Abstract
Background
Pancreatic β-cells are the insulin factory of an organism with a mission to regulate glucose homeostasis in the body. Due to their high secretory activity, β-cells rely on a functional and intact endoplasmic reticulum (ER). Perturbations to ER homeostasis and unmitigated stress lead to β-cell dysfunction and death. Type 1 diabetes (T1D) is a chronic inflammatory disease caused by the autoimmune-mediated destruction of β-cells. Although autoimmunity is an essential component of T1D pathogenesis, accumulating evidence suggests an important role of β-cell ER stress and aberrant unfolded protein response (UPR) in disease initiation and progression.
Scope of review
In this article, we introduce ER stress and the UPR, review β-cell ER stress in various mouse models, evaluate its involvement in inflammation, and discuss the effects of ER stress on β-cell plasticity and demise, and islet autoimmunity in T1D. We also highlight the relationship of ER stress with other stress response pathways and provide insight into ongoing clinical studies targeting ER stress and the UPR for the prevention or treatment of T1D.
Major conclusions
Evidence from ex vivo studies, in vivo mouse models, and tissue samples from patients suggest that β-cell ER stress and a defective UPR contribute to T1D pathogenesis. Thus, restoration of β-cell ER homeostasis at various stages of disease presents a plausible therapeutic strategy for T1D. Identifying the specific functions and regulation of each UPR sensor in β-cells and uncovering the crosstalk between stressed β-cells and immune cells during T1D progression would provide a better understanding of the molecular mechanisms of disease process, and may reveal novel targets for development of effective therapies for T1D.
Keywords: Er stress, Beta cell, Type 1 diabetes, NOD mice, Human islets
1. Introduction
Type 1 diabetes (T1D) is characterized by the destruction of pancreatic β-cells through selective action of autoimmune processes, resulting in insulin deficiency and dependency. Despite decades of research, the initial signals that trigger the inflammatory pathways and the mechanisms leading to β-cell death remain poorly understood.
β-Cells have long been considered as the “innocent victim cells” in T1D pathogenesis. However, in light of emerging data, there is a growing appreciation for their important contribution to disease pathology. The hypothesis that β-cells may play an active role in the initiation or progression of T1D was supported by the findings showing that β-cell dysfunction and metabolic impairment were present in autoantibody-positive (Aab+) donors long before diabetes onset [1]. While the decline in β-cell function is likely exacerbated by genetic predisposition and the environmental conditions during progression to T1D, the intrinsic mechanisms leading to β-cell dysfunction remain largely unknown. Evidence from rodent and human islet studies, as well as data from pre-clinical models, single-cell omics findings, and imaging analyses of the pancreatic tissue from organ donors, indicate endoplasmic reticulum (ER) stress and the aberrant unfolded protein response (UPR) as the key contributors of β-cell dysfunction in T1D. Hence, in this review, the role of ER stress and the UPR in β-cell function and inflammation is discussed and recent data indicating their potential role in T1D progression and the therapeutic potential of targeting the ER stress pathway are highlighted.
2. Endoplasmic reticulum stress
The ER is the core of protein synthesis, proper folding, and processing of newly synthesized proteins as well as lipid synthesis in the cell. The ER has a specialized lumen that maintains an oxidized state compared to the cytosol to facilitate protein folding and processing. It also contains the largest releasable calcium (Ca2+) reserve in the cell [2]. The ER is the residence to chaperones that facilitate the proper folding and structural maturation of proteins, and to enzymes that are responsible for post-translational modifications, including glycosylation, disulfide bond formation, and proteolytic cleavage. This intricate network ensures that newly synthesized proteins remain fully functional before they are transported to their destination.
Although the ER is equipped to achieve proper folding and processing of numerous proteins, it does not always work at optimal efficiency and produces misfolded/unfolded proteins quite often. Environmental insults including hypoxia, oxidative stress, and viral infections as well as intrinsic insults such as increased protein synthesis demand, and mutations in specific proteins cause ER stress, thereby increasing the levels of unfolded/misfolded proteins. One quality control mechanism for proteins that do not fold properly is ER-associated degradation (ERAD), which facilitates the elimination of misfolded proteins with a mechanism involving ubiquitylation and proteosome-mediated degradation [3].
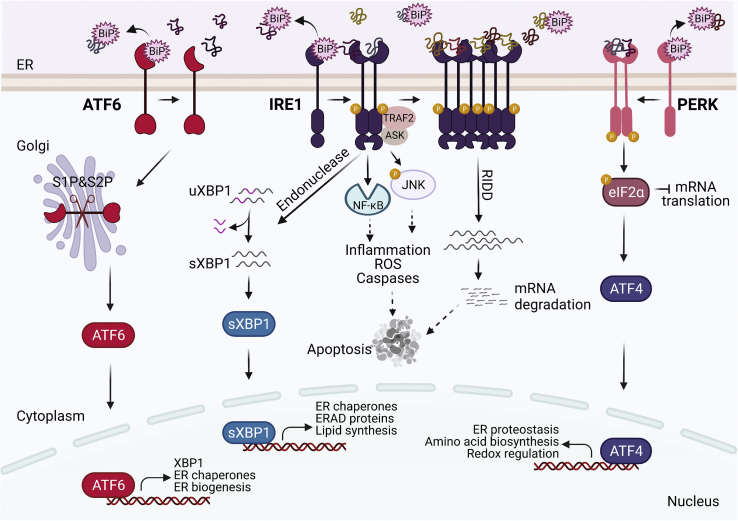
The ER has evolved to have a surveillance system on the unfolded proteins, known as the UPR, that relays the status of the ER to the nucleus [4,5]. In vertebrates, three ER transmembrane proteins, namely protein kinase R-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6), constitute the canonical UPR (Figure 1). When there is no stress, these proteins are stabilized to be inert through binding of binding-immunoglobulin protein (BiP), also known as GRP78, to their luminal domain. Once activated, the outcomes of the UPR mainly depend on the duration and severity of stress experienced by the ER. If the stress is mild and acute, the adaptive UPR facilitates the upregulation of proteins involved in increased ER synthesis, folding capacity, and/or ERAD to alleviate the stress and resume the ER's ability to function. However, if cells are undergoing severe and prolonged stress, the maladaptive/terminal UPR initiates apoptotic signals [[6], [7], [8]].
Figure 1.
The unfolded protein response. Upon ER stress and accumulation of unfolded proteins, BiP (GRP78) dissociates from IRE1α, PERK, and ATF6 leading to their dimerization/oligomerization or translocation to the Golgi apparatus. PERK phosphorylates eIF2α to halt general mRNA translation. ATF4 induces the expression of genes involved in ER protein folding, amino acid metabolism, and redox regulation. IRE1α dimerization and oligomerization activate its endonuclease and kinase domains resulting in XBP1 splicing, activation of NF-kB and JNK, and regulated IRE1-dependent decay (RIDD) activity. XBP1 activates the transcription of genes involved in protein folding, secretion, ERAD, and lipid synthesis. Activation of ATF6α leads to its transport to the Golgi apparatus where its cytosolic domain is released to translocate to the nucleus and activate downstream target genes. Apoptosis is induced during prolonged and unresolved ER stress.
3. The UPR
3.1. PERK
PERK is a type I transmembrane protein belonging to the eIF2α kinase subfamily. While its luminal domain senses ER stress, its cytoplasmic domain carries kinase activity. In the absence of ER stress, PERK resides on the ER membrane as a monomer with its luminal domain bound to BiP. Upon ER stress, dissociation of BiP and binding of accumulated unfolded proteins to its luminal domain initiate dimerization and trans-autophosphorylation of PERK, activating its cytoplasmic kinase domain [9]. PERK phosphorylates α subunit of translation initiation factor, eIF2, inhibiting its activity [10]. The inhibition of eIF2α by PERK is a crucial early response to ER stress to halt general mRNA translation to decrease protein synthesis and the folding load of the ER. Besides PERK, heme-regulated inhibitor (HRI), protein kinase R (PKR), and general control non-depressible 2 (GCN2) can also phosphorylate eIF2α upon heme depletion, viral infection, and amino acid starvation, respectively, forming the integrated stress response [11].
While the general translation rate decreases upon inhibition of eIF2α, translation of a subset of mRNAs that have inhibitory upstream open reading frame (uORF) increases, including activating transcription factor 4 (ATF4), the best-characterized downstream effector of PERK [12]. ATF4 is a transcription factor that upregulates the expression of genes involved in ER proteostasis, amino acid biosynthesis, and redox regulation [[13], [14], [15]]. ATF4 also provides negative feedback on PERK activity by inducing the expression of growth arrest and DNA damage-inducible protein 34 (GADD34), the eIF2α phosphatase regulator subunit. GADD34 dephosphorylates eIF2α and re-initiates the general translation in the cell [13,16]. When severe and chronic ER stress ensues, ATF4 activates apoptotic signaling pathways via induction of the transcription factor C/EBP-homologous protein 10 (CHOP) [17].
3.2. IRE1α
IRE1α is another type I transmembrane protein with a cytoplasmic domain that contains both serine/threonine kinase and endoribonuclease activity. In the absence of ER stress, it resides on the ER membrane as a monomer and BiP stays bound to its luminal domain, stabilizing IRE1α at the inactive state. Upon ER stress and accumulation of unfolded proteins, BiP dissociation from the IRE1α luminal domain and direct binding of accumulated unfolded proteins activate IRE1α [18]. Activation of IRE1α initiates trans-autophosphorylation of its kinase domains, which in turn causes a conformational change to activate its cytoplasmic enzymatic domains [19,20].
The Active RNase domain of IRE1α excises 26 nt intron of the X-box binding protein 1 (XBP1), leading to translation of spliced XBP1 (sXBP1). sXBP1 regulates adaptive stress responses by promoting the expression of ER chaperones and ERAD components [21]. RNase domain of IRE1α also targets other ER-localized mRNAs through a process known as regulated IRE1-dependent decay (RIDD) [22,23]. When cells undergo acute and mild ER stress, RIDD activity helps decrease the folding load of the ER [24]. However, during chronic and severe ER stress, IRE1α monomers oligomerize, leading to hyperactivation of RIDD, consequently degrading a specific subset of mRNAs and miRNAs [25]. Degradation of one such miRNA, miR-17, results in upregulation of thioredoxin-interacting protein (TXNIP), which plays an important role in oxidative stress, inflammasome activation, and apoptosis [26,27].
The serine/threonine kinase activity of IRE1α is independent of its RNase activity. Upon ER stress, phosphorylation of its kinase domain can bind to Ring finger protein (RNF) RING finger protein and tumor necrosis factor receptor-associated factor 2 (TRAF2). This activation initiates the formation of the IRE1α-TRAF2-ASK1 signaling complex to induce c-Jun amino-terminal kinases (JNK), which can trigger apoptosis [28,29]. In addition, the recruitment of TRAF2 can induce nuclear factor kappa-B (NF-κB) to form inflammasomes [30].
3.3. ATF6
Activating transcription factor 6 (ATF6), comprising α and β homologs, is a type-II transmembrane protein with a luminal domain and a cytoplasmic domain that is comprised of a transcriptional activation domain, basic leucine-zipper (b-ZIP) domain, DNA binding domain, and nuclear localization signals [31,32]. Unlike PERK and IRE1α, upon ER stress, ATF6 does not oligomerize; instead, it is translocated to the Golgi apparatus. While the detailed molecular mechanisms by which ATF6 senses ER stress remain largely unknown, dissociation of BiP from its luminal domain triggers its localization to the Golgi apparatus [33]. In the Golgi, the ATF6 monomer is recognized and cleaved sequentially by serine 1 and serine 2 proteases to release its N-terminal cytoplasmic domain, ATF6(N) [31,34]. ATF6 (N) then moves to the nucleus and activates transcription of UPR target genes including Xbp1, Chop, and ER chaperones [21].
Both homologs, ATF6α and ATF6β, reside on the ER membrane and respond to ER stress by forming homodimers and heterodimers [35]. While they are structurally alike with conserved b-ZIP and DNA binding domains, they significantly differ in their transactivation domain. ATF6β lacks eight amino acids that are required for the full transcription activity that ATF6α exerts. Consistently, ATF6β does not induce transcriptional upregulation of target genes as robustly as ATF6α. Rather, ATF6β is more stable but a much weaker transcription factor, whereas ATF6α is unstable but a stronger one whose activity is tightly correlated with the strength and duration of ER stress. Therefore, it has been postulated that the activity of ATF6α and ATF6β together fine tune the transcriptional regulation of target genes in response to ER stress [36].
