Abstract
Background
Home telemonitoring has been used with discharged patients in an attempt to reduce 30-day readmissions with mixed results.
Objective
To assess whether home 30-day telemonitoring after discharge for patients at high risk of readmission would reduce readmissions or mortality.
Design
Prospective, randomized controlled trial.
Patients
We compared 30-day readmission rates and mortality for patients at high risk for readmission who received home telemonitoring versus standard care between November 1, 2014, and November 30, 2018, in 2 tertiary care hospitals.
Interventions
The intervention group received home-installed equipment to measure blood pressure, heart rate, pulse oximetry, weight if heart failure was present, and glucose if diabetes was present. Results were transmitted daily and reviewed by a nurse. Both groups received standard care.
Main Measures
The primary outcome was a composite end point of hospital readmission or death within 30 days after discharge. The secondary outcome was an emergency department visit within 30 days after discharge.
Key Results
A total of 1380 participants (mean [SD] age, 66 [14] years; 722 [52.3%] men and 658 [47.7%] women) participated in this study. Using a modified intention-to-treat analysis, the risk of readmission or death within 30 days among patients at high readmission risk was 23.7% (137/578) in the control group and 18.2% (87/477) in the telemonitoring group (absolute risk difference, − 5.5% [95% CI, − 10.4 to − 0.6%]; relative risk, 0.77 [95% CI, 0.61 to 0.98]; P = .03). Emergency department visits occurred within 30 days after discharge in 14.2% (81/570) of patients in the control group and 8.6% (40/464) of patients in the telemonitoring group (absolute risk difference, − 5.6% [95% CI, − 9.4 to − 1.8%]; relative risk, 0.61 [95% CI, 0.42 to 0.87]; P = .005).
Conclusions
Thirty days of postdischarge telemonitoring may reduce readmissions of high-risk patients.
Trial Registration
ClinicalTrials.gov identifier: NCT02136186.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-020-06589-1.
KEY WORDS: communication, risk assessment, telemedicine
INTRODUCTION
The Centers for Medicare & Medicaid Services Hospital Readmissions Reduction Program (CMS HRRP) has led to a national conversation and prodigious efforts to identify and correct gaps in the current state of health care transitions in the USA.1–4 Rates of hospital 30-day readmissions have decreased slightly over the past 5 years but remain high—approximately 15% for all Medicare patients.5 Strategies to improve patient transitions from hospital to home are critical, given possible financial penalties, the focus on readmissions as a measure of quality of care, and transparent reporting of readmission rates on public websites.
Most efforts to reduce readmissions have focused on improving in-hospital patient education and discharge planning as well as increasing postdischarge follow-up and monitoring with various interventions, including structured telephone calls, telemonitoring, and home or clinic visits occurring within the immediate postdischarge period.6–16 Although these interventions have been extensively studied in various contexts and disease processes, questions remain regarding which interventions truly provide the highest value. One emerging theme is that frequent, health system–initiated contact after discharge has a significant and consistent impact on reducing readmissions.6,7
Telemedicine has been increasingly viewed as an alternative to in-person visits in this context because of the ability to reach more patients and ideally intervene before events occur. However, studies thus far have shown inconsistent results regarding the efficacy of telemedicine in reducing readmissions.8–16 Decreased readmissions with postdischarge home telemonitoring have been shown in some studies of patients hospitalized with diagnoses such as lung cancer,8 chronic obstructive pulmonary disease,9,10 hip and knee arthroplasty,11 and congestive heart failure12,13; yet, other studies of patients with similar diagnoses have shown no benefit.14–16 These studies were either small or very disease specific, making their results difficult to generalize. Because of these complexities, systematic reviews and meta-analyses have reached wide-ranging conclusions regarding the effectiveness of postdischarge telemonitoring in preventing admissions or readmissions.14,17–21
Despite these mixed results, many organizations are using home telemonitoring with the goal of reducing readmissions. However, to our knowledge, no study has prospectively evaluated the outcomes of home telemonitoring for patients at risk for readmission regardless of diagnosis, simulating the current practice in many health care systems. To answer this question, we conducted a prospective randomized clinical trial to determine whether home telemonitoring for 30 days after discharge for patients at high risk for readmission would reduce readmissions or mortality.
