Highlights
-
•
A negative relationship was observed between the level of tannin inclusion and CH4 emission.
-
•
The effect of CH4 mitigation is increasing as the level of tannin inclusion is higher.
-
•
Sub-group analysis revealed differences of tannins supplementation response according to CH4 emission measurements techniques.
Keywords: Enteric methane mitigation, Meta-analyses, Secondary metabolites, Greenhouse gases
Abstract
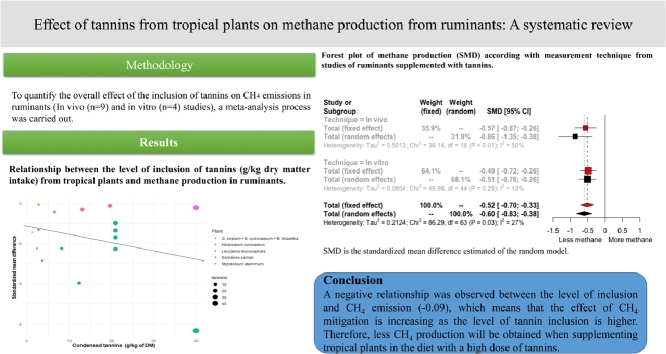
Methane (CH4) is a greenhouse gas generated during the feed fermentation processes in the rumen. However, numerous studies have been conducted to determine the capacity of plant secondary metabolites to enhance ruminal fermentation and decrease CH4 production, especially those plants rich in tannins. This review conducted a descriptive analysis and meta-analysis of the use of tannin-rich plants in tropical regions to mitigate CH4 production from livestock. The aim of this study was to analyse the effect of tannins supplementation in tropical plants on CH4 production in ruminants using a meta-analytic approach and the effect on microbial population. Sources of heterogeneity were explored using a meta-regression analysis. Final database was integrated by a total of 14 trials. The ‘meta’ package in R statistical software was used to conduct the meta-analyses. The covariates defined a priori in the current meta-regression were inclusion level, species (sheep, beef cattle, dairy cattle, and cross-bred heifers) and plant. Results showed that supplementation with tropical plants with tannin contents have the greatest effects on CH4 mitigation . A negative relationship was observed between the level of inclusion and CH4 emission (−0.09), which means that the effect of CH4 mitigation is increasing as the level of tannin inclusion is higher. Therefore, less CH4 production will be obtained when supplementing tropical plants in the diet with a high dose of tannins.
Graphical abstract
1. Introduction
A major problem facing our world today is climate change caused by the emission of greenhouse gases (GHG) of anthropogenic activity (Cardona-Iglesias, Mahecha-Ledesma, and Angulo-Arizala, 2016). Overall, livestock contributes to 14–15% of the anthropogenic GHG emissions, and ruminants are responsible for two-thirds of this production (FAO, 2013; Gerber et al., 2013). These GHG could be methane (CH4), carbon dioxide (CO2), and nitrous oxide (NO2) which are major contributors to global warming (Ugbogu et al., 2019). In particular, ruminants produce around 115 million tons of CH4 per year, a gas generated from rumen fermentation, carried out by a microbial complex of bacteria, archaea, protozoa and fungi, called "ruminal microbiota" (Sandoval-Pelcastre, Ramírez-Mella, Rodríguez-Ávila, & Candelaria-Martínez, 2020).
Because CH4 has a global warming effect 23 times greater than CO2 (Ugbogu et al., 2019), the increase in global temperature is having effects on many species of animals and plants. These effects will increase in the coming years, causing crops and fodder to be affected by extreme weather (Olesen and Bindi, 2002). In the search for solutions to reduce GHG emissions, the use of tropical plants with anti-methanogenic potential has been suggested (Canul-Solis et al., 2020; Jayanegara et al., 2020; Ku-Vera et al., 2020a,b; Rivera et al., 2015). However, due to the great diversity of tropical plant species with an anti-methanogenic effect, more research is needed in order to assess which ones have major impact on CH4 emissions and the amount whereby the need to be included in the diet (Sandoval-Pelcastre, Ramírez-Mella, Rodríguez-Ávila, & Candelaria-Martínez, 2020). Ruminant production systems in the tropics and subtropical areas are characterized by grazing native and introduced grasses varying in quantity and quality throughout the year (Becholie, Tamir, Terrill, Singh, and Kassa, 2005). Tropical trees (TT) as Leucaena leucocephala, Acacia pennatula, Enterolobium cyclocarpum, Gliricidia sepium may contribute to an improvement in ruminants’ feeding due to their high nutritive value (Topps, 1992). Furthermore, TT contain a range of plant secondary metabolites (PSM) (Montoya-Flores et al., 2020; Piñeiro-Vázques et al., 2018), which could alter rumen fermentation and consequently reduce CH4 emissions (El-Zaiat et al., 2020; Piñeiro-Vázques et al., 2018).
Tannins are containing into the PSM, and reduce methane due to their inhibitory effect on methanogens, protozoa and other hydrogen-producing microbes (Patra and Saxena, 2010; Tavendale et al., 2005). Temperate climate plants, rich in tannins such as Lotus pedunculatus, have been shown to reduce methane production up to 30% (Woodward, Waghorn, and Laboyrie, 2004) and can replace other forages in the diet. Therefore, the objective of this review and meta-analysis is to show the main tropical tannin plants that can be used as natural additives for mitigation of CH4 emissions in ruminants.
2. Controlling rumen-level methane production
Reducing the output of CH4 generated by ruminal fermentation is a great challenge for nutritionists. In fact, the digestive system of ruminants has evolved over the years to use cellulose and polysaccharides by means of a pre-gastric fermentation system that produces CH4, however, this system represents a disadvantage for the environment in terms of contamination (Gill, Smith, and Wilkinson, 2010). On the other hand, the level of CH4 yield emitted by ruminant animals related to the amount of feed intake (Monteny, Bannink, and Chadwick, 2006). This means that, although CH4 yield levels increase directly with feed intake (Benchaar, Pomar, and Chiquette, 2001). It is important to consider, that not all feed ingredients will ferment in the same way in the rumen, as different amounts of CH4/unit of fermented carbohydrate are produced. Within concentrate feeding, soluble sugars will produce more CH4 than starch per MJ of GE intake, so replacing sugars with starch in concentrated feeds will decrease CH4 by 15%, as well as the emission of other gases into the environment (Mills et al., 2001). Most studies on dairy cows have reported that increasing the proportion of concentrate in the diet increases milk production since feed digestibility is improved; however, these studies were conducted with cows that produce more than 20 kg of milk per day in temperate climates (Olijhoek et al., 2018; Yan et al., 2010). However, Robles-Jimenez et al. (2021) reported that crossbred F1 dual-purpose cows (½ Bos taurus – ½ Bos indicus) grazing in tropical systems and supplemented with 150 – 450 g of concentrate per kg of daily milk production did not improve milk yield but increased CH4 and N2O production per cow as the concentrate increased in the diet, which agrees with Lawrence, O'Donovan, Boland, Lewis, and Kennedy (2015) and Dale, McGettrick, Gordon, and Ferris (2015).
Another aspect to consider is to know that, in diets rich in concentrates, the ruminal pH decreases (this is due to the yield of a large amount of volatile fatty acids, VFA), which facilitates the production of more propionate, acting as a sink for H2 and, consequently, producing less CH4/unit organic matter fermented (OMF) in the rumen (Monteny et al., 2006). A second approach aims to reduce the production of CH4 by using ingredients or additives specifically intended for that purpose. The function of these ingredients is to directly or indirectly inhibit the process of methanogenesis. Some PSM and plant extracts are included in this category as main secondary compounds that directly inhibit methanogens (García-González, González, and López, 2010).
3. Effect of secondary plant metabolites on CH4 emission
Due to their availability, TT and fodder is often the main ingredient in the diet of animals in tropical and subtropical regions of the world (Ayasan, Cetinkaya, Aykanat, and Celik, 2020; Canul-Solis et al., 2020; Schultze-Kraft et al., 2018). Feed ingredients (tree foliage, grasses, and legumes) from these regions differs from those from temperate regions, due to their structure, chemical composition and digestibility (Assoumaya, Sauvant, and Archimède, 2007). Phytochemicals, circumscribed but appropriately chosen (primarily PSM) are attractive because they are naturally produced by plants and can be include it in feed rations. However, forages from tropical regions may contain secondary metabolites that can alter rumen methanogenesis, decreasing the CH4 yield (Bodas et al., 2012; Canul-Solis et al., 2020; Jouany and Morgavi, 2007; Vélez-Terranova, Campos-Gaona, and Sánchez-Guerrero, 2014). Ruminants fed tropical forage and pasture have been reported to produce more enteric CH4 than ruminants fed temperate forage and pastures under different climatic conditions (Ku-Vera et al., 2020b), because in each climatic region the chemical composition and content of PSM will vary. It should be noted that while the IPCC (2006) provides default values for emission calculations (i.e., Ym 6.5%), which are used in most publications, it also specifies that emission factors need to be precise and validated in each country (Van Lingen et al., 2019). In this sense the values of Ym change (Ym 0.54% of energy intake) under tropical conditions as well as the type of animal´s genetics used in this region (Montoya-Flores et al., 2020).
4. Tannin chemistry
Tannins are natural chemical substances that belong to the group of PSM and are produced by plants in their intermediate metabolism. Plant secondary metabolites play a role of protection from herbivores, pests and pathogens. Secondary metabolites prevent toxicity and act as precursors to physical defence systems (Bennett and and Wallsgrove, 1994).
Tannins are polyphenolic compounds of high molecular weight and are able to precipitate protein (Patra and Saxena, 2009). Tannins found in plants are presented as condensed tannins (CT) and hydrolysable tannins (HT) and both vary between fodders (Naumann, Tedeschi, Zeller, and Huntley, 2017).
Due to their lower risk of toxicity for the animal, anti-methanogenic activity has been studied mainly for CT-rich plants or extracts than HT (Beauchemin, Kreuzer, O'Mara, and McAllister, 2008), However, there are few studies related to the addition of tropical plants containing tannins and their antimethanogenic effect. These polyphenolic compounds chemically have variable molecular weights and the ability to bind to natural polymers such as proteins and carbohydrates, and are found in the wood, bark, fruits, flowers, nuts, leaves, and roots of most plant species (Min et al., 2020; Mueller-Harvey, 2006; Ortiz-Domínguez, Posada, & Noguera, 2014). Compared to tropical plants, temperate climate plants such as Lotus pedunculatus, which are rich in tannins, have also been shown to reduce CH4 excretion by up to 30% (Woodward et al., 2004) and can replace the use of other forages in the diet.
