Abstract
Post-traumatic osteomyelitis (PTO) is a worldwide problem in the field of orthopaedic trauma. So far, there is no ideal treatment or consensus-based gold standard for its management. This paper reviews the representative literature focusing on PTO, mainly from the following four aspects: (1) the pathophysiological mechanism of PTO and the interaction mechanism between bacteria and the body, including fracture stress, different components of internal fixation devices, immune response, occurrence and development mechanisms of inflammation in PTO, as well as the occurrence and development mechanisms of PTO in skeletal system; (2) clinical classification, mainly the etiological classification, histological classification, anatomical classification and the newly proposed new classifications (a brief analysis of their scope and limitations); (3) imaging diagnosis, including non-invasive examination and invasive examination (this paper discusses their advantages and disadvantages respectively, and briefly compares the sensitivity and effectiveness of the current examinations); and (4) strategies, including antibiotic administration, surgical choices and other treatment programs. Based on the above-mentioned four aspects, we try to put forward some noteworthy sections, in order to make the existing opinions more specific.
Keywords: Osteomyelitis, Bone infection, Post-traumatic osteomyelitis, Pathology, Diagnosis, Clinical classification, Treatment
Introduction
Post-traumatic osteomyelitis (PTO) generally refers to osteomyelitis that occurs at the wound site after surgery, open fractures or other injures. The most common cause is postoperative infection of open fractures. In most PTO patients, the bone tissue is contaminated by bacteria after trauma. Bacteria proliferate rapidly in the anaerobic environment in the medullary cavity, resulting in disturbance of blood supply for bone tissue or formation of dead bones. PTO has a high recurrent rate. Complete control of PTO can be hardly achieved because (1) very limited antibiotics can reach the infection focus that locates inner the medullary cavity via intravenous ways,1 (2) the topical curative effects of the medicine can only last for a short duration, and (3) there exists wide bacterial drug resistance.2 The purpose of this study is to systematically review the pathology, clinical classification, imaging characteristics and treatment of PTO.
Pathology of PTO
The occurrence and progression of infection in traumatic osteomyelitis are mainly related to three factors. The first one is the degree of tissue injury, including the size and depth of the wound, the organs involved, any combined injuries, the mode of operation, and the surgical approaches. The second is the bacteria, mainly the number, strain, size of the contaminated area, bacteria virulence and reproduction rate, etc. The third is the host defense, mainly the immunologic status, nutritional condition, concomitant disease, etc. During high-energy trauma, the huge impact destructs the bone integrity and continuity, causing bone exposure and severe tissue damage. Moreover the following surgical procedures and lack of fracture fixation site will further aggravate the periosseous vascular injury. Damage of the periosseous vascular network can easily cause osteonecrosis, while necrotic tissue is an excellent medium for bacteria, which accelerates the infection to spread along the necrotic tissue into the bone marrow cavity and extraosseous tissue: the former forms PTO and the latter induces severe infection with suppurative osteomyelitis. Related reports also suggest that infection in inactivation bone areas has a very high risk of osteomyelitis.3,4
The choice of internal fixation materials and mode of fracture fixation has a significant impact on the prognosis of bone infection management. A correct choice of internal materials and mode for fixation ensures a stable fracture fixation. If the fracture segments cannot be stabilized, their movement will not only aggravate the damage of the vascular network around the fracture block, but also aggravate the immune response. While destruction of the vascular network may induce new tissue necrosis and expand the range of the original necrotic tissue.5 The accumulating necrotic tissue strengthens the inflammatory response of the body, and eventually develops into systemic inflammatory response syndrome and PTO. The abovementioned pathophysiological process further illustrates the necessity and importance of constructing an anatomical reduction following fractures.6 When the fracture is not anatomically stable or fixed, the mild movement of bone fragments will also bring great interference to the formation and regeneration of the vascular network around the bone. If the implant of internal fixation is not stable, screw and plate loosening may happen, which can easily lead to exposure of the fracture internal fixation to form a new source of infection. Clinical studies and animal experiments have proved that the lack of stable internal fixation is one of the most important factors inducing infection follow fractures.7,8 All the factors of time duration from injury to surgery, surgical approaches, minimally invasive or open surgery, and the material and size of the fixation implant have direct impacts on the recovery of trauma patients. At present, the components containing nickel in metal implants are more likely to cause allergies and secondary damage.9, 10, 11
Bacterium is also one of the most important factors affecting the occurrence and development of PTO. Staphylococcus aureus is the most common bacteria in osteomyelitis and the most important bacteria in post-traumatic fracture infection.12, 13, 14 A series of severe complications are also likely to be caused by Staphylococcus aureus. Claro et al.15, Marriott et al.16 and Somayaji et al.17 reported that Staphylococcus aureus promotes osteoclast production by enhancing the expression of pro-inflammatory cytokines and NF-κB, which conversely aggravated the local bone destruction. Alexander et al.18 and Young et al.19 reported that Staphylococcus aureus mediates bone destruction by inducing osteoblast apoptosis. Cassat et al.20 revealed that Sae, a bacterial regulatory site, plays a key role in the pathogenesis of osteomyelitis. Sae promotes pathological bone remodeling and intraosseous bacterial survival, and regulates osteolytic exogenous protein α-phenol soluble modulin to produce cytotoxicity to osteoblasts and induce bone destruction.
