Abstract
Purpose
Platelet-rich plasma (PRP) is a promising noninvasive technique for facial rejuvenation. This systematic literature review aims to appraise the nature and quality of published evidence evaluating the effectiveness and safety of PRP in facial rejuvenation.
Patients and Methods
A systematic literature review was conducted with the search string “Platelet-rich plasma AND Facial rejuvenation” in PubMed and Embase. Clinical studies evaluating the outcomes after PRP-based facial rejuvenation either as monotherapy or in combination with other treatment modalities were included. Studies evaluating wound-healing properties of PRP were excluded. The outcomes included both patient-reported and physician-assessed outcomes. Nonstatistical synthesis of evidence was performed by qualitative assessment. The results are reported by the Synthesis Without Meta-analysis (SWiM) reporting standard.
Results
A total of 36 studies that included a total of 3172 patients were considered for the evidence synthesis. The number of patients in the included studies ranging from 11 to 2005 with a median of 27.5 patients that reflects the challenges in clinically assessing the aesthetic outcomes after PRP-based facial rejuvenation. Among the 36 studies, 17 were observational studies and 18 were interventional studies with 1 being case report PRP was evaluated either alone or in combination with hyaluronic acid, lipofilling, micro-needling technique, and laser-based interventions. Among the studies, 1 study reported the enhanced platelet concentrate in a fibrin matrix to be relatively safe and effective with a maximum benefit observed at 12 weeks suggesting the platelet-rich fibrin matrix may provide desired aesthetic outcomes and it requires further studies to substantiate.
Conclusion
The results suggest very limited clinical evidence, and further clinical studies are warranted to establish the effectiveness of PRP in facial rejuvenation. Furthermore, a consensus for end points used for establishing clinical utility in patients requiring facial rejuvenation is warranted.
Keywords: fibrin, hyaluronic acid, platelet-rich plasma, rejuvenation, wound healing
Introduction
The main purpose of facial rejuvenation is to reverse the aging process either by surgical or noninvasive modalities.1 In recent years, despite the increase in the number of facial rejuvenation cosmetic procedures, there is a decrease in the number of patients opting for surgical facial rejuvenation procedures.2 This emphasizes the importance of noninvasive procedures in improving cosmetic outcomes. Among the noninvasive facial rejuvenation methods, platelet-rich plasma (PRP), which is a platelet concentrate obtained from autologous plasma, has been used in wide dermatological applications including facial rejuvenation.3
PRP concentrate consists of various growth factors that perform diverse functions including but not limited to mitogenic and chemotactic functions. The growth factors in PRP include platelet-derived growth factors (PDGFs), transforming growth factor-β1 (TGF-β1), TGF-β2, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and epithelial growth factor (EGF).3 Although the exact mechanism of action of PRP in bringing about facial rejuvenation is not yet elucidated, it is hypothesized that the growth factors may mediate tissue repair and regulate cellular proliferation and differentiation genes thereby leading to angiogenesis.1 These growth factors facilitate rejuvenation at the cellular level, which may lead to better long-term effects in comparison with invasive procedures.4 The most suitable PRP preparation method is not yet clear. PRP can be prepared by single and double spin systems. The double spin kits have the potential to yield highly concentrated PRP, while single spin designs result in a serum with less platelets than whole blood (platelet poor plasma).5 The use of PRP was further fine-tuned by the introduction of platelet-rich fibrin, which is a modified concentrate of PDGFs extracted from autologous plasma,6 and is prepared by procedure similar to PRP preparation.7
Despite the availability of different extraction procedures for PRP, there is no consensus on the effectiveness of the extraction procedures. Further, clinically PRP is used both as topical and intradermal injectables. This has further complicated the assessment of effectiveness. Due to the lack of high-quality clinical evidence, the usage of PRP is currently governed by the treating physicians’ clinical judgment and discretion. The clinical evidence landscape is also further complicated by the usage of multiple patients- and physician-reported outcomes of qualitative nature. In this context, the main purpose of this systematic literature review is to appraise the available clinical evidence to provide insights and to assist the treating physicians in clinical decision-making.
Methodology
Data Sources and Searches
The systematic literature review was performed according to the Synthesis Without Meta-analysis (SWiM) guidelines. PubMed and Embase were searched from inception to September 14, 2021. The search was performed with the keywords “platelet-rich plasma AND facial rejuvenation.” A literature search was performed in the databases with a general search string to retrieve all possible studies (clinical trials, observational studies). Additionally, search strings relevant to each query were also used to retrieve any study that could have been missed with a general search string.
Study Selection
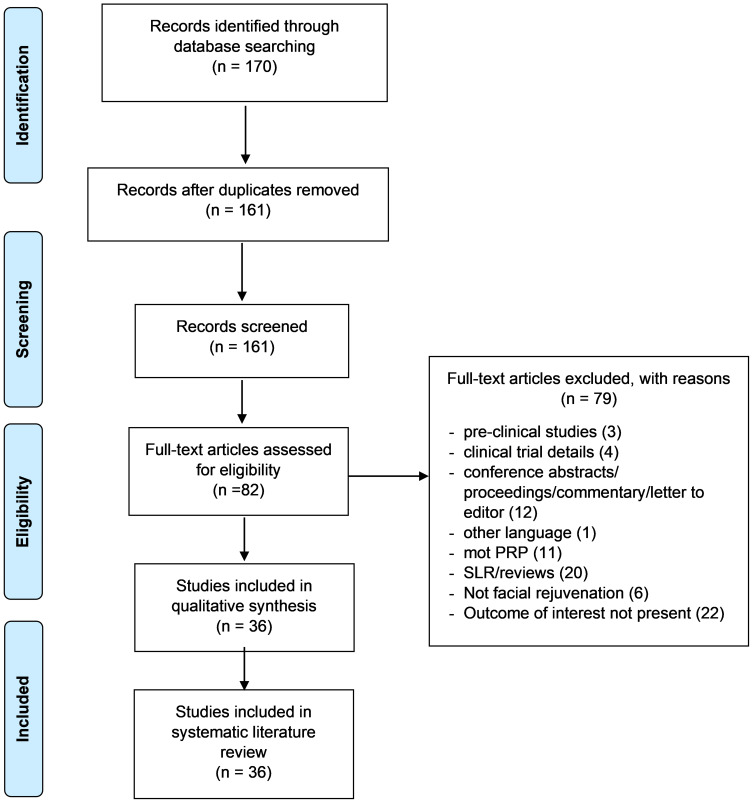
Two reviewers independently screened the abstracts and full texts for eligibility for inclusion. Literature from PubMed and Embase were exported into excel to a single master data document. Reviews, meta-analyses, duplicated publications from the same patient population, in vitro studies, and non-English articles were excluded. Any disagreements regarding study inclusion were resolved by a third independent reviewer. Various study attributes including study design, number of patients, treatment arms, study outcomes, end points, and salient results were extracted for appraisal. The PRISMA guidelines were followed for the development of this review (Figure 1). The qualitative assessment of the included studies was performed by the New Castle Ottawa scale for observational studies and JADAD scale for randomized control studies. The study was prospectively registered on the PROSPERO website (ID:CRD42018112733).
Figure 1.
PRISMA flow chart.
Notes: PRISMA figure adapted from Moher D, Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.60
Results
The systematic search yielded a total of 161 articles that were subjected to preliminary screening based on the title and abstract. A total of 36 studies were included for the evidence synthesis, of which, 18 studies were interventional, whereas 17 were observational studies; the remaining 1 study was a case report. The baseline trial characteristics of the studies included in the analysis are represented in Table 1. Among the 36 studies, 9 (25%) studies used PRP as monotherapy and 23 (80%) studies used PRP in combination with other techniques such as micro-needling, CO2 laser treatment, or hyaluronic acid, growth factors, and Botox. PRP was used for treating acne scars in 17 studies (47.22%), facial aging in 17 studies (48.44%), and loss of tissue volume in 2 studies.
Table 1.
