Highlights
-
•
The PIRATES protocol is a novel tumor radiation dose-escalation approach.
-
•
PIRATES integrates proton therapy, image guided RT and hybrid hyper-fractionation.
-
•
It targets only head and neck cancer patients at high risk of treatment failure.
-
•
This Phase I trial aims to test the feasibility and safety of PIRATES.
Keywords: Head and neck cancer, Radiation dose-escalation, Proton therapy, Image guided RT, Hyper-fractionation, Phase I trial, Toxicity
Abstract
Introduction
Radiation dose-escalation for head and neck cancer (HNC) patients aiming to improve cure rates is challenging due to the increased risk of unacceptable treatment-induced toxicities. With “Proton Image-guided Radiation Assignment for Therapeutic Escalation via Selection of locally advanced head and neck cancer patients” (PIRATES), we present a novel treatment approach that is designed to facilitate dose-escalation while minimizing the risk of dose-limiting toxicities for locally advanced HPV-negative HNC patients. The aim of this Phase I trial is to assess the safety & feasibility of PIRATES approach.
Methods
The PIRATES protocol employs a multi-faceted dose-escalation approach to minimize the risk of dose-limiting toxicities (DLTs): 1) sparing surrounding normal tissue from extraneous dose with intensity-modulated proton therapy, 2) mid-treatment hybrid hyper-fractionation for radiobiologic normal tissue sparing; 3) Magnetic Resonance Imaging (MRI) guided mid-treatment boost volume adaptation, and 4) iso-effective restricted organ-at-risk dosing to mucosa and bone tissues.
The time-to-event Bayesian optimal interval (TITE-BOIN) design is employed to address the challenge of the long DLT window of 6 months and find the maximum tolerated dose. The primary endpoint is unacceptable radiation-induced toxicities (Grade 4, mucositis, dermatitis, or Grade 3 myelopathy, osteoradionecrosis) occurring within 6 months following radiotherapy. The second endpoint is any grade 3 toxicity occurring in 3–6 months after radiation.
Discussion
The PIRATES dose-escalation approach is designed to provide a safe avenue to intensify local treatment for HNC patients for whom therapy with conventional radiation dose levels is likely to fail. PIRATES aims to minimize the radiation damage to the tissue surrounding the tumor volume with the combination of proton therapy and adaptive radiotherapy and within the high dose tumor volume with hybrid hyper-fractionation and not boosting mucosal and bone tissues. Ultimately, if successful, PIRATES has the potential to safety increase local control rates in HNC patients with high loco-regional failure risk.
Trial registration: ClinicalTrials.gov ID: NCT04870840; Registration date: May 4, 2021.
Netherlands Trial Register ID: NL9603; Registration date: July 15, 2021.
Introduction
High risk HPV-negative locally advanced HNC
Worldwide, head and neck cancer (HNC) affects approximately 650,000 people, accounting for 350,000 deaths annually [1]. Radiation oncology plays a pivotal role in HNC treatment, eliminating tumor cells locally with radiation. Typical curative radiation doses of 70 Gy are prescribed to the primary tumor and pathological lymph nodes in 33–35 daily fractions (i.e. 5 or 6 fractions per week over 6 to 7 weeks). Unfortunately, despite efforts to precisely deliver radiation to tumor targets, there is inevitable co-irradiation of surrounding normal tissues, which can lead to toxicities that compromise quality of life [2]. Thus, HNC radiotherapy is challenged by the fine line between maximizing tumor control and dose-limiting toxicities.
While advances in radiotherapy, such as proton therapy, have reduced toxicity risks, local regional tumor recurrence rates have not improved, and occur for the general HNC population in approximately 30% of cases [3], [4], [5], [6], [7], [8], [9]. In particular, outcome rates remain relatively poor for locally advanced (i.e. stage III/IV) human papillomavirus (HPV)-negative HNC patients treated with chemo-radiotherapy (which is the standard-of-care treatment for inoperable locally advanced HNC) [10]. The 5-year local–regional control (LRC) rates are around 50% for locally advanced HPV negative oropharyngeal carcinomas, 30–40% for HNC of the hypopharynx, and 60–70% for larynx carcinomas [10], [11], [12].
Multiple studies have shown that the primary site local regional recurrence originates in the central regions that receive the high curative radiation dose (i.e. 70 Gy) [5], [8], [9]. This suggests that increasing the radiation dose to the tumor volume (e.g. from 70 to 80 Gy) is needed to improve cure rates in high-risk locally advanced HNC patients.