In this way, knowing the plants, tree foliage, legumes and other natural resources with high potential in the mitigation of CH4 would be beneficial for environment protection. However, what is currently known is that these substances are antimicrobial compounds that have the ability to inhibit abundance of some ruminal microorganisms. This is because they have bactericidal or bacteriostatic activities, which prevent growth or activity of methanogens in the rumen, which is due to the binding of microbial cell proteins and enzymes (Liu, Vaddella, and Zhou, 2011; Tavendale et al., 2005). The challenge in ruminant nutrition is to implement the use of these natural resources with high tannin content in arid and subtropical areas, since in production systems where it is possible to use these supplements, today is a viable alternative to reduce environmental pollution. Furthermore, most of the research published today on the use of tannins shows positive results (Albores-Moreno et al., 2018; Alves, Dall-Orsoletta, and Ribeiro-Filho, 2017).
5. Effect of tannins on rumen microbial population
The diet has been reported as a predominant factor affecting the microbial community composition in the rumen on the host and the rumen environment (Henderson et al., 2015). Therefore, when PSM are included in the feed, they alter the availability of nutrients and metabolites and/or inhibit ruminal microbial metabolism of bacteria, protozoa, fungi and archea populations (Bodas et al., 2012; Henderson et al., 2015; Vasta et al., 2019).
5.1. Effect of tannins on rumen bacteria and methanogens
The high molecular weight and polyphenolic nature of tannins result in the formation of complexes with microbial enzymes or cell walls. Thus, the exerted activity may cause the inhibition of cellulolytic or proteolytic bacteria or methanogens (Mannelli et al., 2019; McSweeney, Palmer, Bunch, and Krause, 2001). The mode of action of tannins is strictly dependant on their chemical structure as well as the bacteria species (Vasta et al., 2019). Condensed tannins (CT) were recognized to have a stronger binding with nutrients than hydrolysed tannins (HT), mainly due to the fact they have a higher grade of polymerization, which makes their degradation in the rumen environment more difficult (Jayanegara, Goel, Makkar, and Becker, 2015). On the contrary, HT have been reported to have a greater protein precipitation capacity that has been related to higher biological activity and a higher methane mitigation capacity in comparison to CT. Additionally, the HT activity may be enhanced by the direct toxic methanogenic activity exerted by HT fractions, produced as a consequence of HT degradation by rumen microorganism enzymes, i.e. tannase (Bhat, Singh, and Sharma, 1998; Jayanegara et al., 2015).
The CT have been proposed to directly inhibit some ruminal gram-positive specialized fibrolytic bacteria (Fibrobacter succinogenes, Ruminococcus albus, Ruminococcus flavefaciens, Butyrivrio proteoclasticus) in an in vivo study with fistulated ewes (Costa et al., 2018). In another study, Fibrobacter succinogenes and total methanogens population inhibition (up to 36%), have been reported in vitro, either supplementing CT or HT (Jayanegara et al., 2015).
Salami et al. (2018), included 4% of either CT (Mimosa pudica, Uncaria gambir) or HT (Castanea sp., Caesalpinia spinosa) in lambs’ diet, and did not observe a difference in absolute abundance of bacteria and fungi, while methanogens (−12%) abundance decreased similarly with both types of tannins. In a recent in vitro study, the same concentration of chestnut tannins (HT) was fermented, and methane produced was reduced by 12.5% compared to control, while acetate production increased (Cappucci et al., 2021). Goel and Makkar (2012) suggested that HT directly inhibit methanogens activity, and, therefore, they might affect less fibre digestibility, which can be compromised by the inclusion of CT in the diet. Tavendale et al. (2005) evaluated in broth culture the growth and methane production of tMethanobrevibacter ruminantium testing either polymeric or oligomeric CT fractions from Lotus pedunculatus. The polymeric CT fractions were the only effective in inhibiting the growth, thus demonstrating the importance of PSM chemical structure and synergistic effect of all components to directly inhibiting methanogens along with other rumen microorganism activities (Mannelli et al., 2019). The reduction of fibre digestibility, when CT sources were included in the diet, was thereby supported by the reduction of total VFA production mainly explained by the reduction of acetate production, as evaluated in sheep fed with an inclusion of 16 g/Kg dry matter (DM) intake of quebracho extract (Buccioni et al., 2015). However, total VFA production was not impaired with a level of tannins inclusion less of than 2 g/Kg DM (Table 1). This low dosage might be not always sufficient to achieve a methane mitigating effect. Hence, a dosage above 20 g/Kg of tannins has been proposed by Jayanegara, Leiber, and Kreuzer (2012). In accordance with Salami et al. (2018) both HT and CT extracts could impact the ruminal microbiome when supplemented at moderate levels (<50 g/Kg DM, Mueller-Harvey, 2006), but their detrimental effect on fibrolytic bacteria should be considered when animals are fed with high-fibre diets. The contrasting results concerning rumen fermentation traits, microbial population, and methane production can be at least partially explained by the heterogeneity of tannin chemical structures from plants, the various dosages intake and the feeding regimen (Patra and Saxena, 2011; Vasta et al., 2019). Moreover, microbial adaptation to tannins might occur through mechanisms of some bacteria such as the formation of protective exopolysaccharide layer around the cells, degradation of tannins, and modification of cell membrane (Patra and Saxena, 2011).
Table 1.
Effect of dietary tannins on methane production and other major effects in vitro and in vivo studies.
| Plant | Dosage | Trial type | Unit | Methane reduction potential (% of control) | Other major effects reported | References |
|---|---|---|---|---|---|---|
| Acacia tannins | 50 g/kg DM | In vitro | mL/24h | 15% | −11% of total VFA | Staerfl, Kreuzer, and Soliva, 2010 |
| Chestnut and sumarch (HT) and mimosa and quebracho (CT) | 1 g/L | In vitro | mL/L | 3% CT 7% HT |
−14% CT and −5.8% HT of total VFA |
Jayanegara et al., 2015 |
| Chestnut leaves | ∼24 mg/g DM of HT tannin | in vitro | mL/24h | 28% | −13% total VFA | Terranova, Kreuzer, Braun, and Schwarm, 2018 |
| CT from leaves of Gliricidia sepium, Leucaena leucocephala, and Manihot esculenta. | 0, 0.25, 0.5, 0.75, and 1.0 g CT/Kg, respectively | In vitro and in vivo (rumen-cannulated sheep) | mL/24h | Up to 22% (in vitro) | Up to −25% (in vitro) of total VFA No effect on Methanogens population (in vivo) |
Rira et al., 2015 |
| Vaccinium vitis idaea | 140 g of extract containing 2 g of tannins/kg DM | In vivo (Polish Holstein-Friesian dairy) | mM | 8% | −46% rumen NH3 −35% Protozoa −21% Methanogens No effect on total VFA |
Cieslak, Zmora, Pers-Kamczyc, and Szumacher-Strabel, 2012 |
| Acacia mearnsii tannin extract | 7 g/Kg DMI | In vivo (dairy cows) | g/day | 32% | No effect on milk production | Alves et al., 2017 |
| Chestnut or Chestnut+Quebracho tannin extract | 1.5 g/Kg | In vivo (crossbred steers) | g/day | No effect | No effect on Protozoa population No effect on total VFA production |
Aboagye et al., 2018 |
CT, Condensed tannins; HT, Hydrolysable tannins; VFA, Volatile Fatty Acids.
5.2. Effect of tannins on rumen protozoa
The antiprotozoal activity of some PSM might be relevant since methanogens colonizing ciliate protozoa were suggested to be responsible for 9 – 25% of methanogenesis in rumen fluid (Henderson et al., 2015; Newbold, Lassalas, and Jouany, 1995). The antiprotozoal activity of tannins is contrasting, and Patra and Saxena (2009) suggested that the effect is plant dependant, having the tannin structure-activity relationship a major role in the mechanism of action (Mueller-Harvey, 2006). HT have been proposed to permeate through protozoa membranes, thus compromising methanogens associations (Patra and Saxena, 2011). In the study by Malik et al. (2017), male sheep diets were supplemented with tanniniferous tropical tree leaves (Ficus benghalensis, Artocarpus heterophyllus and Azadirachta indica) containing 7.1–10.8 g/Kg DM of CT. The digestibility was not compromised, whereas methane production was reduced (up to 26%). The authors suggested that methane reduction can be explained by the decrease of protozoa number (−23%). Moreover, CT appeared to affect Entodinimorphs protozoa more than Holotrichs protozoa (Malik et al., 2017). A similar reduction of protozoa number (−21%) was reported by Salami et al. (2018), including 4% of both CT (mimosa, gambier) or HT (chestnut, tara) in lamb's diet. However, other studies conducted in vivo and reported in Table 2 showed that methane reduction was not always related to a decrease of protozoa number.
Table 2.
Effect of dietary tropical taniferous plants on methane production in vivo studies.
| Plant | CT (g kg−1 of DM)1 | Doses (g kg−1 of DM) | Species | CH4 Production | % CH4 reduction2 | Effect on microbial population | References |
|---|---|---|---|---|---|---|---|
| Leucaena leucocephala | 2.70, 8.20 and 12.30 | 120, 240 and 360 | Crossbred heifers | 162.9, 154.8 and 140.00 g/d−1 | 6.49, 11.14 and 19.64 | No changes in Protozoa, Bacteria and Methanogens counts | Montoya-Flores et al. (2020) |
| Samanea saman + Pennisetum purpureum | 1.20, 2.40 and 3.60 | 900, 935 and 965 | Crossbred heifers | 89.63, 72.03 and 59.30 L/d−1 | 25.83, 40.40 and 50.93 | No changes in Protozoa count | (Valencia-Salazar et al., 2017) |
| Leucaena leucocephala | 21.00 in all doses | 200, 400, 600 and 800 | Crossbred heifers | 101.20, 87.40, 74.90 and 53.50 L/d−1 | 26.30, 36.35, 45.45 and 61.03 | No changes in Protozoa count | Piñeiro-Vázques et al. (2018) |
| Lolium perenne | - | 185 | Dairy cattle | 260.00 g/d−1 | 10.34 | - | Woodward et al. (2002) |
| Hedysarum coronarium | 2.72 | 130 | Dairy cattle | 253.90 g/d−1 | 15.37 | - | Woodward et al. (2002) |
| Lolium perenne | - | 161 | Dairy cattle | 360.63 g/d−1 | 10.00 | - | Woodward et al. (2004) |
| Lotus corniculatus | - | 121 | Dairy cattle | 343.24 g/d−1 | 14.19 | - | Woodward et al. (2004) |
| Sericea lespedeza | 153.00 | 881 | Goat | 6.30 g/d−1 | 12.00 | Protozoa count increased in the long period | Puchala et al. (2012) |
| Leucaena leucocephala | 40.00 | 820 | Sheep | 7.80 g/d−1 | 25.71 | Dias-Moreira et al. (2013) | |
| Styzolobium aterrimum | 40.00 | 690 | Sheep | 10.40 g/d−1 | 0.95 | - | Dias-Moreira et al. (2013) |
CT, Condensed tannins (g kg−1 of DM).
% CH4 reduction compared with the control diet, CT, Condensed tannins ((g kg−1 of DM)),.
