Abstract
Background
Recent progress with smaller retrievers has expanded the ability to reach distal brain arteries. We herein report recanalization, bleeding complications and short-term clinical outcomes with the smallest currently known low profile thrombectomy device in patients with primary or secondary distal medium vessel occlusion (DMVO).
Methods
We performed a retrospective analysis of 115 patients receiving mechanical thrombectomy (MT) in DMVO using the extended Thrombolysis in Cerebral Infarction (eTICI), European Cooperative Acute Stroke Study (ECASS) II classification, The National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS) scores at admission and discharge to evaluate outcomes. Patients were stratified into three groups: (1) primary isolated distal occlusion (n=34), (2) secondary distal occlusion after MT of a proximal vessel occlusion (n=71), or (3) during endovascular treatment of aneurysms or arteriovenous malformations (AVMs) (n=10).
Results
Successful distal recanalization, defined as an eTICI score of 2b67, 2c and 3, was achieved in 74.7% (86/115) of patients. More specifically, it was 70.5% (24/34), 73.2% (52/71), and 100% (10/10) of primary DMVO, secondary DMVO after proximal MT, and rescue MT during aneurysm or AVM embolization, respectively. Symptomatic intraparenchymal bleeding occurred in 6.9% (eight patients). In-hospital mortality occurred in 18.1% (19/105) of patients with stroke. The most common cause of death was large infarct, old age, and therapy limitation.
Conclusion
Direct or rescue MT of DMVO using a very low profile thrombectomy device is associated with a high rate of successful recanalization and a reasonable rate of symptomatic hemorrhagic complication, despite a risk of 18.1% hospital mortality in elderly patients. Further trials are needed to confirm our results and assess long-term clinical outcomes.
Keywords: thrombectomy, thrombolysis, stroke, stent
Introduction
Bridging therapy, consisting of combined intravenous thrombolysis (IVT) and mechanical thrombectomy (MT), is the current gold standard treatment in patients with acute ischemic stroke (AIS) presenting with an emergent proximal large vessel occlusion (PLVO) in the anterior brain circulation.1 Although distal, medium vessel occlusions (DMVOs) tend to be less devastating than PLVOs, occlusion of an eloquent peripheral brain vessel may substantially degrade functional outcome and quality of life.1 2 It is estimated that DMVO accounts for 25–40% of all brain vessel occlusions.3 While IVT remains the primary therapy of choice, many contraindications limit its use in clinical practice. Moreover, IVT fails to recanalize one-half to two-thirds of DMVOs.3 4 Furthermore, secondary DMVOs frequently occur after IVT or MT of PLVOs.5 While MT is deemed safe and effective in proximal M2 occlusions of the middle cerebral artery (MCA), its benefit in more distal locations remains unclear.6 Considering the impact of rapid and full or near-complete recanalization on clinical outcome,7 exploring the benefit of MT for primary or secondary DMVO is warranted.3 Moreover, thromboembolic complications during embolization of brain aneurysms or arteriovenous malformation may also benefit from DMVO thrombectomy,8 especially in patients receiving dual antiplatelet medication, where thrombolysis may result in or worsen hemorrhagic complications.
Devices used in PLVO trials require microcatheters with a lumen of 0.021–0.027 inch, which can be difficult to be safely navigated in tortuous sulcal or gyral distal brain arteries measuring 1.5 mm or less in diameter.9 This is especially true when triaxial systems with guiding and intermediate catheters are used. Low profile stent retrievers adapted for DMVO that fit through a 0.017-inch lumen microcatheter include the Catchview mini (Balt, Montmorency, France) and the Mindframe Capture (Medtronic, Dublin, Ireland).10 Lately, a thinner, soft, and adaptive mesh-like stent retriever fitting through a 0.013–0.0165-inch lumen microcatheter was developed (Tigertriever 13, Rapid Medical, Yokneam, Israel). In the present study, we investigated the technical feasibility to perform distal thrombectomy in patients with stroke who were relevantly disabled and in patients who experienced DMVO as a thromboembolic complication during aneurysm or AVM embolization. We assessed recanalization and bleeding rates, as well as short-term clinical outcome in patients with stroke in a consecutive series of patients treated with the Tigertriever 13 device.
Patients and methods
This retrospective study was performed at a single tertiary neurointerventional center in all consecutive AIS and elective patients treated with the Tigertriever 13 device between December 2018 and September 2020 (21 months). Patients were classified into three groups1: primary isolated DMVO,2 secondary DMVO after MT in proximal PLVO,3 and DMVO related to thromboembolic complications during aneurysm or AVM embolization.
