Abstract
Background
Axillary dissection is commonly performed for breast carcinoma. It is uncertain whether insertion of a drain reduces complication rates.
Objectives
To assess the effects of wound drainage after axillary dissection for breast carcinoma on the incidence of postoperative seroma formation. Secondary outcome measures include the incidence of infection and length of hospital stay.
Search methods
We searched the Cochrane Wound and Breast Cancer Group's Specialised Registers (22 February 2013), MEDLINE (1950 to 22 February 2013), EMBASE (1966 to 22 February 2013), the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov (22 February 2013) for all prospectively registered and ongoing trials (22 February 2013). Reference lists of included studies were handsearched by two independent review authors to look for additional eligible trials.
Selection criteria
All randomised controlled trials (RCTs) comparing wound drainage versus no wound drainage in individuals after axillary dissection for the treatment of breast carcinoma were included. All disease stages were considered. Breast‐conserving surgery and mastectomy were considered. Patients undergoing sentinel node biopsy without axillary dissection were not included. No limits were applied to language or study location. Two review authors independently determined the eligibility of each study.
Data collection and analysis
Two review authors independently extracted data for each included study using a predesigned data extraction proforma and assessed risk of bias using The Cochrane Collaboration's 'Risk of bias' tool. Discrepancies were resolved by consensus discussion with a third review author. Dichotomous variables were analysed using a Mantel‐Haenszel model to produce odds ratios (ORs). Continuous variables were analysed using an inverse variance model to produce a mean difference (MD).
Main results
Seven RCTs including 960 participants were identified. The quality of trials was generally low, with several studies at risk of selection bias, and no studies used blinding during treatment or outcome assessment. There was a high level of statistical variation between the studies, which therefore reduces the reliability of the evidence. The OR for seroma formation was 0.46 (95% confidence interval (CI) 0.23 to 0.91, P = 0.03) in favour of a reduced incidence of seroma in participants with drains inserted. There was no significant difference in infection rates between drainage and no drainage groups (OR = 0.70; 95% CI 0.44 to 1.12, P = 0.14). The mean difference in length of hospital stay, reported in four trials consisting of 600 participants, was 1.47 days greater in the drained population (95% CI 0.67 to 2.28, P = 0.0003). A mean difference of 0.79 fewer postoperative seroma aspirations was found in the drained population (95% CI 1.23 to 0.35 fewer, P = 0.0004) in two trials including 212 participants. No significant difference in volume of seroma aspirations was reported (MD ‐19.44, 95% CI ‐59.45 to 20.57, P = 0.34) in three trials including 519 participants. No significant difference in the incidence of lymphoedema was noted (OR 2.31 favouring no drainage, 95% CI 0.47 to 11.37, P = 0.30), with only six instances reported in three trials of 360 participants, nor was any significant difference in the incidence of haematoma observed (OR 1.68, 95% CI 0.33 to 8.51, P = 0.53), with only five instances reported in two trials of 314 participants.
Authors' conclusions
There is limited quality evidence that insertion of a drain following axillary lymphadenectomy reduced the odds of developing a seroma and reduced the number of post‐operative seroma aspirations. These benefits should be balanced against an increased length of hospital stay in the drained population.
Plain language summary
Drainage tube placement after lymph gland removal from the armpit for breast cancer
Breast cancer is one of the most common malignancies in women, accounting for a large number of deaths worldwide each year. Sometimes, an essential part of breast cancer management includes an operation to remove the lymph nodes in the armpit, called an 'axillary dissection', sometimes also known as 'axillary lymphadenectomy'. This operation may be needed if the cancer has spread (metastasised) to the armpit. One consequence of removing some, or all, of these lymph nodes is that a collection of fluid called a seroma can develop in the armpit. This can be uncomfortable and may require drainage (also known as aspiration). Other complications include risks of infection, bleeding and arm lymphoedema. One strategy that is widely used to try to minimise these complications is the insertion of a plastic drainage tube into the armpit during surgery that allows any fluid collecting in the armpit to drain away. However, debate is ongoing amongst surgeons regarding the value of such drains because they can cause pain and discomfort and may delay discharge from hospital.
This Cochrane review aims to determine whether drain tube insertion reduces complication rates or is associated with any risks or harms. We analysed seven randomised controlled trials including 960 participants that compared drain insertion with no drainage after axillary lymphadenectomy for the treatment of breast cancer. We found that the chance of getting a seroma if a drain was inserted was less than if no drain was inserted (0.46 times less likely), and that the number of aspirations required (using a needle to drain seroma fluid in the outpatient clinic) was lower (on average, 0.79 fewer per participant). These benefits must be balanced against a longer average hospital stay of 1.47 days in the drained population, although increasingly patients can be discharged with their drain in place, to be removed at a later date. Risk of infection, volume of fluid aspirated and rates of lymphoedema (arm swelling) or haematoma (bruising) did not differ between drained and undrained participants.
Background
Description of the condition
Breast cancer is one of the most frequent malignant neoplasms in women, with an estimated 230,000 new cases of invasive breast cancer per annum in the United States, causing 40,000 deaths (American Cancer Society 2012). Surgical treatment is the most effective option for loco‐regional control of this disease (Anderson 2006). Axillary dissection (or lymphadenectomy) is frequently performed on individuals with carcinoma of the breast (Axelsson 2007). Sentinel lymph node biopsy is a less invasive alternative procedure to axillary dissection that is used to stage breast cancer in clinically node‐negative patients; this procedure may be associated with reduced postoperative morbidity (Mansel 2007). Axillary dissection is associated with the development of postoperative morbidity such as seroma (a pocket of clear serous fluid after surgery), haematoma, infection, lymphoedema and numbness (mainly in the upper and inner aspect of the affected arm) (Morrow 2002). The development of a seroma has been reported in 15% to 85% of cases (Soon 2005).
A number of factors influence the formation of seromas. These include the presence of tumour‐infiltrated axillary lymphatics, the size of the lymphatic interruption after surgery, a history of previous biopsies, the size of the breast and the residual cavity, the surgical technique used (mastectomy or lumpectomy), the use of electrocautery with a diathermy device and the duration of suction drainage and cicatrisation (the process of wound healing that occurs when scar tissue is produced) (Agrawal 2006; Kuroi 2005; Pogson 2003; Stehbens 2003; van Bemmel 2011).
Description of the intervention
The presence of seromas can impact patient satisfaction in the postoperative period and may lead to the need for medical interventions. In some cases, repeated aspirations and even surgical drainage of the seroma may be required, thus increasing the risks of infection, skin flap necrosis, prolonged hospital stay and delayed commencement of chemotherapy, radiotherapy or both (Hashemi 2004; van Bemmel 2011)
Therapeutic approaches used to prevent the formation of seromas include external compression, use of fibrin glue, use of harmonic scalpels during tissue dissection, immobilisation of the ipsilateral arm and wound drainage through a tube (multiple hole type versus multiple channel type) and a device that acts with closed suction or gravity drainage—all of which have had less than satisfying results (Bohm 2012; Pogson 2003; van Bemmel 2011).
Closed suction drains have been used traditionally in the postoperative period after surgery for carcinoma of the breast to reduce the frequency of the formation of seromas. Recently, this concept has been challenged by several authors, who have proposed that the clinical course of the formation of seroma is not modified by the use of drainage (Classe 2006; Talbot 2002), and that prolonged drainage may have a negative impact on wound healing (Jain 2004). However, other studies show that individuals who do not use drainage have seromas of greater volume that last longer and require additional procedures for drainage (Soon 2005).
Why it is important to do this review
The value of drain insertion after axillary lymphadenectomy remains a controversial topic, given that several papers have disputed the value of drains. This review will help clinicians to make evidence‐based decisions regarding the care provided to the large number of patients undergoing axillary lymphadenectomy each year. Previous systematic reviews have considered this question. This meta‐analysis includes greater numbers of participants and randomised controlled trials than were examined in previous reviews.
Objectives
To assess the effects of wound drainage after axillary dissection for the treatment of breast carcinoma on the incidence of postoperative seroma formation. Secondary outcome measures include the incidence of infection and length of hospital stay.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing wound drainage versus no wound drainage after axillary lymphadenectomy for the treatment of breast carcinoma.
Types of participants
Women treated for carcinoma of the breast for whom an axillary dissection was performed.
Types of interventions
Use versus non‐use of closed suction drainage after axillary dissection for carcinoma of the breast.
Types of outcome measures
Primary outcomes
Incidence of seroma formation
Secondary outcomes
Incidence of wound infection
Incidence of arm lymphoedema
Length of hospital stay
Number of seroma aspirations
Volume of seroma aspirations
Incidence of wound haematoma
Quality of life (measured with validated tools)
Rate of healing of wounds
Search methods for identification of studies
Electronic searches
The following electronic trial registries were searched.
Cochrane Breast Cancer Group's Specialised Register on 22 February 2013 (details of search strategies used by the Group for the identification of studies and the procedure used to code references are outlined in the Group's module at http://www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html). Studies that included any of the text words 'mastectomy', 'lumpectomy', 'breast surgery', 'axillary dissection', 'wound drainage', 'suction', 'axillary', 'lymph node', 'seroma', 'lymph gland' or 'dressing' on the Specialised Register were extracted for consideration.
Cochrane Wounds Group's Specialised Register on 22 February 2013, using the following search string in Procite ("axillary dissection" OR "breast cancer" OR "breast neoplasms" OR "breast neoplasm" OR (breast AND carcinoma)) AND drain* AND ("wound infection" OR "surgical wound dehiscence" OR "cicatrix" OR "skin abscess") (details of search strategies used by the Group for the identification of studies and the procedure used to code references are outlined in the Group's module at http://www.mrw.interscience.wiley.com/cochrane/clabout/articles/WOUNDS/frame.html).
MEDLINE (via OVID) from 1950 until 22 February 2013 (see Appendix 1).
EMBASE (via Embase.com) from 1966 until 22 February 2013 (see Appendix 2).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (http://apps.who.int/trialsearch/Default.aspx) on 22 February 2013 for all prospectively registered and ongoing trials (see Appendix 3).
ClinicalTrials.gov (http://clinicaltrials.gov/ct2/home) on 22 February 2013 (see Appendix 4).
Searching other resources
Trial authors were contacted if data were missing from the published manuscripts.
Data collection and analysis
Selection of studies
Two review authors (DRT and HS) independently determined the eligibility of each study. Both review authors analysed the titles and abstracts of all citations found through the search strategy previously described. A copy of the full article was obtained for each reference reporting a potentially eligible trial, and the two review authors independently applied the eligibility criteria; discrepancies were resolved by consensus discussion with a third review author (DF). Full details of all eligible studies were obtained. When necessary, and possible, additional information was sought from the principal investigator of the trial concerned. Any exclusions from the review of a potentially eligible trial were justified in the final report. The search strategy was not limited by language or location of study.
Data extraction and management
At least two review authors (DRT and HS) independently extracted the data for each included study using a predesigned data extraction proforma. Data were extracted according to the details of the trial (first author, year of publication, journal, publication status, period and country of study, sources of funding, study design, sample size); participant characteristics (age, sex, stage of disease, type of surgery, prior treatment status); quality of the study; details of the intervention (related to use or non‐use of a drain); clinical variables related to participant well‐being; duration of follow‐up; and outcomes. A third review author (DF) was consulted to resolve any discrepancies regarding data extraction.
Data were extracted from included studies and were checked by two review authors (DRT and HS). We involved a statistical consultant as part of the review team, and additional statistical guidance was obtained by contacting the Cochrane Breast Cancer Group.
Assessment of risk of bias in included studies
Assessment of risk of bias and study quality was conducted using The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011). Two review authors (DRT and HS) independently evaluated the methodological quality of studies that met the selection requirements; a third review author (DF) resolved any discrepancies regarding quality.
We described the risk of bias and judged bias in seven specific domains.
Sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessors.
Incomplete outcome data.
Selective outcome reporting.
Other sources of bias (i.e. no intention‐to‐treat analysis, cross‐over or baseline differences regarding the most important prognostic factors in the trials; early stop; non–placebo‐controlled trial; differences in follow‐up examinations).
Interpretation of results incorporated all key outcomes of the risk of bias assessment, and caution was exercised when the risk of bias was deemed unacceptably high for any study.
Measures of treatment effect
For dichotomous variables, a Mantel‐Haenszel model was used to produce an odds ratio (OR). For continuous variables, an inverse variance model was used to produce a mean difference (MD).
Unit of analysis issues
Each participant was individually randomly assigned to one intervention arm, and no RCTs used a cross‐over design.
Dealing with missing data
If the results of an RCT were published, but information on the outcome of interest had not been reported, an attempt was made, whenever possible, to contact the study authors to ask for the missing information. All efforts made to obtain additional information were reported in the completed review.
Assessment of heterogeneity
Heterogeneity between trial results was considered and tested for when appropriate. Chi2 tests for heterogeneity were used to test for gross statistical heterogeneity between all trials. P < 0.05 for Chi2 was the value used to detect statistically significant heterogeneity. The I2 value was interpreted by balancing the direction and magnitude of I2 with its statistical significance, using as a guide the values in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: represents considerable heterogeneity.
If significant heterogeneity was found, the following techniques were undertaken to attempt to explain and resolve it.
Subgroup analyses.
Sensitivity analyses.
Analyses using the random‐effects model.
Assessment of reporting biases
A funnel plot was not analysed, as only seven trials were identified; therefore any asymmetry may be spurious and related to the small number of eligible studies. Trial registries were searched to identify unpublished, ongoing trials, and a range of databases were searched to minimise the risk of reporting bias.
Data synthesis
Results of eligible studies were statistically synthesised (meta‐analysis), if appropriate and possible, using the statistical component of the Review Manager software (RevMan).
When possible, all analyses were performed by intention to treat.
Subgroup analysis and investigation of heterogeneity
To assess heterogeneity, subgroup analysis was conducted for primary outcomes based on the type of breast surgery performed (mastectomy or breast‐conserving surgery).
Sensitivity analysis
A sensitivity analysis was performed to evaluate the effects of risk of bias on estimates of the effects of drain insertion.
Results
Description of studies
Results of the search
See Figure 1.
1.
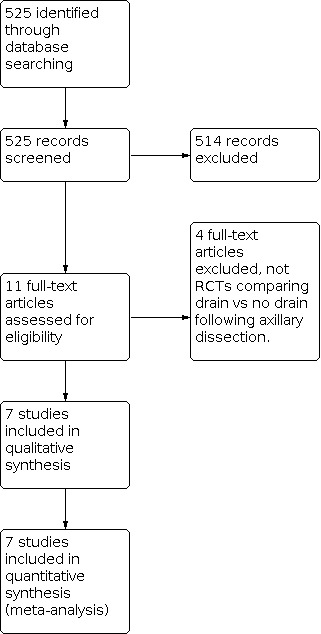
Study flow diagram.
Included studies
A total of 525 records were retrieved (Cochrane Breast Cancer Group's Specialised Register: 294; MEDLINE: 99; EMBASE: 132; in addition to ongoing trials from the WHO ICTRP and ClinicalTrials.gov).
Eleven records were considered as potentially relevant. Of these, seven met our predefined inclusion criteria.
Seven randomised controlled trials were identified that met the inclusion criteria: Cameron 1988; Classe 2006; Jain 2004; Purushotham 2002; Somers 1992; Soon 2005; Zavotksy 1998. See Table 1 for a description of the included studies.
1. Description of the included studies.
| Study | Cameron | Somers | Zavotsky | Purushotham | Jain | Soon | Classe |
| Participants DG NDG | 20 20 |
108 119 |
24 22 |
190 185 |
58 29 |
36 51 |
51 47 |
| Age, years DG NDG | 50.0 ± 2.6 52.0 ± 2.7* |
60.1 ± 12.8 59.3 ± 13.8* |
58.2 59.6 |
57.6 57.7 |
61.9 ± 13.2 62.3 ± 12.3* |
59 58 |
60 ± 10 58 ± 11* |
| Operation | NS | Lumpectomy | BCT | WLE: 185 Mast: 190 |
Mastectomy/WLE | Mastectomy/ partial mastectomy |
BCT |
| Type of drain | Single closed suction (RedivacTM) | Jackson‐Pratt closed suction | Jackson‐Pratt closed suction |
Single Portovac® | Single 14‐Fr vacuum (Medinorm®) | 1 closed suction drain | NS (suction implied) |
| Time of drain removal | < 25 mL/24 h | 24 hours postoperative | < 30 mL/24 h | < 50 mL/24 h or 5 d | < 50 mL/24 h | 24 to 48 hours postop | < 35 mL/24 h |
| Level of ALND | "cleared below axillary vein" | I and II | I, II and a portion of III Complete III if grossly palpable axillary nodes |
II | I and II | "all tissue inferior to axillary vein removed" | I and II |
| Lymph nodes removed DG NDG | NS NS | 15.7 ± 5.7* 16.7 ± 5.7* | 15.3† 15.7† |
NS NS |
7.1 ± 2.8* 8.2 ± 3.4* |
16 17.5 |
NS NS |
*: mean ± standard deviation. †: median. ALND: axillary lymph node dissection. BCT: breast‐conserving therapy. DG: drainage. NDG: no drainage. NS: not stated. WLE: wide local excision. Mast: mastectomy.
Excluded studies
From the eleven records considered potentially relevant, four studies were excluded from the review after reading of the full text because they did not meet the study inclusion criteria (Garbay 2012; Talbot 2002; Warren 1994; Wheeler 1976). After the full manuscripts had been read, it was clear that these four studies were not randomised controlled trials, and they were thus excluded from our analysis.
Risk of bias in included studies
See Figure 2 and Figure 3 for visual summaries of the risk of bias analysis.
2.

