only, 25% (n=1,117) had radiographic knee OA only, and 25% (n=1,139) had neither radiographic knee OA nor knee pain. Compared with those with neither radiographic knee OA nor knee pain, participants with symptomatic knee OA were more likely to be non-white, had lower percentage of college education, higher BMI and disability score, more comorbidities, depressive symptoms, widespread pain, knee injury history, and oral pain medications use. They also had lower PCS and MCS of QoL (Table 1).
Table 1.
Characteristics of Included Participants (n=4,499)
| Overall (n=4,499) | Neither Radiographic Knee OA nor Knee Pain (n=1,139) | Radiographic Knee OA Only (n=1,117) | Knee Pain Only (n=856) | Symptomatic Knee OA (n=1,387) | |
|---|---|---|---|---|---|
| Age, mean (SD), years | 61.2 (9.2) | 60.1 (9.1) | 64.0 (8.8) | 58.3 (8.9) | 61.5 (9.0) |
| Women (%) | 58.1 | 58.1 | 59.4 | 58.4 | 56.8 |
| Non-white (%) | 18.8 | 11.4 | 14.6 | 21.6 | 29.8 |
| College Graduate or Above (%) | 60.3 | 68.3 | 63.3 | 59.4 | 51.8 |
| Smoking Status (%) | |||||
| Never | 79.5 | 79.2 | 80.5 | 79.6 | 78.9 |
| Former | 17.3 | 17.4 | 16.9 | 16.9 | 17.9 |
| Current | 3.2 | 3.5 | 2.6 | 3.6 | 3.2 |
| Drinking Status (%) | |||||
| None | 18.9 | 15.3 | 17.4 | 21.1 | 21.7 |
| <1 drink/week | 37.7 | 36.5 | 38.7 | 36.9 | 38.4 |
| 1–21 drinks/week | 41.9 | 46.5 | 42.0 | 40.8 | 38.6 |
| >21 drinks/week | 1.6 | 1.8 | 1.9 | 1.2 | 1.3 |
| BMI, mean (SD), kg/m 2 | 28.6 (4.8) | 26.9 (4.4) | 29.0 (4.6) | 27.8 (4.6) | 30.2 (4.6) |
| Comorbidity Score, mean (SD) | 0.4 (0.8) | 0.3 (0.7) | 0.4 (0.8) | 0.4 (0.8) | 0.5 (0.9) |
| Depression Score, mean (SD) | 6.5 (6.9) | 5.4 (5.9) | 5.4 (5.9) | 7.3 (7.1) | 7.7 (7.8) |
| History of Knee Injury (%) | 43.4 | 32.3 | 46.0 | 40.2 | 52.0 |
| Widespread Pain (%) | 4.5 | 2.5 | 2.4 | 6.3 | 6.9 |
| Disability Score, mean (SD) | 9.5 (11.7) | 4.1 (7.1) | 6.5 (9.0) | 10.0 (11.5) | 16.0 (13.6) |
| PCS of QoL, mean (SD) | 47.7 (9.7) | 50.7 (8.4) | 48.9 (8.9) | 47.7 (9.8) | 44.0 (10.0) |
| MCS of QoL, mean (SD) | 53.7 (8.5) | 54.1 (7.7) | 54.4 (7.8) | 53.1 (8.7) | 53.3 (9.3) |
| Oral Pain Medications Use (%) | 31.7 | 21.8 | 28.0 | 31.2 | 43.2 |
| Death (%) | 6.3 | 6.3 | 6.5 | 5.0 | 6.9 |
n, number; OA, osteoarthritis; SD, standard deviation; BMI, body mass index; PCS, physical component summary score; MCS, mental component summary score; QoL, quality of life.