4. ER stress and inflammation
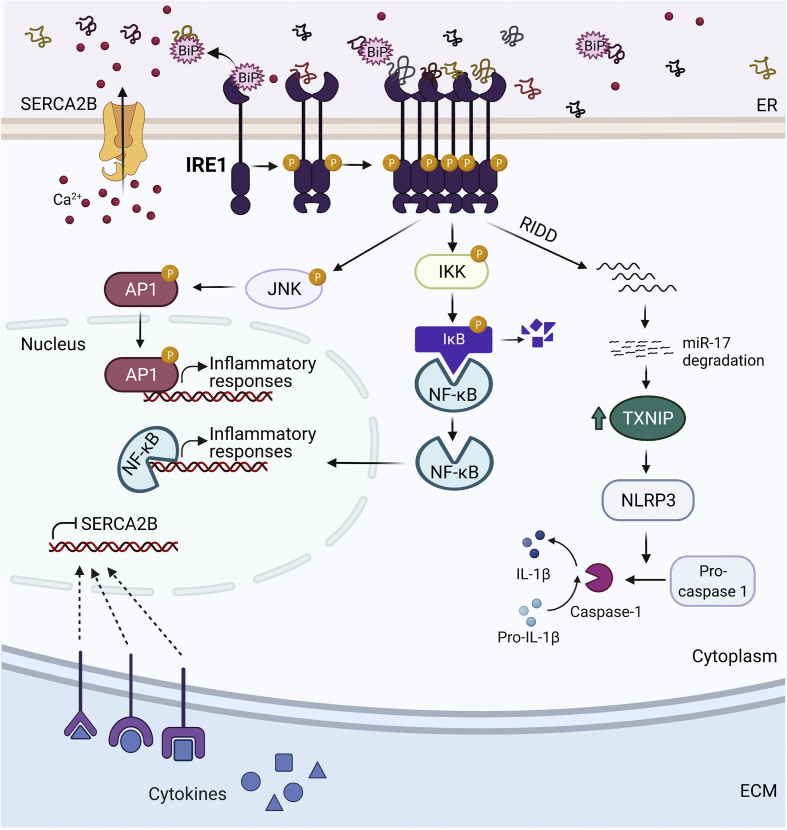
Inflammation is typically a protective response to defend the host against tissue injury or infections. However, defective or uncontrolled immune responses can elicit negative outcomes such as autoimmunity, in which the body's own tissues are attacked and destroyed. Thus, the regulation of key players in the inflammatory pathway is crucial for maintaining immune tolerance and homeostasis. The UPR orchestrates inflammatory responses via multiple signaling molecules including NF-κB, JNK, TXNIP, and the acute phase response proteins (Figure 2). NF-κB in its inactive form resides in the cytosol bound to IκBα. IRE1α recruits IκBα kinase (IKK), which phosphorylates IκBα, leading to its degradation and NF-κB activation. Since targets of NF-κB include pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6), activation of the UPR through chronic ER stress can trigger inflammatory responses [37]. IRE1α also engages inflammatory signals by phosphorylating and activating JNK. Activated JNK phosphorylates activator protein-1 (AP-1), a transcription factor that orchestrates inflammatory responses. Furthermore, under prolonged ER stress, IRE1α-mediated TXNIP activity causes the NLR family pyrin domain containing 3 (NLRP3) inflammasome to cleave pro-caspase 1 into caspase 1, which processes interleukin-1β (IL-1β) for secretion and leads to cell death [26,27].
Figure 2.
Regulation of the inflammatory signals by the UPR. Inflammation activates the UPR, and vice versa. Pro-inflammatory cytokines such as IL-1β and IFN-γ induce ER stress through the downregulation of SERCA2B. In the presence of ER stress, IRE1α downregulates the expression of miR-17, a TXNIP-destabilizing micro-RNA. As a result, the TXNIP level rises and the NLRP3 inflammasome is activated, which leads to the formation of caspase-1 and the cleavage of pro-IL-1β into IL-1β. IRE1α also activates the NF-κB pathway through induction of IKK. NF-κB then translocates to the nucleus to induce inflammatory responses. Finally, IRE1α can induce JNK and AP1 activation.
In addition to IRE1α, ATF4 plays an important role in UPR-mediated inflammatory responses through its ability to increase the expression levels of NF-κB and IL-6 [38]. ATF4 also activates the NLRP1 inflammasome directly in the presence of ER stress [38,39]. Like the NLRP3 inflammasome, NLRP1 activates pro-caspase 1 for proteolytic cleavage of IL-1β [40]. In response to infection, trauma, and inflammation the first reaction of the body is to induce a systemic non-specific innate reaction, called the acute phase response (APR). In the liver, upon ER stress the ER-localized (cAMP)-responsive element-binding protein H (CREBH) is cleaved to induce the APR [41].
The UPR plays a critical role in the development and function of immune cells themselves. IRE1α is required in the early and later stages of terminal differentiation for B lymphocytes, while XBP1 is required for plasma cell differentiation [[42], [43], [44]]. XBP1 has also been shown to be a key transcription factor in the development and survival of dendritic cells, which play a key role in antigen presentation to effector T cells in T1D [45]. Hence, maintaining ER homeostasis is crucial for various cell types that are implicated in autoimmune diseases.
The UPR and the inflammatory response have a reciprocal interaction such that inflammation can induce ER stress [46,47]. For example, cytokines by downregulating SERCA2b, a Ca2+ pump in the ER that transports cytosolic Ca2+ into the ER and induces ER stress. The diminished expression of SERCA2b may occur at the transcriptional or translational level and is proposed to be mediated by the formation of nitric oxide (NO) [46,47]. Though cytokines induce apoptosis in rat, mouse, and human β cell models, the underlying mechanisms by which it does so seem to differ from species to species [47]. Conversely, while inflammatory cytokines such as IL-1β and interferon-gamma (IFN-γ) can induce ER stress, anti-inflammatory cytokines, such as IL-10, have been shown to repress ER stress by promoting protein folding [48]. Administration of antibodies against IL-10 and its receptor, IL-10R1, in Winnie mice, a mouse model for chronic intestinal inflammation, substantially worsens ER stress [48].
5. ER stress in β-cells
Upon glucose sensing, β-cells can produce up to one million molecules of preproinsulin per minute [49], which imposes a tremendous burden on the ER for proper protein folding, trafficking, and secretion. The frequent and routine acute physiological ER stress that long-lived β-cells experience is ameliorated by the adaptive UPR. β-cells exhibit high levels of basal activity of the UPR compared to non-secretory cells, suggesting that active UPR is an integral component of β-cell homeostasis. However, when pathological conditions such as viral infections, β-cell exposure to chemicals and toxins, reactive oxygen species (ROS), and chronic inflammation, inflict a greater degree of stress for a prolonged time, the maladaptive UPR can alter the fate of β-cells.
Elevated ER stress and abnormal UPR are common features of β-cells in both polygenic and monogenic forms of diabetes [50]. For example, leptin-deficient ob/ob mice, a mouse model of type 2 diabetes (T2D) and obesity exhibit markedly reduced protein levels of ATF6α and sXBP1 in their β-cells even before the onset of hyperglycemia [51]. On the other hand, islets isolated from obese and diabetic db/db mice that have defective leptin receptor signaling show considerable upregulation of Atf4, Chop, and Bip mRNA levels, and a significant increase in sXBP1 protein level [52]. In addition to the genetic models of obesity, β-cells of mice fed with a high-fat diet (HFD) show significantly reduced protein levels of ATF6α, markedly increased phosphorylation of eIF2α, and no alterations in sXBP1 protein expression compared to chow-fed control mice [51]. These findings suggest that while the mechanisms of actions might differ, both genetic and diet-induced obesity cause perturbations to ER homeostasis [51]. Consistent with the presence of dysregulated β-cell UPR in animal models of obesity and T2D, β-cells of individuals with T2D exhibit substantially reduced expression of ATF6α, sXBP1, and eIF2α protein levels [51].
Over the last two decades, a variety of mouse models lacking different components of the UPR pathway, either in the whole body or specifically in β-cells, have been generated (Table 1). In the absence of Perk, either in the whole body or at the pancreatic tissue level, mice undergo a rapid decline in endocrine tissue function and progressively develop diabetes [[53], [54], [55]]. While global deletion of Perk causes loss of β-cell mass [53], pancreatic tissue-specific deletion of Perk causes impaired proliferation and differentiation of β-cells [54]. Notably, deletion of other eiF2α kinases [hemin-regulated inhibitor (HRI), PKR, and GCN2] does not impair glucose metabolism [[56], [57], [58]], suggesting that the ER stress response plays a more prominent role for β-cell health compared to the integrated stress response. p58IPK, an ER chaperone, attenuates PERK-mediated eIF2α phosphorylation during ER stress. Whole-body deletion of DnaJ Homolog Subfamily Member C3 (Dnajc3), encoding for p58IPK, leads to β-cell apoptosis and abnormal blood glucose levels in mice [59]. Whole-body deletion of Atf4 decreases diet-induced and age-dependent obesity and diabetes, partly by increasing energy expenditure [60]. Whole-body deletion of ATF4 target, Chop, improves β-cell function and survival by preventing oxidative damage in response to ER stress in both genetic and diet-induced models of insulin resistance [61]. Chop deletion in β-cells decreases ER stress and protects HFD-fed aged mice from liver steatosis [62]. The targeted Chop deletion further remodels the ER in β-cells by modulating glucose-induced cytosolic Ca2+ oscillations [62]. Overall, these studies emphasize the importance of the PERK pathway to resolve chronic ER stress.
Table 1.
Overview of mouse models studied for proteins involved in UPR pathways and their observed phenotypes with respect to β-cell health and function.
| Deletion model | Deletion Tissue | Deletion Time | Genetic Background | Phenotype | Reference |
|---|---|---|---|---|---|
| PERK | Whole body | Embryonic | 129svev(Swiss Webster) | Diabetes with β-cell mass loss | Harding et al., 2001 [53] |
| Pancreas | C57BL/6J or 129SvEvTac | Diabetes with impaired proliferation and differentiation of β-cells | Zhang et al., 2006 [54] | ||
| β-cells | Adulthood | C57BL/6 | Diabetes with increased β-cell death | Gao et al., 2012 [55] | |
| ATF4 | Whole body | Embryonic | C57BL/6 | Protection against diet-induced obesity and diabetes | Seo et al., 2009 [60] |
| CHOP | Whole body | Embryonic | C57BL/6 | Reduced hyperglycemia and glucose intolerance on high-fat diet | Song et al., 2008 [61] |
| db/db | Increased obesity, normal glucose tolerance due to β-cell mass expansion | ||||
| Akita | Delayed diabetes onset | Oyadomari et al., 2002 [77] | |||
| NOD | No effect on diabetes incidence, delayed appearance of insulin autoantibodies | Satoh et al., 2011 [95] | |||
| β-cells | Adulthood | C57BL/6J | Alleviates ER stress and protects from hepatic steatosis | Yong et al., 2021 [62] | |
| p58 | Whole body | Embryonic | C57BL/6J | Hyperglycemia with β-cell death | Ladiges et al., 2005 [59] |
| IRE1α | β-cells | Embryonic | C57BL/6J | Impaired insulin secretion with no apparent change in islet morphology or mass | Xu et al., 2014 [64] |
| Adulthood | C57BL/6 | Diabetes, defects in secretion of proinsulin, increased oxidative stress | Hassler et al., 2015 [65] | ||
| Postnatal | NOD | Transient hyperglycemia followed by protection from diabetes | Lee et al., 2020 [90] | ||
| GRP78 | β-cells | Embryonic | N/A | Insulin-deficient diabetes, impaired β-cell differentiation | Sharma et al., 2018 [72] |
| XBP1 | β-cells | Embryonic | C57BL/6 | Hyperglycemia, glucose intolerance, decreased insulin secretion | Lee et al., 2011 [63] |
| ATF6α | Whole body | Embryonic | C57BL/6 | Hyperglycemia on high-fat diet | Usui et al., 2012 [68] |
| Agouti | Hyperglycemia, improved insulin sensitivity on high-fat diet | ||||
| Akita | |||||
| β-cells | Postnatal | C57BL/6 | Loss of protective effects of TUDCA against diabetes, mild glucose intolerance | Engin et al., 2013 [69] |
The role of the IRE1α/XBP1 pathway in β-cell function and health has been investigated by developing both global and tissue-specific inducible knockout mouse models. Deletion of Xbp1 in β-cells during embryogenesis causes modest hyperglycemia and glucose intolerance. These mice also show impaired β-cell proliferation, disorganized islet structure, reduced pro-insulin processing, and glucose-stimulated insulin secretion [63]. In addition, Xbp1 deficiency causes constitutive hyperactivation of IRE1α leading to degradation of mRNAs that encode proinsulin processing enzymes [63]. β-Cell-specific, embryonic deletion of Ire1α leads to increased fasting glucose levels and impaired glucose clearance [64]. Unlike the β-cell-specific XBP1-deficient mice, these mice do not exhibit alterations in pancreatic architecture or islet morphology, instead, they show comparable islet mass and cell proliferation [64]. On the other hand, deletion of Ire1α in adult, differentiated β-cells causes a diabetic phenotype [65]. IRE1α-deficient mature β-cells show defects in translation, folding, trafficking, and secretion of proinsulin [65,66], and upregulation of genes involved in oxidative stress and inflammation [65].