6. Effect of tannins on CH4 emission
6.1. In vitro studies
The inclusion of tannins directly from plants or as plant extracts, in ruminant diets, has been showed to decrease CH4 above 20 g/kg (Jayanegara et al., 2011). In this sense, Goel and Makkar (2012) reported that CH4 synthesis from ruminal fermentation has been reduced by to 50% in response to tannin or plant extracts containing these polyphenolic compounds (Patra and Saxena, 2010). Authors who conducted experiments on plants with high tannin content (Molina-Botero et al., 2019; Morgavi, Martin, Jouany, and Ranilla, 2012; Patra and Saxena, 2011; Tavendale et al., 2005) agreed that tannin plants reduce CH4 production due to their antimicrobial properties, for example, Jayanegara et al. (2015) found that all tannins decreased CH4 concentration in a linear or quadratic manner, and they also reported that the magnitude of the decrease was greater for plants containing hydrolysable tannins than for those plants rich in condensed tannins. The mode of action and the effects that tannins have on the animal will continue to be the subject of research. Reduction of nematode egg excretion and worm burden have been also reported in small ruminants fed with tanniferous plants (Birhan, Gesses, Kenubih, Dejene, and Yayeh, 2020; Marley, Cook, Keatinge, Barrett, and Lampikin, 2003; Mengistu et al., 2017; Minho, Filippsen, Amarte, & Abdalla, 2010; Naumann et al., 2017; Oliveira et al., 2011). The inclusion of tropical tanniferous plants in vitro studies has been reported (Table 3). For example, Albores-Moreno et al. (2018) reported that supplementation with Leucaena leucocephala at a concentration of 950 g/kg DM in an in vitro study on diets for cattle based on Pennisetum purpureum grass, is a feeding alternative that can promote greater efficiency and synthesis of microbial biomass, increase the proportions of propionic and butyric acid, and decrease the output of enteric CH4 up to 15.6 to 31.6%. Rodríguez, Britos, Rodríguez-Romero, and Fondevila (2011) studied the effect of inclusion of plant tanniferous extracts equivalent to 240 mg of Acacia cornigera or Albizia lebbekoides added to 800 mg Pennisetum purpureum, A. cornigera, and A. lebbekoides and reported that CH4 concentration (ml/ml gas) was lower (14 and 7%, respectively) than Pennisetum Purureaum as a control after 24 h of incubation. Tan et al. (2011) evaluated the effects of CT from Leucaena leucocephala at 15 mg of CT/500 mg DM reducing CH4 excretion by ∼47%, while Carulla, Kreuzer, Machmüller, and Hess (2005) reported that supplementation of 25 g/kg of CT (12.5 mg CT/500 mg DM) from Acacia mearnsii in sheep fed ryegrass with a reduction of CH4 emissions by ∼12%. In an in vitro study, Petlum, Paengkoum, Liang, Vasupen, & Paengkoum, 2019 evaluated the inclusion of CTs of a higher molecular weight as Azadirachta indica, showing stronger effect than those of a lower molecular weight as Leucaena leucocephala on CH4 excretion. The inclusion of Siamese neem suppressed CH4 output at inclusion levels of 2, 4 or 6 mg/100 g DM, while supplementation of Leucaena leaves showed reductions on CH4 production at 6 mg/100 mg DM of supplementation. Huang et al. (2010), 2011) suggested that chemical structure and molecular weight of the CTs influenced their efficacy to manipulate rumen fermentation, with specific effect on CH4 mitigation output. Hassan and Benchaar (2012) added Valonea (Quercus aegilops; Nutriad-Adisseo®) extracts as sources of HT, showing that CH4 excretion reduced up to 11% at 50 g/kg DM. On the other hand, Vandermeulen et al. (2018) evaluated the effect of Desmanthus spp. which emitted less CH4 (mL/g OM incubated) than the reference grass hay at 72 h (C. gayana) up to 23%. In vitro studies vary in their response to CH4 production and that seems to depend on the concentration of CT, which is affected by various management and environmental factors such as nutrient soil composition, light intensity, and temperature (Albores-Moreno et al., 2019; Frutos, Hervas, Giraldez, and Mantecon, 2004; Yang et al., 2018). Thus, we can notice that the differences in the concentrations of CT amongst studies vary with plant species, and geographical locations of plants. It is difficult to extrapolate in vitro to in vivo results, due to the variation between results and doses. Therefore, it is highly recommended to evaluate the effect of the supplementation of tanniferous plants on CH4 mitigation in vivo studies.
Table 3.
Effect of dietary tropical taniferous plants on methane production in vitro studies.
| Plant | CT (g kg−1 of DM | Doses (g kg−1 of DM) | CH4 Production | % CH4 reduction 1 | References |
|---|---|---|---|---|---|
| Pennisetum purpureum + Neomillspaughia emargiata; P. purpureum + Tabernaemontana amygdalifolia; P. purpureum + Piscidia piscipula; P. purpureum + Leucaena leucocephala; P. + Havardia albicans | P. p + N. e = 52.90; P. p + T. a = 0.52, P. p + P. pis=8.19, P. p + L. l = 5.90, P. p + H. a = 5.40 | 950 | 25.80 – 33.00 L/kg−1 of digested DM | 12.47 – 31.57 | Albores-Moreno et al. (2018) |
| Pennisetum purpureum + Acacia cornigera; P. purpureum + Albizia lebbekoides; P. purpureum + Leucaena leucocephala | P. p + A.c = 19.7; P.p + A.l = 88.6; P.p + L.l = 66.0 | 104 | 0.22 mL | 4.35 | Rodríguez et al. (2011) |
| Leucaena leucocephala + Panicum maximum | - | 53 | 5.50 mL/g DM | 63.09 | Tan et al. (2011) |
| Acacia mearnsii, Schinopsis balansae, Castanea sativa, Quercus aegilops | A. m = 820.00; S. b = 904.00; C. s = 57.00; Q. a = 80.00 | 200 | 4.48 – 4.77 mL | 36.4 – 40.27 | Hassanat and Benchaar (2012) |
| Delonix regia seed meal | - | 6.6, 20 and 30 | 114.4, 105.4 and 94.1 mL | 9.07, 16.22 and 25.20 | Supapong et al. (2017) |
| Digitaria eriantha+ Leucaena leucocephala | 4.10 | 60 | 5.8 mL/g DM of substrate | 42.00 | Petlum, Paengkoum, Liang, Vasupen, & Paengkoum, 2019 |
| Digitaria eriantha+ Azadirachta indica A. Juss. | 7.90 | 20, 40 and 60 | 3.3, 1.7 and 0.01 mL/g DM of substrate | 67.00, 83.00 and 99.90 | Petlum, Paengkoum, Liang, Vasupen, & Paengkoum, 2019 |
| Desmanthus leptophyllus, Desmanthus virgatus, Desmanthus bicornutus | - | 1000 | 29.8 – 33.6 mL/g OM fermente | 11.79 – 21.77 | Vandermeulen et al. (2018) |
| Leucaena leucocephala, Acacia saligna, Atriplex halimus | L.l = 67.00; A.s = 72.00; A.h = 5.3.00 | 500 | 9.5 – 9.7 mL / g DM | 22.40 – 24.00 | El-Zaiat et al. (2020) |
| Calliandra calothyrsus, Acacia nilotica, Gliricidia sepium, Leucaena leucocephala, Manihot esculenta, Musa spp | C.c = 58.20; A.n = 73.00; G.s = 94.90; L.l = 77.80; M.e = 88.60; M.spp = 84.30 | 100 | 1.41 mL/d | 64.04 | Rira, Morgavi, Popova, Maxin, and Doreau, 2021 |
| Acacia nilotica leave, Acacia nilotica leaves | A.n.l = 80.00; A.n.p = 157.00 | 25, 50, 75, 100 | 1.41 mL/d | 64.04 | Rira et al., 2019 |
| Castanea sativa, Schinopsis lorentzii | 53.80 | 15 and 30 | 54.70 mL/ g DM | 44.40 | Menci et al., 2021 |
| Arachis pintoi, Cratylia argéntea, Calliandra calothyrsus | 29.00 | 200 | 0.78 g/d | 50.31 | Hess et al., 2003 |
| Brachiaria humidicola, Vigna unguiculata, Calliandra calothyrsus, Flemingia macrophylla | - | 15 | 13.00 mL/d | 80.92 | Tiemann et al., 2008 |
% CH4 reduction compared with the control diet.
7. Meta-analysis: methodology
To quantify the overall effect of the inclusion of tannins on CH4 emissions in ruminants (In vivo studies), a meta-analysis process was carried out. A compressed and structured search of articles was carried out using the search engines Google Scholar, PubMed. Different sets of the following keywords were provided to field experts to integrate the study database: "ruminants", "tropical plants", "secondary metabolites", "tannins", "methane emission", "treatment" (control vs tropical plant), “in vitro”, and “in vivo”.
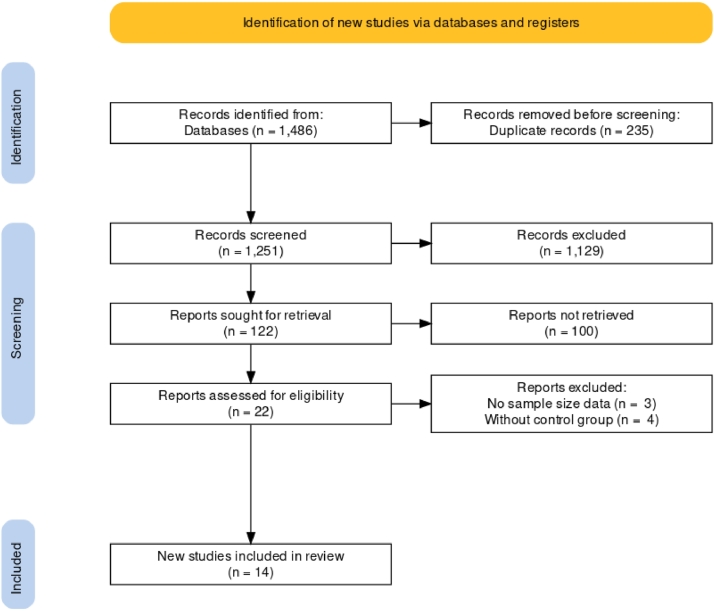
Only articles peer reviewed, written in English containing an experimental set up were included in the current literature review. To be considered, the studies must met the following inclusion and exclusion criteria according with Lean, Thompson, and Dunshea (2014): a) studies published in an international peer-reviewed scientific journal, b) specific procedures for random assignment of animals to each treatment (experimental design), c) report minimum means squares and a measure of variability, and c) report the sample size of each group (Fig. 1).
Fig. 1.
PRISMA flow diagram of the systematic review from initial search and screening of publications included in the meta-analysis.
