Abstract
Background
Postmastectomy radiotherapy (PMRT), as an important regional treatment, improves the survival rate of patients with T3N0M0 breast cancers. However, the therapeutic effect of PMRT on T3N0M0 patients in different age groups is unclear.
Methods
Using Surveillance, Epidemiology, and End Results database, we identified 4840 T3N0M0 patients between 2000 and 2015. The primary and secondary outcomes were overall survival (OS) and breast cancer-specific survival (BCSS). Survival outcomes were compared using Kaplan-Meier survival test, COX regression analysis, propensity score matching and forest plot, which present the relationship between age and PMRT.
Results
Survival analysis demonstrated that for young patients (aged 18–45 and 46–55), there was no significant difference in OS between with and without PMRT. However, for patients older than 65 years, PMRT could significantly improve survival time (P < 0.001). Multivariate Cox analysis of OS showed older patients with PMRT had a lower hazard ratio (HR) than those without PMRT (aged 56–65: HR = 0.67, P = 0.014; aged >65: HR = 0.60, P < 0.001), and little benefit for young patients. The consistent results were also observed in 1:1 matched cohort. Subgroup analysis revealed the survival HRs of with versus without PMRT for patients older than 65 years were significant in most subgroups.
Conclusion
The effect of PMRT in T3N0M0 patients is related to the age. PMRT is associated with improved survival in older patients with T3N0M0 breast cancer, especially those older than 65 years. While the benefit of PMRT is limited in T3N0M0 patients of young age. The observation suggests the importance of age for T3N0M0 patients when individualized treatment is made.
Keywords: Postmastectomy radiotherapy, T3N0M0 breast Cancer, Overall survival, Breast cancer-specific survival, Age
Highlights
-
•
Using SEER database, survival analysis, COX analysis, propensity score matching and forest plot, research presents the relationship between age and postmastectomy radiotherapy in T3N0M0 patients.
-
•
The effect of postmastectomy radiotherapy in T3N0M0 patients is associated to the age.
-
•
Postmastectomy radiotherapy is associated with improved survival in older patients with T3N0M0 breast cancer. While the benefit of PMRT is limited in T3N0M0 patients of young age.
1. Introduction
Breast cancer has become the most common cancer worldwide [1]. Especially, T3N0M0 breast cancer patients are a group who have a primary tumor larger than 50 mm without positive nodes or distant metastases at the time of the diagnosis [2]. Until now, the common clinical practice guidelines did not mention T3N0M0 patients in detail [3]. Given the large tumor load within breast tissue, more research is needed to explore the clinical characteristics and precise treatment of T3N0M0 breast cancer.
PMRT is an important local treatment for breast cancer in addition to surgery [4]. Previous research proves that PMRT was associated with significant improvements in overall survival (OS) of patients with T3N0M0 breast cancers treated with mastectomy, including improving the survival rate and reducing the local recurrence rate [[5], [6], [7]]. Postmastectomy radiotherapy (PMRT) might be suggested for T3N0M0 patients in clinical practice [8].
Several researchers found that the age of diagnosis played a critical role in the prognosis of breast cancer and response to treatment. Although it's reported that PMRT is beneficial for the T3N0M0 patients [8,9], few researches have explored deeply the influence of age on the effect of PMRT on the prognosis of T3N0M0 patients in different age groups.
In this study, we aimed to investigate the effect of PMRT for the different-aged patients with T3N0M0 breast cancer by utilizing the Surveillance, Epidemiology, and End Results (SEER) database, which consists of 18 population-based cancer registries for the period of 1973–2015. Our research tried to unveil the necessity of PMRT for different age groups of T3N0M0 breast cancer patients, and propose the accurate strategy of PMRT treatment for the T3N0M0 patients.
2. Methods
2.1. Database
We used the SEER database (November 2018 submission), a National Cancer Institute-sponsored program, and obtained data from 18 population-based registries with the SEER∗Stat software, version 8.3.8.
2.2. Study population
We derived a dataset of female breast cancer patients diagnosed from 2000 to 2015. The specific inclusion criteria were listed as follows: females aged 18 years or older; microscopically confirmed of breast cancer; diagnosis not obtained from a death certificate or autopsy; one primary only and active follow-up.