Risk of bias graph: review authors' judgements about all risk of bias items presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In two studies, high risk of selection bias was due to inadequate methods of randomisation: Somers et al used a pseudorandomisation method based on the last digit of the participant's admission number, and Soon et al used pseudorandomisation based on the day of the month (Somers 1992; Soon 2005). Furthermore, in four studies, the risk of bias during randomisation was unclear: Cameron et al used “sequentially numbered sealed envelopes”. However, it is unclear whether these envelopes were opaque, and whether the sequence was truly random. Classe et al stated that “randomisation was done in blocks of six”, yet it is unclear whether these blocks were generated randomly. Purushotham et al used "consecutively numbered opaque envelopes". Therefore, in all of these studies, it is unclear whether the sequences were generated randomly. Zavotsky et al state that "patients...were intra‐operatively randomised to drain or no‐drain groups” with no mention of the method of randomisation used. Only one study was assessed as low risk: Jain et al, who used "sealed, opaque envelopes containing computer generated random numbers" (Cameron 1988; Classe 2006; Jain 2004; Purushotham 2002; Zavotksy 1998).
Allocation concealment was at a generally lower risk of bias than randomisation. The two studies that used pseudorandomisation methods were rated as high risk because a surgeon who knew the randomisation method would know perioperatively whether the participant was receiving a drain (Somers 1992; Soon 2005). Two studies were rated as unclear because they did not state when the allocation was revealed (Classe 2006; Purushotham 2002). Three studies revealed the allocation at the end of the operation, so were rated as low risk (Cameron 1988; Jain 2004; Zavotksy 1998).
Blinding
When blinding of participants and personnel is attempted, it is clearly not possible to blind surgeons or participants to the presence or absence of a drain inserted into the axilla; therefore all studies were rated as unclear bias because we cannot be certain what, if any, impact this inability to blind would have.
Despite these obvious difficulties in blinding to the presence or absence of a drain in the axilla, none of the trials mentioned any attempt to blind assessors during postoperative follow‐up after drain removal. Therefore when detection bias was assessed, an important consideration was whether study methodologies objectively defined key outcome measures of seroma and infection and the criteria for aspiration of seroma so as to allow objective assessment of these clinical outcomes. Three studies adequately defined seroma (Classe 2006; Jain 2004; Somers 1992), and four studies adequately defined infection (Classe 2006; Jain 2004; Purushotham 2002; Somers 1992). Among studies that reported the number of aspirations, three of four adequately defined criteria for seroma aspiration (Jain 2004; Somers 1992; Soon 2005). Volume of aspiration was judged to be at low risk of bias because it is an objectively measured quantity of fluid.
Only one study included aspects of participant‐reported outcome measures, including pain scores and measures of psychological distress. The inability to blind to the presence of a drain introduces a high risk of bias for participant self‐assessment of pain and other considerations of morbidity.
Incomplete outcome data
In five studies, no concerns regarding attrition bias were described, and complete outcome data were reported for all participants. Somers et al did not perform an intention‐to‐treat analysis, and 4/103 participants in the drain group were excluded from subsequent analysis because they had drains remaining in situ at > 24 hours, in breach of study protocol. However, reasons for prolonged drain retention are not discussed. Cameron et al was judged to be at high risk because, despite reporting on all 40 participants, no data on the follow‐up period were provided because “follow‐up and treatment were not uniform”.
Selective reporting
In five studies, no concerns regarding reporting bias arose, and all outcomes were reported. Purushotham et al did not record the number of aspirations, despite listing this as a secondary outcome. Cameron et al did not report a follow‐up period because “follow‐up and treatment were not uniform”.
Other potential sources of bias
An important potential methodological bias concerns length of hospital stay. In most studies, discharge protocols differed between drained and undrained groups, generally favouring a longer stay for drained groups. Two studies used length of hospital stay as a primary outcome. Purushotham discharged drain groups when drainage was < 50 mL/24 h or at 5 days postprocedure, whichever was soonest. No drain participants were released 24 to 48 hours postoperatively, creating a potential bias in this design towards keeping drain participants for a longer time. Classe et al did not detail their discharge protocol.
Effects of interventions
Incidence of seroma formation
All seven trials included seroma as an outcome, reporting on 960 participants. Significant statistical heterogeneity between trials was noted (I2 = 68%, P = 0.005). Analysis using a random‐effects model demonstrated a statistically significant odds ratio of 0.46 (95% CI 0.23 to 0.91, P = 0.03) in favour of a reduced incidence of seroma in participants with drains inserted (Figure 4; Analysis 1.1).
4.

