Abstract
SRC family kinases play essential roles in a variety of cellular functions, including proliferation, survival, differentiation, and apoptosis. The activities of these kinases are regulated by intramolecular interactions and by heterologous binding partners that modulate the transition between active and inactive structural conformations. p130CAS (CAS) binds directly to both the SH2 and SH3 domains of c-SRC and therefore has the potential to structurally alter and activate this kinase. In this report, we demonstrate that overexpression of full-length CAS in COS-1 cells induces c-SRC-dependent tyrosine phosphorylation of multiple endogenous cellular proteins. A carboxy-terminal fragment of CAS (CAS-CT), which contains the c-SRC binding site, was sufficient to induce c-SRC-dependent protein tyrosine kinase activity, as measured by tyrosine phosphorylation of cortactin, paxillin, and, to a lesser extent, focal adhesion kinase. A single amino acid substitution located in the binding site for the SRC SH3 domain of CAS-CT disrupted CAS-CT's interaction with c-SRC and inhibited its ability to induce tyrosine phosphorylation of cortactin and paxillin. Murine C3H10T1/2 fibroblasts that expressed elevated levels of tyrosine phosphorylated CAS and c-SRC–CAS complexes exhibited an enhanced ability to form colonies in soft agar and to proliferate in the absence of serum or growth factors. CAS-CT fully substituted for CAS in mediating growth in soft agar but was less effective in promoting serum-independent growth. These data suggest that CAS plays an important role in regulating specific signaling pathways governing cell growth and/or survival, in part through its ability to interact with and modulate the activity of c-SRC.
Homeostasis in multicellular organisms is maintained through the integration of diverse environmental signals for survival, proliferation, differentiation, and apoptosis. These signals are sensed by a variety of cell surface receptors that are coupled to complex networks of cytoplasmic regulatory proteins. SRC family nonreceptor protein tyrosine kinases (PTKs) are important components of many of these signaling networks, including those originating from integrin receptors, receptor PTKs, G-protein-coupled receptors, and cytokine receptors (for reviews, see references 1, 45, 54, 70 and 77). The activities of SRC kinases are tightly regulated and repressed under most circumstances. The importance of this negative regulation is highlighted by the fact that expression of constitutively activated forms of c-SRC results in cellular transformation, characterized by uncontrolled cell proliferation and deregulated cell survival (56, 61).
The unique structure of SRC family kinases allows them to be regulated by substrate availability, as well as by the presence of other interacting proteins (31, 45, 54, 55, 70, 72, 74, 77, 86, 87, 91). Activity is down-modulated by a series of intramolecular interactions that impose conformational constraints on the catalytic domain, making it inaccessible to the substrate. This inactive conformation is established in c-SRC by phosphorylation and subsequent binding of tyrosine 527 to the src homology 2 (SH2) domain and by interaction between the src homology 3 (SH3) domain and a linker region located between the SH2 and kinase domains (74, 86, 91). These intramolecular interactions not only render the protein catalytically inactive but also inhibit other cellular proteins from binding to the SH2 and SH3 domains. Dephosphorylation of tyrosine 527, or engagement of the SH2 and SH3 domains with heterologous proteins, results in enzymatic activation of SRC kinases (56, 61). The association of several proteins with the SH3 and/or SH2 domains of c-SRC has been correlated with increased c-SRC activity. These proteins include focal adhesion kinase (FAK) (76), the tyrosine phosphatase Shp-2 (85), and a member of the CAS family of adapter molecules called Sin (also called Efs) (2). In an analogous fashion, the SRC family member HCK becomes activated by the human immunodeficiency virus protein Nef through a mechanism that involves direct association with the HCK SH3 domain (47).
The adapter protein p130CAS (CAS) may similarly function as a regulator of c-SRC activity. CAS binds to c-SRC through a bipartite binding motif that engages both the SH3 and SH2 domains of SRC (49). Mutational studies suggest that the initial interaction between SRC and CAS occurs between the SH3 domain of SRC and a polyproline motif located in the carboxy terminus of CAS (49). Subsequent phosphorylation of tyrosine 668 then creates a binding site for the SRC SH2 domain. Dual occupancy of both the SH3 and SH2 domains of SRC by CAS may serve to stabilize c-SRC in its active conformation through a mechanism analogous to that of FAK, Shp-2, and Sin. In this way, CAS may function both as a regulator and as a substrate of c-SRC.
In addition to the SRC binding sites, CAS contains several dedicated protein interaction domains (63, 65). The amino-terminal SH3 domain of CAS is capable of interacting with a functionally diverse array of proteins, including FAK (27, 57, 58) and the related kinase PYK2 (41, 80, 89), the guanine nucleotide exchange factor C3G (35), the protein tyrosine phosphatases (PTPases) PTP-1B and PTP-PEST (20, 21, 42), and CMS (for CAS ligand with multiple SH3 domains) (36). The central substrate-binding YXXP domain of CAS contains a stretch of 15 repeats of the amino acid motif YXXP, all of which conform to consensus SH2 domain binding sites. Phosphorylation of the tyrosine residues within this domain results in the recruitment and binding of at least two cellular adapter molecules, Crk and Nck (37, 68, 83). Several other less defined “domains” are also present on CAS, some of which are enriched in proline or serine residues. Although the functions of these regions have not been determined, 14-3-3 has been shown to interact with CAS through a serine-rich domain located adjacent to the substrate-binding YXXP domain (19) and the NSP (for novel SH2-containing protein) family member Chat has been shown to interact with the extreme carboxy terminus (64). The fact that CAS is composed of multiple protein-binding domains that can be modified by tyrosine and perhaps serine phosphorylation suggests that its function may be linked to that of other signaling molecules.
Direct evidence for coordinated functions of SRC and CAS comes from studies focusing on src-mediated transformation. CAS was originally identified in v-src-transformed cells as a tyrosine-phosphorylated protein that associated with v-SRC (33, 59, 60, 63, 65). More recently, it was reported that fibroblasts isolated from CAS−/− mouse embryos were unable to be transformed by activated variants of c-SRC (29), suggesting that CAS is an essential component of the signaling pathways leading to transformation. In a related study, the functional requirement for CAS in src-mediated cellular transformation was addressed by expressing a portion of the carboxy-terminal SRC-binding region of CAS (CAS-CT) in src-transformed Rat 1 fibroblasts (9). Expression of CAS-CT in these cells was shown to effectively inhibit endogenous CAS phosphorylation and v-SRC–CAS interactions, but it had no detectable effect on cellular transformation. A novel v-SRC–CAS-CT complex was formed in place of the v-SRC–CAS complex that is normally present in src-transformed cells, suggesting that the required function of CAS in src transformation may be mediated by the interaction between its carboxy terminus and SRC.
There is considerable, although less direct, evidence that c-SRC and CAS coordinately regulate numerous aspects of cellular behavior. Tyrosine phosphorylation of CAS is induced in response to activation of a variety of cell surface receptors that mediate their effects in part through c-SRC. These include several G-protein-coupled receptors and the receptors for insulin growth factor-1, epidermal growth factor, and platelet-derived growth factor (13, 14, 24, 51, 62, 71, 92). CAS also becomes phosphorylated following integrin engagement to a wide variety of extracellular-matrix (ECM) components, including fibronectin, vitronectin, laminin, and collagen (27, 50, 84). Fibroblast cell lines that are deficient in c-SRC exhibit decreased adhesion-dependent phosphorylation of CAS as well as other focal adhesion proteins (26, 68, 80, 83). Coincident with a lack of CAS phosphorylation, SRC-deficient fibroblasts exhibit marked defects in cell migration (38, 78), similar to the phenotype observed in fibroblasts derived from CAS−/− embryos (28).
While these data demonstrate a strong correlation between the signaling pathways in which c-SRC and CAS participate, the functional significance of the SRC-CAS protein complex remains unclear. We hypothesized that these pathways may be regulated in part through the establishment of a c-SRC–CAS signaling complex and the resultant modulation of c-SRC activity. In this report, we demonstrate that overexpression of c-SRC with either CAS or CAS-CT could induce c-SRC activation and tyrosine phosphorylation of specific c-SRC substrates. Disruption of the interaction between the SH3 domain of SRC and CAS-CT inhibited the ability of CAS-CT to activate c-SRC. Stable association of c-SRC with CAS in cells overexpressing high levels of both of these molecules correlated with the ability of these cells to grow independently of serum and the ECM. CAS-CT fully substituted for CAS in mediating growth in soft agar but was less effective in promoting serum-independent growth. This study thus reveals a novel role for CAS as a regulator of c-SRC and provides evidence that the SRC-CAS protein complex may function to integrate signals emanating from growth factor and adhesion receptors into pathways leading to proliferation and/or cell survival.
MATERIALS AND METHODS
Cell culture and plasmids.
C3H10T1/2-5H murine fibroblasts, which overexpress chicken c-SRC at levels approximately 16-fold over endogenous levels (88), were a generous gift from S. J. Parsons (University of Virginia, Charlottesville). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml), and streptomycin (100 U/ml). pcDNA derivatives encoding wild-type chicken c-SRC (pcDNA-c-SRC) and a kinase-inactive variant of c-SRC containing an alanine-to-valine substitution at residue 430 (pcDNA-c-SRC-KD) were the generous gift of S. J. Parsons (79). pRK5 constructs bearing the genes encoding full-length CAS (CAS-FL) and CAS-CT have been previously described (9).
pcDNA constructs encoding FLAG-tagged paxillin, cortactin, and FAK were generated as follows. The cytomegalovirus-driven expression vector pCDNA3FLAG2AB (18) was used for the generation of all FLAG-tagged SRC substrates. FLAG-cortactin was produced by PCR using the murine cortactin cDNA (46) as the template. A 5′ KpnI restriction site in frame with the initiation codon and an EcoRI restriction site 3′ of the stop codon were used to facilitate subcloning into pCDNA3FLAG2AB. The resultant PCR product was subcloned into pCR-SCRIPT (Stratagene, La Jolla, Calif.), subjected to DNA sequencing to confirm the validity of the cortactin PCR product, and subcloned as a KpnI-EcoRI restriction fragment into pCDNA3FLAG2AB. FLAG-FAK was generated by subcloning the chicken FAK cDNA (67) from pCMV-c-myc FAK (90) into pCDNA3FLAG2AB using the BamHI and NotI sites in the multiple-cloning site of the FLAG expression vector. For FLAG-paxillin, PCR amplification was used to introduce a BamHI site in frame with the chicken paxillin coding sequence (66) and an EcoRI site 3′ of the stop codon. The PCR product was digested with BamHI and EcoRI and ligated into pBluescript (KS+; Stratagene). The paxillin fragment was subsequently cloned into pcDNA3FLAG2AB using the BamHI and XhoI sites in the multiple-cloning site of the FLAG vector.
Construction of CAS variants.