Acquisition of data
Demographic and clinical data were gathered from electronic medical records. Images were reviewed via the Agfa IMPAX picture archiving and communication system and randomly presented to two experienced interventional neuroradiologists (RC, PJM) blinded to the patient’s clinical characteristics. The following DMVOs were included in the present study: A3 segment of the anterior cerebral artery, M3 and M4 segments of the MCA, P2, and P3 segments of the posterior cerebral artery, as well as cerebellar arteries. Occlusions of the proximal and distal M2 segment were excluded because these are generally treated with conventional stent retrievers through 0.021 microcatheters (mostly the Solitaire 6×40 Platinum device; Medtronic). No manual or pump-assisted distal aspiration was performed.
Angiographic results after the MT procedure were interpreted according to the extended Thrombolysis in Cerebral Infarction (eTICI) classification.11 Discrepancy between the two blinded readers was resolved by consensual reading. Technical variables such as type of device, number of passes, and time from groin puncture to reperfusion were collected from the interventional reports. The primary endpoint was successful recanalization, defined as an eTICI score of 2b67, 2c, or 3. Secondary endpoints were safety and short-term clinical outcome. Safety was assessed based on the rate of hemorrhagic transformation or symptomatic bleeding, according to the European Cooperative Acute Stroke Study (ECASS) II classification on follow-up brain imaging 20–30 hours after MT.12 Peri-interventional subarachnoid contrast extravasation or subarachnoid hemorrhage (SAH) depicted on flat-panel cone-beam CT immediately after DMVO thrombectomy was also reported.13 Short-term clinical outcome in patients with stroke was evaluated by certified stroke neurologists who reported the NIHSS scores at admission, 24 hours after MT, and at discharge. Short-term functional outcome in patients with stroke was assessed using the modified Rankin Scale (mRS) at discharge from our institution. Good functional outcome was defined as an mRS of 0–2. Since the score could not be retrieved in four patients, these were included in the analysis assuming the worst-case scenario of bad short-term functional outcome (mRS 3–5). Finally, in-hospital mortality was assessed and, whenever possible, dichotomized into neurological versus other causes of death.
DMVO thrombectomy procedure
All interventions were performed under general anesthesia on biplane angiography systems (Artis Icono and Artis Zee, Siemens, Forchheim, Germany). Nimodipine (3 mg/L) was systematically used in the flushing solution of the guiding catheter to prevent vasospasm with constant care not to influence blood pressure negatively (mean arterial pressure above 90 mm Hg). After systematic placement of an 8 French balloon-guiding catheter (Flowgate 2, Stryker, Portage, MI, USA) in theinternal carotid artery or vertebral artery through a transfemoral access, a 1.3 French 0.0165-inch inner lumen 167 cm Headway Duo (MicroVention, Tustin, CA, USA) or 1.5 French Marathon microcatheter (Medtronic) was navigated over a 0.014 or 0.007 wire (Traxcess, MicroVention or Hybrid 7, Balt, respectively) beyond the occlusion site before deploying the Tigertriever 13 device. The built-in slider was used to incrementally expand the device until its distal part started to invaginate, a sign of overexpansion. The Tigertriever 13 device was then slightly relaxed and partially resheathed to pinch the thrombus before being retracted. In case of excessive tension during pulling, the device was further relaxed to avoid injuring the artery.
All interventional neuroradiologists had at least 2 years of experience. Peri-interventional perforation was managed through blood pressure reduction and temporary or permanent coiling at the operator’s discretion. Figure 1 shows a typical DMVO thrombectomy procedure in a distal MCA branch.
Figure 1.
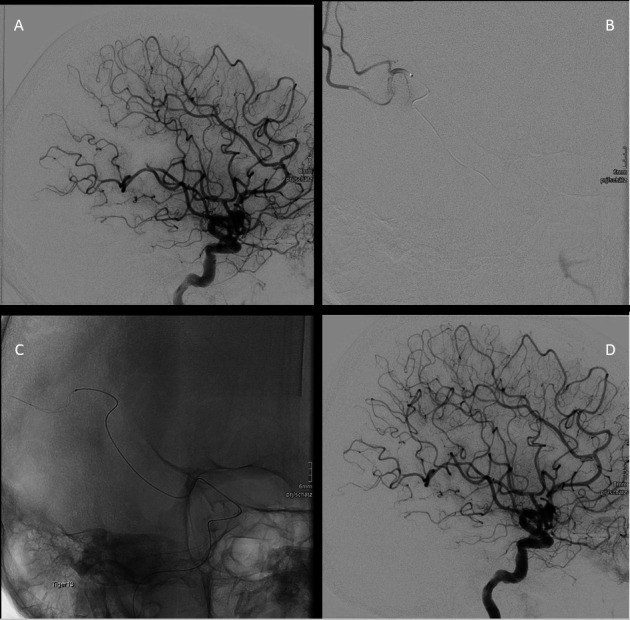
Small vessel occlusion reperfusion by Tigertriever 13 device. (A) Primary distal small vessel occlusion in the M3 branch of the middle cerebral artery. (B) Navigation of Headway duo microcatheter over Traxes wire beyond the thrombosis, and selective injection of contrast agent distal to the occluded vessel. (C) Picture depicts the expanded stent retriever Tigertriever 13, advanced through the Headway27 microcatheter into the target branch artery. (D) Reperfusion of the occluded branch after the procedure.