During the 96-month follow-up period, 282 deaths occurred (Table 2). Participants with symptomatic knee OA had the highest mortality rate (15.7/1,000 person-years), followed by those with radiographic knee OA only (14.1/1,000 person-years) and those with neither radiographic knee OA nor knee pain (9.4/1,000 person-years). Participants with knee pain only had the lowest mortality (7.9/1,000 person-years). Compared with those with neither radiographic knee OA nor knee pain, the multivariable-adjusted hazards ratios (HRs) and their 95% confidence intervals (CIs) of mortality were 2.2 (95%CI: 1.6 to 3.1), 0.9 (95%CI: 0.6 to 1.4), and 2.0 (95%CI: 1.4 to 2.9) for the participants with symptomatic knee OA, knee pain only, and radiographic knee OA only, respectively.
Table 2.
Total, Direct, and Indirect Effect of Radiographic Knee OA Only, Knee Pain Only, and Symptomatic Knee OA on All-cause Mortality
| Neither Radiographic Knee OA nor Knee Pain (n=1,139) | Radiographic Knee OA Only (n=1,117) | Knee Pain Only (n=856) | Symptomatic Knee OA (n=1,387) | |
|---|---|---|---|---|
| Number of Death | 72 | 72 | 43 | 95 |
| Deaths per 1000 Person-years (95% CI) | 9.4 (7.4 to 11.8) | 14.1 (11.1 to 17.8) | 7.9 (5.7 to 10.6) | 15.7 (12.7 to 19.2) |
| Total Effect, HR (95% CI) | 1.0 (reference) | 2.6 (1.8 to 3.6) | 0.9 (0.6 to 1.3) | 2.6 (1.9 to 3.6) |
| Total Effect, HR (95% CI) * | 1.0 (reference) | 2.0 (1.4 to 2.9) | 0.9 (0.6 to 1.4) | 2.2 (1.6 to 3.1) |
| Disability Score | ||||
| Direct, HR (95% CI) * | 1.0 (reference) | 2.0 (1.7 to 2.4) | 0.9 (0.7 to 1.1) | 1.9 (1.6 to 2.3) |
| Indirect, HR (95% CI) * | 1.0 (reference) | 1.0 (0.9 to 1.2) | 1.0 (0.9 to 1.2) | 1.1 (1.0 to 1.4) |
| Mediation effect (%) | - | 2.0 | Not same direction | 22.4 |
| PCS of QoL | ||||
| Direct, HR (95% CI) * | 1.0 (reference) | 2.0 (1.7 to 2.4) | 0.9 (0.7 to 1.1) | 1.9 (1.6 to 2.2) |
| Indirect, HR (95% CI) * | 1.0 (reference) | 1.0 (0.8 to 1.2) | 1.1 (0.9 to 1.3) | 1.2 (1.0 to 1.4) |
| Mediation effect (%) | - | 0.0 | Not same direction | 26.5 |
| MCS of QoL | ||||
| Direct, HR (95% CI) * | 1.0 (reference) | 2.0 (1.7 to 2.4) | 0.9 (0.7 to 1.2) | 2.2 (1.9 to 2.6) |
| Indirect, HR (95% CI) * | 1.0 (reference) | 1.0 (0.8 to 1.2) | 1.0 (0.8 to 1.2) | 1.0 (0.8 to 1.2) |
| Mediation effect (%) | - | 0.0 | Not same direction | 0.0 |
| Oral Pain Medications Use | ||||
| Direct, HR (95% CI) * | 1.0 (reference) | 2.0 (1.7 to 2.4) | 0.9 (0.7 to 1.1) | 2.1 (1.7 to 2.5) |
| Indirect, HR (95% CI) * | 1.0 (reference) | 1.0 (0.9 to 1.2) | 1.0 (0.9 to 1.2) | 1.1 (0.9 to 1.3) |
| Mediation effect (%) | - | 2.0 | Not same direction | 12.0 |
n, number; OA, osteoarthritis; CI, confidence interval; HR, hazard ratio; PCS, physical component summary score; MCS, mental component summary score; QoL, quality of life.