Forest plot of comparison: 1 Seroma, outcome: 1.1 Seroma.
1.1. Analysis.

Comparison 1: Seroma, Outcome 1: Seroma
To identify a potential source for this heterogeneity, a subgroup analysis was performed to analyse the differential effects of breast‐conserving surgery (lumpectomy or wide local excision) and mastectomy. For breast‐conserving surgery, five trials reported results from 595 participants. Significant heterogeneity remained between trials (I² = 78%, P = 0.001). The odds ratio was 0.64 (95% CI 0.23 to 1.76, P = 0.39) (Figure 5; Analysis 1.2). For mastectomy, two trials reported results from 238 participants. Again, there remained significant heterogeneity between the two trials (I2= 86%, P = 0.007). The OR was 0.26 (95% CI 0.02 to 2.82, P = 0.27) (Figure 6Analysis 1.3). One trial did not stratify results by breast‐conserving surgery versus mastectomy (Soon 2005).
5.

Forest plot of comparison: 1 Drain inserted versus No drain inserted, outcome: 1.4 Seroma—breast‐conserving therapy.
1.2. Analysis.

Comparison 1: Seroma, Outcome 2: Seroma—breast‐conserving therapy
6.

Forest plot of comparison: 1 Seroma, outcome: 1.3 Seroma—mastectomy.
1.3. Analysis.

Comparison 1: Seroma, Outcome 3: Seroma—mastectomy
We attempted to probe this heterogeneity by conducting a sensitivity analysis that excluded Soon and Somers because of the high risk of selection bias (i.e. random sequence generation and allocation concealment bias) due to the use of pseudorandomisation methods in these studies. Significant heterogeneity remained (I2= 73%, P = 0.006). The OR was 0.44 (95% CI 0.18 to 1.11, P = 0.08) (Analysis 1.4).
1.4. Analysis.