Statistical analysis
Statistical analysis was performed using SPSS version 23. Qualitative data were represented as frequencies and medians, while quantitative data were presented as mean±SD. Paired Student T-test and Wilcoxon signed-rank tests were used to compare means of variables. A p-value of less than 0.05 was considered to be statistically significant.
The study was performed in agreement with institutional guidelines and according to the latest version of the Helsinki Declaration. The review board waived the need for specific patient informed consent due to the retrospective nature of the study.
Results
During the 21 month study period, intracranial endovascular treatment was performed in 2056 patients, of whom 690 received MT. A total of 115 patients with DMVO were consecutively treated with the Tigertriever 13 device, 105 in the context of AIS (see table 1).
Table 1.
Demographic, clinical characteristics and treatment outcome
| Primary isolated distal occlusion (group 1) | Secondary DMVO following MT of a proximal vessel occlusion (group 2) | Distal thrombectomy during elective AVM or aneurysm embolization (group 3) | |
| Patient characteristics | n=34 | n=71 | n=10 |
| Age, mean (SD) | 76 (12) | 74 (15) | 46 (17) |
| Female sex, n (%) | 17 (50) | 41 (57.8) | 5 (50) |
| Prior IVT, n (%) | 23 (67.6) | 27 (38.0) | NA |
| NIHSS at admission, median (range) | 7 (0–22) | 11 (0–31) | NA |
| NIHSS at 24 hours, median (range) | 6 (0–31) | 8 (0–31) | NA |
| NIHSS at discharge, median (range) | 3 (0–31) | 5 (0–23) | NA |
| Successful recanalization (eTICI 2b67-3), n (%) | 24 (70.5) | 52 (73.2) | 10 (100) |
| No of passes with Tigertriever 13, median (range) | 2 (1–4) | 1 (1–5) | 1 (1–3) |
| Duration of MT procedure (min), mean (SD) | 71 (50.1) | 104 (68.2) | NA |
| Contrast agent extravasation in CBCT immediately after thrombectomy, n (%) | 16 (47.0) | 31 (43.6) | 0 |
| Asymptomatic intracranial bleeding,* n (%) | 7 (20.6) | 12 (16.9) | 0 |
| Symptomatic intraparenchymal bleeding, n (%) | 3 (8.8) | 5 (7.0) | 0 |
| In-hospital mortality, n (%) | 4 (11.8) | 15 (21.1) | NA |
*Including subarachnoid and intra-parenchymal.
AVM, arteriovenous malformation; CBCT, cone-beam CT; DMVO, distal medium vessel occlusion; eTICI, extended Thrombolysis in Cerebral Infarction; IVT, intravenous thrombolysis; MT, mechanical thrombectomy.
Of the 115 patients, 34 had a primary isolated DMVO (group 1), while 71 presented with a secondary DMVO after failed or incomplete MT of a PLVO (group 2). The remaining 10 patients experienced distal thromboembolic events during elective aneurysm or arteriovenous malformation embolization (group 3). Mean age was 72±16.5 years (range 23–99, median 77 years), 63 patients (54.8%) were female. Fifty patients with AIS (48%) received IVT prior to MT. The mean number of passes with the Tigertriever 13 device was 1.6±0.8 in all patients, 1.6±0.8 in group 1, 1.6±0.9 in group 2, and 1.6±0.9 in group 3, respectively. No relevant vasospasms requiring intra-arterial therapy were observed.
Overall, successful recanalization was achieved in 86 (74.7%) of the 115 patients (table 1). Prior IVT was equally used in patients with AIS with and without successful recanalization (p=0.51).
Of the 34 patients with a primary DMVO (group 1), successful recanalization was achieved in 24 (70.5%) patients. The most common clot location was M3 (tables 2 and 3).
Table 2.
Clot location in DMVO
| A3 | M3 | M4 | P2 | P3 | PICA | SCA | Total | |
| Primary isolated DMVO, n (%) | 1 (2.9) | 28 (82.4) | 0 (0) | 2 (5.9) | 1 (2.9) | 0 (0.0) | 2 (5.9) | 34 |
| Secondary DMVO after MT in proximal PLVO, n (%) | 9 (12.7) | 48 (67.6) | 6 (8.5) | 0 (0.0) | 2 (2.8) | 1 (1.4) | 5 (7.0) | 71 |
| DMVO related to secondary causes, n (%) | 0 (0) | 8 (80.0) | 1 (10) | 1 (10) | 0 (0.0) | 0 (0.0) | 0 (0) | 10 |
DMVO, distal medium vessel occlusion; MT, mechanical thrombectomy; PLVO, proximal large vessel occlusion.