Adjusted for age, race, sex, body mass index, education level, weekly alcohol consumption, smoking habits, history of knee injury, comorbidities, depressive symptoms and widespread pain.
As shown in Table 2 and Figure 1, the indirect effect (HR) of symptomatic knee OA on mortality through PCS of QoL was 1.2 (95%CI: 1.0 to 1.4) and the percentage of mediation was 26.5%. The indirect effect of symptomatic knee OA on mortality via disability was borderline level of statistically significant (HR=1.1, 95%CI: 1.0 to 1.4, percentage of mediation: 22.4%). No apparent mediation effect was observed via either MCS of QoL or the use of oral pain-relief medications. An increased risk of mortality observed among participants with radiographic knee OA only was mediated through none of three aforementioned mediators.
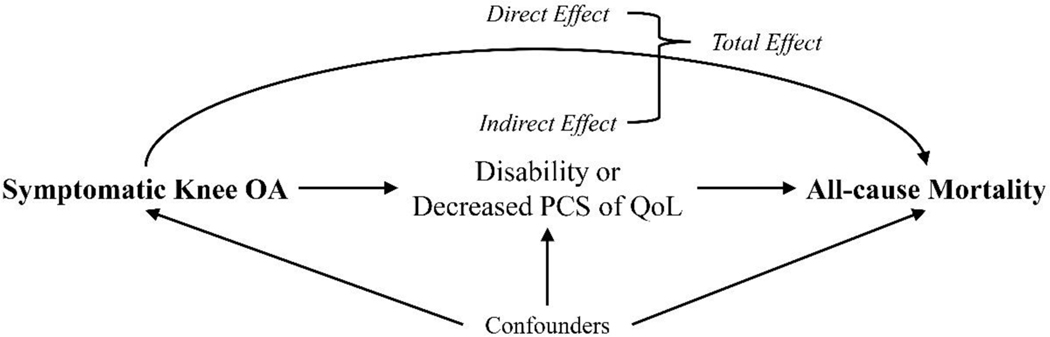
Figure 1. A Directed Acyclic Graph to Describe the Mediation Effect between Symptomatic Knee OA and All-cause Mortality.
OA, osteoarthritis; PCS of QoL, physical component summary score of quality of life
Indirect effect: effect of symptomatic knee OA on all-cause mortality through its effect on mediators (i.e., disability and PCS of QoL)
Direct effect: effect of symptomatic knee OA on all-cause mortality not through its effect on mediators (i.e., disability and PCS of QoL)
Total effect: sum of indirect and direct effects
Confounders: confounders of association between symptomatic knee OA and all-cause mortality (i.e., age, race, sex, body mass index, education level, weekly alcohol consumption, smoking habits, history of knee injury, comorbidities, depressive symptom and widespread pain)
The sensitivity analysis with the new algorithm, where the order of “radiographic knee OA only” preceded that of “knee pain only” to define a participant’s knee OA status at baseline, showed that the results did not change materially (Table 3). When we used updated data to define knee OA status, participants with symptomatic knee OA and those with radiographic knee OA only still experienced a higher mortality than those with neither radiographic knee OA nor knee pain (Table 4). The multivariable-adjusted HRs were 2.0 (95% CI: 1.4 to 2.9) and 1.7 (95% CI: 1.2 to 2.4), respectively. The indirect effects of symptomatic knee OA on mortality through PCS of QoL (HR=1.2, 95%CI: 1.0 to 1.5, percentage of mediation: 38.9%) and disability (HR=1.7, 95%CI: 1.5 to 2.0, percentage of mediation: 85.5%) were greater than previous findings. However, no apparent mediation effect was observed via either MCS of QoL or the use of oral pain-relief medications. The association between radiographic knee OA only and risk of mortality was mediated by none of the three aforementioned mediators. There was no statistically significant relation of knee pain only to the risk of mortality; however, its direct and indirect effects via two mediators (i.e., disability score and PCS of QoL) showed an apparent opposite direction when a participant’s knee OA status was defined right before the mediators were assessed, indicating a potential inconsistent mediation.