Patients with T3N0M0 breast cancer were enrolled according to “Breast - Adjusted AJCC 6th T/N/M (1988–2015)”. Then, we enrolled patients treated with mastectomy according to “RX Summ--Surg Prim Site (1998+)”, excluded patients with ambiguous data like “PMRT recommended, but unknown if administered”, and excluded patients with unknown race, marital status, grade, unknown ER or PR, and other laterality. The flow diagram for the selection of the study cohort was presented in Figure S1. We classified “Refused (1988+)” and “None/Unknown” into the group without PMRT, and classified “Beam radiation”, “Combination of beam with implants or isotopes”, “Radiation, NOS method or source not specified” and “Radioactive implants (includes brachytherapy) (1988+)” into the group with PMRT. Finally, overall 4840 patients were enrolled into the cohort, including 2243 patients (46.34%) with PMRT and 2597 patients (53.66%) without PMRT. Among these patients with PMRT, there were 416, 570, 512, and 1099 in 18–45, 46–55, 56–65, and older than 65 years, respectively. Among patients without PMRT, there were 588, 606, 495, and 554 in 18–45, 46–55, 56–65, and older than 65 years, respectively.
3. Ethics statement
The data released by the Surveillance, Epidemiology, and End Results (SEER) database was publicly available and deidentified, and it did not require patients’ informed consent.
3.1. Statistical analysis
The relationships between listed variables and PMRT were analyzed by the Pearson Chi-square test or Fisher's exact test, if appropriate. The primary and secondary outcomes of our study were overall survival (OS) and breast cancer-specific survival (BCSS), respectively. The OS means the survival time from the diagnosis date of breast cancer to the date of death caused by any cause, and the BCSS means the survival time from the diagnosis date of breast cancer to the date of death caused by breast cancer.
Log-rank tests and Kaplan-Meier survival estimates were conducted to compare the differences in the survival analyses of OS and BCSS. COX analyses and propensity score matching (PSM) methods were utilized to examine the influence of age in PMRT. These methods analyzed the relationship between the influences of PMRT and different age groups for patients with T3N0M0 breast cancer. And the forest plots of the hazard ratios (HRs) of different subgroups were utilized to describe the stratified prognosis value of PMRT for T3N0M0 breast cancer in different age groups. All the statistical analyses were performed by using Stata statistical software, version 14.0, R 4.0.5, and RStudio 1.4.1106. Two-sided P < 0.05 was considered significant.
4. Results
4.1. Demographic and clinical characteristics of patients
All 4840 patients’ demographic and clinical characteristics according to age are summarized in Table 1. The patients were divided into four different age groups, that is 18–45, 46–55, 56–65, and older than 65 years cohorts. In the four groups, significant differences (P < 0.001) were observed in most variables, except laterality. The baseline of T3N0M0 patients showed that the patients with different ages had distinct clinicopathological characteristics, which inferred that the benefit of PMRT could be discussed in different aged cohorts respectively. Therefore, we analyzed the survival data and risk factors in the overall population and the four different age cohorts subsequently.
Table 1.
Baseline Characteristics of T3N0M0 patients diagnosed in 2000–2015 from the SEER database.
