Abstract
Background
The effectiveness of endovascular treatment (EVT) for large vessel occlusion (LVO) stroke severely depends on time to treatment. However, it remains unclear what the value of faster treatment is in the years after index stroke. The aim of this study was to quantify the value of faster EVT in terms of health and healthcare costs for the Dutch LVO stroke population.
Methods
A Markov model was used to simulate 5-year follow-up functional outcome, measured with the modified Rankin Scale (mRS), of 69-year-old LVO patients. Post-treatment mRS was extracted from the MR CLEAN Registry (n=2892): costs per unit of time and Quality-Adjusted Life Years (QALYs) per mRS sub-score were retrieved from follow-up data of the MR CLEAN trial (n=500). Net Monetary Benefit (NMB) at a willingness to pay of €80 000 per QALY was reported as primary outcome, and secondary outcome measures were days of disability-free life gained and costs.
Results
EVT administered 1 min faster resulted in a median NMB of €309 (IQR: 226;389), 1.3 days of additional disability-free life (IQR: 1.0;1.6), while cumulative costs remained largely unchanged (median: -€15, IQR: −65;33) over a 5-year follow-up period. As costs over the follow-up period remained stable while QALYs decreased with longer time to treatment, which this results in a near-linear decrease of NMB. Since patients with faster EVT lived longer, they incurred more healthcare costs.
Conclusion
One-minute faster EVT increases QALYs while cumulative costs remain largely unaffected. Therefore, faster EVT provides better value of care at no extra healthcare costs.
Keywords: economics, stroke, thrombectomy, intervention, artery
Introduction
Occlusions of the intracranial carotid artery and middle cerebral artery, commonly referred to as large vessel occlusions (LVO), are severe causes of acute ischemic stroke (AIS), frequently resulting in persisting neurological deficits affecting patients’ health and healthcare costs.1–3 Mortality of the LVO stroke population is high and a large portion of the survivors remain dependent on care.2 3 These outcomes have drastically improved in recent years with the introduction of endovascular treatment (EVT).4 The beneficial effect of EVT is, however, highly time-dependent.5 As a result, various policies that aim at reducing time delay from onset of neurologic deficit to EVT have been investigated.6 However, it remains unclear what the health and cost effects of faster EVT are in the years following treatment.
Health outcomes and costs have a strong association in the case of LVO stroke as the high disease burden results in a lower perceived quality of life and higher demand on healthcare facilities.3 7 Besides the short- and long-term health benefits, several studies have proven that EVT reduces healthcare costs in the years after treatment.4 8 9 Although EVT has become standard practice in developed countries worldwide,10 various time-consuming inefficiencies remain present in current practice.11 12 With the growing use of advanced diagnostics the negative side-effects in terms of added delay to treatment should be known.13 With several policies aiming at faster EVT delivery, proposed and promising yet time-consuming advanced diagnostics available,6 13 so understanding the value of each minute has become increasingly important.
Studies that evaluate workflow improvements mainly used time saved as primary outcome and thus do not include long-term health and cost effects.6 A recent study determined the health and cost effects of faster EVT in the case of LVO stroke based on US guidelines and data.14 However, extrapolation of US data on a population level to the Netherlands and other European countries is difficult: besides differences in healthcare costs, geographical differences cause different onset to EVT times,15 16 and US guidelines apply stricter imaging selection criteria for EVT eligibility.17 18 The use of similar guidelines and empirical findings suggest that Dutch data is more comparable to other European countries.19 Furthermore, the adaptation of the guidelines in the US is not universal, resulting in a more comparable situation in regions of the US to the Dutch setting. The aim of this study was to quantify the value of faster EVT in terms of health and healthcare costs for the Dutch LVO stroke population with a Markov model.
Methods
In this study, simulations with a Markov model were performed to compute expected health and costs during follow-up for each hour of delay to EVT of patients with intracranial carotid artery, M1, and proximal M2 occlusions. Subsequently, the differences in health and cost outcomes between each hour of delay was used to represent the effect of faster EVT per minute.
Modeling procedure
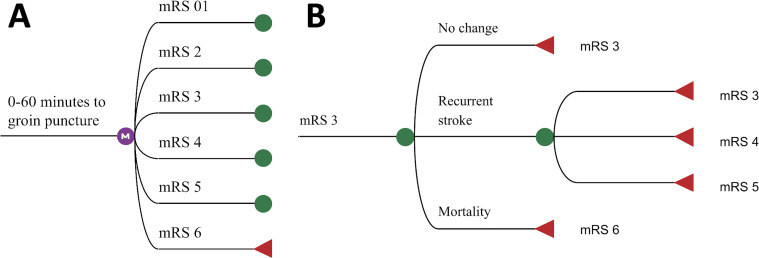
A two-staged Markov model was developed with a short-term and long-term part. For each time step, simulated patients could be in one of six Markov states defined by modified Rankin Scale (mRS) sub-scores: mRS 0 and 1 were combined in one Markov state since the sample size was otherwise too low to retrieve accurate estimates. The mRS is a seven-point scale for disability, ranging from 0 (no disabilities) to 6 (death). The short-term model was used to simulate 90-day mRS per hour delay of time from onset to the start of EVT, which was defined by the time of groin puncture. Time to groin puncture was preferred to time to end-of-intervention as the resulting time related mRS would include an effect related to LVO and thus intervention complexity: furthermore it is unlikely that intervention time can be saved to achieve faster EVT. Simulated cohorts were assumed to exist of an even split of males and females aged 69, the median age of patients in the MR CLEAN registry,15 at the beginning of the long-term simulations deterioration of mRS could only occur after stroke recurrence or due to death. In the long-term model changes in mRS due to stroke recurrence and all-cause mortality were simulated over 5 years of follow-up with a cycle length of 1 year. Figure 1 contains the short- and long-term model architecture graphically. Quality-adjusted life years (QALYs) and costs related to each Markov state were discounted at a 1.5% and 4% compounded annual rate, respectively, according to Dutch guidelines for cost effectiveness research.20 A yearly inflation rate of 1.7% was used to adjust future costs based on forecasts from the Dutch Ministry of Health, Welfare and Sport.21 TreeAge software (TreeAge Pro 2019, version R2.1; TreeAge, Williamstown MA, USA) was used for implementing the model and simulations.
Figure 1.
Markov model architecture: Pane A: Short-term model used for each hour to simulate 90-day mRS (modified Rankin Scale). In this example, 0–60 minutes of onset time to groin puncture was presented. Pane B: Long-term model for patients (example for mRS 3 at 90-days' post-index stroke). After recurrent stroke an equal or higher mRS score can be achieved. After stroke recurrence, death (mRS 6) is not possible to prevent duplicate mortality rates in the model. mRS after stroke recurrence was based on normalized values from the MR CLEAN trial control arm.