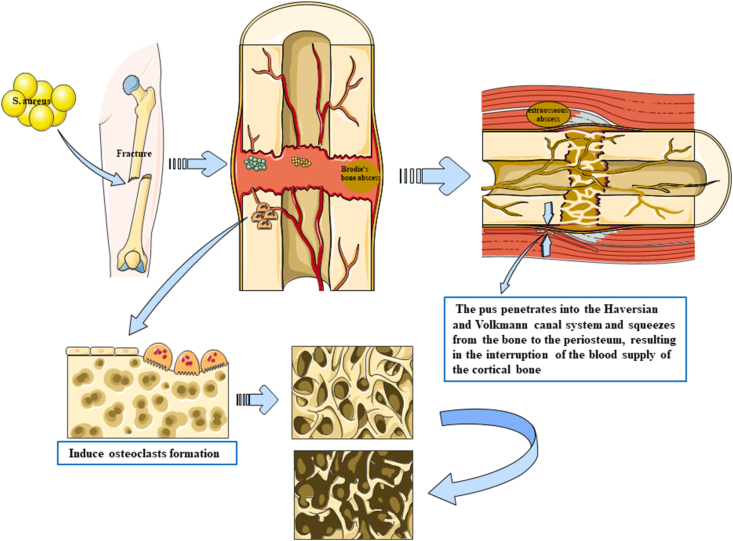
Bacteria invade the body to cause inflammation, which induces macrophages to produce hydrolytic protease, but hydrolytic protease can also destroy the body's own tissue (Fig. 1). The leucocidin in Staphylococcus aureus further promotes and induces this reaction.21 Tissue necrosis, inflammatory infiltration and increased edema can lead to aggravation of bone marrow injury, bone trabecular necrosis, bone marrow infarction, and then localized formation of Brodie's bone abscess. The pus penetrates into the Haversian and Volkmann canal system and squeezes from the bone to the periosteum, resulting in interruption of the blood supply of the cortical bone and formation of a new extraosseous abscess. Extraosseous infection gradually forms a new soft tissue abscess or is accompanied by skin rupture and pus outflow. In open fractures, periosteum is often detached from bone tissue due to trauma, which further leads to cortical necrosis.
Fig. 1.
Pathological pictures of PTO induced by Staphylococcus aureus. Staphylococcus aureus itself can enhance the expression of pro-inflammatory cytokines and osteolytic protein, induce osteoclast production, and aggravate bone destruction. Staphylococcus aureus infection of bone marrow produces inflammatory reaction and induces macrophages to produce hydrolytic protease, but hydrolytic protease itself can also aggravate tissue injury; on the other hand, tissue necrosis, inflammatory infiltration and edema can lead to aggravation of bone marrow injury, bone trabecular necrosis, bone marrow infarction, and then localized formation of Brodie's bone abscess in bone. The pus penetrates into the Haversian and Volkmann canal system and squeezes from the bone to the periosteum, resulting in the interruption of the blood supply of the cortical bone and the formation of a new extraosseous abscess.