Comparison 1: Seroma, Outcome 4: Seroma—Somers and Soon excluded
Another potential source for the heterogeneity is significant methodological differences regarding definitions of seroma. As discussed previously, only three trials mentioned the criteria used to define seroma.
Incidence of wound infection
All seven trials reported infection rates in 960 participants. A low level of heterogeneity was observed (I2 = 26%, P = 0.25). Analysis using a fixed‐effect model revealed no significant difference in the number of infections with or without drain insertion (OR 0.70, 95% CI 0.44 to 1.12, P =0.14; Figure 7; Analysis 2.1).
7.

Forest plot of comparison: 2 Infection, outcome: 2.1 Infection.
2.1. Analysis.

Comparison 2: Infection, Outcome 1: Infection
Lymphoedema
Only three trials recorded rates of arm lymphoedema, with a total of just six reported incidences in 360 participants. No significant difference in the incidence of lymphoedema using drains versus no drainage was reported, despite an OR of 2.31 favouring no drainage but with a very wide 95% CI of 0.47 to 11.37 (P = 0.30) (Analysis 3.1).
3.1. Analysis.

Comparison 3: Lymphoedema, Outcome 1: Lymphoedema
Length of hospital stay
Four trials reported length of hospital stay for 600 participants. Analysis using a random‐effects model showed a significant mean difference of 1.47 days longer in the drained population (95% CI 0.67 to 2.28, P = 0.0003; Figure 8; Analysis 4.1). However, heterogeneity was significant (I2 = 89%, P < 0.0001). One explanation for this significant heterogeneity may be the variability in trial protocols regarding dates of discharge. Two studies (Cameron 1988; Classe 2006) did not detail the criteria used by clinicians in deciding when to discharge participants. Both of the remaining studies (Jain 2004; Purushotham 2002) used different criteria to determine discharge dates for drained and undrained participants. A subgroup analysis of 138 participants after the two clearly biased studies were excluded showed a non‐significant mean difference of 1.62 days longer in the drained population with a wide 95% CI of ‐0.54 to 3.77 (P = 0.14) and significant heterogeneity (I2 = 94%, P < 0.0001; Analysis 4.2).
8.

Forest plot of comparison: 4 Length of hospital stay, outcome: 4.1 Length of hospital stay.
4.1. Analysis.

Comparison 4: Length of hospital stay, Outcome 1: Length of hospital stay
4.2. Analysis.

Comparison 4: Length of hospital stay, Outcome 2: Length of hospital stay (Cameron and Classe)
Number of seroma aspirations
Three trials reported data on the number of aspirations of postoperative seroma from 240 participants who developed seroma (Jain 2004; Somers 1992; Zavotksy 1998). The number of aspirations may be considered as a sign of the severity of a seroma and its burden to both participant and clinician. Analysis could be performed only for the former two studies (Jain and Somers, n = 212), as Zavotsky's group reported zero aspirations in the drain group (n = 24), producing a mean of 0. This could not be compared with the 14 participants who required at least one aspiration. The forest plot therefore is based on two studies; a fixed‐effect model showed a mean difference of 0.79 fewer aspirations per participant in the drained group (95% CI 0.35 to 1.23, P = 0.0004; Figure 9; Analysis 5.1).
9.

Forest plot of comparison: 5 Postoperative seroma aspirations, outcome: 5.1 Number of postoperative seroma aspirations.
5.1. Analysis.

Comparison 5: Postoperative seroma aspirations, Outcome 1: Number of postoperative seroma aspirations
Volume of seroma aspirated
Five trials reported data on the volume aspirated from postoperative seromas; three provided appropriate data for meta‐analysis. These three trials—Zavotksy 1998, Purushotham 2002 and Classe 2006—included 519 participants. Analysis using a fixed‐effect model revealed no significant mean difference between groups (MD ‐19.44, 95% CI ‐59.45 to 20.57, P = 0.34; Analysis 5.2) with low heterogeneity (I2 = 0%, P < 0.56). For the two trials that could not be used in the meta‐analysis for this outcome, Soon et al reported total volumes of 538.8 mL in the drained group versus 856.7 mL in the undrained group, but they did not report standard deviations or the results of any statistical tests (Soon 2005); Jain et al reported median volumes of 140 mL (interquartile range (IQR) 125 to 205) in the drained group versus 300 mL (IQR 245 to 660) in the undrained group (Jain 2004).
5.2. Analysis.

Comparison 5: Postoperative seroma aspirations, Outcome 2: Volume of postoperative seroma aspirations
Incidence of wound haematomas
Two trials reported data on the incidence of haematoma, with five reported incidences in 314 participants. A low level of heterogeneity was observed (I2 = 0%, P = 0.48). Analysis using a fixed‐effect model revealed no significant difference in the number of infections with or without drain insertion (OR 1.68, 95% CI 0.33 to 8.51, P = 0.53; Analysis 6.1).
6.1. Analysis.