METHODS
Patients and Settings
We performed a 1:1 randomization of adults at high risk for hospital readmission at discharge to a control group consisting of standard care and to an intervention group consisting of home telemonitoring in addition to standard care. We compared 30-day readmission rates for both groups between November 1, 2014, and November 30, 2018. Mayo Clinic hospitals in Florida and Arizona were included in the study. Florida began November 1, 2014, and Arizona was added as a site January 1, 2017. The study was approved by the Mayo Clinic Institutional Review Board. The reporting of this study is in compliance with the CONSORT (Consolidated Standards of Reporting Trials) statement.22
Both study sites are tertiary care teaching hospitals with approximately 300 beds each that use the same electronic health record (EHR). After admission, all patients ≥ 18 years were scored for readmission risk that were computed with a validated electronic tool23 and based on 13 criteria assessed during hospitalization. These 13 criteria included payer source, poor health literacy, lack of social support or the inability to self-care, an admission within the previous 12 months, emergent admission, a hospitalization > 5 days, or history of a major medical comorbid condition (diabetes mellitus, myocardial infarction, stroke, peripheral artery disease, congestive heart failure, chronic obstructive pulmonary disease, substance abuse, depression, acute delirium, receiving dialysis, previous or active cancer, end-stage liver disease, or HIV) (Supplemental Table 1). The electronic tool obtains objective data from patients’ documented comorbid conditions and medications and social data (e.g., health literacy, social support) from the nurse assessment at admission using standardized questions. Patients were divided into 3 risk groups based on their readmission risk score: < 10 = low risk, 10–14 = medium risk, and > 14 = high risk. Approximately 38% of hospitalized patients were considered high risk for readmission; 16%, intermediate risk; and 46%, low risk. A study coordinator met with the high-risk patients, explained the study, and gave them the opportunity to participate. Written informed consent was obtained and documented. Patients were excluded if they lived outside the US, were < 18 years, were discharged to hospice, were discharged to a subacute care hospital, were transferred to an acute care hospital, or had a planned readmission within 30 days. Having physical and mental capability to handle the home telemonitoring device and to communicate over the phone with a nurse was a prerequisite. If none of these exclusions was present, patients were contacted by a study coordinator and offered participation. Those who consented were randomized at discharge.
Interventions
Standard care included but was not limited to teach-back education, medication reconciliation, and a follow-up phone call within 72 h of discharge by a trained nurse for patients designated high risk for readmission. These standard interventions were established before the start of the study to improve patient education and intervene with patients after discharge to help reduce readmissions. Patients randomized to the telemedicine group received standard care plus monitoring for 30 days. Other than telemonitoring in the intervention group, there were no differences in care in relation to standard interventions to reduce rehospitalization. In addition, the initial program that developed the standardized care interventions to reduce readmission was used at all sites; therefore, standard care was the same at each study site. Because this program was in place before the start of the study, these interventions were not adjusted for the study.
Patients in the intervention group were contacted by the equipment company and provided with monitoring devices including a blood pressure cuff, heart rate monitor, pulse oximeter, scale if they had a history of congestive heart failure, and glucose monitor if they had diabetes mellitus, as well as a console used for contacting patients. The cost of the monitoring equipment was approximately $70 per patient. In-home installation was required to link the monitoring devices to the communication unit using cellular or phone line connections. The home installation was completed within 24 to 72 h after discharge and included patient education for completing the monitoring. The patient used the equipment each day to determine vital signs, and the monitoring devices transmitted information daily for the next 30 days to a cloud-based program; a nurse was responsible for monitoring the data daily. Changes in vital signs outside a preset range, determined by a standard protocol provided by the device company, triggered an alert for the nurse. The nurse then sent an automatically generated questionnaire to the patient through the console if data were not submitted or phoned the patient if data were out of ranges. Depending on the patient’s clinical condition, additional interventions were recommended, which consisted of a nurse visit, referral to the emergency department, or referral for an outpatient appointment. All patient communications and nursing recommendations were documented in the EHR. Home telemonitoring was continued for a total of 30 days after discharge or until the first hospital readmission, whichever occurred first.
Outcome Measures
The primary outcome measure was a composite end point of hospital readmission or death within 30 days after hospital discharge. The secondary outcome was an emergency department visit within 30 days after discharge. These outcomes were chosen because they are the outcomes tracked by CMS HRRP for penalty assessment. Readmission or death was documented from the EHR. All patients whose EHR did not include a documented readmission were called to determine if the study participants had been readmitted to another facility or died within the 30 days after discharge.