Table 3.
Sensitive Analysis (Participants with Discordant Knee Pain and Radiographic Knee OA Assigned to the Radiographic Knee OA Only Group)
| Neither Radiographic Knee OA nor Knee Pain (n=1,139) | Radiographic Knee OA Only (n=1,180) | Knee Pain Only (n=793) | Symptomatic Knee OA (n=1,387) | |
|---|---|---|---|---|
| Number of Death | 72 | 75 | 40 | 95 |
| Deaths per 1000 Person-years (95% CI) | 9.4 (7.4 to 11.8) | 13.8 (10.9 to 17.3) | 7.8 (5.6 to 10.6) | 15.7 (12.7 to 19.2) |
| Total Effect, HR (95% CI) | 1.0 (reference) | 2.4 (1.7 to 3.4) | 0.9 (0.6 to 1.3) | 2.6 (1.9 to 3.6) |
| Total Effect, HR (95% CI) * | 1.0 (reference) | 2.0 (1.4 to 2.8) | 0.9 (0.6 to 1.4) | 2.2 (1.6 to 3.1) |
| Disability Score | ||||
| Direct, HR (95% CI) * | 1.0 (reference) | 2.0 (1.7 to 2.4) | 0.9 (0.7 to 1.1) | 1.9 (1.6 to 2.3) |
| Indirect, HR (95% CI) * | 1.0 (reference) | 1.0 (0.9 to 1.2) | 1.0 (0.9 to 1.2) | 1.1 (1.0 to 1.4) |
| Mediation effect (%) | - | 2.0 | Not same direction | 22.4 |
| PCS of QoL | ||||
| Direct, HR (95% CI) * | 1.0 (reference) | 2.0 (1.7 to 2.4) | 0.8 (0.6 to 1.0) | 1.9 (1.6 to 2.2) |
| Indirect, HR (95% CI) * | 1.0 (reference) | 1.0 (0.8 to 1.2) | 1.1 (0.9 to 1.3) | 1.2 (1.0 to 1.4) |
| Mediation effect (%) | - | 0.0 | Not same direction | 26.5 |
| MCS of QoL | ||||
| Direct, HR (95% CI) * | 1.0 (reference) | 2.0 (1.7 to 2.4) | 0.9 (0.7 to 1.1) | 2.2 (1.9 to 2.6) |
| Indirect, HR (95% CI) * | 1.0 (reference) | 1.0 (0.8 to 1.2) | 1.0 (0.8 to 1.2) | 1.0 (0.8 to 1.2) |
| Mediation effect (%) | - | 0.0 | Not same direction | Not same direction |
| Oral Pain Medications Use | ||||
| Direct, HR (95% CI) * | 1.0 (reference) | 2.0 (1.6 to 2.4) | 0.9 (0.7 to 1.1) | 2.1 (1.7 to 2.5) |
| Indirect, HR (95% CI) * | 1.0 (reference) | 1.0 (0.9 to 1.2) | 1.0 (0.9 to 1.2) | 1.1 (0.9 to 1.3) |
| Mediation effect (%) | - | 3.9 | Not same direction | 12.0 |
n, number; OA, osteoarthritis; CI, confidence interval; HR, hazard ratio; PCS, physical component summary score; MCS, mental component summary score; QoL, quality of life.
Adjusted for age, race, sex, body mass index, education level, weekly alcohol consumption, smoking habits, history of knee injury, comorbidities, depressive symptoms and widespread pain.
Table 4.