Myc epitope-tagged CAS variants consisting of the SH3 domain (amino acids 6 to 64), the YXXP region (amino acids 117 to 418), and the carboxy terminus (amino acids 544 to 874) were derived using PCR to synthesize cDNAs, which were then cloned in frame into pRK5-myc (52) to generate the expression plasmids pRK5-CAS-SH3, pRK5-CAS-YXXP, and pRK5-CAS-CT, respectively. The single point mutation substituting an alanine residue for proline residue 642 (P642A) was generated in the context of CAS-FL using the Altered Sites in vitro mutagenesis system (Promega, Madison, Wis.). A PCR fragment corresponding to amino acid residues 544 to 874 of CASP642A was then generated using primers that contained appropriate restriction sites and cloned into pRK5, to yield pRK5-CAS-CTP642A. The presence of this mutation and all PCR-derived DNA sequences were confirmed by automated DNA sequencing.
The deletion variants of CAS were generated as follows. dlSH3 (deletion of amino acid residues 1 to 64) was engineered by synthesizing a PCR product using a 5′ primer that contained a unique BamHI restriction site just upstream from the codon for amino acid residue 65 and a specific 3′ primer identical to sequences beyond the unique NheI restriction site of the CAS cDNA. The PCR product was digested with BamHI and NheI and ligated into pRK5-CAS-FL in place of the analogous wild-type restriction fragment. The dlYXXP construct (deletion of amino acid residues 119 to 421) was generated by ligating together a PCR product encoding the first 118 amino acids of CAS with a PCR product encoding amino acids 422 to 780, which was then digested with BamHI and BglII. This PCR product was engineered into pRK5myc (52) by replacing the resident BamHI-BglII restriction fragment of pRK5myc-CAS-FL. As a consequence of these manipulations, a novel SalI restriction site was engineered at the position of the deletion, resulting in the insertion of an aspartic acid residue at the splice junction. Deletion of the carboxy terminus of CAS to produce dlCT (deletion of amino acid residues 546 to 874) was accomplished by digesting pRK5-CAS-FL with HindIII, which cut once at a site 3′ of codon 545 and again in the multiple-cloning site of pRK5 downstream of the CAS cDNA. The plasmid was then religated to produce pRK5-dlCT. All PCR-generated sequences were confirmed by DNA sequencing as described above.
Antibodies.
Polyclonal CAS-B and CAS-F antisera have been previously described (6). Anti-SRC monoclonal antibody (MAb) EC10 was kindly provided by S. J. Parsons, and anti-SRC MAb 2-17 was purchased from Quality Biotech Inc. (Camden, N.J.). Anti-phosphotyrosine (anti-pTyr) MAb 4G10 was purchased from Upstate Biotech Inc. (Lake Placid, N.Y.). Anti-cortactin MAb 4F11 (32) was kindly provided by J. T. Parsons. Anti-Myc MAb 9E10 and anti-FLAG MAb M5 were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.) and Sigma Chemicals (St. Louis, Mo.) respectively. Anti-FLAG M2 affinity gel was purchased from Sigma Chemicals.
Protein expression.
Transient transfections in COS-1 cells were performed using Superfect (Qiagen, Valencia, Calif.) as specified by the manufacturer. For analysis of c-SRC activation by CAS, cells were transfected with 5 μg of pRK5-CAS-FL and 5 μg of pcDNA-c-SRC or pcDNA-c-SRC-KD. When necessary, the total amount of DNA used was brought up to 10 μg with parental pRK5 or pcDNA vector DNA. For analysis of the CAS variants, 5 μg of pRK5-dlSH3, 10 μg of pRK5-dlYXXP, 10 μg of pRK5-dlCT, 10 μg of pRK5-CAS-SH3, 10 μg of pRK5-CAS-YXXP, or 10 μg of pRK5-CAS-CT was cotransfected with 5 μg of pcDNA-c-SRC. When necessary, pRK5 vector DNA was used to bring the total amount of transfected DNA to 15 μg. Triple transfections in COS-1 cells were performed using 1 μg of pcDNA-c-SRC; 5 μg of pcDNAFLAG2AB-paxillin, -cortactin, or -FAK; and 5 μg of either pRK5-CAS-CT or pRK5-CAS-CTP642A. Transient transfections into C3H10T1/2-5H cells were performed using Lipofectamine Plus (Life Technologies, Rockville, Md.) as specified by the manufacturer. Cells were transfected with 2.5 μg of pcDNA3FLAG2AB-paxillin, -cortactin, or -FAK and 2.5 μg of either pRK5-CAS-CT or pRK5-CAS-CTP642A. Total DNA was brought up to 5 μg with vector DNA when necessary.
In order to isolate protein, cells were lysed 24 h (COS-1) or 48 h (C3H10T1/2-5H) posttransfection in modified radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 50 mM Tris-HCl [pH 7.5], 1% NP-40, 0.5% sodium deoxycholate) containing protease and phosphatase inhibitors (100 mM leupeptin, 1 mM phenylmethylsulfonyl fluoride, 0.15 U of aprotinin per ml, 1 mM sodium vanadate) as previously described (10). Protein concentrations were determined using the bicinchoninic acid assay (Pierce, Rockford, Ill.).
Generation of cell lines expressing CAS and CAS-CT.
In order to generate C3H10T1/2-5H murine fibroblast cell lines that overexpressed either CAS-FL or CAS-CT in the context of overexpressed c-SRC, pRK5, pRK5-CAS-FL, or pRK5-CAS-CT was cotransfected with pBABEpuro (48) at a 4:1 molar ratio using Lipofectamine Plus according to the manufacturer's specifications. Cells were split into DMEM supplemented with 5 μg of puromycin (Sigma Chemicals) per ml 24 h following transfection. Puromycin-resistant colonies were expanded and screened for expression of CAS-FL, CAS-CT, and c-SRC by immunoblot analysis.
Immunoprecipitation and immunoblotting.
For immunoprecipitation experiments, cell extracts were incubated on ice with the indicated antibodies for 1 to 2 h (EC10, 4F11) or overnight at 4°C (9E10), and immune complexes were recovered by incubation for 1 h with protein A-Sepharose (Amersham Pharmacia Biotech, Piscataway, N.J.) that had been preincubated with rabbit anti-mouse immunoglobulin (Jackson ImmunoResearch, West Grove, Pa.). For immunoprecipitation of FLAG-epitope-tagged proteins, lysates were incubated for 1 h at 4°C with M2 resin (25 μl/mg of protein). Recovered immune complexes were washed two times in modified RIPA buffer and two times in ice-cold phosphate-buffered saline (PBS), resuspended in Laemmli sample buffer (40), and boiled for 5 min. For immunoblotting, proteins were resolved by sodium dodecyl sulfate–8% polyacrylamide gel electrophoresis (SDS–8% PAGE), transferred to nitrocellulose, and probed with the antibodies indicated on each figure. Anti-pTyr MAb 4G10 was detected using 125I-anti-mouse immunoglobulin (NEN Life Science Products, Boston, Mass.); all other primary antibodies were detected with horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin (Amersham Pharmacia Biotech), followed by enhanced chemiluminescence.
BrdU incorporation assay.
Cells were seeded onto fibronectin-coated coverslips in six-well tissue culture dishes at subconfluent density (105 cells per well) as described previously (9). Cells were allowed to adhere in serum-containing medium for 2 h and then washed twice with PBS before the addition of serum-free DMEM. 5-Bromo-2′deoxy-uridine (BrdU; 0.1 mM) was added directly to the medium at 16, 40, and 64 h following plating, and cells were incubated for an additional 8 h. Coverslips were washed in PBS, incubated in 100% methanol for 10 min at 4°C and then in 2 M HCl for 1 h at 37°C, and neutralized by two 5-min incubations in borate buffer (0.1 M borate, pH 8.5) for 5 min each time at room temperature. Cells were then incubated with fluorescein-conjugated anti-BrdU antibodies (Chemicon International, Inc., Temecula, Calif.) (2 μg/ml) for 45 min at room temperature. The percentage of cells incorporating BrdU was determined by immunofluorescence microscopy.
Colony formation assay.
Colony formation assays were performed as previously described (9). Briefly, 105 cells were resuspended in DMEM containing 10% FCS, 16% 2× Ham's F-10 medium (Sigma Chemicals), and 16% agar (FMC BioProducts, Rockland, Maine) and plated in triplicate on a bottom agar layer consisting of DMEM, 10% FCS, 33% Ham's F-10 medium, and 33% agar. Colonies were visualized after 14 days by adding p-iodonitrotetrazolium violet (1 mg/ml) for 24 h and counted using EagleSight software (Stratagene). Gating parameters for each of three independent experiments were determined based on colonies obtained from vector control cells (5H-RK5).
RESULTS
Coexpression of CAS and c-SRC leads to increased PTK activity.
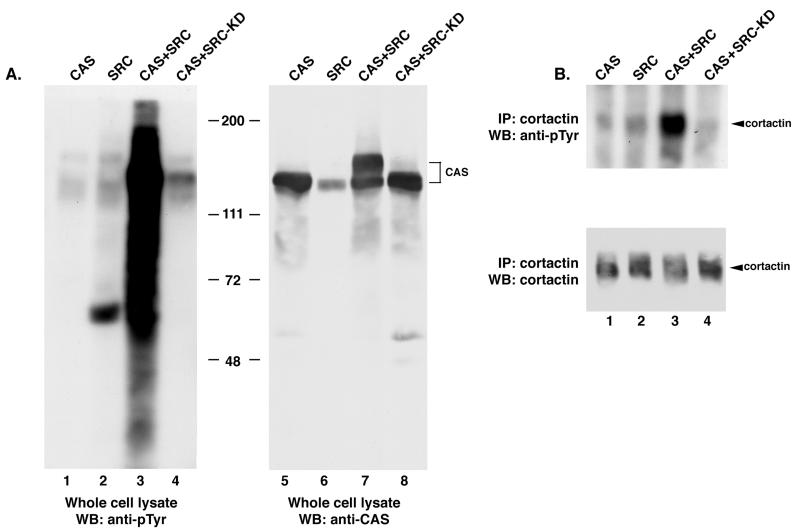
To determine whether c-SRC activity could be modulated by CAS expression, plasmids bearing the genes encoding c-SRC (pcDNA-c-SRC) and/or full-length CAS (pRK5-CAS-FL) were cotransfected into COS-1 cells. Cell lysates were prepared 24 h posttransfection and examined by Western blot analysis for the presence of tyrosine-phosphorylated proteins. Cells overexpressing either CAS or c-SRC alone contained a limited number of proteins that were reactive with anti-pTyr antibodies (Fig. 1A, lanes 1 and 2). These were identical to the pTyr-containing proteins present in COS-1 cells transfected with the corresponding parental plasmids (pRK5 or pcDNA [data not shown]). In contrast, coexpression of both CAS and c-SRC resulted in a significant qualitative and quantitative increase in pTyr reactivity (lane 3). This effect was dependent on SRC kinase activity, as coexpression of a kinase-inactive mutant of c-SRC (SRC-KD) with CAS failed to induce increased levels of pTyr in these cells (lane 4). Thus, even though CAS was expressed well above endogenous levels in each of the transfections (compare lanes 5, 7, and 8 with lane 6), the induction of tyrosine phosphorylation was absolutely dependent on coexpression of c-SRC and on c-SRC kinase activity.