Data sources
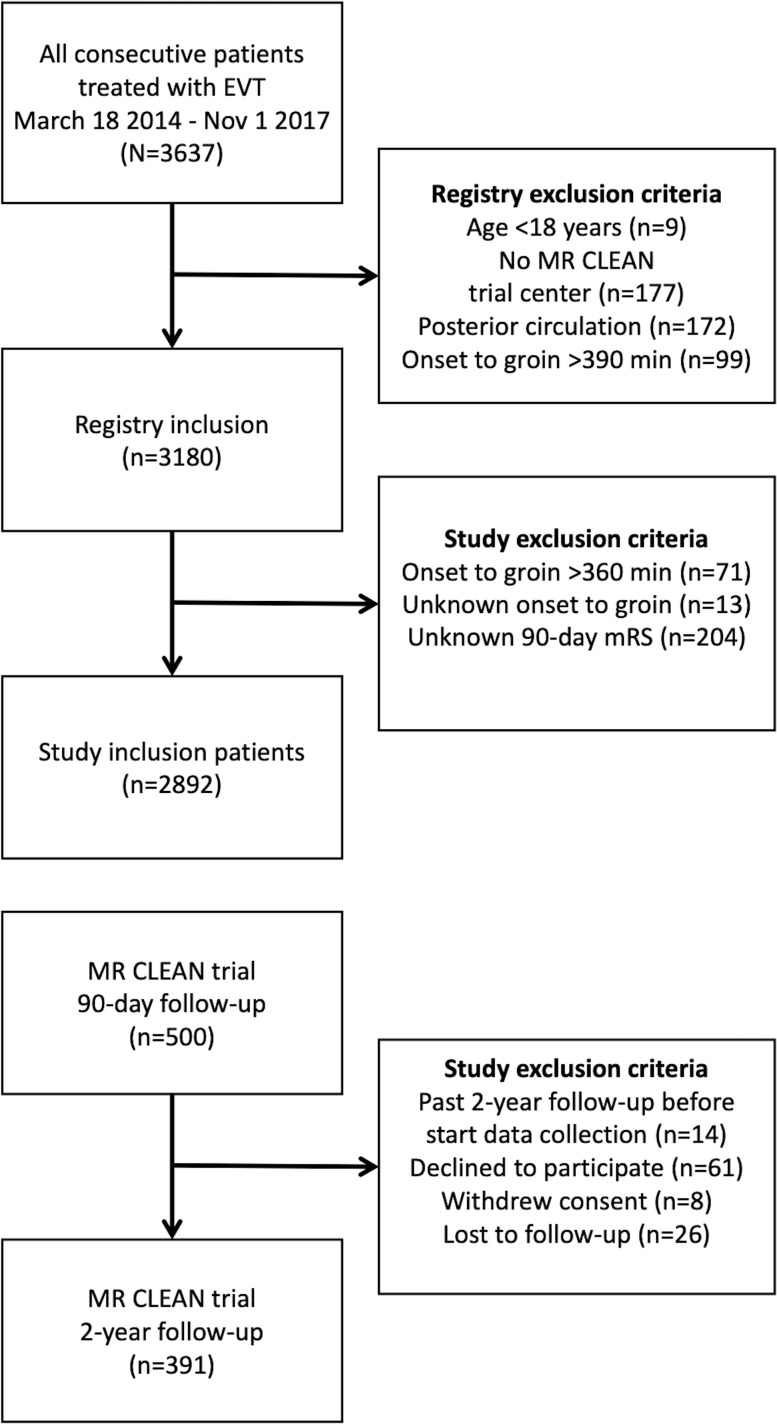
Input parameters of the Markov model were retrieved retrospectively from the MR CLEAN Registry part 1 and 2,15 and 2-year follow-up data from the MR CLEAN trial,22 23 public data,24 and literature.25 26 Patients from the MR CLEAN Registry were included based on the following criteria: LVO in the anterior circulation, treatment in MR CLEAN trial center, age ≥18 years, available 90-day mRS, available time from onset to groin puncture, and time from onset to groin puncture ≤360 min (figure 2). The data collection protocols have been published, and ethics committee and research board approval has previously been received in compliance with the Declaration of Helsinki.15 27 28
Figure 2.
Data and inclusion. In this study data from the MR CLEAN Registry (part 1 and 2), MR CLEAN trial, and 2-year follow-up from the MR CLEAN trial were used. For this study, additional exclusion criteria were formulated for the MR CLEAN Registry data. EVT, endovascular treatment; mRS, modified Rankin Scale.
Probabilities
The data of 2892 patients in the MR CLEAN Registry were used to retrieve the per-hour of time from onset to groin puncture and the probability of mRS sub-scores 90 days after EVT (figure 1A). Yearly mortality rates of the Dutch population by age and gender were retrieved from forecasts of the Royal Dutch Actuarial Society starting in 2021.24 Subsequently, published hazard rates (HR) of additional mortality related to mRS and years after index stroke were used to compute all-cause, including recurrent stroke-related, mortality rates.25 The probability of a recurrent stroke for each year after an index stroke was retrieved from a population study.26 To prevent double inclusion of stroke-related mortality in the model, death (mRS 6) was not modeled as an outcome after recurrent stroke. Instead, the HR by Hong et al on all-cause mortality was used, which included recurrent stroke-related mortality and other causes of death.25 Functional outcome at 90 days excluding death (mRS6) of the MR CLEAN trial control arm (n=267) was used to compute the mRS distribution after stroke recurrence (figure 1).22 As death could not occur and mRS could only remain equal or deteriorate, the outcomes of the MR CLEAN trial control arm were normalized for the available outcome options (online supplemental table S1).
neurintsurg-2020-017017supp001.pdf (270.4KB, pdf)
Costs and QALYs
Two-year follow-up data of the MR CLEAN trial (n=391) was used to estimate QALYs and costs per unit of time in separate mRS sub-scores. Data was collected at 3, 6, 12, 18, and 24 months' post-index stroke and included utility scores computed from EuroQoL 5D and cost questionnaires. A QALY generally is a value between 0 and 1 where 0 implies death and 1 perfect health during 1 year. However, in our reference data it was also possible to have a negative QALY since some health situations were perceived by the patients as worse than death. Per patient, the average of each mRS/utility score combination across different time points was used as the QALY estimate. The online supplemental material contains more information on the data collection and computations with costs and utility scores previously collected by van den Berg et al.27 In short, costs were computed from a healthcare payer perspective and included: acute setting treatment cost, in-hospital costs, outpatient clinic visits, rehabilitation, formal homecare, and long-term institutionalized costs. The mRS at 90 days and 18 months were used as reference points for the calculation of costs per mRS-related health state for the first and second year after index AIS, respectively. Follow-up costs per mRS from the third year onward were assumed equal to costs of the second year excluding rehabilitation costs (online supplemental table S1). Mean costs and QALY values were used to perform baseline simulations. Historical inflation rates from the Dutch Central Bureau of Statistics were used to adjust the reported costs with reference year 2015 to the reference simulation start year of this study (2021).27
Sensitivity analyses
One-way sensitivity analyses were performed to assess the degree of change in outcome due to change of a single input parameter. Outcome measures were reported for an increase and decrease of 10% with respect to the baseline input parameters. For age of the simulated population, a deviation of 4 years from the baseline age was used. Deviations in age were only used in the long-term model, but the effect of age on 90-day mRS was not included in this study. A probabilistic sensitivity analysis was performed to assess input parameter uncertainty. Online supplemental table S1 includes the distribution type per input parameter. 10 000 s order Monte Carlo simulations were used to estimate the certainty of the estimates. External validation with respect to simulated mRS and reported mRS in contemporary follow-up data of endovascular treated patients was performed.23 29 30 In the external validation the proportion of patients with good functional outcome (mRS ≤2), poor functional outcome but not deceased (mRS 3–5), or death (mRS 6) were compared with follow-up results from REVASCAT (1 year),29 MR CLEAN (2 years),23 and a study by Clua-Espuny et al (5 years).30
Population effects
An estimate was made to represent the effects of 1 min of faster EVT for the Dutch population based on the PSA results. For this estimate the total population in the MR CLEAN registry part 1 and 2 (n=3279) included in 43 months was used to compute the yearly number of LVO patients that receive EVT (n=887) and thus could benefit from faster EVT. The outcome on the population level was computed by taking a weighted average based on the prevalence of each hour delay to groin puncture in the MR CLEAN registry.