Sensitive Analysis (OA Status Updated before Mediators)
| Neither Radiographic Knee OA nor Knee Pain (n=1,302) | Radiographic Knee OA Only (n=1,462) | Knee Pain Only (n=525) | Symptomatic Knee OA (n=1,209) | |
|---|---|---|---|---|
| Number of Death | 81 | 88 | 28 | 86 |
| Deaths per 1000 Person-years (95% CI) | 9.3 (7.4 to 11.5) | 12.8 (10.3 to 15.7) | 8.7 (5.8 to 12.5) | 15.8 (12.6 to 19.5) |
| Total Effect, HR (95% CI) | 1.0 (reference) | 2.0 (1.5 to 2.7) | 1.0 (0.6 to 1.5) | 2.4 (1.8 to 3.3) |
| Total Effect, HR (95% CI) * | 1.0 (reference) | 1.7 (1.2 to 2.4) | 0.9 (0.6 to 1.3) | 2.0 (1.4 to 2.9) |
| Disability Score | ||||
| Direct, HR (95% CI) * | 1.0 (reference) | 1.7 (1.4 to 2.0) | 0.6 (0.5 to 0.8) | 1.1 (0.9 to 1.4) |
| Indirect, HR (95% CI) * | 1.0 (reference) | 1.0 (0.9 to 1.2) | 1.4 (1.2 to 1.6) | 1.7 (1.5 to 2.0) |
| Mediation effect (%) | - | 2.4 | Not same direction | 85.5 |
| PCS of QoL | ||||
| Direct, HR (95% CI) * | 1.0 (reference) | 1.7 (1.5 to 2.0) | 0.7 (0.6 to 0.9) | 1.6 (1.4 to 1.9) |
| Indirect, HR (95% CI) * | 1.0 (reference) | 1.0 (0.8 to 1.2) | 1.2 (1.0 to 1.4) | 1.2 (1.0 to 1.5) |
| Mediation effect (%) | - | 0.0 | Not same direction | 38.9 |
| MCS of QoL | ||||
| Direct, HR (95% CI) * | 1.0 (reference) | 1.7 (1.5 to 2.0) | 0.8 (0.7 to 1.1) | 2.0 (1.7 to 2.4) |
| Indirect, HR (95% CI) * | 1.0 (reference) | 1.0 (0.8 to 1.2) | 1.0 (0.8 to 1.2) | 1.0 (0.8 to 1.2) |
| Mediation effect (%) | - | 2.4 | Not same direction | 0.0 |
| Oral Pain Medications Use | ||||
| Direct, HR (95% CI) * | 1.0 (reference) | 1.7 (1.5 to 2.0) | 0.8 (0.6 to 1.1) | 1.9 (1.6 to 2.3) |
| Indirect, HR (95% CI) * | 1.0 (reference) | 1.0 (0.8 to 1.2) | 1.1 (0.9 to 1.3) | 1.1 (0.9 to 1.3) |
| Mediation effect (%) | - | 2.4 | Not same direction | 11.4 |
n, number; OA, osteoarthritis; CI, confidence interval; HR, hazard ratio; PCS, physical component summary score; MCS, mental component summary score; QoL, quality of life.
Adjusted for age, race, sex, body mass index, education level, weekly alcohol consumption, smoking habits, history of knee injury, comorbidities, depressive symptoms and widespread pain.
DISCUSSION
Using the data collected from OAI, we found that all-cause mortality among participants with either symptomatic or radiographic knee OA only was higher than those with neither knee OA nor knee pain; however, no such an association was observed among participants with knee pain only. An increased mortality observed among participants with symptomatic knee OA was partly mediated through its effect on either disability or PCS of QoL.
To date, at least nine studies have examined the relation of knee OA to all-cause mortality (7-11, 13, 14, 29, 30). Of them, seven studies reported that knee OA was associated with an increased risk of mortality (7-11, 13, 14). Of the studies that reported a positive association between knee OA and mortality, four (7-9, 11) showed that patients with symptomatic knee OA had an increased mortality compared with those with neither radiographic knee OA nor knee pain; two studies (13, 14) found that individuals with radiographic knee OA experienced a higher risk of mortality than those without radiographic knee OA, and one study reported an excess mortality in people with either symptomatic or radiographic OA of the knee or hip compared with the general population (10). Our findings are consistent with those of previous studies, indicating that knee OA, regardless of their symptomatic status, was associated with an increased risk of all-cause mortality.