FIG. 1.
Tyrosine phosphorylation of cellular proteins in COS-1 cells coexpressing CAS and c-SRC. COS-1 cells were transfected with a construct encoding CAS (lanes 1 and 5), SRC (lanes 2 and 6), both SRC and CAS (lanes 3 and 7), or a catalytically inactive SRC variant (SRC-KD) together with CAS (lanes 4 and 8). (A) Expression of CAS and catalytically active c-SRC induces PTK activity. Total cell protein (25 μg) was resolved by SDS–8% PAGE, transferred to nitrocellulose, and immunoblotted with anti-pTyr MAb 4G10 (lanes 1 to 4) or anti-CAS-F antiserum (lanes 5 to 8). (B) Expression of CAS and c-SRC induces tyrosine phosphorylation of cortactin. Cell lysate (350 μg) was incubated with the anti-cortactin MAb 4F11. The collected immune complexes were divided into two equal parts, separated by SDS–8% PAGE, and immunoblotted with either MAb 4G10 to determine pTyr levels (top blot) or MAb 4F11 to determine the amount of cortactin present in each immune complex (bottom blot). WB, Western blotting; IP, immunoprecipitation.
The intense staining with anti-pTyr antibodies in the 120- to 130-kDa region of the pTyr immunoblot (Fig. 1A, lane 3), as well as the appearance of an additional slower-migrating species of CAS in the CAS immunoblot (lane 7), indicated that CAS was highly tyrosine phosphorylated in cells coexpressing c-SRC and CAS. To determine whether overexpression of c-SRC and CAS resulted in phosphorylation of substrates other than CAS, the SRC substrate cortactin was selectively immunoprecipitated from COS-1 cell extracts generated 24 h posttransfection. Immune complexes were then immunoblotted with pTyr and cortactin antibodies (Fig. 1B). Coexpression of full-length CAS with c-SRC resulted in significantly elevated levels of tyrosine-phosphorylated cortactin relative to those observed when CAS or c-SRC was expressed alone (in upper blot in Fig. 1B, compare lane 3 with lanes 1 and 2). This increase in cortactin phosphorylation was dependent on c-SRC kinase activity, as coexpression of c-SRC-KD with CAS failed to induce this effect (lane 4). Immunoblot analysis of the immune complexes confirmed that equal amounts of cortactin were present (lower blot). Thus, coexpression of CAS and c-SRC in COS-1 cells resulted in elevated tyrosine phosphorylation of several cellular proteins, including at least two established substrates of c-SRC, CAS and cortactin.
The carboxy-terminal region of CAS is sufficient to induce c-SRC-dependent PTK activity.
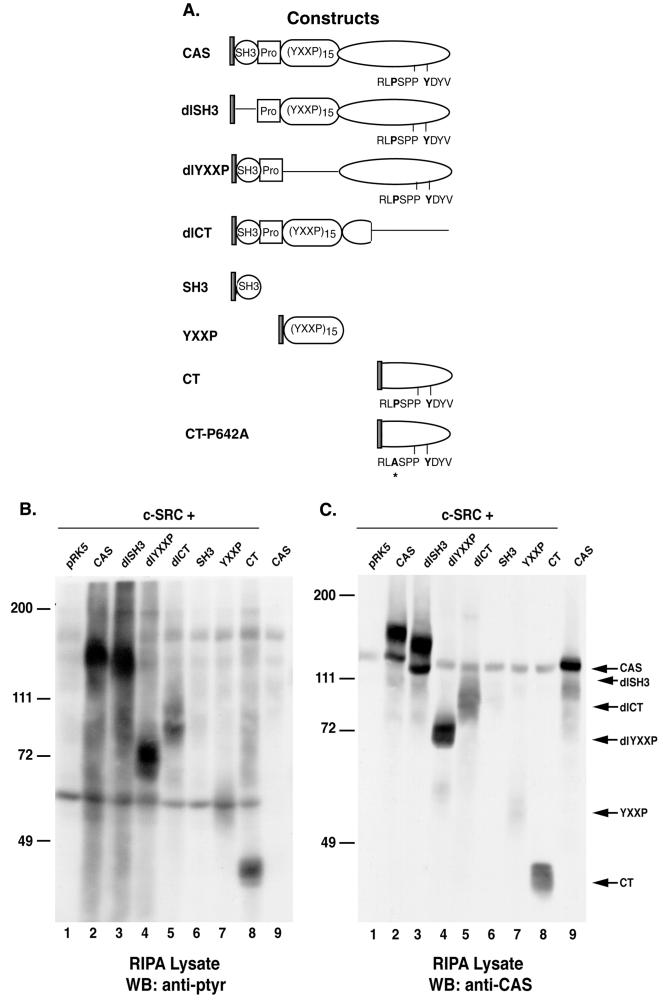
As indicated in Fig. 1A, CAS can serve as an inducer of c-SRC kinase activity. To determine the structural features of CAS that were required for the induction of PTK activity, a panel of CAS variants (Fig. 2A) was coexpressed with c-SRC in COS-1 cells. Cellular proteins from lysates obtained 24 h posttransfection were examined by immunoblotting with anti-pTyr antibodies (Fig. 2B). Deletion of the SH3 domain (dlSH3) did not significantly alter the induction of PTK activity by CAS, as determined by overall cellular levels of tyrosine phosphorylation (Fig. 2B, compare lanes 2 and 3). In contrast, deletion of the substrate-binding domain (dlYXXP) appeared to reduce the ability of CAS to induce PTK activity and deletion of the carboxy-terminal region (dlCT) had an even greater effect (compare lane 2 with lanes 4 and 5). Immunoblot analysis using anti-CAS antibodies indicated that all of the CAS deletion variants were expressed at equivalent levels with the exception of dlCT, whose expression was approximately 50% less than that of the other CAS variants (Fig. 2C). The most prominent tyrosine-phosphorylated protein in each cell extract corresponded to the CAS construct that was coexpressed with c-SRC (Fig. 2B). Interestingly, dlYXXP appeared to be efficiently phosphorylated, despite the fact that the majority of potential phosphorylation sites were deleted in this molecule (lane 4).
FIG. 2.
Induction of PTK activity by coexpression of c-SRC and CAS variants. (A) Schematic representation of Myc-tagged CAS and CAS variants. The circle represents the SH3 domain of CAS. The square represents a proline-rich region, and the oval designated (YXXP)15 represents the substrate-binding YXXP domain. The elongated oval represents the carboxy-terminal domain of CAS. RPLP642SPP and Y668DYV are the single-letter symbols for the amino acid residues contributing to the SRC binding sites located in the carboxy terminus of CAS (49). Single black lines indicate regions of the CAS protein that were deleted in each CAS variant. The asterisk indicates the site of the proline-to-alanine substitution in CAS-CTP642A. (B and C) Protein expression and phosphorylation in COS-1 cells coexpressing c-SRC and the indicated CAS variants. Total cell protein (25 μg) was resolved by SDS–8% PAGE, transferred to nitrocellulose, and immunoblotted either with anti-pTyr MAb 4G10 (B) or a mixture of polyclonal CAS-B and CAS-F antisera (C). WB, Western blot.
To determine whether sequences within individual protein-binding domains of CAS were sufficient to induce PTK activity, isolated domains of CAS were coexpressed with c-SRC in COS-1 cells. Expression of the isolated SH3 or YXXP domains had little effect on c-SRC-dependent tyrosine phosphorylation of cellular proteins (Fig. 2B, lanes 6 and 7). In contrast, coexpression of CAS-CT and c-SRC resulted in a pattern of pTyr-containing cellular proteins that was qualitatively similar to that seen in cells expressing CAS-FL, albeit with a reduced magnitude (Fig. 2B, compare lane 8 with lane 2). Since the level of expression of the YXXP domain was routinely lower than that of the CT domain (Fig. 2C, compare lanes 7 and 8), it was difficult to fully assess the activity of the isolated YXXP domain relative to that of CAS-CT. Nevertheless, these data suggest that the carboxy terminus of CAS plays a key role in the induction of PTK activity by CAS.
Expression of CAS-CT is sufficient to induce tyrosine phosphorylation of multiple c-SRC substrates.
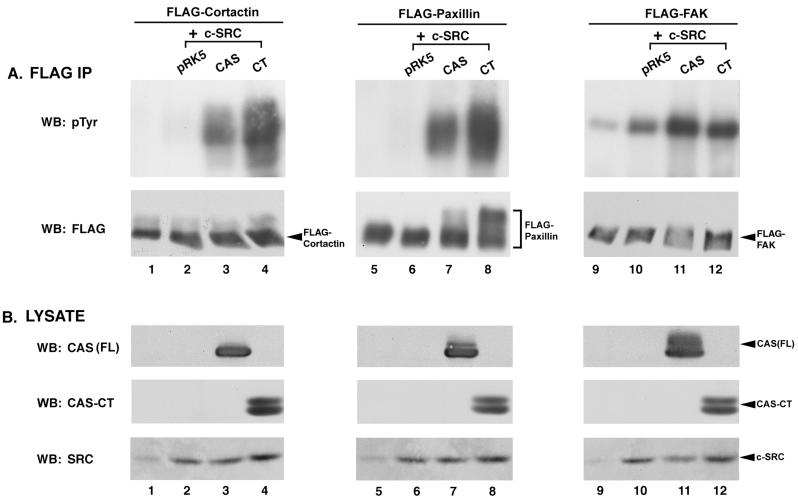
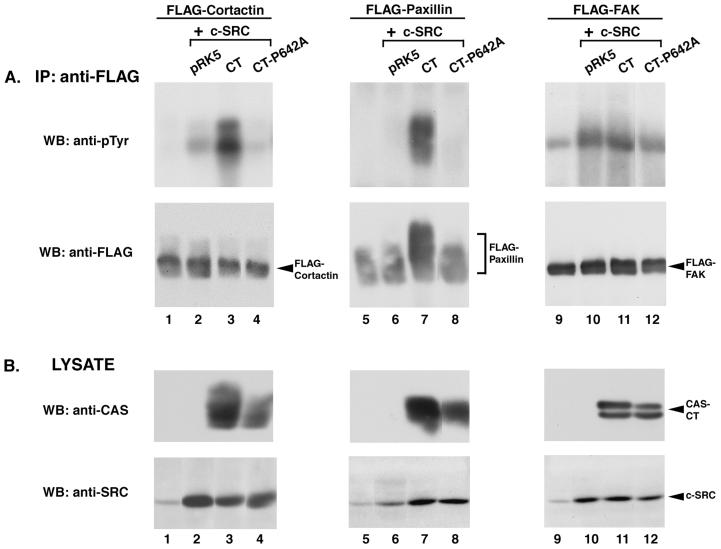
To further characterize the induction of PTK activity by CAS and CAS-CT, we performed triple transfections that resulted in the coexpression of c-SRC, one of three FLAG epitope-tagged substrates, and either CAS-FL or CAS-CT. Each FLAG-tagged substrate was then selectively immunoprecipitated from cell lysates using an anti-FLAG MAb and analyzed by immunoblotting with anti-pTyr antibodies. Coexpression of FLAG-cortactin with c-SRC alone resulted in a modest induction of FLAG-cortactin phosphorylation to a level above that seen in the absence of c-SRC (Fig. 3A, lanes 1 and 2). However, tyrosine phosphorylation of FLAG-cortactin was significantly elevated in cells coexpressing CAS-FL and c-SRC, and the level of phosphorylation was even greater in cells coexpressing CAS-CT (lanes 3 and 4). Similarly, tyrosine phosphorylation of FLAG-paxillin was dramatically increased in cells coexpressing c-SRC and CAS, and CAS-CT appeared to induce phosphorylation better than CAS (lanes 7 and 8). Immunoblot analysis confirmed that equivalent levels of FLAG-cortactin and FLAG-paxillin were present in the immune complexes (Fig. 3A, lanes 1 to 8, bottom blots), that CAS and CAS-CT were expressed at roughly equivalent levels (Fig. 3B, lanes 1 to 8, top and middle blots), and that c-SRC was expressed appropriately (Fig. 3B, lanes 1 to 8, bottom blots).