While global deletion of both α and β isoforms of Atf6 in mice leads to embryonic lethality [67], mice with whole-body knockout of Atf6α on an HFD present glucose intolerance, reduced pancreatic insulin content, and morphological signs of ER stress [68]. Mice with β-cell-specific deletion of Atf6 (Atf6β−/−) on C57BL/6J background showed mild glucose tolerance and a decrease in glucose-stimulated insulin secretion [69]. When crossed with a virally-induced diabetes mouse model (RIP-LCMV-GP), Atf6α deletion in β-cells does not alter diabetes incidence [69]. In addition to these genetic studies, findings from cultured mice and human islets demonstrate a significant role for ATF6α in mediating the glucose-induced β-cell proliferation [70]. Homozygous deletion of ATF6 target, Grp78, results in early embryonic lethality; however, Grp78+/− mice are viable and fertile [71]. Deletion of Grp78 in β-cells results in insulin-deficient diabetes by 2 weeks of age. Knockout mice exhibit bi-hormonal (glucagon and insulin-positive) cells in their islets within the first postnatal week suggesting defective fetal maturation, de-differentiation, or trans-differentiation of β-cells [72].
Wolfram syndrome is an autosomal-recessive genetic disorder, caused by mutations in the Wolfram Syndrome 1 (WFS1) gene, that manifests as a combination of severe neurodegenerative disease and early onset insulin-dependent diabetes [73]. The lack of functional WFS1 significantly affects secretory β-cells and neurons due to the elevated ER stress. WFS1 plays an important role in the regulation of ER stress by enhancing ubiquitination and proteasome-mediated degradation of ATF6α, thereby preventing UPR hyperactivity [74].
In Akita mice, another monogenic diabetes mouse model expressing a dominant-negative mutant proinsulin 2 protein, compromised ER function and ER stress led to β-cell loss [75,76]. The accumulation of misfolded proinsulin 2 protein triggers pro-apoptotic UPR signaling, including CCAAT/enhancer-binding protein homologous protein (CHOP) induction. However, deletion of Chop in Akita mice only slows down the onset of diabetes but does not prevent it [77]. Interestingly, β-cell-specific deletion of p85α, a protein that binds to sXBP1 to facilitate its nuclear location, decreases the activation of ER stress-dependent apoptotic pathways and preserves β-cell mass and function in Akita mice [78]. Recently, lactogenic hormones have been demonstrated to modulate the ER stress pathway. Overexpression of placental lactogen in β-cells of Akita mice significantly reduces β-cell apoptosis, hyperglycemia, and diabetes incidence [79]. These studies demonstrate that β-cell apoptosis in Akita mice is mediated by a combination of several pro-apoptotic pathways triggered by the UPR.
6. β-Cell ER stress in T1D
ER stress-induced β-cell dysfunction has been outlined in various genetic models as outlined in the previous section. The role of β-cell ER stress in autoimmune diabetes pathogenesis has been supported by in vitro and ex vivo studies performed on human and rodent β-cells using cytokine cocktails mimicking the inflammatory milieu associated with T1D. These studies have shown that cytokine-mediated impairment of β-cell function and survival is mediated by ER stress [[80], [81], [82]]. Mechanistically, cytokines predominantly induce IRE1α-dependent activation of JNK in human β-cells, and inhibition of JNK protects these cells against apoptosis when exposed to cytokines [47]. A recent study shows that when exposed to inflammatory stress, β-cells translocate GRP78 to the cell surface where it acts as a pro-apoptotic signaling receptor [83]. This suggests multiple mechanisms whereby ER stress can trigger or amplify T1D pathogenesis [84]. Furthermore, genome-wide association studies performed on cultured human β-cells reveal changes in the transcription profile of β-cells and their heterogeneity after exposure of these cells to T1D-associated proinflammatory cytokines [85].
The non-obese diabetic (NOD) mouse is a widely used and well-established mouse model of T1D that shares similar features to the human disease, including genetic susceptibility in their major histocompatibility complex (MHC) genes that ultimately lead to immune-dependent infiltration in their islets [86,87]. β-Cells of NOD mice display markedly reduced expression of the key UPR genes [69,88]. Consistent with this finding, insulin-positive β-cells of individuals with T1D exhibit significantly downregulated protein levels of ATF6 and sXBP1 compared to β-cells of healthy donors [69]. Moreover, increased expressions of CHOP and BiP have been reported in islets from individuals with T1D [89].
A direct link for β-cell ER stress and T1D was provided by showing that mitigating β-cell ER stress in two different mouse models of T1D (NOD and RIP-LCMV-GP) via administration of the chemical chaperone tauroursodeoxycholic acid (TUDCA) protects mice against T1D [69]. Furthermore, by using a β-cell-specific ATF6-RIP-LCMV-GP deletion model, ATF6 was shown to mediate the diabetes-protective effects of TUDCA [69].
Until recently, β-cell-specific function of the UPR sensors during T1D development was unknown. An inducible β-cell-specific IRE1α knockout mouse model (IRE1αβ−/−) on NOD background [90] revealed that deletion of IRE1α in β-cells prior to islet inflammation confers protection against T1D. IRE1αβ−/− mice exhibit transient hyperglycemia starting from weaning age, yet within ∼2 weeks they recover from hyperglycemia and remain normoglycemic for up to a year. Further analyses indicated that β-cells of IRE1αβ−/− mice redifferentiate and remarkably restore their mature identity and function. These data suggest that temporary dedifferentiation of β-cells during a critical window (i.e., prior to insulitis) results in diminished expression of autoantigens and antigen presentation genes, increased expression of immune inhibitory markers, and altered chemokine expression. Altogether, these intrinsic changes in β-cells blunt T cell diabetogenic activity and allow β-cells to escape immune attack [90].
Induction of immune tolerance through reduced immunogenicity has previously been observed in NOD mice [91]. A recent study shows that a subpopulation of β-cells from NOD mice is resistant to autoimmune attack and exhibits decreased expression of β-cell maturity markers (including Ucn3 and MafA) and β-cell autoantigens [91], a phenotype that is reminiscent of β-cells of IRE1αβ−/− NOD mice. Therefore, markedly diminished expression of sXBP1 in the residual β-cells of individuals with T1D [69] and histological evidence supporting the presence of dedifferentiated β-cells in islets at T1D onset [92] suggest the contribution of diminished IRE1α/XBP1 to the survival of these cells. Together, these findings suggest that modulating the β-cell UPR can protect these cells against immune assault.
Due to IRE1α′s dual role as a kinase and RNase, the question arises whether the diabetes-protection phenotype observed in IRE1αβ−/− NOD mice results from a loss of its kinase or its RNase activity. The deficiency in XBP1-dependent regulation of transcriptional programs or lack of RIDD activity could have played a role in the immune evasion of β-cells. In support of this notion, pharmacological inhibition of the IRE1α RNase hyperactivity leads to a reversal of T1D in NOD mice [93]. Coxsackievirus B (CVB), associated with T1D development, exploits the IRE1α-JNK pathway to support its replication in rat and human β-cells [94], hence inhibition of IRE1α′s kinase activity may be important for diabetes protection. These findings underscore the need to understand the specific signaling mechanism downstream of IRE1α to achieve specific therapeutic targeting.
In addition to the IRE1αβ−/− mouse model, whole-body deletion of Chop on the NOD mouse background was generated [95]. CHOP deficiency does not alter the diabetes incidence, insulitis, or β-cell apoptosis in NOD mice. However, these mice exhibit a delayed appearance of insulin autoantibodies compared to wild-type mice suggesting that chronic ER stress may execute its apoptotic effects in a Chop-independent manner in NOD mice.
As deletion of IRE1α in β-cells prior to islet inflammation prevents autoimmune diabetes, it raises the question of whether β-cell ER stress can initiate the immune responses, a question that is controversial in the field. The contribution of ER dysfunction to β-cell death has been demonstrated in monogenic forms of diabetes including Wolfram syndrome, Walcott-Rallison syndrome, mutant insulin gene-induced diabetes, and microcephaly, epilepsy and diabetes syndrome (MEDS) caused by mutations in WFS1, EIF2AK3, INS, and IER3IP1, respectively [[96], [97], [98], [99], [100]]. However, there is no evidence that autoimmunity exists in these monogenic ER stress-associated disorders, suggesting that ER stress alone is not sufficient to initiate an immune response in the absence of other genetic factors. In line with this, it is important to note that the phenotype of IRE1αβ−/− NOD mice are in stark contrast to β-cell IRE1α-deficient mice on a non-stressed genetic background, which develops hyperglycemia due to impaired proinsulin processing. This suggests that the IRE1α/XBP1 pathway can have protective or disruptive effects depending on genetic make-up [65,66]. Indeed, the difference in phenotype between these models could be attributed to the genetic predisposition of immune-independent β-cell fragility in NOD mice, in which their β-cells respond differently to ER stress than non-autoimmune models due to genetic variations in the Glis3 and Xrcc4 genes [101]. The view that inherited abnormalities causing β-cell dysfunction and frailty may contribute to T1D risk is further supported by clinical data highlighting that family members of individuals with T1D compared to non-relative controls may present β-cell dysfunction (reviewed in detail in [1]). Moreover, compared to nondiabetic control donors, pancreatic sections from Ab + individuals exhibit expanded proinsulin positive areas. The pancreatic proinsulin-to-insulin area ratio (indicative of increased secretory demand and ER stress) is also markedly increased in donors with prediabetes [102]. Taken together, these intrinsic β-cells abnormalities, combined with a genetic predisposition to autoimmunity and environmental insults can constitute the multiple hits needed to initiate T1D.
While ER stress has been detected in β-cells of individuals with T1D, how chronic and severe ER stress affects β-cell plasticity in T1D remains unknown. Chen et al. showed that healthy β-cells undergo cycles of stress and recovery from physiological ER stress, a process called “adaptive plasticity”, which is required for proper β-cell function. By using ribosome profiling combined with RNA sequencing analysis in cultured β-cells, they further show that chronic ER stress decreases the expression of β-cell maturity markers as a necessary response for β-cell survival during prolonged ER stress. When stress is relieved, the β-cells can regain their mature identity and restore their homeostasis. These data suggest that repetitive episodes of pathological ER stress can induce gradual loss of β-cells' adaptive plasticity. Based on their findings from single-cell gene expression signatures of β-cells from T1D patients, the authors propose that loss of adaptive β-cell plasticity results in accumulation of immature β-cells which contribute to the loss of functional β-cell mass in T1D [103].
7. β-Cell ER stress: the nexus between autoimmunity and other stress responses in T1D
Stressed β-cells can alter immune cell recruitment, function, and survival through multiple mechanisms. For example, regulation of antigen processing and presentation, key processes of autoimmunity, takes place in the ER. MHC class I molecules bind peptide antigens, transporting them to the cell surface for presentation [104]. The ER plays an important role in protein degradation and peptide antigen production, and ER stress can interfere with MHC class I surface expression and peptide presentation by differentially regulating the expression of ER-vs. cytosol-derived peptides [105,106]. Any disruption in ER homeostasis or an abnormal UPR can dysregulate the processing of MHC class I molecules and other antigens that have been shown to accelerate T1D disease progression [105,107]. Consistent with this notion, altered expression of MHC class I molecule, β2-microglobulin, and MHC class I peptide loading pathway genes are detected in β-cells of IRE1αβ−/− NOD mice.
Besides changing the expression levels of known autoantigens, ER stress can incite post-translational modifications (PTMs) and alternative mRNA splicing in β-cells that may result in the production of neo-antigens recognized by T cells [108]. Elevated cytosolic Ca2+ levels due to ER stress can over-activate Ca2+-dependent PTM enzymes such as tissue transglutaminase 2, peptidyl arginine deaminase, and several cysteine proteases [109]. These enzymes can also promote transpeptidation reactions to produce hybrid insulin peptides (HIPs) that are recognized by T cells in T1D patients [110,111]. Furthermore, unusual PTMs can produce immunogenic peptides by changing their tertiary structures, modifying their functions and proteolytic processes. The large repertoire of PTMs produced by a stressed ER, and their possible impacts on the structure and function of known and novel autoantigens in β-cells, further emphasizes the importance of a well-balanced ER in maintaining immune tolerance [109,112].