Comparison 6: Haematoma, Outcome 1: Haematoma
Quality of Life
Only one study reported on quality of life outcomes. Purushotham et al found no significant differences in psychological morbidity between drained and undrained participants (Purushotham 2002).
Rate of wound healing
None of the included studies reported on the rate of wound healing.
Discussion
Summary of main results
Insertion of a drain after axillary lymphadenectomy reduces the risk of seroma formation (OR 0.46, 95% CI 0.23 to 0.91, P = 0.03). Subanalysis by type of breast operation demonstrated an odds ratio of 0.64 (95% CI 0.23 to 1.76, P = 0.39) after breast‐conserving surgery and 0.26 (95% CI 0.02 to 2.82, P = 0.27) after mastectomy; both showed a non‐significant reduction in seroma formation after drain insertion, with wider confidence intervals due to smaller subgroup sample sizes.
No difference in infection rates was reported between drained versus undrained populations with an OR 0.70 (95% CI 0.44 to 1.12, P = 0.14). No difference was noted in the OR for lymphoedema in the drained versus undrained groups (OR 2.31, 95% CI 0.47 to 11.37, P = 0.30) or for haematoma (OR 1.68, 95% CI 0.33 to 8.51, P = 0.53).
A mean difference of 1.47 days longer hospital stay was noted in the drained population (95% CI 0.67 to 2.28, P = 0.0003), and a mean difference of 0.79 fewer aspirations per drained participant was observed (95% CI 0.35 to 1.23, P = 0.0004). However, no difference in the volume of aspirations was reported (MD ‐19.44, 95% CI ‐59.45 to 20.57, P = 0.34).
In summary, we have identified limited quality evidence to support a clinically significant reduction in the odds of developing a seroma if a drain is inserted, and in the number of postoperative seroma aspirations required. However, evidence supports a clinically significant increase in the duration of hospital stay for drained versus undrained participants.
Overall completeness and applicability of evidence
The seven studies identified for inclusion in this review include a heterogeneous population of breast cancer patients. It is important to note that the studies include participants undergoing a range of different breast operations, including lumpectomy, wide local excision and mastectomy; as well as a variable extent of axillary clearance procedures. All studies conducted axillary dissection up to level II lymph nodes, and three studies extended this dissection to level III nodes/nodes inferior to the axillary vein (Cameron 1988; Soon 2005; Zavotksy 1998). The average age of participants in these studies ranged from 50.0 to 62.3 years. The heterogeneity of breast carcinoma pathology included in this review lends validity to our conclusions across a wide range of patients.
However, more complicated cases of breast carcinoma may not have been included in the study populations. The inclusion/exclusion criteria for participants are not detailed in some earlier studies; however, Jain, Soon and Classe all excluded patients who had undergone previous breast or axillary surgery (excluding sentinel lymph node biopsy), Classe et al excluded those who received neoadjuvant therapy, and Soon et al excluded patients with prior axillary or chest wall irradiation (Classe 2006; Jain 2004; Soon 2005). Therefore our findings may have reduced validity in more complicated or advanced breast cancer patients.
It is important to note that none of the seven studies included male participants. Although men account for less than 1% of cases of breast cancer, the disease is often more advanced at presentation (Miao 2011), and the effects of axillary drainage in men remain unclear.
A substantial debate continues between surgeons regarding the value of drain insertion, with some authors questioning the use of drains at all (Jeffrey 1995). An important evolution in surgical practice has been an increasing trend towards discharging patients with drains in situ. Some surgeons have argued that inserting a drain unnecessarily delays discharge and results in longer hospital stays (Jeffrey 1995)—a finding supported by this review. However, the increasing empowerment of patients and the presence of community support have led surgeons to discharge patients with drains in situ; this will reduce the validity of this finding for future practice.
Another important issue concerns the timing of axillary drain removal. Within the seven studies included in this meta‐analysis, fixed‐time removal ranged from 24 hours to 5 days postoperatively, and fixed‐volume removal ranged from < 50 mL to < 25 mL over 24 hours postoperatively. A systematic review has evaluated this issue, attempting to develop guidelines for timing of drain removal (Kelley 2012). Whilst addressing this issue is beyond the scope of this review, the range of drain removal protocols used in this review adds validity to our findings by encompassing the variety of drainage choices made by surgeons.
Quality of the evidence
This review analyses data from seven randomised controlled trials of 960 participants, published between 1988 and 2006. A total of 487 participants were randomly assigned to drain insertion, and 473 participants were randomly assigned to no drain. All trials were single‐centre studies. Sample size across studies ranged from 40 to 375 participants.
Outcomes were generally consistent between studies. Six of seven trials demonstrated some reduction in seroma incidence among drained participants. Four of five trials showed some reduction in infection rates among drained participants; two trials reported no infection in either group, and one did not report infection rates. Two trials reported lower numbers of seroma aspirations in drained participants. All five trials reporting length of hospital stay showed longer stay lengths in the drained participant groups. Rates of lymphoedema were very low in these studies, as would be expected with short follow‐up times, meaning that firm conclusions cannot be drawn on this issue.
There was significant evidence of heterogeneity for seroma incidence, which remained despite efforts to explain it using sub‐group analyses. One explanation may be the wide range of seroma incidence reported: 0% to 94% in drained participants and 17% to 96% in undrained participants. This variation is due primarily to differences in clinical definitions of seroma and variations in local surgical practice. The key issue for surgical practice is whether axillary drainage reduces clinically relevant seroma; however, information derived from the studies concerning this important distinction is limited. It may be argued that aspiration of a seroma is a surrogate marker of clinical relevance, and when this secondary outcome measure was included, use of a drain reduced the number of aspirations.
The key methodological limitation of all seven studies is the inability to blind participants or outcome assessors to the presence or absence of a drain in the axilla. However, it is unclear whether this will have a substantial effect on study results because most key outcome assessments were likely to be made after drain removal. As was discussed earlier, studies with robust, objective outcome definitions of outcomes were assessed as low risk of bias. However, studies that did not detail criteria for outcome assessment were assessed as having high risk of bias because the judgement of surgeons could have been affected by personal bias in favour of or against drain insertion.
Potential biases in the review process
The potential for bias in the review process is low. The main potential for bias lies in the statistical heterogeneity of the included studies, in particular with regard to length of hospital stay, for which the magnitude of effect varies substantially. We think this is likely secondary to the different protocols used in the studies to determine the day of discharge. Despite this, the direction of effect in all studies favoured non‐drainage to allow earlier discharge from hospital. Our literature search was conducted by the Cochrane Breast Cancer Group according to accepted standards; thus we are confident that no risk of bias was detected in the included studies. Study selection was undertaken by two independent review authors (DRT and HS), working according to agreed upon inclusion/exclusion criteria. Although no language limits were applied to our search, all three review authors are English language speakers, thus there is a potential risk that non–English language material could have been missed, although our literature search should have identified any such studies. No non–English language studies were identified. We think the risk of publication bias is low, owing to the fact that researchers are unlikely to have a commercial interest in the results of trials. However, it is difficult to rule out publication bias secondary to non‐publication of studies for other reasons, for example, because the results were not significant, or because the trials were not accepted for publication by journal editors.
Agreements and disagreements with other studies or reviews
Two previous systematic reviews have addressed the question of drain insertion after axillary lymphadenectomy. He et al reviewed drainage after axillary lymphadenectomy; they analysed results from six trials including 585 participants. The review of He et al includes six of the trials included in this Cochrane review, but the review authors did not include Purushotham et al, a large trial that studied 385 participants. They concluded that drain insertion reduced the rate of seroma formation and the number of aspirations yet resulted in increased length of hospital stay, with no effect on infection rates (He 2011). Droeser et al conducted a meta‐analysis of six trials comparing volume‐controlled versus no or short‐term drainage and including 561 participants, in which short‐term drainage was defined as ≤ 3 days (Droeser 2009). This Cochrane review includes the three trials in Droeser's review that had a no drainage arm (Cameron 1988; Jain 2004; Zavotksy 1998). Droeser et al concluded that drain insertion reduced seroma incidence yet increased length of hospital stay, with no effect on infection rates, although it is important to note that this analysis excludes studies that used a fixed‐time removal protocol.
This Cochrane review is larger than either of the previously published systematic reviews, increasing the study population by more than 60%, with 960 included participants. The results of this study concur with previous evidence, chiefly that axillary drainage reduces seroma rates at the expense of longer hospital stay, with no effect on infection rates.
