Abstract
Objective
To explore if changes over time of plasma phosphorylated tau (p-tau)181 and neurofilament light chain (NfL) predict future tau and amyloid β (Aβ) PET load and cognitive performance, we studied a subsample of the Alzheimer’s disease (AD) neuroimaging cohort with longitudinal blood peptide assessments.
Methods
Eight hundred and sixty-five AD Neuroimaging Initiative participants were included. Using established AD cut-points for the cerebrospinal fluid concentrations of Aβ42, total-tau and p-tau181, subjects were classified according to the National Institute on Aging-Alzheimer’s Association research framework, grouping markers into those of Aβ deposition (A), tau pathology (T) and neurodegeneration (N). Analysis of variance was used to compare the plasma biomarker data between the ATN groups. The rate of change over time of p-tau181 and NfL was obtained from linear mixed effects models and compared between the ATN groups. Linear regression analysis was used to investigate the association of baseline plasma biomarker concentrations and rates of change with future PET tau and Aβ load and cognitive performance.
Results
P-tau181 and NfL plasma concentrations increased along the AD spectrum, but only NfL showed greater rates of change in AD patients versus controls. Cognitive performance was associated cross-sectionally with NfL in all subgroups, and with p-tau181 only in AD spectrum individuals. The baseline concentrations of both plasma markers predicted PET Aβ and tau load and cognitive performance. The rate of change of NfL predicted future PET tau and cognitive performance.
Conclusions
P-tau and NfL behave differently within the same individuals over time and may therefore offer complementary diagnostic information.
Trial registration number
Introduction
Alzheimer’s disease (AD) is characterised by the cerebral accumulation of the misfolded amyloid-β (Aβ) and phosphorylated tau (p-tau) proteins in extracellular senile plaques and intraneuronal neurofibrillary tangles respectively. A diagnosis of definite AD requires the confirmation of those pathological hallmarks at autopsy, but an accurate ascertainment of AD diagnosis in vivo has become available due to the development of reliable cerebrospinal fluid (CSF) and PET measures of Aβ and tau burden. Those markers have excellent diagnostic accuracy in clinical populations, but they involve an invasive lumbar puncture or exposure to ionising radiation, and they are not readily available outside of specialist settings.
As the clinical use of AD-modifying drugs to reduce Aβ accumulation has recently turned into reality, a widely applicable and accurate method for risk-adapted, personalised prevention and therapy becomes imperative. A blood-based biomarker algorithm could be of critical importance, due to the ease of measurement and cost-effectiveness compared with the current gold-standard assessments. Recent work demonstrated promising results of new ultrasensitive assay technology-based measures of Aβ42/40,1 p-tau181,2–4 p-tau2175 (but not total tau, (t-tau))6 and neurofilament light chain (NfL)7 in clinical and population-based cohorts8; however, many aspects of the blood-based measures remain to be explored in further research, including the longitudinal trajectories of the measurements within the same individuals and their relation to cognitive decline and changes of PET tau load.9
We used the biomarker-based classification system of the National Institute on Aging-Alzheimer’s Association (NIA-AA) research framework10 to contrast groups with different biomarker profiles. Our main aim was to explore the changes over time of p-tau181 and NfL measured in plasma within the NIA-AA biomarker groups and to explore how the peptide baseline concentrations and temporal trajectories were related to memory performance, tau and PET Aβ accumulation up to 6 years later.
Methods
Study design
The dataset analysed here was obtained from the ongoing AD Neuroimaging Initiative (ADNI) multisite observational study, launched in October 2004. Between September 2010 and July 2016, plasma NfL/p-tau181 and CSF biomarkers were quantified at baseline and in up to 4 yearly follow-up (FU) assessments (NfL and p-tau181 only). After approximately 5 years from baseline, tau and Aβ-PET were acquired (mean 5.1, SD=1.6 years) and cognitive performance (mean=5.8, SD=1.2 years) was assessed.
Participants
All available ADNI participants with baseline plasma p-tau181 and NfL and CSF Aβ42, t-tau and p-tau181 concentration measurements were included, resulting in a final dataset of N=865. The detailed ADNI inclusion/exclusion criteria can be found elsewhere (https://adni.loni.usc.edu/wp-content/uploads/2008/07/adni2-procedures-manual.pdf). The participants were divided into subgroups following the recommendations of the NIA-AA research framework.10 At baseline each participant was assigned to a group defined by their CSF biomarker profile according to the A(Aβ)/T(tau)/N(neurodegeneration) classification system, irrespective of clinical status.
CSF biomarker cut-points used for group definitions were: Aβ42, 980 pg/mL; t-tau, 266 pg/mL; p-tau181, 24 pg/mL.11 Individuals with an A−TN− profile (N=260, 129 female, mean age=71 years) were considered as healthy controls. To study plasma p-tau181 and NfL along the AD spectrum, A+TN− (N=173, 62 females, mean age=73 years) and A+TN+ (N=300, 143 females, mean age=74 years) groups were defined. The A−TN+ (N=132, 65 females, mean age=74 years) group was considered as suspected non-AD pathophysiology (SNAP). Study dropouts at the yearly FU visits resulted in the following missing biomarker assessments (total number of individuals in the study): FU1, 104 (761); FU2, 198 (563); FU3, 192 (371); FU4, 223 (148). Aβ- and tau-PET results and cognitive scores were only available for 103, 196 and 503 individuals respectively.
Memory performance was evaluated using the validated summary metric for memory ADNI-mem12 (derived from: Rey-Auditory-Verbal-Learning-Test, AD-Assessment-Scale-cognitive-subscale, MMSE and Wechsler-Memory-Test-logical-memory-I).