Study Power and Randomization
On the basis of an historical 30-day readmission rate of 20%, we estimated that 950 patients would be required to enroll in each study group to achieve 80% power at the 5% significance level (2-sided) to detect an absolute reduction of 5% (i.e., 20% in the control group and 15% in the telemonitoring group). The study was stopped before the total accrual because we could no longer obtain monitoring devices. The reduction in total sample size from 1900 to 1380 decreased the power from 80 to 68% when all other assumptions remained the same as the original sample-size calculation.
A computer algorithm was written in SAS (SAS Institute Inc.) for each of the 2 hospital sites by the study statistician to generate a block randomization with randomly selected block sizes of 4, 6, and 8. The randomization scheme was uploaded into the REDCap (Research Electronic Data Capture) study database using the randomization module. After enrolling patients into the study, the study coordinator logged into the REDCap study database and randomized patients to the control group or the telemonitoring group. Because of the nature of the intervention, it was not possible to blind patients to the intervention type.
Statistical Methods
Patient data were analyzed according to randomized group assignment (modified intention-to-treat) unless otherwise indicated. Separately for the control group and telemonitoring group, we estimated the proportion of patients who experienced the primary study end point (hospital readmission or death within 30 days after hospital discharge) along with 95% CIs using the Wilson score method. We compared these proportions between the 2 study groups using a χ2 test along with the estimated relative risk (RR) and 95% CI. One preplanned interim analysis for efficacy was performed after half of the study patients had completed the 30-day follow-up period, the point at which the study would be stopped early for efficacy if there was overwhelming evidence (2-sided P < .001) of differences in 30-day readmissions between the 2 groups. With an overall 5% significance level for the study, a 2-sided P ≤ .049 was considered statistically significant for the primary analysis. We evaluated the impact of potential confounding variables on our results using a post hoc multivariable log-binomial regression model adjusting for all baseline characteristics in Table 1. In the multivariable model, we included an indicator variable for race (1 = Black, 0 = non-Black or unknown) and ethnicity (1 = Hispanic or Latino, 0 = non-Hispanic/Latino or unknown).
Table 1.
Baseline Characteristics of All Randomized Study Participants
| Variable | Control group (n = 690) | Telemonitoring group (n = 690) |
|---|---|---|
| Demographic characteristics | ||
| Age, mean (SD), y | 66 (15) | 67 (14) |
| Sex, no. (%) | ||
| Female | 329 (47.7) | 329 (47.7) |
| Male | 361 (52.3) | 361 (52.3) |
| Race, no. (%) | ||
| Black or African American | 83 (12.0) | 83 (12.0) |
| Asian | 7 (1.0) | 9 (1.3) |
| White | 571 (82.8) | 575 (83.3) |
| Other | 11 (1.6) | 10 (1.4) |
| Unknown | 18 (2.6) | 13 (1.9) |
| Ethnicity, no. (%) | ||
| Hispanic/Latino | 24 (3.5) | 29 (4.2) |
| Non-Hispanic/Latino | 643 (93.2) | 640 (92.8) |
| Unknown | 23 (3.3) | 21 (3.0) |
| Body mass index, mean (SD), kg/m2 | 28.9 (7.1) | 29.0 (7.7) |
| Comorbid conditions, no. (%) | ||
| Myocardial infarction | 101 (14.6) | 82 (11.9) |
| Congestive heart failure | 185 (26.8) | 200 (29.0) |
| Peripheral vascular disease | 179 (25.9) | 170 (24.6) |
| Cerebrovascular disease | 150 (21.7) | 166 (24.1) |
| Dementia | 33 (4.8) | 26 (3.8) |
| Chronic pulmonary disease | 219 (31.7) | 224 (32.5) |
| Ulcer | 40 (5.8) | 31 (4.5) |
| Mild liver disease | 104 (15.1) | 112 (16.2) |
| Diabetes mellitus | 180 (26.1) | 185 (26.8) |
| Hemiplegia | 24 (3.5) | 33 (4.8) |
| Moderate to severe renal disease | 241 (34.9) | 245 (35.5) |
| Metastatic solid tumor | 80 (11.6) | 92 (13.3) |
| AIDS | 5 (0.7) | 2 (0.3) |
| Rheumatologic disease | 26 (3.8) | 27 (3.9) |
| Other cancer | 227 (32.9) | 224 (32.5) |
Three post hoc sensitivity analyses were performed for our primary outcome of 30-day death or hospital readmission. The first sensitivity analysis was a per-protocol analysis where we excluded all patients randomized to the telemonitoring arm who did not access the home telemonitoring device at least once. The second and third sensitivity analyses were used to evaluate the impact of missing outcomes on our results by assuming the rates of 30-day death or readmission among those missing the 30-day end point were the same as the study cohort in the second analysis and the control group in the third analysis. All statistical tests were 2-sided. Statistical analyses were performed using SAS (version 9.4).