| Characteristics | Total (n = 4840) n (%) | 18–45 (n = 1004) n (%) | 46–55 (n = 1176) n (%) | 56–65 (n = 1007) n (%) | >65 (n = 1653) n (%) | P |
|---|---|---|---|---|---|---|
| Year | 0.004 | |||||
| 2000–2009 | 2457(50.76) | 530(52.79) | 602(51.19) | 461(45.78) | 864(52.27) | |
| 2010–2015 | 2383(49.24) | 474(47.21) | 574(48.81) | 546(54.22) | 789(47.73) | |
| Race | <0.001 | |||||
| White | 3665(75.72) | 688(68.53) | 880(74.83) | 776(77.06) | 1321(79.92) | |
| Black | 746(15.41) | 206(20.52) | 179(15.22) | 148(14.70) | 213(12.89) | |
| Others | 429(8.86) | 110(10.96) | 117(9.95) | 83(8.24) | 119(7.20) | |
| Marital status | <0.001 | |||||
| Married | 2452(50.66) | 588(58.57) | 688(58.50) | 531(52.73) | 645(39.02) | |
| Not married | 2388(49.34) | 416(41.43) | 488(41.50) | 476(47.27) | 1008(60.98) | |
| Laterality | 0.499 | |||||
| Left | 2416(49.92) | 500(49.80) | 581(49.40) | 487(48.36) | 848(51.30) | |
| Right | 2424(50.08) | 504(50.20) | 595(50.60) | 520(51.64) | 805(48.70) | |
| Histology | <0.001 | |||||
| IDC | 2702(55.83) | 724(72.11) | 695(59.10) | 540(53.62) | 743(44.95) | |
| ILC and others | 2138(44.17) | 280(27.89) | 481(40.90) | 467(46.38) | 910(55.05) | |
| Grade | <0.001 | |||||
| I and II | 2477(51.18) | 375(37.35) | 584(49.66) | 537(53.33) | 981(59.35) | |
| III and others | 2363(48.82) | 629(62.65) | 592(50.34) | 470(46.67) | 672(40.65) | |
| Tumor size | 0.002 | |||||
| 5–6 cm | 1799(37.17) | 373(37.15) | 439(37.33) | 365(36.25) | 622(37.63) | |
| 6–8 cm | 1426(29.46) | 288(28.69) | 338(28.74) | 319(31.68) | 481(29.10) | |
| >8 cm | 732(15.12) | 151(15.04) | 207(17.60) | 164(16.29) | 210(12.70) | |
| Others/Unknown | 883(18.24) | 192(19.12) | 192(16.33) | 159(15.79) | 340(20.57) | |
| HoR | <0.001 | |||||
| Negative | 1555(32.13) | 409(40.74) | 430(36.56) | 304(30.19) | 412(24.92) | |
| Positive | 3285(67.87) | 595(59.26) | 746(63.44) | 703(69.81) | 1241(75.08) | |
| Chemotherapy | <0.001 | |||||
| Yes | 2880(59.50) | 154(15.34) | 268(22.79) | 331(32.87) | 1207(73.02) | |
| No/Unknown | 1960(40.50) | 850(84.66) | 908(77.21) | 676(67.13) | 446(26.98) | |
| Radiation | <0.001 | |||||
| Yes | 2243(46.34) | 416(41.43) | 570(48.47) | 512(50.84) | 1099(66.49) | |
| No | 2597(53.66) | 588(58.57) | 606(51.53) | 495(49.16) | 554(33.51) |
Abbreviations: HoR, hormone receptor; IDC, infiltrating duct carcinoma; ILC, infiltrating lobular carcinoma.
5. Comparison of survival between patients with and without PMRT
Kaplan-Meier survival estimates were utilized to evaluate the OS and BCSS in patients between with and without PMRT. Patients with PMRT had better survival than patients without PMRT for OS (P < 0.001) (Fig. 1 A), as well as for BCSS (P < 0.001) (Figure S2 A).
Fig. 1.
Kaplan-Meier survival estimates of overall survival with or without PMRT in different age groups. (A) OS Overall, (B) OS of aged 18–45 years, (C) OS of aged 46–55 years, (D) OS of aged 56–65 years, (E) OS of aged >65 years.
Survival analysis in different age groups showed there was no statistical significance between with and without PMRT for patients aged 18–45 years and 46–55 years (aged 18–45: OS P = 0.130, BCSS P = 0.158; aged 46–55: OS P = 0.352, BCSS P = 0.735, respectively). For the patients aged 56–65 years, there was statistical significance between with and without PMRT for OS, but no significant difference for BCSS (OS, P = 0.002; BCSS, P = 0.220). Furthermore, patients older than 65 years with PMRT had better survival than patients without PMRT in both OS and BCSS (log-rank P < 0.001) (Fig. 1 E and Figure S2 E).
5.1. Cox analysis of different variables
Due to the distinct clinicopathological characteristics of T3N0M0 patients of different ages, we utilized the univariate and multivariate cox regression model to evaluate the effect of the variables in the four aged cohorts respectively. All variables in the univariate analysis (Table S1) were included in the multivariate analysis (Table 2), such as year of diagnosis, race, marital status, laterality, histologic type, tumor grade, tumor size, HoR status, chemotherapy, and radiation. After balancing the effect of others, PMRT was confirmed as an independent protective factor in OS for the patients aged 56–65 and aged older than 65 years (aged 56–65: HR = 0.67, 95%CI: 0.49–0.92, P = 0.014; aged >65: HR = 0.60, 95%CI: 0.50–0.72, P < 0.001). However, PMRT did not show the same efficacy in the younger cohorts (aged 18–45: HR = 0.74, 95%CI: 0.50–1.08, P = 0.119; aged 46–55: HR = 0.91, 95%CI: 0.65–1.28, P = 0.603).
