Learning objectives.
By reading this article, you should be able to:
-
•
Distinguish normal physiological changes from pathological findings in a compromised newborn at the time of birth.
-
•
Describe the steps recommended for neonatal resuscitation at the time of birth.
-
•
Explain the reasons for recent changes in neonatal resuscitation recommendations.
Key points.
-
•
The unique physiology of transition from fetal to neonatal life informs resuscitation recommendations.
-
•
Preparation is essential for teams and individuals caring for newborn infants at the time of birth.
-
•
Ventilation is the most important intervention in neonatal resuscitation.
Resuscitation at the time of birth is different from all other forms of resuscitation because of the physiological transition from fetal to neonatal life. The vast majority of neonates will make this transition without any significant medical interventions. However, for those neonates who do need assistance at birth, resuscitation guidelines emphasise priorities that account for these unique physiological circumstances.1 Table 1 provides a summary of current key recommendations for neonatal resuscitation. The guidelines also stress the importance of effective communication, preparedness and teamwork.
Table 1.
Summary of key recommendations for neonatal resuscitation. PPV, positive-pressure ventilation.
| Category | Recommendation |
|---|---|
| Cord management | Defer umbilical cord clamping for 1–2 min, if feasible. |
| Initial steps |
|
| Monitoring |
|
| PPV |
|
| Alternate airway |
|
| Chest compressions |
|
| Medications |
|
| Temperature management |
|
For the anaesthetist actively caring for a mother, one of the most dreaded quandaries is being called upon to lead the resuscitation of her struggling neonate at the same time. Multiple national and international practice guidelines strongly recommend that an anaesthetist caring for a mother should not be responsible for neonatal resuscitation.1,2 Despite this recommendation, survey data demonstrate that anaesthetists are commonly called upon to assist or lead resuscitation of the newborn.3 Anaesthetists' involvement in neonatal resuscitation is unsurprisingly quite variable based on the practice setting, with an increased likelihood of involvement in hospitals with fewer deliveries.3
Should the anaesthetist be asked to care for both a mother and her neonate simultaneously, the anaesthetist's first responsibility is to the mother, and the first step should always be to call for help. If a second anaesthesia provider is unavailable but the mother is stable, it may be reasonable to consider assisting with the resuscitation of the newborn while another healthcare provider (e.g. midwife or operating theatre nurse) monitors for any change in the mother's vital signs or clinical status.
As the anaesthetist's involvement in neonatal resuscitation is often unplanned and sporadic, any anaesthetist providing obstetric care should maintain familiarity with current practices in neonatal resuscitation. As the adage goes, neonates are not ‘tiny adults’, and adult or paediatric life support does not account for the physiological considerations pertinent to the transition from fetal to neonatal life.4 This article discusses the most up-to-date recommendations for neonatal resuscitation with a focus on the most recent evidence for suggested interventions.
Preparation
The birth of a neonate usually occurs with enough warning that a team of providers has time to prepare for a resuscitation if necessary. Risk factors known before birth can help determine the likelihood that resuscitation may be needed (Table 2).5 Clear communication with the obstetric and anaesthesia teams is essential to alert the neonatal resuscitation team of the level of risk for resuscitation. Neonatal resuscitation teams should be organised and have a plan for how to manage varying levels of resuscitation requirements. This plan includes determining which team members will be present and which tasks team members should be ready to perform. At the time the team is called to attend the birth of a newborn who may need resuscitation, the team members should be briefed with clear communication about all potential risk factors, roles should be assigned and equipment should be prepared. The neonatal resuscitation team may also initiate communication with the parents to begin preparing them for the events that will occur. The anaesthetist can often assist with communication with the mother as events progress.
Table 2.