Authors' conclusions
Implications for practice.
We have found evidence of limited quality that drain insertion after axillary lymphadenectomy reduces seroma formation and the requirement for postoperative aspiration. Key limitations of the evidence summarised in this review include risk of bias and high levels of statistical variation between study results. The use of a drain does not by any means eliminate the risk of developing seroma and the need for subsequent aspiration. Use of a drain has been shown to increase duration of hospital stay; however, the implications of this finding will be influenced by local practice regarding discharge of patients with drains in situ.
Implications for research.
Whilst this review offers good evidence for some conclusions, the limited number of trials reporting certain outcomes means that some analyses lack statistical power, particularly concerning rates of infection, lymphoedema and haematoma and subanalysis of breast‐conserving therapy versus mastectomy. Several methodological concerns and flaws were highlighted earlier. Breast cancer is one of the most common malignancies in women; consequently a large number of axillary lymphadenectomies are performed every year. It is therefore vital that we have good quality evidence regarding best practice management of these patients. Future research should include adequately powered randomised controlled trials, reported in accordance with CONSORT guidelines.
The most important improvement needed to existing trials should be the use of blinded outcome assessment. To add further validity, outcome measures must be objectively defined and ideally objectively measured. A distinction should be made between all seromas and "clinically significant" seromas. These definitions must be objectively measurable, ideally through a system of independent ultrasound‐aided diagnosis, although this may prove unfeasible in the clinical setting. Similarly, criteria for seroma aspiration should be objectively defined. The definition of wound infection could be improved through the use of validated scoring scales, such as those used by Purushotham et al (Purushotham 2002).
Finally, further studies should also include participant‐reported outcome measures, as relatively few trials have investigated these.
What's new
| Date | Event | Description |
|---|---|---|
| 18 November 2021 | Review declared as stable | After a search of the evidence, it appears that one randomised controlled trial has been conducted since review publication and the results are unlikely to change the overall findings of this review. Therefore we do not expect to update this review. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 10, 2013
Notes
After a search of the evidence, it appears that one randomised controlled trial has been conducted since review publication and the results are unlikely to change the overall findings of this review. Therefore we do not expect to update this review.
Acknowledgements
The authors would like to acknowledge Dr Daniel Lunn, University of Oxford, for his advice concerning statistical analysis.
Appendices
Appendix 1. MEDLINE (via OVID)
| # ▲ | Searches |
| 1 | randomized controlled trial.pt. |
| 2 | controlled clinical trial.pt. |
| 3 | randomized.ab. |
| 4 | randomised.ab. |
| 5 | placebo.ab. |
| 6 | randomly.ab. |
| 7 | trial.ab. |
| 8 | groups.ab. |
| 9 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 |
| 10 | Breast Neoplasms/ |
| 11 | (breast cancer$ or breast tumor$ or breast tumour$ or breast neoplasm$ or breast carcinoma$ or breast adenocarcinoma$).tw,sh. |
| 12 | 10 or 11 |
| 13 | exp Drainage/ |
| 14 | drainage.tw,sh. |
| 15 | wound drainage.tw,sh. |
| 16 | exp Suction/ |
| 17 | suction.tw,sh. |
| 18 | closed aspirative drainage.tw,sh. |
| 19 | aspirat*.tw,sh. |
| 20 | 13 or 14 or 15 or 16 or 17 or 18 or 19 |
| 21 | exp Seroma/ |
| 22 | exp Hematoma/ |
| 23 | (seroma$ or hematoma$ or haematoma$).tw,sh. |
| 24 | seroma formation.tw,sh. |
| 25 | 21 or 22 or 23 or 24 |
| 26 | 9 and 12 and 20 and 25 |
| 27 | limit 26 to humans |
Appendix 2. EMBASE (via Embase.com)
#28 #27 AND [humans]/lim AND [embase]/lim
#27 #8 AND #15 AND #21 AND #26
#26 #22 OR #23 OR #24 OR #25
#25 'seroma formation'
#24 'haematoma'/exp OR haematoma
#23 'hematoma'/exp OR hematoma
#22 'seroma'/exp OR seroma
#21 #16 OR #17 OR #18 OR #19 OR #20
#20 aspirat*
#19 'closed aspirative drainage'
#18 'suction'/exp OR suction
#17 'wound drainage'/exp OR 'wound drainage'
#16 drainage
#15 #9 OR #10 OR #11 OR #12 OR #13 OR #14
#14 'breast adenocarcinoma'/exp OR 'breast adenocarcinoma'
#13 'breast tumor'/exp OR 'breast tumor'
#12 'breast tumour'
#11 'breast neoplasm'
#10 'breast carcinoma'/exp OR 'breast carcinoma'
#9 'breast cancer'/exp OR 'breast cancer'
#8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
#7 groups:ab
#6 trial:ab
#5 randomly:ab
#4 placebo:ab
#3 randomi*ed:ab
#2 controlled AND clinical AND trial
#1 randomised AND controlled AND trial
Appendix 3. WHO ICTRP search portal
Basic Searches:
1. Wound drainage after axillary dissection for carcinoma of the breast
2. Wound drainage AND axillary dissection AND breast cancer
3. Wound drainage AND axillary dissection AND seroma
Advanced Searches:
1. Title: Wound drainage after axillary dissection for carcinoma of the breast
Recruitment Status: All
2. Condition: breast cancer% and seroma%
Recruitment:All
3. Condition: breast cancer% AND (hematoma% OR haematoma%)
Recruitment:All
4. Condition: breast cancer
Intervention: wound drainage OR drainage OR suction OR closed aspirative drainage OR aspirat%
Recruitment:
All
Appendix 4. ClinicalTrials.gov
Basic Searches:
1. Wound drainage after axillary dissection for carcinoma of the breast
2. Wound drainage AND axillary dissection AND breast cancer
3. Wound drainage AND axillary dissection AND seroma
Advanced Searches:
1. Title: Wound drainage after axillary dissection for carcinoma of the breast
Recruitment: All studies
Study Results: All studies
Study Type: All studies
Gender: All studies
2. Condition: breast cancer and seroma
Recruitment: All studies
Study Results: All studies
Study Type: All studies
Gender: All studies
3. Condition: breast cancer AND (hematoma OR haematoma)
Recruitment: All studies
Study Results: All studies
Study Type: All studies
Gender: All studies
4. Condition: breast cancer
Intervention: wound drainage OR drainage OR suction OR closed aspirative drainage OR aspiration OR aspirate
Recruitment: All studies
Study Results: All studies
Study Type: All studies
Gender: All studies
Data and analyses
Comparison 1. Seroma.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Seroma | 7 | 960 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.23, 0.91] |
| 1.2 Seroma—breast‐conserving therapy | 5 | 595 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.23, 1.76] |
| 1.3 Seroma—mastectomy | 2 | 238 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.02, 2.82] |
| 1.4 Seroma—Somers and Soon excluded | 5 | 646 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.18, 1.11] |
Comparison 2. Infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Infection | 7 | 960 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.44, 1.12] |
Comparison 3. Lymphoedema.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Lymphoedema | 3 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.47, 11.37] |
Comparison 4. Length of hospital stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Length of hospital stay | 4 | 600 | Mean Difference (IV, Random, 95% CI) | 1.47 [0.67, 2.28] |
| 4.2 Length of hospital stay (Cameron and Classe) | 2 | 138 | Mean Difference (IV, Random, 95% CI) | 1.62 [‐0.54, 3.77] |
Comparison 5. Postoperative seroma aspirations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Number of postoperative seroma aspirations | 2 | 212 | Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐1.23, ‐0.35] |
| 5.2 Volume of postoperative seroma aspirations | 3 | 519 | Mean Difference (IV, Fixed, 95% CI) | ‐19.44 [‐59.45, 20.57] |
Comparison 6. Haematoma.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Haematoma | 2 | 314 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.33, 8.51] |
Characteristics of studies
Characteristics of included studies [ordered by year]
Cameron 1988.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised trial of 40 consecutive participants | |
| Participants | n = 40. Drain 20, no drain 20. Age of participants, years: drain 50 ± 2.6, no drain 52 ± 2.7 | |
| Interventions | Drain versus no drain. Single closed suction Redivac drain | |
| Outcomes | Seroma, duration of hospital stay, infection | |
| Notes | Setting: Department of Surgery, King’s College Hospital, UK Funding source not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Sequentially numbered sealed envelope”—doesn't say 'opaque', not clear if this was a random sequence |
| Allocation concealment (selection bias) | Low risk | Allocation revealed only after haemostasis had been achieved with diathermy. After allocation, no further use of diathermy allowed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Cannot blind surgeons or participants to presence/absence of drain. Unclear what effect, if any, this would have |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Do not define seroma or infection Authors state, “after discharge from hospital, follow‐up and treatment were not uniform: therefore data have not been collected concerning the subsequent incidence of late complications” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes reported on all 40 participants. No dropouts whilst in hospital. No data on follow‐up period because “follow‐up and treatment were not uniform” |
| Selective reporting (reporting bias) | High risk | Report on all participants on hospital stay, seroma and infection. No data on follow‐up period because “follow‐up and treatment were not uniform” |
| Other bias | Unclear risk | No other biases |
Somers 1992.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled trial on 227 participants undergoing breast‐conserving therapy with level I and level II axillary dissection | |
| Participants | n = 227. Drain 108, no drain 119. Age of participants, years: drain 60.1 ± 12.8, no drain 59.3 ± 13.8 (mean ± SD) | |
| Interventions | Drain versus no drain. Jackson‐Pratt closed suction | |
| Outcomes | Seroma, total volume of drainage, total number of aspirations, number of days from surgery to final aspiration, wound infection, lymphoedema, impaired shoulder movement, haematoma, wound dehiscence | |