Table 2.
Multivariate Cox regression model analysis of OS in different age groups.
| Characteristics | 18–45 |
46–55 |
56–65 |
>65 |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| Year | ||||||||
| 2000–2009 | Reference | Reference | Reference | Reference | ||||
| 2010–2015 | 0.97(0.61–1.53) | 0.880 | 0.75(0.50–1.13) | 0.165 | 1.04(0.73–1.47) | 0.831 | 0.78(0.65–0.95) | 0.011 |
| Race | ||||||||
| White | Reference | Reference | Reference | Reference | ||||
| Black | 1.19(0.78–1.82) | 0.425 | 0.91(0.59–1.41) | 0.679 | 1.56(1.08–2.25) | 0.016 | 0.95(0.76–1.18) | 0.623 |
| Others | 1.34(0.72–2.49) | 0.353 | 0.89(0.49–1.61) | 0.692 | 0.66(0.35–1.27) | 0.215 | 0.89(0.65–1.22) | 0.476 |
| Marital status | ||||||||
| Married | Reference | Reference | Reference | Reference | ||||
| Not married | 1.81(1.24–2.64) | 0.002 | 1.41(1.01–1.96) | 0.045 | 1.04(0.77–1.41) | 0.779 | 1.74(1.47–2.06) | <0.001 |
| Laterality | ||||||||
| Left | Reference | Reference | Reference | Reference | ||||
| Right | 0.93(0.65–1.34) | 0.713 | 0.97(0.71–1.34) | 0.869 | 1.12(0.84–1.50) | 0.430 | 0.90(0.78–1.05) | 0.187 |
| Histology | ||||||||
| IDC | Reference | Reference | Reference | Reference | ||||
| ILC and others | 0.92(0.59–1.44) | 0.707 | 0.70(0.47–1.06) | 0.089 | 0.95(0.68–1.33) | 0.760 | 0.95(0.81–1.11) | 0.488 |
| Grade | ||||||||
| I and II | Reference | Reference | Reference | Reference | ||||
| III and others | 1.53(0.93–2.52) | 0.092 | 1.38(0.88–2.16) | 0.155 | 2.24(1.57–3.21) | <0.001 | 1.70(1.43–2.03) | <0.001 |
| Tumor size | ||||||||
| 5–6 cm | Reference | Reference | Reference | Reference | ||||
| 6–8 cm | 1.62(0.98–2.68) | 0.058 | 1.32(0.85–2.04) | 0.222 | 1.24(0.85–1.79) | 0.259 | 0.92(0.76–1.13) | 0.438 |
| >8 cm | 1.75(0.97–3.17) | 0.065 | 1.70(1.05–2.73) | 0.029 | 1.09(0.69–1.73) | 0.715 | 1.02(0.80–1.31) | 0.862 |
| Others/Unknown | 1.95(1.18–3.24) | 0.009 | 1.43(0.91–2.27) | 0.124 | 1.19(0.78–1.80) | 0.428 | 0.99(0.81–1.21) | 0.936 |
| HoR | ||||||||
| Negative | Reference | Reference | Reference | Reference | ||||
| Positive | 0.74(0.49–1.11) | 0.146 | 0.51(0.34–0.77) | 0.001 | 0.79(0.57–1.10) | 0.165 | 0.85(0.70–1.02) | 0.088 |
| Chemotherapy | ||||||||
| No/Unknown | Reference | Reference | Reference | Reference | ||||
| Yes | 1.07(0.62–1.84) | 0.803 | 0.86(0.57–1.31) | 0.486 | 0.75(0.54–1.04) | 0.086 | 0.53(0.43–0.65) | <0.001 |
| Radiation | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.74(0.50–1.08) | 0.119 | 0.91(0.65–1.28) | 0.603 | 0.67(0.49–0.92) | 0.014 | 0.60(0.50–0.72) | <0.001 |
Abbreviations: HoR, hormone receptor; HR, hazard ratio; IDC, infiltrating duct carcinoma; ILC, infiltrating lobular carcinoma.