Table 3.
Recanalization results after distal thrombectomy according to eTICI score
| 0 | 1 | 2A | 2B | 2C | 3 | Total | |
| Primary isolated DMVO, n (%) | 7 (20.6) | 0 (0.0) | 3 (8.8) | 4 (11.8) | 7 (20.6) | 13 (38.2) | 34 |
| Secondary DMVO after MT in proximal PLVO, n (%) | 10 (14.1) | 1 (1.4) | 8 (11.3) | 10 (14.1) | 16 (22.5) | 26 (36.6) | 71 |
| DMVO related to secondary causes, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 9 (90.0) | 10 |
DMVO, distal medium vessel occlusion; eTICI, extended Thrombolysis in Cerebral Infarction; MT, mechanical thrombectomy; PLVO, proximal large vessel occlusion.
Median NIHSS score at admission and discharge was 7 and 3, respectively (p=0.102 for change from admission to discharge; table 1). Fifteen of the 34 patients (44.1%) had a good functional outcome at discharge from our institution. Four patients died in hospital.
Seventy-one patients (group 2) had a DMVO after PLVO thrombectomy or presented simultaneously with PLVO and DMVO on the initial digital subtraction angiography after partial recanalization (spontaneous or after intravenous tissue plasminogen activator). Successful recanalization of such secondary DMVOs was achieved in 52 patients (73.2%). The most common occlusion site was the M3 segment of the MCA (48 patients, 67.6%; see table 2). In seven patients, a dual retriever maneuver with a Solitaire X retriever (Medtronic) and Tigertriever 13 device was necessary after two unsuccessful retrievals with the Tigertriever 13 device alone. In these cases, the Solitaire and the Tigertriever 13 device were placed in the larger and smaller division branch, respectively. Successful recanalization was achieved in four (57%) of these seven patients without peri-interventional complications. Median NIHSS at admission, after 24 hours, and at discharge was 11, 8, and 5, respectively (p=0.001 for change from admission to discharge). A good functional outcome was observed in 28 of the 71 patients (39.4%) in the combined PLVO/DMVO thrombectomy group.
All patients with distal vessel thromboembolic complications during cerebral aneurysm or AVM embolization (group 3) were successfully recanalized (10/10). The most common clot location was M3 (80%). There were no deaths or thrombectomy-related morbidity.
Active SAH caused by guidewire perforation or after a thrombectomy attempt was angiographically identified during the MT procedure in seven (6.7%) of the 105 patients with AIS. Three ceased spontaneously within 10 min after systolic blood pressure reduction below 80 mm Hg. Two needed temporary coiling, while the remaining two required definitive coil occlusion.
Subarachnoid contrast medium extravasation after DMVO MT was observed on immediate postoperative cone-beam CT (CBCT) in 47 (44.7%) of the 105 patients with AIS (groups 1 and 2). No bleeding occurred in elective patients with thromboembolic events treated by distal thrombectomy (group 3).
Symptomatic and asymptomatic intracranial hemorrhage on control brain imaging was observed in 8 (7.6%) and 19 (18.1%) of the 105 patients with AIS, respectively, independently of prior use of IVT (p=0.9).
Nineteen patients with AIS (18.1%) died during their hospital stay (table 4).
Table 4.
Cause of death in patients with acute ischemic stroke (groups 1 and 2)
| Cause | No of patients |
| Large infarction due to proximal LVO (ICA, carotid T, basilar artery, combined MCA/AC), age >80 years, and therapy limitation (palliative care) | 9 |
| Failed mechanical thrombectomy | 5 |
| Symptomatic ICH | 2 |
| Other reason (acute coronary syndrome with pulmonary edema, sepsis) | 3 |
ICA, internal carotid artery; ICH, intracranial hemorrhage; LVO, large vessel occlusion; MCA, middle cerebral artery.
Mean age at death was 81.1±11.9 years (range 54–99 years; 13 (68%) older than 80 years). The mean duration of intervention in the deceased patients with AIS was 146±83.6 min compared with 82.4±53.6 in stroke survivors. The difference was statistically significant (p=0.001). Only six of the 19 deceased patients with AIS received IVT, the remainder having presented outside of the eligible time window. Two died due to a symptomatic brain hemorrhage, one having received prior IVT.
Figure 2 shows representative examples of different contrast extravasation or bleeding types observed.
Figure 2.