Baseline Characteristics of the Included Studies
| Authors | No. of Patients | Therapy | Condition | Assessment Period | Mean Age/Range (Years) |
|---|---|---|---|---|---|
| Lee 201850 | 31 | PRP monotherapy | Facial aging | 5.7 weeks | 21–80 |
| Cameli 201751 | 12 | PRP monotherapy | Facial aging | 1 month | 45–65 |
| Abuaf 201652 | 20 | PRP monotherapy | Skin aging: sagging, wrinkles, pigmentation | – | 43.65 ± 2.43 |
| Elnehrawy 201753 | 20 | PRP monotherapy | Facial wrinkles [Nasolabial fold (NLFs), crows’ feet wrinkles, and transverse forehead lines] | 8 weeks | 36.90 ± 8.21 |
| Gordon 201654 | 1 | PRP monotherapy | Severe actinic scarring around lips, with generalized loss of lip volume. In addition, the lip and perioral area presented with sun damage | – | 44 |
| Yuksel 201444 | 10 | PRP monotherapy | Skin aging: sagging, wrinkles, pigmentation | 3 months | 50 ± 8 |
| Mehryan 201455 | 10 | PRP monotherapy | Facial aging, infraorbital dark circles, and crow’s feet wrinkles | 3 months | 41.2 |
| Kang 201456 | 20 | PRP monotherapy | Facial aging: wrinkles and darkened skin tone | 3 months | 50.6 ± 3.7 |
| Redaelli 201057 | 23 | PRP monotherapy | Acne scar, forehead scar, wrinkles | 1 month | 47 |
| Sevilla 201558 | 80 | PRP monotherapy | Facial folds (nasolabial folds) | 1 year | 35–65 |
| Ibrahim 201813 | 35 | Combination therapy | Atrophic post-acne scars | 1 year | 24.7 ± 6.8 |
| Min 201815 | 25 | Combination therapy | Acne scars | 28 days | 31.9 |
| Abdel Aal 201816 | 30 | Combination therapy | Acne scars | 6 months | 24.73 |
| El-Domyati 201811 | 24 | Combination therapy | Atrophic acne scars | 3 months | 27.33 ± 4.05 |
| Al Taweel 201821 | 40 | Combination therapy | Atrophic acne scars | 3 months | Group A: 28.65 ± 7.74 |
| Group B: 25.10 ± 6.70 | |||||
| Tenna 201725 | 30 | Combination therapy | Atrophic acne scars | 6 months | 18–52 |
| Asif 201612 | 50 | Combination therapy | Atrophic scars | 3 months | 25.72 |
| Faghihi 201617 | 16 | Combination therapy | Atrophic acne scars: | 4 months | 36.8 |
| Zhu 201322 | 22 | Combination therapy | Acne or acne scar | 3 months | 28 |
| Lee 201118 | 14 | Combination therapy | Acne scars | 4 months | 28.1 |
| El-Domyati 201810 | 24 | Combination therapy | Facial wrinkles and other signs of photoaging | 3 months | 50.87 ± 4.35 |
| Deshmukh 201839 | 40 | Combination therapy | Atrophic acne scars | 2 months | 26.9 in males and 26.7 in females |
| Kar 201720 | 30 | Combination therapy | Atrophic acne scars | 1 month | 25.06 ± 4.44 |
| Willemsen 201826 | 32 | Combination therapy | Loss of skin elasticity and tissue volume | 1 year | 52 ±6.75 |
| Ali 201832 | 63 | Combination therapy | Facial aging | 24 months | Female: 40; male: 45–55 |
| Ibrahim 20179 | 90 | Combination therapy | Atrophic post-acne scars, atrophic post-traumatic scars, post chickenpox scars | 3 months | - |
| Ulusal 201735 | 94 | Combination therapy | Facial aging | - | 53.0 ± 5.6. |
| Hui 201719 | 13 | Combination therapy | Facial aging | 3 months | 42.08 ± 7.37 |
| Kamakura 201536 | 2005 | Combination therapy | Wrinkles and depression in the skin | 3 months | 48.2 |
| Willemsen 201427 | 82 | Combination therapy | Loss of tissue volume, significant ptosis, and the subsequent descent of tissues | 3 months | 35–65 |
| van Dongen 202159 | 28 | Combination therapy | Facial aging | 1 year | - |
| Abdel-Maguid 202137 | 33 | Combination therapy | Atrophic acne scars | - | - |
| Sasaki 201930 | 10 | Combination therapy | Facial aging | 1 year | - |
| El-Taieb 201923 | 75 | Combination therapy | Atrophic acne scars | 1 year | 26 ± 5.1 |
| Rigotti 201628 | 13 | Combination therapy | Facial aging | 3 years | - |
| Alam 201814 | 27 | Combination therapy | Facial aging | 1 year | 46.37 ± 10.88 |
Abbreviation: PRP, platelet-rich plasma.
Except for 7 studies (19.44%), all studies reported a significant improvement after PRP application either as monotherapy or combination therapy in various conditions of facial rejuvenation including facial wrinkles, aging, pigmentation, nasolabial folds, acne scars, and tissue volume.
Administration of PRP
Due to the non-specific nature of autologous PRP, the dose of administration of PRP is not standardized in clinical practice. Since the active component of PRP is the growth promoters released by the platelets upon activation, it may not be feasible to standardize the same. Among the 36 studies, 20 studies reported the volume of PRP administered in the respective cohort of patients, and it ranged from 0.1 to 4 mL (Table 2). Although the purpose and desired outcome in each study were different, the rationale for using a particular volume of platelet concentrate was not explained in any of the studies. Further, platelet counts and leukocyte cell counts were provided only in 7 and 1 studies, respectively, which suggests a lack of a rational approach in determining the PRP administration volume. Because PRP is the first generation of platelet concentrate and other blood-derived platelet concentrates like the platelet-rich fibrin is also used in clinical practice, it is essential to use a surrogate marker for determining the amount of growth factors in the PRP.
Table 2.
Components of Platelet-Rich Plasma Preparation in 36 Included Studies
| Author, Year | Platelets | |||||||
|---|---|---|---|---|---|---|---|---|
| The Method of PRP Preparation | The Volume Injected per Session | Frequency of PRP | Interval of PRP Administration | Platelet Count | Leukocyte Content | Red Blood Cell Content | Activation Yes/No | |
| Lee 201850 | Eight mL of blood was drawn from the antecubital vein. Blood was collected in 3 tubes containing 7.2 mg of EDTA each. Samples were centrifuged at 3200 rpm for 5 minutes, following which 2 layers were formed over the parser gel and a bottom layer of erythrocytes, a middle layer containing the buffy coat of PRP, and a top layer of PPP. The yellow fluid of PPP was gently collected using a syringe and set aside. The buffy coat was then collected and combined with enough PPP to produce 4 mL of PRP. | 0.33 mL at each site (2 mL total) | 1 | – | – | – | – | No |
| Cameli 201751 | Nine mL of venous blood was collected in a sterile kit from each patient. Blood tubes were immediately processed by centrifugation for 8 minutes at 1100 rpm. The PRP obtained remained for 30 to 45 minutes at room temperature to dissolve the platelet aggregates before splitting into 4 one milliliters aliquots. | 4 (1 mL into forehead and crow’s feet area; 2 mL into the cheeks [1mL per side]; and 1 mL into the nasolabial folds) | 3 | 3 sessions of treatment at 1-month intervals | 885–3760 × 106/mL (mean: 1680 × 106/mL) | 0.03 × 10/µL-7.6 × 10/µL, (range 1.9) | - | No |
| Abuaf 201652 | Eight mL blood sample was aspirated from the patient’s peripheral vein in tubes containing sodium citrate anticoagulant. The test tube was centrifuged at 3000 rpm during 5 minutes. Red blood cells were discarded from the plasma at the bottom of the gel. Platelets and white blood cells were pellet on top of the gel and re-suspended in plasma by gently mixing the tube. The 2 mL of cell suspension was called the PRP | 2 mL | 1 | - | - | - | - | - |
| Elnehrawy 201753 | Eighteen milliliters of subject blood was collected into special vacuum tubes pre-equipped with sodium citrate solution as an anticoagulant followed by centrifugation for 7 min at 388 g resulting in a yellow upper part of plasma and a lower red part of erythrocytes. The plasma part was aspirated and placed in another vacuum tube to be centrifuged for the second time at 1376g for 5 min. PPP was first gently aspirated to avoid its mixing with the lower part and the PRP (the residual part) was subsequently aspirated and prepared for activation. | - | 1 | - | - | - | - | Yes |
| Gordon 201654 | Blood was drawn from the cubital fossa area. An amount of 32 mL was harvested and centrifuged at 450 g for 9 minutes using the BTI Endoret system. Visual manual separation of PRP was carried out. The PPP was separated out to be prepped for topical placement. The PRP was extracted and activated with calcium chloride for injection and topical injection. | 0.1 mL at each site | 2 | 2 sessions of treatment at 4-week intervals | - | - | - | Yes |
| Yuksel 201444 | Eight mL of blood was collected from each volunteer. The tube with cell extraction kit and ficoll was centrifuged at 3200 rpm for 8 min following which two layers were formed over the parser gel and erythrocytes remained under it. PPP was the yellow fluid at the top of the tube and collected using a syringe. PRP was the buffy coat over the parser gel and withdrawn with a long cannula. | - | 3 | 2-week intervals | - | - | - | - |
| Mehryan 201455 | Ten mL of each participant’s venous blood was drawn and emptied into 10-mL tubes and centrifuged 1600–1800 g /6 min. A second cycle of centrifugation was performed using 2000 g for 5 min. Then, the bottom 3 milliliters of plasma in the upper chamber was taken out gently. After that, the extracted PRP was transferred into 1-mL insulin syringes and activated by adding 0.1 mL of calcium chloride to 0.9 mL of PRP. | 1.5 mL | 1 | - | - | - | - | Yes |
| Kang 201456 | Twelve mL of blood and the MyCells® kit were used for PRP preparation. PPP was carefully aspirated from the supernatant fluid. A total volume of 1 mL of PRP was finally concentrated. | 1 mL | 1 | 3 treatment sessions at 4-week intervals | - | - | - | No |
| Redaelli 201057 | - | 4 mL | 3 | 3 treatment sessions at 1-month intervals | - | - | - | Yes |
| Ibrahim 201813 | Ten mL of autologous whole blood was collected into tubes containing acid citrate dextrose and centrifuged at 2500 rpm for 10. Then, the PRP was further centrifuged at 3500 rpm for 10 min at room temperature to obtain a platelet rich count. PPP was partly removed and partly used to resuspend the platelets and activated using calcium gluconate. | 0.1 mL at each site | 6 (maximum 6 or till patient satisfaction) | 3-week intervals | - | - | - | Yes |
| Min 201815 | Ten mL of venous blood was drawn in a syringe prefilled with 1.5 mL of anticoagulant solution. The blood was centrifuged at 160 g for 10 minutes. After the first spin, the lower red blood cell portion was discarded, and the supernatant was centrifuged at 400 g for 10 minutes. The resulting pellet of platelets was mixed with 1.5 mL of supernatant, which made 1.5 mL of PRP. | 0.02 mL at each site | 2 | 4-week intervals | - | - | - | Yes |
| Abdel Aal 201816 | Ten mL of blood was collected in special five sterile vacutainer tubes containing an anticoagulant Na Citrate 3.8%. Each tube was centrifuged at 3000 rpm for 7 minutes at room temperature in order to separate red blood cells from plasma, which contains “buffy coat” (white blood cells and platelets). The plasma and buffy coat were gently aspirated from each tube and transferred to a second tube (plain tube without anticoagulant). Further centrifugation was carried out at 4000 rpm for 5 minutes at room temperature, thus obtaining a two-part plasma: the uppermost part, consisting of PPP, and the lower part, consisting of PRP. | 0.1 mL at each site | 2 | 3 to 4-week intervals | - | - | - | Yes |