Outcome measures
The primary outcome measures used were Net Monetary Value (NMV), a single aggregated value for costs, and QALYs where QALYs are converted to a monetary value NMV=QALYs*willingness to pay - costs, per hour delay from onset to groin puncture, and Net Monetary Benefit (NMB, the NMV difference between each hour): at a willingness to pay threshold of €80 000 per QALY.20 Secondary outcome measures were QALYs and costs. Results were presented as cumulative values over the 5-year simulated period. Outcome measures were computed per hour delay of time from onset to groin puncture and reported for 1 min, 10 min, and 1 hour of faster EVT. Differences in outcome measures between the hours were used to compute outcome measures of faster EVT. Transforming outcomes per hour of faster EVT to per minute includes an assumption of constant differences between hours. Per minute of faster EVT, additional days of disability-free life (additional days in perfect health; QALYs/365), change in costs, and NMB were computed. PSA results were reported as median with IQR. For descriptive statistics (online supplemental table S2) distributions across the 6-hour delay were statistically compared with ANOVA, Kruskall–Wallis, and Chi-squared tests for normal distributed continuous, non-normal distributed continuous, and categorical distributed baseline variables, respectively.
Model input parameters
Online supplemental table S1 in the online supplementary material depicts all parameters used for baseline simulations and probabilistic sensitivity analyses.
Results
Descriptive statistics
Online supplemental table S2 contains descriptive statistics of 2892 MR CLEAN registry patients per hour of delay from onset to groin puncture. Age, clinical (NIHSS), and radiological (ASPECTS) parameters were significantly associated with delay to EVT. The proportion of directly referred patients was significantly lower in the 4th–6th hours of delay from onset to groin puncture. Furthermore, intravenous thrombolysis was administered to 77.4%, and 61.3% of the entire population were directly referred to an EVT-capable center.
Baseline simulations and one-way sensitivity analyses
Costs per hour of delay remained roughly equal, while QALYs and thus NMV decreased (online supplemental table S3). Each minute of faster EVT resulted in an NMB of €242 (per hour: €14,519). This was mainly due to a 1.4 gain of disability free life-days (0.224 QALYs per hour). Costs changed with -€41 per minute of faster EVT (per hour: -€2,433).
The one-way sensitivity analysis revealed that age at the start of simulations, QALYs per year in mRS 0–4, and costs per year in mRS 3–4 affect NMB most. Costs in the first year in mRS 2–4 affected NMB more than costs made in the subsequent 4 years, except for costs in mRS 4. Online supplemental figure S1 in the online supplementary material depicts the one-way sensitivity analyses results.
Probabilistic sensitivity analysis
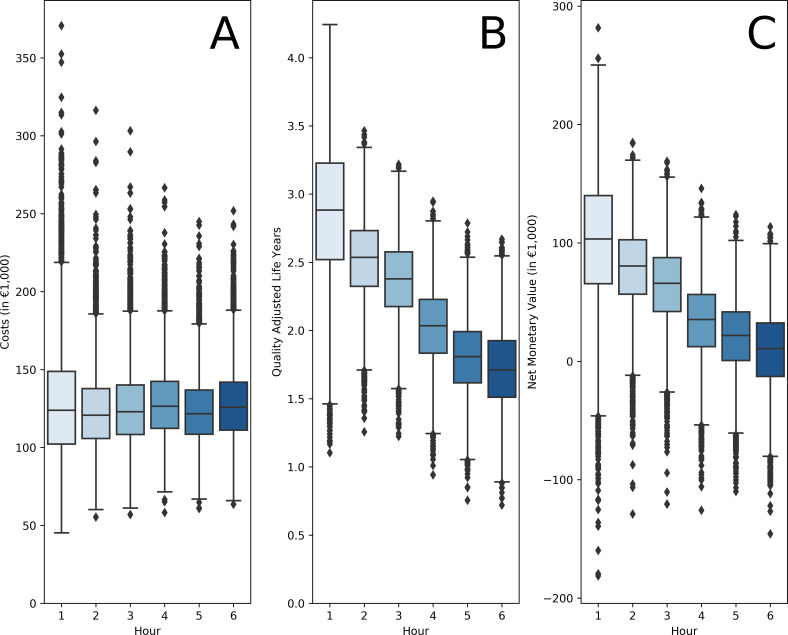
Similar to the baseline simulation results, costs remained stable while QALYs and thus NMV decreased per hour of delay in the PSA (figure 3). Faster EVT resulted in no change in costs (per minute median: -€15, IQR: -€65;€33 – per hour median: -€969, IQR: -€3,897;€1,972), a gain in health (per minute disability-free life-days gained median: 1.3, IQR: 1.0;1.6 – per hour QALYs gained median: 0.22, IQR: 0.17;0.27), resulting in a positive NMB (per minute median: €309, IQR: €226;389 – per hour median: €18 513 IQR: €13,574;€23,376). Results of faster EVT for differences between separate hours of time to groin and the outcome for the Dutch population are depicted in table 1. Faster EVT between the third and fourth hour had a more profound effect on disability-free life-days and NMB. For an expected number of 887 EVT-eligible AIS patients in the Dutch population yearly, delivery of EVT by 1 min faster would result in a median QALY gain of 3.5 (IQR: 2.1;4.9 – per hour median: 210.1, IQR: 124.0;294.8), and a per minute median NMB of €287 324 (IQR: €146,270;€428,547 – per hour median: €17,239,435, IQR: €8,776,181;€25,712,803).
Figure 3.
Probabilistic sensitivity analysis results per hour of delay from onset to groin puncture: Costs (A), quality-adjusted life-year (QALY) (B), and Net Monetary Value (C) per hour time from onset to groin puncture.
Table 1.