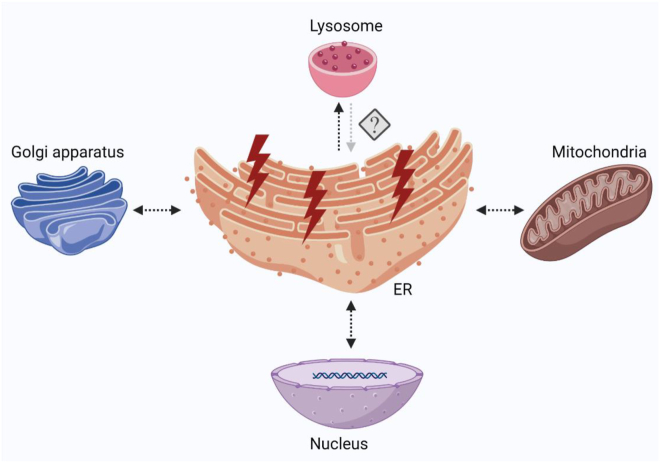
The ER can physically interact with other organelles and communicate with multiple stress response pathways including oxidative stress, autophagy, and cellular senescence (Figure 3). ER stress and oxidative stress are highly interrelated stress responses and can occur concomitantly in cells undergoing stress. The disulfide bond formation during protein folding in the ER involves ER-resident protein disulfide isomerase (PDI) and ER oxidoreductin 1 (ERO1). ERO1 uses oxygen as an electron acceptor during disulfide bond formation, which leads to the generation of hydrogen peroxide (H2O2) [113]. During adaptive stress, H2O2 produced by the PDI-Ero1α pathway is locally detoxified via antioxidant defense mechanisms. However, under prolonged and severe stress conditions, ERO1 activity can account for a potential source of ER-derived oxidative stress. ER stress can also induce mitochondrial-derived oxidative stress. ER and mitochondria form physical interactions at sites called mitochondria-associated ER-membranes (MAMs) through tethering proteins [114,115]. MAMs act as signaling hubs and are enriched in proteins that regulate Ca2+ transport from ER to the mitochondria. A significant increase in the number of MAMs leads to mitochondrial Ca2+ overload, compromised mitochondrial oxidative capacity, and augmented oxidative stress in hepatocytes of a mouse model of obesity and type 2 diabetes [116]. Both IRE1α and PERK are located at MAMs and mediate ER-mitochondria interactions [117,118]. ATF6 physically interacts with the peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α) and promotes mitochondrial biogenesis, which can ultimately affect cellular response to stress [119]. Finally, ATF4 can also link mitochondrial stress and ER stress by regulating the transcription of the ubiquitin ligase Parkin, which plays a key role in the elimination of damaged mitochondria through mitophagy [120]. Whether altered organization of MAMs and ER stress-induced oxidative stress and/or impaired mitochondrial activity contribute to β-cell dysfunction in T1D remain to be investigated.
Figure 3.
ER stress impacts the function of other organelles and stress pathways. ER stress increases reactive oxygen species and imbalance in Ca2+ levels, both of which directly affect mitochondria through MAMs. The UPR activity may induce or inhibit autophagy depending on the cell type and context. The UPR pathway proteins may affect and be affected by cellular senescence. ER stress and Golgi stress may disrupt ER-to-Golgi and Golgi-to-ER protein transport that may impair insulin production and secretion in β-cells.
Autophagy, an intracellular lysosome-mediated degradation pathway that can facilitate the recycling and elimination of misfolded proteins, protein aggregates, and damaged organelles, serves as an essential protective mechanism during ER stress. All three branches of the UPR can induce autophagy under duress [121]. In addition, Ca2+ depletion in the ER can activate calcium/calmodulin-dependent protein kinase kinase-β (CaMKKβ), which in turn induces the activity of 5′ adenosine monophosphate-activated protein kinase (AMPK) [122]. AMPK through the mammalian Target of Rapamycin (mTOR) pathway regulates autophagy. Conversely, ER stress can inhibit autophagy in a cell type or context-dependent manner. However, these two stress responses have concurrently been detected in various pathologies including chronic inflammatory diseases, cardiovascular diseases, neurodegenerative disorders, cancer, and diabetes. However, little remains known about the role of autophagy in T1D pathogenesis. In a recent study, autophagy has been reported to be impaired in the islets of diabetic NOD mice and in residual proinsulin-positive β-cells of human organ donors with T1D [123]. Furthermore, the increased autophagosomes and telolysosomes in the β-cells of Aab + individuals suggest the likelihood of defective autophagic flux prior to disease onset. How impaired autophagy affects β-cell ER stress, or whether β-cell ER stress alters autophagic responses in T1D remains largely unknown.
Senescence is a stress response induced by various stressors including DNA damage and oxidative stress. Senescent cells undergo permanent cell cycle arrest, are protected from apoptosis, and secrete proinflammatory cytokines and components of the extracellular matrix. Targeting senescent cells with senolytics is emerging as a promising therapeutic strategy for many chronic inflammatory diseases [124]. Senescent β-cells have been observed in the islets of both NOD and human T1D donors as they progress through T1D [125]. Pharmacological clearance of senescent cells in NOD mice prevents diabetes [125]. Since NOD mice and human islets exhibit ER stress and senescence, a crosstalk between the UPR and senescence is plausible in β-cells during T1D progression. Emerging data from in vitro systems suggest that ATF6 and IRE1α play a significant role in cellular senescence induced by various stress conditions [126,127]. Consistent with this, ER stress in osteoarthritis chondrocytes stimulates increased activity of senescence-associated β-galactosidase, a marker of senescence [128,129]. Reciprocally, cellular senescence can also regulate the UPR, as shown in therapy-induced senescence in lymphoma cells to increase the levels of sXBP1 and ATF4 [130]. Whether the UPR is activated because of senescence, or if it acts as a driver of cellular senescence, remains unknown. Additional mechanistic studies are required to uncover the crosstalk between ER stress and senescence in β-cells in the context of T1D.
Overall, in addition to ER dysfunction, emerging data suggest that impaired activity of several other organelles including mitochondria (oxidative stress), lysosomes (autophagy), and Golgi [131] promotes β-cell dysfunction in T1D.
8. Therapeutic targeting of ER stress and the UPR in T1D
Targeting the immune system has long been the mainstream therapeutic approach for T1D, yet to date immunotherapy alone at best delays the progression of T1D [132]. While this is an important step towards successful therapy, clearly other mechanisms are also involved in the disease pathology; understanding these mechanisms may provide novel and effective therapeutic opportunities. In light of the recent evidence indicating that β-cells experience elevated ER stress during the progression of T1D, and that β-cell ER stress might play a role in the progression of T1D [90,133], restoration of ER homeostasis at various stages of disease presents a feasible therapeutic strategy.
It is well-established that high secretory demand can cause ER stress and reducing this demand can alleviate ER stress. First shown in 1940, in the suppression of insulin release from β-cells, known as ‘β-cell rest’, intensive insulin therapy markedly decreased exogenous insulin requirements in children with T1D [134]. Later, preclinical and clinical findings from patients with T1D and T2D proposed that insulin treatment leads to a recovery of β-cell dysfunction likely due to β-cell rest and/or redifferentiation [[135], [136], [137]]. In an in vitro study, diazoxide-induced β-cell rest was shown to reduce ER stress in β-cells exposed to palmitate [138]. Induction of β-cell rest either pharmacologically or via bariatric surgery was reported to normalize the secreted proinsulin: insulin ratio [139,140], suggesting that β-cell rest shows its beneficial effects, at least in part, by mitigating ER stress.
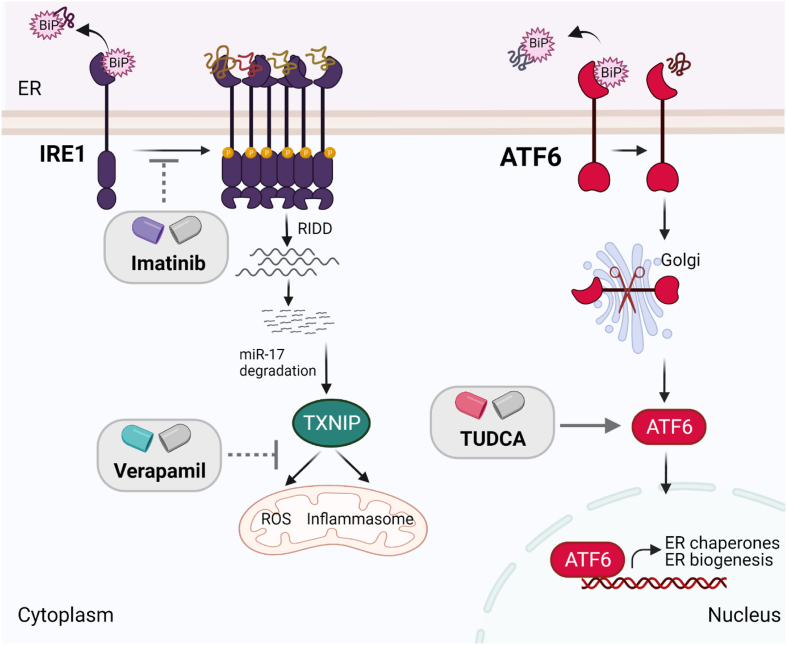
In addition to β-cell rest, interest is growing in targeting β-cell ER stress and the UPR and its downstream signaling in T1D. TUDCA, imatinib, and verapamil, all of which modulate ER stress pathway components, are currently being tested in clinical trials (Figure 4).
Figure 4.
Clinical trials with drugs that can target the UPR pathway components in T1D. Imatinib, a tyrosine kinase inhibitor, may blunt IRE1α′s hyperactivation and inhibit pro-apoptotic pathways in β-cells. Verapamil, a calcium channel blocker, may decrease expression of TXNIP that otherwise promotes apoptosis by increasing oxidative stress and inflammasome formation in β-cells. TUDCA alleviates ER stress in β-cells in an ATF6-dependent manner.
TUDCA is a naturally occurring bile acid that has been used in the clinic for the treatment of cholestatic liver disease [141]. Though it has an unclear mechanism of action, TUDCA is predicted to serve as a chemical chaperone and alleviate ER stress by stabilizing protein folding [141]. While TUDCA treatment at a prediabetic stage prevents diabetes in two different animal models of T1D, it fails to revert diabetes in mice with new-onset diabetes (Engin et al., unpublished data). TUDCA has also been shown to protect human β-cells against cytokine- and ER stress-induced apoptosis [47], suggesting the translatability of TUDCA treatment to individuals who are at high risk for T1D. TUDCA is currently under investigation in phase II clinical trials in patients with new-onset T1D, and the findings of this study have yet to be reported. As this study is designed for patients with new-onset T1D, TUDCA's effects as a preventive therapy in high-risk individuals remain to be investigated.
Imatinib, a specific tyrosine kinase inhibitor used as an anti-cancer agent in the clinic, has been shown to prevent and reverse diabetes in NOD mice [142,143]. The mechanism of imatinib-mediated β-cell protection has been linked to inhibition of IRE1α RNAse hyperactivity and significant reduction of TXNIP levels [93]. Interestingly, in a recent study, besides modulating the UPR, imatinib has been shown to increase B lymphocyte antioxidant capacity and improve reactive oxygen species handling in NOD mice, which plays a crucial role to reverse overt diabetes in these mice [144]. These studies attest that imatinib abolishes maladaptive UPR and elevates immune cell antioxidant capacity to provide a more favorable microenvironment for β-cells to survive and function. Consistent with this, in a recent phase II clinical trial, 26 weeks of treatment with imatinib slowed the decrease in β-cell function for up to 12 months in adults with recent-onset type T1D, although the effect was not sustained out to 24 months [145].
Verapamil, a calcium-channel blocker, has been shown to decrease the expression of TXNIP by decreasing intracellular Ca2+ levels. It has been suggested that a decrease in intracellular Ca2+ levels by verapamil inhibits calcineurin signaling, which would otherwise induce carbohydrate response element-binding protein (ChREBP)-mediated upregulation of TXNIP [146]. Verapamil-induced reduction in TXNIP levels increases β-cell survival and function, protects against streptozotocin (STZ)-induced diabetes, and improves glucose homeostasis in ob/ob mice [146,147]. A recent phase II clinical trial using verapamil in recent-onset T1D patients over a 12-month period demonstrated enhanced preservation of β-cell function and decreased insulin requirements [147].