The final database included the publications from 2002 to 2021 and comprised the following information of least squares means, variability measures [standard error of mean (SEM), standard error of differences (SE) or standard deviation (SD)] and number of experimental units for both groups to each output variables, animal species as sheep (Ovis aries), goat (Capra hircus), cattle (Bos Taurus and Bos indicus), beef and dairy cattle and crossbred heifers, plant, dose, CH4 emission from the control and tannin groups, as well as the number of repetitions. The CH4 values from in vitro studies were homogenized to mL/g DM, g/d, or mL/d. With regard to in vivo studies all were adjusted and expressed in g CH4/d. Current analysis, random effects models were fitted to estimate the effect size (ES), the 95% confidence interval and the statistical significance of ES for each outcome variable, using the 'meta' package version 4.6–0 (Schwarzer, Carpenter, & Rücker, 2015) in the R statistic software version 3.3.1 (R Core Team, 2016). The ES was calculated as standardized mean difference (SMD) using the methods described by Hedges (1981) for the fixed effects and by DerSimonian and Laird, (2015) for random effects models. The studies that reported outcome variables in the same unit of measure aid to calculate the raw mean difference (RMD), which permits ES interpretation under original measures units (Appuhamy et al., 2013). The current systematic review analyses studies performed in different places with different methods and under different animal management; hence, the heterogeneity was needed (Higgins, 2008). Heterogeneity of results amongst trials was reported using the I2 statistic (Higgins & Green, 2011). The I2 represents the approximate proportion of total variability and indicate estimates that can be attributed to heterogeneity, which was calculates as:
Where Q is the X2 heterogeneity statistic and K is the number of trials. I2 values of 25%, 50% and 75% represented small, moderate, and high levels of heterogeneity, respectively. For output variables that showed substantial heterogeneity (I2>50%), mixed effects regression models (meta-regression analysis) were constructed to explore sources of heterogeneity using the 'metaphor' package (Viechtbauer, 2010). The covariates defined a priori in the current meta-regression were inclusion level, species (sheep, beef cattle, dairy cattle, and cross-bred heifers) and plant.
8. Results from meta-analysis
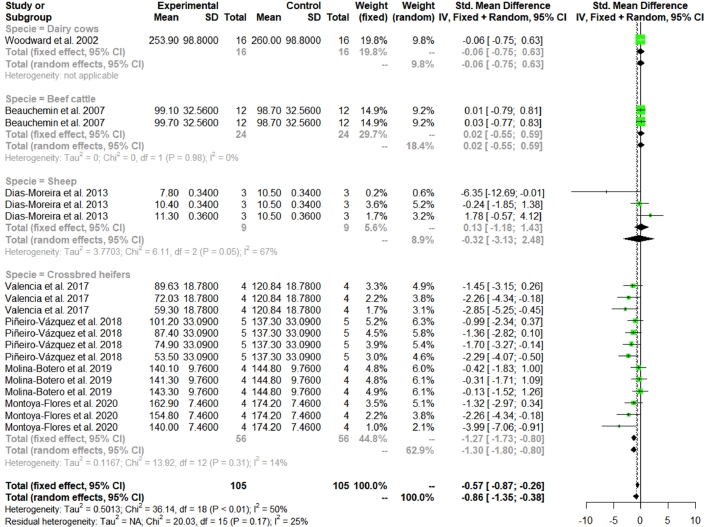
A total of 14 articles were analysed to assess the effect of tannins supplementation on CH4 emissions of ruminants (Fig. 2). According to the obtained database, two meta-analysis were carried out in the current work. The first meta-analyses assessed the effect of tannins supplementation on CH4 enteric emission in ruminant using in vivo studies (n = 19 trials). In vivo technique´s database allowed to estimate the raw mean difference (RMD) and standardized mean difference (SMD) because all studies reported the CH4 emission in the same unit (g/d). The second meta-analysis evaluated the effect of tannins supplementation on CH4 emissions of overall studies (in vivo = 19; vitro = 45 trials). However, because those studies reported CH4 emission in different units of measurement, only the SMD was estimated (Table 4). In both meta-analyses the values of heterogeneity (I2) were greater than 25%, therefore the sources of heterogeneity were explored.
Fig. 2.
Forest plot of methane production, expressed as Dry Matter Intake (DMI, g tannins/d) from studies focused on tannins supplementation in ruminants.
Table 4.
Standardized mean difference (SMD) and 95% CI of enteric CH4 emissions of ruminants supplemented with tannins.
| Source | Effect size (SMD) | 95% CI | |
|---|---|---|---|
| Lower | Upper | ||
| Acacia cornigera | −0.49 | −1.92 | 0.92 |
| Acacia mearnsii | −0.58 | −1.24 | 0.06 |
| Acacia nilotica | −0.76 | −5.53 | 3.9 |
| Albizia lebbekoides | −0.78 | −2.26 | 0.70 |
| Caesalpinia gaumeri | 0.15 | −0.98 | 1.28 |
| Calliandra calothyrsus | −0.93 | −1.96 | 0.09 |
| Castanea sativa | −0.67 | −1.32 | −0.01 |
| Flemingia macrophylla | −2.21 | −3.53 | −0.89 |
| Gliricidia sepium | −0.13 | −2.23 | 1.96 |
| Havardia albicans | 0.21 | −0.91 | 1.35 |
| Hedysarum coronarium | −0.06 | −0.75 | 0.63 |
| Leucaena leucocephala | −1.46 | −1.95 | −0.97 |
| Manihot esculenta | 0.02 | −1.93 | 1.99 |
| Mimosa caesalpiniaefolia | 1.77 | −0.56 | 4.12 |
| Musa spp | −0.88 | −6.28 | 4.50 |
| Neomillspaughia emargiata | 0.04 | −1.08 | 1.17 |
| Piscidia piscipula | −0.03 | −1.16 | 1.09 |
| Quercus aegilops | −0.50 | −1.15 | 0.14 |
| Samanea saman | −2.02 | −3.17 | −0.86 |
| Schinopsis balansae | −0.54 | −1.20 | 0.10 |
| Schinopsis lorentzii | −3.31 | −6.88 | 0.26 |
| Schinopsis quebracho | 0.021 | −0.54 | 0.58 |
| Styzolobium aterrimum | −0.23 | −1.85 | 1.38 |
| Tabernaemontana amygdalifolia | −0.12 | −1.26 | 1.00 |
| Vigna unguiculata | 2.07 | 0.79 | 3.32 |
| Gliricidia sepium+Enterolobium cyclocarpum+Brachiaria brizantha sepium | −0.28 | −1.09 | 0.52 |
SMD is the standardized mean difference estimated of the random model.
8.1. In vivo studies meta-analysis
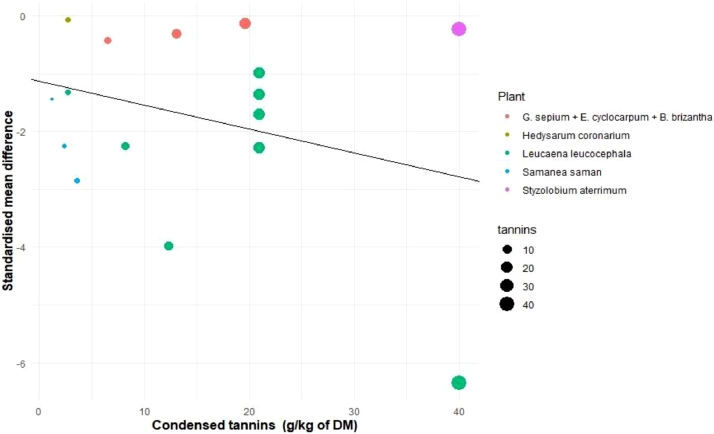
The in vivo studies showed a positive response in mitigating CH4 emission due to the inclusion of tannins in the diets of ruminants through the feeding of tropical plants (SMD = −0.86; P = 0.005) (Fig. 2). The response to tannin content has a moderate heterogeneity (I2 = 50.5%) that can be explained by the type of plant offered, level of inclusion and animal species. With regard to the type of animal that was fed Leucaena leucocephala and the combination of Samanea saman + Pennisetum purpureum, showed the greatest mitigation effects of CH4 according to the meta-regression analysis (Fig. 3, Table 4). The effect of tannins was most evident in heifers with an effect size of −1.3 compared to dairy cows (ES = −0.06), beef cattle (ES = 0.02) and sheep (ES = −0.32) (Fig. 2). Finally, a negative relationship was observed between the level of inclusion of tannins and CH4 emission (−0.09), by increasing the dose of tannins, the difference between control and treatment increases, although in a negative direction (Fig. 3). This means that, the higher the dose of tannins, the treatment group will emit less CH4 compared with control, showing differences between the type of plant used, with a rather interesting effect on Leucaena leucocephala and Samanea saman, being mostly condensed tannins in ruminant animal production.
Fig. 3.
Relationship between the level of inclusion of tannins (g/kg dry matter intake) from tropical plants and methane production in ruminants.
8.2. Overall meta-analysis
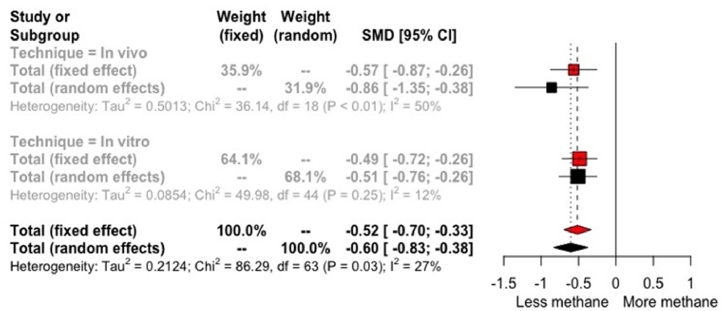
The global response of tannins supplementation in ruminants (in vivo and in vitro studies) when all available studies where analysed depicts a SMD of −0.60 to the random effect model. The heterogeneity was considerably lower than overall meta-analysis (I2 = 27%) in comparison with the meta-analysis of in vivo studies (I2 = 50%). Sub-group analysis revealed differences of tannins supplementation response according with the measure technique of CH4 emission. The effect size of in vitro studies was lower (−0.51; 95% CI −0.76 – −0.26) compared with in vivo studies (−0.86; 95% CI −1.35 – −0.38) (Fig. 4). With regard to sources of tannins (Table 4), the highest mitigation response was observed in Flemingia macrophylla (−2.21; 95% CI −3.53 – −0.89) followed by Samanea saman (−2.02; 95% CI −3.17 – −0.86) and Leucaena leucocephala (−1.46; 95% CI −1.95 – −0.97). The studies that supplemented Schinopsis lorentzii showed a higher effect size (−3.31; 95% CI −6.88 – −0.26), however the confidence intervals were wide and included zero value.
Fig. 4.
Forest plot of methane production (SMD) according with measurement technique from studies of ruminants supplemented with tannins. SMD is the standardized mean difference estimated of the random model.