Fluid biomarker analysis methods
Plasma p-tau181 and NfL concentrations were both analysed using the ultrasensitive Single-Molecule-Array (SIMOA) technology platform by Professors Henrik Zetterberg and Kaj Blennow at the University of Gothenburg, Sweden (details available elsewhere, http://adni.loni.usc.edu). Briefly, plasma p-tau181 was analysed with an in-house assay to measure N-terminal to mid domain forms of p-tau181 using two monoclonal antibodies. Plasma samples were measured in singlicate, calibrators as duplicates.3 For the plasma NfL assay a combination of monoclonal antibodies and purified bovine NfL as calibrator was used with all samples measured as duplicate. All samples were above the limit of detection, analytical sensitivity was <1 pg/mL.13
The CSF concentrations of Aβ42, t-tau and p-tau181 were measured in aliquoted samples using an electrochemiluminescence immunoassay on an Elecsys Cobas e 601 analyzer (Roche Diagnostics, Penzberg, Germany) with a single lot of each reagent for each of the three measured biomarkers (details available elsewhere, http://adni.loni.usc.edu). TaqMan quantitative PCR assays were used for genotyping the APOE nucleotides 334 TC and 472 CT with an ABI 7900 real-time thermocycler (Applied Biosystems, Foster City, California, USA) using DNA freshly prepared from whole blood samples. The results were dichotomised into carriers and non-carriers of the APOE ε4 allele.
PET acquisition and analysis
Whole brain anatomical T1-MPRAGE or IR-SPGR MRI (Slice thickness 1–1.2 mm; TR, 2300–3000 ms; TE 2.9–3.5 ms; FoV, 256×256 cm2) and 18F-florbetapir Aβ-PET (AV45) and 18F-flortaucipir (AV-1451) tau-PET were acquired. PET scans were conducted using the following parameters: florbetapir, 370 MBq (10.0 mCi)±10%, 20 min (4×5 min frames) acquisition at 50–70 min postinjection; flortaucipir, 370 MBq (10.0 mCi)±10%, 30 min (6×5 min frames) acquisition at 75–105 min postinjection (details available elsewhere, http://adni.loni.usc.edu/).
All T1-weighted images were processed in the FreeSurfer (V.6.0) recon-all pipeline, including registration to standard space, intensity normalisation, brain extraction, tissue type classification, surface reconstruction and probabilistic anatomical labelling.14 The segmentations and parcellations were visually inspected for accuracy in anatomic labelling and surface reconstruction. N=45 individuals were excluded due to severe inaccuracies.
Cortical tau and Aβ depositions were assessed by 18F-AV1451/AV45 PET imaging using the PETSurfer tool in FreeSurfer.15 16 We selected only PET scans with an available anatomical MRI within a period±180 days from the date of the corresponding PET. All 18F-AV1451/AV45 scans were downloaded in the most fully preprocessed format available on LONI (https://ida.loni.usc.edu/; series description: AV1451/AV45 Coreg, Avg, Std Img and Vox Siz, Uniform Resolution) and were coregistered to the corresponding anatomical MRI. Partial volume correction was performed using the previously created high-resolution segmentation of the anatomical MRI using the Muller-Gartner method.17 Cerebellar cortex was used as the reference region for intensity scaling.
To perform surface-based analysis, the results were sampled onto the FreeSurfer fsaverage surface, halfway between the white and pial surface via the individual surface. The results were spatially smoothed on the surface using a 5 mm FWHM Gaussian kernel. We calculated a composite meta region of interest (ROI) using the standardised uptake value ratio in FreeSurfer-derived atlas regions (Desikan-Killiany Atlas), extracted for each participant for tau-PET and Aβ-PET. We calculated composite meta ROIs for tau and Aβ PET. The tau PET meta ROI included the median uptake of voxels in the entorhinal, amygdala, parahippocampal, fusiform, inferior temporal and middle-temporal-ROIs.18 The Aβ PET meta ROI included four large brain regions (frontal, anterior/posterior cingulate, lateral parietal, lateral temporal) as previously shown.19
Statistical analysis
All statistical analyses were performed in R (https://www.r-project.org/). The concentration measures of all CSF and blood markers were not normally distributed and were therefore log(10) transformed for the further analyses. We used analysis of variance (ANOVA) to compare the baseline sociodemographic and biomarker data between the Aβ deposition, tau pathology and neurodegeneration (ATN) groups and chi square test to compare the dichotomous variables APOE and sex. All post hoc tests were Bonferroni corrected for multiple comparisons, as appropriate.
To assess the rate of change over time of plasma p-tau181 and NfL concentrations, in a first step we obtained individual slopes for each subject to be included in the subsequent analyses, that is, ANOVA, partial correlations and linear regressions, using a linear mixed effects model (LMEM, lme4 R package). LMEM results in the best estimates of slopes for all subjects in the study even if FU data in a few participants is incomplete due to missed FU visits or dropouts. The model included time (ie, years from baseline) and subject as a random factor and ATN group as fixed factor. Additionally, the interaction between time and ATN group was included. We performed the same model within the ATN groups, omitting the factor ATN group and the interaction between ATN group and time.