Factors associated with need for resuscitation at birth. Based on data from Aziz and colleagues5 and Weiner.6
| Factor | |
|---|---|
| Antepartum | Preterm birth <36 weeks or post-term birth >40 weeks |
| Multiple pregnancy at <35 weeks' gestation | |
| Maternal hypertension, pre-eclampsia or eclampsia | |
| Maternal infection | |
| Polyhydramnios or oligohydramnios | |
| Fetal anaemia | |
| Fetal hydrops | |
| Intrauterine growth restriction or fetal macrosomia | |
| Major fetal anomalies or malformations | |
| No prenatal care | |
| Intrapartum | Emergency Caesarean section |
| Meconium-stained amniotic fluid | |
| Breech or other malpresentation | |
| Maternal general anaesthesia | |
| Maternal magnesium therapy | |
| Placental abruption | |
| Intrapartum bleeding | |
| Chorioamnionitis | |
| Narcotics administered to mother within 4 h of delivery | |
| Prolapsed umbilical cord | |
| Abnormal fetal HR pattern | |
| Shoulder dystocia | |
Neonatal resuscitation equipment must be readily available whenever it may be needed (Table 3). Equipment will be needed to maintain warmth, clear the airway, monitor the infant, provide ventilation, place an alternate airway and provide circulatory support. It is critical to ensure that correct sizes of each piece of equipment needed are available. Equipment should also be checked to ensure its function and that it is primed for use (i.e. radiant warmer turned on to full power, and suction and gas flow turned on and ready to use). For particularly high-risk situations, such as emergency Caesarean section, the team members should consider having the emergency supplies set out and prepared to be used quickly, rather than simply available. In these situations, having an umbilical catheter opened, prepared and flushed could save precious minutes during a critical resuscitation.
Table 3.
Equipment checklist for neonatal resuscitation. PIP, peak inspiratory pressure.
| Procedure | Equipment |
|---|---|
| Warm | Preheated warmer Warm towels/blankets Temperature sensor and cover Hat Plastic bag/wrap (for preterm <32 weeks) Thermal mattress (for preterm <32 weeks) |
| Clear airway | Bulb syringe 10 Fr or 12 Fr suction catheter Meconium aspirator |
| Monitor | Stethoscope ECG leads and monitor Pulse oximeter and sensor |
| Ventilation | Flowmeter set to 10 L min−1 Oxygen blender set to 21% for term; 21–30% for preterm T-piece set to PIP 20–30; PEEP 5 cmH2O Self-inflating bag or flow-inflating bag Appropriately sized masks 8 Fr feeding tube and large syringe |
| Alternative airway | Laryngoscope handle Laryngoscope blades (Miller 00, 0 and 1) Stylet Tracheal tubes (sizes 2.5, 3 and 3.5) End-tidal CO2 detector Tape Scissors Supraglottic airway sizes 1 and 5 ml syringe |
| Circulation | Adrenaline 100 mcg ml−1 Normal saline 5 Fr umbilical catheter Stopcock Syringes 1, 3, 5 and 20 ml Saline flushes |
Resuscitation training occurring in frequent small amounts seems necessary to maintain strong resuscitation skills.7 Regular practice through interdisciplinary simulation helps teams work well together, enabling resuscitation team members to be comfortable performing all roles and to work together in an organised and efficient manner.8 Such simulated practice is ideally performed in situ (where resuscitations normally occur) during the workday so that teams may get to know their environment and team members as well as possible. A team that works well together is likely to perform resuscitation in a highly effective manner when these skills become necessary. Figure 1 demonstrates the typical position for a newborn on the radiant warmer, and Figure 2 shows the positioning of team members around the bed.
Fig 1.
Neonatal resuscitation team positions and tasks. The typical positioning of the newborn on the radiant warmer is shown. The oval represents the clinician at the head of the bed. This team member is often responsible for airway management. The square represents the clinician on the left side of the baby. This clinician may be responsible for auscultating the HR and temperature management. The polygon represents the clinician on the right side of the baby. This clinician may be responsible for placing the Spo2 probe and ECG leads.
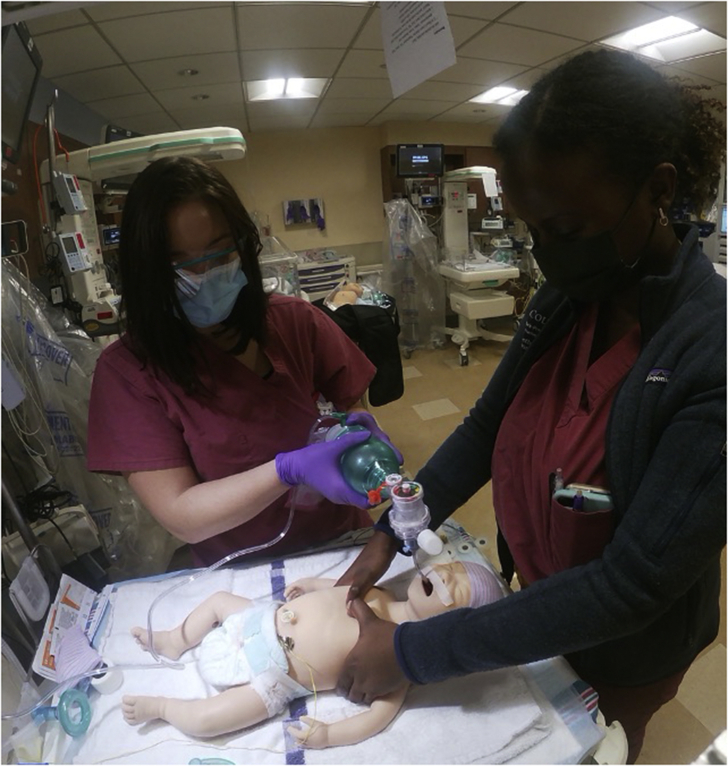
Fig 2.