Background radiation dose-escalation
Previous studies have tested radiation tumor dose-escalation by either increasing: 1) the tumor dose per fraction, or 2) the total dose by using smaller doses per fraction yet treating multiple times per day (hyper-fractionation). Photon radiotherapy-based phase I-II studies showed that performing dose-escalation by increasing doses per fraction (e.g. 35x 2.3 Gy instead of 35x 2.0 Gy) results in unacceptable severe toxicity in the high dose area [13]. Specifically, the study by Madani et al. observed a severe mucosal ulcer (mucositis grade 4) in a single patient with tumor dose-escalation up to 80.9 Gy, and in 5 out of 14 patients with dose level II of 85.9 Gy [13]. While 4 out of total 6 mucosal ulcers healed with a median time of 4 months, one patient presented with a mucosal ulcer of which the consequences resulted in death. In contrast, delivering 80.5 Gy with hyper-fractionation (i.e. 70x 1.15 Gy) resulted in significantly lower tumor recurrence rates without severe mucosal ulceration [14], [15]. Nevertheless, the higher doses to surrounding normal tissues that accompany photon therapy-based hyper-fractionation still translated to higher toxicity rates, and the (near) doubling of fractions increased clinical workflow burden.
PIRATES approach for proton therapy-based dose-escalation
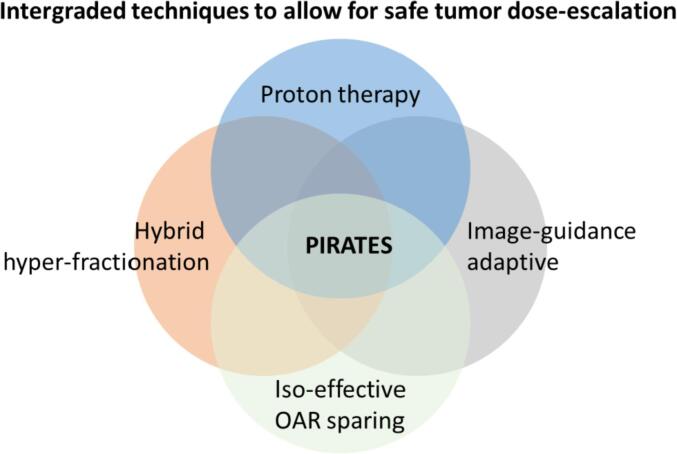
This protocol aims to improve the treatment of high risk HNC patients (i.e. locally advanced HPV negative HNC patients). For this group only, we propose a novel method for safe dose-escalation: Proton Image-guided Radiation Assignment for Therapeutic Escalation via Selection of locally advanced head and neck cancer patients (PIRATES). We reasonably expect PIRATES to be a safe method for dose-escalation, as damage to surrounding normal tissues is kept low in order to prevent severe toxicity and severe mucosal ulceration, based on the following principles (Fig. 1):
-
1.
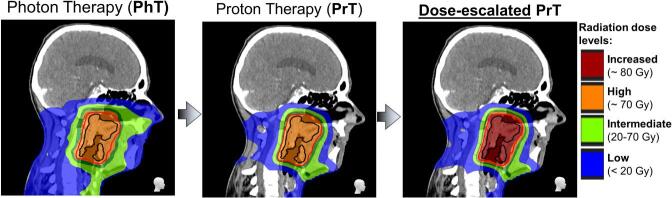
Using proton therapy to limit dose surrounding normal tissues while escalating the dose to the tumor compared to photon therapy. Proton therapy offers the possibility of tumor dose-escalation without increasing, or even lowering, toxicity compared to conventional IMRT/VMAT (Fig. 2), due to the unique properties of accelerated protons.
-
2.
Applying a hybrid hyper-fractionation strategy, meaning that dose-escalation will be delivered with two daily fractions but only for part of the treatment period (i.e. from radiotherapy weeks 5 to 7), while therapy will be with conventional dose levels in the first 4 weeks. Hyper-fractionation allows for biological normal tissue recovery between morning and afternoon fractions. An interval of 6–8 h between fractions is preferred.
-
3.
Defining the dose-escalation “boost” target volume with MR-guided mid-treatment adaptation based on tumor shrinkage. This facilitates the boost dose levels being applied to the (usually) smaller volume of still visible tumor. The initial target volume at start of treatment, not overlapping with the boost dose, will only receive the conventional target dose (i.e. 70 Gy).
-
4.
Using iso-effective organ-at-risk sparing to prevent the mucosa and mandible within the target area from receiving the boost dose, i.e. these organ-at-risk (OAR) should receive similar doses as with conventional treatment.
Fig. 1.
State-of-the-art technology integration used in PIRATES to allow for safe dose-escalation.
Fig. 2.
Dose-escalation planning example with proton therapy. Compared to photon therapy plan (left), both proton therapy plans without (middle) and with tumor dose-escalation (right) show much lower doses to the surrounding normal tissues.