We used ANOVA in a second analysis step to compare biomarker slopes between ATN groups. Linear regression models were calculated to assess if the baseline concentration and the rate of change of the plasma concentrations of p-tau181 and NfL predicted ADNI-mem and PET tau and Aβ load. All analyses were adjusted for age, sex and the time difference between baseline and FU measurements, as appropriate. When analysing longitudinal cognitive deterioration using ADNI-mem score, the regression model was adjusted for ADNI-mem baseline score. In a separate model we tested how the results change when considering clinical stage in the analysis using clinical dementia rating (CDR) global score. All analyses in this model were additionally adjusted for CDR global score (online supplemental eTable 2).
jnnp-2020-325537supp001.pdf (414.2KB, pdf)
Vertex-wise partial correlation analyses of the normalised tau and Aβ-PET data were performed for the entire cohort and within the individual ATN groups using permutation-based Pearson correlation (N=5000) in FSL-PALM (Permutation Analysis of Linear Models),20 applying threshold free cluster enhancement21 and family-wise error rate (FWE) to control for false positives (p<0.05). All analyses were adjusted for age, sex and the time difference between baseline biomarker assessment and PET scan (mean=5.1 years, SD=1.2).
Results
Baseline differences of plasma p-Tau181 and NFL concentrations between the ATN groups
Sociodemographic and clinical characteristics of the study population are shown in table 1. Significant differences at baseline between the ATN groups were detected for p-tau181 (p<0.001) and NfL (p<0.001) plasma concentrations. The post hoc pairwise comparisons revealed significant differences between the ATN groups for p-tau181, showing increasing mean concentrations with A−TN−<A+TN−<A−TN+<A+TN+ (p<0.001 for all comparisons except A+TN vs A−TN+ with p=0.43)). NfL followed that same pattern of increasing plasma concentrations with A−TN−<A+TN−<A−TN+<A+TN+ (p<0.001 for all comparisons except A+TN vs A−TN+ with p=0.96). The baseline differences for p-tau181 and NfL plasma concentrations are shown in online supplemental eFigure 1A, B and table 1.
Table 1.
Characteristics of the study cohort
| Variable | Entire cohort | A−TN– | A+TN– | A+TN+ | A−TN+ | P value |
| Numbers (N) | 865 | 260 | 173 | 300 | 132 | |
| Age at bl, mean (SD) in years | 73 (7) | 71 (7) | 73 (7) | 74 (7) | 74 (8) | <0.001 * |
| Female no (%) | 399 (46) | 129 (50) | 62 (36) | 143 (48) | 65 (49) | 0.02* |
| Education, mean (SD) years | 16.3 (2.6) | 16.4 (2.6) | 16.5 (2.7) | 16 (2.7) | 16.3 (2.4) | 0.12* |
| APOE genotype positive, no (%) | 478 (55) | 211 (81) | 88 (51) | 88 (30) | 91 (69) | <0.001† |
| Plasma p-tau181 (pg/mL), mean (SD) | 19 (18) | 13 (9) | 17 (10) | 24 (11) | 20 (39) | <0.001* |
| Plasma NfL (pg/mL), mean (SD) | 39 (21) | 33 (19) | 38 (17) | 45 (19) | 40 (30) | <0.001* |
| CSF Aβ42 (pg/mL), mean (SD) | 1106 (607) | 1567 (396) | 665 (197) | 662 (166) | 1784 (641) | <0.001* |
| CSF t-tau (pg/ml), mean (SD) | 282 (131) | 191 (40) | 181 (44) | 389 (124) | 349 (106) | <0.001* |
| CSF p-tau181 (pg/mL), mean (SD) | 27 (14) | 17 (4) | 17 (5) | 39 (13) | 32 (11) | <0.001* |
| MMSE, score, mean (SD) | 27 (3) | 29 (2) | 28 (2) | 26 (3) | 28 (2) | <0.001* |
| ADNI-mem, score, mean (SD) | 0.4 (0.9) | 0.9 (0.7) | 0.4 (0.8) | −0.3 (0.9) | 0.7 (0.8) | <0.001* |
| CDR score, mean (SD) | 0.39 (0.32) | 0.28 (0.26) | 0.37 (0.29) | 0.55 (0.35) | 0.27 (0.29) | <0.001* |
*ANOVA.
†χ² test on independence.
Aβ, amyloid β; A+/−, amyloid-β positive/negative; ADNI, Alzheimer’s Disease Neuroimaging Initiative; ANOVA, analysis of variance; ATN, Aβ deposition, tau pathology and neurodegeneration; bl, baseline; CDR, clinical dementia rating; CSF, cerebrospinal fluid; mem, memory; MMSE, Mini-Mental State Examination; NfL, neurofilament light protein; p-tau181, plasma phosphorylated tau 181; sob, sum of boxes; TN+/−, tau/neurodegeneration positive/negative.
Baseline associations between cognitive performance and p-tau181 and NfL
Using a linear model corrected for age and sex, we tested if baseline p-tau181 and NfL plasma concentrations predicted ADNI-mem within the ATN subgroups. The analysis indicated that p-tau181 was predictive of ADNI-mem in the A+TN and A+TN+ group but not in the A−TN− and A−TN+ group. NfL predicted ADNI-mem in the A−TN−, A+TN and A+TN+ group but not in the A−TN+ group (online supplemental eTable 1 and eFigure 1C, D).