| El-Domyati 201711 | Ten milliliters of blood was drawn from each patient into conical tubes and centrifuged at 252 g for 10 minutes (1st spin). Precipitation of RBCs occurred at the bottom of the tube and the plasma-containing platelets at the rest of the tube. The plasma was gently transferred to an empty tube and centrifuged again at a higher spin at 1792 g for 5 minutes (2nd spin) to precipitate the platelets at the bottom of the tube, after which sample was divided into 2 parts; the PRP (the lower one-third) and the PPP (the remaining upper portion). | - | 6 | 2-week intervals | - | - | - | Yes |
| Al Taweel 201821 | Fifteen mL of autologous blood was withdrawn from each patient into tube containing 4% sodium citrate. The tubes were centrifuged at 1500 rpm for 6 minutes at room temperature resulting in three basic components, red blood cells (bottom of the tube), PRP (middle of the tube), and PPP (top of the tube). Separated PRP with PPP was collected with the help of pipette in another test tube. This tube was rotated in a second centrifugation at 2500 rpm for 15 minutes. The upper layer containing PPP was discarded, and the lower layer of PRP was loaded in an insulin syringe. | - | 3 | 1-month intervals | - | - | - | - |
| Tenna 201725 | The production of PRP was achieved by the RegenLab THT tube® method. | - | 2 | 6 months | - | - | - | - |
| Asif 201612 | Seventeen milliliters of blood was withdrawn in a 20-mL syringe prefilled with 3 mL of acid-citrate-dextrose anticoagulant. First centrifugation was performed at 293.88 g for 5 min (soft spin). Both buffy coat and plasma layer were taken for further centrifugation and red cell sediments were discarded. Second centrifugation was performed at 690.94 g for 17 min (hard spin) resulting in the formation of PPP above and platelet-rich zone at the bottom, then the PPP was removed and discarded leaving behind a solution of 2 mL PRP. | 2 mL | 3 | 1-month intervals | 1173 × 107 platelets/mL | - | - | Yes |
| Faghihi 201617 | Twenty mL of blood was drawn from the participant’s medial cubital vein and transferred to a tube prefilled with 2.4 mL anticoagulant (citrate phosphate dextrose) solution. The mixture was then centrifuged at 2000 g for 3 min. After the first spin, the lower red blood cell portion was discarded and the supernatant that contained PPP and buffy coat was centrifuged at 5000 g for 5 min. The resulting pellet of platelets (lower portion) was mixed with 4 mL of supernatant. | 0.2 mL at each site | 2 | 1-month intervals | - | - | - | Yes |
| Sevilla 201558 | Acid citrate dextrose vacutainer containing whole blood was centrifuged at 380 g for 15 minutes. The upper layer of plasma was removed in another sterile centrifuge tube leaving behind buffy coat in the middle and packed red blood cells at the bottom. PRP was centrifuged again at 2700 g for 10 minutes. Supernatant containing PPP was then removed and collected in other sterile centrifuge tube for subsequent use. Platelet-rich pellet at the bottom was then suspended in 5 mL of plasma to make platelet-rich suspension. | 2.5 mL | 1 | - | 625 × 106/mL | - | - | - |
| Zhu 201322 | Ten mL of blood was drawn from the participant’s medial cubital vein and collected in a sterile tube containing 1 mL anticoagulant. After measuring the blood platelet concentration, the tubes were centrifuged at 1500 rpm for 10 min. The first spin separated PPP from RBCs and PRP. The PPP, PRP and a few RBCs were aspirated into a new tube, mixed, platelet concentration was detected again, and in the second spin, the tubes were centrifuged at 3000 rpm for 20 min. The upper section consisted of PPP and the PRP collected at the bottom of the tube. | - | 2 | - | 700–1000 × 106/mL | - | - | Yes |
| Lee 201118 | Sixty mL of blood was drawn from the participant’s medial cubital vein. Blood was aliquoted into the anticoagulant over a period of 10 seconds. The anticoagulated blood was then gently pipetted into the separation kit to minimize red blood cell damage. The mixture was then centrifuged at a speed of 3000 rpm for 3 minutes. The blood was separated into PPP, buffy coat, and RBCs. Because PRP is a mixture of buffy coat and plasma, RBCs were extracted from the kit. For further concentration, the separated fraction composed of PPP and buffy coat was centrifuged with the concentration kit for 3 minutes at 4000 rpm. | 0.3 mL per site | 2 | 1-month interval | - | - | - | - |
| El-Domyati 201810 | Ten milliliters of blood was drawn from each patient under sterile condition, collected, and put into conical tubes that contained 2 mL acid citrate dextrose solution, then, the tube was centrifuged at 252 g for 10 minutes (first spin). Precipitation of RBCs happened at the bottom of the tube and the plasma containing platelets at the rest of the tube. The plasma was gently transferred to an empty tube and then re-centrifuged at a higher spin of 1792 g for 5 minutes (second spin) to precipitate the platelets at the bottom of the tube. After the second spin, the sample was divided into two parts; the PRP (the lower one-third) and the PPP (the remaining upper portion). | - | 6 | 2-week intervals | - | - | - | Yes |
| Deshmukh 201839 | Twenty milliliter of blood was withdrawn and initially centrifuged at 800 rpm for 8 minutes (slow spin). The separated plasma was collected along with superficial layer of RBCs and centrifuged again at 1200 rpm for 12 minutes [heavy spin] to obtain a small pellet of platelet concentrate. Upper 2/3rd plasma was collected as PPP, and the bottom pellet was resuspended in the residual lower 1/3rd plasma and used as PRP. | 1.5 to 3 mL | 4 | 4-week intervals | - | - | - | Yes |
| Kar 201720 | Whole blood samples (10 mL) were drawn from patient’s medial cubital vein and transferred to a vial containing an anticoagulant. It was centrifuged at 1500 rpm for 10 min. PPP, PRP, and a few RBCs were aspirated into a new tube and centrifuged at 3000 rpm for 20 min. The middle layer that consists of the PRP was aspirated for topical application. | - | 3 | - | - | - | - | - |
| Willemsen 2018 | - | 3 mL | 1 | - | - | - | - | - |
| Ali 2018 | - | - | 7 | 1-month intervals | - | - | - | - |
| Ibrahim 20179 | Ten to 20 cc of venous blood was collected from the antecubital vein. The whole blood sample was collected into tubes containing sodium citrate (10:1) as an anticoagulant. Then, the citrated whole blood was subjected to two centrifugation steps. The initial centrifugation (“soft” spin) at 1419 g for 7 minutes to separate the plasma and platelets from the red and white cells. The resulting plasma supernatant, which contains the suspended platelets was harvested to a second centrifugation step (“hard” spin) at 2522 g for 5 minutes, leading to separation of the plasma into two portions: PPP and PRP. | 0.1 mL per site | 6 | - | - | - | - | Yes |
| Ulusal 201735 | About 12.5 cc of patients’ blood was collected in a syringe. Then, whole blood was instilled to a PRP kit and centrifuged at 1800 rpm for 20–50 min until all RBCs separate from plasma and the buffy coat became clearly visible. The buffy coat (PRP and PPP) were collected in 5 cc syringes. | - | 11.7% = 1, 20.2% = 2, 31.9% = 3, 5.31% = 4, 12.7% = 5, 7.44% = 6, 2.12% = 7, 8.51% = 8, Mean = 3.6±2 | 3 to 4-week intervals | - | - | - | No |
| Hui 201719 | About 30 mL venous blood were drawn in a sterile syringe containing 600 U heparin calcium. The blood sample was centrifuged at 1200 rpm for 10 minutes. Subsequently, plasma, buffy coat, and 2–3 mm RBCs were collected, mixed, and then centrifuged at 3500 rpm for 5 minutes. About 1/2 volume of PPP was discarded, and the remaining PPP was resuspended to obtain PRP. | 0.1 mL per site (2.2 mL total) | 3 | 3-month intervals | 700–1000 × 106/mL | - | - | Yes |
| Kamakura 201536 | Nine mL of blood was collected and in the first round, centrifugation was performed at 1800 rpm for 10 minutes, after which the top layer of plasma was collected. In the second round, centrifugation was performed at 3200 rpm for 10 minutes, after which the buffy coat and bottom layer of plasma were collected. | Varies with every patient | 1 | - | - | - | - | - |
| Willemsen 201427 | - | 3 mL (1.7 mL each side) | 1 | - | - | - | - | Yes |
| van Dongen 202159 | Prior to the surgery, 62 mL of whole blood was drawn from each subject and 8 mL of anticoagulant citrate dextrose solution A was added to 52 mL of whole blood and prepared following the Arthrex Angel system™ instructions. This resulted in 6 mL of non-activated PRP with a platelet concentration of 4 times the baseline. | Varies with each patient | - | - | - | - | - | Yes |
| Abdel-Maguid 202137 | - | Not mentioned (venous blood 10 mL taken) | 12 sessions (at 2 weeks interval) | 2 weeks interval | 200,000 ± 500,000 cells/mL | - | - | Yes |
| Sasaki 201930 | Fifty-four mL of whole blood was withdrawn from an antecubital arm vein mixing with 6 mL of adenosine-citrate-dextrose. Through floating shelf and double spin centrifuge technology, PPP was used to resuspend the buffy-coat pellet to a final volume suspension of approximately 7 mL PRP. | 7 mL (Final concentration: 6 mL) | 3, 6- and 12-month sessions | - | 1.214 ± 3.9 × 106/µL | - | - | Yes |
| El-Taieb 201923 | Ten mL of blood was obtained and collected in sterile tubes containing sodium citrate 3.8%. Each tube was centrifuged at 2000 rpm for 7 min. The plasma and buffy coat were gently aspirated from each tube and transferred to another tube (plain tube without anticoagulant). Further centrifugation was carried out at 4000 rpm for 7–10 min, thus obtaining a two-part plasma: an uppermost part consisting PPP, and a lower part consisting of PRP. | Not mentioned | - | - | - | - | ||
| Rigotti 201628 | Peripheral blood was collected using blood collection tubes containing 0.5 mL 3.2% sodium citrate solution. Whole blood was centrifuged at 300 g for 5 minutes. After the first centrifugation, the whole plasma above the buffy coat was collected, separating platelets from RBCs and leukocytes (PRP1). PRP1 was centrifuged at 700 g during 17 minutes, after which the platelet pellet was suspended in 300 μL of PPP (new fraction named PRP2). | - | - | - | - | - | - | Yes |
| Alam 201814 | The blood sample was combined with acid citrate dextrose A, and spun in a centrifuge with 2 spins (a hard spin and a soft spin) to separate the PRP from PPP. The remaining PRP was then injected into the cheek of the participant within the next 7 minutes. | 0.1 mL per site | 12 months | - | - | - | - | Yes |
Abbreviations: EDTA, ethylenediamine tetraacetic acid; g, gravitational forces; rpm, revolutions per minutes; PPP, platelet-poor plasma; PRP, platelet-rich plasma; RBCs, red blood cells.