5.2. Survival analysis in the matched group
Both survival analysis and the COX model showed the importance of PMRT for the older T3N0M0 patients. To confirm the conclusion and balance the bias from other variables, a 1:1 (with and without PMRT) matched case-control analysis, by the propensity score matching (PSM), was carried out to balance the differences of baseline characteristics in the four aged cohorts, respectively. 660 patients aged 18–45, 776 patients aged 46–55, 592 patients aged 56–65, and 998 patients older than 65 years were obtained with known information (Table S2). Ten factors were included in the PSM, including radiation, year of diagnosis, race, marital status, laterality, histologic type, tumor grade, tumor size, HoR status, and chemotherapy. The survival analysis of the matched groups in different age groups showed that PMRT could significantly improve OS compared with those without PMRT for the older patients (aged 56–65: log-rank P = 0.110; aged >65: log-rank P < 0.001). And there was no significant difference in the OS and BCSS between patients with and without PMRT for the younger patients (aged 18–45: OS P = 0.282, BCSS P = 0.515; aged 46–55: OS P = 0.998, BCSS P = 0.928) (Fig. 2 and Figure S3). These outcomes were consistent with the foregoing results.
Fig. 2.
Kaplan-Meier survival estimates of overall survival comparing 1:1 matched Without vs With PMRT in different age groups. (A) PSM OS of aged 18–45 years, (B) PSM OS of aged 46–55 years, (C) PSM OS of aged 56–65 years, (D) PSM OS of aged >65 years.
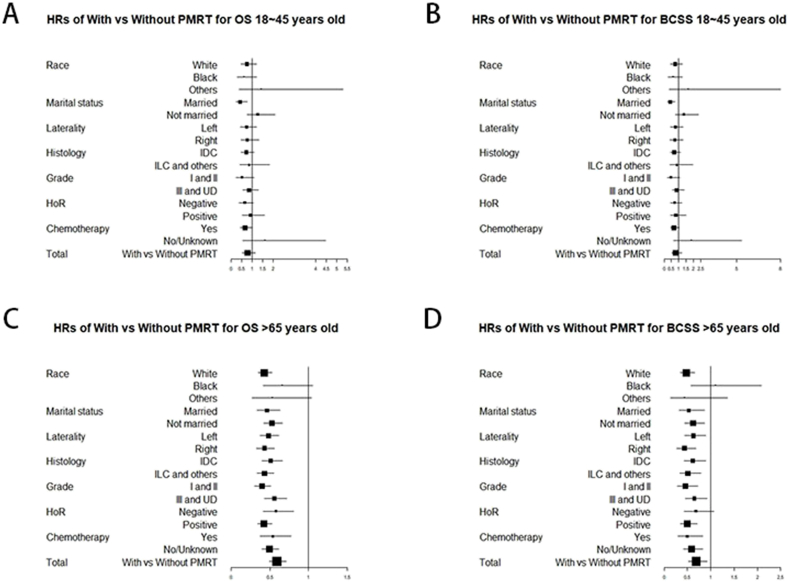
5.3. Subgroup analysis by forest plot
The above analysis, including Kaplan-Meier plots, Cox analysis, and PSM model, indicated that PMRT could improve the survival of the older T3N0M0 patients. The forest plots of the hazard ratios (HRs) for OS and BCSS in different-aged cohorts were plotted to describe the stratified protective value of PMRT for T3N0M0 breast cancer (Fig. 3 and Figure S4). The HRs of patients aged 18–45 years with PMRT versus those without PMRT for OS and BCSS were significant only in the two subgroups (Fig. 3A and B). Detailedly, compared with patients without PMRT, patients aged 18–45 years with PMRT presented lower HRs for OS of married status (HR = 0.44, 95%CI: 0.26–0.76) and with chemotherapy (HR = 0.67, 95%CI: 0.45–0.99). By contrast, for the patients aged 56–65 and the patients older than 65 years, the HRs with PMRT versus without PMRT for OS and BCSS were significant in most subgroups (Fig. 3C and D). Particularly, compared with patients without PMRT, the patients older than 65 years with PMRT presented lower HRs for OS of white race (HR = 0.43, 95%CI: 0.35–0.53), both married and not married (married: HR = 0.46, 95%CI: 0.34–0.63; not married: HR = 0.53, 95%CI: 0.42–0.66, respectively), all histologic types (IDC: HR = 0.51, 95%CI: 0.40–0.67; ILC and others: HR = 0.43, 95%CI: 0.34–0.55, respectively), all grades (grade I and II: HR = 0.40, 95%CI: 0.31–0.51; grade III and UD: HR = 0.56, 95%CI: 0.43–0.72, respectively), and both negative positive HoR status (negative: HR = 0.58, 95%CI: 0.42–0.81; positive: HR = 0.43, 95%CI: 0.35–0.53, respectively). These data support that PMRT could play a protective role in the elder patients and benefit the most T3N0M0 patients aged older than 65 years.