Probabilistic sensitivity analysis results per hour of faster treatment
| Hour difference | Costs in € median (IQR) | QALY gained median (IQR) | NMB in € median (IQR) |
| First – second hour | 2633 (−10,957;18,613) | 0.36 (0.09;0.61) | 23 799 (−3004;49,133) |
| Second – third hour | −2219 (−6,651;2,240) | 0.15 (0.07;0.23) | 14 052 (6,809;21,714) |
| Third – fourth hour | −3228 (−7,274;1,026) | 0.34 (0.28;0.40) | 30 143 (23,732;36,679) |
| Fourth – fifth hour | 4666 (440;9,078) | 0.22 (0.16;0.29) | 13 125 (6,353;19,682) |
| Fifth – sixth hour | −3860 (−10,108;2,261) | 0.09 (−0.01;0.18) | 10 560 (1,708;19,971) |
| Per hour faster treatment* | −969 (−3,897;1,972) | 0.22 (0.17;0.27) | 18 513 (13,574;23,376) |
| Per 10 min faster treatment* | −151 (−649; 329) | 13.5 (10.5;16.4)† | 3085 (2,262;3,896) |
| Per minute faster treatment* | −15 (−65;33) | 1.3 (1.0;1.6)† | 309 (226;389) |
| Population outcome per hour of earlier treatment‡ | −865,387 (−5,848,900;4,459,552) | 210.1 (124.0;294.8) | 17 239 435 (8,776,181;25,712,803) |
| Population outcome per 10 min of earlier treatment‡ | −144,231 (−974,817;743,258) | 35.0 (20.7;49.1) | 2 873 239 (1,462,696;4,285,467) |
| Population outcome per minute of earlier treatment§ | −14,423 (−97,482;74,326) | 3.5 (2.1;4.9) | 287 324 (146,270;428,547) |
*For each simulation the median value of the five differences between 6 hours was taken.
†The per minute QALY results are depicted as disability-free life days gained (DALY). This is the daily value of a QALY (DALY=QALY/365).
‡Outcome for the Dutch LVO stroke population if faster EVT is performed.
External validation of simulated MRS distributions
In online supplemental figure S2 in the online supplementary material the external validation results are depicted. Good functional outcome (mRS ≤2; online supplemental figure S2A) and mortality (mRS 6; online supplemental figure S2C) at 1 and 2 years were comparable with results from the REVASCAT and MR CLEAN follow-up studies.23 29 Observed values were similar to simulated values of 3-hour delay to groin puncture. Mortality (mRS 6; online supplemental figure S2C) at 5= year follow-up was higher in the simulated data compared with the study by Clua-Espuny et al.30
Discussion
Delay of time from onset to groin puncture results in a loss of health (QALYs) that accumulates over the years following an acute LVO stroke setting. Faster EVT will result in a gain in health, while costs from a healthcare payer perspective remain stable. Thus, faster EVT is cost effective. We have indicated that an NMB of €309 per minute faster EVT may be achieved, which, extrapolated to the Dutch population, equals an annual NMB of €287 324. Since the treatment effect of EVT in the Dutch MR CLEAN trial and Registry was lower than that of its international peers, it seems likely that the NMB per minute faster EVT is even higher in populations with higher treatment effect of EVT.
Even though only the potential benefits and not the costs of realizing faster EVT were considered, this study provides a strong argument to invest more in stroke logistics. Depending on the approach to realize faster EVT it seems likely that additional costs will be made. For example, better ambulance coverage or public awareness of stroke symptoms might be used for faster EVT delivery, which requires investments in ambulance coverage and educational programs that are not included in this study. Furthermore, it should be considered that in per euro invested in expediting EVT there might be diminishing returns with respect to the realized minutes of faster EVT.
The paradox of higher lifetime healthcare costs due to longer survival caused no cost savings as a result of faster EVT in this study. Patients with faster EVT had an improved functional outcome (mRS) and life expectancy, however, additional healthcare costs were made in the long term. Faster EVT in the population treated between the third and fourth hour of onset to groin puncture was related to a larger health effect than that of differences between other hours. This could be related to the proportion of directly referred patients to an EVT-capable stroke center (online supplemental table S2): a potential selection of indirectly referred patients could have occurred. Although a clear relationship between faster EVT and NMB was found, this result was very sensitive to age. An increase in age resulted in an exponential decrease of the cost effectiveness of faster EVT in terms of NMB, which is in line with the findings by Wu et al where the cost effectiveness of reperfusion after EVT decreased with an increase in age.31 In addition, the effect of age in this study is likely to be an underestimation since the modeled effect of age only considered mRS changes in the long run (after 90 days' post-stroke), whereas it is known that age is also related to poor functional outcome at 90 days.4
Findings from this study are in accordance with the US-based population study by Kunz et al,14 and the indirect effect of time on the cost-effectiveness analyses for M2 occlusions by Khunte et al.32 The exact quantitative value of faster EVT was, however, much less (NMB median: $1,059; 95% prediction interval: $555-$1,485) than the study by Kunz et al. This difference in NMB per minute of faster EVT was mainly due to differences in follow-up simulation time and cost computations: Kunz et al used life-time simulations and included both societal and healthcare costs, while 5-year follow-up and only direct healthcare costs were used in this study. This timespan was chosen to retrieve a more conservative and certain estimation of the value of time, since the simulation of longer follow-up results in the accumulation of probability estimate errors. Kunz et al also found that age was the major factor affecting NMB of faster treatment.14 The comparison of simulated results with observed long-term mRS distributions revealed a comparable proportion of good functional outcome but a higher mortality rate in the baseline simulations than observed rates in the validation studies considered.23 29 30 Although this might implicate an overestimated mortality rate, a lower age in two of three validation studies (age in Clua-Espuny et al30; MR CLEAN; REVASCAT: 69.5;65.5;67.2) could explain the lower mortality rate in these studies. In addition, these validation studies either were retrospective in design and thus prone to selection bias (Clua-Espuny et al30), had a large loss to follow-up (MR CLEAN, 16.7%), or had a relatively low sample size (REVASCAT, n=103).
The lack of data regarding the long-term decline of mRS, stroke recurrence, and mortality in patients with LVO undergoing EVT remains a shortcoming of this study. If survival and time in different mRS states differ in the long-run costs and health effects also alter significantly. Another limitation of this study was the lack of recent data on healthcare spending after a 2-year follow-up period and societal costs. To improve the accuracy of cost-effectiveness studies in stroke, future research should aim at retrieving better cost estimates from a healthcare payer and societal perspective related to mRS during multiple years after an initial stroke. However, if the relative cost differences related to the mRS Markov states remain similar, it is in the line of expectation that the results of this study would not differ. Furthermore, mRS decline and mortality related to mRS in time should be studied more extensively.
The results of this study should be seen as the potential benefit of faster EVT at a population level: 1 min of faster EVT does not necessarily benefit every patient equally. Since age has a large effect on the cost effectiveness of faster treatment, the extrapolation of findings from this study to other, potentially much older, populations should be performed with caution. Furthermore, the high percentage of direct referral to an EVT-capable center and intravenous thrombolysis administration were not included in this study. Deviations of those percentages in other populations might result in a different EVT treatment effect/time relationship which, in turn, affects cost effectiveness and clinical outcomes. However, randomized controlled trial-based cost effectiveness analyses are required to determine the relationship between direct referral to EVT-capable centers, thrombolysis administration, and faster EVT. Nevertheless, we believe that with the available (public) information this study gives a fine-grained representation of mRS and a conservative cost and health effect estimate that stresses the necessity for healthcare payers to extensively invest in faster EVT delivery.
Conclusion
Saving time to groin puncture has the potential to improve health while healthcare costs may be expected to remain stable over 5-year follow-up. At a willingness to pay of €80 000 per QALY, 1 min of faster treatment is equivalent to 1.3 disability-free life-days saved and a NMB of €309 in a median 69-year-old Dutch population.
neurintsurg-2020-017017supp002.pdf (83.5KB, pdf)
Footnotes
Correction notice: This article has been corrected since it appeared Online First. Author names and affiliations have been corrected.