Methods
Overview of the study design
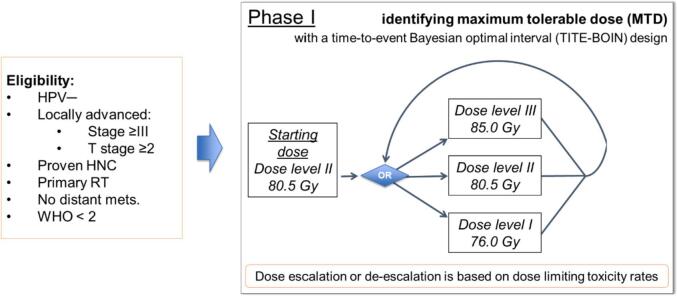
This phase I study is based on the time-to-event Bayesian optimal interval (TITE-BOIN) design [16], a novel dose-finding design that accommodates late-onset toxicities. A total of 18 HNC patients will be included according to their eligibility. The MTD with proton therapy will be tested with 3 possible dose levels (Fig. 3).
Fig. 3.
Phase I trial schema and dose-escalation levels.
Primary objective
To assess the safety & feasibility of image guided mid-treatment hyper-fractioned dose-escalation with proton therapy and identify the maximum tolerable dose (MTD) for the treatment of locally advanced HPV negative HNC.
Primary endpoint
Severe unacceptable local adverse events which are radiotherapeutically attributable. Specifically, CTCAEv5 grade 4 mucositis or dermatitis that does not resolve to a grade ≤ 3 in 3 months, and CTCAEv5 grade ≥ 3 myelopathy, and/or osteoradionecrosis.
Secondary and exploratory endpoints
Rates of grade 3 toxicity at 3 to 6 months after radiation oncology. Specifically, CTCAEv5 grade 3 mucositis, dermatitis, aspiration, dysphagia, hearing impaired, xerostomia, weight loss, trismus, hoarseness, oropharyngeal pain. Local and regional control will be analyzed as exploratory endpoints.
Inclusion and exclusion criteria
Conditions for patient eligibility
-
•
Biopsy proven diagnosis of squamous cell carcinoma of HNC originating in the oropharynx, hypopharynx, larynx, or oral cavity
-
•
Primary radiotherapy with curative intent, either in combination with chemotherapy or not
-
•Inoperable locally advanced disease, defined as:
-
oAJCC 8th stage ≥ III
-
oT stage ≥ 2
-
oNegative for HPV by p16 IHC or ISH
-
o
Conditions for patient ineligibility
-
•
Previous radiation treatment in the head and neck region
-
•
Head and neck surgery of the primary tumor or lymph nodes except for incisional or excisional biopsies
-
•
Pregnant or breast-feeding females
-
•
Patients younger than 18 years
-
•
Patients with ECOG performance score of 2 or lower
-
•
Contraindications to MRI
Additional exclusion criteria: continuation of smoking and/or alcohol abuse
A recent in-depth analysis of contribution factors related to the development of mucosal ulcers with dose-escalation showed that the development of ulcers is highly correlated with smoking and alcohol abuse (>5 unit/d) [17]. These results strongly advise against smoking and alcohol use during and after treatment, due to the increased risk of developing mucosal ulcers. Therefore, current smokers or extensive alcohol users are asked to stop during and after treatment. Continuation of the use of tobacco or alcohol will be tested with urine analyses during and after treatment, if patients fail to refrain from these substances, they will be excluded from the study for safety purposes, even is patient is already being treated.
Radiotherapy treatment description
Image-guided hybrid hyper-fractionation dose-escalation with proton therapy
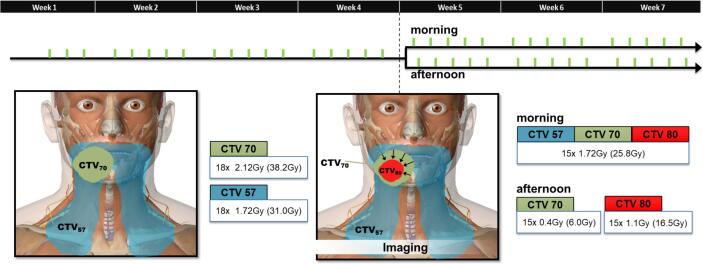
The radiation regimen will be administered in a two-part schedule (see schematic overview and dose levels in Fig. 4 and Table 1, respectively).
Fig. 4.
Two-part radiation schedule (for boost dose level 80.5 Gy). Three target volumes are defined: conventional low dose level to elective lymph nodes (in blue CTV57), conventional high dose clinical tumor volume determined at start (in green CTV70) and the boost adapted, after shrinkage (arrows), to gross tumor volume at week 4 (in red GTV80). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
PIRATES dose levels for phase I trial for Clinical Target Volumes (CTV).