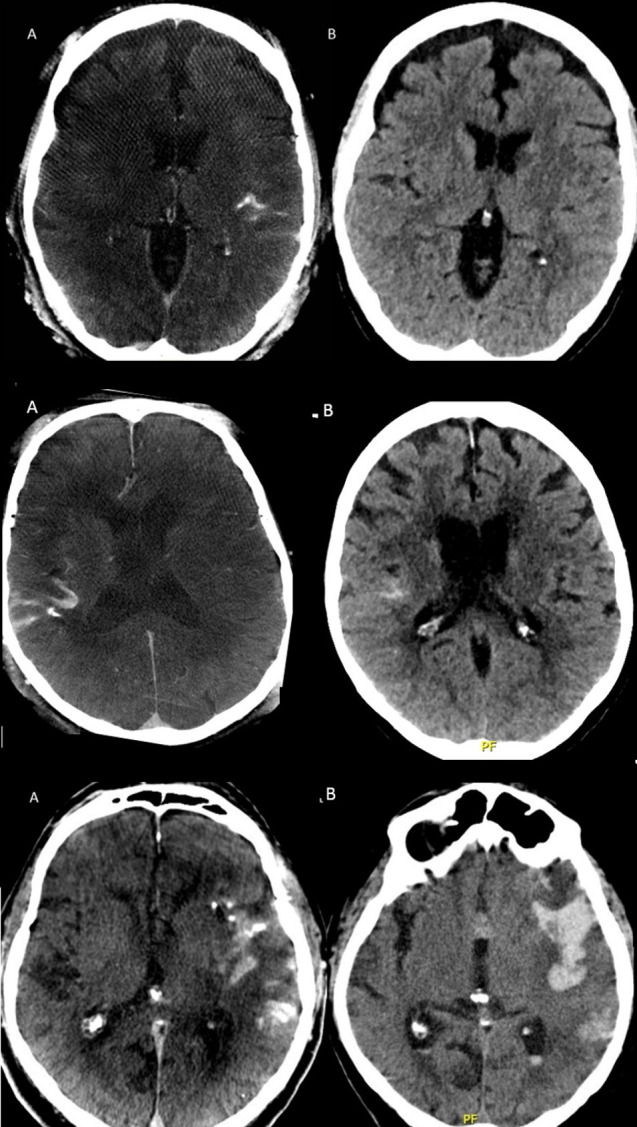
Column A (left) and B (right) displaying CBCT immediately after thrombectomy and regular CT scan after 24 hours, respectively. Upper row: minimal contrast agent extravasation in the left sylvian fissure initially with complete resolution on follow-up. Middle row: moderate contrast agent extravasation in the right sylvian fissure with marked but incomplete resorption at 24 hours (rated as asymptomatic intracranial bleeding). Lower row: important contrast agent extravasation in the left sylvian fissure with an associated temporal parenchymal hemorrhage and intraventricular extension on the control CT the following day (rated as symptomatic intraparenchymal hemorrhage). CBCT=flat panel cone-beam CT.
Discussion
The main finding of our study is that stent retriever thrombectomy in DMVO is technically feasible by experienced neurointerventionalists, as successful mechanical recanalization was achieved in 74.7% of patients overall, matching the results of PLVO thrombectomy studies.14
The literature on distal MT is scarce. Piergallini and colleagues15 reported a series of eight patients (mean age 84±11 years) with distal occlusions successfully recanalized with a Catchview Mini stent-retriever through a Headway Duo microcatheter. No treatment-related complications were reported, and only one patient underwent general anesthesia while the remainder received conscious sedation. Our cohort represents a more diverse set of patients, including rescue therapy for distal iatrogenic emboli after LVO thrombectomy and patients with distal thromboembolic complications during elective embolization procedures. Our results show that mechanically removing the distal clots resulted in a high rate of successful recanalization, which is known to be one of the strongest predictors for good functional outcome.16 Similarly, Dobrocky and colleagues17 reported on 38 patients with isolated M2 occlusions using the low profile Mindframe Capture retriever under balanced general anesthesia and conscious sedation. Successful recanalization was achieved in 74% (28 patients) and only a single case of symptomatic intracranial hemorrhage was observed. An adjusted mRS score of 0–2 at 3 months was observed in 65% of the 38 patients when taking pre-stroke disability into account. We observed a favorable short-term clinical outcome with an adjusted mRS score of 0–2 at discharge in only 41% of our patients with AIS. Since more than half of our patients with AIS (56/105) were transferred back to the referring hospitals within 72 hours after the MT procedure, it is possible that we underestimated the rate of favorable outcomes.