9. Discussion from meta-analysis
The combination of Samanea saman and Pennisetum purpureum (Valencia-Salazar et al., 2017) in cattle diets have been shown to contribute to the reduction of CH4 up to 50.9% (Table 2), showing the greatest mitigation effects of CH4 according to the results of the meta-regression analysis. On the other hand, in an in vivo study with lambs, Dias-Moreira et al. (2013) evaluated the effect of three forages, Leucaena leucocephala, Styzolobium terrimum and Mimosa caesalpiniaefolia , reporting that with the use of Leucaena leucocephala there is a greater reduction in CH4 emissions (∼25%). El-Zaiat et al. (2020) carried out in vitro and in vivo studies on sheep (Table 3), confirming that in the in vitro study, the supplementation of Leucaena leucocephala, Atriplex halimus or Acacia saligna to the diet (50/50) reduced CH4 output to almost 23% compared with the control group, and in the in vivo study in sheep diets, showed reductions of 11.45% in the CH4 production. Tiemann et al. (2008) in an in vitro study found an 80% reduction of CH4 by including Flemingia macrophyla, followed by Leucaena leucocephala with variations in CH4 reduction (30–60%) in vitro studies (Table 3), which may be due to the different levels of inclusion, which coincided with our results (Table 4).
Ku-Vera et al. (2020a) confirmed that the use of Leucaena leucocephala in beef cattle has a mitigating effect on CH4 when fed at levels of up to 30–35% DM. Furthermore, Ku-Vera et al. (2020a) mentioned that the legume Samanea saman which contains saponins, has demonstrated to have a mitigating effect on enteric CH4 in cattle and sheep housed in respiration chambers, since saponins break the membrane of the rumen protozoa thus decreasing the number of methanogenic protozoa and archaea. This result of the use of tropical plants was confirmed by Ku-Vera et al. (2020b) who, by incorporating ground foliage and pods from tropical trees and shrubs into beef cattle rations, obtained a decrease of between 10% and 25% in CH4 (g CH4/kg DM intake), and those responses depended on the species of plant and the level of intake of the ration.
Piñeiro-Vázques et al. (2018) evaluated the use of Leucaena leucocephala in crossed heifers and reported that, the higher the dose concentration, the lower the CH4 emission, indeed they obtained a 61% decrease in CH4 at a dose of 800 g/kg DM of Leucaena leucocephala. This result agrees with Montoya-Flores et al. (2020) and Valencia-Salazar et al. (2017) in another study with crossed heifers, reporting that the use of Samanea saman + Pennisetum purpureum pod meal decreases CH4 emissions as its inclusion increases, since, from the inclusion of 0, 10, 20 and 30%, the latter decreased 50.9% of CH4 in L/d.
Rumen CH4 yield represents an energy loss of up to 0.12 of the total feed intakes (Olijhoek et al., 2018). In this sense, if the inclusion of tannins reduce CH4 output (Ku-Vera et al., 2020a,b), plants containing these compounds should have a positive impact on energy utilization, as well as a reduction of the environmental impact of livestock production (Vázquez-Carrillo, Montelongo-Pérez, González-Ronquillo, Castillo-Gallegos, & Castelán-Ortega, 2020). However, a selective effect of tannins on fibrolytic bacteria occurred, with Ruminococcus albus being most affected, in agreement with the negative effects of saponins on this species (Galindo et al., 2016). These different bacterial responses to tannins might be due to the specific attachment mechanisms to the substrate and the fermentation pattern (Koike and Kobayashi, 2009), as well as by the different modes of action of tannins depending on their source (Tiemann et al., 2008). In the present study, the inclusion of tropical forage rich in tannins seems to reduce CH4 emission in vivo trials, but responses vary amongst plant sources, doses and animal species (Figs. 2, 3).
Since the concentration of tannins varies depending on the plant, (Fig. 3, Table 4) it is observed that Leucaena leucocephala shows a better effect in terms of CH4 reduction compared with Styzolobium térimum at the same concentration. Likewise, it was observed that Leucaena leucocephala and Samanea saman showed a greater effect in the decrease of CH4, compared to other plants such as Brachiaria brizantha, Gliricidia sepium, Enterolobium cyclocarpum, this effect was found in cross breed cattle. Puchala et al. (2012) found a 12% decrease in CH4 in goats when adding Sericea lespedeza (Table 2). Likewise, Dias-Moreira et al. (2013) obtained a reduction of CH4 in sheep up to 25% when supplementing Leucaena leucocephala, being lower when supplementing Styzolobium térimum (0.99%), although both plants contained the same concentration of tannins (40 g CT/kg−1 of DM), this effect may be due to the fact that 19% more L. Leucocephala was administered compared to Styzolobium térimum. On the contrary, when supplementing Mimosa caesalpiniaefolia there was no effect on the reduction of CH4, possibly due to the amount administered in the diet (530 g/kg DM), being 34% less TC compared with L. leucocephala. Beauchemin, McGinn, Martinez, and McAllister (2007) when supplementing Schinopsis balansae in beef cattle, found a CH4 reduction of 0.96%, being very similar to that found by Puchala et al. (2012) when supplementing Mimosa caesalpiniaefolia. When supplementing Hedysarum coronarium, Woodward, Waghorn, Lassey, and Laboyrie (2002) found CH4 reductions of 14%, with a concentration of condensed tannins of 2.72 g/100 g DM in Hedysarum coronarium. However, in general a negative relationship was observed between the level of tannin inclusion and CH4 emission. The reduction in CH4 production observed with the use of tannins could be attributed to the fact that they inhibit the activity of microbial enzymes, decrease the populations of protozoa and cellulolytic bacteria and form links with forage proteins, reducing the degradation of ruminal protein (Jakhmola, Taruna, and Raghuvans, 2010; Moscoso et al., 2017). However, an important factor to consider is that the concentration of plant tannins (HT and CT) are known to have both adverse and beneficial effects depending on their concentration and nature, besides other factors such as season, geographical region, animal species and genetics, animal physiological stage and dietary composition (Goel and Makkar, 2012; Piluzza, Sulas, and Bullitta, 2014) and derived from it, the effect on the decrease of CH4 excretion in ruminants (Fig. 2). Therefore, supplementing tropical and subtropical plants in the diet with a high dose of tannins will result in less CH4 production.
10. Final remarks
The efficacy of CTs from plant materials to reduce CH4 emission depends on the plant species and possibly to the environment in which they are grown. Supplementation of tannin-rich plants such as Leucaena leucocephala, Flemingia macrophylla and Samanea saman in vitro and in vivo studies, have a positive effect on the reduction of CH4 in ruminants. Other tropical tannin-rich plants such as Shinopsis lorentzii, Musa spp, Acacia spp., and Albizia spp. can reduce CH4 , but further in vivo studies are suggested to determine rumen microbiome and rumen metabolites.
Funding
This project was financed by UAEMex 4335/ 2017
Compliance with ethical standards
Due to the nature of the work (systematic review), the authors have nothing to declare.
Ethical statements
The authors state that no animals were used in this study, as it is a review of previous work in the tannin supplementation in ruminants and the effect on CH4
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Dr. Lizbeth Robles was supported by a grant of the CONACyT during her studies of Doctorate, in the Programa de Doctorado en Ciencias Agropecuarias y Recursos Naturales, Universidad Autonoma del Estado de Mexico. During the study, Dr. Einar Vargas-Bello-Pérez was a visiting scholar, also supported by project number 4974/2020CIB, and Dr Angeles-Hernandez was granted by Secretaria de Educacion Publica Mexico,Grant numbers: UAEH-PTC-823
Contributor Information
E. Vargas-Bello-Pérez, Email: evargasb@sund.ku.dk.
M. González-Ronquillo, Email: mrg@uaemex.mx.
References
- Aboagye I.A., Oba M., Castillo A.R., Koenig K.R., Iwaasa A.D., Beauchemin K.A. Effects of hydrolyzable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high-forage diet. Journal of Animal Science. 2018;96(12):5276–5286. doi: 10.1093/jas/sky352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albores-Moreno S., Alayón-Gamboa J.A., Miranda-Romero L.A., Alarcón-Zúñiga B., Jiménez-Ferrer G., Ku-Vera J.C., Piñeiro-Vázquez A.T. Effect of tree foliage supplementation of tropical grass diet on in vitro digestibility and fermentation, microbial biomass synthesis and enteric methane production in ruminants. Tropical Animal Health and Production. 2018;51(4):893–904. doi: 10.1007/s11250-018-1772-7. [DOI] [PubMed] [Google Scholar]
- Albores-Moreno S., Alayón-Gamboa J.A., Miranda-Romero L.A., Alarcón-Zúñiga B., Jiménez-Ferrer G., Ku-Vera J.C., Piñeiro-Vázquez A.T. Effect of supplementation with tree foliage on in vitro digestibility and fermentation, synthesis of microbial biomass and methane production of cattle diets. Agroforestry Systems. 2019;94(4):1469–1480. doi: 10.1007/s10457-019-00416-1. [DOI] [PubMed] [Google Scholar]
- Alves T.P., Dall-Orsoletta A.C., Ribeiro-Filho H.M.N. The effects of supplementing acacia mearnsii tannin extract on dairy cow dry matter intake, milk production, and methane emission in a tropical pasture. Tropical Animal Health and Production. 2017;49(8):1663–1668. doi: 10.1007/s11250-017-1374-9. [DOI] [PubMed] [Google Scholar]
- Appuhamy J.A.D.R.N., Strathe A.B., Jayasundara S., Dijkstra J., France J., Kebreab E. Anti-methanogenic effects of monensin in dairy and beef cattle: a meta-analysis. Journal of Dairy Sciences. 2013;96(8):5161–5173. doi: 10.3168/jds.2012-5923. [DOI] [PubMed] [Google Scholar]
- Assoumaya C., Sauvant D., Archimède H. Etude comparative de l'ingestion et de la digestion des fourrages tropicaux et tempérés. INRA Productions Animales. 2007;20(5):383–392. [Google Scholar]
- Ayasan T., Cetinkaya N., Aykanat S., Celik C. Nutrient contents and in vitro digestibility of different parts of corn plant. South African Journal of Animal Science. 2020;50(2):302–309. doi: 10.4314/sajas.v50i2.13. [DOI] [Google Scholar]
- Beauchemin K.A., McGinn S.M., Martinez T.F., McAllister T.A. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. Journal of Animal Science. 2007;85(8):1990–1996. doi: 10.2527/jas.2006-686. [DOI] [PubMed] [Google Scholar]
- Beauchemin K.A., Kreuzer M., O'Mara F., McAllister T.A. Nutritional management for enteric methane abatement: a review. Australian Journal of Experimental Agriculture. 2008;48(2):21–27. doi: 10.1071/EA07199. [DOI] [Google Scholar]