Outcomes and End Point of Interest in PRP Treatment
Apart from the administration dose of PRP, the lack of uniform assessment of efficacy is another important aspect of PRP treatment. The end points for determining efficacy were based on the desired treatment outcome. Among the included studies, the end points of efficacy ranged from the usage of the FACE Q questionnaire to an independent photographic assessment of wrinkles and acne (Table 3). The findings of combination therapy are presented in Table 4. A qualitative assessment of the included studies is provided in Table 5.
Table 3.
Studies with Platelet-Rich Monotherapy for Facial Rejuvenation
| Author, Year | No. of Patients | Condition | Outcomes | Method of Assessment |
|---|---|---|---|---|
| Lee 201850 | 31 | Facial aging and photodamage | FACEQ appearance appraisal scales scores showed a significant increase in satisfaction with appearance and cheeks. | Photography before and after treatment |
| The degree of facial wrinkles using the 5-grade Wrinkle Severity Rating Scale (WSRS). | ||||
| Patient-reported outcomes by FACEQ | ||||
| Cameli 201751 | 12 | Facial aging | - A volume increase of fine wrinkles, improvement in skin gross elasticity, improvement in skin barrier function and capacitance. | Investigators and patients’ assessment by a 4-point scale: excellent, good, sufficient, and insufficient. – instrumental evaluation by transepidermal water loss, Corneometry, Cutometer, Visioscan, and Visioface |
| - Visioscan texture parameters energy, contrast, and variance documented good skin condition, Skin smoothness, scaliness. | ||||
| - A leukocyte population (mainly CD3+) and neutrophils depletion were documented in all the PRP samples | ||||
| Abuaf 201652 | 20 | Skin aging: sagging, wrinkles, pigmentation | The mean density of collagen fibers was greatest on the PRP side. The increase of collagen was statistically significant (P<0.001). | Photography before and after treatment and mean optical density of collagen fibers. |
| Histopathologic evaluation of collagen (by Masson’s trichrome staining) | ||||
| Elnehrawy 201753 | 20 | Facial wrinkles Nasolabial fold, crows’ feet wrinkles, and transverse forehead lines | PRP treatment was significant (P<0.001) as the severity grades decreased. | Wrinkle severity rating scale, Skin homogeneity and texture scale, physician assessment scale, and subject satisfaction scale |
| The improvement between fine, moderate, and deep types of wrinkles (P = 0.002). | ||||
| Two dermatologists compared the digital photos of subjects before and after treatment | ||||
| Improvement in skin homogeneity and texture after PRP treatment (P<0.001). | ||||
| Nasolabial fold was the most vulnerable wrinkle type for improvement with single PRP injection followed by that of crows’ feet wrinkles and the transverse forehead lines (P = 0.013). | ||||
| Gordon 201654 | 1 | Severe actinic scarring around lips, with generalized loss of lip volume. Lip and perioral area presented with sun damage | Significant dermal enhancement, which consisted of dermal thickness, associated with zone A. - rejuvenation and tightening of zone A was also clinically evident. | Digital photography was used to record the preoperative and postoperative clinical presentations |
| Zone B demonstrated a remarkable enhancement in tonicity, thickness, and color, changing from a dull pink to a richer red. | ||||
| There was also a distinct reduction in associated sun damage around zone A | ||||
| Yuksel 201444 | 10 | Skin aging: sagging, wrinkles, pigmentation | There was a statistically significant difference between the grading scale of the patients before and after 3 PRP applications regarding the general appearance, skin firmness, sagging, and wrinkle state (p < 0.001). | Skin aging index |
| Mehryan 201455 | 10 | Facial aging, infraorbital dark circles, and crow’s feet wrinkles | Improvement in infraorbital color homogeneity was statistically significant (P = 0.010) | Digital photography |
| The melanin content of the infraorbital area was measured using the Mexameter probe (MX18) and the epidermal stratum corneum hydration was evaluated using the Corneometer CM825 probe of the Cutometer MPA 580 device | ||||
| Kang 201456 | 20 | Facial aging: wrinkles and darkened skin tone | Infraorbital skin treated with PRP showed significant improvement of wrinkles and skin tone - After PRP treatment, the erythema and melanin indices significantly decreased from 8.52 to 7.37 (P = 0.01) and from 34.42 to 31.86 (P<0.01), respectively after PRP treatment. | photographs were obtained at baseline and the last follow-up. |
| The erythema and melanin indices were also evaluated by spectrophotometry | ||||
| Redaelli 201057 | 23 | Acne scar, forehead scar, wrinkles (canthal periocular and neck) | An average 29% improvement was obtained (variable improvement range, 6–50%). The average score for spider improvement was 4.6, ranging from good to very good. | A special spider improvement score, a photograph score, a patient’s satisfaction score, and a doctor’s satisfaction score |
| Sevilla 201546 | 80 | Facial folds (nasolabial folds) | Physician assessment improvement score with GFC was better than PRP (P<0.001). | Wrinkle severity rating scale (WSRS) (1–5), Global aesthetic improvement scale (GAIS), and atlas photographic grading at rest and at the full smile |
| The objective assessment score by the blinded investigators was significantly better in GFC than PRP (P<0.001). | ||||
| Patient assessment score, for improvement, was better in GFC compared to PRP (P<0.0001). | ||||
| Overall improvement score analysis showed that GFC was significantly superior to PRP (P<0.001). | ||||
| El-Taieb 201923 | 75 | Facial aging with atrophic post-acne scars | PRP treatment in 3 groups with pretreatment scar grade was not statistically significant (P = 0.831). The treated groups showed significant improvement in acne scar grading with a significant decrease in the severity of scars | Photography before and after treatment |
| Safety assessments before and after treatment | ||||
| Acne scar grading according to Goodman and Baron scale |
Abbreviations: GFC, growth factor concentrate; PRP, platelet-rich plasma.
Table 4.