| week | 1–4 | week | 5–7 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Target | fraction dose | # | sub total | morning fraction dose | # | afternoon fraction dose | # | sub total | Total | Description |
| CTV70 | 2.12 | 18 | 38.16 | 1.72 | 15 | 0.40 | 15 | 31.80 | 70.0 Gy | Conventional primary tumor dose |
| CTV57 | 1.72 | 18 | 30.96 | 1.72 | 15 | ˠ | ˠ | 25.80 | 56.8 Gy | Conventional elective lymph node level dose |
| GTVboost-80 | * | * | 38.16 | 1.72 | 15 | 1.10 | 15 | 42.30 | 80.5 Gy | Boost dose level II – initial escalation dose |
| Alternatives: | ||||||||||
| GTVboost-85 | * | * | 38.16 | 1.72 | 15 | 1.40 | 15 | 46.80 | 85.0 Gy | If no adverse event, GTVboost dose will be increased |
| GTVboost-76 | * | * | 38.16 | 1.72 | 15 | 0.80 | 15 | 37.80 | 76.0 Gy | If adverse event, GTVboost dose will be decreased |
*GTVboost will be determined in week 4, yet is assumed to be within CTV70 for week 1–4.
ˠCTV57 is not included in the afternoon fraction.
The first 4 weeks of the radiation treatment will be according to the conventional clinical standard (i.e. first 18 fractions). Throughout the entire treatment, the primary tumor target volumes, referred to as CTV70, will receive the conventional 70 Gy (daily fraction dose of 2.12 Gy) and the elective lymph node levels will receive 57 Gy (1.72 Gy/fraction) according to current clinical practice at MD Anderson Cancer Center (MDACC).
For weeks 5 to 7, a boost dose will be administered to the mid-treatment tumor volume. This volume will be determined on CT and MR imaging in treatment position acquired at week 4; PET-imaging is optional. Only the adapted tumor volume (GTVboost) will receive the dose-escalation (e.g. 80.5 Gy for the first patients entering the trial, and either 76 Gy or 85 Gy, which will be decided by the statistical TITE-BOIN design detailed below). Since the tumor typically reduces in size during treatment [18], the GTVboost will be within CTV70, thus having received the conventional prescribed dose for first 18 fractions. Patients will be radiated twice per day (i.e. hyper-fractionation), as illustrated in Fig. 4. In the morning, both the CTV57, CTV70 and GTVboost will receive the conventional elective of 1.72 Gy/fraction. In the afternoon, only the primary CTV will receive dose: the CTV70 will receive additional 0.4 Gy to get in total to its conventional daily dose of 2.12 Gy (=1.72 + 0.40 Gy) and the GTVboost will receive 1.1 Gy for a total daily dose of 2.82 Gy (=1.72 + 1.10 Gy). Subsequently, this leads to a total radiation dose of 80.5 Gy (Table 1). For subsequent phases of the TITE-BOIN, the additional second fraction dose of the GTVboost will be either 1.4 Gy or 0.8 Gy depending on interval evaluation, resulting in total doses of 85.0 or 76.0 Gy respectively (Table 1-Alternatives).
Specific organ constraints
For this protocol, the mucosal area is defined as the 2 mm mucosal rim around the airway. For the mucosal rim, mandible, and other bone structures dose, the objective is to keep the maximum dose below 74 Gy (appendix A).
Technical factors
Proton therapy will be delivered with Intensity Modulated Proton Therapy (IMPT) technique on the gantry that allows for pencil beam scanning in the MDACC proton therapy center. The Varian Medical Systems synchrotron produces protons with energies varying between 70 and 250 MeV in order to allow for 3D dose delivery. IMPT plans will be developed in Eclipse (from Varian Medical Systems) or Raystation (Raysearch), with robust optimization to ensure for adequate dose delivery in case of setup or range errors.
Specification of safety parameters: adverse events
Safety of phase I trial will be assessed by monitoring severe local (i.e. in the escalated radiation field) adverse radiation-attributable side effects, which includes the following toxicities: 1) Mucositis, either oral or laryngeal – Grade 4: severe mucosal ulcers; 2) Dermatitis – Grade 4: skin necrosis or ulceration of full thickness dermis; 3) Cervical myelopathy – Grade ≥ 2: Severe weakness or sensory loss or paraplegia; 4) Osteoradionecrosis – Grade ≥ 3: elective or major surgery required. For grade 5 (i.e. Death) any grade 5 local radiation attributable Adverse Event is considered.
Not adverse events: additionally monitored symptoms
Common not severe acute (≤90 days) radiation attributable adverse events will be monitored: weight loss, xerostomia, dysphagia, dysgeusia, hoarseness, fatigue, regional alopecia, radiation dermatitis (G ≤ 3), radiation mucositis (G ≤ 3), and aspiration. Standard of care radiotherapy routinely demonstrates acute (i.e. <90 days post-therapy) Grade 3 toxicity rates are nearly ubiquitous (almost 90% for mucosal squamous carcinomas stage II-IV). Consequently, a 0.75 MTD rate at 3 months represents a conservative assessment of toxicity which is beginning to resolve still for many patients at 3 months post-chemoradiation. Less common not severe or treatable long-term (>90 days) adverse events include: hypothyroidism, hearing loss, and dysphagia (i.e. chronic swallowing dysfunction that may require permanent feeding tube placement).