FIG. 3.
CAS and CAS-CT modulate c-SRC PTK activity toward specific SRC substrates. (A) Immunoblot analysis of FLAG immune complexes. One milligram of lysate from cells expressing the indicated proteins was incubated with anti-FLAG MAb M2-conjugated resin, and the collected immune complexes were divided into two equal parts. One half was immunoblotted with MAb 4G10 to determine pTyr levels of the indicated FLAG-tagged constructs (top blots), while the other half was immunoblotted with the anti-FLAG MAb M5 to verify that equal amounts of protein were present in the immune complexes (bottom blots). (B) Verification of recombinant protein expression. Total cell lysate (50 μg) from the indicated cells was separated by SDS–8% PAGE and immunoblotted with either CAS-F antiserum to determine expression levels of CAS-FL and CAS-CT (top blots) or the anti-SRC MAb 2-17 to determine levels of expression of c-SRC (bottom blots). IP, immunoprecipitation; WB, Western blotting.
A third substrate of c-SRC, FAK, was also examined in this assay. Although the autophosphorylation site is the major tyrosine phosphorylation site on FAK, several additional phosphorylation sites have been shown to be dependent on c-SRC activity (23, 69, 82). FLAG-FAK was found to contain detectable levels of pTyr when it was expressed in COS-1 cells, and its phosphorylation was significantly enhanced by c-SRC overexpression (Fig. 3A, lanes 9 and 10). Coexpression of CAS-FL with c-SRC had a modest additional effect on FLAG-FAK phosphorylation, and CAS-CT had a lesser effect (lanes 11 and 12). Thus, in contrast to what was observed for FLAG-cortactin and FLAG-paxillin, phosphorylation of FLAG-FAK was increased in cells expressing c-SRC alone and coexpression of CAS or CAS-CT had only a modest supplementary effect.
The association of c-SRC with CAS is required for modulation of PTK activity.
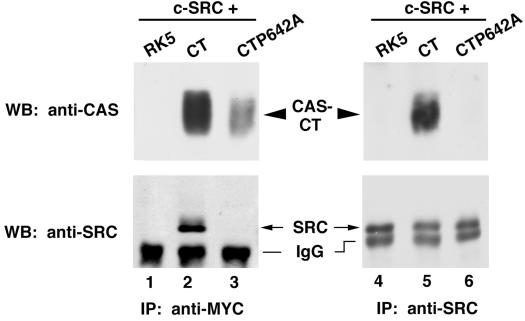
The previous experiments indicated that the carboxy terminus of CAS, which contains a bipartite binding site for SRC (49), has the necessary sequences to induce c-SRC-dependent kinase activity. To investigate the mechanism of PTK activation by CAS-CT, a single amino acid substitution (proline 642 to alanine) was engineered at the site on CAS-CT that interacts with the SRC SH3 domain (49). Wild-type (CAS-CT) and mutant (CAS-CTP642A) proteins were coexpressed with c-SRC in COS-1 cells to determine the effect of this mutation on the ability of c-SRC and CAS-CT to associate. As seen in Fig. 4, c-SRC was readily detected in CAS-CT immune complexes generated using an antibody that recognized the Myc epitope located at the amino terminus of the CAS constructs (lane 2, bottom blot). CAS-CT was also readily detected in c-SRC immune complexes (lane 5, top blot). In contrast, c-SRC and CAS-CTP642A were not detected in reciprocal immune complexes (lanes 3 and 6). Although less CAS-CTP642A was recovered in the Myc immune complexes than wild-type CAS-CT (compare lanes 2 and 3, top blot), extended exposure of the immunoblots still showed no evidence of an interaction between c-SRC and CAS-CTP642A (data not shown). Thus, substitution of an alanine for proline 642 in the SRC SH3-binding site of CAS-CT inhibited its interaction with c-SRC.
FIG. 4.
Inhibition of the association between CAS-CT and c-SRC by a single amino acid substitution of CAS, proline642 to alanine (P642A). Cell lysate (500 μg) was incubated with either the anti-Myc MAb 9E10 (lanes 1 to 3) or the anti-SRC MAb EC10 (lanes 4 to 6), and the collected immune complexes were divided into two equal parts. Proteins were separated by SDS–8% PAGE and immunoblotted using either CAS-F antiserum (top blot) or the SRC MAb 2-17 (bottom blots). IP, immunoprecipitation; WB, Western blotting; IgG, immunoglobulin G.
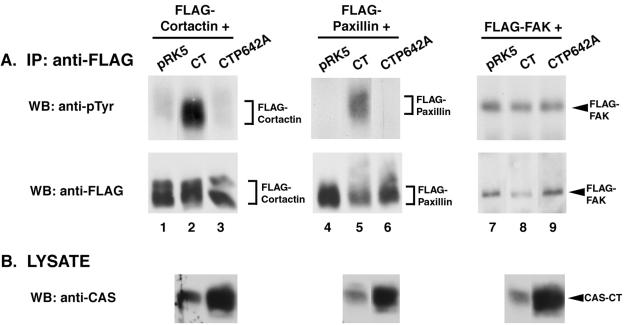
To determine the effect of disrupting the association between CAS-CT and c-SRC on the ability of CAS-CT to induce PTK activity, a triple-transfection procedure was again used to examine tyrosine phosphorylation of FLAG-tagged cortactin, paxillin, and FAK (Fig. 5). In contrast to what was observed in the case of expression of wild-type CAS-CT, coexpression of CAS-CTP642A with c-SRC failed to induce significant levels of tyrosine phosphorylation of FLAG-cortactin or FLAG-paxillin (Fig. 5A, compare lanes 3 and 4 and lanes 7 and 8). Similar amounts of FLAG-cortactin and FLAG-paxillin were present in each immune complex (Fig. 5A, lanes 1 to 8, lower blots), and c-SRC and CAS-CT or CAS-CTP642A were expressed appropriately (Fig. 5B, lanes 1 to 8). The shift in the apparent molecular weight of paxillin that was seen in the FLAG immunoblot was indicative of an increase in the pTyr content of paxillin (Fig. 5A, lane 7, bottom blot). Thus, the direct association of CAS-CT and c-SRC appeared to be required for the phosphorylation of FLAG-cortactin and FLAG-paxillin. Similar experiments using FLAG-FAK as a substrate revealed that expression of CAS-CT did not significantly enhance the level of pTyr above that induced by c-SRC alone, in agreement with the data presented in Fig. 3 (Fig. 5A, lanes 10 and 11, upper blot). c-SRC-dependent FAK phosphorylation was also not enhanced in cells expressing CAS-CTP642A (lane 12). Therefore, in contrast to FLAG-cortactin and FLAG-paxillin, the mechanism by which c-SRC phosphorylates FLAG-FAK appears to be independent of CAS.
FIG. 5.
Tyrosine phosphorylation of SRC substrates requires the SRC-binding site of CAS-CT. (A) Immunoblot analysis of FLAG immune complexes. One milligram of lysate from cells expressing the indicated proteins was incubated with anti-FLAG MAb M2-conjugated resin, and the collected immune complexes were divided into two equal parts. One half was immunoblotted with MAb 4G10 to determine pTyr levels of the indicated FLAG-tagged constructs (top blots), while the other half was immunoblotted with the anti-FLAG MAb M5 to verify that equal amounts of protein were present in the immune complexes (bottom blots). (B) Verification of recombinant protein expression. Fifty micrograms of total cell lysate from the indicated cells was separated by SDS–8% PAGE and immunoblotted with either CAS-F antiserum to determine expression levels of CAS-CT and CAS-CTP642A (top blots) or anti-SRC MAb 2-17 to determine levels of expression of c-SRC (bottom blots). IP, immunoprecipitation; WB, Western blotting.
PTK activity can be modulated by CAS-CT in the context of stable overexpression of c-SRC.
A number of factors can influence substrate specificity, including differences in substrate availability, substrate function, and cellular context. Consequently, it was important to verify that the previous findings were not unique to transient overexpression of c-SRC in COS-1 cells. To determine whether CAS-CT expression could modulate c-SRC activity in a second cell system, CAS-CT or CAS-CTP642A was cotransfected with one of the FLAG-tagged SRC substrates into C3H10T1/2-5H murine fibroblasts (88). This cell line stably overexpresses chicken c-SRC approximately 16-fold over endogenous levels. FLAG immune complexes were isolated from cell lysates 48 h posttransfection and examined for pTyr content. As seen in Fig. 6, FLAG-cortactin and FLAG-paxillin expressed in the absence of CAS-CT were not significantly tyrosine phosphorylated in C3H10T1/2-5H cells, even though c-SRC was overexpressed in these cells (Fig. 6A, lanes 1 and 4). Coexpression of CAS-CT induced a significant increase in the tyrosine phosphorylation of both FLAG-cortactin and FLAG-paxillin (Fig. 6A, lanes 2 and 5), but expression of the SRC-binding mutant CAS-CTP642A had no such effect (lanes 3 and 6). Examination of anti-FLAG immune complexes from these cells confirmed that roughly equal amounts of protein were present (Fig. 6A, lanes 1 to 6, lower blots), and immunoblot analysis confirmed expression of CAS-CT and CAS-CTP642A (Fig. 6B). Interestingly, expression of CAS-CT was routinely lower than that of CAS-CTP642A, which makes the induced phosphorylation of FLAG-cortactin and FLAG-paxillin in these cells even more striking. In contrast to what was observed for cortactin and paxillin, tyrosine phosphorylation of FLAG-FAK in C3H10T1/2-5H cells was unaffected by expression of CAS-CT or CAS-CTP642A (Fig. 6A, lanes 7 to 9). Thus, CAS-CT-induced PTK activity in the C3H10T1/2-5H cells showed a substrate specificity similar to that observed in COS-1 cells. These results prompted us to develop the C3H10T1/2-5H cells into a model system with which to address the role of the c-SRC–CAS complex in the regulation of cell growth and survival (see below).