Notably, none of these drugs selectively target β-cells. Hence, an improved β-cell function may stem from not only the effects of these drugs on β-cells but also their potential effects on immune cells. Furthermore, while endpoints like C-peptide assess β-cell function, the status of β-cell ER function remains unclear. Inclusion of endpoints such as proinsulin: insulin ratio can further inform about β-cell ER homeostasis. Nonetheless, these limited clinical studies suggest that targeting pathways involved in downstream UPR signaling may potentially provide novel therapeutic options for patients. Combining immunotherapies with β-cell therapies and taking into consideration disease stage and patient endotypes [148], the chances for prevention or treatment of this disease can be improved. As underlying pathogenic mechanisms of autoimmune diseases show a great degree of similarities, and emerging data point to an important role for “target cell” dysfunction in autoimmune diseases [149], filling the current knowledge gap on molecular mechanisms of β-cell dysfunction in T1D will undoubtedly be beneficial for identifying novel therapeutic approaches not only for T1D but also for other autoimmune and chronic inflammatory diseases that share a similar etiology.
9. Future directions
A large body of in vitro and ex vivo studies using cell lines, rodent β-cells, and human islets have paved the way for our current understanding of the role of β-cell ER stress in T1D pathogenesis. However, this research field is still in its infancy on understanding the role of β-cell ER stress during different stages of the disease, the function and regulation of each UPR sensor, and the crosstalk between stressed β-cells and immune cells. Generation of in vivo genetic models of the UPR pathway components in T1D pre-clinical models and humanized mice is required to uncover the mechanisms of stress-driven pathology of T1D. Once identified, these mechanisms need further investigation for their translatability to human islets. It is important to acknowledge that stress caused by isolation, transportation, and culture conditions can alter the phenotype of human islet cells [150,151]. Thus, complementing these studies with organoids, live pancreatic tissue slices, and pseudo islets will help accurately define the impact of stress on β-cell plasticity, function, and survival in T1D.
Finally, the interaction of stressed β-cells with other islet cells as well as with other cell types in addition to immune cells including endocrine, exocrine, ductal, vascular, perivascular, stromal, and neuronal cells in the islet microenvironment has been receiving increasing attention. With recent data indicating that cells can transmit their ER stress to their neighboring cells [152], understanding the intercellular transmission of ER stress between β-cells and their neighboring non-β-islet cells and islet-infiltrating immune cells during T1D gains more importance. New advances in single-cell measurements and tissue-based cell–cell interaction network analyses hold promise to refine our understanding of how various cell populations of the pancreas crosstalk under conditions of stress.
Author contribution
Each of the authors made contributions to this work by preparing figures, writing, and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We thank Dr. James P. Zacny for the critical reading and editing of the manuscript and apologize to those colleagues whose work could not be cited here due to space constraints. All figures were created with BioRender.com. H.L. is supported by a University of Wisconsin Stem Cell and Regenerative Medicine Center Graduate Fellowship. F.E. is supported by grants from the NIH 1R01DK130919-01 and 1R56DK128136-01.
Conflict of interest
F.E. serves as a Review Editor in Molecular Metabolism.
References
- 1.Sims E.K., DiMeglio L.A. Cause or effect? A review of clinical data demonstrating beta cell dysfunction prior to the clinical onset of type 1 diabetes. Molecular Metabolism. 2019;27S:S129–S138. doi: 10.1016/j.molmet.2019.06.010. Epub 2019/09/11. PubMed PMID: 31500824; PMCID: PMC6768572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Anken E., Braakman I. Versatility of the endoplasmic reticulum protein folding factory. Critical Reviews in Biochemistry and Molecular Biology. 2005;40(4):191–228. doi: 10.1080/10409230591008161. Epub 2005/08/30. PubMed PMID: 16126486. [DOI] [PubMed] [Google Scholar]
- 3.Hwang J., Qi L. Quality control in the endoplasmic reticulum: crosstalk between ERAD and UPR pathways. Trends in Biochemical Sciences. 2018;43(8):593–605. doi: 10.1016/j.tibs.2018.06.005. Epub 2018/07/31. PubMed PMID: 30056836; PMCID: PMC6327314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernales S., Papa F.R., Walter P. Intracellular signaling by the unfolded protein response. Annual Review of Cell and Developmental Biology. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. Epub 2006/07/07. PubMed PMID: 16822172. [DOI] [PubMed] [Google Scholar]
- 5.Schroder M., Kaufman R.J. ER stress and the unfolded protein response. Mutation Research. 2005;569(1–2):29–63. doi: 10.1016/j.mrfmmm.2004.06.056. Epub 2004/12/18. PubMed PMID: 15603751. [DOI] [PubMed] [Google Scholar]
- 6.Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. Epub 2011/11/26. PubMed PMID: 22116877. [DOI] [PubMed] [Google Scholar]
- 7.Hetz C., Zhang K., Kaufman R.J. Mechanisms, regulation and functions of the unfolded protein response. Nature Reviews Molecular Cell Biology. 2020;21(8):421–438. doi: 10.1038/s41580-020-0250-z. Epub 2020/05/28. PubMed PMID: 32457508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hetz C., Papa F.R. The unfolded protein response and cell fate control. Mol Cell. 2018;69(2):169–181. doi: 10.1016/j.molcel.2017.06.017. Epub 2017/11/07. PubMed PMID: 29107536. [DOI] [PubMed] [Google Scholar]
- 9.Marciniak S.J., Garcia-Bonilla L., Hu J., Harding H.P., Ron D. Activation-dependent substrate recruitment by the eukaryotic translation initiation factor 2 kinase PERK. The Journal of Cell Biology. 2006;172(2):201–209. doi: 10.1083/jcb.200508099. Epub 2006/01/19. PubMed PMID: 16418533; PMCID: PMC2063550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnamoorthy T., Pavitt G.D., Zhang F., Dever T.E., Hinnebusch A.G. Tight binding of the phosphorylated alpha subunit of initiation factor 2 (eIF2alpha) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Molecular and Cellular Biology. 2001;21(15):5018–5030. doi: 10.1128/MCB.21.15.5018-5030.2001. Epub 2001/07/05. PubMed PMID: 11438658; PMCID: PMC87228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnelly N., Gorman A.M., Gupta S., Samali A. The eIF2alpha kinases: their structures and functions. Cellular and Molecular Life Sciences. 2013;70(19):3493–3511. doi: 10.1007/s00018-012-1252-6. Epub 2013/01/29. PubMed PMID: 23354059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu P.D., Harding H.P., Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. The Journal of Cell Biology. 2004;167(1):27–33. doi: 10.1083/jcb.200408003. Epub 2004/10/14. PubMed PMID: 15479734; PMCID: PMC2172506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y., Hendershot L.M. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. Journal of Biological Chemistry. 2003;278(37):34864–34873. doi: 10.1074/jbc.M301107200. Epub 2003/07/04. PubMed PMID: 12840028. [DOI] [PubMed] [Google Scholar]
- 14.Han J., Back S.H., Hur J., Lin Y.H., Gildersleeve R., Shan J., et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nature Cell Biology. 2013;15(5):481–490. doi: 10.1038/ncb2738. Epub 2013/04/30. PubMed PMID: 23624402; PMCID: PMC3692270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harding H.P., Zhang Y., Zeng H., Novoa I., Lu P.D., Calfon M., et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–633. doi: 10.1016/s1097-2765(03)00105-9. Epub 2003/04/02. PubMed PMID: 12667446. [DOI] [PubMed] [Google Scholar]
- 16.Novoa I., Zeng H., Harding H.P., Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. The Journal of Cell Biology. 2001;153(5):1011–1022. doi: 10.1083/jcb.153.5.1011. Epub 2001/05/31. PubMed PMID: 11381086; PMCID: PMC2174339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marciniak S.J., Yun C.Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes & Development. 2004;18(24):3066–3077. doi: 10.1101/gad.1250704. Epub 2004/12/17. PubMed PMID: 15601821; PMCID: PMC535917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Credle J.J., Finer-Moore J.S., Papa F.R., Stroud R.M., Walter P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proceedings of the National Academy of Sciences of the U S A. 2005;102(52):18773–18784. doi: 10.1073/pnas.0509487102. Epub 2005/12/21. PubMed PMID: 16365312; PMCID: PMC1316886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karagoz G.E., Acosta-Alvear D., Nguyen H.T., Lee C.P., Chu F., Walter P. An unfolded protein-induced conformational switch activates mammalian IRE1. Elife. 2017;6 doi: 10.7554/eLife.30700. Epub 2017/10/04. PubMed PMID: 28971800; PMCID: PMC5699868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali M.M., Bagratuni T., Davenport E.L., Nowak P.R., Silva-Santisteban M.C., Hardcastle A., et al. Structure of the Ire1 autophosphorylation complex and implications for the unfolded protein response. The EMBO Journal. 2011;30(5):894–905. doi: 10.1038/emboj.2011.18. Epub 2011/02/15. PubMed PMID: 21317875; PMCID: PMC3049214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida H., Matsui T., Yamamoto A., Okada T., Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–891. doi: 10.1016/s0092-8674(01)00611-0. Epub 2002/01/10. PubMed PMID: 11779464. [DOI] [PubMed] [Google Scholar]
- 22.Hollien J., Weissman J.S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313(5783):104–107. doi: 10.1126/science.1129631. Epub 2006/07/11. PubMed PMID: 16825573. [DOI] [PubMed] [Google Scholar]
- 23.Hollien J., Weissman J.S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313(5783):104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 24.Han D., Lerner A.G., Vande Walle L., Upton J.P., Xu W., Hagen A., et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138(3):562–575. doi: 10.1016/j.cell.2009.07.017. Epub 2009/08/12. PubMed PMID: 19665977; PMCID: PMC2762408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Upton J.P., Wang L., Han D., Wang E.S., Huskey N.E., Lim L., et al. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science. 2012;338(6108):818–822. doi: 10.1126/science.1226191. Epub 2012/10/09. PubMed PMID: 23042294; PMCID: PMC3742121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lerner A.G., Upton J.P., Praveen P.V., Ghosh R., Nakagawa Y., Igbaria A., et al. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metabolism. 2012;16(2):250–264. doi: 10.1016/j.cmet.2012.07.007. Epub 2012/08/14. PubMed PMID: 22883233; PMCID: PMC4014071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oslowski C.M., Hara T., O'Sullivan-Murphy B., Kanekura K., Lu S., Hara M., et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metabolism. 2012;16(2):265–273. doi: 10.1016/j.cmet.2012.07.005. Epub 2012/08/14. PubMed PMID: 22883234; PMCID: PMC3418541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H.P., et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. Epub 2000/01/29. PubMed PMID: 10650002. [DOI] [PubMed] [Google Scholar]
- 29.Nishitoh H., Matsuzawa A., Tobiume K., Saegusa K., Takeda K., Inoue K., et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes & Development. 2002;16(11):1345–1355. doi: 10.1101/gad.992302. Epub 2002/06/07. PubMed PMID: 12050113; PMCID: PMC186318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tam A.B., Mercado E.L., Hoffmann A., Niwa M. ER stress activates NF-kappaB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0045078. Epub 2012/10/31. PubMed PMID: 23110043; PMCID: PMC3482226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haze K., Yoshida H., Yanagi H., Yura T., Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Molecular Biology of the Cell. 1999;10(11):3787–3799. doi: 10.1091/mbc.10.11.3787. Epub 1999/11/17. PubMed PMID: 10564271; PMCID: PMC25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida H., Haze K., Yanagi H., Yura T., Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. Journal of Biological Chemistry. 1998;273(50):33741–33749. doi: 10.1074/jbc.273.50.33741. Epub 1998/12/05. PubMed PMID: 9837962. [DOI] [PubMed] [Google Scholar]