Several mechanisms linking knee and hip OA to an increased mortality have been postulated, including physical function impairment (e.g., walking disability) and pain-relief medications use (e.g., NSAIDs use). For example, results from the Wuchuan OA study showed that the relation of symptomatic knee OA to all-cause mortality was almost entirely (96.8%) mediated through its effect on walking disability (11). Similar finding was also observed in the Studies of Osteoporotic Fracture although the percentage of the total effect of hip OA through the mediation of physical function was smaller (42.9%) (12). Our study found that 26.5% of mortality among the patients with symptomatic knee OA were mediated through the poor physical function.
Interestingly, neither Wuchuan OA study nor Studies of Osteoporotic Fracture found that NSAIDs use, a potential risk factor for cardiovascular or gastrointestinal diseases, mediated the relation of OA to the risk of mortality. The lack of statistical power was also evident in the current study, which showed that only 12.0% of mortality from symptomatic knee OA were mediated through oral pain-relief medications use (i.e., NSAIDs and opioids). Since participants recruited into OAI consisted of those who had either knee OA or were at an increased risk of knee OA (i.e., a high-risk population), NSAIDs and opioids use was common even among the participants with neither radiographic knee OA nor knee pain (21.8%), making it difficult to assess the indirect effect of symptomatic knee OA on all-cause mortality through oral pain-relief medications use. Moreover, safety profiles of individual oral pain-relief medications varied considerably, with some pain-relief medications, such as naproxen, showing no or relatively low risk of all-cause mortality compared with non-use of pain-relief medications (31, 32). Since OAI didn’t specifically collect the data for each individual pain-relief medication use, we were unable to examine the mediation effect via each specific pain-relief medication. Further studies are needed to provide a more definitive examination of this association.
Although there was no apparent relation of knee pain only to the risk of all-cause mortality we observed significantly inconsistent mediation effects of knee pain only on the risk of all-cause mortality via two mediators (i.e., disability score and PCS of QoL) when we used an updated information to define a participant’s knee OA status. The exact biological mechanisms for such a phenomenon are not fully understood. We postulate that the effect of knee OA status on all-cause mortality not only depends on its symptoms, such as pain, but also depends on degenerative changes in joint structure, and the latter may represent, to some extent, the degenerative process of joint structure or other organs. A few studies reported that the telomere length was shorter in chondrocytes at the osteoarthritic lesion site in the knee than those at the intact regions of cartilage in the same joint (33-35), and telomere shortening has been linked to mortality (36-39), suggesting that structural change of radiographic knee OA might really impact mortality. In the current study we also found that participants with radiographic knee OA only experienced higher all-cause mortality than those with neither radiographic knee OA nor knee pain. This finding was consistent with other reports (13, 14) although none of the aforementioned mediators were able to explain the association.
Several characteristics of the current study are worth commenting. First, we used data from OAI, a large and well-executed population-based cohort study, to examine the association between knee OA status and risk of mortality. The exposure variable (i.e., knee OA status), potential confounders, mediators, and outcome variable (i.e., all-cause mortality) in OAI were assessed using the validated methods. Second, the selected mediators were measured at the closest visit prior to death or to the final follow-up time point, ensuring the valid time sequence from knee OA status to mediators to death. Third, when potential misclassification of knee OA status was addressed in our sensitivity analysis, the associations of symptomatic knee OA and radiographic knee OA only in relation to the mortality became stronger, and the indirect effects of symptomatic knee OA through the disability and PCS of QoL mechanisms were also greater than the findings based on the knee OA status assessed at baseline. Nevertheless, as in any observational epidemiologic studies we cannot rule out residual confounding even if a number of potential confounders have been adjusted, including Charlson comorbidities index, depressive symptoms and widespread pain. Moreover, data on the cause-specific mortality were not collected in OAI; thus, we were unable to examine the relation of knee OA status to the risk of cause-specific mortality. In addition, the detailed information on specific pain-relief medications taken by the participants was not collected as well; thus, we were unable to examine the potential medication effect via the specific pain-relief medications.