Collaborators: This study was performed for the MR CLEAN Registry investigators. Group authors MR CLEAN Registry: Executive committee: Diederik W.J. Dippel (Department of Neurology, Erasmus MC University Medical Center); Aad van der Lugt (Department of Radiology, Erasmus MC University Medical Center); Charles B.L.M. Majoie (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam);Yvo B.W.E.M. Roos (Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Robert J. van Oostenbrugge (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Wim H. van Zwam (Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Jelis Boiten (Department of Neurology, Haaglanden MC, The Hague); Jan Albert Vos (Department of Radiology, Sint Antonius Hospital, Nieuwegein). Study coordinators: Ivo G.H. Jansen (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Maxim J.H.L. Mulder (Department of Neurology, Radiology, Erasmus MC University Medical Center); Robert- Jan B. Goldhoorn (Department of Neurology, Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Kars C.J. Compagne (Department of Radiology, Erasmus MC University Medical Center); Manon Kappelhof (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Josje Brouwer (Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Sanne J. den Hartog (Department of Neurology, Radiology, Public Health, Erasmus MC University Medical Center;); Wouter H. Hinsenveld (Department of Neurology, Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)). Local principal investigators: Diederik W.J. Dippel (Department of Neurology, Erasmus MC University Medical Center); Bob Roozenbeek (Department of Neurology, Erasmus MC University Medical Center); Aad van der Lugt (Department of Radiology, Erasmus MC University Medical Center); Adriaan C.G.M. van Es (Department of Radiology, Erasmus MC University Medical Center); Charles B.L.M. Majoie (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Yvo B.W.E.M. Roos (Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Bart J. Emmer (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Jonathan M. Coutinho (Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Wouter J. Schonewille (Department of Neurology, Sint Antonius Hospital, Nieuwegein); Jan Albert Vos (Department of Radiology, Sint Antonius Hospital, Nieuwegein); Marieke J.H. Wermer (Department of Neurology, Leiden University Medical Center); Marianne A.A. van Walderveen (Department of Radiology, Leiden University Medical Center); Julie Staals (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Robert J. van Oostenbrugge (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Wim H. van Zwam (Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Jeannette Hofmeijer (Department of Neurology, Rijnstate Hospital, Arnhem); Jasper M. Martens (Department of Radiology, Rijnstate Hospital, Arnhem); Geert J. Lycklama à Nijeholt (Department of Radiology, Haaglanden MC, The Hague); Jelis Boiten (Department of Neurology, Haaglanden MC, The Hague); Sebastiaan F. de Bruijn (Department of Neurology, HAGA Hospital, The Hague); Lukas C. van Dijk (Department of Radiology, HAGA Hospital, The Hague); H. Bart van der Worp (Department of Neurology, University Medical Center Utrecht); Rob H. Lo (Department of Radiology, University Medical Center Utrecht); Ewoud J. van Dijk (Department of Neurology, Radboud University Medical Center, Nijmegen); Hieronymus D. Boogaarts (Department of Neurosurgery, Radboud University Medical Center, Nijmegen); J. de Vries (Department of Neurology, Isala Klinieken, Zwolle); Paul L.M. de Kort (Department of Neurology, Elisabeth-TweeSteden ziekenhuis, Tilburg); Julia van Tuijl (Department of Neurology, Elisabeth-TweeSteden ziekenhuis, Tilburg); Jo P. Peluso (Department of Radiology, Elisabeth-TweeSteden ziekenhuis, Tilburg); Puck Fransen (Department of Neurology, Isala Klinieken, Zwolle); Jan S.P. van den Berg (Department of Neurology, Isala Klinieken, Zwolle); Boudewijn A.A.M. van Hasselt (Department of Radiology, Isala Klinieken, Zwolle); Leo A.M. Aerden (Department of Neurology, Reinier de Graaf Gasthuis, Delft); René J. Dallinga (Department of Radiology, Reinier de Graaf Gasthuis, Delft); Maarten Uyttenboogaart (Department of Neurology, University Medical Center Groningen); Omid Eschgi (Department of Radiology, University Medical Center Groningen); Reinoud P.H. Bokkers (Department of Radiology, University Medical Center Groningen);Tobien H.C.M.L. Schreuder (Department of Neurology, Atrium Medical Center, Heerlen); Roel J.J. Heijboer (Department of Radiology, Atrium Medical Center, Heerlen); Koos Keizer (Department of Neurology, Catharina Hospital, Eindhoven); Lonneke S.F. Yo (Department of Radiology, Catharina Hospital, Eindhoven); Heleen M. den Hertog (Department of Neurology, Isala Klinieken, Zwolle); Emiel J.C. Sturm (Department of Radiology, Medical Spectrum Twente, Enschede); Paul J.A.M. Brouwers (Department of Neurology, Medical Spectrum Twente, Enschede). Imaging assessment committee: Charles B.L.M. Majoie (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam) (chair); Wim H. van Zwam (Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Aad van der Lugt (Department of Radiology, Erasmus MC University Medical Center); Geert J. Lycklama à Nijeholt (Department of Radiology, Haaglanden MC, The Hague); Marianne A.A. van Walderveen (Department of Radiology, Leiden University Medical Center); Marieke E.S. Sprengers (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Sjoerd F.M. Jenniskens (Department of Radiology, Radboud University Medical Center, Nijmegen); René van den Berg (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Albert J. Yoo (Department of Radiology, Texas Stroke Institute, Texas, United States of America); Ludo F.M. Beenen (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Alida A. Postma (Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Stefan D. Roosendaal (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Bas F.W. van der Kallen (Department of Radiology, Haaglanden MC, The Hague); Ido R. van den Wijngaard (Department of Radiology, Haaglanden MC, The Hague); Adriaan C.G.M. van Es (Department of Radiology, Erasmus MC University Medical Center); Bart J. Emmer (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam;); Jasper M. Martens (Department of Radiology, Rijnstate Hospital, Arnhem); Lonneke S.F. Yo (Department of Radiology, Catharina Hospital, Eindhoven); Jan Albert Vos (Department of Radiology, Sint Antonius Hospital, Nieuwegein); Joost Bot (Department of Radiology, Amsterdam UMC, Vrije Universiteit van Amsterdam, Amsterdam); Pieter-Jan van Doormaal (Department of Neurology, Erasmus MC University Medical Center); Anton Meijer (Department of Radiology, Radboud University Medical Center, Nijmegen); Elyas Ghariq (Department of Radiology, Haaglanden MC, The Hague); Reinoud P.H. Bokkers (Department of Radiology, University Medical Center Groningen); Marc P. van Proosdij (Department of Radiology, Noordwest Ziekenhuisgroep, Alkmaar); G. Menno Krietemeijer (Department of Radiology, Catharina Hospital, Eindhoven); Jo P. Peluso (Department of Radiology, Elisabeth-TweeSteden ziekenhuis, Tilburg); Hieronymus D. Boogaarts (Department of Neurosurgery, Radboud University Medical Center, Nijmegen); Rob Lo (Department of Radiology, University Medical Center Utrecht); Dick Gerrits (Department of Radiology, Medical Spectrum Twente, Enschede); Wouter Dinkelaar (Department of Radiology, Erasmus MC University Medical Center); Auke P.A. Appelman (Department of Radiology, University Medical Center Groningen); Bas Hammer (Department of Radiology, HAGA Hospital, The Hague); Sjoert Pegge (Department of Radiology, Radboud University Medical Center, Nijmegen); Anouk van der Hoorn (Department of Radiology, University Medical Center Groningen); Saman Vinke (Department of Neurosurgery, Radboud University Medical Center, Nijmegen). Writing committee: Diederik W.J. Dippel (Department of Neurology, Erasmus MC University Medical Center) (chair); Aad van der Lugt (Department of Radiology, Erasmus MC University Medical Center); Charles B.L.M. Majoie (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Yvo B.W.E.M. Roos (Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Robert J. van Oostenbrugge (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Wim H. van Zwam (Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Geert J. Lycklama à Nijeholt (Department of Radiology, Haaglanden MC, The Hague;); Jelis Boiten (Department of Neurology, Haaglanden MC, The Hague); Jan Albert Vos (Department of Radiology, Sint Antonius Hospital, Nieuwegein); Wouter J. Schonewille (Department of Neurology, Sint Antonius Hospital, Nieuwegein); Jeannette Hofmeijer (Department of Neurology, Rijnstate Hospital, Arnhem); Jasper M. Martens (Department of Radiology, Rijnstate Hospital, Arnhem); H. Bart van der Worp (Department of Neurology, University Medical Center Utrecht); Rob H. Lo (Department of Radiology, University Medical Center Utrecht). Adverse event committee: Robert J. van Oostenbrugge (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)) (chair); Jeannette Hofmeijer (Department of Neurology, Rijnstate Hospital, Arnhem); H. Zwenneke Flach (Department of Radiology, Isala Klinieken, Zwolle). Trial methodologist: Hester F. Lingsma (Department of Public Health, Erasmus MC University Medical Center). Research nurses and local trial coordinators: Naziha el Ghannouti (Department of Neurology, Erasmus MC University Medical Center); Martin Sterrenberg (Department of Neurology, Erasmus MC University Medical Center); Wilma Pellikaan (Department of Neurology, Sint Antonius Hospital, Nieuwegein); Rita Sprengers (Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Marjan Elfrink (Department of Neurology, Rijnstate Hospital, Arnhem); Michelle Simons (Department of Neurology, Rijnstate Hospital, Arnhem); Marjolein Vossers (Department of Radiology, Rijnstate Hospital, Arnhem); Joke de Meris (Department of Neurology, Haaglanden MC, The Hague); Tamara Vermeulen (Department of Neurology, Haaglanden MC, The Hague); Annet Geerlings (Department of Neurology, Radboud University Medical Center, Nijmegen); Gina van Vemde (Department of Neurology, Isala Klinieken, Zwolle); Tiny Simons (Department of Neurology, Atrium Medical Center, Heerlen); Gert Messchendorp (Department of Neurology, University Medical Center Groningen); Nynke Nicolaij (Department of Neurology, University Medical Center Groningen); Hester Bongenaar (Department of Neurology, Catharina Hospital, Eindhoven); Karin Bodde (Department of Neurology, Reinier de Graaf Gasthuis, Delft); Sandra Kleijn (Department of Neurology, Medical Spectrum Twente, Enschede); Jasmijn Lodico (Department of Neurology, Medical Spectrum Twente, Enschede); Hanneke Droste (Department of Neurology, Medical Spectrum Twente, Enschede); Maureen Wollaert (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Sabrina Verheesen (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); D. Jeurrissen (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Erna Bos (Department of Neurology, Leiden University Medical Center); Yvonne Drabbe (Department of Neurology, HAGA Hospital, The Hague); Michelle Sandiman (Department of Neurology, HAGA Hospital, The Hague); Nicoline Aaldering (Department of Neurology, Rijnstate Hospital, Arnhem); Berber Zweedijk (Department of Neurology, University Medical Center Utrecht); Jocova Vervoort (Department of Neurology, Elisabeth-TweeSteden ziekenhuis, Tilburg); Eva Ponjee (Department of Neurology, Isala Klinieken, Zwolle); Sharon Romviel (Department of Neurology, Radboud University Medical Center, Nijmegen); Karin Kanselaar (Department of Neurology, Radboud University Medical Center, Nijmegen); Denn Barning (Department of Radiology, Leiden University Medical Center). PhD and Medical students: Esmee Venema (Department of Public Health, Erasmus MC University Medical Center); Vicky Chalos (Department of Neurology, Public Health, Erasmus MC University Medical Center); Ralph R. Geuskens (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Tim van Straaten (Department of Neurology, Radboud University Medical Center, Nijmegen); Saliha Ergezen (Department of Neurology, Erasmus MC University Medical Center); Roger R.M. Harmsma (Department of Neurology, Erasmus MC University Medical Center); Daan Muijres (Department of Neurology, Erasmus MC University Medical Center); Anouk de Jong (Department of Neurology, Erasmus MC University Medical Center); Olvert A. Berkhemer (Department of Neurology, Erasmus MC University Medical Center; Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Anna M.M. Boers (Department of Radiology and Nuclear Medicine, Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam); J. Huguet (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); P.F.C. Groot (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Marieke A. Mens (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Katinka R. van Kranendonk (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Kilian M. Treurniet (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Manon L. Tolhuisen (Department of Radiology and Nuclear Medicine, Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam); Heitor Alves (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Annick J. Weterings (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam), Eleonora L.F. Kirkels (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam), Eva J.H.F. Voogd (Department of Neurology, Rijnstate Hospital, Arnhem); Lieve M. Schupp (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Sabine Collette (Department of Neurology, Radiology, University Medical Center Groningen); Adrien E.D. Groot (Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Natalie E. LeCouffe (Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Praneeta R. Konduri (Department of Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam); Haryadi Prasetya (Department of Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam); Nerea Arrarte-Terreros(Department of Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam); Lucas A. Ramos(Department of Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam).
Contributors: HvV: First author, writing of the manuscript, merging of all data, and constructing the final model. WGK: Clinical rationale, motivation, and scope of study. Development of initial cost-effectiveness model and combining of multiple public and private data sources. LAvd: Data collection and interpretation of long-term follow-up, cost and EQ5D data used for estimating mRS-cost, and utility estimates. MK: Data collection and interpretation of MR CLEAN registry data, mRS, and time parameters. Adjustments in writing in the final manuscripts’ introduction and discussion sections. FMEP: Long-term model development and choosing of multiple data sources. MG: Clinical rationale, motivation, and scope of study. MGMH: Specifying and explaining the bridge between clinical reasoning and methodology. Refining writing of the cost-effectiveness methodology. BJE: Clinical rationale, motivation, and scope of study. Assisted with writing of the Introduction. MJM: Reviewing and adjusting the population-based estimate of primary outcome. DWJD: Clinical rationale, motivation, scope of study, and adjustments in the Introduction and Methods sections. JMC: Clinical rationale, motivation, and scope of study. HAM: Clinical rationale, motivation, and scope of study. Additional final review of the study. HDB: Clinical rationale, motivation, and scope of study. Development of cost effectiveness model and combining of multiple public and private data sources. AvdL: Clinical rationale, motivation, and scope of study. Additional final review of the study. WHvZ: Rationale and methodological review. YBWEM: Rationale and methodological review. EB: Cost-effectiveness modeling, and explanation of modeling procedures. MGWD: Cost-effectiveness modeling, and estimating mRS-cost and mRS-utility relationship. Final methodological review and adjustments. CBLMM: Clinical rationale, clinical motivation of study design, and initializing the study set-up. All authors reviewed and made adjustments to the final version of the submitted manuscript.