Neonatal resuscitation team. The photograph shows how the team members described in Figure 1 function at the bedside.
Cord management
Over the last several years, much attention has been paid to the practice of umbilical cord clamping after birth. The obstetric practice of immediate clamping of the umbilical cord was initiated as a component of the active management of the third stage of labour in an effort to prevent postpartum haemorrhage.9 However, clamping the cord immediately after birth as compared with allowing the cord to stay intact for a longer period of time (‘delayed or deferred’ cord clamping) results in lower neonatal blood volumes after birth. A significant portion of the feto-placental blood volume will remain in the placenta instead of infusing into the newborn when the cord is clamped immediately. The duration of time that the cord is left intact after birth affects the amount of blood that is transferred to the newborn. The respiratory status of the infant will also affect the transfer of blood from placenta to the newborn so that a newborn who begins breathing spontaneously will receive a greater volume of blood transfer from the placenta before cord clamping compared with a newborn who does not initiate spontaneous breathing.10
In term infants, the effect of the increased blood volume that is transferred from placenta to the newborn in response to later cord clamping can lead to improved iron stores in infancy, which has the potential to improve neurological development.11 In preterm infants, the effect can be seen more immediately in the newborn period with improved haemodynamic stability during the transition from fetal to neonatal life. Meta-analysis of available clinical trials also demonstrated lower mortality rates in infants treated with delayed cord clamping, fewer blood transfusions and lower incidence of intraventricular haemorrhage.12
Based on physiological observations in animal models that demonstrate a more stable haemodynamic transition from fetal to newborn life when ventilation begins before the cord is clamped, several ongoing studies are evaluating the practice of providing respiratory support before the cord is clamped in infants who do not breathe spontaneously after birth.10 Other studies have evaluated ‘milking’ or ‘stripping’ the cord, in which the obstetrician attempts to transfer blood more quickly from the placenta to a newborn who is not breathing spontaneously. Unfortunately, this practice may be harmful to more preterm infants who are at risk of intraventricular haemorrhage.13
The Royal College of Obstetricians & Gynaecologists currently recommends deferring cord clamping whenever feasible for at least 2 min after birth.9 The European Resuscitation Council (ERC) recommends that cord clamping be deferred until at least 1 min of life for infants who do not need immediate resuscitation.1 Adequate communication between the obstetrician, paediatrician and anaesthetist as to the plan for cord management is essential to providing appropriate care. The timing of giving uterotonic drugs in relation to cord clamping has not been adequately studied, but does not appear to affect maternal or newborn outcomes.10
Initial assessment
In the first moments after birth, a quick assessment of the newborn will help determine the next steps. If the baby is term, crying and breathing, and has good tone, the newborn may stay with the mother and engage in skin-to-skin care. However, if all of those criteria are not present, the baby should be assessed more carefully. After the umbilical cord has been clamped, the baby should be brought to a radiant warmer and placed in a neutral position. Newborns over 32 weeks' gestation should be dried immediately. Those newborns under 32 weeks' gestation should be wrapped in plastic without drying (except for the face) to maintain adequate temperature.14 The ERC recommends maintaining the delivery room (DR) temperature between 23°C and 25°C and greater than 25°C for infants less than 28 weeks' gestation.1 The use of thermal mattresses can also help preterm infants maintain normothermia.14 If the newborn is not breathing well during the initial assessment, gentle stimulation, such as that associated with drying the baby, should be attempted to encourage the baby to breathe adequately and consistently.