- Becholie D., Tamir B., Terrill T.H., Singh B.P., Kassa H. Suitability of tagasaste (Chamaecytisus palmensis L.) as a source of protein supplement to a tropical grass hay fed to lambs. Small Ruminant Research. 2005;56:55–64. doi: 10.1016/j.smallrumres.2004.02.012. [DOI] [Google Scholar]
- Benchaar C., Pomar C., Chiquette J. Evaluation of dietary strategies to reduce methane production in ruminants: a modelling approach. Canadian Journal of Animal Science. 2001;81(4):563–574. doi: 10.4141/A00-119. [DOI] [Google Scholar]
- Bhat T.K., Singh B., Sharma O.P. Microbial degradation of tannins–a current perspective. Biodegradation. 1998;9(5):343–357. doi: 10.1023/a:1008397506963. [DOI] [PubMed] [Google Scholar]
- Bodas R., Prieto N., García-González R., Andrés S., Giráldez F.J., López S. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Animal Feed Science and Technology. 2012;176(1–4):78–93. doi: 10.1016/j.anifeedsci.2012.07.010. [DOI] [Google Scholar]
- Buccioni A., Pauselli M., Viti C., Minieri S., Pallara G., Roscini V., Rapaccini S., Trabalza Marinnucci M., Lupi P., Conte G., Mele M. Milk fatty acid composition, rumen microbial population, and animal performances in response to diets rich in linoleic acid supplemented with chestnut or quebracho tannins in dairy ewes. Journal of Dairy Science. 2015;98(2):1145–1156. doi: 10.3168/jds.2014-8651. [DOI] [PubMed] [Google Scholar]
- Bennett R.N., Wallsgrove R.M. Secondary metabolites in plant defence mechanisms. New Phytologist. 1994;127(4):617–633. doi: 10.1111/j.1469-8137.1994.tb02968.x. [DOI] [PubMed] [Google Scholar]
- Birhan M., Gesses T., Kenubih A., Dejene H., Yayeh M. Evaluation of anthelminthic activity of tropical taniferous plant extracts against haemonchus contortus. Veterinary Medicine: Research and Reports. 2020;11:109–117. doi: 10.2147/VMRR.S225717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canul-Solis J., Campos-Navarrete M., Piñeiro-Vázquez A., Casanova-Lugo F., Barros-Rodríguez M., Chay-Canul A.…Castillo-Sánchez L. Mitigation of rumen methane emissions with foliage and pods of tropical trees. Animals. 2020;10(5):1–14. doi: 10.3390/ani10050843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappucci A., Mantino A., Buccioni A., Casarosa L., Conte G., Serra A., Mannelli F., Luciano G., Foggi G., Mele M. Diets supplemented with condensed and hydrolysable tannins affected rumen fatty acid profile and plasmalogen lipids, ammonia and methane production in an in vitro study. Italian Journal of Animal Science. 2021;20(1):935–946. doi: 10.1080/1828051X.2021.1915189. [DOI] [Google Scholar]
- Cardona-Iglesias J.L., Mahecha-Ledesma L., Angulo-Arizala J. Arbustivas forrajeras y ácidos grasos: estrategias para disminuir la producción de metano entérico en bovinos. Agronomía Mesoamericana. 2016;28(1):273–288. [Google Scholar]
- Carulla J.E., Kreuzer M., Machmüller A., Hess H.D. Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Australian Journal of Agricultural Research. 2005;56(9):961–970. doi: 10.1071/AR05022. [DOI] [Google Scholar]
- Cieslak A., Zmora P., Pers-Kamczyc E., Szumacher-Strabel M. Effects of tannins source (Vaccinium vitis idaea L.) on rumen microbial fermentation in vivo. Animal Feed Science and Technology. 2012;176(1–4):102–106. 10.1016%2FJ.anifeedsci.2012.07.012. [Google Scholar]
- Costa M., Alves S.P., Cabo Â., Guerreiro O., Stilwell G., Dentinho M.T., Bessa R.J. Modulation of in vitro rumen biohydrogenation by Cistus ladanifer tannins compared with other tannin sources. Journal of the Science Food and Agriculture. 2018;97(2):629–635. doi: 10.1002/jsfa.7777. [DOI] [PubMed] [Google Scholar]
- Dale A.J., McGettrick S., Gordon A.W., Ferris C.P. The effect of two contrasting concentrate allocation strategies on the performance of grazing dairy cows. Grass and Forage Science. 2015;71(3):379–388. doi: 10.1111/gfs.12185. [DOI] [Google Scholar]
- DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemporary Clinical Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Moreira G., Lima P.M.T., Borges B.O., Primavesi O., Longo C., McManus C., Abdalla A., Louvandini H. Tropical tanniniferous legumes used as an option to mitigate sheep enteric methane emission. Tropical Animal Health and Production. 2013;45(3):879–882. doi: 10.1007/s11250-012-0284-0. [DOI] [PubMed] [Google Scholar]
- El-Zaiat H.M., Kholif A.E., Moharam M.S., Attia M.F., Abdalla A.L., Sallam S.M.A. The ability of tanniniferous legumes to reduce methane production and enhance feed utilization in Barki rams: in vitro and in vivo evaluation. Small Ruminant Research. 2020;193:106259. doi: 10.1016/j.smallrumres.2020.106259. [DOI] [Google Scholar]
- FAO. (2013). Tackling climate change through livestock: a global assessment of emissions and mitigation opportunities. Available online at http://www.fao.org/3/i3437e/i3437e.pdf.
- Frutos P., Hervas G., Giraldez F.J., Mantecon A.R. Review: tannins and ruminant nutrition. Spanish Journal of Agricultural Research. 2004;2(2):191–202. doi: 10.5424/sjar/2004022-73. [DOI] [Google Scholar]
- García-González R., González J.S., López S. Decrease of ruminal methane production in Rusitec fermenters through the addition of plant material from rhubarb (Rheum spp.) and alder buckthorn (Frangula alnus) Journal of Dairy Science. 2010;93(8):3755–3763. doi: 10.3168/jds.2010-3107. [DOI] [PubMed] [Google Scholar]
- Gerber P.J., Steinfeld H., Henderson B., Mottet A., Opio C., Dijkman J.…Tempio G. Food and Agriculture Organization of the United Nations (FAO); Rome, Italy: 2013. Tackling climate change through livestock - A global assessment of emissions and mitigation opportunities. ISBN: 9789251079201. [Google Scholar]
- Gill M., Smith P., Wilkinson J.M. Mitigating climate change: the role of domestic livestock. Animal. 2010;4(3):323–333. doi: 10.1017/S1751731109004662. [DOI] [PubMed] [Google Scholar]
- Goel G., Makkar H.P.S. Methane mitigation from ruminants using tannins and saponins. Tropical Animal Health and Production. 2012;44(4):729–739. doi: 10.1007/s11250-011-9966-2. [DOI] [PubMed] [Google Scholar]
- Galindo J., González N., Abdalla A.L., Alberto M., Lucas R.C., Dos Santos K.C., Santos M.R., Louvandini P., Moreira O., Sarduy L. Effect of a raw saponin extract on ruminal microbial population and in vitro methane production with star grass (Cynodon nlemfuensis) substrate. Cuban Journal of Agricultural Science. 2016;50(1):77–88. ISSN 2079-3480. [Google Scholar]
- Hassanat F., Benchaar C. Assessment of the effect of condensed (acacia and quebracho) and hydrolysable (chestnut and valonea) tannins on rumen fermentation and methane production in vitro. Journal of Science of Food Agriculture. 2012;93(2):332–339. doi: 10.1002/jsfa.5763. [DOI] [PubMed] [Google Scholar]
- Hedges L.V. Distribution theory for glass's estimator of effect size and related estimators. Journal of Educational and Behavioral Statistics. 1981;6(2):107–128. doi: 10.2307/1164588. [DOI] [Google Scholar]
- Henderson G., Cox F., Ganesh S., Jonker A., Young W., Janssen P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Scientific Reports. 2015;5(1):1–15. doi: 10.1038/srep14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess H.D., Monsalve L.M., Lascano C.E., Carulla J.E., Díaz T.E., Kreuzer M. Supplementation of a tropical grass diet with forage legumes and Sapindus saponaria fruits: effects on in vitro ruminal nitrogen turnover and methanogenesis. Australian Journal Agricultural Research. 2003;54(7):703–713. doi: 10.1071/AR02241. [DOI] [Google Scholar]
- Higgins J.P.T. Commentary: heterogeneity in meta-analysis should be expected and appropriately. International Journal of Epidemiology. 2008;37(5):1158–1160. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
- Huang X.D., Liang J.B., Tan H.Y., Yahya R., Khamseekhiew B., Ho Y.W. Molecular weight and protein binding affinity of Leucaena condensed tannins and their effects on in vitro fermentation parameters. Animal Feed Science and Technology. 2010;159(3–4):81–87. doi: 10.1016/j.anifeedsci.2010.05.008. [DOI] [Google Scholar]
- Higgins J.P.T., Green S. Cochrane Collaboration; London, UK: 2011. Cochrane handbook for systematic reviews of interventions version 5.1.0.Www.handbook.cochrane.org.quantified Available online at: (accessed February 3, 2021) [Google Scholar]
- Huang X.D., Liang J.B., Tan H.Y., Yahya R., Ho Y.W. Effects of Leucaena condensed tannins of differing molecular weights on in vitro CH4 production. Animal Feed Science and Technology. 2011;166-167:373–376. doi: 10.1016/j.anifeedsci.2011.04.026. [DOI] [Google Scholar]
- IPCC . IGES; Japan: 2006. Guidelines for National Greenhouse Gas Inventories. National Greenhouse Gas Inventories Programme. [Google Scholar]
- Jakhmola R.C., Taruna P., Raghuvans S.K.S. Feeding strategies to reduce enteric methane production in ruminants: a review. The Indian Journal of Small Ruminants. 2010;16(1):1–17. ISSN: 0971-9857. [Google Scholar]
- Jayanegara A., Wina E., Soliva C.R., Marquardt S., Kreuzer M., Leiber F. Dependence of forage quality and methanogenic potential of tropical plants on their phenolic fractions as determined by principal component analysis. Animal Feed Science and Technology. 2011;163(2–4):231–243. doi: 10.1016/j.anifeedsci.2010.11.009. [DOI] [Google Scholar]
- Jayanegara A., Leiber F., Kreuzer M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. Journal of Animal Physiology and Animal Nutrition (Berl) 2012;96(3):365–375. doi: 10.1111/j.1439-0396.2011.01172.x. [DOI] [PubMed] [Google Scholar]
- Jayanegara A., Goel G., Makkar H.P.S., Becker K. Divergence between purified hydrolysable and condensed tannins effects on methane emission, rumen fermentation and microbial population in vitro. Journal of Animal Feed Science and Technology. 2015;209:60–68. doi: 10.1016/j.anifeedsci.2015.08.002. [DOI] [Google Scholar]
- Jayanegara A., Yogianto Y., Wina E., Sudarman A., Kondo M., Obitsu T., Kreuzer M. Combination effects of plant extracts rich in tannins and saponins as feed additives for mitigating in vitro ruminal methane and ammonia formation. Animals. 2020;10(9):1–14. doi: 10.3390/ani10091531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouany J.P., Morgavi D.P. Use of ‘natural’ products as alternatives to antibiotic feed additives in ruminant production. Animal. 2007;1(10):1443–1466. doi: 10.1017/S1751731107000742. [DOI] [PubMed] [Google Scholar]
- Koike S., Kobayashi Y. Fibrolytic rumen bacteria: their ecology and functions. Asian-Australasian Journal of Animal Sciences. 2009;22(1):131–138. doi: 10.5713/ajas.2009.r.01. [DOI] [Google Scholar]