Studies with Platelet-Rich Combination Therapy for Facial Rejuvenation
| Author, Year | No. of Patients | Condition | Treatment | Outcomes | Methods of Assessment |
|---|---|---|---|---|---|
| Ibrahim 201813 | 35 | Atrophic post acne scars | PRP with micro-needling | Significant improvement in acne scars and facial aging. | Goodman and Baron’s global acne scarring system |
| PRP with micro-needling had significantly lesser erythema and edema compared to than micro-needling alone | |||||
| Min 201815 | 25 | Acne scars | PRP with fractional CO2 laser treatment | Significant improvement, Patient satisfaction scores were also significantly higher in PRP and CO2 laser treatment than CO2 treatment alone. | Investigator global assessment scale, Patient satisfaction scores |
| Abdel Aal 201816 | 30 | Acne scars | PRP with fractional CO2 laser treatment | Combination of fractional CO2 laser resurfacing and intradermal PRP was superior to CO2 laser alone for acne scar treatment | Evaluation was carried out through operating physicians, two blinded physicians as well as through patient’s satisfaction. |
| El-Domyati 201811 | 24 | Atrophic acne scars | PRP with micro-needling | Improvement of dermal structures, combination with trichloroacetic acid more effective than PRP | Photography and punch biopsies |
| Al Taweel 201821 | 40 | Atrophic acne scars | PRP combined with carboxytherapy and PRP with CO2 laser treatment | PRP combined with CO2 laser treatment significantly improved acne scars compared with PRP combined with carboxytherapy treatment. | Photographs and patient satisfaction score |
| Tenna 201725 | 30 | Atrophic acne scars | Fat grafts with PRP | Improved scars by increasing skin and subcutaneous tissue thickness | FACE-Q postoperative module was administered to analyze each patient’s satisfaction |
| Asif 201612 | 50 | Atrophic scars | PRP with micro-needling | Significant improvement in atrophic acne scars. Patient satisfaction scores were also significantly different between PRP and micro-needling | Goodman’s qualitative and quantitative scales |
| Faghihi 201617 | 16 | Atrophic acne scars: | PRP with fractional CO2 laser treatment | Clinical improvement of acne scars was higher on the platelet-rich plasma-fractional CO2 laser treated side but the difference was not statistically significant. | Patient satisfaction and the objective evaluation of serial photographs |
| Zhu 201322 | 22 | Acne or acne scar | PRP with fractional CO2 laser treatment | Improvement of >50%, and 91% of the patients were satisfied with the treatment. | Comparing pre- and post-treatment photographs of the patients |
| Lee 201118 | 14 | Acne scars | PRP with fractional CO2 laser treatment | Enhanced recovery of laser-damaged skin and synergistically improved the clinical appearance of acne scarring. | Dermatologists evaluated clinical improvement using a quartile grading scale |
| El-Domyati 201810 | 24 | Facial wrinkles and other signs of photoaging | PRP with micro-needling | Significant clinical improvement after treatment | Photography and punch biopsies |
| Deshmukh 201839 | 40 | Atrophic acne scars | PRP with subcutaneous incision-less surgery (subcision) | Significant improvement was observed in PRP plus subcision compared with subcision alone. | Goodman and Baron’s qualitative acne scar grading system |
| Kar 201720 | 30 | Atrophic acne scars | PRP with fractional CO2 laser treatment | Significant improvement on both sides of the face. | Goodman and Barons quantitative global acne scar grading system |
| Willemsen 201826 | 32 | Loss of skin elasticity and tissue volume | PRP with lipofilling | No improvement in the outcome about skin elasticity, graft volume maintenance, and patient satisfaction, however, the recovery time was significantly reduced due to addition of PRP | Changes in skin elasticity, volumetric changes of the nasolabial fold, recovery time, and patient satisfaction |
| Ali 201832 | 63 | Facial aging | PRP with thread-lifting | Highly augmented results are observed when combined with PRP | Patient satisfaction |
| Ibrahim 20179 | 90 | Atrophic post-acne scars, atrophic post-traumatic scars, post chickenpox scars | PRP with micro-needling | Significant improvement in the appearance of atrophic scars | Clinical evaluation scale score |
| Ulusal 201735 | 94 | Facial aging | PRP with hyaluronic acid | Significant improvement was observed regarding the general appearance | 5-grade wrinkle severity rating scale and by patient satisfaction scores. |
| Hui 201719 | 13 | Facial aging | PRP with fractional CO2 laser treatment | Subjective scores of facial wrinkles, skin texture, and skin elasticity were higher | Satisfaction scores, dermatologists’ double-blind evaluation and the VISIA skin analysis system |
| Kamakura 201536 | 2005 | Wrinkles and depression in the skin | PRP with growth factor | Level of patient satisfaction was 97.3% and the level of investigator satisfaction was 98.4%. | Global Aesthetic Improvement Scale, Wrinkle Severity Rating Scale |
| Willemsen 201427 | 82 | Loss of tissue volume, significant ptosis, and the subsequent descent of tissues | PRP with fat grafting | Significant drop in the number of days needed to recover. | Questionnaire based evaluation |
| van Dongen 202159 | 28 | Facial aging | PRP supplemented lipofilling with stromal vascular fraction | In comparison to PRP-supplemented lipofilling, PRP-supplemented lipofilling combined with stromal vascular fraction does not improve facial skin quality or patient satisfaction. | Changes in skin elasticity and transepidermal water loss, changes in skin-aging-related features, ie, superficial spots, wrinkles, skin texture, pores, vascularity, and pigmentation, as well as patient satisfaction (FACE-Q), recovery, and number of complications |
| Abdel-Maguid 202137 | 33 | Atrophic acne scars. | PRP with fractional CO2 laser | Better and faster improvement, dermal collagen and procollagen type 1 was increased after PRP therapy | Clinical assessment and Skin biopsies |
| Sasaki 201930 | 10 | Facial aging | PRP with fat grafting | Improvement in the average percent change in mean volume assessments at the fat/PRP sites from baseline values | 3D Vectra Analysis, VISIA, and Cortex facial skin analyses |
| El-Taieb 201923 | 75 | Atrophic acne scars | PRP with erbium laser | Improvement with combined treatment was better than that with erbium-YAG laser or platelet-rich plasma alone | Acne scar grading, photography, and subjective evaluation. |
| Rigotti 201628 | 13 | Facial aging | PRP with fat grafting | Presence of more pronounced inflammatory infiltrates and a greater vascular reactivity, increasing in vascular permeability and a certain reactivity of the nervous component. | Clinical observation, optical and electron microscopy |
| Alam 201814 | 27 | Facial aging | PRP with micro-needling | Improved the visual appearance in individuals with photoaged skin, fine and coarse texture improved significantly. | Photoaging scores, self-assessment scores of improvements on a 5-point scale |
Abbreviations: CO2, carbon-dioxide; PRP, platelet-rich plasma.
Table 5.
Evidence Grading of the Studies Included in the Literature Review
| Author | No. of Patients | Therapy | Quality Assessment | |
|---|---|---|---|---|
| Newcastle-Ottawa Scale (NOS) | JADAD Scale | |||
| Lee 201850 | 31 | PRP monotherapy | 4 | - |
| Cameli 201751 | 12 | PRP monotherapy | 4 | - |
| Abuaf 201652 | 20 | PRP monotherapy | 8 | - |
| Elnehrawy 201753 | 20 | PRP monotherapy | 3 | - |
| Gordon 201654 | 1 | PRP monotherapy | - | - |
| Yuksel 201444 | 10 | PRP monotherapy | 4 | - |
| Mehryan 201455 | 10 | PRP monotherapy | 4 | - |
| Kang 201456 | 20 | PRP monotherapy | - | 2 |
| Redaelli 201057 | 23 | PRP monotherapy | 4 | - |
| Sevilla 201558 | 80 | PRP monotherapy | 5 | - |
| Ibrahim 201813 | 35 | Combination therapy | 6 | - |
| Min 201815 | 25 | Combination therapy | 4 | - |
| Abdel Aal 201816 | 30 | Combination therapy | 6 | - |
| El-Domyati 201811 | 24 | Combination therapy | 6 | - |
| Al Taweel 201821 | 40 | Combination therapy | 5 | - |
| Tenna 201725 | 30 | Combination therapy | 5 | - |
| Asif 201612 | 50 | Combination therapy | 5 | |
| Faghihi 201617 | 16 | Combination therapy | 6 | |
| Zhu 201322 | 22 | Combination therapy | 5 | |
| Lee 201118 | 14 | Combination therapy | 5 | |
| El-Domyati 201810 | 24 | Combination therapy | 6 | - |
| Deshmukh 201839 | 40 | Combination therapy | 6 | - |
| Kar 201720 | 30 | Combination therapy | 4 | - |
| Willemsen 201826 | 32 | Combination therapy | - | 5 |
| Ali 201832 | 63 | Combination therapy | 4 | - |
| Ibrahim 20179 | 90 | Combination therapy | 5 | - |
| Ulusal 201735 | 94 | Combination therapy | 5 | - |
| Hui 201719 | 13 | Combination therapy | 6 | - |
| Kamakura 201536 | 2005 | Combination therapy | 4 | - |
| Willemsen 201427 | 82 | Combination therapy | 5 | - |
| van Dongen 202159 | 28 | Combination therapy | - | 2 |
| Abdel-Maguid 202137 | 33 | Combination therapy | - | 2 |
| Sasaki 201930 | 10 | Combination therapy | - | 1 |
| El-Taieb 201923 | 75 | Combination therapy | - | 2 |
| Rigotti 201628 | 13 | Combination therapy | - | 2 |
| Alam 201814 | 27 | Combination therapy | - | 2 |
Note: OS was used for assessing the quality of the non-randomized study; the JADAD scale is used for assessing randomized trials.
Application of PRP in Facial Rejuvenation
Among the studies that used PRP monotherapy, the mode of administration of PRP was an intradermal injection in all the studies except for one study in which it was used as a topical application. All studies reported significant improvement after PRP application although there is a lack of uniformity in assessment and reporting of the outcome measures. This makes it difficult to draw definitive conclusions based on the results reported in these studies. Results in 7 studies were assessed based on the photographs obtained at baseline and follow-up (Table 3).