Funding: This study was partly funded by the CONTRAST consortium. The CONTRAST consortium is supported by the Netherlands Cardiovascular Research Initiative (CVON), an initiative of the Dutch Heart Foundation, the Brain Foundation Netherlands, Medtronic, and Cerenovus. The MR CLEAN Registry was partly funded by the TWIN Foundation and by Erasmus MC University Medical Centre, Maastricht University Medical Centre, and Amsterdam UMC.
Competing interests: DD and AvdL received funding from the Dutch Heart Foundation, Brain Foundation Netherlands, The Netherlands Organization for Health Research and Development, Health Holland Top Sector Life Sciences & Health, and unrestricted grants from Penumbra Inc., Stryker, Medtronic, Thrombolytic Science, and Cerenovus for research, all paid to institution. Consultation fees were received by DD and AvdL from Stryker and Bracco Imaging. Amsterdam UMC received a grant from Stryker for research led by CM and YR. CM, YR, and HM are shareholders of Nico.lab. Maastricht University MC received funds from Stryker and Cerenovus for consultations by WvZ. MG received consultation fees from Medtronic, Stryker, Microvention, and Mentice. Radboud UMC received funds from Stryker for consultations by Hieronymus Boogaarts.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
For the MR CLEAN Registry investigators:
Diederik W J Dippel, Aad van der Lugt, Charles B L M Majoie, Yvo BWEM Roos, Robert J van Oostenbrugge, Wim H van Zwam, Jelis Boiten, Jan Albert Vos, Ivo G H Jansen, Maxim J H L Mulder, Robert-Jan B Goldhoorn, Kars C J Compagne, Manon Kappelhof, Josje Brouwer, Sanne J den Hartog, Wouter H Hinsenveld, Bob Roozenbeek, Adriaan C G M van Es, Bart J Emmer, Jonathan M Coutinho, Wouter J Schonewille, Marieke J H Wermer, Marianne A A van Walderveen, Julie Staals, Jeannette Hofmeijer, Jasper M Martens, Diederik W J Dippel, Aad van der Lugt, Charles B L M Majoie, Yvo BWEM Roos, Robert J van Oostenbrugge, Wim H van Zwam, Jelis Boiten, Jan Albert Vos, Ivo G H Jansen, Maxim J H L Mulder, Robert-Jan B Goldhoorn, Kars C J Compagne, Manon Kappelhof, Josje Brouwer, Sanne J den Hartog, Wouter H Hinsenveld, Bob Roozenbeek, Adriaan C G M van Es, Bart J Emmer, Jonathan M Coutinho, Wouter J Schonewille, Marieke J H Wermer, Marianne A A van Walderveen, Julie Staals, Jeannette Hofmeijer, Jasper M Martens, Geert Lycklama Nijeholt, Sebastiaan F de Bruijn, Lukas C van Dijk, H Bart van der Worp, Rob H Lo, Ewoud J van Dijk, Hieronymus D Boogaarts, J de Vries, Paul L M de Kort, Julia van Tuijl, Jo P Peluso, Puck Fransen, Diederik W J Dippel, Aad van der Lugt, Charles B L M Majoie, Yvo BWEM Roos, Robert J van Oostenbrugge, Wim H van Zwam, Jelis Boiten, Jan Albert Vos, Ivo G H Jansen, Maxim J H L Mulder, Robert-Jan B Goldhoorn, Kars C J Compagne, Manon Kappelhof, Josje Brouwer, Sanne J den Hartog, Wouter H Hinsenveld, Bob Roozenbeek, Adriaan C G M van Es, Bart J Emmer, Jonathan M Coutinho, Wouter J Schonewille, Marieke J H Wermer, Marianne A A van Walderveen, Julie Staals, Jeannette Hofmeijer, Jasper M Martens, Geert Lycklama Nijeholt, Sebastiaan F de Bruijn, Lukas C van Dijk, H Bart van der Worp, Rob H Lo, Ewoud J van Dijk, Hieronymus D Boogaarts, J de Vries, Paul L M de Kort, Julia van Tuijl, Jo P Peluso, Puck Fransen, Jan van den Berg, Boudewijn van Hasselt, Leo A M Aerden, René J Dallinga, Maarten Uyttenboogaart, Omid Eschgi, Reinoud P H Bokkers, Tobien H C M L Schreuder, Roel J J Heijboer, Koos Keizer, Lonneke S F Yo, Heleen M den Hertog, Emiel J C Sturm, Paul J A M Brouwers, Marieke E S Sprengers, Sjoerd F M Jenniskens, René van den Berg, Albert J Yoo, Ludo FM Beenen, Alida A Postma, Stefan D Roosendaal, Bas FW van der Kallen, Ido R van den Wijngaard, Joost Bot, Pieter-Jan van Doormaal, Anton Meijer, Elyas Ghariq, Marc P van Proosdij, G Menno Krietemeijer, Dick Gerrits, Wouter Dinkelaar, Auke P A Appelman, Bas Hammer, Sjoert Pegge, Anouk van der Hoorn, Saman Vinke, H Zwenneke Flach, Hester F Lingsma, Naziha el Ghannouti, Martin Sterrenberg, Wilma Pellikaan, Rita Sprengers, Marjan Elfrink, Michelle Simons, Marjolein Vossers, Joke de Meris, Tamara Vermeulen, Annet Geerlings, Gina van Vemde, Tiny Simons, Gert Messchendorp, Nynke Nicolaij, Hester Bongenaar, Karin Bodde, Sandra Kleijn, Jasmijn Lodico, Hanneke Droste, Maureen Wollaert, Sabrina Verheesen, D Jeurrissen, Erna Bos, Yvonne Drabbe, Michelle Sandiman, Nicoline Aaldering, Berber Zweedijk, Jocova Vervoort, Eva Ponjee, Sharon Romviel, Karin Kanselaar, Denn Barning, Esmee Venema, Vicky Chalos, Ralph R Geuskens, Tim van Straaten, Saliha Ergezen, Roger RM Harmsma, Daan Muijres, Anouk de Jong, Olvert A Berkhemer, Anna MM Boers, J Huguet, PFC Groot, Marieke A Mens, Katinka R van Kranendonk, Kilian M Treurniet, Manon L Tolhuisen, Heitor Alves, Annick J Weterings, Eleonora LF Kirkels, Eva JHF Voogd, Lieve M Schupp, Sabine Collette, Adrien ED Groot, Natalie E LeCouffe, Praneeta R Konduri, Haryadi Prasetya, Nerea Arrarte-Terreros, and Lucas A Ramos
Data availability statement
Data are available upon reasonable request. Data used in this study can be accessed upon request, more information is available at https://www.mrclean-trial.org/home.html.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
MR CLEAN Registry: The MR CLEAN Registry is a quality registry that assessed the safety of endovascular treatment in routine clinical practice. At the time of data acquisition endovascular treatment was standard practice for the population studied and thus the requirement for informed consent was waived. Attached in the author statement is a supporting document of the waiver granted by the institutional review board. MR CLEAN trial and 2-year follow-up of the MR CLEAN trial: Institutional board approval has been granted for the MR CLEAN trial and 2-year follow-up of the MR CLEAN trial and is referred to in the articles that were attached as author statements. In the author statements submitted an institutional review board (IRB) waiver for use of patient data is added and the required CHEERS checklist.