- Ku-Vera J.C., Jiménez-Ocampo R., Valencia-Salazar S.S., Montoya-Flores M.D., Molina-Botero I.C., Arango J., Gómez-Bravo C.A., Aguilar-Pérez C.F., Solorio-Sánchez F.J. Role of secondary plant metabolites on enteric methane mitigation in ruminants. Frontiers in Veterinary Science. 2020;7:584. doi: 10.3389/fvets.2020.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku-Vera J.C., Castelán-Ortega O.A., Galindo-Maldonado F.A., Arango J., Chirinda N., Jiménez-Ocampo R., Valencia-Salazar S.S., Flores-Santiago E.J., Montoya-Flores M.D., Molina-Botero I.C., Piñeiro-Vázquez A.T., Arceo-Castillo J.I., Aguilar-Pérez C.F., Ramírez-Avilés L., Solorio-Sánchez F.J. Review: strategies for enteric methane mitigation in cattle fed tropical forages. Animal. 2020;14(3):453–463. doi: 10.1017/s1751731120001780. [DOI] [PubMed] [Google Scholar]
- Lawrence D.C., O'Donovan M., Boland T.M., Lewis E., Kennedy E. The effect of concentrate feeding amount and feeding strategy on milk production, dry matter intake, and energy partitioning of autumn-calving Holstein-Friesian cows. Journal of Dairy Science. 2015;98(1):348–388. doi: 10.3168/jds.2014-7905. [DOI] [PubMed] [Google Scholar]
- Lean, I.J., .Thompson, J.M., .& Dunshea, F.R. (.2014). A meta-analysis of zilpaterol and ractopamine effects on feedlot performance, carcass traits and shear strength of meat in cattle. PloS ONE, 10.1371/journal.pone.0115904. [DOI] [PMC free article] [PubMed]
- Liu H., Vaddella V., Zhou D. Effects of chestnut tannins and coconut oil on growth performance, methane emission, ruminal fermentation, and microbial populations in sheep. Journal of Dairy Science. 2011;94(12):6069–6077. doi: 10.3168/jds.2011-4508. [DOI] [PubMed] [Google Scholar]
- Malik P.K., Kolte A.P., Baruah L., Saravanan M., Bakshi B., Bhatta R. Enteric methane mitigation in sheep through leaves of selected tanniniferous tropical tree species. Livestock Science. 2017;200:29–34. doi: 10.1016/j.livsci.2017.04.001. [DOI] [Google Scholar]
- Mannelli F., Daghio M., Alves S.P., Bessa R.J., Minieri S., Giovannetti L.…Buccioni A. Effects of chestnut tannin extract, vescalagin and gallic acid on the dimethyl acetals profile and microbial community composition in rumen liquor: an in vitro study. Microorganisms. 2019;7(7):1–16. doi: 10.3390/microorganisms7070202. 10.3390%2Fmicroorganisms7070202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney C., Palmer B., Bunch R., Krause D. Effect of the tropical forage calliandra on microbial protein synthesis and ecology in the rumen. Journal of Applied Microbiology. 2001;90(1):78–88. doi: 10.1046/j.1365-2672.2001.01220.x. [DOI] [PubMed] [Google Scholar]
- Menci R., Coppa M., Torrent A., Natalello A., Valenti B., Luciano G., Priolo A., Niderkorn V. Effects of two tannin extracts at different doses in interaction with a green or dry forage substrate on in vitro rumen fermentation and biohydrogenation. Animal Feed Science and Technology. 2021;278:1–13. doi: 10.1016/j.anifeedsci.2021.114977. [DOI] [Google Scholar]
- Mills J.A., Djikstra J., Bannink A., Cammell S.B., Kebreab E., France J. A mechanistic model of whole-tract digestion and methanogenesis in the lactating cow: model development, evaluation and application. Journal of Animal Science. 2001;79(6):1584–1597. doi: 10.2527/2001.7961584x. [DOI] [PubMed] [Google Scholar]
- Min B.R., Solaiman S., Waldrip H.M., Parker D., Todd R.W., Brauer D. Dietary mitigation of enteric methane emissions from ruminants: a review of plant tannins mitigation options. Animal Nutrition Journal. 2020;6(3):231–246. doi: 10.1016/j.aninu.2020.05.002. 10.1016%2Fj.aninu.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minho A.P., Filippsen L.F., Amarte A.F.T., Abdalla A.L. Efficacy of condensed tannin presents in acacia extract on the control of Trichostrongylus colubriformis in sheep. 2010;40(6):1360–1365. doi: 10.1590/S0103-84782010005000088. [DOI] [Google Scholar]
- Molina-Botero I.C., Arroyave-Jaramillo J., Valencia-Salazar S., Barahona-Rosales R., Aguilar-Pérez C.F., Ayala Burgos A., Jacobo A., Ku-Vera J.C. Effects of tannins and saponins contained in foliage of Gliricidia sepium and pods of Enterolobium cyclocarpum on fermentation, methane emissions and rumen microbial population in crossbred heifers. Animal Feed Science and Technology. 2019;251:1–11. doi: 10.1016/j.anifeedsci.2019.01.011. [DOI] [Google Scholar]
- Montoya-Flores M.D., Molina-Botero I.C., Arango J., Romano-Muñoz J.L., Solorio-Sánchez F.J., Aguilar-Pérez C.F., Ku-Vera J.C. Effect of dried leaves of Leucaena leucocephala on rumen fermentation, rumen microbial population, and enteric methane production in crossbred heifers. Animals. 2020;10:1–17. doi: 10.3390/ani10020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteny G.J., Bannink A., Chadwick D. Greenhouse gas abatement strategies for animal husbandry. Agriculture, Ecosystems and Environment, 2006;112(2–3):163–170. doi: 10.1016/j.agee.2005.08.015. [DOI] [Google Scholar]
- Morgavi D.P., Martin C., Jouany J.P., Ranilla M.J. Rumen protozoa and methanogens: not a simple cause effect relationship. British Journal of Nutrition. 2012;107(3):388–397. doi: 10.1017/S0007114511002935. [DOI] [PubMed] [Google Scholar]
- Moscoso M.J.E., Franco F.F., San Martín H.F., Olazábal L.J., Chino V.L.B., Pinares-Patiño C. Producción de metano en vacunos al pastoreo suplementados con ensilado, concentrado y taninos en el altiplano peruano en época seca. Revista de Investigaciones Veterinarias del Perú. 2017;28(4):822–833. doi: 10.15381/rivep.v28i4.13887. [DOI] [Google Scholar]
- Mueller-Harvey I. Unravelling the conundrum of tannins in animal nutrition and health. Journal Science Food Agriculture. 2006;86(3):2010–2037. doi: 10.1002/jsfa.2577. [DOI] [Google Scholar]
- Marley C.L., Cook R., Keatinge R., Barrett J., Lampikin N.H. The effect of birdsfoot trefoil (Lotus corniculatus) and chicory (Cichorium intybus) on parasite intensities and performance of lambs naturally infected with helminth parasites. Vetinary Parasitology. 2003;112(1–2):147–155. doi: 10.1016/S0304-4017(02)00412-0. [DOI] [PubMed] [Google Scholar]
- Mengistu G., Hoste H., Karonen M., Salminen J.P., Hendriks W.H., Pellikaan W.F. The in vitro anthelmintic properties of browse plant species against Haemonchus contortus is determined by the polyphenol content and composition. Vetinary Parasitology. 2017;237:110–116. doi: 10.1016/j.vetpar.2016.12.020. [DOI] [PubMed] [Google Scholar]
- Naumann H.D., Tedeschi L.O., Zeller W.E., Huntley N.F. The role of condensed tannins in ruminant animal production: advances, limitations and future directions. Revista Brasileria Zootecnia. 2017;46(12):929–949. doi: 10.1590/S1806-92902017001200009. [DOI] [Google Scholar]
- Newbold C.J., Lassalas B., Jouany J.P. The importance of methanogens associated with ciliate protozoa in ruminal methane production in vitro. Letters Applied Microbiology. 1995;21(4):230–234. doi: 10.1111/j.1472-765X.1995.tb01048.x. [DOI] [PubMed] [Google Scholar]
- Olesen J.E., Bindi M. Consequences of climate change for European agricultural productivity, land use and policy. European Journal of Agronomy. 2002;16(4):239–262. doi: 10.1016/S1161-0301(02)00004-7. [DOI] [Google Scholar]
- Olijhoek D.W., Løvendahl P., Lassen J., Hellwing A.L.F., Höglund J.K., Weisbjerg M.R., Noel S.J., McLean F., Højberg O., Lund P. Methane production, rumen fermentation, and diet digestibility of Holstein and Jersey dairy cows being divergent in residual feed intake and fed at 2 forage-to-concentrate ratios. Journal of Dairy Science. 2018;101(11):9926–9940. doi: 10.3168/jds.2017-14278. [DOI] [PubMed] [Google Scholar]
- Oliveira L.M.B., Bevilaqua C.M.L., Macedo I.T.F., Morais S.M., Monteiro M.V.B., Campello C.C., Ribeiro W.L.C., Batista E.K.F. Effect of six tropical tanniferous plant extracts on larval exsheathment of Haemonchus contortus. Revista Brasileira de Parasitologia Veterinária. 2011;20(2):155–160. doi: 10.1590/S1984-29612011000200011. [DOI] [PubMed] [Google Scholar]
- Ortiz-Domínguez M., Posada S.L., Noguera R.R. Efecto de metabolitos secundarios de las plantas sobre la emisión entérica de metano en rumiantes. Livestock Research for Rural Development. 2014;26(11):1–16. [Google Scholar]
- Patra A.K., Saxena J. Dietary phytochemicals as rumen modifiers: a review of the effects on microbial populations. Antonie Van Leeuwenhoek. 2009;96(4):363–375. doi: 10.1007/s10482-009-9364-1. [DOI] [PubMed] [Google Scholar]
- Patra A.K., Saxena J. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry. 2010;71(11–12):1198–1222. doi: 10.1016/j.phytochem.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Patra A.K., Saxena J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. Journal of Science Food Agriculture. 2011;91(1):24–37. doi: 10.1002/jsfa.4152. [DOI] [PubMed] [Google Scholar]
- Piñeiro-Vázques A.T., Canul-Solis J.R., Jiménez-Ferrer G.O., Alayón-Gamboa J.A., Chay-Canul A.J., Ayala-Burgos A.J., Aguilar-Pérez C.F., Ku-Vera J.C. Effect of condensed tannins from Leucaena leucocephala on rumen fermentation, methane production and population of rumen protozoa in heifers fed low-quality forage. Asiuan-Australas Journal of Animal Science. 2018;31(11):1738–1746. doi: 10.5713/ajas.17.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchala R., Animut G., Patra A.K., Detweiler G.D., Wells J.E., Varel V.H., Sahlu T., Goetsch A.L. Methane emissions by goats consuming Sericea lespedeza at different feeding frequencies. Animal Feed Science and Technology. 2012;175(1–2):76–84. doi: 10.1016/j.anifeedsci.2012.03.015. [DOI] [Google Scholar]
- Petlum A., Paengkoum P., Liang J.B., Vasupen K., Paengkoum S. Molecular weight of condensed tannins of some tropical feed-leaves and their effect on in vitro gas and methane production. Animal Production Science. 2019;59(12):1–7. doi: 10.1071/AN17749. [DOI] [Google Scholar]
- Piluzza G., Sulas L., Bullitta S. Tannins in forage plants and their role in animal husbandry and environmental sustainability: a review. Grass and Forage Science. 2014;69(1):32–48. doi: 10.1111/gfs.12053. [DOI] [Google Scholar]
- Rira M., Morgavi D.P., Archimède H., Marie-Magdeleine C., Popova M., Bousseboua H., Doreau M. Potential of tannin-rich plants for modulating ruminal microbes and ruminal fermentation in sheep. Journal of Animal Science. 2015;93(1):334–347. doi: 10.2527/jas.2014-7961. [DOI] [PubMed] [Google Scholar]
- Rira M., Morgavi D.P., Genestoux L., Djibiri S., Sekhri I., Doreau M. Methanogenic potential of tropical feeds rich in hydrolysable tannins. Journal of Animal Science. 2019;97(7):2700–2710. doi: 10.1093/jas/skz199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rira, M., Morgavi, D.P., .Popova, M., Maxin, G., & Doreau, M. (2021). Rumen disappearance of tannins from tropical tannin-rich plants: interplay between degradability, methane production and adherent rumen microbiota. BioRxiv, 1–50. 10.1101/2021.08.12.456105.