PRP has several benefits in facial rejuvenation and due to its enormous benefits, it is being used in several conditions such as atrophic acne scars, pigmentation, facial wrinkles, facial folds, loss of elasticity, and loss of tissue volume.8
PRP with Micro-Needling Applications
PRP in conjunction with the micro-needling procedure was explored in 5 studies reporting significant effects in treating acne scars and facial aging.9–13 Ibrahim et al compared the efficacy and safety of micro-needling with PRP and micro-needling alone in 35 patients with atrophic acne scars. Although significant improvement was observed after both the treatment modalities there was no significant difference between the 2 modalities as assessed by Goodman and Baron’s global acne scarring system. However, regarding safety, PRP with micro-needling had significantly lesser erythema and edema than micro-needling alone, which could plausibly be attributed to the healing and tissue repair properties of PRP.13 A randomized study evaluated the effects of micro-needling and PRP both, alone and in combination, in 90 patients. There was a statistically significant improvement in the PRP and micro-needling group compared with PRP and micro-needling alone. Moreover, the patient satisfaction was also statistically significant in the combination group compared with PRP and micro-needling groups alone. The authors suggest that the combination therapy is efficacious and safe in treating atrophic scars of different etiologies with minimal downtime and affordable cost.9 Asif et al assessed the combination efficacy of PRP with micro-needling in managing atrophic acne scars in 50 patients. Goodman’s qualitative and quantitative scales showed significant improvement by micro-needling alone and a combination of PRP and micro-needling. There was a significant difference between the 2 treatments modalities. Patient satisfaction scores were also significantly different between PRP and micro-needling and micro-needling alone. The authors propose a hypothesis that in combination therapy of micro-needling and PRP, the damage caused due to micro-needling is cured by the healing mechanism of the activated platelets, cytokines, and the growth factors from PRP.12 Another study compared a combination of PRP and micro-needling with micro-needling and trichloroacetic acid (TCA) peeling in 24 subjects with atrophic acne scars. Both the combinations showed significant improvement in acne scars, however, there was no significant difference by histometric evaluation of epidermal thickness. Although both the combination techniques were efficacious, micro-needling with TCA was preferred by the subjects over micro-needling with PRP for acne scars.11 A similar study of combination techniques for photoaging and facial wrinkles showed a significant increase in evaluation scores of PRP and micro-needling compared with micro-needling and TCA. Additionally, histological results also revealed improvement of dermal structures in micro-needling and PRP combination compared with micro-needling and TCA.10 In a recent randomized trial, Alam et al showed that PRP therapy improved the visual appearance in individuals with photoaged skin, the results showed that both fine and coarse texture improved significantly more with a single treatment of PRP than the individuals treated with normal saline.14
PRP with Laser Applications
Laser treatment with PRP has been reported to produce superior results in treating acne scars and facial aging. Seven studies reported the efficacy of CO2 laser treatment in conjunction with PRP. Min et al evaluated the molecular mechanism of increased clinical efficacy of PRP combined with CO2 laser treatment in 25 subjects with acne scars. PRP in combination with CO2 laser treatment showed significant improvement as assessed by the investigator global assessment scale. Patient satisfaction scores were also significantly higher in PRP and CO2 laser treatment than CO2 treatment alone. Immunohistochemical results suggest that there was increased TGFβ1 and C-myc levels, and collagen 1 expression was significantly higher in combination treatment suggestive of the clinical efficacy of the combination therapy.15 Similar results were reported in other studies,16–19 Kar et al reported significant improvement on both sides of the face with combination treatment of fractional CO2 laser treatment along with PRP and treatment with CO2 for atrophic acne scars as assessed by Goodman and Barons quantitative global acne scar grading system, but there was no significant difference between right and left sides of the face. The authors conclude that in the PRP treatment there is a significant reduction in the downtime and the inflammation caused by laser treatment.20 AI Taweel et al investigated the efficacy of PRP combined with carboxytherapy compared with PRP with fractional CO2 laser treatment in 40 patients with atrophic acne scars. PRP combined with CO2 laser treatment significantly improved acne scars compared with PRP combined with carboxytherapy treatment. However, it was observed that carboxytherapy had fewer side effects than CO2 treatment and could be a beneficial treatment option for acne scars.21 Zhu et al evaluated the efficacy and safety of erbium fractional laser treatment combined with PRP in treating acne scars. Combination therapy was given to 22 subjects and 90.9% of the patients showed an improvement of >50%, and 91% of the patients were satisfied with the treatment. The assessment was performed by comparing pre- and post-treatment photographs of the patients. The results suggest the efficacy and safety of the combination therapy in producing synergic benefits in the treatment of acne scars.22 Another recent clinical trial conducted by El-Taieb showed that combination treatment of PRP with the addition of erbium laser showed superior efficacy as compared with 12 sessions of single plasma-rich therapy over the same period. The clinical trial participants were assessed by acne scar grading, photography, and subjective evaluation.23
PRP with Fat Grafting/Lipofilling Applications
Fat grafting or lipofilling has gained importance as a surgical alternative due to its long-term results in treating acne scars.24 A study comparing the efficacy of nano fat and PRP with or without fractional CO2 laser in treating atrophic scars revealed that both the combination treatments were efficacious in improving scars by increasing skin and subcutaneous tissue thickness with no significant difference between the 2 types of combinations.25 A double-blind, placebo-controlled, randomized trial on the efficacy of PRP with lipofilling in patients with loss of skin elasticity and tissue volume revealed no improvement in the outcome about skin elasticity, graft volume maintenance, and patient satisfaction, however, the recovery time was significantly reduced due to addition of PRP.26 A retrospective study evaluated the effects of PRP on the recovery time and aesthetic outcome in treating loss of tissue volume, significant ptosis, and the subsequent descent of tissues. The patients were divided into 4 groups consisting of fat grafts only, fat grafting and PRP, minimal access cranial suspension (MACS) lift and fat grafting, and MACS-lift, fat grafting, and PRP. It was observed that there was a significant drop in the recovery days after the addition of PRP to lipofilling and additionally the aesthetic outcome was also better in fat grafting and PRP group. The results of the assessment were based on pre- and post-photographs evaluated by plastic surgeons.27 A study by Rigotti et al28 demonstrated that treatment with expanded adipose-derived stem cells or stromal vascular fraction (SVF) enriched fat modified the pattern of the subcutaneous dermis and thus producing a skin rejuvenation effect. SVF represents a rich reservoir of regenerative precursor cells.29 However, the PRP therapy did not have any significant advantages in producing any skin rejuvenation effect over the adipose-derived stem cells or enriched fat. However, another study conducted by Van Dongen showed that PRP supplemented lipofilling did not improve the facial skin quality or patient satisfaction in a population consisting of healthy individuals. Concerning the safety profile, the investigators concluded that PRP supplemented lipofilling supplemented with a SVF could be considered a safe procedure. Additionally, in a study conducted by Sasaki et al, fat grafting techniques combined with PRP therapy showed improvement in the average percent change in mean volume assessments at the fat/PRP sites from baseline values, as profiled by 3D Vectra Analysis, demonstrated a higher, but statistically nonsignificant value over 1 year than the percent value changes at the fat/normal saline sites in the opposing face or hand.30 The large variation in the outcomes of fat grafting when PRP was added might be attributed to the presence or absence of inflammatory leukocytes or reactive oxygen species in erythrocytes.31
PRP with Thread-Lifting Applications
Ali et al evaluated the 2-year outcome of thread lifting with absorbable barbed polydioxanone (PDO) threads with or without PRP in the treatment of facial aging. Thread lifting had significant effects on skin lifting and patient satisfaction. Significant results were also seen with thread lifting combined with PRP.32
PRP with Hyaluronic Acid Applications
Research studies have shown that by combining hyaluronic acid (HA) and PRP, the synergic effect of the treatment would be associated with the enhanced function of dermal fibrosis, thus providing better outcomes in facial rejuvenation.33,34 Ulusal et al studied the effect of HA with PRP in facial aging. Significant improvement was observed regarding the general appearance as graded by the physicians on the 5-grade wrinkle severity rating scale and by patient satisfaction scores. However, there was no significant difference in the aesthetic improvement scores provided by patients and physicians after treatment.35
PRP with Growth Factor Applications
A combination of growth factors with PRP has shown to be an effective treatment in facial rejuvenation. Kamakura et al studied the efficacy and safety of PRP with growth factors for treating wrinkles and depressed areas of the skin of 2005 patients. Patient and investigator satisfaction was 97.3% and 98.4%, respectively, as assessed by the global aesthetic improvement scale. Ratings by wrinkle severity rating scale also showed improvement in the grade with PRP and growth factor combination suggesting that this is an effective and safe treatment of wrinkles with minimal complications.36 A recent prospective clinical trial conducted by Abdel-Maguid et al in a head-to-head trial to determine the efficacy of stem cell-conditioned medium after fractional CO2 laser (FCL) compared with combined FCL and PRP, the results showed better and faster improvement with FCL plus PRP than FCL. In 33 patients dermal collagen and procollagen type 1 was increased after PRP therapy in 3 sessions.37
PRP with Subcision Applications
Subcutaneous incision-less surgery (subcision) is also a widely used technique for treating acne scars.38 Deshmukh et al studied the effect of subcision adjunct to PRP in treating atrophic acne scars in 44 patients. Significant improvement was observed in PRP plus subcision compared with subcision alone. Goodman and Baron’s qualitative acne scar grading system revealed statistically significant improvement with PRP plus subcision compared with subcision alone (32.08% vs 8.33%, P = 0.04). Patients’ objective scores also increased significantly in combination therapy suggestive of the synergic effect of the treatment. However, post-inflammatory pigmentation was reported as a side effect of the treatment.39
Implementation of the Standard Classification System for PRP
The optimal PRP platelet concentration is unclear; however, the current methods40,41 by which PRP is prepared reported >1×106 platelets/µL having 300% to 700% enrichment.42 In most cosmetic procedures for dermatological conditions such as acne scar, facial rejuvenation, and graft survival in hair transplantation, higher concentrations of platelet-derived growth factors are appropriate to use for obtaining better results.43,44 Even though there is enormous evidence of the benefits of PRP in facial rejuvenation, conflicting results are reported in the literature. Moreover, there is no standard reporting system for PRP to deduce and replicate the results of the published literature. PRP preparation includes various steps such as platelet concentration, white blood cells, red blood cells, and use of activators. Details of these along with the volume delivered are not reported in all the published literature evaluating the efficacy and safety of PRP. Although there are a few recommendations and classifications proposed for the adoption of reporting important characteristics of the PRP preparation in the scientific literature,45–47 as evident from our systematic review, none of these are implemented at large. Additionally, following a standard method to measure the outcomes is also an important factor for clinicians and researchers for interpretation and reproduction of the published literature to obtain the optimal results of PRP as the need for its use continues to grow in facial rejuvenation. Similar interpretations were made by Hausauer and Humphrey in a two-part systematic review and expert analysis where they performed an in-depth analysis of PRP in hair restoration, soft-tissue remodeling, resurfacing, and rejuvenation. They found a wide variation in study methodologies with different protocols and differences in outcomes measured in different studies for indication of PRP in facial rejuvenation.48,49 Hence, in the future, it is of utmost importance to standardize the methods of PRP preparation and optimize treatment methods in order to further improve its usefulness.