RESULTS
Participants
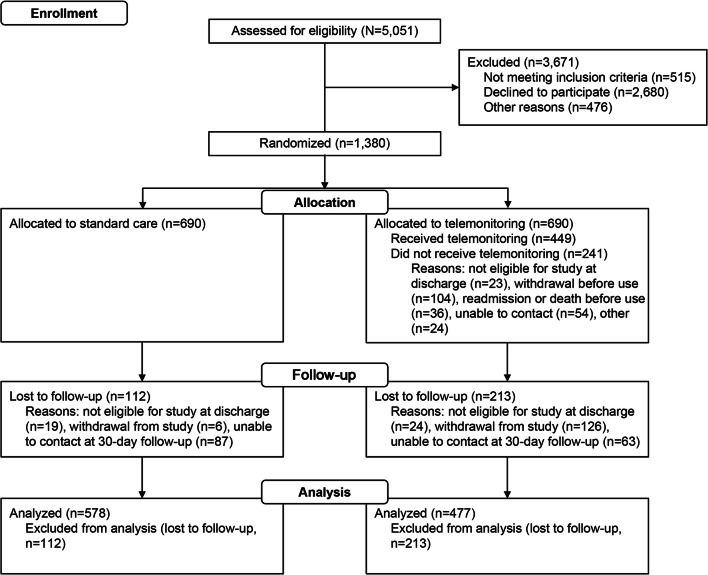
A total of 1380 high-risk participants (mean [SD] age, 66 [14] years; 722 [52.3%] men and 658 [47.7%] women) gave consent to participate between December 1, 2014, and November 27, 2018; the last day of follow-up was January 2, 2019. Data analysis was completed in May 2019. Patients were randomized to standard care (n = 690) or telemonitoring (n = 690). No meaningful differences were found in patient demographic characteristics or comorbid conditions across groups at baseline (Table 1). The study was completed by 578 patients in the standard care group and 477 patients in the telemonitoring group (Fig. 1). A descriptive summary of demographic characteristics and comorbid conditions of patients who completed the study is shown in Supplemental Table 2. As comorbid conditions were similar between the 2 groups, no cohort matching was necessary.
Figure 1.
Participant flow diagram.
Primary Outcome
The risk of readmission or death within 30 days after discharge from the hospital among patients at high risk for readmission was 23.7% (137/578) in the control group and 18.2% (87/477) in the telemonitoring group (absolute risk difference, − 5.5% [95% CI, − 10.4 to − 0.6%]; RR, 0.77 [95% CI, 0.61 to 0.98]; P = .03) (Table 2), showing significant improvement in outcomes in the telemonitoring group. We found consistent results when we adjusted for all baseline characteristics in Table 1 using a post hoc multivariable log-binomial model (adjusted RR, 0.76 [95% CI, 0.60 to 0.96]; P = .02). In the telemetry arm, there were a total of 8543 events triggered in 405 individual patients, most of which (57%) were missing measurements that required only a reminder to the patient.
Table 2.