The susceptibility to infection varies with the degree of injuries to soft tissue and bone, and is closely related to the location of the fracture and pathophysiological status of the local wound.22,23 The release of body-related inflammatory factors such as local injury, hypoxia and acidosis mediates the body's non-specific immune defense response, and the body stress response and emergency response are activated.24, 25, 26, 27 Excessive immune response eventually leads to sepsis or systemic inflammatory response syndrome.28, 29, 30
Clinical classification of PTO
The development of PTO is complex due to different injury mechanisms. At present, there is no recognized expert consensus on the clinical classification of PTO. It is difficult to classify PTO only by imaging examination. Now the clinical classification of PTO is mainly based on the clinical classification of osteomyelitis. Because of the complex characteristics of osteomyelitis, it is difficult to have a simple clear classification system.31 Previous classification systems are more based on the perspective of pathophysiology,32, 33, 34 pathogen specificity and the duration of infection.12,32,33,35 Since the classification of osteomyelitis was first put forward by Waldvogel et al. in 1970,33 more and more scholars have proposed a variety of classification methods and basis. The following is a summary of them.
In 1970, Waldvogel and colleagues33 first reported the classification of osteomyelitis, mainly hematogenous osteomyelitis, secondary osteomyelitis, functional osteomyelitis (common in patients with diabetes). Kelly36 reported that the clinical types of osteomyelitis were hematogenous osteomyelitis, fracture healing with osteomyelitis, fracture nonunion with osteomyelitis and no fracture osteomyelitis. This classification standard puts more emphasis on the diagnosis of the etiology. The clinical classification of osteomyelitis reported by Gordon et al.37 can be divided into three types, i.e. type A: tibia defect and nonunion, no obvious segmental loss; type B: tibia defect >3 cm, fibula intact; type C: tibia defect >3 cm, involving both tibia and fibula. Lew and Waldvogel12 reported osteomyelitis secondary to adjacent infection (trauma, surgery or insertion of a joint prosthesis), secondary to vascular insufficiency (diabetic foot infection), or hematogenous osteomyelitis. This classification was based on the treatment. May et al.35,38 reported PTO classified by bone infection, soft tissue and bone defect in tibia, i.e. type Ⅰ: tibia and fibula intact and load-bearing stable; type Ⅱ: fibula intact, tibia continuous but need bone graft to have structural support; type Ⅲ: fibula intact, tibia defect ≤6 cm; type IV: fibula intact, tibia defect >6 cm; type V: tibia defect >6 cm, fibula fracture or apraxia.
According to Cierny-Mader classification, the classification criteria of osteomyelitis were the combination of anatomical classification and physiological classification (Table 1).39, 40, 41 Anatomical classification is medulla, superficial limitation, diffuse; while physiological classification is mainly based on the evaluation of the patient's own condition: (1) patients with normal physiological function, normal immune and blood circulatory system; and (2) patients with abnormal or local physiological function, poor systemic condition, poor prognosis and worse treatment effect. The point of this staging is that it can make a better distinction between different types of patients and select targeted treatment programs for osteomyelitis at different stages of development. The disadvantage of this classification is that it does not include the pathogenic microorganism and factors affecting fracture stability, and thus more suitable for the diagnosis of long bone osteomyelitis.
Table 1.
| Classification | Description |
|---|---|
| Anatomic type | |
| Stage 1 | Medullary osteomyelitis |
| Stage 2 | Superficial osteomyelitis |
| Stage 3 | Localized osteomyelitis |
| Stage 4 | Diffuse osteomyelitis |
| Physiological class | |
| A host | Normal host |
| B host | Systemic compromise (Bs), local compromise (Bl), or systemic and local compromise (Bls) |
| C host | Treatment worse than the disease |
In 2014, Schmidt et al.42 reported that they classified PTO by eight items, respectively source of the infection, the anatomical region, stability of the affected fracture (bone continuity), foreign body (internal fixation, prosthesis), infection range (structures involved), activity of the infection (acute, chronic, quiescent), pathogenic microorganisms (non-specific and specific bacteria, fungi) and comorbidity (systemic and localized immunosuppressive diseases). The classification of PTO was finally determined through different items. The Schmidt's classification separately classifies the source of infection, infection scope and infection activity, which is helpful to highlight the characteristics of traumatic osteomyelitis and take into account the previous project setting for osteomyelitis classification. It provides a good reference value for the clinical classification of PTO. However, the setting of these items is complex, and thus limits its use in clinic.