Rate of change of plasma p-Tau181 and NFL concentrations in the ATN groups
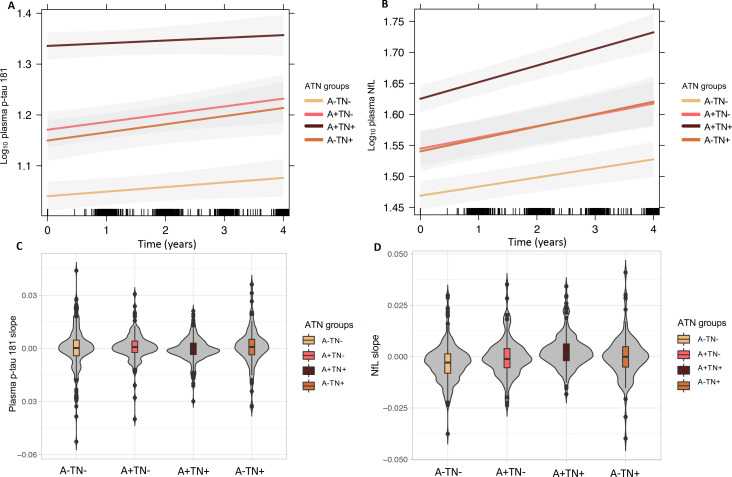
We calculated the predicted trajectories of plasma p-tau181 and NfL concentrations over a FU period of approximately 4 years in the different ATN groups using an LMEM, adjusted for age and sex (figure 1A, B). The ANOVA analysis comparing plasma biomarker slopes between the ATN groups revealed no significant differences for p-tau181 (p=0.13), whereas NfL rate of change differed significantly between the groups (p<0.001). Bonferroni corrected post hoc pairwise comparisons revealed the highest rate of change in A+TN+ (mean=2.5×10−3, SD=7×10−3) with a significantly higher rate of change compared with A−TN− (mean=−2.7×10−3, SD=8.6×10−3; p<0.001), A+TN− (mean=−3.2×10−4, SD=8.3×10−3; p=0.002) and A−TN+ (mean=−4.8×10−5, SD=9.8×10−3; p=0.02). The plasma NfL rate of change was also higher in A+TN− compared with A−TN− (p=0.02) and in A−TN+ compared with to A−TN− (p=0.02). A boxplot comparing the normalised rate of change between the ATN groups is presented in figure 1C, D.
Figure 1.
Longitudinal trajectories of plasma p-Tau181 and plasma NfL concentrations in the different ATN groups (A) log10 plasma p-Tau181 (B) log10 plasma NFL. Rate of change over time (slope) obtained using a LMEM (C) plasma p-Tau181 (normalised) and (D) plasma NfL concentrations (normalised) comparing the ATN groups. A+/−, amyloid-β positive/negative; ATN, Aβ deposition, tau pathology and neurodegeneration; LMEM, linear mixed effects model; NfL, neurofilament light protein, picogram per millilitre; p-Tau181, tau phosphorylated at threonine 181; TN+/−, tau/neurodegeneration.
Prediction of cognitive deterioration by plasma p-Tau181 and NFL
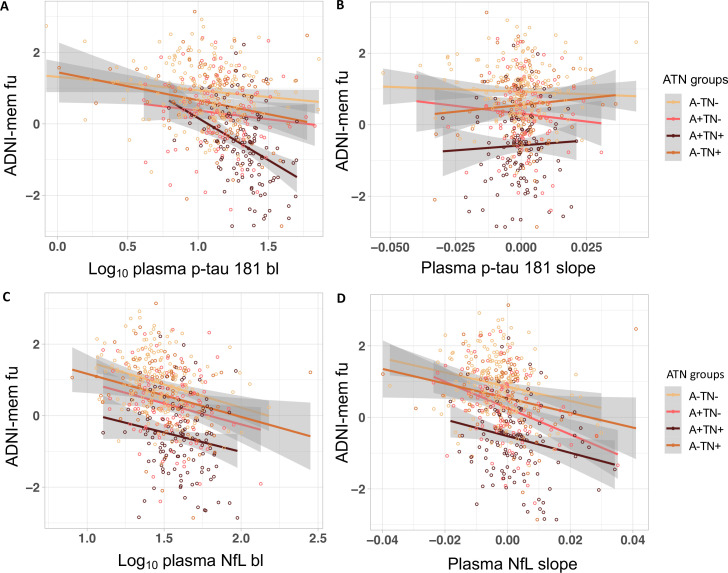
We analysed if the baseline plasma concentrations and rates of change over time of p-tau181 and NfL predicted ADNI-mem 5.8 years after baseline on average. For the entire study cohort linear regression analyses, corrected for age, sex, baseline ADNI-mem score and the time difference between ADNI-mem baseline and FU showed that plasma p-tau181 baseline concentration, plasma NfL baseline concentration and plasma NfL rate of change was predictive of ADNI-mem. Within the ATN groups, significant associations were found within A−TN− for plasma NfL rate of change, within A+TN+ for plasma p-tau181 baseline concentration and plasma NfL rate of change and within A−TN+ for plasma NfL baseline concentration (figure 2, table 2). When adjusting for CDR in a second model, the results for the entire study cohort remained unchanged in the ATN groups. NfL baseline was additionally predictive of cognitive deterioration in A+TN+ (online supplemental eTable 2).
Figure 2.
Associations between (A) log10 plasma p-Tau181 baseline concentration (B) plasma p-Tau181 rate of change (slope) (C) log10 plasma NfL baseline concentration and (D) plasma NfL rate of change (slope) and ADNI-mem after a mean of 5.8 (SD=1.6) Years in the different ATN groups. ADNI-mem, composite memory score. A+/−, amyloid-β positive/negative; ADNI, Alzheimer’s Disease Neuroimaging Initiative; bL, baseline; Fu, follow-up; NFL, neurofilament light protein; p-Tau181, tau phosphorylated at threonine 181; TN+/−, Tau/Neurodegeneration positive/negative.