Although previously recommended for all newborns, suctioning the oro- and nasopharynx is now recommended only when there are clearly excessive secretions present, especially if the airway seems obstructed.15 Suctioning should also be performed when positive-pressure ventilation (PPV) is indicated and should be attempted if the PPV provided with a face mask does not result in effective ventilation as noted by chest rise. Of note, suctioning the trachea through a tracheal tube (TT) is no longer recommended in newborns born through meconium-stained amniotic fluid unless resuscitation is ineffective because of airway obstruction. This change in practice is based on the lack of evidence for benefit from universal tracheal suctioning and the potential risk from delaying resuscitation and performing unnecessary laryngoscopy.16
Monitoring
Assessment of HR and oxygen saturation (Spo2) is an integral part of neonatal resuscitation and guides clinical management. Heart rate is the most important vital sign in determining the effectiveness of resuscitative interventions. Acceptable methods of assessing HR during neonatal resuscitation include ECG, pulse oximetry monitoring, cardiac auscultation and umbilical cord palpation.1 However, palpation of pulses, including the umbilical cord, is less reliable in the immediate newborn period.17 The ideal tools used to help the neonatal resuscitation team measure vital signs in the DR can also provide continuous monitoring of the newborn.18 Pulse oximetry has the advantage of simultaneously providing both HR and Spo2. The pulse oximeter sensor should be applied to the right wrist or hand of a newborn to obtain pre-ductal Spo2, which represents the oxygenation of cerebral blood flow. There is a normal pre-to post-ductal difference of 10–15% during the first 10 min of life. Motion artefact, acrocyanosis, skin oedema or a low-volume state can lead to delays or inaccurate readings.18 This can be mitigated by warming and drying the newborn and using sensors designed for neonatal resuscitation, which have maximal sensitivity and minimal averaging time.
Because of its reliability and efficiency, ECG is currently the gold standard for assessment of the newborn's HR during advanced neonatal resuscitations requiring advanced airway for ventilation or more interventions.18 Importantly, use of ECG also allows the newborn's HR to be displayed continuously on a monitor that is available to the whole team during the resuscitation. Although ECG is an accurate and efficient tool, it should not be the only method used during resuscitation to assess a newborn's HR, as pulseless electrical activity, although rarely reported in newborns, can occur. Other difficulties with ECG can arise if the leads do not adhere well to the newborn's chest because of amniotic fluid, vernix or meconium. The primary barrier to use of ECG for neonatal resuscitation is its availability outside of the neonatal ICU in many institutions.
Temperature management has also been shown to be an essential part of neonatal resuscitation, particularly with preterm neonates. Hypothermia at the time of admission increases the risk of morbidity and mortality in neonates.14 The ideal temperature targets are between 36.5°C and 37.5°C.1 Setting a temperature target on the radiant warmer and use of a skin temperature probe will help ensure that a newborn meets the minimum target temperatures to maintain normothermia without developing hyperthermia.
Positive pressure ventilation
Once the initial steps of resuscitation are completed, reassessment of the newborn will help determine the next steps. If a newborn is apnoeic, gasping or has HR <100 beats min−1 after a brief period of drying and stimulation has been performed, PPV should be initiated. An appropriate-sized mask that covers the newborn's mouth and nose should be chosen that allows the provider to minimise mask leak without compressing the eyes. Ventilation can be provided using a self-inflating bag, flow-inflating bag or a neonatal T-piece resuscitator. Neonatal T-piece resuscitators are devices that use a constant flow of blended air and oxygen to provide controlled assisted inflations.
The goal of assisted ventilation during resuscitation is to help the newborn infant transition from the fetal state of fluid-filled lungs to the neonatal state of air-filled lungs. This transition allows the fetus to develop a functional residual capacity (FRC) that will allow the lungs to stay inflated. The optimal levels of peak inspiratory pressure (PIP) or PEEP for starting resuscitation are unknown, but current ERC recommendations suggest starting with a PIP of 30 cmH2O for term newborns and 20–25 cmH2O for preterm newborns.1 The use of PEEP during ventilation has been shown to facilitate the development of FRC after birth in animal studies.19 The flow-inflating bag and T-piece can both provide PEEP effectively, but the self-inflating bag requires an additional PEEP valve to provide PEEP, and it may not be as effective.