| Notes | Setting: Albert Einstein Medical Centre, Philadelphia, Pennsyvania, USA Funding source not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation method based on the last digit of the participant's admission number—pseudorandomisation. Code could easily be broken by surgeon performing several operations |
| Allocation concealment (selection bias) | High risk | Method only pseudorandomisation, so easy to crack code |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Cannot blind surgeons or participants to presence/absence of drain. Unclear what effect, if any, this would have |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Seroma defined: "any palpable or ballottable fluid collection" Aspiration defined: "all collections were aspirated...regardless of size or symptoms" Wound infection defined: "erythema, warmth or purulent drainage at incision site" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Clearly state why four patients were excluded from drain group because drains were left in for > 1 day, in breach of study protocol. 4/109—affects 3% of study population, but unclear why they went > 1 day—all could have developed infection, lots of aspirations required |
| Selective reporting (reporting bias) | Low risk | No unreported outcomes |
| Other bias | Low risk | No other biases in study design. Good write‐up with lots of detail and good objective definitions of study outcomes |
Zavotksy 1998.
| Study characteristics | ||
| Methods | Prospective randomised control trial on 46 participants undergoing breast‐conserving surgery | |
| Participants | n = 46. Drain 24, no drain 22. Age of participants, years: drain 58.2, no drain 59.6 (median age). All participants undergoing treatment for stage I or stage II breast cancer | |
| Interventions | Drain versus no drain. Jackson‐Pratt closed suction | |
| Outcomes | Number of lymph nodes removed, number of tumour‐positive lymph nodes, primary tumour size, postop seroma aspiration, mean aspirate volume, mean duration of drainage in drain group, postoperative drain insertion, infection, haematoma, lymphoedema, arm circumference, number of office visits, pain score on a 0 to 10 scale | |
| Notes | Setting: John Wayne Cancer Institute, a tertiary cancer centre, USA Funding source: Ben B. and Joyce E. Eisenberg Foundation (Los Angeles) and the Fashion Footwear Association This paper studied 115 participants, of whom 46 were studied prospectively and 69 retrospectively. This meta‐analysis includes only the 46 randomly assigned, prospective participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “At the completion of the dissection, patients in the prospective group were intra‐operatively randomised to drain or no‐drain groups”. No discussion of method of randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation revealed at end of operation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Cannot blind surgeons or participants to presence/absence of drain. Unclear what effect, if any, this would have |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants followed up as required, no discussion of randomisation for 4 follow‐up visits for measurement of pain and arm circumference No definitions of seroma, criteria for aspiration or infection Theoretical bias risk that assessment of postoperative seroma and decisions to aspirate could be biased for different groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/24 participants in drain group did not have duration of drainage recorded, no reason given No other study dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other biases |
Purushotham 2002.
| Study characteristics | ||
| Methods | Two parallel randomised trial arms looking at wide local excision (breast‐conserving surgery) and mastectomy participants separately | |
| Participants | n = 375. Mastectomy arm: drain 96, no drain 94. BCT arm: drain 94, no drain 91. Age of participants, years: mastectomy arm: drain 58.1 ± 12.1, no drain 58.4 ± 12.2. BCT arm: drain 57.1 ± 9.1, no drain: 56.9 ± 10.2 (mean ± SD). All participants undergoing treatment for primary breast cancer | |
| Interventions | Drain versus no drain. Portovac drains. Single axillary drain for both arms, plus a second drain under mastectomy flaps, no second drain for BCT participants | |
| Outcomes | Primary outcome: length of hospital stay Secondary outcomes: surgical morbidity—seroma, volume and number of aspirations, infection, shoulder movement |
|
| Notes | Setting not clearly stated, presumed Western Infirmary, UK Funding source: grant from Chief Scientist Office of the Department of Health, Scottish Executive |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Consecutively numbered opaque envelopes"—not stated what was put into the envelopes |
| Allocation concealment (selection bias) | Unclear risk | Authors do not state when allocation was revealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Cannot blind surgeons or participants to presence/absence of drain. Unclear what effect, if any, this would have |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants discharged after drain removal, so follow‐up could be blind to treatment, although surgeons may recognise participants from wards/theatre Infection defined: ASEPSIS score for infection > 10 No description of seroma definition or aspiration threshold—HIGH bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear CONSORT diagram, no dropouts |
| Selective reporting (reporting bias) | High risk | Number of aspirations not reported |
| Other bias | High risk | Study design However, trial primarily looking at hospital stay length. Drain groups released when < 50 mL/24 h or 5 days, whenever soonest. No drains released 24 to 48 hours later, inherent bias in this design towards keeping drain participants for longer time based on protocol Trial excluded women not thought suitable for early discharge—surely these women would make no drain + early discharge more psychologically damaging and potentially alter trial conclusions—cannot generalise from this trial to ALL women undergoing breast surgery |
Jain 2004.
| Study characteristics | ||
| Methods | Single‐centre randomised controlled trial on 116 participants undergoing breast‐conserving surgery or mastectomy with level I/II axillary dissection. No drain group stratified into 29 treated with Tisseel fibrin sealant and 29 without. Only 29 participants treated without Tisseel included in no drain meta‐analysis group | |
| Participants | n = 87. Drain 58, no drain 29. Mastectomy: drain 9, no drain 10. BCT arm: drain 22, no drain 17. Age of participants, years: drain: 61.9 ± 13.2, no drain: 62.3 ± 12.3 (mean ± SD) | |
| Interventions | Drain versus no drain. Single 14‐Fr vacuum drain | |
| Outcomes | Primary outcome: incidence of symptomatic seroma Secondary outcomes: hospital stay, pain scores, total volume of aspirate, number of aspirations, infection |
|
| Notes | Setting: Department of Surgery, Scarborough General Hospital, UK Funding source: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed, opaque envelope containing computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Envelope opened by theatre nursing staff at the end of the operation to reveal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Cannot blind surgeons or participants to presence/absence of drain. Unclear what effect, if any, this would have |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Seroma defined as "palpable fluid collection under the wound" Wound infection defined as "erythema, tenderness and/or purulent discharge from the incision site" Only clinically symptomatic seromas aspirated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other biases |
Soon 2005.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled trial on 87 participants undergoing breast cancer surgery with level III axillary dissection | |
| Participants | n = 87. Drain 36, no drain 51. Age of participants, years: drain 59, no drain 58 (no SD reported) Previous axillary lymph node surgery (except SLNB) and prior axillary or chest wall irradiation are exclusion criteria |
|
| Interventions | Drain for 24 to 48 hours postoperatively versus no drain. Closed suction drain | |
| Outcomes | Mean number of lymph nodes involved and total number of lymph nodes excised (check other studies for these data to look for a trend), seroma, total seroma volume, number of aspirations, length of seroma persistence, inpatient stay length, infection—cellulitis oral antibiotics, cellulitis IV antibiotics, abscess requiring drainage, haematoma, lymphoedema, skin necrosis | |
| Notes | Setting: Breast/Endocrine Unit, St. George’s Hospital, UK Funding source: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudorandomisation based on day of the month. If operation happened on odd day—drain inserted, even day—no drain |
| Allocation concealment (selection bias) | High risk | Pseudorandomisation method used very easily known by surgeons. Cannot conceal allocation, as surgeon will know from the start whether participant is to receive a drain |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Cannot blind surgeons or participants to presence/absence of drain. Unclear what effect, if any, this would have |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Seroma and infection not defined Any seroma was aspirated, so limited scope for subjective interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No explicit description of dropouts, but apparent from data that full outcome assessment was performed on all participants |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other biases |
Classe 2006.
| Study characteristics | ||
| Methods | Single‐centre randomised controlled trial on participants undergoing breast‐conserving surgery with level I/II axillary dissection | |
| Participants | n = 98. Drain 51, no drain 47. Age of participants, years: drain 60 ± 10, no drain 58 ± 11 (mean ± SD) | |
| Interventions | Drain till flow < 35 mL/24 hours versus no drain with axillary padding. Type of drain not stated | |
| Outcomes | Primary outcome: reduction in length of hospital stay Secondary outcomes: seroma, aspiration, volume of aspiration, total drainage, time from surgery to final drainage, infection, shoulder movement, pain, quality of life |
|
| Notes | Setting: Nantes Saint Herblain, France Funding source: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not state method used. Say that “randomisation was done in blocks of six” |