Table 2.
Prediction of ADNI-mem, tau (flortaucipir) and Aβ (florbetapir) PET meta ROI SUVR tracer uptake for plasma p-Tau181 and plasma NfL baseline concentrations and rates of change over time (slope)
| Variable | Entire cohort (N=503) | A−TN− (N=192) | A+TN− (N=101) | A+TN+ (N=121) | A−TN+ (N=89) | |||||
| ADNI-mem | B (s.B) | p value | B (s.B) | P value | B (s.B) | P value | B (s.B) | P value | B (s.B) | P value |
| Plasma p-tau181 log10 baseline | −0.64 (-0.16) | <0.001 | −0.13 (-0.05) | 0.30 | −0.28 (-0.07) | 0.29 | −1.59 (-0.26) | <0.001 | −0.42 (-0.12) | 0.12 |
| Plasma NfL log10 baseline | −0.88 (-0.16) | <0.001 | −0.38 (-0.09) | 0.08 | −0.22 (-0.04) | 0.56 | −0.88 (-0.14) | 0.07 | −1.23 (-0.26) | <0.001 |
| Plasma p-tau181 slope | −2.49 (-0.02) | 0.38 | −3.47 (-0.05) | 0.23 | −5.39 (-0.05) | 0.40 | 0.82 (0.01) | 0.93 | −2.77 (-0.03) | 0.69 |
| Plasma NfL slope | −12.89 (-0.11) | <0.001 | −10.61 (-0.12) | 0.01 | −8.16 (-0.08) | 0.24 | −19.30 (-0.17) | 0.02 | 0.94 (0.01) | 0.91 |
| Tau PET | Entire cohort (N=196) | A−TN− (N=83) | A+TN− (N=40) | A+TN+ (N=41) | A−TN+ (N=32) | |||||
| Plasma p-tau181 log10 baseline | 0.68 (0.28) | <0.001 | 0.03 (0.05) | 0.68 | 0.15 (0.10) | 0.59 | 2.23 (0.35) | 0.03 | 0.24 (0.14) | 0.50 |
| Plasma NfL log10 baseline | 0.66 (0.18) | 0.02 | 0.08 (0.08) | 0.55 | −0.09 (-0.04) | 0.82 | 1.22 (0.18) | 0.29 | 0.07 (0.03) | 0.89 |
| Plasma p-tau181 slope | 2.92 (0.04) | 0.55 | 0.14 (0.01) | 0.93 | 10.04 (0.21) | 0.17 | 7.69 (0.05) | 0.77 | 2.46 (0.04) | 0.82 |
| Plasma NfL slope | 23.21 (0.27) | <0.001 | 0.83 (0.04) | 0.75 | 0.82 (0.25) | 0.12 | 49.20 (0.32) | 0.047 | 19.52 (0.31) | 0.097 |
| Amyloid PET | Entire cohort (N=103) | A−TN− (n=51) | A+TN− (n=19) | A+TN+ (n=16) | A−TN+ (N=17) | |||||
| Plasma p-tau181 log10 baseline | 0.76 (0.26) | 0.01 | −0.04 (-0.03) | 0.86 | 0.77 (0.18) | 0.52 | 0.56 (0.21) | 0.45 | 0.07 (0.04) | 0.88 |
| Plasma NfL log10 baseline | 1.08 (0.23) | 0.03 | 0.31 (0.14) | 0.81 | 0.53 (0.07) | 0.80 | 0.35 (0.10) | 0.70 | 0.10 (0.04) | 0.91 |
| Plasma p-tau181 slope | 5.88 (0.07) | 0.50 | 0.01 (0.12) | 0.44 | 57.49 (0.36) | 0.11 | −13.94 (-0.15) | 0.65 | −5.45 (-0.10) | 0.71 |
| Plasma NfL slope | 14.92 (0.14) | 0.16 | 8.76 (0.17) | 1.18 | 12.24 (0.09) | 0.69 | 10.04 (-0.13) | 0.62 | 19.69 (0.32) | 0.15 |
Analyses adjusted for age, sex and the time difference between baseline and ADNI-mem assessment/PET scan. Baseline cognition was included in the model when predicting ADNI-mem at follow-up.
Aβ, amyloid β; A+/−, Amyloid-β positive/negative; ADNI, Alzheimer’s Disease Neuroimaging Initiative; ATN, Aβ deposition, tau pathology and neurodegeneration; B, beta (slope); NfL, neurofilament light protein; p-tau181, plasma phosphorylated tau 181; s.B, standardised beta; TN+/−, Tau/Neurodegeneration positive/negative.