FIG. 6.
Modulation of c-SRC activity by CAS-CT in C3H10T1/2-5H cells. (A) Immunoblot analysis of FLAG immune complexes. Total cell lysate (1 mg) was incubated with anti-FLAG MAb M2-conjugated resin to specifically immunoprecipitate the indicated FLAG-tagged constructs. Collected immune complexes were divided into two equal parts and immunoblotted using either the anti-pTyr MAb 4G10 (top blots) or the anti-FLAG MAb M5 to verify that equal amounts of FLAG-tagged protein were present in the immune complexes (bottom blots). (B) Verification of recombinant protein expression. Total cell protein (50 μg) from the indicated cells was separated by SDS–8% PAGE and immunoblotted with CAS-F antiserum. IP, immunoprecipitation; WB, Western blotting.
Enhanced activation of PTK activity by coexpression of CAS and c-SRC results in alterations in growth-regulatory pathways.
To assess whether the coordinated overexpression of both CAS and c-SRC had an effect on growth-regulatory pathways in C3H10T1/2-5H cells, we generated independent cell lines that expressed various levels of the vector (5H-pRK5), CAS-FL (5H-CAS), or CAS-CT (5H-CT). Cell lines expressing equal amounts of overexpressed c-SRC (Fig. 7A, bottom blot) in the presence of differing levels of CAS or CAS-CT (top and middle blots) were chosen for further study in order to demonstrate possible dose-dependent effects on cell growth.
FIG. 7.
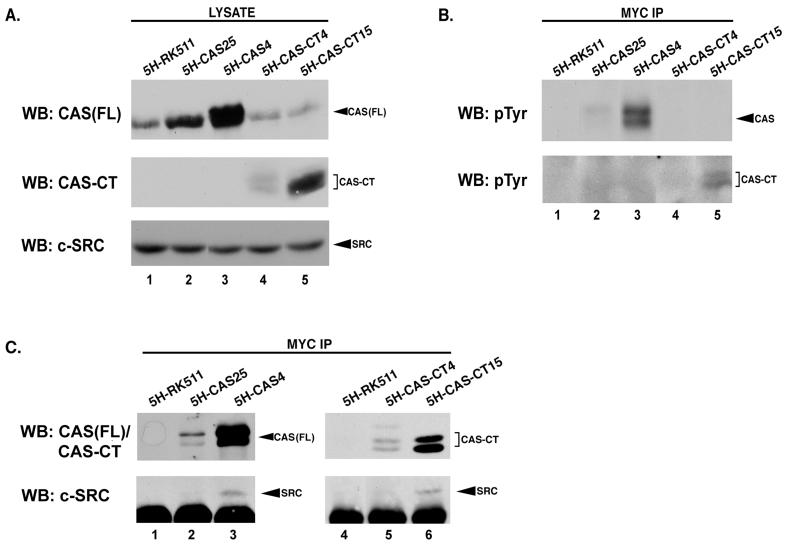
Association of c-SRC with CAS or CAS-CT in C3H10T1/2-5H clonal cell lines. (A) Protein expression in C3H10T1/2-5H stable cell lines. Total cell protein (50 μg) derived from representative C3H10T1/2-5H stable cell lines was resolved by SDS–8% PAGE, transferred to nitrocellulose, and immunoblotted using either CAS-F antiserum to determine relative levels of CAS or CAS-CT present in each cell line (top and middle blots) or anti-SRC MAb 2-17 to determine the amount of SRC in each cell line (bottom blot). The top two blots represent identical exposures of different regions of the same gel, allowing expression levels of CAS and CAS-CT to be directly compared. (B and C) CAS and CAS-CT are tyrosine phosphorylated and associate with c-SRC in cells expressing high levels of these proteins. Proteins from 1 mg of the indicated cell lysate were incubated with anti-Myc MAb 9E10 to selectively immunoprecipitate Myc-tagged CAS-FL or CAS-CT. Immune complexes were divided in half, resolved by SDS–8% PAGE, transferred to nitrocellulose, and immunoblotted with anti-pTyr MAb 4G10 (B), CAS-F antiserum (C, top blots), or anti-SRC MAb 2-17 (C, bottom blots). IP, immunoprecipitation; WB, Western blotting.
Based on analogy with the transient-expression experiments described above, we predicted that CAS and CAS-CT would be tyrosine phosphorylated and in association with c-SRC in the stable C3H10T1/2-5H clones. To determine whether this was the case, cell extracts were subjected to immunoprecipitation with Myc MAb 9E10 to selectively isolate the ectopic CAS constructs and the immune complexes were examined by immunoblotting to assess pTyr and c-SRC contents (Fig. 7B and C). In cells that expressed high levels of CAS and CAS-CT, both of these molecules were found to be tyrosine phosphorylated (Fig. 7B, lanes 3 and 5) and in association with c-SRC (Fig. 7C, bottom blot, lanes 3 and 6). Tyrosine phosphorylation and association with c-SRC were not evident when these molecules were expressed at lower levels (Fig. 7B, lanes 2 and 4, and 7C, lanes 2 and 5). Immunoblot analysis with CAS antibodies confirmed the presence of each CAS construct in the Myc immune complexes (Fig. 7C, top blot). These data suggest that the ratio of CAS to c-SRC may be a critical factor in determining phosphorylation and SRC-CAS complex formation, since the variability in CAS and CAS-CT expression existed within a background of equivalent levels of c-SRC (Fig. 7A, lower blot).
We hypothesized that the growth-regulatory phenotype of cells expressing different levels of CAS and CAS-CT might also be sensitive to the SRC/CAS ratio, since phosphorylation and complex formation occurred only in the presence of high CAS or CAS-CT expression. This hypothesis was addressed in a series of experiments that examined the dependence of each cell line on growth and survival signals emanating from serum and the ECM. In order to determine whether the presence of c-SRC–CAS and/or c-SRC–CAS-CT complexes in C3H10T1/2-5H cells resulted in serum-independent growth, serum was removed from actively proliferating cell monolayers and the number of cells incorporating BrdU was determined 24, 48, and 72 h later (Fig. 8A). By 24 h in serum-free medium, approximately 38% of vector control cells incorporated BrdU, and this level steadily decreased over the remaining course of the experiment until approximately 15% of the cells incorporated BrdU at 72 h. A statistically greater number of cells expressing high levels of c-SRC and either CAS-FL (5H-CAS4) or CAS-CT (5H-CT15) incorporated BrdU after 24 h in serum-free medium (66 and 62%, respectively). The high-level CAS expressors continued to show enhanced BrdU uptake in the absence of serum, as measured by a sustained statistical increase in the number of cells incorporating BrdU over the number in vector control cells at 48 and 72 h. Cells expressing high levels of CAS-CT behaved more like vector control cells at these later time points. The two cell lines that expressed lower levels of ectopic CAS (5H-CAS25) or CAS-CT (5H-CT4) and that did not exhibit detectable increases in CAS phosphorylation or enhanced c-SRC–CAS interactions were similar to vector control cells throughout the course of the experiment. Thus, serum-independent growth of C3H10T1/2-5H fibroblasts expressing c-SRC and CAS correlated with a high level of CAS expression, CAS phosphorylation, and the presence of c-SRC–CAS complexes. Expression of CAS-CT at levels sufficient to form stable c-SRC–CAS-CT complexes conferred a measure of serum-independent growth during the first 24 hours in serum-free medium, but it did not have a prolonged effect on the ability of C3H10T1/2-5H cells to grow in the absence of serum.
FIG. 8.
Biological activities of the c-SRC–CAS complex. (A) Serum-independent growth. The percentages of cells exhibiting nuclear staining of BrdU at 24, 48, and 72 h after serum withdrawal are presented for the indicated cell lines. The results for 5H-RK5 are representative of the combined results for two vector control cell lines. Values are averages (± standard deviations) of results of four independent experiments, and asterisks indicate a P value of ≤0.01. (B) Anchorage-independent growth. Colony number per 105 cells plated in soft agar is presented for each of the designated cell lines. Colonies were counted using EagleSight software, and the results presented are the averages of results of three independent experiments.
To determine whether the c-SRC–CAS complex may be involved in modulating growth signals derived from the ECM, anchorage-dependent growth was analyzed by a colony formation assay (Fig. 8B). Cell lines expressing high levels of CAS or CAS-CT (5H-CAS4 or 5H-CT15) in the context of overexpressed c-SRC exhibited a dramatic increase in their ability to form colonies in soft agar, compared to that of vector control cells that overexpressed only c-SRC (5H-RK5). The efficiency of colony formation increased approximately 10-fold in the 5H-CAS4 cell line and 18-fold in the 5H-CT15 cell line. The ability to grow in soft agar correlated with CAS or CAS-CT tyrosine phosphorylation and the presence of stable c-SRC–CAS or c-SRC–CAS-CT complexes. Clones that contained lower levels of CAS or CAS-CT (5H-CAS25 or 5H-CT4) did not demonstrate enhanced growth in soft agar compared with that seen in control cells. In these cells, the CAS constructs were not phosphorylated to high levels and they were not found to be associated with c-SRC. Thus, the induction of anchorage-independent growth appeared to be extremely sensitive to both CAS and CAS-CT levels.
DISCUSSION
SRC kinases are expressed in virtually every cell type, where they function in pathways that regulate cell cycle progression, cell survival, cell adhesion, and differentiation (1, 31, 45, 54, 55, 70, 72, 77, 87). For homeostasis to be maintained in a multicellular organism, these events must be precisely controlled and integrated. This regulation is achieved in part through modulation of the enzymatic activity of SRC family kinases, which is mediated by cell-specific expression, intracellular compartmentalization, substrate and/or binding protein availability, and the molecular structure of the kinases (1, 31, 45, 54, 70, 72, 74, 77, 86, 87, 91). In this study, we identify a novel role for the adapter molecule CAS in the regulation of c-SRC activity. We demonstrate that overexpression of c-SRC with either CAS or CAS-CT was sufficient to activate c-SRC-dependent PTK activity in COS-1 cells, as measured by the induction of tyrosine phosphorylation of multiple endogenous cellular proteins. We show that CAS-CT was more efficient than CAS-FL at inducing c-SRC-dependent tyrosine phosphorylation of two c-SRC substrates, cortactin and paxillin, and that phosphorylation of a third substrate, FAK, was largely independent of CAS or CAS-CT expression. The direct interaction between CAS-CT and c-SRC was absolutely required for enhanced tyrosine phosphorylation of cortactin and paxillin, since expression of a CAS-CT mutant that was unable to bind to c-SRC did not induce phosphorylation of these proteins. The stable association of c-SRC with CAS in C3H10T1/2-5H murine fibroblasts correlated with an enhanced ability to grow independently of serum and the ECM. CAS-CT could substitute for CAS in mediating growth in soft agar but was less effective in promoting serum-independent growth. These results suggest that CAS has the ability to modulate c-SRC enzymatic and biological activity through the establishment of a c-SRC–CAS signaling complex.