In conclusion, participants with either symptomatic or radiographic knee OA were at an increased risk of all-cause mortality. The increased risk of mortality from symptomatic knee OA was partially mediated through its effect on disability and PCS of QoL.
SIGNIFICANCE AND INNOVATIONS.
Both symptomatic knee OA and radiographic knee OA were associated with an increased risk of all-cause mortality. These findings underscore the importance of developing appropriate preventive and treatment strategies to reduce the risk of knee OA.
The increased risk of all-cause mortality from symptomatic knee OA was mediated through disability and PCS of QoL, suggesting that more efforts should focus on improving function and QoL to reduce all-cause mortality among patients with symptomatic knee OA.
Acknowledgement:
The contributions of study participants of the OAI are gratefully acknowledged.
Funding Sources: This work was supported by the National Natural Science Foundation of China (81772413, 81601941, 81702207, 81702206) and the innovation Foundation of the Central South University for Postgraduate (2018zzts045). The Osteoarthritis Initiative (OAI) is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline and Pfizer. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use dataset and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
Footnotes
Ethical Approval: This study received approval from the medical ethical committee, and participants gave written informed consent.
Conflict of interests: The authors declare that they have no competing interests.
References
- 1.GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1260–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–56. [PMC free article] [PubMed] [Google Scholar]
- 4.Puig-Junoy J, Ruiz ZA. Socio-economic costs of osteoarthritis: a systematic review of cost-of-illness studies. Semin Arthritis Rheum. 2015;44(5):531–41. [DOI] [PubMed] [Google Scholar]
- 5.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–30. [DOI] [PubMed] [Google Scholar]
- 6.Institute for Health Metrics and Evaluation. The global burden of disease: Generating evidence, guiding policy. Washington: Institute for Health Metrics and Evaluation, 2013:1–70. [Google Scholar]
- 7.Cleveland RJ, Alvarez C, Schwartz TA, Losina E, Renner JB, Jordan JM, et al. The impact of painful knee osteoarthritis on mortality: a community-based cohort study with over 24 years of follow-up. Osteoarthritis Cartilage. 2019;27(4):593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kluzek S, Sanchez-Santos MT, Leyland KM, Judge A, Spector TD, Hart D, et al. Painful knee but not hand osteoarthritis is an independent predictor of mortality over 23 years follow-up of a population-based cohort of middle-aged women. Ann Rheum Dis. 2016;75(10):1749–56. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Niu J, Huang J, Ke Y, Tang X, Wu X, et al. Knee osteoarthritis and all-cause mortality: the Wuchuan Osteoarthritis Study. Osteoarthritis and Cartilage. 2015;23(7):1154–7. [DOI] [PubMed] [Google Scholar]
- 10.Nüesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Jüni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ. 2011;342:d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, Niu J, Li H, Ke Y, Li R, Zhang Y, et al. Knee Symptomatic Osteoarthritis, Walking Disability, NSAIDs Use and All-cause Mortality: Population-based Wuchuan Osteoarthritis Study. Sci Rep. 2017;7(1):3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbour KE, Lui LY, Nevitt MC, Murphy LB, Helmick CG, Theis KA, et al. Hip Osteoarthritis and the Risk of All-Cause and Disease-Specific Mortality in Older Women: A Population-Based Cohort Study. Arthritis Rheum. 2015;67(7):1798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuboi M, Hasegawa Y, Matsuyama Y, Suzuki S, Suzuki K, Imagama S. Do musculoskeletal degenerative diseases affect mortality and cause of death after 10 years in Japan? J Bone Miner Metab. 2011;29(2):217–23. [DOI] [PubMed] [Google Scholar]