Symptom assessment, baseline and follow-up proceedings
Toxicities will be monitored by the treating physician/s for every consult, which is clinical practice, before, weekly during, and at 8–10 weeks, 3, 6, 9, 12 months after therapy in the first year, subsequently every 4 months in the second year and then every 6 months. During treatment, when patients are seen, a dedicated physician assistant will independently rate CTC-AEs, blinded to physician rating as a secondary check. When CTC-AEs ≥ 3 are divergent, a third clinician will rate the case. A REDCap database will contain: physician-rated toxicity scores (CTCAEv5), quality of life scores (FACT-HNSI-10), and patient-reported toxicity (MDADI, MDASI, XeQOLS and EQ-5D-3L). Refer to Appendix B for more details.
Statistical considerations
The statistical hypothesis is that image-guided hybrid hyper-fractionated dose-escalation with mucosal sparing proton therapy is a feasible and safe treatment for locally advanced HNC patients. Feasibility is defined as at least 80% of the patients receiving the MTD complete treatment. Safety is defined that none of the patients that receive the MTD develop severe unacceptable toxicities (Grade 4, mucositis, dermatitis, or Grade 3 myelopathy, osteonecrosis) within 6 months following radiotherapy (primary endpoint) and rates of any grade 3 toxicity do not exceed 80% of patients in 3 to 6 months following treatment.
The time-to-event Bayesian optimal interval (TITE-BOIN) design [16] is used to find the MTD and address the challenge that the DLT window is as long as 6 months. Unlike the majority of existing phase I designs, which require suspending the accrual after treating each cohort of patients, the TITE-BOIN design allows for real-time dose assignment decisions for new patients while some enrolled patients’ toxicity data are still pending. This shortens the trial duration and reduces the logistic difficulties caused by repeatedly suspending accrual. The TITE-BOIN works by predicting the dose-limiting toxicity (DLT) outcome for patients whose DLT data are pending based on their follow-up time. It is implemented in a simple way similar to the traditional 3 + 3 design, but is more flexible and possesses superior operating characteristics that are comparable to those of the more complex model-based designs, such as the time-to-event continual reassessment method (TITE-CRM) [19]. Refer to Appendix C for statistical plan.
Discussion
If this Phase I trial is successful, PIRATES dose-escalation approach will be the first to provide a safe avenue to intensify treatment for locally advanced HNC patients for whom therapy with conventional radiation dose levels is more likely to be unsuccessful. The recent technical advances that allow for more precise radiation dose delivery have not been deployed to improve poor tumor control and survival odds of the HNC subgroup of locally advanced HPV-negative HNC patients [10], [11], [12]. Since nearly all tumor recurrences occur in the high radiation dose region (i.e. within the CTV70), increasing radiation dose to the pathological tumor sites has the potential to improve the tumor control for these high risk patients [8]. However, this was previously not feasible, as it caused devastating toxicity [20]. This proposal details the novel PIRATES approach of integrating state-of-the-art: 1) delivery with proton therapy, 2) adaptive radiotherapy with MR-guided boost definitions, 3) biological recovery strategy with hybrid hyper-fractionation and 4) physical dose constraints, in order to keeping toxicity levels at acceptable tolerance. Several large clinical trials have reported toxicity incidence rates for locally advanced HNC patients caused by ‘conventional’ chemo-radiation, either with cisplatin, cetuximab or carboplatin [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]. Despite high rates of toxicity, these studies demonstrate that acute grade 4 are very rare (∼1–3% depending on the side effects). With the PIRATES approach, we believe that we can aim to have similar rates of these severe side effects.
Proton therapy and adaptive radiotherapy can reduce the radiation damage to the tissue surrounding the tumor volume. Proton therapy has the capacity to deliver dose to tumor tissue without depositing dose at the distal end of the beam, allowing for more conformal dose to the tumor only [32], [33]. Furthermore, by not dose-escalating the entire tumor from the start of treatment, but rather using an adapted boost volumes that conform to visually detectable tumor tissue on mid-treatment CT and MRI scans, we expect to decrease the volume that is boosted and thus minimize unnecessary dose delivery to the surrounding normal tissues.
On the other hand, hybrid hyper-fractionation (i.e. hyper-fractionation for the last 3 weeks) and not boosting mucosal and bone tissues can minimize severe toxicity within the high dose tumor volume. A hyper-fractionation approach allows for in-field normal tissue recovery, as these normal tissues tend to recover more rapidly than tumor tissue [15]. Moreover, rather than full treatment hyper-fractionation (i.e. 2 × 33 = 66 fractions), this hybrid hyper-fractionation (48 fractions) design balances the (logistic) burden on the patient and the department.