Primary and Secondary Outcomesa,b
| Control group | Telemonitoring group | Difference % (95% CI) | |||
|---|---|---|---|---|---|
| Outcomes | % (n/N) | 95% CI | % (n/N) | 95% CI | |
| Primary outcome | |||||
| 30-day death or hospital readmission | 23.7 (137/578) | 20.4 to 27.3 | 18.2 (87/477) | 15.0 to 22.0 | − 5.5 (− 10.4 to − 0.6) |
| Components of primary outcome | |||||
| 30-day death | 1.9 (11/574) | 1.1 to 3.4 | 1.7 (8/463) | 0.9 to 3.4 | − 0.2 (− 1.8 to 1.4) |
| 30-day hospital readmission | 22.5 (129/574) | 19.3 to 26.1 | 17.0 (81/476) | 13.9 to 20.7 | − 5.5 (− 10.3 to − 0.7) |
| Secondary outcome | |||||
| 30-day emergency department visit | 14.2 (81/570) | 11.6 to 17.3 | 8.6 (40/464) | 6.4 to 11.5 | − 5.6 (− 9.4 to − 1.8) |
an = number of patients with the outcome; N = number of patients with available data
bThe 95% CIs are estimated by using the Wilson score method
Table 3 shows the results of post hoc sensitivity analyses of the primary outcome. In the first sensitivity analysis, we excluded the 58 patients in the telemonitoring group who did not access the home telemonitoring device at least once (36 of the excluded patients had 30-day death or readmission, and 22 had neither death nor readmission within 30 days). The RR in this per-protocol analysis was 0.51 (95% CI, 0.38 to 0.69; P < .001), confirming the improvement seen in the telemonitoring arm. In the second sensitivity analysis, we gave all the missing primary outcomes the same rate as the cohort of study patients (21.2%), resulting in an RR of 0.82 (95% CI, 0.6 to 1.01; P = .056). In the third sensitivity analysis, we gave all missing primary outcomes the same rate as the standard of care group (23.7%), resulting in an RR of 0.83 (95% CI, 0.68 to 1.02; P = .08).
Table 3.
Post Hoc Sensitivity Analyses of the Primary Outcome
| Control group, % (n/N) | Telemonitoring group, % (n/N) | RR (95% CI) | P value | |
|---|---|---|---|---|
| Original analysis of the primary outcomea | 23.7 (137/578) | 18.2 (87/477) | 0.77 (0.62–0.98) | .03 |
| Post hoc sensitivity analyses | ||||
| 1. Excluding patients in the telemonitoring group who did not access the telemonitoring device at least onceb | 23.7 (137/578) | 12.2 (51/419) | 0.51 (0.38–0.69) | < .001 |
| 2. Assuming the rate of the primary outcome is the same as the cohort of study participants—21.2%c | 23.3 (161/690) | 19.1 (132/690) | 0.82 (0.6–1.01) | .056 |
| 3. Assuming the rate of the primary outcome is the same as the control group—23.7%c | 23.8 (164/690) | 19.8 (137/690) | 0.83 (0.68–1.02) | .08 |
Abbreviation: RR, relative risk
aThe primary outcome was a composite of 30-day death or hospital readmission
bExcluded 58 patients in telemonitoring group who did not access the telemonitoring device at least once; 36 of the 58 excluded patients had 30-day death or hospital readmission
cTwo post hoc sensitivity analyses were used to evaluate the impact of missing outcomes on our results. In the first scenario, the missing composite outcomes were assigned the rate of the cohort of study participants. In the second scenario, the missing composite outcomes were assigned the rate of the control arm
Secondary Outcome
Emergency department visits occurred within 30 days after discharge for 14.2% (81/570) of patients in the control group and 8.6% (40/464) of patients in the telemonitoring group (absolute risk difference, − 5.6% [95% CI, − 9.4 to −1.8%]; RR, 0.61 [95% CI, 0.42 to 0.87]; P = .005) (Table 2), again showing fewer visits for the telemonitoring arm.
Interim Analysis for Efficacy
A preplanned interim analysis for efficacy was conducted after more than half of our original, planned sample size completed the 30-day follow-up period. Evidence of efficacy was not overwhelming (χ2 P = .16); therefore, the trial was not stopped early for efficacy.
Harms
In the telemonitoring group, 115 (16.7%) patients withdrew from the study after discharge from the hospital. The most common reason specified by study participants for withdrawing was patient request because of the daily monitoring obligation.
DISCUSSION
This 2-center randomized clinical trial is, to our knowledge, the first large trial involving remote patient telemonitoring for high-risk patients discharged with any diagnosis. It was designed to assess the effectiveness of the intervention in reducing hospital readmission for any patient who was deemed to be at high risk for readmission during an index admission. We found a greater than 5% decrease for both the primary outcome of 30-day readmission and all-cause mortality, as well as for the secondary outcome of an emergency department visit within 30 days of discharge, using per-protocol analysis.