Fig. 3.
Forest plot of the hazard ratios for With vs Without PMRT in 18–45 years group and older than 65 years group.
6. Discussion
PMRT has a favorable effect on the survival of T3N0M0 patients. Previous researches revealed that using PMRT to treat breast cancer patients with clinical T3N0 disease who received neoadjuvant chemotherapy (NAC) and mastectomy can decrease the local-regional recurrence (LRR) risk [8,[10], [11], [12]]. However, these researches did not explore the effect of age on the efficacy of PMRT. We utilized 4840 patients (including 2243 patients with PMRT) diagnosed in 2000–2015. As the largest analysis of effects of PMRT on survival in different age groups for patients with T3N0M0 breast cancer to date, the research inferred that in different age groups, the effects of PMRT are different. Especially for patients older than 65 years, PMRT could significantly improve survival time compared with those without PMRT.
The radiation therapy developed quickly, and the new technology of RT, such as hypofractionated RT, CT-based simulation, 3-dimensional beam modulation, showed more efficacy and lower toxicity. Both CALGB 9394 and PRIME II trials demonstrated for the certain older patients, those with early and low-risk breast cancer, radiotherapy is ineffective and can be exempted [13,14]. Whereas certain researches indicated that PMRT was associated with significant improvements in overall survival (OS) in patients with high-risk breast cancers [[5], [6], [7]]. But the value of PMRT in T3N0M0 breast cancer patients of different age groups has not been deeply studied. Our study put forward a new point of view that age is an important factor in T3N0M0 breast cancer.
Kim and Liu et al. reported that the pathogenesis and clinical treatment of breast cancer vary by age [15,16]. Similarly, in our study, the effect of PMRT for T3N0M0 breast cancer patients exhibited a various influence in the four age groups. For the patients older than 65 years, PMRT could significantly improve survival time compared with those without PMRT (P < 0.001). Furthermore, the multivariate Cox analysis, the propensity score matching model, and forest plot demonstrated that PMRT was significantly effective in patients with T3N0M0 older than 56 years, especially older than 65 years. On the contrary, for the young patients (aged 18–45 years and 46–55 years), there was no difference in the OS and BCSS between patients with and without PMRT (aged 18–45: OS P = 0.130, BCSS P = 0.158; aged 46–55: OS P = 0.352, BCSS P = 0.735, respectively).
The discrepancy between previous studies, which showed the low efficacy of radiation in elder women with early breast cancer, and our finding, that the elder patients seemed to derive benefit from radiation, was probably due to the different value of radiation in locoregional control. PRIME II and CALGB 9394 trial recruited the elder patients with early breast cancer (>64 and > 69 respectively, T1-2N0). The two trials found that the postoperative radiation resulted in a significant but modest improvement in local control, such as 5-year ipsilateral recurrence from 4.1% without radiation to 1.3% with radiation in PRIME II trial, and the small improvement in locoregional control did not translate into survival benefit [13,14]. In contrast, the randomized trial conducted by the Danish Breast Cancer Collaborative Group evaluated the role of PMRT in high-risk patients with N0 and showed postmastectomy radiation decreased locoregional recurrence rate hugely from 23% to 6% in postmenopausal patients and from 17% to 3% in premenopausal patients [17,18]. We inferred that the improvement of locoregional control by radiation in the elder T3N0 patients, which was much larger than the counterpart with early cancer, could translate into survival benefit. Compared with the elder patients, the young patients with better physical state may treat with the locoregional recurrence in the more progressive and radical way, which partially explained why the better regional control by radiation did not translate to survival benefit in the younger T3N0 patients.