The ERC recommends that the first five inflations be held for 2–3 s each to help open the lung.1 Once PPV has begun, the team should look for signs of effective and adequate ventilation evidenced by observable chest rise and an increase in HR. Alternatively, a CO2 detector can be used to demonstrate ventilation with colour change.1
Providing ventilation with excessive tidal volume over even a few inflations can cause lung injury especially in extremely preterm infants.20 The T-piece offers the benefit of consistent PIP delivery, which may help avoid excessive tidal volume delivery. Another approach aimed at minimising PIP delivery has been to provide a recruitment manoeuvre, such as a sustained inflation (holding the PIP at a constant level for more than 5 s). This approach has not been shown to be safe and is not currently recommended.21
Physiologically, newborns have an Spo2 of about 60% at 1 min of life, which increases to 85% at 5 min of life.22 Oxygen use during neonatal resuscitation has been studied extensively. Use of a lower Fio2 (0.21–0.30) compared with higher Fio2 (0.6–1.0) during neonatal resuscitation decreases short-term mortality in term newborns.23 In preterm newborns, the best initial Fio2 remains unclear, but most will require some supplemental oxygen during initial stabilisation.23 Therefore, initial Fio2 is set at 0.21 in term and 0.21–0.30 for preterm newborns. If the newborn's HR does not increase with PPV and 0.21 Fio2, the Fio2 should be increased.1 If an infant requires chest compressions at any time during resuscitation, the Fio2 should be increased immediately to 1.0.
Placement of an advanced airway (TT or supraglottic airway [SGA]) is indicated if the HR does not improve despite use of corrective steps to optimise mask ventilation. The team should choose the appropriately sized TT and laryngoscope or SGA based on the newborn's size.6 Although an SGA should always be readily available in case of a difficult intubation, size limitations may preclude the use of an SGA in preterm or growth-restricted neonates.
For newborns who are spontaneously breathing but have signs of respiratory distress, such as use of accessory muscles or grunting, CPAP can be considered to help stabilise the newborn. In preterm newborns, the use of CPAP can help avoid the need for mechanical ventilation and improve longer-term outcomes, such as death or bronchopulmonary dysplasia.20
Chest compressions
The vast majority of newborns respond to resuscitation when ventilation is provided effectively. Therefore, neonatal resuscitation guidelines support the establishment of effective ventilation, including placing an advanced airway before starting chest compressions.1 If a newborn has severe bradycardia with HR <60 beats min−1 despite effective ventilation for 30 s, chest compressions are indicated.
Chest compressions should be coordinated with breaths in a 3:1 pattern. The ideal method of providing chest compressions is to use the two-thumb technique and compress the newborn's chest to one-third of the anterior–posterior diameter at the lower third of the sternum. The two-thumb technique has been shown to provide consistent compressions of the appropriate depth when compared with the two-finger technique in manikin studies.24 Figure 3 shows placement of the hands using the two-thumb technique. With either technique, the provider giving chest compressions should keep their thumbs or fingers in contact with the newborn's chest at all times.
Fig 3.
Chest compressions. The photograph demonstrates the proper hand position for providing chest compressions using the two-thumb technique. Note that the clinician providing chest compressions stands at the head of the bed when the infant's trachea is intubated, and the person to the right of the baby performs lung inflations coordinated in a 3:1 manner with compressions.
During chest compressions, ECG and pulse oximetry can provide continuous monitoring of the HR to the entire resuscitation team. Once an advanced airway is established, the person providing ventilation should stand to the side to provide inflations, allowing the provider performing chest compressions to stand at the head of the bed to provide the most effective chest compressions (see Fig. 3). The provider performing chest compressions should count out loud to help with coordination of chest compressions and ventilation. Heart rate should be assessed after 60 s of chest compressions. During HR assessment, chest compressions should stop. Heart rate is a sensitive indicator of a newborn's clinical status and will improve with effective resuscitation.