Prediction of Tau and Aβ-PET load by p-tau181 and NfL plasma concentrations
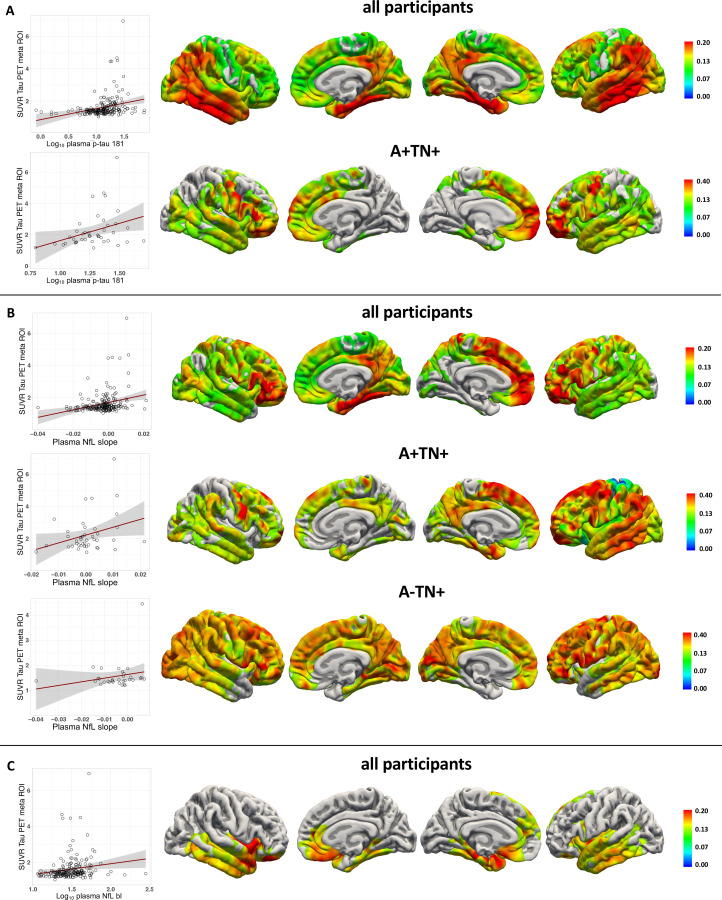
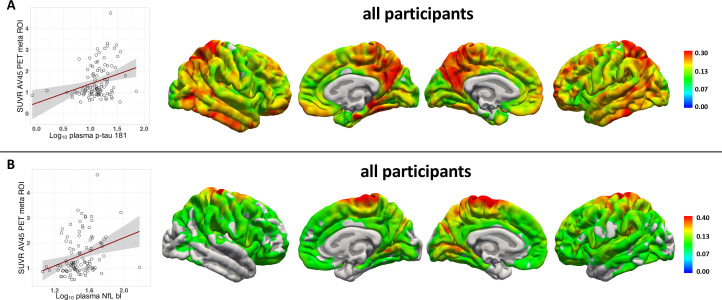
We analysed if the baseline plasma concentrations and rates of change over time of p-tau181 and NfL predicted PET tau and Aβ load after 5.1 years on average in a linear regression analysis, adjusted for age, sex, and the time difference between plasma sampling and PET scan. In the entire cohort, p-tau181 and NfL baseline plasma concentration and plasma NfL rate of change predicted tau-PET tracer uptake. Baseline plasma p-tau181 and baseline plasma NfL concentration predicted Aβ-PET tracer uptake. Furthermore, in the A+TN+ group, plasma p-tau181 baseline concentration and plasma NfL rate of change were predictive of tau-PET tracer uptake (figure 3, table 2 for tau-PET and figure 4 for Aβ-PET). In a second model CDR was included to consider clinical severity in the analysis. In the entire cohort, the results remained largely unchanged except that NfL baseline was not predictive for both tau-PET and Aβ-PET. Within the ATN groups, p-tau181 in the A+TN+ group and NfL slope in the A−TN+ group was not predictive for tau-PET tracer uptake (online supplemental eTable 2). To determine the spatial associations of plasma biomarkers with tau- and Aβ-PET tracer uptake distributions, a vertex-wise Pearson correlation, adjusted for sex, age and the time difference between baseline plasma sampling and PET scan, was calculated. Significant associations following FWE correction were detected for plasma p-tau181 baseline concentration, plasma NfL baseline concentration and plasma NfL rate of change, shown in figure 3 for tau-PET and figure 4 for Aβ-PET, respectively.
Figure 3.
Association of plasma biomarkers with flortaucipir uptake after approximately 5 years time. (A) Log10 plasma p-Tau181 (B) plasma NfL rate of change (slope) (C) log10 plasma NfL baseline in the whole study cohort and within the ATN groups. Left: linear regression between biomarker and SUVR PET uptake in a meta ROI*. Right: Vertex-wise Pearson correlations adjusted for age, sex and the time difference between plasma sampling and flortaucipir PET (tfce FWE corrected p<0.05). AV45, 18F-florbetapir amyloid-β PET. *Flortaucipir meta ROI: entorhinal, amygdala, parahippocampal, fusiform, inferior temporal and middle-temporal-ROIs. A+/−, Aβ positive/negative; ATN, Aβ deposition, tau pathology and neurodegeneration; FWE, family-wise error corrected; NfL, neurofilament light chain; p-Tau181, tau phosphorylated at threonine 181; ROI, region of interest; SUVR, standardised uptake value; tfce, threshold free cluster enhancement; TN+/−, Tau/Neurodegeneration.
Figure 4.
Association of plasma biomarkers with florbetapir uptake after approximately 5 years time. (A) Log10 plasma p-Tau181 B) log10 plasma NfL baseline. Left: linear regression between biomarker and SUVR PET uptake in a meta ROI*. Right: Vertex-wise Pearson correlations adjusted for age, sex and the time difference between plasma sampling and florbetapir PET (tfce FWE corrected p<0.05). *Florbetapir meta ROI: frontal, anterior/posterior cingulate, lateral parietal, lateral temporal regions. bL, baseline; FWE, family-wise error corrected; NFfL, neurofilament light protein; ROI, region of interest; SUVR, standardised uptake value; tfce, threshold free cluster enhancement;
Discussion
The blood concentrations of p-tau181 and NfL are promising new biomarker candidates for AD diagnosis and prognosis, but their longitudinal profiles have not been explored in detail so far. Here we show that (1) both plasma p-tau181 and NfL concentrations increase across the AD severity spectrum as represented by ATN classification; (2) in contrast, the two markers show different temporal trajectories with greater rates of change in AD spectrum individuals versus controls only for plasma NfL, but not for plasma p-tau181; (3) cognitive performance is associated cross-sectionally with plasma NfL in all ATN categories, including biomarker negative and SNAP individuals, whereas a specific association with plasma p-tau181 is present only in AD spectrum patients; and (4) baseline plasma p-tau181 and plasma NfL rate of change predict cognitive performance and tau-PET load after approximately 5 years’ time in the most advanced AD group (A+TN+), and Aβ-PET in the entire cohort.