| Allocation concealment (selection bias) | Unclear risk | Does not state when random assignment was revealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Cannot blind surgeons or participants to presence/absence of a drain. Unclear what effect, if any, this would have |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both groups followed‐up for 1 month using the same schedule of postop visits Seroma defined as "palpable fluid accumulation causing discomfort and needing aspiration" Wound infection defined as "inflamed wound with pyrexia and positive microbiology that needed antibiotic treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts noted, full follow‐up of all participants |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | High risk | Not a fair comparison—external compression versus drain with no attempt to close dead space |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Garbay 2012 | Not an RCT, data obtained from two successive prospective studies |
| Talbot 2002 | Not an RCT, participants treated in consecutive series, no randomisation |
| Warren 1994 | Study of breast biopsy wounds, not axillary dissection |
| Wheeler 1976 | Study of breast biopsy wounds, not axillary dissection |
Differences between protocol and review
The protocol of this review was originally published by V Pineda, C Manterola, M Vial and P Astudillo.
The original protocol proposed that the type of drain used (with or without aspiration) should be considered for subanalysis. However, all seven papers used closed suction drains, so this subanalysis was not possible.
Originally, microbiologically confirmed wound infection was listed; we changed this to infection, because microbiological results were rarely reported.
A number of changes were made to the list of secondary outcomes listed in the published protocol. These include the following.
'Type of breast surgery (mastectomy or lumpectomy)' was removed from the outcome list and instead was integrated as part of the subgroup analysis, when possible.
Arm lymphoedema rate was added to the secondary outcomes.
'Quantity of fluid aspirated' is now referred to as 'Volume of seroma aspirated'.
'Frequency of seroma aspirations' is now referred to as 'Number of seroma aspirations'.
Contributions of authors
We would like to acknowledge the following authors who developed and published the protocol version of this Cochrane review.
Viviana Pineda – Protocol development and writing. Carlos Manterola – Protocol development and writing. Manuel Vial – Protocol development and writing. Paula Astudillo – Protocol development and writing.
David R Thomson (DRT) – Initial concept, protocol development and writing, literature searching, data extraction, data analysis and interpretation, drafting of manuscript, editing of manuscript, and approval of final version of manuscript.
Hazim Sadiddeen (HS) – Literature searching, data extraction, data analysis and interpretation, editing of manuscript, and approval of final version of manuscript.
Dominic Furniss (DF) – Initial concept, assistance with data analysis and interpretation, editing of manuscript, and approval of final version of manuscript.
Sources of support
Internal sources
-
Cochrane Breast Group, Other
We gratefully acknowledge the technical support and guidance provided by the Cochrane Breast Cancer Group.
External sources
No sources of support provided
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Cameron 1988 {published data only}
- Cameron AE, Ebbs SR, Wylie F, Baum M. Suction drainage of the axilla: a prospective randomized trial. British Journal of Surgery 1988;75(12):1211. [PMID: 3069178] [DOI] [PubMed] [Google Scholar]
Classe 2006 {published data only (unpublished sought but not used)}
- Classe JM, Berchery D, Campion L, Pioud R, Dravet F, Robard S. Randomized clinical trial comparing axillary padding with closed suction drainage for the axillary wound after lymphadenectomy for breast cancer. British Journal of Surgery 2006;93(7):820-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jain 2004 {published and unpublished data}
- Jain PK, Sowdi R, Anderson AD, MacFie J. Randomized clinical trial investigating the use of drains and fibrin sealant following surgery for breast cancer. British Journal of Surgery 2004;91(1):54-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Purushotham 2002 {published data only (unpublished sought but not used)}
- Purushotham AD, McLatchie E, Young D, George WD, Stallard S, Doughty J, et al. Randomized clinical trial of no wound drains and early discharge in the treatment of women with breast cancer. British Journal of Surgery 2002;89(3):286-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Somers 1992 {published data only (unpublished sought but not used)}
- Somers RG, Jablon LK, Kaplan MJ, Sandler GL, Rosenblatt NK. The use of closed suction drainage after lumpectomy and axillary node dissection for breast cancer. A prospective randomized trial. Annals of Surgery 1992;215(2):146-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soon 2005 {published data only (unpublished sought but not used)}
- Soon PS, Clark J, Magarey CJ. Seroma formation after axillary lymphadenectomy with and without the use of drains. Breast 2005;14(2):103-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zavotksy 1998 {published and unpublished data}
- Zavotsky J, Jones RC, Brennan MB, Giuliano AE. Evaluation of axillary lymphadenectomy without axillary drainage for patients undergoing breast-conserving therapy. Annals of Surgical Oncology 1998;5(3):227-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Garbay 2012 {published data only}
- Garbay JR, Thoury A, Moinon E, Cavalcanti A, Palma MD, Karsenti G, et al. Axillary padding without drainage after axillary lymphadenectomy—a prospective study of 299 patients with early breast cancer. Breast Care (Basel) 2012;7(3):231-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Talbot 2002 {published data only}
- Talbot ML, Magarey CJ. Reduced use of drains following axillary lymphadenectomy for breast cancer. ANZ Journal of Surgery 2002;72(7):488-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Warren 1994 {published data only}
- Warren HW, Griffith CD, McLean L, Angerson WJ, Kaye B, McElroy M. Should breast biopsy cavities be drained? Annals of the Royal College of Surgeons of England 1994;76(1):39-41. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Wheeler 1976 {published data only}
- Wheeler MH, Lakhany Z. Breast biopsy: a trial of wound drainage. American Journal of Surgery 1976;131(5):581-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Additional references
Agrawal 2006
- Agrawal A, Ayantunde AA, Cheung KL. Concepts of seroma formation and prevention in breast cancer surgery. ANZ Journal of Surgery 2006;76:1088-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
American Cancer Society 2012
- American Cancer Society. Breast Cancer Facts & Figures 2011-2012. American Cancer Society, Inc., Atlanta 2012.
Anderson 2006
- Anderson BO, Shyyan R, Eniu A, Smith RA, Yip CH, Bese NS, et al. Breast cancer in limited-resource countries: an overview of the Breast Health Global Initiative 2005 guidelines. The Breast Journal 2006;12(Suppl 1):3-15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Axelsson 2007
- Axelsson CK, Mouridsen HT, During M, Moller S. Axillary staging during surgery for breast cancer. British Journal of Surgery 2007;94(3):304-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bohm 2012
- Bohm D, Kubitza A, Lebrecht A, Schmidt M, Gerhold-Ay A, Battista M, et al. Prospective randomized comparison of conventional instruments and the Harmonic Focus(®) device in breast-conserving therapy for primary breast cancer. European Journal of Surgical Oncology 2012;38(2):118-24. [PMID: 22152942 ] [DOI] [PubMed] [Google Scholar]
Droeser 2009
- Droeser RA, Frey DM, Oertli D, Kopelman D, Baas-Vrancken Peeters MJ, Giuliano AE, et al. Volume-controlled vs no/short-term drainage after axillary lymph node dissection in breast cancer surgery: a meta-analysis. Breast 2009;18:109-14. [PMID: 19289285] [DOI] [PubMed] [Google Scholar]
Hashemi 2004
- Hashemi E, Kaviani A, Najafi M, Ebrahimi M, Hooshmand H, Montazeri A. Seroma formation after surgery for breast cancer. World Journal of Surgical Oncology 2004;2:44-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
He 2011
- He XD, Guo ZH, Tian JH, Yang KH, Xie XD. Whether drainage should be used after surgery for breast cancer? A systematic review of randomized controlled trials. Medical Oncology 2011;28(Suppl 1):S22-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane-handbook.org.
Jeffrey 1995
- Jeffrey SS, Goodson WH, Ikeda DM, Birdwell RL, Bogetz MS. Axillary lymphadenectomy for breast cancer without axillary drainage. Archives of Surgery 1995;130(8):909-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kelley 2012
- Kelley TA, Thomson DR, Furniss D. When should axillary drains be removed post axillary dissection? A systematic review of randomised control trials. Surgical Oncology 2012;21(4):247-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kuroi 2005
- Kuroi K, Shimozuma K, Taguchi T, Imai H, Yamashiro H, Ohsumi S, et al. Pathophysiology of seroma in breast cancer. Breast Cancer 2005;12:288-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mansel 2007
- Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. Journal of the National Cancer Institute 2006;98(9):599-609. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miao 2011
- Miao H, Verkooijen HM, Chia KS, Bouchardy C, Pukkala E, Larønningen S, et al. Incidence and outcome of male breast cancer: an international population-based study. Journal of Clinical Oncology 2011;29(33):4381-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Morrow 2002
- Morrow M, Strom EA, Bassett LW, Dershaw DD, Fowble B, Giuliano A, et al. Standard for breast conservation therapy in the management of invasive breast carcinoma. CA: A Cancer Journal for Clinicians 2002;52:277-300. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pogson 2003
- Pogson CJ, Adwani A, Ebbs SR. Seroma following breast cancer surgery. European Journal of Surgical Oncology 2003;29:711-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan [Computer program]
- Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Stehbens 2003
- Stehbens WE. Postmastectomy serous drainage and seroma: probable pathogenesis and prevention. ANZ Journal of Surgery 2003;73:877-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Bemmel 2011
- Bemmel AJ, de Velde CJ, Schmitz RF, Liefers GJ. Prevention of seroma formation after axillary dissection in breast cancer: a systematic review. European Journal of Surgical Oncology 2011;37:829-35. [PMID: ] [DOI] [PubMed] [Google Scholar]