Medications
Infants who are born severely compromised and remain bradycardic with a HR >60 beats min−1 despite effective ventilation and chest compressions may respond to adrenaline (epinephrine). During neonatal resuscitation, it is recommended that adrenaline concentration be standardised to 0.1 mg ml−1. The i.v. route is the most effective.25 Adrenaline can also be given via an i.o. catheter or through a TT if no other route is available. The i.v. or i.o. dose is 10–30 mcg kg−1 (0.1–0.3 ml kg−1), whereas the intratracheal dose of adrenaline is 50–100 mcg kg−1 (0.5–1 ml kg−1).1
When needed, placing an umbilical venous catheter quickly is an essential skill of neonatal resuscitation. To place an umbilical venous catheter during resuscitation quickly, the catheter must be readily available and flushed with saline. The umbilical cord should be cleaned with povidone iodine, a tie should be secured around the cord to prevent bleeding and the cord should be cut to allow access to the vessels. The clinician identifies the vein as one vessel with a thinner wall and larger lumen compared with the two thicker-walled arteries. The clinician then advances the umbilical catheter to a position just beyond the cord at a point where blood return is confirmed. Medications and volume can then be given rapidly.
Although rare, some newborns are hypovolaemic at birth and respond to infusion of fluids. For newborns with a history consistent with possible hypovolaemia (e.g. from blood loss), infusions of saline or O-negative blood may be given in 10 ml kg−1 boluses. Emergency transfusion with blood is preferred in newborns with suspected severe blood loss to both replenish the lost volume and correct the resultant anaemia, but this may take longer to prepare.
For any newborn who is not responding to resuscitation efforts, other causes of newborn compromise should be considered. Pneumothorax or unrecognised anomalies, such as congenital diaphragmatic hernia, may prevent successful resuscitation. Such causes should be considered in an infant who remains persistently bradycardic before determining that resuscitation has been unsuccessful. For newborns who are born with no detectable HR and have not responded to effective resuscitation efforts for more than 20 min, a decision may be made to discontinue resuscitation.23 It is critical to communicate with the family, and allow them time to see and hold their child when appropriate.
Conclusions
Most newborns can be stabilised at birth with minimal intervention. When needed, resuscitation is usually successful if adequate ventilation is provided. The events around the time of birth can have important long-term implications for the health and neurological outcome of the child. All providers who may help resuscitate newborns at the time of birth should be aware of current recommendations and the unique needs of the newborn transitioning from fetal life. Ongoing training and improvement practices are important to maintain resuscitation skills.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Declaration of interests
The authors declare that they have no conflicts of interest.
Biographies
Elizabeth Kariuki MD is a fellow in neonatal–perinatal medicine at New York-Presbyterian Morgan Stanley Children's Hospital, Columbia University Irving Medical Center. Dr Kariuki is interested in neonatal resuscitation and is currently performing both observational and interventional studies in newborn infants at the time of birth.
Caitlin Sutton MD is assistant professor of paediatrics and chief of the Division of Maternal-Fetal Anesthesia at Texas Children's Hospital, Baylor School of Medicine. She trained in both paediatric and obstetric anaesthesia, and brings a unique level of expertise to her role.
Tina Leone MD is associate professor of paediatrics and director of the neonatal–perinatal medicine programme at Columbia University Vagelos School of Medicine. She is a member of the Neonatal Resuscitation Program Steering Committee.
Matrix codes: 1B04, 2B07, 3D00
References
- 1.Wyllie J., Bruinenberg J., Roehr C.C., Rudiger M., Trevisanuto D., Urlesberger B. European Resuscitation Council guideline for resuscitation 2015: section 7. Resuscitation and support of transition of babies at birth. Resuscitation. 2015;95:249–263. doi: 10.1016/j.resuscitation.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 2.Bogod D., Mushambi M., Craig S.K., Plaat F., Lucas N. 2020. Guidelines for the provision of anaesthesia services for an obstetric population 2020.https://www.rcoa.ac.uk/sites/default/files/documents/2020-02/GPAS-2020-09-OBSTETRICS.pdf Available from: [Google Scholar]