- Rivera J.E., Molina I.C., Donneys G., Villegas G., Chará J., Barahona R. Dinámica de fermentación y producción in vitro de metano en dietas de sistemas silvopastoriles intensivos con L. leucocephala y sistemas convencionales orientados a la producción de leche. Livestock Research for Rural Development. 2015;27(4):1–15. doi: 10.2527/jas.2014-7961. [DOI] [Google Scholar]
- Robles-Jimenez L.E., Xochitemol-Hernandez A., Benaouda M., Osorio-Avalos J., Corona L., Castillo-Gallegos E.…Gonzalez-Ronquillo M. Concentrate supplementation on milk yield, methane and CO2 production in crossbred dairy cows grazing in tropical climate regions. Journal of Animal Behaviour and Biometeorology. 2021;9(2):1–8. doi: 10.31893/jabb.21018. [DOI] [Google Scholar]
- Rodríguez R., Britos A., Rodríguez-Romero N., Fondevila M. Effect of plant extracts from several tanniferous browse legumes on in vitro microbial fermentation of the tropical grass Pennisetum purpureum. Animal Feed Science and Technology. 2011;168(3–4):188–195. doi: 10.1016/j.anifeedsci.2011.04.095. [DOI] [Google Scholar]
- Salami S.A., Valenti B., Bella M., O’Grady M.N., Luciano G., Kerry J.P.…Newbold C.J. Characterisation of the ruminal fermentation and microbiome in lambs supplemented with hydrolysable and condensed tannins. FEMS Microbiology. Ecology. 2018;94(5):1–13. doi: 10.1093/femsec/fiy061. [DOI] [PubMed] [Google Scholar]
- Sandoval-Pelcastre A.A., Ramírez-Mella M., Rodríguez-Ávila N.L., Candelaria-Martínez B. Árboles y arbustos tropicales con potencial para disminuir la producción de metano en rumiantes. Tropical and Subtropical Agroecosystems. 2020;23:1–16. [Google Scholar]
- Schultze-Kraft R., Rao I.M., Peters M., Clements R.J., Bai C., Liu G. Tropical forage legumes for environmental benefits: an overview. Tropical Grasslands-Forrajes Tropicales. 2018;6(1):1–14. doi: 10.17138/TGFT(6)1-14. [DOI] [Google Scholar]
- Schwarzer G, Carpenter JR, Rücker G. Meta-Analysis with R. Springer, Cham; Switzerland: 2015. pp. 1–252. [Google Scholar]
- Staerfl S.M., Kreuzer M., Soliva C.R. In vitro screening of unconventional feeds and various natural supplements for their ruminal methane mitigation potential when included in a maize-silage based diet. Journal of Animal and Feed Science. 2010;19:651–664. doi: 10.22358/jafs/66338/2010. [DOI] [Google Scholar]
- Supapong C., Cherdthong A., Seankamsorn A., Khonkhaeng B., Wanapat M., Uriyapongson S., Gunun N., Gunun P., Chanjula P., Polyorach S. In vitro fermentation, digestibility and methane production as influenced by Delonix regia seed meal containing tannins and saponins. Journal of Animal and Feed Science. 2017;26(2):123–130. doi: 10.22358/jafs/73890/2017. [DOI] [Google Scholar]
- Tan H.Y., Sieo C.C., Abdullah N., Liang J.B., Huang X.D., Ho Y.W. Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Animal and Feed Sciences and Technology. 2011;169(3–4):185–193. doi: 10.1016/j.anifeedsci.2011.07.004. [DOI] [Google Scholar]
- Tavendale M.H., Meagher L.P., Pacheco D., Walker N., Attwood G.T., Sivakumaran S. Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Animal Feed Science and Technology. 2005;123(1):403–419. doi: 10.1016/j.anifeedsci.2005.04.037. [DOI] [Google Scholar]
- Terranova M., Kreuzer M., Braun U., Schwarm A. In vitro screening of temperate climate forages from a variety of woody plants for their potential to mitigate ruminal methane and ammonia formation. The Journal Agricultural Science. 2018;156(7):929–941. doi: 10.1017/S0021859618000989. [DOI] [Google Scholar]
- Tiemann T.T., Avila P., Ramirez G., Lascano C.E., Kreuzer M., Hess H.D. In vitro ruminal fermentation of tanniferous tropical plants: plant-specific tannin effects and counteracting efficiency of PEG. Animal Feed Science and Technology. 2008;146(3):222–241. doi: 10.1016/j.anifeedsci.2007.12.009. [DOI] [Google Scholar]
- Topps J.H. Potential, composition and use of legume shrubs and trees as fodders for livestock in the tropics. Journal of Agricultural Science. 1992;118:1–8. doi: 10.1017/S0021859600067940. [DOI] [Google Scholar]
- Ugbogu E.A., Elghandour M.M.M.Y., Ikpeazu V.O., Buendía G.R., Molina O.M., Arunsi U.O., Emmanuel O., Salem A.Z.M. The potential impacts of dietary plant natural products on the sustainable mitigation of methane emission from livestock farming. Journal of Cleaner Production. 2019;213:915–925. doi: 10.1016/j.jclepro.2018.12.233. [DOI] [Google Scholar]
- Valencia-Salazar S.S., Piñeiro V.A.T., Molina B.I.C., Lazos B.F.J., Uuh N.J.J., Segura C.M.R., Ramírez A.L., Solorio S.F.J., Ku-Vera J.C. Potential of Samanea saman pod meal for enteric methane mitigation in crossbred heifers fed low-quality tropical grass. Agricultural and Forest Meteorology. 2017;258:108–116. doi: 10.1016/j.agrformet.2017.12.262. [DOI] [Google Scholar]
- Vandermeulen S., Singh S., Ramírez-Restrepo C.A., Kinley R.D., Gardiner C.P., Holtum J.A.M., Hannah I., Bindelle J. In vitro assessment of ruminal fermentation, digestibility and methane production of three species of desmanthus for application in northern Australian grazing systems. Crop and Pasture Science. 2018;69(8):797–807. doi: 10.1071/cp17279. [DOI] [Google Scholar]
- Van Lingen H.J., Niu M., Kebreab E., Valadares F.S.C., Rooke J.A., Duthie C.A.…Chaves A.V. Prediction of enteric methane production, yield and intensity of beef cattle using an intercontinental database. Agriculture, Ecosystems and Environment, 2019;283:1–19. doi: 10.1016/j.agee.2019.106575. 106575. [DOI] [Google Scholar]
- Vasta V., Daghio M., Cappucci A., Buccioni A., Serra A., Viti C., Mele M. Invited review: plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: experimental evidence and methodological approaches. Journal of Dairy Science. 2019;102(5):3781–3804. doi: 10.3168/jds.2018-14985. [DOI] [PubMed] [Google Scholar]
- Vázquez-Carrillo M.F., Montelongo-Pérez H.D., González-Ronquillo M., Castillo-Gallegos E., Castelán-Ortega O.A. Effects of three herbs on methane emissions from beef cattle. Animals. 2020;10(9):1–17. doi: 10.3390/ani10091671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez-Terranova M., Campos-Gaona R., Sánchez-Guerrero H. Use of plant secondary metabolites to reduce ruminal methanogenesis. Tropical and Subtropical Agroecosystems. 2014;17(3):489–499. [Google Scholar]
- Yan T., Mayne C.S., Gordon F.G., Porter M.G., Agnew R.E., Patterson D.C., Ferris C.P., Kilpatrick D.J. Mitigation of enteric methane emissions through improving efficiency of energy utilization and productivity in lactating dairy cows. Journal of Dairy Science. 2010;93(6):2630–2638. doi: 10.3168/jds.2009-2929. [DOI] [PubMed] [Google Scholar]
- Yang L., Wen K.S., Ruan X., Zhao Y.X., Wei F., Wang Q. Response of plant secondary metabolites to environmental factors. Molecules. 2018;23(4):1–26. doi: 10.3390/molecules23040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward S.L., Waghorn G.C., Lassey K.R., Laboyrie P.G. Does feeding sulla (Hedysarum coronarium) reduce methane emissions from dairy cows? Proceedings of the New Zealand Society of Animal Production. 2002;62:227–230. [Google Scholar]
- Woodward S.L., Waghorn G.C., Laboyrie P.G. Condensed tannins in birdsfoot trefoil (Lotus corniculatus) reduce methane emissions from dairy cows. Proceedings of the New Zealand Society of Animal Production. 2004;64:160–164. [Google Scholar]
- Viechtbauer, W. (2010). “Metafor: Meta-Analysis Package for R.” R package version 1.4-0, URL http://CRAN.R-project.org/package=metafor.