The mechanism by which telemonitoring was impactful in this study could be a result of several factors. Telemedicine and telemonitoring are complex interventions that are associated with greater contact with a patient outside of the traditional clinical setting. The benefits of the technology are likely multifactorial and related to the interventions associated with abnormal triggers, increased patient engagement with a clinical system, or the patient’s perception of having additional health care resources available. It is possible that study participants obtained greater insight into their disease process through the intervention, which may have enhanced self-care and compliance with other disease-management modalities. Patients may have also received some comfort from the presence of remote monitoring, which could have resulted in less anxiety (i.e., knowing that a health care provider was “watching over them”).
The importance of actual interventions triggered by remote monitoring should not be discounted. Intervention when vital signs were out of reference ranges could have prevented worsening of underlying medical conditions that otherwise would have resulted in a return to the hospital or emergency department within the 30-day window. The telemonitoring may have signaled which patients needed to have direct provider contact.
Most prior studies have been directed to patient populations with specific diagnoses impacted by the CMS HRRP (pneumonia, congestive heart failure, chronic obstructive pulmonary disease, total knee arthroplasty/total hip arthroplasty, and acute myocardial infarction). Several studies showed reduced readmission rates for patients with congestive heart failure, chronic obstructive pulmonary disease, lung cancer, and total knee arthroplasty/total hip arthroplasty6–16; however, other studies with similar cohorts of patients and disease processes have shown no benefit in reducing readmissions with telemonitoring. Home telemonitoring has also been studied for at-risk community-dwelling adults, also with mixed results.13,24–27 Congestive heart failure has been the most widely studied condition, and the reported effect of telemonitoring on readmissions and mortality has been mixed.15,21 Possibly, telemonitoring for certain disease processes, such as congestive heart failure, may be less impactful than telemonitoring in a general patient population. This study targeted a different patient population than these other studies—patients at high risk for readmission with any diagnosis—thus, the different results.
This study is important because we are in an era of greater availability of technological innovations to assist with complex disease management. In addition, financial incentives have been established to limit the use of hospital resources, reduce hospital admissions and readmissions, and transition a greater proportion of patient care to the outpatient environment. Hospitals routinely implement strategies designed to reduce readmissions in high-risk patient populations. Telemedicine and remote monitoring are 2 technologies that have held promise in reducing the use of hospital resources and limiting readmissions of high-risk patients. As our study targeted patients with any diagnosis who were considered high risk for readmission, the results could be applicable to a wide range of patients in other hospitals and health systems.
Several limitations to our study may have affected the outcome. In the telemonitoring group, 115 of the enrolled patients (16.7%) withdrew from the study after hospital discharge. Although this may have affected the results, this is a level of adherence that is consistent with the rates observed previously.15 Patients who withdrew most frequently said the requirement for taking daily vital signs was cumbersome. Also, patients were reluctant to enroll in the study because many already had a prolonged hospital stay and did not want to continue medical care at home. We did not evaluate nonadherence in the telemetry arm, but this could be an important consideration if telemonitoring technology is to become more widely used beyond clinical studies. Early identification of patients likely to be nonadherent would assist in managing the early costs associated with the intervention. In addition, sensitivity analysis did not show the same significance for reduction in readmissions; however, assumptions in these analyses may have affected the results. We believe using the per-protocol analysis gives the most accurate depiction of real-world results, indicating that patients who can complete 30 days of monitoring could have fewer readmissions and emergency room visits. In addition, the intensity of remote monitoring interventions was limited to daily business hours, although patient data was uploaded to a cloud-based system 24 h per day. A Cochrane review of the impact of telemonitoring on patients with congestive heart failure showed improvement in all-cause hospitalization in studies where patients were monitored during regular business hours, whereas no benefit was associated with telemonitoring 7 days a week, 24 h per day21; thus, our business-hour monitoring may have had little impact on the results of our study. Finally, our study was stopped before reaching our accrual target (target = 1900, actual = 1380). This may have resulted in a type II error (false-negative finding) when we conducted our sensitivity analysis to evaluate the impact of missing outcomes on our results, where the RR was 0.83 (95% CI, 0.68 to −1.02; P = .08).