The PIRATES protocol is only directed to the small sub-cohort of the HNC population that is at high risk of treatment failure loco-regionally, as treatment intensification should only be applied to patients that are likely to benefit from this proposed sub-volume dose-escalation. If applied to patients that have lower risk, the desired effect of improved treatment efficiency may be counterproductive, as increase in symptoms has been shown to be related to overall survival in HNC [34]. Even more exemplary, the RTOG 0617 non–small-cell lung cancer (NSCLC) dose-escalation trial showed that dose-escalation to unselected populations can potentially result in lower overall survival rates [35]. While an overall consensus has not been reached, this result is likely caused by the increased dose to surrounding normal tissues (e.g. the heart and lung). As described throughout, the PIRATES approach takes extensive dosimetric and radiobiologic considerations to limit the dose to the normal tissues surrounding and within the high dose receiving volume, while selecting for eligibility only high risk (e.g. HPV-negative, large-volume, tobacco-associated) locally advanced HNC patients, for whom tumor progression is their highest risk of oncologic modal recurrence and thus the greatest mortality-modulating potential risk factor.
While overall tumor staging is a useful prognostic marker of overall survival [36], identification of high risk patients for particular modes of oncologic recurrence may be improved by using more advanced outcome prediction models. As shown by Ang et al. [37], other factors that are not incorporated in the AJCC tumor staging are of influence for overall survival and progression-free survival, such as smoking pack years and age. Moreover, several studies have shown that tumor-specific characteristics quantified from imaging, so-called radiomics features, can aid in treatment outcome prediction [38], [39], [40], [41], [42]. These predictive radiomics features are generally associated with the tumor tissue heterogeneity and the size/shape, but have also shown to be able to subside clinical variables such as N stage [40]. The next years require dedicated efforts to design robust treatment outcome prediction models to facilitate improved selection of high-risk patients for dose-escalation approaches so that only these at excessive risk of local or regional recurrence can be effectively slated for potential local escalation strategies.
Current status and planned timeline
Institutional review board (IRB) approval was obtained in October 2020, and study is monitored by Data and Safety Monitoring Committee at MD Anderson Cancer Center. Start of the trial was delayed due to COVID-19 pandemic; the accrual of patients at MDACC started in May 2021. Two patients have been enrolled in the study at current standing. The total accrual time of the 18 HNC patients is expected to be within 2 years.
Patient consent statement
Patients were only included in the study if they provided written informed consent before treatment and after receiving comprehensive written and verbal information of the study. This included the potential risk concerning severe toxicities.
Conflict of interest
The authors state that the research presented in this manuscript is free of conflicts of interest.
Funding statement
Dr. van Dijk, received/receives funding and salary support for initiation and execution of this trial from the Dutch organization NWO ZonMw via the Rubicon Individual career development grant.
Dr. Mohamed and Fuller received/receives funding and salary support unrelated to this project during the period of study execution from: the NIH National Cancer Institute (NCI) Early Phase Clinical Trials in Imaging and Image-Guided Interventions Program (R01CA218148), the National Institutes of Health (NIH) National Institute for Dental and Craniofacial Research (NIDCR) Establishing Outcome Measures Award (R01DE025248/R56DE025248).
Dr. Fuller received funding unrelated to this project during the period of study execution from NIH/NCI Cancer Center (P30CA016672, P50CA097007, and R01CA2148250); from NIH/NIBIB (R25EB025787-01); from NIH/NSF (NSF1557679); NSF-CMMI grant (NSF1933369); and the Sabin Family Foundation.
Acknowledgments
We thank Taylor R. Box for her excellent ongoing efforts in patient accrual and care. We thank Tyler D. Williamson for his ongoing insightful input and efforts for proton plan optimization.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2021.11.003.
Contributor Information
Lisanne V. van Dijk, Email: lvvan@mdanderson.org.