There are a few limitations in this systematic review, firstly most of the studies included were of observational design with very few being RCTs. The reporting of the PRP preparation was improper and a few of the studies did not include a standard outcome measurement tool and hence statistical synthesis of the outcomes could not be performed.
Conclusion
PRP has beneficial effects on facial rejuvenation either alone or in combination with other treatment modalities such as laser treatment, fat grafting, subcision, growth factors, and thread lifting. This systematic literature review reveals the high potential of PRP in generating growth factors combined with platelets that render healing effect is essentially a minimally invasive technique for the treatment of skin acne scars and aging. However, due to the lack of uniformity in reporting PRP preparation and employing standard valid assessment methods to report outcomes, there is paucity in proving the therapeutic benefits of PRP using meta-analysis with the available data. Large scale, randomized, control studies employing standard PRP preparation protocols and standard reporting systems are warranted to further validate the use of PRP in facial rejuvenation.
Acknowledgments
Medical writing assistance was provided by Dr. Kaushik Subramanian and Dr. Amit, Indegene Pvt Ltd, Bengaluru, India.
Funding Statement
This study did not receive any specific grant from any funding agency or non-profit organization.
Ethics Approval and Informed Consent
Since this is a systematic literature review, the studies included had obtained informed consent as well as approval from their respective ethics committee.
Consent for Publication
All the authors have given their consent for the publication of this study.
Disclosure
The authors have no conflicts of interest in this work.
References
- 1.Goel S. Facial rejuvenation: an evolving world. Off Sci J Delhi Ophthalmol Soc. 2016;27(2):132–135. doi: 10.7869/djo.226 [DOI] [Google Scholar]
- 2.American SOciety of plastic surgeons. 2018 plastic surgery statistics. Available from: https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf. Accessed September 14, 2021.
- 3.Lei X, Xu P, Cheng B. Problems and solutions for platelet-rich plasma in facial rejuvenation: a systematic review. Aesth Plast Surg. 2019;43(2):457–469. doi: 10.1007/s00266-018-1256-1 [DOI] [PubMed] [Google Scholar]
- 4.Motosko CC, Khouri KS, Poudrier G, Sinno S, Hazen A. Evaluating platelet-rich therapy for facial aesthetics and alopecia: a critical review of the literature. Plast Reconstr Surg. 2018;141(5):1115–1123. doi: 10.1097/PRS.0000000000004279 [DOI] [PubMed] [Google Scholar]
- 5.El-Husseiny RM, Saleh HM, Moustafa AA, Salem SA. Comparison between single- versus double-spin prepared platelet-rich plasma injection in treatment of female pattern hair loss: clinical effect and relation to vascular endothelial growth factor. Arch Dermatol Res. 2021;313(7):557–566. doi: 10.1007/s00403-020-02134-6 [DOI] [PubMed] [Google Scholar]
- 6.Sclafani AP. Platelet-rich fibrin matrix for improvement of deep nasolabial folds. J Cosmet Dermatol. 2010;9(1):66–71. doi: 10.1111/j.1473-2165.2010.00486.x [DOI] [PubMed] [Google Scholar]
- 7.Reksodiputro MH, Harahap AR, Setiawan L, Yosia M. A modified preparation method of ideal platelet-rich fibrin matrix from whole blood. Front Med (Lausanne). 2021;8:724488. doi: 10.3389/fmed.2021.724488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33(5):381–394. doi: 10.2165/00007256-200333050-00004 [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim ZA, El-Ashmawy AA, Shora OA. Therapeutic effect of microneedling and autologous platelet-rich plasma in the treatment of atrophic scars: a randomized study. J Cosmet Dermatol. 2017;16(3):388–399. doi: 10.1111/jocd.12356 [DOI] [PubMed] [Google Scholar]
- 10.El-Domyati M, Abdel-Wahab H, Hossam A. Combining microneedling with other minimally invasive procedures for facial rejuvenation: a split-face comparative study. Int J Dermatol. 2018;57:1324–1334. doi: 10.1111/ijd.14172 [DOI] [PubMed] [Google Scholar]
- 11.El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison. J Cosmet Dermatol. 2018;17(1):73–83. doi: 10.1111/jocd.12459 [DOI] [PubMed] [Google Scholar]
- 12.Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15(4):434–443. doi: 10.1111/jocd.12207 [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatol Treat. 2018;29(3):281–286. doi: 10.1080/09546634.2017.1365111 [DOI] [PubMed] [Google Scholar]
- 14.Alam M, Hughart R, Champlain A, et al. Effect of platelet-rich plasma injection for rejuvenation of photoaged facial skin: a randomized clinical trial. JAMA Dermatol. 2018;154(12):1447. doi: 10.1001/jamadermatol.2018.3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min S, Yoon JY, Park SY, Moon J, Kwon HH, Suh DH. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation: PRP WITH FRACTIONAL CO 2 LASER FOR ACNE SCAR. Lasers Surg Med. 2018;50(4):302–310. doi: 10.1002/lsm.22776 [DOI] [PubMed] [Google Scholar]
- 16.Abdel Aal AM, Ibrahim IM, Sami NA, Abdel Kareem IM. Evaluation of autologous platelet-rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther. 2018;20(2):106–113. doi: 10.1080/14764172.2017.1368667 [DOI] [PubMed] [Google Scholar]
- 17.Faghihi G, Keyvan S, Asilian A, Nouraei S, Behfar S, Nilforoushzadeh M. Efficacy of autologous platelet-rich plasma combined with fractional ablative carbon dioxide resurfacing laser in treatment of facial atrophic acne scars: a split-face randomized clinical trial. Indian J Dermatol Venereol Leprol. 2016;82(2):162. doi: 10.4103/0378-6323.174378 [DOI] [PubMed] [Google Scholar]
- 18.Lee JW, Kim BJ, Kim MN, Mun SK. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37(7):931–938. doi: 10.1111/j.1524-4725.2011.01999.x [DOI] [PubMed] [Google Scholar]
- 19.Hui Q, Chang P, Guo B, Zhang Y, Tao K. The clinical efficacy of autologous platelet-rich plasma combined with ultra-pulsed fractional CO2 laser therapy for facial rejuvenation. Rejuvenation Res. 2017;20(1):25–31. doi: 10.1089/rej.2016.1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kar BR, Raj C. Fractional CO2 laser vs fractional CO2 with topical platelet-rich plasma in the treatment of acne scars: a split-face comparison trial. J Cutan Aesthet Surg. 2017;10(3):136–144. doi: 10.4103/JCAS.JCAS_99_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Taweel A, Al Refae A, Hamed AM, Kamal AM. Comparative study of the efficacy of platelet-rich plasma combined with carboxytherapy vs its use with fractional carbon dioxide laser in atrophic acne scars. J Cosmet Dermatol. 2018. doi: 10.1111/jocd.12561 [DOI] [PubMed] [Google Scholar]
- 22.Zhu J-T, Xuan M, Zhang Y-N, et al. The efficacy of autologous platelet-rich plasma combined with erbium fractional laser therapy for facial acne scars or acne. Mol Med Rep. 2013;8(1):233–237. doi: 10.3892/mmr.2013.1455 [DOI] [PubMed] [Google Scholar]
- 23.El-Taieb MA, Ibrahim HM, Hegazy EM, Ibrahim AK, Gamal AM, Nada EA. Fractional erbium-YAG laser and platelet-rich plasma as single or combined treatment for atrophic acne scars: a randomized clinical trial. Dermatol Ther (Heidelb). 2019;9(4):707–717. doi: 10.1007/s13555-019-00318-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barret JP, Sarobe N, Grande N, Vila D, Palacin JM. Maximizing results for lipofilling in facial reconstruction. Clin Plast Surg. 2009;36(3):487–492. doi: 10.1016/j.cps.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 25.Tenna S, Cogliandro A, Barone M, et al. Comparative study using autologous fat grafts plus platelet-rich plasma with or without fractional CO2 laser resurfacing in treatment of acne scars: analysis of outcomes and satisfaction with FACE-Q. Aesthetic Plast Surg. 2017;41(3):661–666. doi: 10.1007/s00266-017-0777-3 [DOI] [PubMed] [Google Scholar]
- 26.Willemsen JCN, Van Dongen J, Spiekman M, et al. The addition of platelet-rich plasma to facial lipofilling: a double-blind, placebo-controlled, randomized trial. Plast Reconstr Surg. 2018;141(2):331–343. doi: 10.1097/PRS.0000000000004081 [DOI] [PubMed] [Google Scholar]