CONCLUSION
In conclusion, we showed that 30 days of telemonitoring after discharge can reduce readmissions and emergency department visits for high-risk patients. To our knowledge, this is the first study to focus on this population, one that is often the target of interventions because of the known risk of readmission. Further studies verifying the cost-effectiveness of this intervention could confirm the value of this technology to improve transitions of care.
Supplementary Information
(DOC 218 kb)
(DOCX 22 kb)
Acknowledgments
Contributors
All contributors to the manuscript meet authorship requirements (as below).
Data Access and Responsibility
Nancy L. Dawson, MD, and Colleen T. Ball had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Nancy L. Dawson takes responsibility for the integrity of the work as a whole, from inception to published article.
Abbreviations
- CMS HRRP
Centers for Medicare & Medicaid Services Hospital Readmissions Reduction Program
- CONSORT
Consolidated Standards of Reporting Trials
- EHR
Electronic health record
- REDCap
Research Electronic Data Capture
- RR
Relative risk
Author Contributions
Nancy L. Dawson, MD: Study concept and design, drafting of the manuscript, critical revision of the manuscript for important intellectual content, obtained funding, and study supervision.
Bryan P. Hull, MD: Drafting of the manuscript and study supervision.
Priyanka Vijapura, MD: Drafting of the manuscript.
Adrian G. Dumitrascu, MD: Drafting of the manuscript.
Colleen T. Ball, MS: Study concept and design; drafting of the manuscript; statistical analysis; and administrative, technical, and material support.
Kay M. Thiemann, MBA: Study concept and design and obtained funding.
Michael J. Maniaci, MD: Drafting of the manuscript.
M. Caroline Burton, MD: Drafting of the manuscript, critical revision of the manuscript for important intellectual content.
All authors reviewed the final version of the submitted manuscript.
Funding
Funding was received from Brooks Health Foundation and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Disclaimer
Sponsors had no role in the design or implementation of the study.
Footnotes
Prior Presentations
The material in this manuscript has not been presented previously.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gupta A, Fonarow GC. The Hospital Readmissions Reduction Program-learning from failure of a healthcare policy. Eur J Heart Fail. 2018;20(8):1169–1174. doi: 10.1002/ejhf.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, Observation, and the Hospital Readmissions Reduction Program. N Engl J Med. Apr 21 2016;374(16):1543-51. 10.1056/NEJMsa1513024 [DOI] [PubMed]
- 3.Desai NR, Ross JS, Kwon JY, et al. Association Between Hospital Penalty Status Under the Hospital Readmission Reduction Program and Readmission Rates for Target and Nontarget Conditions. JAMA. Dec 27 2016;316(24):2647-2656. 10.1001/jama.2016.18533 [DOI] [PMC free article] [PubMed]
- 4.Dharmarajan K, Wang Y, Lin Z, et al. Association of Changing Hospital Readmission Rates With Mortality Rates After Hospital Discharge. JAMA. Jul 18 2017;318(3):270-278. 10.1001/jama.2017.8444 [DOI] [PMC free article] [PubMed]
- 5.America's Health Rankings. Analysis of the Dartmouth Atlas of Health Care. Internet. United Health Foundation. Accessed Aug 29, 2019. https://www.americashealthrankings.org/explore/senior/measure/hospital_readmissions_sr/state/ALL
- 6.Branowicki PM, Vessey JA, Graham DA, et al. Meta-Analysis of Clinical Trials That Evaluate the Effectiveness of Hospital-Initiated Postdischarge Interventions on Hospital Readmission. J Healthc Qual. 2017;39(6):354–366. doi: 10.1097/JHQ.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 7.Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675–84. doi: 10.1111/j.1532-5415.2004.52202.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen YJ, Narsavage GL, Frick KD, Petitte TM. Home-Telemonitoring Lung Cancer Intervention in Appalachia: A Pilot Study. Int J Chronic Dis Ther. 2016;2(2):21–30. [PMC free article] [PubMed] [Google Scholar]
- 9.Ho TW, Huang CT, Chiu HC, et al. Effectiveness of Telemonitoring in Patients with Chronic Obstructive Pulmonary Disease in Taiwan-A Randomized Controlled Trial. Sci Rep. Mar 31 2016;6:23797. 10.1038/srep23797 [DOI] [PMC free article] [PubMed]