- 14.Mendy A, Park J, Vieira ER. Osteoarthritis and risk of mortality in the USA: a population-based cohort study. Int J Epidemiol. 2018;47(6):1821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr SJ, Rowett DS, Sayer GP, Whicker SD, Saltman DC, Mant A. All-cause mortality of elderly Australian veterans using COX-2 selective or non-selective NSAIDs: a longitudinal study. Br J Clin Pharmacol. 2011;71(6):936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee TA, Bartle B, Weiss KB. Impact of NSAIDS on mortality and the effect of preexisting coronary artery disease in US veterans. Am J Med. 2007;120(1):98.e9–16. [DOI] [PubMed] [Google Scholar]
- 17.Sattler M, Dannhauer T, Hudelmaier M, Wirth W, Sanger AM, Kwoh CK, et al. Side differences of thigh muscle cross-sectional areas and maximal isometric muscle force in bilateral knees with the same radiographic disease stage, but unilateral frequent pain - data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2012;20(6):532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckstein F, Maschek S, Wirth W, Hudelmaier M, Hitzl W, Wyman B, et al. One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative progression subcohort: association with sex, body mass index, symptoms and radiographic osteoarthritis status. Ann Rheum Dis. 2009;68(5):674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veronese N, Stubbs B, Noale M, Solmi M, Vaona A, Demurtas J, et al. Fried potato consumption is associated with elevated mortality: an 8-y longitudinal cohort study. Am J Clin Nutr. 2017;106(1):162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. [DOI] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 22.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–72. [DOI] [PubMed] [Google Scholar]
- 23.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 24.Ware JE, Kosinski M, Keller SD. SF-12: how to score the SF-12 physical and mental health summary scales. The Health Institute, New England Medical Centre. 1995. [Google Scholar]
- 25.Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. [DOI] [PubMed] [Google Scholar]
- 26.Nevitt M, Felson D, Lester G. The Osteoarthritis Initiative: Protocol for the Cohort Study. 2006. [Google Scholar]
- 27.Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol. 2012;176(3):190–5. [DOI] [PubMed] [Google Scholar]
- 28.VanderWeele TJ. Mediation Analysis: A Practitioner’s Guide. Annu Rev Public Health. 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 29.Turkiewicz A, Neogi T, Bjork J, Peat G, Englund M. All-cause Mortality in Knee and Hip Osteoarthritis and Rheumatoid Arthritis. Epidemiology. 2016;27(4):479–85. [DOI] [PubMed] [Google Scholar]
- 30.Veronese N, Cereda E, Maggi S, Luchini C, Solmi M, Smith T, et al. Osteoarthritis and mortality: A prospective cohort study and systematic review with meta-analysis. Semin Arthritis Rheum. 2016;46(2):160–7. [DOI] [PubMed] [Google Scholar]
- 31.Coxib and traditional NSAID Trialists’ Collaboration, Bhala N, Emberson J, Merhi A, Abramson S, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piera-Velazquez S, Jimenez SA, Stokes D. Increased life span of human osteoarthritic chondrocytes by exogenous expression of telomerase. Arthritis Rheum. 2002;46(3):683–93. [DOI] [PubMed] [Google Scholar]
- 34.Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1(1):57–65. [DOI] [PubMed] [Google Scholar]
- 35.Zhai G, Aviv A, Hunter DJ, Hart DJ, Gardner JP, Kimura M, et al. Reduction of leucocyte telomere length in radiographic hand osteoarthritis: a population-based study. Ann Rheum Dis. 2006;65(11):1444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–5. [DOI] [PubMed] [Google Scholar]
- 37.Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, et al. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66(4):421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstatter A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69–75. [DOI] [PubMed] [Google Scholar]
- 39.Weischer M, Bojesen SE, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Nordestgaard BG. Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arterioscler Thromb Vasc Biol. 2012;32(3):822–9. [DOI] [PubMed] [Google Scholar]