References
- 1. GBD 2016 Neurology Collaborators . Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 2019;18:459–80. 10.1016/S1474-4422(18)30499-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malhotra K, Gornbein J, Saver JL. Ischemic strokes due to large-vessel occlusions contribute disproportionately to stroke-related dependence and death: a review. Front Neurol 2017;8. 10.3389/fneur.2017.00651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rajsic S, Gothe H, Borba HH, et al. Economic burden of stroke: a systematic review on post-stroke care. Eur J Health Econ 2019;20:107–34. 10.1007/s10198-018-0984-0 [DOI] [PubMed] [Google Scholar]
- 4. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 5. Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316:1279–88. 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 6. Janssen PM, Venema E, DiWJ DIppel. Effect of workflow improvements in endovascular stroke treatment: a systematic review and meta-analysis. Stroke 2019;50:665–74. [DOI] [PubMed] [Google Scholar]
- 7. Chaisinanunkul N, Adeoye O, Lewis RJ, et al. Adopting a patient-centered approach to primary outcome analysis of acute stroke trials using a utility-weighted modified Rankin Scale. Stroke 2015;46:2238–43. 10.1161/STROKEAHA.114.008547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sevick LK, Ghali S, Hill MD, et al. Systematic review of the cost and cost-effectiveness of rapid endovascular therapy for acute ischemic stroke. Stroke 2017;48:2519–26. 10.1161/STROKEAHA.117.017199 [DOI] [PubMed] [Google Scholar]
- 9. McCarthy DJ, Diaz A, Sheinberg DL, et al. Long-term outcomes of mechanical thrombectomy for stroke: a meta-analysis. Sci World J 2019;2019:1–9. 10.1155/2019/7403104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Am J Neuroradiol 2018;29:441–53. 10.1016/j.jvir.2017.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Venema E, Groot AE, Lingsma HF, et al. Effect of interhospital transfer on endovascular treatment for acute ischemic stroke. Stroke 2019;50:923–30. 10.1161/STROKEAHA.118.024091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamal N, Sheng S, Xian Y, et al. Delays in door-to-needle times and their impact on treatment time and outcomes in get with the Guidelines–Stroke. Stroke 2017;48:946–54. 10.1161/STROKEAHA.116.015712 [DOI] [PubMed] [Google Scholar]
- 13. Caldwell J, Heran MKS, McGuinness B, et al. Imaging in acute ischaemic stroke: pearls and pitfalls. Pract Neurol 2017;17:349–58. 10.1136/practneurol-2016-001569 [DOI] [PubMed] [Google Scholar]
- 14. Kunz WG, Hunink MG, Almekhlafi MA, et al. Public health and cost consequences of time delays to thrombectomy for acute ischemic stroke. Neurology 2020;95:e2465–75. 10.1212/WNL.0000000000010867 [DOI] [PubMed] [Google Scholar]
- 15. Jansen IGH, Mulder MJHL, Goldhoorn R-JB, et al. Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN registry). BMJ 2018;360:k949. 10.1136/bmj.k949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zaidat OO, Castonguay AC, Gupta R, et al. North American Solitaire stent retriever acute stroke registry: post-marketing revascularization and clinical outcome results. J Neurointerv Surg 2018;10:i45–9. 10.1136/neurintsurg-2013-010895.rep [DOI] [PubMed] [Google Scholar]
- 17. Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) – European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg 2019;11:535–8. 10.1136/neurintsurg-2018-014568 [DOI] [PubMed] [Google Scholar]
- 18. Ackerson T, Adeoye OM, Brown M, et al. AHA/ASA Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke 2019:344–418.
- 19. Campbell BCV, Majoie CBLM, Albers GW, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol 2019;18:46–55. 10.1016/S1474-4422(18)30314-4 [DOI] [PubMed] [Google Scholar]
- 20. Roijen LH, der LNV. Richtlijn voor Het uitvoeren van economische evaluaties in de gezondheidszorg, 2016. [Google Scholar]
- 21. Zorguitgaven . Volksgezondheid Toekomst Verkenning [Internet]. Available: https://www.vtv2018.nl/zorguitgaven [Accessed 1 Apr 2020].
- 22. Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 23. van den Berg LA, Dijkgraaf MGW, Berkhemer OA, et al. Two-year outcome after endovascular treatment for acute ischemic stroke. N Engl J Med 2017;376:1341–9. 10.1056/NEJMoa1612136 [DOI] [PubMed] [Google Scholar]
- 24. Genootschap KA. Prognosetafel AG2018. www ag-ai nl [Internet], 2018. Available: https://www.ag-ai.nl/view.php?action=view&Pagina_Id=888
- 25. Hong K-S, Saver JL. Years of disability-adjusted life gained as a result of thrombolytic therapy for acute ischemic stroke. Stroke 2010;41:471–7. 10.1161/STROKEAHA.109.571083 [DOI] [PubMed] [Google Scholar]
- 26. Pennlert J, Eriksson M, Carlberg B, et al. Long-term risk and predictors of recurrent stroke beyond the acute phase. Stroke 2014;45:1839–41. 10.1161/STROKEAHA.114.005060 [DOI] [PubMed] [Google Scholar]
- 27. van den Berg LA, Dijkgraaf MGW, Berkhemer OA, et al. Two-year clinical follow-up of the multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands (MR CLEAN): design and statistical analysis plan of the extended follow-up study. Trials 2016;17:555. 10.1186/s13063-016-1669-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fransen PSS, Beumer D, Berkhemer OA, et al. MR CLEAN, a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands: study protocol for a randomized controlled trial. Trials 2014;15:343. 10.1186/1745-6215-15-343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dávalos A, Cobo E, Molina CA, et al. Safety and efficacy of thrombectomy in acute ischaemic stroke (REVASCAT): 1-year follow-up of a randomised open-label trial. Lancet Neurol 2017;16:369–76. 10.1016/S1474-4422(17)30047-9 [DOI] [PubMed] [Google Scholar]
- 30. Clua-Espuny JL, Abilleira S, Queralt-Tomas L, et al. Long-term survival after stroke according to reperfusion therapy, cardiovascular therapy and gender. Cardiol Res 2019;10:89–97. 10.14740/cr839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu X, Khunte M, Gandhi D, et al. Implications of achieving TICI 2B vs TICI 3 reperfusion in patients with ischemic stroke: a cost-effectiveness analysis. J Neurointerv Surg 2020;12:neurintsurg-2020-015873. 10.1136/neurintsurg-2020-015873 [DOI] [PubMed] [Google Scholar]
- 32. Khunte M, Wu X, Payabvash S, et al. Cost-effectiveness of endovascular thrombectomy in patients with acute stroke and M2 occlusion. J Neurointerv Surg 2020. 10.1136/neurintsurg-2020-016765. [Epub ahead of print: 19 Oct 2020]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
neurintsurg-2020-017017supp001.pdf (270.4KB, pdf)
neurintsurg-2020-017017supp002.pdf (83.5KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data used in this study can be accessed upon request, more information is available at https://www.mrclean-trial.org/home.html.