- 3.Gaiser R., Lewin S.B., Cheek T.G., Guttsche B.B. Anesthesiologists’ interest in neonatal resuscitation certification. J Clin Anesth. 2001;13:374–376. doi: 10.1016/s0952-8180(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 4.Gillis J., Loughlan P. Not just small adults: the metaphors of paediatrics. Arch Dis Child. 2007;92:946–947. doi: 10.1136/adc.2007.121087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aziz K., Chadwick M., Baker M., Andrews W. Ante- and intra-partum factors that predict increased need for neonatal resuscitation. Resuscitation. 2008;79:444–452. doi: 10.1016/j.resuscitation.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Weiner G.M., editor. Textbook of neonatal resuscitation. 7th Edn. American Academy of Pediatrics; Elk Grove Village, IL: 2016. [Google Scholar]
- 7.Anderson R., Sebalt A., Lin Y., Cheng A. Optimal training frequency for acquisition and retention of high-quality CPR skills: a randomized trial. Resuscitation. 2019;135:153–161. doi: 10.1016/j.resuscitation.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Fung L., Boet S., Bould M.D., et al. Impact of crisis resource management simulation-based training for interprofessional and interdisciplinary teams: a systematic review. J Interprof Care. 2015;29:433–444. doi: 10.3109/13561820.2015.1017555. [DOI] [PubMed] [Google Scholar]
- 9.Duley L.M.M., Drife J.O., Soe A. Clamping of the umbilical cord and placental transfusion. 2015. Weeks AD for the royal College of obstetricians & Gynaecologists. Scientific impact paper no. 14, London. [Google Scholar]
- 10.Katheria A.C., Lakshminrusimha S., Rabe H., McAdams R., Mercer J.S. Placental transfusion: a review. J Perinatol. 2017;37:105–111. doi: 10.1038/jp.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald S.J., Middleton P., Dowswell T., Morris P.S. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2013;2013 doi: 10.1002/14651858.CD004074.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabe H., Gyte G.M.L., Díaz-Rossello J.L., Duley L. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2019;9 doi: 10.1002/14651858.CD003248.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katheria A., Reister F., Essers J., et al. Association of umbilical cord milking or delayed cord clamping with death or severe intraventricular haemorrhage among preterm infants. JAMA. 2019;322:1877–1886. doi: 10.1001/jama.2019.16004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCall E.M., Alderdice F., Halliday H.L., Vohra S., Johnston L. Interventions to prevent hypothermia at birth in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2018;2018 doi: 10.1002/14651858.CD004210.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster J.P., Dawson J.A., Davis P.G., Dahlen H.G. Routine oropharyngeal suction versus no suction at birth. Cochrane Database Syst Rev. 2017;2017 doi: 10.1002/14651858.CD010332.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trevisanuto D., Strand M.L., Kawakami M.D., et al. On behalf of the International Liaison Committee on Resuscitation Neonatal Life Support Task Force. Tracheal suctioning of meconium at birth for non-vigorous infants: a systematic review and meta-analysis. Resuscitation. 2020;149:117–126. doi: 10.1016/j.resuscitation.2020.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Owen C.J., Wyllie J.P. Determination of heart rate in the baby at birth. Resuscitation. 2004;60:213–217. doi: 10.1016/j.resuscitation.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Dawson J.A., Schmolzer G.M., Wyllie J. Monitoring heart rate in the delivery room. Semin Fetal Neonatal Med. 2018;23:327–332. doi: 10.1016/j.siny.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Siew M.L., Te Pas A.B., Wallace M.J., et al. Positive end-expiratory pressure enhances development of functional residual capacity in preterm rabbits ventilated from birth. J Appl Physiol. 2009;106:1487–1493. doi: 10.1152/japplphysiol.91591.2008. [DOI] [PubMed] [Google Scholar]
- 20.Foglia E.E., Jensen E.A., Kirpalani H. Delivery room interventions to prevent bronchopulmonary dysplasia in extremely preterm infants. J Perinatol. 2017;37:1171–1179. doi: 10.1038/jp.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruschettini M., O’Donnell C.P.F., Davis P.G., Morley C.J., Moja L., Calevo M.G. Sustained versus standard inflations during neonatal resuscitation to prevent mortality and improve respiratory outcomes. Cochrane Database Syst Rev. 2017;7 doi: 10.1002/14651858.CD004953.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawson J.A., Kamlin C.O., Vento M., et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics. 2010;125:e1340–e1347. doi: 10.1542/peds.2009-1510. [DOI] [PubMed] [Google Scholar]
- 23.Wyckoff M.H., Wyllie J., Aziz K., et al. Neonatal life support 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2020;156:A156–A187. doi: 10.1016/j.resuscitation.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Mildenhall L.F.J., Huynh T.K. Factors modulating effective chest compressions in the neonatal period. Semin Fetal Neonatal Med. 2013;18:352–356. doi: 10.1016/j.siny.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Kapadia V.S., Wyckoff M.H. Drugs during delivery room resuscitation—what, when and why? Semin Fetal Neonatal Med. 2013;18:357–361. doi: 10.1016/j.siny.2013.08.001. [DOI] [PubMed] [Google Scholar]