- 27.Willemsen JCN, van der Lei B, Vermeulen KM, Stevens HP. The effects of platelet-rich plasma on recovery time and aesthetic outcome in facial rejuvenation: preliminary retrospective observations. Aesthetic Plast Surg. 2014;38(5):1057–1063. doi: 10.1007/s00266-014-0361-z [DOI] [PubMed] [Google Scholar]
- 28.Rigotti G, Charles-de-sá L, Gontijo-de-amorim NF, et al. Expanded stem cells, stromal-vascular fraction, and platelet-rich plasma enriched fat: comparing results of different facial rejuvenation approaches in a clinical trial. Aesthet Surg J. 2016;36(3):261–270. doi: 10.1093/asj/sjv231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gir P, Oni G, Brown SA, Mojallal A, Rohrich RJ. Human adipose stem cells: current clinical applications. Plast Reconstr Surg. 2012;129(6):1277–1290. doi: 10.1097/PRS.0b013e31824ecae6 [DOI] [PubMed] [Google Scholar]
- 30.Sasaki GH. A preliminary clinical trial comparing split treatments to the face and hand with autologous fat grafting and Platelet-Rich Plasma (PRP): a 3D, IRB-approved study. Aesthet Surg J. 2019;39(6):675–686. doi: 10.1093/asj/sjy254 [DOI] [PubMed] [Google Scholar]
- 31.Modarressi A. Platelet Rich Plasma (PRP) improves fat grafting outcomes. World J Plast Surg. 2013;2(1):6–13. [PMC free article] [PubMed] [Google Scholar]
- 32.Ali YH. Two years’ outcome of thread lifting with absorbable barbed PDO threads: innovative score for objective and subjective assessment. J Cosmet Laser Ther. 2018;20(1):41–49. doi: 10.1080/14764172.2017.1368562 [DOI] [PubMed] [Google Scholar]
- 33.Anitua E, Sanchez M, De la Fuente M, Zalduendo MM, Orive G. Plasma rich in growth factors (PRGF-Endoret) stimulates tendon and synovial fibroblasts migration and improves the biological properties of hyaluronic acid. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1657–1665. doi: 10.1007/s00167-011-1697-4 [DOI] [PubMed] [Google Scholar]
- 34.Anitua E, Sánchez M, Nurden AT, et al. Platelet-released growth factors enhance the secretion of hyaluronic acid and induce hepatocyte growth factor production by synovial fibroblasts from arthritic patients. Rheumatology (Oxford). 2007;46(12):1769–1772. doi: 10.1093/rheumatology/kem234 [DOI] [PubMed] [Google Scholar]
- 35.Ulusal BG. Platelet-rich plasma and hyaluronic acid - an efficient biostimulation method for face rejuvenation. J Cosmet Dermatol. 2017;16(1):112–119. doi: 10.1111/jocd.12271 [DOI] [PubMed] [Google Scholar]
- 36.Kamakura T, Kataoka J, Maeda K, et al. Platelet-rich plasma with basic fibroblast growth factor for treatment of wrinkles and depressed areas of the skin. Plast Reconstr Surg. 2015;136(5):931–939. doi: 10.1097/PRS.0000000000001705 [DOI] [PubMed] [Google Scholar]
- 37.Abdel-Maguid EM, Awad SM, Hassan YS, El-Mokhtar MA, EL-Deek HE, Mekkawy MM. Efficacy of stem cell-conditioned medium vs. platelet-rich plasma as an adjuvant to ablative fractional CO 2 laser resurfacing for atrophic post-acne scars: a split-face clinical trial. J Dermatol Treat. 2021;32(2):242–249. doi: 10.1080/09546634.2019.1630701 [DOI] [PubMed] [Google Scholar]
- 38.Chandrashekar B, Nandini A. Acne scar subcision. J Cutan Aesthet Surg. 2010;3(2):125–126. doi: 10.4103/0974-2077.69029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deshmukh NS, Belgaumkar VA. Platelet-rich plasma augments subcision in atrophic acne scars: a split-face comparative study. Dermatol Surg. 2018. doi: 10.1097/DSS.0000000000001614 [DOI] [PubMed] [Google Scholar]
- 40.Weibrich G, Kleis WKG, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17(2):184–190. [PubMed] [Google Scholar]
- 41.Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58(3):297–300;discussion 300–301. doi: 10.1016/s0278-2391(00)90058-2 [DOI] [PubMed] [Google Scholar]
- 42.Li ZJ, Choi H-I, Choi D-K, et al. Autologous platelet-rich plasma: a potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38(7):1040–1046. doi: 10.1111/j.1524-4725.2012.02394.x [DOI] [PubMed] [Google Scholar]
- 43.Uebel CO, da Silva JB, Cantarelli D, Martins P. The role of platelet plasma growth factors in male pattern baldness surgery. Plast Reconstr Surg. 2006;118(6):1458–1466; discussion 1467. doi: 10.1097/01.prs.0000239560.29172.33 [DOI] [PubMed] [Google Scholar]
- 44.Yuksel EP, Sahin G, Aydin F, Senturk N, Turanli AY. Evaluation of effects of platelet-rich plasma on human facial skin. J Cosmet Laser Ther. 2014;16(5):206–208. doi: 10.3109/14764172.2014.949274 [DOI] [PubMed] [Google Scholar]
- 45.Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012;13(7):1185–1195. doi: 10.2174/138920112800624283 [DOI] [PubMed] [Google Scholar]
- 46.Mautner K, Malanga GA, Smith J, et al. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. PM&R. 2015;7(4):S53–S59. doi: 10.1016/j.pmrj.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 47.DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28(7):998–1009. doi: 10.1016/j.arthro.2012.04.148 [DOI] [PubMed] [Google Scholar]
- 48.Hausauer AK, Humphrey S. The physician’s guide to platelet-rich plasma in dermatologic surgery part I: definitions, mechanisms of action, and technical specifications. Dermatol Surg. 2020;46(3):348–357. doi: 10.1097/DSS.0000000000002147 [DOI] [PubMed] [Google Scholar]
- 49.Hausauer AK, Humphrey S. The physician’s guide to platelet-rich plasma in dermatologic surgery part II: clinical evidence. Dermatol Surg. 2020;46(4):447–456. doi: 10.1097/DSS.0000000000002148 [DOI] [PubMed] [Google Scholar]
- 50.Lee Z-H, Sinno S, Poudrier G, et al. Platelet rich plasma for photodamaged skin: a pilot study. J Cosmet Dermatol. 2018. doi: 10.1111/jocd.12676 [DOI] [PubMed] [Google Scholar]
- 51.Cameli N, Mariano M, Cordone I, Abril E, Masi S, Foddai ML. Autologous pure platelet-rich plasma dermal injections for facial skin rejuvenation: clinical, instrumental, and flow cytometry assessment. Dermatol Surg. 2017;43(6):826–835. doi: 10.1097/DSS.0000000000001083 [DOI] [PubMed] [Google Scholar]
- 52.Abuaf OK, Yildiz H, Baloglu H, Bilgili ME, Simsek HA, Dogan B. Histologic evidence of new collagen formulation using platelet rich plasma in skin rejuvenation: a prospective controlled clinical study. Ann Dermatol. 2016;28(6):718. doi: 10.5021/ad.2016.28.6.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elnehrawy NY, Ibrahim ZA, Eltoukhy AM, Nagy HM. Assessment of the efficacy and safety of single platelet-rich plasma injection on different types and grades of facial wrinkles. J Cosmet Dermatol. 2017;16(1):103–111. doi: 10.1111/jocd.12258 [DOI] [PubMed] [Google Scholar]
- 54.Gordon R. Platelet-rich growth factor for lip and perioral rejuvenation. a case study on “the kiss shot”. Dent Today. 2016;35(5):114, 116. [PubMed] [Google Scholar]
- 55.Mehryan P, Zartab H, Rajabi A, Pazhoohi N, Firooz A. Assessment of efficacy of platelet-rich plasma (PRP) on infraorbital dark circles and crow’s feet wrinkles. J Cosmet Dermatol. 2014;13(1):72–78. doi: 10.1111/jocd.12072 [DOI] [PubMed] [Google Scholar]
- 56.Kang BK, Shin MK, Lee JH, Kim NI. Effects of platelet-rich plasma on wrinkles and skin tone in Asian lower eyelid skin: preliminary results from a prospective, randomised, split-face trial. Eur J Dermatol. 2014;1–2(1):100–101. doi: 10.1684/ejd.2014.2267 [DOI] [PubMed] [Google Scholar]
- 57.Redaelli A, Romano D, Marcianó A. Face and neck revitalization with platelet-rich plasma (PRP): clinical outcome in a series of 23 consecutively treated patients. J Drugs Dermatol. 2010;9(5):466–472. [PubMed] [Google Scholar]
- 58.Sevilla G, Dhrat R, Shetty G, Kadam P, Totey S. Safety and efficacy of growth factor concentrate in the treatment of nasolabial fold correction: split face pilot study. Indian J Dermatol. 2015;60(5):520. doi: 10.4103/0019-5154.159628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Dongen JA, Boxtel JV, Willemsen JC, et al. The addition of tissue stromal vascular fraction to platelet-rich plasma supplemented lipofilling does not improve facial skin quality: a prospective randomized clinical trial. Aesthet Surg J. 2021;sjab109. doi: 10.1093/asj/sjab109 [DOI] [PubMed] [Google Scholar]
- 60.Moher D, Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10) [DOI] [PubMed] [Google Scholar]