Past researches recommended PMRT as a supplement treatment for high-risk breast cancer patients including T3N0M0 patients. According to the previously validated nomogram, the 88% risk of local lymph node metastasis exists for T3 patients [19]. T3N0 patients, although lack nodal metastasis at initial diagnosis, could suffer the high-risk local and regional recurrence. Besides our study, Benjamin et al. reported that for older women with high-risk breast cancer (such as T3/T4 and/or N2/3), PMRT was associated with significant benefits in survival (HR = 0.85, 95%CI: 0.75–0.97) [20]. A similar conclusion was also inferred in Truong's studies [21]. However, Bertolo et al. reported that fewer patients in the old-aged group, compared to the young patients, had PMRT as part of their treatment [22].
This study has some potential implications for clinical practice. Firstly, our observations, that for T3N0M0 patients older than 65 years, the advantage of PMRT for OS is significant, proposed the access to PMRT for the older T3N0M0 patients in the clinical practice. Radiotherapy is a common postoperative treatment for breast cancer. Our research indicated that older women with T3N0M0 could obtain a benefit of both OS and BCSS from PMRT after balancing other interfering factors, as we discussed above. Secondly, as for patients aged 18–45 years and 46–55 years, there was no difference in survival time whether they received PMRT or not in our research. Therefore, more clinicopathological factors like age should be considered when making the treatment plan. Certainly, randomized clinical trials are urgently needed to confirm these findings and define optimal treatment strategies for the different aged groups. And more work is needed to increase the patients’ acceptance of PMRT.
Inevitably, our research has some limitations. Firstly, some important variables are missing in the SEER database, including Her2 status (available since 2010), disease-free survival information, etc. Secondly, the method of PMRT was not detailed and defined in our research due to the limitation of the database. Thirdly, the specific systemic therapy of patient is not available from the SEER database. Not accounting for any systemic therapy is another significant limitation. Therefore, further and more detailed research is needed to investigate the relationship between PMRT and age in the survival analysis, including well-designed clinical trials.
7. Conclusion
In conclusion, we analyzed the effect of PMRT on patients diagnosed with T3N0M0 in different age groups. PMRT is associated with improved survival in older patients with T3N0M0 breast cancer, especially those older than 65 years. While the benefit of PMRT is inapparent in the T3N0M0 patients of younger age. We hope that these findings may provide clinicians with some ideas and help evaluate the effect of PMRT more effectively in the individualized treatment.
Ethical approval
The data released by the Surveillance, Epidemiology, and End Results (SEER) database was publicly available and deidentified, and does not require patient's informed consent. The ethical approval is not required.
Declaration of competing interest
No potential conflicts of interest were disclosed.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.11.006.
Abbreviations: OS, overall survival.
Abbreviations: OS, overall survival; PSM, propensity score matching.
Abbreviations: BCSS, breast cancer-specific survival; HR, hazard ratio; OS, overall survival.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Edge SBB D.R., Compton C.C., Fritz A.G., Greene F.L.T.A. seventh ed. Springer; 2011. AJCC cancer staging manual. [Google Scholar]
- 3.National Comprehensive Cancer Network Breast cancer (version 5.2021) 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [DOI] [PubMed]
- 4.Hennequin C., Barillot I., Azria D., et al. Radiothérapie du cancer du sein. Cancer Radiother. 2016;20:S139–S146. doi: 10.1016/j.canrad.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Johnson M.E., Handorf E.A., Martin J.M., Hayes S.B. Postmastectomy radiation therapy for T3N0: a SEER analysis. Cancer. 2014;120(22):3569–3574. doi: 10.1002/cncr.28865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassidy R.J., Liu Y., Kahn S.T., et al. The role of postmastectomy radiotherapy in women with pathologic T3N0M0 breast cancer. Cancer. 2017;123(15):2829–2839. doi: 10.1002/cncr.30675. [DOI] [PubMed] [Google Scholar]