- 33.Sato Y., Nadanaka S., Okada T., Okawa K., Mori K. Luminal domain of ATF6 alone is sufficient for sensing endoplasmic reticulum stress and subsequent transport to the Golgi apparatus. Cell Structure and Function. 2011;36(1):35–47. doi: 10.1247/csf.10010. PubMed PMID: 21150130. [DOI] [PubMed] [Google Scholar]
- 34.Ye J., Rawson R.B., Komuro R., Chen X., Dave U.P., Prywes R., et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6(6):1355–1364. doi: 10.1016/s1097-2765(00)00133-7. Epub 2001/02/13. PubMed PMID: 11163209. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida H., Okada T., Haze K., Yanagi H., Yura T., Negishi M., et al. Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factors 6alpha and 6beta that activates the mammalian unfolded protein response. Molecular and Cellular Biology. 2001;21(4):1239–1248. doi: 10.1128/MCB.21.4.1239-1248.2001. Epub 2001/02/07. PubMed PMID: 11158310; PMCID: PMC99577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thuerauf D.J., Marcinko M., Belmont P.J., Glembotski C.C. Effects of the isoform-specific characteristics of ATF6 alpha and ATF6 beta on endoplasmic reticulum stress response gene expression and cell viability. Journal of Biological Chemistry. 2007;282(31):22865–22878. doi: 10.1074/jbc.M701213200. Epub 2007/05/25. PubMed PMID: 17522056. [DOI] [PubMed] [Google Scholar]
- 37.Hayden M.S., Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–362. doi: 10.1016/j.cell.2008.01.020. Epub 2008/02/13. PubMed PMID: 18267068. [DOI] [PubMed] [Google Scholar]
- 38.Iwasaki Y., Suganami T., Hachiya R., Shirakawa I., Kim-Saijo M., Tanaka M., et al. Activating transcription factor 4 links metabolic stress to interleukin-6 expression in macrophages. Diabetes. 2014;63(1):152–161. doi: 10.2337/db13-0757. Epub 2013/08/31. PubMed PMID: 23990363. [DOI] [PubMed] [Google Scholar]
- 39.D'Osualdo A., Anania V.G., Yu K., Lill J.R., Kaufman R.J., Matsuzawa S., et al. Transcription factor ATF4 induces NLRP1 inflammasome expression during endoplasmic reticulum stress. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0130635. Epub 2015/06/19. PubMed PMID: 26086088; PMCID: PMC4472728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. Epub 2002/08/23. PubMed PMID: 12191486. [DOI] [PubMed] [Google Scholar]
- 41.Zhang K., Shen X., Wu J., Sakaki K., Saunders T., Rutkowski D.T., et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124(3):587–599. doi: 10.1016/j.cell.2005.11.040. Epub 2006/02/14. PubMed PMID: 16469704. [DOI] [PubMed] [Google Scholar]
- 42.Reimold A.M., Iwakoshi N.N., Manis J., Vallabhajosyula P., Szomolanyi-Tsuda E., Gravallese E.M., et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412(6844):300–307. doi: 10.1038/35085509. Epub 2001/07/19. PubMed PMID: 11460154. [DOI] [PubMed] [Google Scholar]
- 43.Iwakoshi N.N., Lee A.H., Vallabhajosyula P., Otipoby K.L., Rajewsky K., Glimcher L.H. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nature Immunology. 2003;4(4):321–329. doi: 10.1038/ni907. Epub 2003/03/04. PubMed PMID: 12612580. [DOI] [PubMed] [Google Scholar]
- 44.Zhang K., Wong H.N., Song B., Miller C.N., Scheuner D., Kaufman R.J. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. Journal of Clinical Investigation. 2005;115(2):268–281. doi: 10.1172/JCI21848. Epub 2005/02/04. PubMed PMID: 15690081; PMCID: PMC546421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwakoshi N.N., Pypaert M., Glimcher L.H. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. Journal of Experimental Medicine. 2007;204(10):2267–2275. doi: 10.1084/jem.20070525. Epub 2007/09/19. PubMed PMID: 17875675; PMCID: PMC2118458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tong X., Kono T., Evans-Molina C. Nitric oxide stress and activation of AMP-activated protein kinase impair beta-cell sarcoendoplasmic reticulum calcium ATPase 2b activity and protein stability. Cell Death & Disease. 2015;6 doi: 10.1038/cddis.2015.154. Epub 2015/06/19. PubMed PMID: 26086963; PMCID: PMC4669835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brozzi F., Nardelli T.R., Lopes M., Millard I., Barthson J., Igoillo-Esteve M., et al. Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia. 2015;58(10):2307–2316. doi: 10.1007/s00125-015-3669-6. Epub 2015/06/24. PubMed PMID: 26099855. [DOI] [PubMed] [Google Scholar]
- 48.Hasnain S.Z., Tauro S., Das I., Tong H., Chen A.C., Jeffery P.L., et al. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology. 2013;144(2):357–368 e9. doi: 10.1053/j.gastro.2012.10.043. Epub 2012/11/06. PubMed PMID: 23123183. [DOI] [PubMed] [Google Scholar]
- 49.Schuit F.C., In't Veld P.A., Pipeleers D.G. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proceedings of the National Academy of Sciences of the U S A. 1988;85(11):3865–3869. doi: 10.1073/pnas.85.11.3865. Epub 1988/06/01. PubMed PMID: 3287379; PMCID: PMC280320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eizirik D.L., Cardozo A.K., Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocrine Reviews. 2008;29(1):42–61. doi: 10.1210/er.2007-0015. Epub 2007/12/01. PubMed PMID: 18048764. [DOI] [PubMed] [Google Scholar]
- 51.Engin F., Nguyen T., Yermalovich A., Hotamisligil G.S. Aberrant islet unfolded protein response in type 2 diabetes. Scientific Reports. 2014;4:4054. doi: 10.1038/srep04054. Epub 2014/02/12. PubMed PMID: 24514745; PMCID: PMC3920274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laybutt D.R., Preston A.M., Akerfeldt M.C., Kench J.G., Busch A.K., Biankin A.V., et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50(4):752–763. doi: 10.1007/s00125-006-0590-z. Epub 2007/02/03. PubMed PMID: 17268797. [DOI] [PubMed] [Google Scholar]
- 53.Harding H.P., Zeng H., Zhang Y., Jungries R., Chung P., Plesken H., et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7(6):1153–1163. doi: 10.1016/s1097-2765(01)00264-7. Epub 2001/06/30. PubMed PMID: 11430819. [DOI] [PubMed] [Google Scholar]
- 54.Zhang W., Feng D., Li Y., Iida K., McGrath B., Cavener D.R. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metabolism. 2006;4(6):491–497. doi: 10.1016/j.cmet.2006.11.002. Epub 2006/12/05. PubMed PMID: 17141632. [DOI] [PubMed] [Google Scholar]
- 55.Gao Y., Sartori D.J., Li C., Yu Q.-C., Kushner J.A., Simon M.C., et al. PERK is required in the adult pancreas and is essential for maintenance of glucose homeostasis. Molecular and Cellular Biology. 2012;32(24):5129–5139. doi: 10.1128/mcb.01009-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han A.P., Yu C., Lu L., Fujiwara Y., Browne C., Chin G., et al. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. The EMBO Journal. 2001;20(23):6909–6918. doi: 10.1093/emboj/20.23.6909. Epub 2001/12/01. PubMed PMID: 11726526; PMCID: PMC125753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang P., McGrath B.C., Reinert J., Olsen D.S., Lei L., Gill S., et al. The GCN2 eIF2α kinase is required for adaptation to amino acid deprivation in mice. Molecular and Cellular Biology. 2002;22(19):6681–6688. doi: 10.1128/mcb.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abraham N., Stojdl D.F., Duncan P.I., Méthot N., Ishii T., Dubé M., et al. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. Journal of Biological Chemistry. 1999;274(9):5953–5962. doi: 10.1074/jbc.274.9.5953. [DOI] [PubMed] [Google Scholar]
- 59.Ladiges W.C., Knoblaugh S.E., Morton J.F., Korth M.J., Sopher B.L., Baskin C.R., et al. Pancreatic beta-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes. 2005;54(4):1074–1081. doi: 10.2337/diabetes.54.4.1074. Epub 2005/03/29. PubMed PMID: 15793246. [DOI] [PubMed] [Google Scholar]
- 60.Seo J., Fortuno E.S., 3rd, Suh J.M., Stenesen D., Tang W., Parks E.J., et al. Atf4 regulates obesity, glucose homeostasis, and energy expenditure. Diabetes. 2009;58(11):2565–2573. doi: 10.2337/db09-0335. Epub 2009/08/20. PubMed PMID: 19690063; PMCID: PMC2768187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song B., Scheuner D., Ron D., Pennathur S., Kaufman R.J. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. Journal of Clinical Investigation. 2008;118(10):3378–3389. doi: 10.1172/JCI34587. Epub 2008/09/09. PubMed PMID: 18776938; PMCID: PMC2528909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yong J., Parekh V.S., Reilly S.M., Nayak J., Chen Z., Lebeaupin C., et al. Chop/Ddit3 depletion in beta cells alleviates ER stress and corrects hepatic steatosis in mice. Science Translational Medicine. 2021;13(604) doi: 10.1126/scitranslmed.aba9796. Epub 2021/07/30. PubMed PMID: 34321322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee A.H., Heidtman K., Hotamisligil G.S., Glimcher L.H. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proceedings of the National Academy of Sciences of the U S A. 2011;108(21):8885–8890. doi: 10.1073/pnas.1105564108. Epub 2011/05/11. PubMed PMID: 21555585; PMCID: PMC3102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu T., Yang L., Yan C., Wang X., Huang P., Zhao F., et al. The IRE1alpha-XBP1 pathway regulates metabolic stress-induced compensatory proliferation of pancreatic beta-cells. Cell Research. 2014;24(9):1137–1140. doi: 10.1038/cr.2014.55. Epub 2014/05/07. PubMed PMID: 24797433; PMCID: PMC4152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hassler J.R., Scheuner D.L., Wang S., Han J., Kodali V.K., Li P., et al. The IRE1alpha/XBP1s pathway is essential for the glucose response and protection of beta cells. PLoS Biology. 2015;13(10) doi: 10.1371/journal.pbio.1002277. Epub 2015/10/16. PubMed PMID: 26469762; PMCID: PMC4607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsuchiya Y., Saito M., Kadokura H., Miyazaki J.I., Tashiro F., Imagawa Y., et al. IRE1-XBP1 pathway regulates oxidative proinsulin folding in pancreatic beta cells. The Journal of Cell Biology. 2018;217(4):1287–1301. doi: 10.1083/jcb.201707143. Epub 2018/03/07. PubMed PMID: 29507125; PMCID: PMC5881499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamoto K., Sato T., Matsui T., Sato M., Okada T., Yoshida H., et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Developmental Cell. 2007;13(3):365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 68.Usui M., Yamaguchi S., Tanji Y., Tominaga R., Ishigaki Y., Fukumoto M., et al. Atf6alpha-null mice are glucose intolerant due to pancreatic beta-cell failure on a high-fat diet but partially resistant to diet-induced insulin resistance. Metabolism. 2012;61(8):1118–1128. doi: 10.1016/j.metabol.2012.01.004. Epub 2012/03/06. PubMed PMID: 22386934. [DOI] [PubMed] [Google Scholar]
- 69.Engin F., Yermalovich A., Nguyen T., Hummasti S., Fu W., Eizirik D.L., et al. Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Science Translational Medicine. 2013;5(211):211ra156. doi: 10.1126/scitranslmed.3006534. Epub 2013/11/15. PubMed PMID: 24225943; PMCID: PMC4169117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma R.B., O'Donnell A.C., Stamateris R.E., Ha B., McCloskey K.M., Reynolds P.R., et al. Insulin demand regulates beta cell number via the unfolded protein response. Journal of Clinical Investigation. 2015;125(10):3831–3846. doi: 10.1172/JCI79264. Epub 2015/09/22. PubMed PMID: 26389675; PMCID: PMC4607122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo S., Mao C., Lee B., Lee A.S. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Molecular and Cellular Biology. 2006;26(15):5688–5697. doi: 10.1128/MCB.00779-06. Epub 2006/07/19. PubMed PMID: 16847323; PMCID: PMC1592753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma R.B., Zheng X., Gablaski B., Kim J.K., Lee A.S., Alonso L.C. Grp78 is critical for beta-cell maturation and survival. Diabetes. 2018;67(Supplement 1) doi: 10.2337/db18-85-OR. [DOI] [Google Scholar]