Enzymatic activation of c-SRC by CAS.
Several important factors contribute to the regulation of SRC family kinases (16, 55, 74, 86, 91). These enzymes are generally maintained in a repressed state through a network of intramolecular interactions involving both the SH2 and SH3 domains. Activation of these kinases is proposed to occur through dephosphorylation of the tyrosine residue located in the carboxy-terminal negative regulatory domain (tyrosine 527 of chicken c-SRC) and/or by engagement of the SH3 and SH2 domains with heterologous proteins. This model has been supported by several recent studies that demonstrated a correlation between activation of either c-SRC or HCK and the interaction of these kinases with specific SH3 and SH2 ligands (2, 47, 76, 85). The findings that CAS-CT bound to c-SRC and induced PTK activity but that a mutant that was impaired in its ability to associate with SRC failed to do so provide evidence that the direct association between c-SRC and CAS contributes to the regulation of c-SRC. Because CAS binding to c-SRC engages both the SH3 and SH2 domains of SRC, we propose that the ability of CAS to activate c-SRC arises from the disruption of intramolecular interactions and the resulting stabilization of an enzymatically active conformation.
The demonstration that c-SRC activation by CAS-CT required an intact SRC SH3 domain-binding site suggests that increased c-SRC activity was a direct result of CAS binding. Based on the finding that CAS can serve as both a regulator and a substrate of c-SRC, we propose a two-phase model for the regulation of c-SRC catalytic activity by CAS. The initial phase would involve a stoichiometric, direct interaction between c-SRC and CAS that would likely require a high localized concentration of each component. When some threshold level of c-SRC–CAS complexes is reached, the number of active c-SRC molecules would be sufficient to induce the second phase of catalysis. During this phase, phosphorylation of c-SRC substrates would follow more conventional enzyme kinetics, such that a single kinase molecule would phosphorylate multiple targets. Further experimentation using purified components in vitro will help to clarify the precise mechanism of regulation of c-SRC activity and function by CAS.
In addition to the structural stabilization of c-SRC by the carboxy terminus of CAS, there are several indications that CAS may modulate PTK activities in other ways as well. First, coexpression of full-length CAS with c-SRC reproducibly resulted in a higher level of tyrosine phosphorylation of total cellular proteins than did coexpression of CAS-CT with c-SRC (Fig. 2B). Second, CAS-FL was a more effective inducer of FAK phosphorylation than was CAS-CT. Third, deletion of the YXXP substrate-binding domain from CAS resulted in a decrease in the phosphorylation of cellular proteins relative to the level of phosphorylation observed in the presence of CAS-FL. These data suggest that interactions between CAS-FL and proteins that bind to domains other than its carboxy terminus may contribute to modulation of PTK activity in vivo. The SH3 domain of CAS can interact with PTKs (FAK and PYK2) (27, 41, 57, 58, 80, 89) and PTPases (PTP1B and PTP-PEST) (20, 21, 42), and the adapter proteins Crk and Nck bind to the YXXP substrate-binding domain in response to a number of extracellular stimuli (37, 68, 83). Thus, through interactions of this nature, CAS-FL may modulate the activity of PTPases and PTKs other than c-SRC by bringing substrates or other enzymatic regulators into the c-SRC–CAS complex. Alternatively, CAS-FL may effectively target enzymatically active complexes to sites within the cell that are enriched for particular substrates. Whatever the mechanism, these results indicate that the ability of the carboxy-terminal region of CAS to bind to and activate c-SRC is likely to become integrated with additional aspects of CAS function during the regulation of intracellular singling events.
Regulation of substrate phosphorylation by the c-SRC–CAS complex.
Multiple proteins were phosphorylated in COS-1 cells coexpressing CAS and c-SRC, but the most prominent tyrosine-phosphorylated protein was CAS itself. This finding may reflect the fact that CAS was overexpressed in these cells, whereas other SRC substrates were present at endogenous levels. However, CAS may also be a preferred substrate of c-SRC under the conditions of this assay. Differences in substrate phosphorylation induced by CAS or CAS-CT were observed under several different experimental conditions, suggesting that this may be a real aspect of c-SRC regulation in vivo. For example, when cotransfected with c-SRC alone, FLAG-cortactin and FLAG-paxillin were not significantly tyrosine phosphorylated in either COS-1 or C3H10T1/2-5H cells. Instead, phosphorylation required expression of CAS or CAS-CT and the physical association of these molecules with c-SRC. In both cell types, CAS-CT induced a more potent pTyr signal than CAS-FL, which may reflect the fact that the c-SRC–CAS-CT complex was more stable or active toward these substrates than the c-SRC–CAS complex.
Unlike cortactin and paxillin, phosphorylation of FLAG-FAK was greatly enhanced by overexpression of c-SRC alone, and coexpression with CAS or CAS-CT had little additional effect. There is considerable evidence that c-SRC plays an important role in regulating FAK phosphorylation and function in vivo. This is perhaps most apparent in the context of integrin-mediated signaling (11, 12, 22, 23, 27, 30, 37, 39, 43, 50, 58, 69, 82, 84), where integrin engagement results in activation of FAK and autophosphorylation of tyrosine residue 397 (Y397). Integrin-induced autophosphorylation of FAK is observed in c-SRC-deficient fibroblasts but not in triple SRC-Yes-Fyn knockout cells (26, 38, 68, 83), suggesting that SRC family kinases play an important role in maintaining significant pTyr levels on FAK. Phosphorylation of Y397 provides a docking site for the SRC SH2 domain, thus recruiting c-SRC to focal adhesions (23, 69). The direct association of FAK with c-SRC has also been shown to activate c-SRC (76). Thus, the enhanced phosphorylation on FAK that was observed in the presence of overexpressed c-SRC likely reflects both increased phosphorylation of SRC-dependent phosphorylation sites and the physical protection of phospho-Y397 from PTPases. The finding that overexpression of CAS with c-SRC did not substantially increase phosphorylation of FAK above the levels induced by c-SRC alone suggests that FAK may be maximally phosphorylated under conditions of overexpressed c-SRC. Alternatively, the c-SRC–CAS complex may have less specificity for FAK than it has for cortactin or paxillin.
The biological activity of the c-SRC–CAS complex.
The finding that individual clones of C3H10T1/2-5H murine fibroblasts overexpressing either CAS or CAS-CT demonstrated enhanced serum- and anchorage-independent growth suggests an important biological role for the c-SRC–CAS protein complex. Cells expressing v-SRC or constitutively activated variants of c-SRC exhibit similar growth characteristics (54, 55, 77). An essential role for CAS in src-mediated anchorage-independent growth was demonstrated by Honda et al., who reported that CAS−/− fibroblasts expressing constitutively activated SRC were unable to form colonies in soft agar (29). A related study demonstrated that the transformed phenotype was unaffected when v-SRC–CAS complexes were effectively reduced in src-transformed cells and replaced by v-SRC–CAS-CT complexes (9). Thus, modulation of c-SRC catalytic activity (and perhaps substrate specificity) by its interaction with CAS or CAS-CT may fulfill the requirement for CAS function in src-mediated transformation.
The ability of individual C3H10T1/2-5H clones to grow independently of serum and ECM correlated with high expression of CAS or CAS-CT, tyrosine phosphorylation of these proteins, and the establishment of c-SRC–CAS or c-SRC–CAS-CT complexes. It is noteworthy that clones expressing lower levels of CAS or CAS-CT did not show detectable differences in growth regulation, as measured by serum- and anchorage-independent growth. Overexpression of CAS in the context of endogenous levels of c-SRC can activate signaling pathways involved in cell migration (37), but it has yet to be correlated with direct changes in cell growth. These results suggest that an important facet of the modulation of the biological function of c-SRC by CAS is the stoichiometry of the interaction between the two proteins. In physiological settings, high local concentrations of cell surface proteins and associated signaling molecules (including SRC family members) occur at specialized regions of the cell, such as focal adhesions, caveoli, cell-cell junctions, and lipid rafts (3, 8, 73, 75). CAS localizes to focal adhesions following integrin ligation, and tyrosine phosphorylation of CAS is observed in response to activation of several types of cell surface receptors that mediate their effects in part through SRC family kinases (13, 14, 24, 27, 50, 51, 62, 71, 84, 92). Additionally, the c-SRC negative regulatory kinase Csk (C-terminal c-SRC kinase) is primarily cytoplasmic but can be recruited to focal adhesions and to detergent-insoluble membrane domains by FAK and the Csk-binding protein Cbp (17, 34). Thus, the colocalization of CAS with other molecules that can modulate c-SRC activity provides a putative mechanism for the regulation of SRC family members within specific cellular microenvironments and in response to specific extracellular stimuli.
The ability of C3H10T1/2-5H cells overexpressing high levels of CAS and c-SRC to grow independently of serum for prolonged periods of time is consistent with a model in which the presence of c-SRC–CAS complexes partially circumvents proliferative signals that are otherwise provided by serum. The mechanisms that may be involved in serum-independent growth include autocrine production of growth factors and/or the direct activation of signaling components that function downstream of growth factor receptors (1, 44, 54, 70, 77). In this regard, Hakak and Martin showed that CAS functioned to augment v-SRC-dependent transcriptional activation of a serum response element reporter construct, suggesting that the c-SRC–CAS complex may be involved in the regulation of early-response genes (25). This function of CAS required the presence of the CAS SH3 domain. Recent evidence also suggests that CAS is involved in promoting cell survival by protecting cells from apoptosis (15). This activity required the association of Crk with the substrate-binding YXXP domain of CAS and subsequent activation of the small GTP-binding protein Rac. Thus, the serum-independent growth exhibited by the C3H10T1/2-5H cells that express high levels of CAS-FL may result from the concerted activity of multiple protein interaction sites on CAS. The fact that CAS-CT is missing both the SH3 and substrate-binding YXXP domains may account for the lower biological activity of this molecule in promoting serum-independent growth.