Clifton D. Fuller, Email: cdfuller@mdanderson.org.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Langendijk J.A., Doornaert P., Verdonck-de Leeuw I.M., Leemans C.R., Aaronson N.K., Slotman B.J. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–3776. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 3.Romesser P.B., Cahlon O., Scher E., Zhou Y., Berry S.L., Rybkin A., et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol. 2016;118:286–292. doi: 10.1016/j.radonc.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Felice F., Polimeni A., Valentini V., Brugnoletti O., Cassoni A., Greco A., et al. Radiotherapy controversies and prospective in head and neck cancer: a literature-based critical review. Neoplasia (United States) 2018;20:227–232. doi: 10.1016/j.neo.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunn G.B., Blanchard P., Garden A.S., Zhu X.R., Fuller C.D., Mohamed A.S., et al. Clinical outcomes and patterns of disease recurrence after intensity modulated proton therapy for oropharyngeal squamous carcinoma. Int J Radiat Oncol Biol Phys. 2016;95:360–367. doi: 10.1016/j.ijrobp.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W., Zhang X., Yang P., Blanchard P., Garden A.S., Gunn B., et al. Intensity-modulated proton therapy and osteoradionecrosis in oropharyngeal cancer. Radiother Oncol. 2017;123:401–405. doi: 10.1016/j.radonc.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanchard P., Garden A.S., Gunn G.B., Rosenthal D.I., Morrison W.H., Hernandez M., et al. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer – a case matched analysis. Radiother Oncol. 2016;120:48–55. doi: 10.1016/j.radonc.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamed A.S.R., Cardenas C.E., Garden A.S., Awan M.J., Rock C.D., Westergaard S.A., et al. Patterns-of-failure guided biological target volume definition for head and neck cancer patients: FDG-PET and dosimetric analysis of dose escalation candidate subregions. Radiother Oncol. 2017;124:248–255. doi: 10.1016/j.radonc.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Felice F., Thomas C., Barrington S., Pathmanathan A., Lei M., Urbano T.G. Analysis of loco-regional failures in head and neck cancer after radical radiation therapy. Oral Oncol. 2015;51:1051–1055. doi: 10.1016/j.oraloncology.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Salama J.K., Seiwert T.Y., Vokes E.E. Chemoradiotherapy for locally advanced head and neck cancer. J Clin Oncol. 2007;25:4118–4126. doi: 10.1200/JCO.2007.12.2697. [DOI] [PubMed] [Google Scholar]
- 11.Lefebvre J.-L., Andry G., Chevalier D., Luboinski B., Collette L., Traissac L., et al. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann Oncol. 2012;23:2708–2714. doi: 10.1093/annonc/mds065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vignoud J., Peuvrel P., Bourdin S., Beauvillain C., Bergerot P., Deraucourt D., et al. Final results of a randomized trial comparing chemotherapy plus radiotherapy with chemotherapy plus surgery plus radiotherapy in locally advanced resectable hypopharyngeal carcinomas. Laryngoscope. 2004;107:648–653. doi: 10.1097/00005537-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Madani I., Duprez F., Boterberg T., Van de Wiele C., Bonte K., Deron P., et al. Maximum tolerated dose in a phase I trial on adaptive dose painting by numbers for head and neck cancer q. Radiother Oncol. 2011;101:351–355. doi: 10.1016/j.radonc.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Bourhis J., Overgaard J., Audry H., Ang K.K., Saunders M., Bernier J., et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368:843–854. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 15.Horiot J.C., Le Fur R., N'Guyen T., Chenal C., Schraub S., Alfonsi S., et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomized trial of the EORTC cooperative group of radiotherapy. Radiother Oncol. 1992;25:231–241. doi: 10.1016/0167-8140(92)90242-m. [DOI] [PubMed] [Google Scholar]
- 16.Fellman B.M., Yuan Y. Bayesian optimal interval design for phase I oncology clinical trials. Stata J. 2015;15:110–120. [Google Scholar]
- 17.Olteanu L.A.M., Duprez F., De Neve W., Berwouts D., Vercauteren T., Bauters W., et al. Late mucosal ulcers in dose-escalated adaptive dose-painting treatments for head-and-neck cancer. Acta Oncol (Madr) 2018;57:262–268. doi: 10.1080/0284186X.2017.1364867. [DOI] [PubMed] [Google Scholar]
- 18.Marzi S., Pinnarò P., D’Alessio D., Strigari L., Bruzzaniti V., Giordano C., et al. Anatomical and dose changes of gross tumour volume and parotid glands for head and neck cancer patients during intensity-modulated radiotherapy: effect on the probability of xerostomia incidence. Clin Oncol (R Coll Radiol) 2012;24:e54–e62. doi: 10.1016/j.clon.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Cheung Y.K., Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2000;56:1177–1182. doi: 10.1111/j.0006-341x.2000.01177.x. [DOI] [PubMed] [Google Scholar]
- 20.Madani I., Duprez F., Boterberg T., Van de Wiele C., Bonte K., Deron P., et al. Maximum tolerated dose in a phase i trial on adaptive dose painting by numbers for head and neck cancer. Radiother Oncol. 2011;101:351–355. doi: 10.1016/j.radonc.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Forastiere A.A., Goepfert H., Maor M., Pajak T.F., Weber R., Morrison W., et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 22.Hitt R., López-Pousa A., Martínez-Trufero J., Escrig V., Carles J., Rizo A., et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005;23:8636–8645. doi: 10.1200/JCO.2004.00.1990. [DOI] [PubMed] [Google Scholar]