- 7.McCammon R., Finlayson C., Schwer A., Rabinovitch R. Impact of postmastectomy radiotherapy in T3N0 invasive carcinoma of the breast: a Surveillance, Epidemiology, and End Results database analysis. Cancer. 2008;113(4):683–689. doi: 10.1002/cncr.23611. [DOI] [PubMed] [Google Scholar]
- 8.Almahariq M.F., Quinn T.J., Siddiqui Z.A., et al. Post-mastectomy radiotherapy is associated with improved overall survival in T3N0 patients who do not receive chemotherapy. Radiother Oncol. 2020;145:229–237. doi: 10.1016/j.radonc.2020.01.022. [DOI] [PubMed] [Google Scholar]
- 9.SR F.J.F., Ke K., Dk G., Mm P. Outcomes and utilization of postmastectomy radiotherapy for T3N0 breast cancers. Breast. 2017;32:156–161. doi: 10.1016/J.BREAST.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Francis S.R., Frandsen J., Kokeny K.E., Gaffney D.K., Poppe M.M. Outcomes and utilization of postmastectomy radiotherapy for T3N0 breast cancers. Breast. 2017;32:156–161. doi: 10.1016/j.breast.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J.-Q., Lu C.-Y., Qin L., Chen H.-M., Wu S.-Y. Outcome of post-mastectomy radiotherapy after primary systemic treatment in patients with different clinical tumor and nodal stages of breast cancer: a cohort study. Am J Cancer Res. 2020;10(7):2185–2198. www.ajcr.us/ [PMC free article] [PubMed] [Google Scholar]
- 12.Nagar H., Mittendorf E.A., Strom E.A., et al. Local-regional recurrence with and without radiation therapy after neoadjuvant chemotherapy and mastectomy for clinically staged T3N0 breast cancer. Int J Radiat Oncol Biol Phys. 2011;81(3):782–787. doi: 10.1016/j.ijrobp.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Kunkler I.H., Williams L.J., Jack W.J.L., Cameron D.A., Dixon J.M. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266–273. doi: 10.1016/S1470-2045(14)71221-5. [DOI] [PubMed] [Google Scholar]
- 14.Hughes K.S., Schnaper L.A., Bellon J.R., et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–2387. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J.-Y., Kang D., Seok, et al. Clinical features and outcomes of invasive breast cancer: age-specific analysis of a modern hospital-based registry. J Glob Oncol. 2019 doi: 10.1200/JGO.19.00034. https://ascopubs.org/go/authors/open-access [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y.R., Jiang Y.Z., Yu K Da, Shao Z.M. Different patterns in the prognostic value of age for breast cancer-specific mortality depending on hormone receptor status: a SEER population-based analysis. Ann Surg Oncol. 2015;22(4):1102–1110. doi: 10.1245/s10434-014-4108-5. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen H.M., Overgaard M., Grau C., Jensen A.R., Overgaard J. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol. 2006;24(15):2268–2275. doi: 10.1200/JCO.2005.02.8738. [DOI] [PubMed] [Google Scholar]
- 18.Barrientos R., Samtani S., Frelinghuysen M., Sotomayor C., Gormaz J.G., Burotto M. Clinical decision making in postmastectomy radiotherapy in node negative breast cancer. Ecanceramed Sci. 2018;12 doi: 10.3332/ECANCER.2018.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bevilacqua J.L.B., Kattan M.W., Fey J.V., Cody H.S., Borgen P.I., Zee KJ Van. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol. 2007;25(24):3670–3679. doi: 10.1200/JCO.2006.08.8013. [DOI] [PubMed] [Google Scholar]
- 20.Smith B.D., Haffty B.G., Hurria A., Galusha D.H., Gross C.P. Postmastectomy radiation and survival in older women with breast cancer. J Clin Oncol. 2006;24(30):4901–4907. doi: 10.1200/JCO.2006.06.5938. [DOI] [PubMed] [Google Scholar]
- 21.Truong P.T., Lee J., Kader H.A., Speers C.H., Olivotto I.A. Locoregional recurrence risks in elderly breast cancer patients treated with mastectomy without adjuvant radiotherapy. Eur J Cancer. 2005;41(9):1267–1277. doi: 10.1016/j.ejca.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 22.Bertolo A., Rosso C., Voutsadakis I.A. Breast cancer in patients 80 Years-old and older. Eur J Breast Heal. 2020;16(3):208–212. doi: 10.5152/ejbh.2020.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.