In summary, this study identifies a novel role for the c-SRC–CAS protein complex in regulating aspects of cellular proliferation and survival. Although c-SRC–CAS complexes were generated in these studies through overexpression, we propose that these complexes also play an important role in determining how a cell responds to regulatory input originating from growth factors and the ECM under more physiological conditions. This function may be achieved through the regulated assembly of c-SRC–CAS complexes at specific microenvironments within the cell or in cases where c-SRC and/or CAS is naturally overexpressed. Recent reports indicate that CAS is overexpressed in many breast tumors (81), and carcinomas of the colon, breast, lung, and other tissues contain elevated c-SRC activity that is frequently caused by overexpression of c-SRC rather than oncogenic mutation (4, 5, 53). Because overexpression of c-SRC in fibroblast model systems is not sufficient to induce elevated PTK activity and cellular transformation (7, 44, 61, 88), we propose that additional factors are required for enzymatic activation in the context of these cancers. We suggest that CAS, and perhaps other SRC-binding proteins that function in a similar manner, may serve this function. Future studies will address the potential contribution of the c-SRC–CAS complex and its role in the process of carcinogenesis.
ACKNOWLEDGMENTS
We thank J. Thomas Parsons, Sarah J. Parsons, David Brautigan, Tim Bender, Michael Weber, and Michael Cox for their willingness to embark on numerous scientific discussions and provide critical comments. We also thank S. J. Parsons and J. T. Parsons for providing antibodies. Finally, we thank members of the lab for their contributions and critical comments.
This work was supported by grants from the National Science Foundation (MCB9723820) and the Thomas F. Jeffress and Kate Miller Jeffress Memorial Trust (J-421) to A.H.B. A portion of this work was supported by the DHHS-NIH/NCI with a grant to J. T. Parsons (CA40042 and CA29243). M.T.H. was supported by a postdoctoral fellowship from the National Institutes of Health (F32 CA 72142) and S.A.W. is supported by NIH postdoctoral fellowship CA 75695.
REFERENCES
- 1.Abram C L, Courtneidge S A. Src family tyrosine kinases and growth factor signaling. Exp Cell Res. 2000;254:1–13. doi: 10.1006/excr.1999.4732. [DOI] [PubMed] [Google Scholar]
- 2.Alexandropoulos K, Baltimore D. Coordinate activation of c-Src by SH3- and SH2-binding sites on a novel, p130CAS-related protein, Sin. Genes Dev. 1996;10:1341–1355. doi: 10.1101/gad.10.11.1341. [DOI] [PubMed] [Google Scholar]
- 3.Anderson R G W. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 4.Biscardi J S, Belsches A P, Parsons S J. Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Mol Carcinog. 1998;21:261–272. doi: 10.1002/(sici)1098-2744(199804)21:4<261::aid-mc5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Biscardi J S, Tice D A, Parsons S J. c-Src, receptor tyrosine kinases, and human cancer. Adv Cancer Res. 1999;76:61–119. doi: 10.1016/s0065-230x(08)60774-5. [DOI] [PubMed] [Google Scholar]
- 6.Bouton A H, Burnham M R. Detection of distinct pools of the adapter protein p130CAS using a panel of monoclonal antibodies. Hybridoma. 1997;16:403–411. doi: 10.1089/hyb.1997.16.403. [DOI] [PubMed] [Google Scholar]
- 7.Bouton A H, Kanner S B, Vines R R, Parsons J T. Tyrosine phosphorylation of three cellular proteins correlates with transformation of Rat 1 cells by pp60src. Mol Carcinog. 1991;4:145–152. doi: 10.1002/mc.2940040210. [DOI] [PubMed] [Google Scholar]
- 8.Brown D A, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 9.Burnham M R, Harte M T, Bouton A H. The role of SRC-CAS interactions in cellular transformation: ectopic expression of the carboxy terminus of CAS inhibits SRC-CAS interaction but has no effect on cellular transformation. Mol Carcinog. 1999;26:20–31. doi: 10.1002/(sici)1098-2744(199909)26:1<20::aid-mc3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Burnham M R, Harte M T, Richardson A, Parsons J T, Bouton A H. The identification of p130CAS-binding proteins and their role in cellular transformation. Oncogene. 1996;12:2467–2472. [PubMed] [Google Scholar]
- 11.Cary L A, Chang J F, Guan J-L. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 12.Cary L A, Han D C, Polte T R, Hanks S K, Guan J-L. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casamassima A, Rozengurt E. Tyrosine phosphorylation of p130cas by bombesin, lysophosphatidic acid, phorbol esters, and platelet-derived growth factor. J Biol Chem. 1997;272:9363–9370. doi: 10.1074/jbc.272.14.9363. [DOI] [PubMed] [Google Scholar]
- 14.Casamassima A, Rozengurt E. Insulin-like growth factor I stimulates tyrosine phosphorylation of p130Cas, focal adhesion kinase, and paxillin. J Biol Chem. 1998;273:26149–26156. doi: 10.1074/jbc.273.40.26149. [DOI] [PubMed] [Google Scholar]
- 15.Cho S Y, Klemke R L. Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J Cell Biol. 2000;149:223–236. doi: 10.1083/jcb.149.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobb B S, Payne D M, Reynolds A B, Parsons J T. Regulation of the oncogenic activity of the cellular src protein requires the correct spacing between the kinase domain and the C-terminal phosphorylated tyrosine (Tyr-527) Mol Cell Biol. 1991;11:5832–5838. doi: 10.1128/mcb.11.12.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeNichilo M O, Katz B Z, O'Connell B, Yamada K M. De novo expression of pp125FAK in human macrophages regulates CSK distribution, MAP kinase activation but does not affect focal contact structure. J Cell Physiol. 1999;178:164–172. doi: 10.1002/(SICI)1097-4652(199902)178:2<164::AID-JCP5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 18.Devarajan P, Stabach P R, De Matteis M A, Morrow J S. Na,K-ATPase transport from endoplasmic reticulum to Golgi requires the Golgi spectrin-ankyrin G119 skeleton in Madin-Darby canine kidney cells. Proc Natl Acad Sci USA. 1997;94:10711–10716. doi: 10.1073/pnas.94.20.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Guzman M, Dolfi F, Russello M, Vuori K. Cell adhesion regulates the interaction between the docking protein p130Cas and the 14-3-3 proteins. J Biol Chem. 1999;274:5762–5768. doi: 10.1074/jbc.274.9.5762. [DOI] [PubMed] [Google Scholar]
- 20.Garton A J, Flint A J, Tonks N K. Identification of p130CAS as a substrate for the cystolic protein tyrosine phosphatase PTP-PEST. Mol Cell Biol. 1996;16:6408–6418. doi: 10.1128/mcb.16.11.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garton A J, Tonks N K. Regulation of fibroblast motility by the protein tyrosine phosphatase PTP-PEST. J Biol Chem. 1999;274:3811–3818. doi: 10.1074/jbc.274.6.3811. [DOI] [PubMed] [Google Scholar]
- 22.Giancotti F G. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- 23.Guan J-L. Role of focal adhesion kinase in integrin signaling. Int J Biochem Cell Biol. 1997;29:1085–1096. doi: 10.1016/s1357-2725(97)00051-4. [DOI] [PubMed] [Google Scholar]
- 24.Gutkind J S. Cell growth control by G protein-coupled receptors: from signal transduction to signal integration. Oncogene. 1998;17:1331–1342. doi: 10.1038/sj.onc.1202186. [DOI] [PubMed] [Google Scholar]
- 25.Hakak Y, Martin G S. Cas mediates transcriptional activation of the serum response element by Src. Mol Cell Biol. 1999;19:6953–6962. doi: 10.1128/mcb.19.10.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamasaki K, Mimura T, Morino N, Furuya H, Nakamoto T, Aizawa S-I, Morimoto C, Yazaki Y, Hirai H, Nojima Y. Src kinase plays an essential role in integrin-mediated tyrosine phosphorylation of Crk-associated substrate p130CAS. Biochem Biophys Res Commun. 1996;222:338–343. doi: 10.1006/bbrc.1996.0745. [DOI] [PubMed] [Google Scholar]
- 27.Harte M T, Hildebrand J D, Burnham M R, Bouton A H, Parsons J T. p130CAS, a substrate associated with v-src and v-crk, localizes to focal adhesions and binds to focal adhesion kinase. J Biol Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- 28.Honda H, Nakamoto T, Sakai R, Hirai H. p130(Cas), an assembling molecule of actin filaments, promotes cell movement, cell migration, and cell spreading in fibroblasts. Biochem Biophys Res Commun. 1999;262:25–30. doi: 10.1006/bbrc.1999.1162. [DOI] [PubMed] [Google Scholar]
- 29.Honda H, Oda H, Nakamoto T, Honda Z-I, Sakai R, Suzuki T, Saito T, Nakamura K, Nakao K, Ishikawa T, Katsuki M, Yazak Y, Hirai H. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- 30.Howe A, Aplin A E, Alahari S K, Juliano R L. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard S R, Mohammadi M, Schlessinger J. Autoregulatory mechanisms in protein-tyrosine kinases. J Biol Chem. 1998;273:11987–11990. doi: 10.1074/jbc.273.20.11987. [DOI] [PubMed] [Google Scholar]
- 32.Kanner S B, Reynolds A B, Vines R R, Parsons J T. Monoclonal antibodies to individual tyrosine phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci USA. 1990;87:3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanner S B, Reynolds A B, Wang H-R, Vines R R, Parsons J T. The SH2 and SH3 domains of pp60src direct stable association with tyrosine phosphorylated proteins p130 and p110. EMBO J. 1991;10:1689–1698. doi: 10.1002/j.1460-2075.1991.tb07693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 35.Kirsch K H, Georgescu M-M, Hanafusa H. Direct binding of p130Cas to the guanine nucleotide exchange factor C3G. J Biol Chem. 1998;273:25673–25679. doi: 10.1074/jbc.273.40.25673. [DOI] [PubMed] [Google Scholar]
- 36.Kirsch K H, Georgescu M-M, Ishimaru S, Hanafusa H. CMS: an adapter molecule involved in cytoskeletal rearrangements. Proc Natl Acad Sci USA. 1999;96:6211–6216. doi: 10.1073/pnas.96.11.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klemke R L, Leng J, Molander R, Brooks P C, Vuori K, Cheresh D A. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klinghoffer R A, Sachsenmaier C, Cooper J A, Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 1999;18:2459–2471. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar C C. Signaling by integrin receptors. Oncogene. 1998;17:1365–1373. doi: 10.1038/sj.onc.1202172. [DOI] [PubMed] [Google Scholar]
- 40.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 41.Lakkakorpi P T, Nakamura I, Nagy R M, Parsons J T, Rodan G A, Duong L T. Stable association of PYK2 and p130Cas in osteoclasts and their co-localization in the sealing zone. J Biol Chem. 1999;274:4900–4907. doi: 10.1074/jbc.274.8.4900. [DOI] [PubMed] [Google Scholar]