- 23.Hitt R., Grau J.J., López-Pousa A., Berrocal A., García-Girón C., Irigoyen A., et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. 2014;25:216–225. doi: 10.1093/annonc/mdt461. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen-Tan P.F., Zhang Q., Ang K.K., Weber R.S., Rosenthal D.I., Soulieres D., et al. Randomized phase III Trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol. 2014;32:3858–3867. doi: 10.1200/JCO.2014.55.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magrini S.M., Buglione M., Corvò R., Pirtoli L., Paiar F., Ponticelli P., et al. Cetuximab and radiotherapy versus cisplatin and radiotherapy for locally advanced head and neck cancer: a randomized phase II trial. J Clin Oncol. 2016;34:427–435. doi: 10.1200/JCO.2015.63.1671. [DOI] [PubMed] [Google Scholar]
- 26.Ghi M.G., Paccagnella A., Ferrari D., Foa P., Alterio D., Codecà C., et al. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial. Ann Oncol. 2017;28:2206–2212. doi: 10.1093/annonc/mdx299. [DOI] [PubMed] [Google Scholar]
- 27.Noronha V., Joshi A., Patil V.M., Agarwal J., Ghosh-Laskar S., Budrukkar A., et al. Once-a-week versus once-every-3-weeks cisplatin chemoradiation for locally advanced head and neck cancer: a phase III randomized noninferiority trial. J Clin Oncol. 2018;36:1064–1072. doi: 10.1200/JCO.2017.74.9457. [DOI] [PubMed] [Google Scholar]
- 28.Tao Y., Auperin A., Sire C., Martin L., Khoury C., Maingon P., et al. Improved outcome by adding concurrent chemotherapy to cetuximab and radiotherapy for locally advanced head and neck carcinomas: results of the GORTEC 2007–01 phase III randomized trial. J Clin Oncol. 2018;36:3084–3090. doi: 10.1200/JCO.2017.76.2518. [DOI] [PubMed] [Google Scholar]
- 29.Geoffrois L., Martin L., De Raucourt D., Sun X.S., Tao Y., Maingon P., et al. Induction chemotherapy followed by cetuximab radiotherapy is not superior to concurrent chemoradiotherapy for head and neck carcinomas: Results of the GORTEC 2007–02 Phase III Randomized Trial. J Clin Oncol. 2018;36:3077–3083. doi: 10.1200/JCO.2017.76.2591. [DOI] [PubMed] [Google Scholar]
- 30.Gillison M.L., Trotti A.M., Harris J., Eisbruch A., Harari P.M., Adelstein D.J., et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen E.E.W., Karrison T.G., Kocherginsky M., Mueller J., Egan R., Huang C.H., et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32:2735–2743. doi: 10.1200/JCO.2013.54.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lomax A.J. Charged particle therapy: the physics of interaction. Cancer J. 2009;15:285–291. doi: 10.1097/PPO.0b013e3181af5cc7. [DOI] [PubMed] [Google Scholar]
- 33.Water T.A., Bijl H.P., Schilstra C., Pijls‐Johannesma M., Langendijk J.A. The potential benefit of radiotherapy with protons in head and neck cancer with respect to normal tissue sparing: a systematic review of literature. Oncologist. 2011;16:366–377. doi: 10.1634/theoncologist.2010-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shune S.E., Karnell L.H., Karnell M.P., Van Daele D.J., Funk G.F. Association between severity of dysphagia and survival in patients with head and neck cancer. Head Neck. 2012;34:776–784. doi: 10.1002/hed.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley J.D., Paulus R., Komaki R., Masters G., Blumenschein G., Schild S., et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial p. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cramer J.D., Hicks K.E., Rademaker A.W., Patel U.A., Samant S. Validation of the eighth edition American Joint Committee on Cancer staging system for human papillomavirus-associated oropharyngeal cancer. Head Neck. 2018;40:457–466. doi: 10.1002/hed.24974. [DOI] [PubMed] [Google Scholar]
- 37.Ang K.K., Harris J., Wheeler R., Weber R., Rosenthal D.I., Nguyen-Tân P.F., et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aerts H.J.W.L., Velazquez E.R., Leijenaar R.T.H., Parmar C., Grossmann P., Carvalho S., et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5 doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallières M., Freeman C.R., Skamene S.R., El Naqa I. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol. 2015;60:5471–5496. doi: 10.1088/0031-9155/60/14/5471. [DOI] [PubMed] [Google Scholar]
- 40.Zhai T.-T., van Dijk L.V., Huang B.-T., Lin Z.-X., Ribeiro C.O., Brouwer C.L., et al. Improving the prediction of overall survival for head and neck cancer patients using image biomarkers in combination with clinical parameters. Radiother Oncol. 2017;124:256–262. doi: 10.1016/j.radonc.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Zhai T., Langendijk J., van Dijk L., Halmos G., Witjes M., Oosting S., et al. Prognostic value of CT-based image-biomarkers for treatment outcomes of head and neck cancer patients. Int J Radiat Oncol Biol Phys. 2018 doi: 10.1016/j.oraloncology.2019.06.020. Submitted. [DOI] [PubMed] [Google Scholar]
- 42.Kumar V., Gu Y., Basu S., Berglund A., Eschrich S.A., Schabath M.B., et al. Radiomics: the process and the challenges. Magn Reson Imaging. 2012;30:1234–1248. doi: 10.1016/j.mri.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.