Interestingly, eight patients (21%) presented minor focal SAH on follow-up imaging at 24 hours in the study by Dobrocky and colleagues.17 In our cohort, cone-beam CT was systematically performed and disclosed a higher rate of immediate postoperative subarachnoid contrast extravasation in more than a third of patients with AIS. Nonetheless, this should be considered as a benign epiphenomenon related to the mechanical straightening of the distal sulcal vessels due to the tearing of some cortical arterioles during device retraction. Indeed, these short-lived SAHs either resolved or remained stable at 24 hours. More importantly, the rate of symptomatic intracranial hemorrhage remains acceptably low. While the incidence of minor SAH reported here is higher than on CT following PLVO thrombectomy, immediate postoperative cone-beam CT in PLVO studies was rarely performed and virtually never reported.18 No immediate or delayed vasospasm was observed in our series and no patient experienced further related cerebral infarction no received preventive or therapeutic oral nimodipine, further illustrating the benign nature of contrast extravasation after DMVO MT.
Of all the patients presenting with AIS (groups 1 and 2), 47.6% received intravenous thrombolysis prior to distal thrombectomy. While this did not statistically influence the rate of hemorrhagic complications in our series, it is too early to conclude that prior use of intravenous thrombolysis is safe whenever distal thrombectomy is considered. Furthermore, a recent study covering 21 hospitals showed that DMVO occurred in one in four patients receiving intravenous tissue plasminogen activator in whom distal MT was not attempted in 41.3%, thereby depriving a significant number of patients of a possible better clinical outcome.19
Intra-arterial thrombolysis may be achieved as an alternative to DMVO.20 In the Bernese experience, the TICI rate was improved in 34% of patients treated by adjunctive intra-arterial Urokinase after incomplete or failed thrombectomy. However, the absence of hemorrhagic complications in both studies does not reflect the 10% risk of symptomatic hemorrhage reported in PROACT 2.21
Distal aspiration techniques focused on DMVO have also been reported. Grieb and colleagues22 used contact aspiration (ADAPT) with the Sofia 5 French and Catalyst six access catheters for M2 occlusions in 52 patients. Additional stent retriever use increased their rate of successful recanalization from 86.5% to 92.3%. Haussen and colleagues23 used another retriever named the Trevo XP 3×20 mm retriever (Baby Trevo), studying eight patients with distal occlusions. After one to three passes, recanalization was achieved in all cases. One of the patients had a parenchymal hemorrhage type 1, and the other had a parenchymal hemorrhage type 2. None of the patients had SAH or vessel perforation. Five patients experienced intraoperative vasospasm. The same authors also conducted an observational study where they compared 3MAX aspiration with 3 mm Trevo retriever in patients with AIS and distal occlusions. They included 92 vessels that were treated with the Trevo retriever and 52 vessels with the 3MAX device. Of all the patients treated with the Trevo retriever, 58 patients (62%) had an mTICI 2b-3 grade compared with only 23 patients (44%) in the distal aspiration group, which was statistically significant. Similarly, mortality was lower in the stentretriever group (19% compared with aspiration at 24%) despite a lack of statistical significance.24 In contrast to the aforementioned studies, we did not perform additional distal aspiration in our study.
Anesthesia management during MT remains controversial.25 Based on our experience and encouraging results of prospective trials, the overwhelming majority of our patients having thrombectomy undergo general anesthesia. In the present cohort, only one patient was treated under conscious sedation. While respiratory complications remain a concern, particularly in older patients, we are convinced that general anesthesia improves the safety of distal thrombectomy by reducing the risk of wire perforation due to optimal roadmap imaging without patient movement. Nevertheless, SAH caused by guidewire perforation or after a thrombectomy attempt occurred in 6.7% patients. It is not known whether these SAHs are associated with a higher rate of post-stroke seizures or other complications as we are not able to provide long-term outcome data in our patient cohort. The rate of symptomatic brain hemorrhage in our study (7.6%) was higher compared with the rates reported in PLVO thrombectomy (HERMES, ASTER)26 27 but lower compared with the study by Haussen and colleagues using the 'Baby Trevo' device.23 It is therefore important to acknowledge that MT of distal brain arteries is technically challenging and should be performed only by experienced neurointerventionalists at higher volume centers. This is especially true with the Tigertriever 13 device which requires a different learning curve than the more classical stent retrievers.
In our study, 19 patients with AIS and DMVO died during hospitalization (18.0%). This number is similar to the reports of previous large vessel occlusion studies.28 It should be noted that the majority of deaths in our study occurred in patients who were at least 80 years old and mostly with pre-existing conditions. Since it is known that octogenarians are at greater risk of mortality,29 the safety profile of the Tigertriever 13 device may be higher in younger patients.
Finally, we previously reported the use of Tigertriever 13 device8 to retrieve misplaced embolic agents during brain AVM embolization. We herein confirm the usefulness of this device in 10 patients with iatrogenic thromboembolic complications during elective embolization of arteriovenous malformations in whom full recanalization was obtained, followed by an eventful clinical course.