- 10.de Toledo P, Jimenez S, del Pozo F, Roca J, Alonso A, Hernandez C. Telemedicine experience for chronic care in COPD. IEEE Trans Inf Technol Biomed. 2006;10(3):567–73. doi: 10.1109/TITB.2005.863877. [DOI] [PubMed] [Google Scholar]
- 11.Rosner BI, Gottlieb M, Anderson WN. Effectiveness of an Automated Digital Remote Guidance and Telemonitoring Platform on Costs, Readmissions, and Complications After Hip and Knee Arthroplasties. J Arthroplasty. 2018;33(4):988–996. doi: 10.1016/j.arth.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 12.Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. Sep 22 2018;392(10152):1047-1057. 10.1016/S0140-6736(18)31880-4 [DOI] [PubMed]
- 13.Nouryan CN, Morahan S, Pecinka K, et al. Home Telemonitoring of Community-Dwelling Heart Failure Patients After Home Care Discharge. Telemed J E Health. 2019;25(6):447–454. doi: 10.1089/tmj.2018.0099. [DOI] [PubMed] [Google Scholar]
- 14.Van Spall HGC, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail. 2017;19(11):1427–1443. doi: 10.1002/ejhf.765. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. Dec 9 2010;363(24):2301-9. 10.1056/NEJMoa1010029 [DOI] [PMC free article] [PubMed]
- 16.Ong MK, Romano PS, Edgington S, et al. Effectiveness of Remote Patient Monitoring After Discharge of Hospitalized Patients With Heart Failure: The Better Effectiveness After Transition -- Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016;176(3):310–8. doi: 10.1001/jamainternmed.2015.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandor A, Gomersall T, Stevens JW, et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta-analysis. Heart. 2013;99(23):1717–26. doi: 10.1136/heartjnl-2013-303811. [DOI] [PubMed] [Google Scholar]
- 18.Jaana M, Pare G, Sicotte C. Home telemonitoring for respiratory conditions: a systematic review. Am J Manag Care. 2009;15(5):313–20. [PubMed] [Google Scholar]
- 19.Yun JE, Park JE, Park HY, Lee HY, Park DA. Comparative Effectiveness of Telemonitoring Versus Usual Care for Heart Failure: A Systematic Review and Meta-analysis. J Card Fail. 2018;24(1):19–28. doi: 10.1016/j.cardfail.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Tse G, Chan C, Gong M, et al. Telemonitoring and hemodynamic monitoring to reduce hospitalization rates in heart failure: a systematic review and meta-analysis of randomized controlled trials and real-world studies. J Geriatr Cardiol. Apr 2018;15(4):298-309. 10.11909/j.issn.1671-5411.2018.04.008 [DOI] [PMC free article] [PubMed]
- 21.Inglis SC, Clark RA, Dierckx R, Prieto-Merino D, Cleland JG. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev. Oct 31 2015;(10):CD007228. 10.1002/14651858.CD007228.pub3 [DOI] [PMC free article] [PubMed]
- 22.The CONSORT Statement. Internet. Accessed Aug 29, 2019. http://www.consort-statement.org/
- 23.Dawson N, Chirila R, Bhide V, Thomas C, Cannon K, Burton M. An automated electronic tool to assess the risk of 30-day readmission: validation of predictive performance. J Clin Outcomes Manag. Oct 1 2016;23(10):449-454.
- 24.Blum K, Gottlieb SS. The effect of a randomized trial of home telemonitoring on medical costs, 30-day readmissions, mortality, and health-related quality of life in a cohort of community-dwelling heart failure patients. J Card Fail. 2014;20(7):513–21. doi: 10.1016/j.cardfail.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Kessler R, Casan-Clara P, Koehler D, et al. COMET: a multicomponent home-based disease-management programme versus routine care in severe COPD. Eur Respir J. Jan 2018;51(1). 10.1183/13993003.01612-2017 [DOI] [PubMed]
- 26.Segrelles Calvo G, Gomez-Suarez C, Soriano JB, et al. A home telehealth program for patients with severe COPD: the PROMETE study. Respir Med. 2014;108(3):453–62. doi: 10.1016/j.rmed.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Soriano JB, Garcia-Rio F, Vazquez-Espinosa E, et al. A multicentre, randomized controlled trial of telehealth for the management of COPD. Respir Med. 2018;144:74–81. doi: 10.1016/j.rmed.2018.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 218 kb)
(DOCX 22 kb)