- 73.Urano F. Wolfram syndrome: diagnosis, management, and treatment. Current Diabetes Reports. 2016;16(1) doi: 10.1007/s11892-015-0702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fonseca S.G., Ishigaki S., Oslowski C.M., Lu S., Lipson K.L., Ghosh R., et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. Journal of Clinical Investigation. 2010;120(3):744–755. doi: 10.1172/JCI39678. Epub 2010/02/18. PubMed PMID: 20160352; PMCID: PMC2827948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J., Takeuchi T., Tanaka S., Kubo S.K., Kayo T., Lu D., et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. Journal of Clinical Investigation. 1999;103(1):27–37. doi: 10.1172/JCI4431. Epub 1999/01/12. PubMed PMID: 9884331; PMCID: PMC407861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Izumi T., Yokota-Hashimoto H., Zhao S., Wang J., Halban P.A., Takeuchi T. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes. 2003;52(2):409–416. doi: 10.2337/diabetes.52.2.409. Epub 2003/01/24. PubMed PMID: 12540615. [DOI] [PubMed] [Google Scholar]
- 77.Oyadomari S., Koizumi A., Takeda K., Gotoh T., Akira S., Araki E., et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. Journal of Clinical Investigation. 2002;109(4):525–532. doi: 10.1172/JCI14550. Epub 2002/02/21. PubMed PMID: 11854325; PMCID: PMC150879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winnay J.N., Dirice E., Liew C.W., Kulkarni R.N., Kahn C.R. p85alpha deficiency protects beta-cells from endoplasmic reticulum stress-induced apoptosis. Proceedings of the National Academy of Sciences of the U S A. 2014;111(3):1192–1197. doi: 10.1073/pnas.1322564111. Epub 2014/01/08. PubMed PMID: 24395790; PMCID: PMC3903202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li R., Kondegowda N.G., Filipowska J., Hampton R.F., Leblanc S., Garcia-Ocana A., et al. Lactogens reduce endoplasmic reticulum stress-induced rodent and human beta-cell death and diabetes incidence in akita mice. Diabetes. 2020;69(7):1463–1475. doi: 10.2337/db19-0909. Epub 2020/04/26. PubMed PMID: 32332156; PMCID: PMC7306119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oyadomari S., Takeda K., Takiguchi M., Gotoh T., Matsumoto M., Wada I., et al. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proceedings of the National Academy of Sciences of the U S A. 2001;98(19):10845–10850. doi: 10.1073/pnas.191207498. Epub 2001/08/30. PubMed PMID: 11526215; PMCID: PMC58562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cardozo A.K., Ortis F., Storling J., Feng Y.M., Rasschaert J., Tonnesen M., et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54(2):452–461. doi: 10.2337/diabetes.54.2.452. Epub 2005/01/29. PubMed PMID: 15677503. [DOI] [PubMed] [Google Scholar]
- 82.Chan J.Y., Cooney G.J., Biden T.J., Laybutt D.R. Differential regulation of adaptive and apoptotic unfolded protein response signalling by cytokine-induced nitric oxide production in mouse pancreatic beta cells. Diabetologia. 2011;54(7):1766–1776. doi: 10.1007/s00125-011-2139-z. Epub 2011/04/08. PubMed PMID: 21472432. [DOI] [PubMed] [Google Scholar]
- 83.Vig S., Buitinga M., Rondas D., Crevecoeur I., van Zandvoort M., Waelkens E., et al. Cytokine-induced translocation of GRP78 to the plasma membrane triggers a pro-apoptotic feedback loop in pancreatic beta cells. Cell Death & Disease. 2019;10(4):309. doi: 10.1038/s41419-019-1518-0. Epub 2019/04/07. PubMed PMID: 30952835; PMCID: PMC6450900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Engin F. ER stress and development of type 1 diabetes. Journal of Investigative Medicine. 2016;64(1):2–6. doi: 10.1097/JIM.0000000000000229. Epub 2015/08/01. PubMed PMID: 26230493; PMCID: PMC7185224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramos-Rodriguez M., Raurell-Vila H., Colli M.L., Alvelos M.I., Subirana-Granes M., Juan-Mateu J., et al. The impact of proinflammatory cytokines on the beta-cell regulatory landscape provides insights into the genetics of type 1 diabetes. Nature Genetics. 2019;51(11):1588–1595. doi: 10.1038/s41588-019-0524-6. Epub 2019/11/05. PubMed PMID: 31676868; PMCID: PMC7040466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. Epub 1980/01/01. PubMed PMID: 6995140. [DOI] [PubMed] [Google Scholar]
- 87.Serreze D.V., Chapman H.D., Varnum D.S., Gerling I., Leiter E.H., Shultz L.D. Initiation of autoimmune diabetes in NOD/Lt mice is MHC class I-dependent. The Journal of Immunology. 1997;158(8):3978–3986. Epub 1997/04/15. PubMed PMID: 9103469. [PubMed] [Google Scholar]
- 88.Tersey S.A., Nishiki Y., Templin A.T., Cabrera S.M., Stull N.D., Colvin S.C., et al. Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61(4):818–827. doi: 10.2337/db11-1293. Epub 2012/03/24. PubMed PMID: 22442300; PMCID: PMC3314371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marhfour I., Lopez X.M., Lefkaditis D., Salmon I., Allagnat F., Richardson S.J., et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia. 2012;55(9):2417–2420. doi: 10.1007/s00125-012-2604-3. Epub 2012/06/16. PubMed PMID: 22699564. [DOI] [PubMed] [Google Scholar]
- 90.Lee H., Lee Y.S., Harenda Q., Pietrzak S., Oktay H.Z., Schreiber S., et al. Beta cell dedifferentiation induced by IRE1alpha deletion prevents type 1 diabetes. Cell Metabolism. 2020;31(4):822–836 e5. doi: 10.1016/j.cmet.2020.03.002. Epub 2020/03/30. PubMed PMID: 32220307; PMCID: PMC7346095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rui J., Deng S., Arazi A., Perdigoto A.L., Liu Z., Herold K.C. Beta cells that resist immunological attack develop during progression of autoimmune diabetes in NOD mice. Cell Metabolism. 2017;25(3):727–738. doi: 10.1016/j.cmet.2017.01.005. Epub 2017/02/14. PubMed PMID: 28190773; PMCID: PMC5342930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seiron P., Wiberg A., Kuric E., Krogvold L., Jahnsen F.L., Dahl-Jorgensen K., et al. Characterisation of the endocrine pancreas in type 1 diabetes: islet size is maintained but islet number is markedly reduced. Journal of Pathology: Clinical Research. 2019;5(4):248–255. doi: 10.1002/cjp2.140. Epub 2019/09/08. PubMed PMID: 31493350; PMCID: PMC6817830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morita S., Villalta S.A., Feldman H.C., Register A.C., Rosenthal W., Hoffmann-Petersen I.T., et al. Targeting ABL-IRE1alpha signaling spares ER-stressed pancreatic beta cells to reverse autoimmune diabetes. Cell Metabolism. 2017;25(5):1207. doi: 10.1016/j.cmet.2017.04.026. Epub 2017/05/04. PubMed PMID: 28467938. [DOI] [PubMed] [Google Scholar]
- 94.Colli M.L., Paula F.M., Marselli L., Marchetti P., Roivainen M., Eizirik D.L., et al. Coxsackievirus B tailors the unfolded protein response to favour viral amplification in pancreatic beta cells. Journal of Innate Immunity. 2019;11(4):375–390. doi: 10.1159/000496034. Epub 2019/02/26. PubMed PMID: 30799417; PMCID: PMC6738210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Satoh T., Abiru N., Kobayashi M., Zhou H., Nakamura K., Kuriya G., et al. CHOP deletion does not impact the development of diabetes but suppresses the early production of insulin autoantibody in the NOD mouse. Apoptosis. 2011;16(4):438–448. doi: 10.1007/s10495-011-0576-2. Epub 2011/01/29. PubMed PMID: 21274633. [DOI] [PubMed] [Google Scholar]
- 96.Inoue H., Tanizawa Y., Wasson J., Behn P., Kalidas K., Bernal-Mizrachi E., et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome) Nature Genetics. 1998;20(2):143–148. doi: 10.1038/2441. [DOI] [PubMed] [Google Scholar]
- 97.Strom T. Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Human Molecular Genetics. 1998;7(13):2021–2028. doi: 10.1093/hmg/7.13.2021. [DOI] [PubMed] [Google Scholar]
- 98.Delépine M., Nicolino M., Barrett T., Golamaully M., Mark Lathrop G., Julier C. EIF2AK3, encoding translation initiation factor 2-α kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nature Genetics. 2000;25(4):406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 99.Stoy J., Edghill E.L., Flanagan S.E., Ye H., Paz V.P., Pluzhnikov A., et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proceedings of the National Academy of Sciences. 2007;104(38):15040–15044. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Poulton Cathryn J., Schot R., Kia Sima K., Jones M., Verheijen Frans W., Venselaar H., et al. Microcephaly with simplified gyration, epilepsy, and infantile diabetes linked to inappropriate apoptosis of neural progenitors. The American Journal of Human Genetics. 2011;89(2):265–276. doi: 10.1016/j.ajhg.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dooley J., Tian L., Schonefeldt S., Delghingaro-Augusto V., Garcia-Perez J.E., Pasciuto E., et al. Genetic predisposition for beta cell fragility underlies type 1 and type 2 diabetes. Nature Genetics. 2016;48(5):519–527. doi: 10.1038/ng.3531. Epub 2016/03/22. PubMed PMID: 26998692; PMCID: PMC5584070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodriguez-Calvo T., Zapardiel-Gonzalo J., Amirian N., Castillo E., Lajevardi Y., Krogvold L., et al. Increase in pancreatic proinsulin and preservation of β-cell mass in autoantibody-positive donors prior to type 1 diabetes onset. Diabetes. 2017;66(5):1334–1345. doi: 10.2337/db16-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen C.-W., Guan B.-J., Alzahrani M.R., Gao Z., Gao L., Bracey S., et al. Adaptation to chronic ER stress enforces pancreatic β-cell plasticity. bioRxiv. 2021 doi: 10.1101/2021.05.24.445193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qian S.B., Reits E., Neefjes J., Deslich J.M., Bennink J.R., Yewdell J.W. Tight linkage between translation and MHC class I peptide ligand generation implies specialized antigen processing for defective ribosomal products. The Journal of Immunology. 2006;177(1):227–233. doi: 10.4049/jimmunol.177.1.227. PubMed PMID: 16785518. [DOI] [PubMed] [Google Scholar]
- 105.Granados D.P., Tanguay P.L., Hardy M.P., Caron E., de Verteuil D., Meloche S., et al. ER stress affects processing of MHC class I-associated peptides. BMC Immunology. 2009;10:10. doi: 10.1186/1471-2172-10-10. Epub 2009/02/18. PubMed PMID: 19220912; PMCID: PMC2657905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ulianich L., Terrazzano G., Annunziatella M., Ruggiero G., Beguinot F., Di Jeso B. ER stress impairs MHC Class I surface expression and increases susceptibility of thyroid cells to NK-mediated cytotoxicity. Biochimica et Biophysica Acta. 2011;1812(4):431–438. doi: 10.1016/j.bbadis.2010.12.013. PubMed PMID: 21199669. [DOI] [PubMed] [Google Scholar]
- 107.Marre M.L., Piganelli J.D. Environmental factors contribute to beta cell endoplasmic reticulum stress and neo-antigen formation in type 1 diabetes. Frontiers in Endocrinology. 2017;8:262. doi: 10.3389/fendo.2017.00262. Epub 2017/10/17. PubMed PMID: 29033899; PMCID: PMC5626851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gonzalez-Duque S., Azoury M.E., Colli M.L., Afonso G., Turatsinze J.V., Nigi L., et al. Conventional and neo-antigenic peptides presented by beta cells are targeted by circulating naive cd8+ t cells in type 1 diabetic and healthy donors. Cell Metabolism. 2018;28(6):946–960 e6. doi: 10.1016/j.cmet.2018.07.007. Epub 2018/08/07. PubMed PMID: 30078552. [DOI] [PubMed] [Google Scholar]
- 109.Piganelli J.D., Mamula M.J., James E.A. The role of beta cell stress and neo-epitopes in the immunopathology of type 1 diabetes. Frontiers in Endocrinology. 2020;11:624590. doi: 10.3389/fendo.2020.624590. Epub 2021/03/09. PubMed PMID: 33679609; PMCID: PMC7930070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Delong T., Wiles T.A., Baker R.L., Bradley B., Barbour G., Reisdorph R., et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science. 2016;351(6274):711–714. doi: 10.1126/science.aad2791. Epub 2016/02/26. PubMed PMID: 26912858; PMCID: PMC4884646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baker R.L., Rihanek M., Hohenstein A.C., Nakayama M., Michels A., Gottlieb P.A., et al. Hybrid insulin peptides are autoantigens in type 1 diabetes. Diabetes. 2019;68(9):1830–1840. doi: 10.2337/db19-0128. Epub 2019/06/09. PubMed PMID: 31175101; PMCID: PMC6702640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marre M.L., Profozich J.L., Coneybeer J.T., Geng X., Bertera S., Ford M.J., et al. Inherent ER stress in pancreatic islet beta cells causes self-recognition by autoreactive T cells in type 1 diabetes. Journal of Autoimmunity. 2016;72:33–46. doi: 10.1016/j.jaut.2016.04.009. Epub 2016/05/14. PubMed PMID: 27173406; PMCID: PMC4958612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tu B.P., Weissman J.S. The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol Cell. 2002;10(5):983–994. doi: 10.1016/s1097-2765(02)00696-2. Epub 2002/11/28. PubMed PMID: 12453408. [DOI] [PubMed] [Google Scholar]