An electrochemiluminescence study suggested that plasma p-tau181 concentrations differentiate between AD versus healthy ageing and other neurodegenerative conditions. Moreover, plasma p-tau181 tracks with p-tau181 in CSF and on tau-PET, and it predicts further cognitive deterioration in individuals with preexisting minor deficits.2 Using the same assay technology, another study showed that plasma p-tau181 distinguished AD patients from individuals with clinical and autopsy-confirmed frontotemporal dementia (FTD), and that plasma p-tau181 was more specific for AD-type changes than NfL.4 These findings were confirmed more recently by studies using a SIMOA assay, also applied in the present work. In cross-sectional analyses, plasma p-tau181 concentrations increased gradually along the AD severity continuum. Plasma p-tau181 also differentiated between AD versus FTD and other primary tauopathies.3
In contrast to p-tau181, NfL is a non-specific marker of neurodegeneration, reflecting axonal damage. NfL concentrations in CSF and blood are increased, among others, in AD, FTD22 and chronic traumatic encephalopathy.23 While NfL shows changes up to 22 years before symptoms onset in presymptomatic carriers of pathogenic AD mutations,7 24 25 it has limited usefulness for the differentiation of distinct neurodegenerative entities, with the exception of disorders with high axonal damage load, such as amyotrophic lateral sclerosis.26
We extend the existing body of evidence by reporting on longitudinal plasma peptide changes within the same individuals. Our findings highlight that plasma p-tau181 and plasma NfL share increased concentrations across the AD continuum, in line with previous cross-sectional reports.3 4 The clearly increased concentrations of NfL in the SNAP group compared with only slightly increased p-tau181 levels underline their previously suggested differences in specificity for AD pathophysiology. Furthermore, the markers behave differently over time. While plasma p-tau181 concentrations remained relatively stable within each ATN group, NfL showed dynamic increases. Consistent with our cross-sectional findings, we report that baseline measurements of both plasma peptides predicted future cognitive performance, tau and Aβ-PET load, but the rate of change was only predictive for plasma NfL, in line with results in familial AD.7 When considering CDR in the analysis, NfL baseline measurement is not predictive for tau and Aβ-PET load with highly significant predictive value remaining for ptau181 baseline and NfL slope, emphasising the robustness of these results. Taken together, these results support the roles of p-tau181 and NfL as markers of stage vs progression respectively.
It was demonstrated before that plasma p-tau181 identifies individuals with positive Aβ-PET scans, independent from their clinical diagnosis. In previous studies cortical tau deposition on PET was associated with plasma p-tau181 concentrations cross-sectionally.4 In keeping with the idea that p-tau181 is an early response to Aβ toxicity, plasma levels in tau-PET-negative participants were higher if the individual was Aβ-positive.3 For NfL it was shown that CSF and plasma concentrations predict Aβ-PET load27 and cognitive deterioration,28 but are not associated necessarily with tau-PET cross-sectionally.4 Our findings show that baseline plasma concentrations of p-tau181 and the temporal dynamics of NfL predict tau- and Aβ-PET load up to 5 years later.
The availability of a large dataset from the prospective ADNI cohort, including deep clinical and biomarker phenotyping, multimodal PET and MR imaging and longitudinal p-tau181 and NfL measurements in the same participants over a period of almost 5 years are major strengths of this study. But a few limitations related to the ADNI study design must be considered. This cohort is mainly white, educated, middle class, without major comorbidities, and there was no post mortem verification of the clinical diagnoses; however, the ADNI is enriched on purpose with probable predementia AD cases and we used CSF biomarkers to stratify the cohort according to the ATN classification system. Since tau-PET and Aβ-PET was not available at ADNI baseline, only predictive associations with blood biomarkers are shown, and data acquired simultaneously should be explored in more detail. Aβ-PET was only available for a sub-set of participants, potentially limiting statistical power and detection of specific effects in the individual ATN groups.
To conclude, our study suggests that plasma p-tau181 and NfL concentrations both increase across the AD severity continuum but show different temporal dynamics and longitudinal associations with cognitive performance and tau-PET and Aβ-PET. These biomarker candidates may provide complementary information and their combined use may offer an added value for AD prediction, diagnosis and differentiation from other neurodegenerative disorders. The great promise of these markers lies in their potential to ascertain or to rule out AD pathophysiology in primary care to inform subsequent decisions about specialist referral. Hence, research is urgently required to characterise plasma p-tau181 and NfL in unbiased cohorts representing populations in primary care settings.
Footnotes
Collaborators: Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Contributors: B-SR: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; Statistical analysis. TS-A: analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis. RP: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; administrative, technical and material support; study supervision.