The limitations of our study are its retrospective nature with inherent biases but also the self-adjudicated imaging results (no core lab evaluation) and many confounding factors that prevented us to correlate angiographic data with long-term clinical outcome (that was also lacking), although the 24 hour NIHSS appears to be an acceptable surrogate marker.30 Another concern is the learning curve and the possible over-expansion of the mesh during clot pinching and retrieval, which may increase the hemorrhagic risk compared with the usual stent retrievers. Despite these limitations, our data indicate that the Tigertriever 13 device may improve the rate of full or near-complete recanalization in patients with primary or secondary DMVO without exposing them to significant symptomatic hemorrhagic complications.
Conclusion
Direct or rescue thrombectomy of distal, medium vessel occlusion with a very low profile adaptive mesh device seems to be associated with a high rate of successful recanalization and a reasonable rate of symptomatic hemorrhagic complications. Multicenter trials are needed to confirm these results and better assess long-term clinical outcome.
Footnotes
RR and PJM contributed equally.
Contributors: RR: writing of the manuscript, a systemic search of the literature, acquisition of data, study concept, statistical analysis. PJM: gathering of information, a systemic search of the literature, study conception, writing of the manuscript, interpretation of imaging findings, acquisition of data, statistical analysis. MW: gathering of information, a systemic search of the literature, writing of the manuscript, interpretation of imaging findings. EY: gathering of information, a systemic search of literature, writing of the manuscript, interpretation of imaging findings. MM-A-A: statistical analysis, systemic search of literature, writing of the manuscript. RW: neurological examination, acquisition of data, statistical analysis, writing of the manuscript. RC: study concept, overall supervision, final edit of the manuscript, interpretation of imaging findings.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: RC received consulting fee or speaker honoraria from Balt, Medtronic, Microvention, Rapid Medical, Siemens and Stryker. PJM receives public funding related to intracranial aneurysm and vasospasm therapy from the Swiss National Science Foundation and the Innosuisse agency. RW received speaker honoria from Bristol Myers Squibb. RR received a travel grant from Boehringer Ingelheim and Microvention.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The review board waived the need for specific patient informed consent due to the retrospective nature of the study.
References
- 1. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2. Munsch F, Sagnier S, Asselineau J, et al. Stroke location is an independent predictor of cognitive outcome. Stroke 2016;47:66–73. 10.1161/STROKEAHA.115.011242 [DOI] [PubMed] [Google Scholar]
- 3. Saver JL, Chapot R, Agid R, et al. Thrombectomy for distal, medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke 2020;51:2872–84. 10.1161/STROKEAHA.120.028956 [DOI] [PubMed] [Google Scholar]
- 4. Ospel JM, Menon BK, Demchuk AM, et al. Clinical course of acute ischemic stroke due to medium vessel occlusion with and without intravenous alteplase treatment. Stroke 2020;51:3232–40. 10.1161/STROKEAHA.120.030227 [DOI] [PubMed] [Google Scholar]
- 5. Goeggel Simonetti B, Hulliger J, Mathier E, et al. Iatrogenic vessel dissection in endovascular treatment of acute ischemic stroke. Clin Neuroradiol 2019;29:143–51. 10.1007/s00062-017-0639-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sweid A, Head J, Tjoumakaris S, et al. Mechanical Thrombectomy in Distal Vessels: Revascularization Rates, Complications, and Functional Outcome. World Neurosurg 2019;130:e1098–104. 10.1016/j.wneu.2019.07.098 [DOI] [PubMed] [Google Scholar]
- 7. Mokin M, Ansari SA, McTaggart RA, et al. Indications for thrombectomy in acute ischemic stroke from emergent large vessel occlusion (ELVO): report of the SNIS standards and guidelines Committee. J Neurointerv Surg 2019;11:215–20. 10.1136/neurintsurg-2018-014640 [DOI] [PubMed] [Google Scholar]
- 8. Ho FL, Chapot R. Removal of distal fragments of liquid embolic agents during arteriovenous malformation embolization using the TIGERTRIEVER 13: a technical report. J Neurointerv Surg 2020;12:794–7. 10.1136/neurintsurg-2019-015474 [DOI] [PubMed] [Google Scholar]
- 9. Caroff J, King RM, Arslanian R, et al. Microcatheter navigation through the clot: does size matter? J Neurointerv Surg 2019;11:271–4. 10.1136/neurintsurg-2018-014105 [DOI] [PubMed] [Google Scholar]