- 114.Vance J.E. Phospholipid synthesis in a membrane fraction associated with mitochondria. Journal of Biological Chemistry. 1990;265(13):7248–7256. Epub 1990/05/05. PubMed PMID: 2332429. [PubMed] [Google Scholar]
- 115.Giorgi C., Missiroli S., Patergnani S., Duszynski J., Wieckowski M.R., Pinton P. Mitochondria-associated membranes: composition, molecular mechanisms, and physiopathological implications. Antioxidants and Redox Signaling. 2015;22(12):995–1019. doi: 10.1089/ars.2014.6223. Epub 2015/01/06. PubMed PMID: 25557408. [DOI] [PubMed] [Google Scholar]
- 116.Arruda A.P., Pers B.M., Parlakgul G., Guney E., Inouye K., Hotamisligil G.S. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nature Medicine. 2014;20(12):1427–1435. doi: 10.1038/nm.3735. Epub 2014/11/25. PubMed PMID: 25419710; PMCID: PMC4412031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Verfaillie T., Rubio N., Garg A.D., Bultynck G., Rizzuto R., Decuypere J.P., et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death & Differentiation. 2012;19(11):1880–1891. doi: 10.1038/cdd.2012.74. Epub 2012/06/19. PubMed PMID: 22705852; PMCID: PMC3469056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carreras-Sureda A., Jana F., Urra H., Durand S., Mortenson D.E., Sagredo A., et al. Publisher correction: non-canonical function of IRE1alpha determines mitochondria-associated endoplasmic reticulum composition to control calcium transfer and bioenergetics. Nature Cell Biology. 2019;21(7):913. doi: 10.1038/s41556-019-0355-9. Epub 2019/06/16. PubMed PMID: 31201389; PMCID: PMC7292237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu J., Ruas J.L., Estall J.L., Rasbach K.A., Choi J.H., Ye L., et al. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1alpha/ATF6alpha complex. Cell Metabolism. 2011;13(2):160–169. doi: 10.1016/j.cmet.2011.01.003. Epub 2011/02/03. PubMed PMID: 21284983; PMCID: PMC3057411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bouman L., Schlierf A., Lutz A.K., Shan J., Deinlein A., Kast J., et al. Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death & Differentiation. 2011;18(5):769–782. doi: 10.1038/cdd.2010.142. Epub 2010/11/30. PubMed PMID: 21113145; PMCID: PMC3131924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rashid H.O., Yadav R.K., Kim H.R., Chae H.J. ER stress: autophagy induction, inhibition and selection. Autophagy. 2015;11(11):1956–1977. doi: 10.1080/15548627.2015.1091141. Epub 2015/09/22. PubMed PMID: 26389781; PMCID: PMC4824587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hoyer-Hansen M., Bastholm L., Szyniarowski P., Campanella M., Szabadkai G., Farkas T., et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25(2):193–205. doi: 10.1016/j.molcel.2006.12.009. Epub 2007/01/25. PubMed PMID: 17244528. [DOI] [PubMed] [Google Scholar]
- 123.Muralidharan C., Conteh A.M., Marasco M.R., Crowder J.J., Kuipers J., de Boer P., et al. Pancreatic beta cell autophagy is impaired in type 1 diabetes. Diabetologia. 2021;64(4):865–877. doi: 10.1007/s00125-021-05387-6. Epub 2021/01/31. PubMed PMID: 33515072; PMCID: PMC7940272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Di Micco R., Krizhanovsky V., Baker D., d'Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nature Reviews Molecular Cell Biology. 2021;22(2):75–95. doi: 10.1038/s41580-020-00314-w. Epub 2020/12/18. PubMed PMID: 33328614; PMCID: PMC8344376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thompson P.J., Shah A., Ntranos V., Van Gool F., Atkinson M., Bhushan A. Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metabolism. 2019;29(5):1045–1060e10. doi: 10.1016/j.cmet.2019.01.021. Epub 2019/02/26. PubMed PMID: 30799288. [DOI] [PubMed] [Google Scholar]
- 126.Blazanin N., Son J., Craig-Lucas A.B., John C.L., Breech K.J., Podolsky M.A., et al. ER stress and distinct outputs of the IRE1alpha RNase control proliferation and senescence in response to oncogenic Ras. Proceedings of the National Academy of Sciences of the U S A. 2017;114(37):9900–9905. doi: 10.1073/pnas.1701757114. Epub 2017/08/30. PubMed PMID: 28847931; PMCID: PMC5603998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim H.S., Kim Y., Lim M.J., Park Y.G., Park S.I., Sohn J. The p38-activated ER stress-ATF6alpha axis mediates cellular senescence. The FASEB Journal. 2019;33(2):2422–2434. doi: 10.1096/fj.201800836R. Epub 2018/09/28. PubMed PMID: 30260700. [DOI] [PubMed] [Google Scholar]
- 128.Liu Y., Zhu H., Yan X., Gu H., Gu Z., Liu F. Endoplasmic reticulum stress participates in the progress of senescence and apoptosis of osteoarthritis chondrocytes. Biochemical and Biophysical Research Communications. 2017;491(2):368–373. doi: 10.1016/j.bbrc.2017.07.094. Epub 2017/07/22. PubMed PMID: 28728848. [DOI] [PubMed] [Google Scholar]
- 129.Druelle C., Drullion C., Desle J., Martin N., Saas L., Cormenier J., et al. ATF6alpha regulates morphological changes associated with senescence in human fibroblasts. Oncotarget. 2016;7(42):67699–67715. doi: 10.18632/oncotarget.11505. Epub 2016/08/27. PubMed PMID: 27563820; PMCID: PMC5356513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dorr J.R., Yu Y., Milanovic M., Beuster G., Zasada C., Dabritz J.H., et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013;501(7467):421–425. doi: 10.1038/nature12437. Epub 2013/08/16. PubMed PMID: 23945590. [DOI] [PubMed] [Google Scholar]
- 131.Bone R.N., Oyebamiji O., Talware S., Selvaraj S., Krishnan P., Syed F., et al. A computational approach for defining a signature of beta-cell golgi stress in diabetes. Diabetes. 2020;69(11):2364–2376. doi: 10.2337/db20-0636. Epub 2020/08/21. PubMed PMID: 32820009; PMCID: PMC7576569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sims E.K., Bundy B.N., Stier K., Serti E., Lim N., Long S.A., et al. Type 1 diabetes trialnet study G. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Science Translational Medicine. 2021;13(583) doi: 10.1126/scitranslmed.abc8980. Epub 2021/03/05. PubMed PMID: 33658358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sims E.K., Chaudhry Z., Watkins R., Syed F., Blum J., Ouyang F., et al. Elevations in the fasting serum proinsulin-to-c-peptide ratio precede the onset of type 1 diabetes. Diabetes Care. 2016;39(9):1519–1526. doi: 10.2337/dc15-2849. Epub 2016/07/08. PubMed PMID: 27385327; PMCID: PMC5001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jackson R.L. Stabilization of the diabetic child. Archives of Pediatrics and Adolescent Medicine. 1940;59(2) doi: 10.1001/archpedi.1940.01990130115008. [DOI] [Google Scholar]
- 135.Harrison L.B., Adams-Huet B., Raskin P., Lingvay I. Beta-cell function preservation after 3.5 years of intensive diabetes therapy. Diabetes Care. 2012;35(7):1406–1412. doi: 10.2337/dc11-2170. Epub 2012/06/23. PubMed PMID: 22723578; PMCID: PMC3379585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Z., York N.W., Nichols C.G., Remedi M.S. Pancreatic beta cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metabolism. 2014;19(5):872–882. doi: 10.1016/j.cmet.2014.03.010. Epub 2014/04/22. PubMed PMID: 24746806; PMCID: PMC4067979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brown R.J., Rother K.I. Effects of beta-cell rest on beta-cell function: a review of clinical and preclinical data. Pediatric Diabetes. 2008;9(3 Pt 2):14–22. doi: 10.1111/j.1399-5448.2007.00272.x. Epub 2008/01/29. PubMed PMID: 18221429; PMCID: PMC3417290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sargsyan E., Ortsäter H., Thorn K., Bergsten P. Diazoxide-induced β-cell rest reduces endoplasmic reticulum stress in lipotoxic β-cells. Journal of Endocrinology. 2008;199(1):41–50. doi: 10.1677/joe-08-0251. [DOI] [PubMed] [Google Scholar]
- 139.Laedtke T., Kjems L., Pørksen N., Schmitz O., Veldhuis J., Kao P.C., et al. Overnight inhibition of insulin secretion restores pulsatility and proinsulin/insulin ratio in type 2 diabetes. American Journal of Physiology-Endocrinology and Metabolism. 2000;279(3):E520–E528. doi: 10.1152/ajpendo.2000.279.3.E520. [DOI] [PubMed] [Google Scholar]
- 140.Purnell J.Q., Johnson G.S., Wahed A.S., Dalla Man C., Piccinini F., Cobelli C., et al. Prospective evaluation of insulin and incretin dynamics in obese adults with and without diabetes for 2 years after Roux-en-Y gastric bypass. Diabetologia. 2018;61(5):1142–1154. doi: 10.1007/s00125-018-4553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Engin F., Hotamisligil G.S. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes, Obesity and Metabolism. 2010;12(Suppl 2):108–115. doi: 10.1111/j.1463-1326.2010.01282.x. Epub 2010/11/05. PubMed PMID: 21029307. [DOI] [PubMed] [Google Scholar]
- 142.Louvet C., Szot G.L., Lang J., Lee M.R., Martinier N., Bollag G., et al. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proceedings of the National Academy of Sciences of the U S A. 2008;105(48):18895–18900. doi: 10.1073/pnas.0810246105. Epub 2008/11/19. PubMed PMID: 19015530; PMCID: PMC2596241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hagerkvist R., Sandler S., Mokhtari D., Welsh N. Amelioration of diabetes by imatinib mesylate (Gleevec): role of beta-cell NF-kappaB activation and anti-apoptotic preconditioning. The FASEB Journal. 2007;21(2):618–628. doi: 10.1096/fj.06-6910com. Epub 2006/12/01. PubMed PMID: 17135364. [DOI] [PubMed] [Google Scholar]
- 144.Wilson C.S., Spaeth J.M., Karp J., Stocks B.T., Hoopes E.M., Stein R.W., et al. B lymphocytes protect islet beta cells in diabetes prone NOD mice treated with imatinib. JCI Insight. 2019;5 doi: 10.1172/jci.insight.125317. Epub 2019/04/10. PubMed PMID: 30964447; PMCID: PMC6538336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gitelman S.E., Bundy B.N., Ferrannini E., Lim N., Blanchfield J.L., DiMeglio L.A., et al. Imatinib therapy for patients with recent-onset type 1 diabetes: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet Diabetes and Endocrinology. 2021 doi: 10.1016/S2213-8587(21)00139-X. Epub 2021/07/03. PubMed PMID: 34214479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu G., Chen J., Jing G., Shalev A. Preventing beta-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61(4):848–856. doi: 10.2337/db11-0955. Epub 2012/03/24. PubMed PMID: 22442301; PMCID: PMC3314354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ovalle F., Grimes T., Xu G., Patel A.J., Grayson T.B., Thielen L.A., et al. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nature Medicine. 2018;24(8):1108–1112. doi: 10.1038/s41591-018-0089-4. Epub 2018/07/11. PubMed PMID: 29988125; PMCID: PMC6092963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Battaglia M., Ahmed S., Anderson M.S., Atkinson M.A., Becker D., Bingley P.J., et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care. 2020;43(1):5–12. doi: 10.2337/dc19-0880. Epub 2019/11/23. PubMed PMID: 31753960; PMCID: PMC6925574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Szymczak F., Colli M.L., Mamula M.J., Evans-Molina C., Eizirik D.L. Gene expression signatures of target tissues in type 1 diabetes, lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. Science Advances. 2021;7(2) doi: 10.1126/sciadv.abd7600. Epub 2021/02/02. PubMed PMID: 33523973; PMCID: PMC7787485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Teo A.K.K., Lim C.S., Cheow L.F., Kin T., Shapiro J.A., Kang N.Y., et al. Single-cell analyses of human islet cells reveal de-differentiation signatures. Cell Death & Disease. 2018;4:14. doi: 10.1038/s41420-017-0014-5. Epub 2018/03/14. PubMed PMID: 29531811; PMCID: PMC5841351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Negi S., Jetha A., Aikin R., Hasilo C., Sladek R., Paraskevas S. Analysis of beta-cell gene expression reveals inflammatory signaling and evidence of dedifferentiation following human islet isolation and culture. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030415. Epub 2012/02/03. PubMed PMID: 22299040; PMCID: PMC3267725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tirosh A., Tuncman G., Calay E.S., Rathaus M., Ron I., Tirosh A., et al. Intercellular transmission of hepatic ER stress in obesity disrupts systemic metabolism. Cell Metabolism. 2021;33(8):1716. doi: 10.1016/j.cmet.2021.07.005. Epub 2021/08/05. PubMed PMID: 34348100; PMCID: PMC8388259. [DOI] [PMC free article] [PubMed] [Google Scholar]