- 42.Liu F, Hill D E, Chernoff J. Direct binding of the proline-rich region of protein tyrosine phosphatase 1B to the src homology 3 domain of p130CAS. J Biol Chem. 1996;271:31290–31295. doi: 10.1074/jbc.271.49.31290. [DOI] [PubMed] [Google Scholar]
- 43.Longhurst C M, Jennings L K. Integrin-mediated signal transduction. Cell Mol Life Sci. 1998;54:514–526. doi: 10.1007/s000180050180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maa M-C, Leu T-H, McCarley D J, Schatzman R C, Parsons S J. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc Natl Acad Sci USA. 1995;92:6981–6985. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayer B B. Signal transduction: clamping down on Src activity. Curr Biol. 1997;7:R295–R298. doi: 10.1016/s0960-9822(06)00141-2. [DOI] [PubMed] [Google Scholar]
- 46.Miglarese M R, Hannion-Henderson J, Wu H, Parsons J T, Bender T P. The protein tyrosine kinase substrate cortactin is differentially expressed in murine B lymphoid tumors. Oncogene. 1994;9:1989–1997. [PubMed] [Google Scholar]
- 47.Moarefi I, Lafevre-Bernt M, Sicheri F, Huse M, Lee C-H, Kuriyan J, Miller W T. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 48.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3590. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamoto T, Sakai R, Ozawa K, Yazaki Y, Hirai H. Direct binding of C-terminal region of p130CAS to SH2 and SH3 domains of src kinase. J Biol Chem. 1996;271:8959–8965. doi: 10.1074/jbc.271.15.8959. [DOI] [PubMed] [Google Scholar]
- 50.Nojima Y, Morino N, Mimura T, Hamasaki K, Furuya H, Sakai R, Sato T, Tachibana K, Morimoto C, Yazaki Y, Hirai H. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130CAS, a src homology 3-containing molecule having multiple src homology 2-binding motifs. J Biol Chem. 1995;270:15398–15402. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- 51.Ojaniemi M, Vuori K. Epidermal growth factor modulates tyrosine phosphorylation of p130Cas. J Biol Chem. 1997;272:25993–25998. doi: 10.1074/jbc.272.41.25993. [DOI] [PubMed] [Google Scholar]
- 52.Olson M F, Pasteris N G, Gorski J L, Hall A. Faciogenital dysplasia protein (FGD1) and Vav, two related proteins required for normal embryonic development, are upstream regulators of Rho GTPases. Curr Biol. 1996;6:1628–1633. doi: 10.1016/s0960-9822(02)70786-0. [DOI] [PubMed] [Google Scholar]
- 53.Ottenhalff-Kalff A E, Rijksen G, van Beurden E A C M, Hennipman A, Michels A A, Staal G E J. Characterization of protein tyrosine kinases from human breast cancer: involvement of the c-src oncogene product. Cancer Res. 1992;52:4773–4778. [PubMed] [Google Scholar]
- 54.Parsons J T, Parsons S J. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol. 1997;9:187–192. doi: 10.1016/s0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- 55.Parsons J T, Weber M J. Genetics of src: structure and functional organization of a protein tyrosine kinase. Curr Topics Microbiol Immunol. 1989;147:79–127. doi: 10.1007/978-3-642-74697-0_3. [DOI] [PubMed] [Google Scholar]
- 56.Piwnica-Worms H, Saunders K B, Roberts T M, Smith A E, Cheng S H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987;49:75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- 57.Polte T R, Hanks S K. Interaction between focal adhesion kinase and crk-associated tyrosine kinase substrate p130Cas. Proc Natl Acad Sci USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polte T R, Hanks S K. Complexes of focal adhesion kinase (FAK) and crk-associated substrate p130CAS are elevated in cytoskeleton-associated fractions following adhesion and src transformation—requirements for SRC kinase activity and FAK proline-rich motifs. J Biol Chem. 1997;272:5501–5509. doi: 10.1074/jbc.272.9.5501. [DOI] [PubMed] [Google Scholar]
- 59.Reynolds A B, Kanner S B, Wang H-R, Parsons J T. Stable association of activated pp60src with two tyrosine-phosphorylated cellular proteins. Mol Cell Biol. 1989;9:3951–3958. doi: 10.1128/mcb.9.9.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reynolds A B, Roesel D J, Kanner S B, Parsons J T. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol. 1989;9:629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynolds A B, Vila J, Lansing T J, Potts W M, Weber M J, Parsons J T. Activation of the oncogenic potential of the avian cellular src protein by specific structural alteration of the carboxy terminus. EMBO J. 1987;6:2359–2364. doi: 10.1002/j.1460-2075.1987.tb02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rozengurt E. Signal transduction pathways in the mitogenic response to G protein-coupled neuropeptide receptor agonists. J Cell Physiol. 1998;177:507–517. doi: 10.1002/(SICI)1097-4652(199812)177:4<507::AID-JCP2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 63.Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakakibara A, Hattori S. Chat, a Cas/HEF1-associated adaptor protein that integrates multiple signaling pathways. J Biol Chem. 2000;275:6404–6410. doi: 10.1074/jbc.275.9.6404. [DOI] [PubMed] [Google Scholar]
- 65.Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanake T, Nishida J, Yazaki Y, Hirai H. Characterization, partial purification, and peptide sequencing of p130, the main phosphoprotein associated with v-crk oncoprotein. J Biol Chem. 1994;269:32740–32746. [PubMed] [Google Scholar]
- 66.Salgia R, Li J L, Lo S H, Brunkhorst B, Kansas G S, Sobhany E S, Sun Y, Pisick E, Hallek M, Ernst T, et al. Molecular cloning of human paxillin, a focal adhesion protein phosphorylated by p210BCR/ABL. J Biol Chem. 1995;270:5039–5047. doi: 10.1074/jbc.270.10.5039. [DOI] [PubMed] [Google Scholar]
- 67.Schaller M D, Borgman C A, Cobb B S, Vines R R, Reynolds A B, Parsons J T. pp125FAK: a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schlaepfer D D, Broome M A, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adapter proteins. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schlaepfer D D, Hauck C R, Sieg D J. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 70.Schwartzberg P. The many faces of Src: multiple functions of a prototypical tyrosine kinase. Oncogene. 1998;17:1463–1468. doi: 10.1038/sj.onc.1202176. [DOI] [PubMed] [Google Scholar]
- 71.Seufferlein T, Rozengurt E. Lysophosphatidic acid stimulates tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130. J Biol Chem. 1994;269:9345–9351. [PubMed] [Google Scholar]
- 72.Shalloway D, Taylor S J. SRC—more than the sum of its parts. Trends Cell Biol. 1997;7:215–217. doi: 10.1016/S0962-8924(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 73.Shaul P W, Anderson R G W. Role of plasmalemmal caveolae in signal transduction. Am J Physiol. 1998;275:L843–L851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- 74.Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- 75.Singer S J. Intercellular communication and cell-cell adhesion. Science. 1992;255:1671–1677. doi: 10.1126/science.1313187. [DOI] [PubMed] [Google Scholar]
- 76.Thomas J W, Ellis B, Boerner R J, Knight W B, White G C, Schaller M D. SH2- and SH3-mediated interactions between focal adhesion kinase and Src. J Biol Chem. 1998;273:577–583. doi: 10.1074/jbc.273.1.577. [DOI] [PubMed] [Google Scholar]
- 77.Thomas S M, Brugge J S. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 78.Thomas S M, Soriano P, Imamoto A. Specific and redundant roles of Src and Fyn in organizing the cytoskeleton. Nature. 1995;376:267–271. doi: 10.1038/376267a0. [DOI] [PubMed] [Google Scholar]
- 79.Tice D A, Biscardi J S, Nickles A L, Parsons S J. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA. 1999;96:1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ueki K, Mimura T, Nakamoto T, Sasaki T, Aizawa S, Hirai H, Yano S, Naruse T, Nojima Y. Integrin-mediated signal transduction in cells lacking focal adhesion kinase p125FAK. FEBS Let. 1998;432:197–201. doi: 10.1016/s0014-5793(98)00862-x. [DOI] [PubMed] [Google Scholar]
- 81.Van der Flier S, Brinkman A, Look M P, Kok E M, Meijer-van Gelder M E, Klijn J G M, Dorssers L C J, Foekens J A. Bcar1/p130Cas protein and primary breast cancer: prognosis and response to tamoxifen treatment. J Natl Cancer Inst. 2000;92:120–127. doi: 10.1093/jnci/92.2.120. [DOI] [PubMed] [Google Scholar]
- 82.Vuori K. Integrin signaling: tyrosine phosphorylation events in focal adhesion. J Membr Biol. 1998;165:191–199. doi: 10.1007/s002329900433. [DOI] [PubMed] [Google Scholar]
- 83.Vuori K, Hirai H, Aizawa S, Ruoslahti E. Induction of p130cas signaling complex formation upon integrin-mediated cell adhesion: a role for src family kinases. Mol Cell Biol. 1996;16:2606–2613. doi: 10.1128/mcb.16.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vuori K, Ruoslahti E. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J Biol Chem. 1995;270:22259–22262. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- 85.Walter A O, Peng Z-Y, Cartwright C A. The Shp-2 tyrosine phosphatase activates the Src tyrosine kinase by a non-enzymatic mechanism. Oncogene. 1999;18:1911–1920. doi: 10.1038/sj.onc.1202513. [DOI] [PubMed] [Google Scholar]
- 86.Williams J C, Weijland A, Gonfloni S, Thompson A, Courtneidge S A, Supertifurga G, Wierenga R K. The 2.35 angstrom crystal structure of the inactivated form of chicken src—a dynamic molecule with multiple regulatory interactions. J Mol Biol. 1997;274:757–775. doi: 10.1006/jmbi.1997.1426. [DOI] [PubMed] [Google Scholar]
- 87.Williams J C, Wierenga R K, Saraste M. Insights into Src kinase functions: structural comparisons. Trends Biochem Sci. 1998;23:179–184. doi: 10.1016/s0968-0004(98)01202-x. [DOI] [PubMed] [Google Scholar]
- 88.Wilson L K, Parsons S J. Enhanced EGF mitogenic response is associated with enhanced tyrosine phosphorylation of specific cellular proteins in fibroblasts overexpressing c-src. Oncogene. 1990;5:1471–1580. [PubMed] [Google Scholar]
- 89.Xiong W-C, Macklem M, Parsons J T. Expression and characterization of splice variants of PYK2, a focal adhesion kinase-related protein. J Cell Sci. 1998;111:1981–1991. doi: 10.1242/jcs.111.14.1981. [DOI] [PubMed] [Google Scholar]
- 90.Xiong W-C, Parsons J T. Induction of apoptosis after expression of PYK2, a tyrosine kinase structurally related to focal adhesion kinase. J Cell Biol. 1997;139:529–539. doi: 10.1083/jcb.139.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu W Q, Harrison S C, Eck M J. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 92.Zachary I, Stinnett-Smith J, Rozengurt E. Bombesin, vasopressin, and endothelin stimulation of tyrosine phosphorylation in Swiss 3T3 cells. J Biol Chem. 1992;267:19031–19034. [PubMed] [Google Scholar]