Funding: Funding: Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of DefenseDefence award number W81XWH-12-2-0012). ADNI is funded by the National Institute on AgingAgeing, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir; Cogstate; Eisai; Elan Pharmaceuticals; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche and its affiliated company Genentech; Fujirebio; GE Healthcare; IXICO; Janssen Alzheimer Immunotherapy Research & Development; Johnson & Johnson Pharmaceutical Research & Development; Lumosity; Lundbeck; Merck & Co; Meso Scale Diagnostics; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organisation is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. All ADNI data are deposited in a publicly accessible repository and can be accessed at adni.loni.usc.edu.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
ADNI was reviewed and approved by all host study site institutional review boards and participants completed informed consent after receiving a comprehensive description of the ADNI. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. Vergallo A, Mégret L, Lista S, et al. Plasma amyloid β 40/42 ratio predicts cerebral amyloidosis in cognitively normal individuals at risk for Alzheimer's disease. Alzheimers Dement 2019;15:764–75. 10.1016/j.jalz.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 2. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma p-Tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med 2020;26:379–86. 10.1038/s41591-020-0755-1 [DOI] [PubMed] [Google Scholar]
- 3. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 2020;19:422–33. 10.1016/S1474-4422(20)30071-5 [DOI] [PubMed] [Google Scholar]
- 4. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med 2020;26:387–97. 10.1038/s41591-020-0762-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma Phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 2020;324:772. 10.1001/jama.2020.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mattsson N, Zetterberg H, Janelidze S, et al. Plasma tau in Alzheimer disease. Neurology 2016;87:1827–35. 10.1212/WNL.0000000000003246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med 2019;25:277–83. 10.1038/s41591-018-0304-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Wolf F, Ghanbari M, Licher S, et al. Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study. Brain 2020;143:1220–32. 10.1093/brain/awaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jack CR. The transformative potential of plasma phosphorylated tau. Lancet Neurol 2020;19:373–4. 10.1016/S1474-4422(20)30112-5 [DOI] [PubMed] [Google Scholar]
- 10. Jack CR, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018;14:535–62. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blennow K, Shaw LM, Stomrud E, et al. Predicting clinical decline and conversion to Alzheimer's disease or dementia using novel Elecsys Aβ(1-42), pTau and tTau CSF immunoassays. Sci Rep 2019;9:19024. 10.1038/s41598-019-54204-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crane PK, Carle A, Gibbons LE, et al. Development and assessment of a composite score for memory in the Alzheimer's disease neuroimaging Initiative (ADNI). Brain Imaging Behav 2012;6:502–16. 10.1007/s11682-012-9186-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mattsson N, Andreasson U, Zetterberg H, et al. Alzheimer’s Disease Neuroimaging I. Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol 2017;74:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–55. 10.1016/s0896-6273(02)00569-x [DOI] [PubMed] [Google Scholar]
- 15. Greve DN, Salat DH, Bowen SL, et al. Different partial volume correction methods lead to different conclusions: An (18)F-FDG-PET study of aging. Neuroimage 2016;132:334–43. 10.1016/j.neuroimage.2016.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greve DN, Svarer C, Fisher PM, et al. Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. Neuroimage 2014;92:225–36. 10.1016/j.neuroimage.2013.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Müller-Gärtner HW, Links JM, Prince JL, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab 1992;12:571–83. 10.1038/jcbfm.1992.81 [DOI] [PubMed] [Google Scholar]
- 18. Jack CR, Wiste HJ, Schwarz CG, et al. Longitudinal tau PET in ageing and Alzheimer's disease. Brain 2018;141:1517–28. 10.1093/brain/awy059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mormino EC, Kluth JT, Madison CM, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain 2009;132:1310–23. 10.1093/brain/awn320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Winkler AM, Ridgway GR, Webster MA, et al. Permutation inference for the general linear model. Neuroimage 2014;92:381–97. 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salimi-Khorshidi G, Smith SM, Nichols TE. Adjusting the effect of nonstationarity in cluster-based and TFCE inference. Neuroimage 2011;54:2006–19. 10.1016/j.neuroimage.2010.09.088 [DOI] [PubMed] [Google Scholar]
- 22. Forgrave LM, Ma M, Best JR, et al. The diagnostic performance of neurofilament light chain in CSF and blood for Alzheimer's disease, frontotemporal dementia, and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Alzheimers Dement 2019;11:730–43. 10.1016/j.dadm.2019.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dickstein DL, De Gasperi R, Gama Sosa MA, et al. Brain and blood biomarkers of tauopathy and neuronal injury in humans and rats with neurobehavioral syndromes following blast exposure. Mol Psychiatry 2020. 10.1038/s41380-020-0674-z. [Epub ahead of print: 25 Feb 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quiroz YT, Zetterberg H, Reiman EM, et al. Plasma neurofilament light chain in the presenilin 1 E280A autosomal dominant Alzheimer's disease kindred: a cross-sectional and longitudinal cohort study. Lancet Neurol 2020;19:513–21. 10.1016/S1474-4422(20)30137-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sánchez-Valle R, Heslegrave A, Foiani MS, et al. Serum neurofilament light levels correlate with severity measures and neurodegeneration markers in autosomal dominant Alzheimer's disease. Alzheimers Res Ther 2018;10:113. 10.1186/s13195-018-0439-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol 2019;76:1035–48. 10.1001/jamaneurol.2019.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mielke MM, Syrjanen JA, Blennow K, et al. Plasma and CSF neurofilament light: relation to longitudinal neuroimaging and cognitive measures. Neurology 2019;93:e252–60. 10.1212/WNL.0000000000007767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mattsson N, Cullen NC, Andreasson U, et al. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2019;76:791–9. 10.1001/jamaneurol.2019.0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2020-325537supp001.pdf (414.2KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. All ADNI data are deposited in a publicly accessible repository and can be accessed at adni.loni.usc.edu.