- 10. Cerejo R, John S, Bauer A, et al. Emergent mechanical thrombectomy for acute stroke using the Mindframe capture Lp system: initial single-center experience. J Neurointerv Surg 2016;8:1178–80. 10.1136/neurintsurg-2015-012078 [DOI] [PubMed] [Google Scholar]
- 11. Zaidat OO, Lazzaro MA, Liebeskind DS, et al. Revascularization grading in endovascular acute ischemic stroke therapy. Neurology 2012;79:S110–6. 10.1212/WNL.0b013e3182695916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). second European-Australasian acute stroke study Investigators. Lancet 1998;352:1245–51. 10.1016/s0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 13. Shi Z-S, Duckwiler GR, Jahan R, et al. Early blood-brain barrier disruption after mechanical thrombectomy in acute ischemic stroke. J Neuroimaging 2018;28:283–8. 10.1111/jon.12504 [DOI] [PubMed] [Google Scholar]
- 14. Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316:1279–88. 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 15. Piergallini L, Cervo A, Macera A, et al. Distal thrombectomy with Headway duo 167 cm and Catchview mini stent retriever: a technical note. World Neurosurg 2020;137:425–8. 10.1016/j.wneu.2020.01.205 [DOI] [PubMed] [Google Scholar]
- 16. Jang KM, Nam TK, Ko MJ, et al. Thrombolysis in cerebral infarction grade 2C or 3 represents a better outcome than 2B for endovascular thrombectomy in acute ischemic stroke: a network meta-analysis. World Neurosurg 2020;136:e419–39. 10.1016/j.wneu.2020.01.020 [DOI] [PubMed] [Google Scholar]
- 17. Dobrocky T, Bellwald S, Kurmann R, et al. Stent retriever thrombectomy with Mindframe capture Lp in isolated M2 occlusions. Clin Neuroradiol 2020;30:51–8. 10.1007/s00062-018-0739-4 [DOI] [PubMed] [Google Scholar]
- 18. Pfaff J, Herweh C, Pham M, et al. Mechanical thrombectomy using a combined CT/C-arm X-ray system. J Neurointerv Surg 2016;8:621–5. 10.1136/neurintsurg-2015-011744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flint AC, Avins AL, Eaton A, et al. Risk of distal embolization from tPA (tissue-type plasminogen activator) administration prior to endovascular stroke treatment. Stroke 2020;51:2697–704. 10.1161/STROKEAHA.120.029025 [DOI] [PubMed] [Google Scholar]
- 20. Kaesmacher J, Bellwald S, Dobrocky T, et al. Safety and efficacy of intra-arterial urokinase after failed, unsuccessful, or incomplete mechanical thrombectomy in anterior circulation large-vessel occlusion stroke. JAMA Neurol 2020;77:318–26. 10.1001/jamaneurol.2019.4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Furlan A, Higashida R, Wechsler L, et al. Intra-Arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA 1999;282:2003–11. 10.1001/jama.282.21.2003 [DOI] [PubMed] [Google Scholar]
- 22. Grieb D, Schlunz-Hendann M, Brinjikji W, et al. Mechanical thrombectomy of M2 occlusions with distal access catheters using adapt. J Neuroradiol 2019;46:231–7. 10.1016/j.neurad.2019.01.096 [DOI] [PubMed] [Google Scholar]
- 23. Haussen DC, Lima A, Nogueira RG. The Trevo XP 3×20 mm retriever ('Baby Trevo') for the treatment of distal intracranial occlusions. J Neurointerv Surg 2016;8:295–9. 10.1136/neurintsurg-2014-011613 [DOI] [PubMed] [Google Scholar]
- 24. Haussen DC, Eby B, Al-Bayati AR, et al. A comparative analysis of 3MAX aspiration versus 3 MM Trevo retriever for distal occlusion thrombectomy in acute stroke. J Neurointerv Surg 2020;12:279–82. 10.1136/neurintsurg-2019-014990 [DOI] [PubMed] [Google Scholar]
- 25. Schönenberger S, Möhlenbruch M, Pfaff J, et al. Sedation vs. Intubation for Endovascular Stroke TreAtment (SIESTA) - a randomized monocentric trial. Int J Stroke 2015;10:969–78. 10.1111/ijs.12488 [DOI] [PubMed] [Google Scholar]
- 26. Lapergue B, Blanc R, Gory B, et al. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the aster randomized clinical trial. JAMA 2017;318:443–52. 10.1001/jama.2017.9644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Román LS, Menon BK, Blasco J, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol 2018;17:895–904. 10.1016/S1474-4422(18)30242-4 [DOI] [PubMed] [Google Scholar]
- 28. Villwock MR, Padalino DJ, Deshaies EM. Trends in mortality following mechanical thrombectomy for the treatment of acute ischemic stroke in the USA. J Neurointerv Surg 2016;8:457–60. 10.1136/neurintsurg-2015-011674 [DOI] [PubMed] [Google Scholar]
- 29. Awad A-W, Kilburg C, Ravindra VM, et al. Predicting death after thrombectomy in the treatment of acute stroke. Front Surg 2020;7:16. 10.3389/fsurg.2020.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meyer L, Broocks G, Bechstein M, et al. Early clinical surrogates for outcome prediction after stroke thrombectomy in daily clinical practice. J Neurol Neurosurg Psychiatry 2020;91:1055–9. 10.1136/jnnp-2020-323742 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.