The abovementioned methods for classification of osteomyelitis and PTO are mainly aimed at the anatomical area of the patient and the source of infection. The Schmidt's classification integrates the previous classifications for osteomyelitis and further refines the classification items, and roughly disassembles the causes affecting bone stability and infection-related indicators in the past. The Cierny-Mader classification is one of the most classical classifications of osteomyelitis at present, and the adopted classification criteria for physiological and anatomical indexes are still in use. The limitation of Cierny-Mader classification is that it is unable to well reflect the clinical characteristics of PTO. Looking for golden classifications of PTO, which not only highlights the clinical characteristics, but also facilitates its clinical application, is still our long-term goal.
Imaging diagnosis of PTO
The difficulty to diagnose PTO is also reflected in imaging examination. So far, no consensus has been reached on the imaging diagnosis of PTO. Timely and accurate diagnosis of PTO is still very challenging.43, 44, 45, 46 At present, the imaging diagnosis of PTO is developing rapidly, including MRI, three-phase bone scintigraphy (TPBS), white blood cell (WBC) scintigraphy, antigranulocyte antibody (AGA) scintigraphy, 2-deoxy-2 [F] fluoro-d-glucose positron emission tomography (FDG-PET) and CT.
TPBS
TPBS is an examination that injects radionuclides or labeled compounds into the human body as tracers to analyze the blood flow, function and metabolism of different organs and tissues according to the spatial distribution of the tracers in specific organs and tissues. TPBS includes (1) blood flow phase (perfusion phase): dynamic image is obtained immediately after the injection of radiopharmaceuticals, reflecting the tissue blood perfusion and infection, if has, at the lesion site (blood supply is enhance and blood flow phase is positive); (2) blood pool phase (soft tissue phase): 5 min after drug injection, which reveals positive results if soft tissue infection occurs; and (3) delayed phase (bone phase): imaging obtained 2–4 h after drug injection. Typical hyperemia and increased blood pool activity at soft tissue phase and focal bone uptake at bone phase are sensitive and specific signs for the diagnosis of osteomyelitis by TPBS.47,48 99mTc-methylene diphosphate (99mTc-MDP) is one of the most commonly used bone imaging agents in clinic, mainly used to reflect the activity of bone metabolism. 99mTc-MDP can be used in the diagnosis and functional evaluation of bone tumor, femoral head osteonecrosis, PTO, prosthesis loosening or infection following artificial joint replacement, and diabetic foot. Although TPBS is highly sensitive to infection, it is not specific and accurate. For example, Devillers et al.49 reported that the overall sensitivity, specificity and accuracy of 99mTc-MDP bone imaging for PTO were 100%, 17% and 53.3%, respectively.
99mTc-hexamethylpropyleneamine oxime (HMPAO)
The principle of WBC scintigraphy lies in that the radioactivity distribution of 111In labeled leukocytes is different from that of 99mTc labeled sulfur colloids in vivo. The radioactivity of sulfur colloids is mainly distributed in bone marrow, and the radioactivity carried by labeled leukocytes is mainly distributed in blood and infected tissues. The sensitivity, specificity and accuracy of this method are higher than those of other WBC scintigraphies, but the results of 99mTc-HMPAO can still be easily disturbed by non-specific infection, chronic infection and abnormal hematopoietic function of bone marrow. Devillers et al.49 reported that the overall sensitivity, specificity and accuracy of 99mTc-HMPAO for PTO were 93%, 100% and 96%, respectively. While Maugendre and Poirier50 reported that 99mTc-HMPAO is an accurate method for the diagnosis of osteomyelitis at the site of bone destruction, with the sensitivity of 100% and the specificity of more than 95%. De Lima Ramos et al.51 reported that the sensitivity, specificity and accuracy of 99mTc-HMPAO for the location of bone and joint infection were 72.7%, 78.2% and 76.4%, respectively. Krznaric et al.52 reported the sensitivity, specificity and accuracy of 99mTc-HMPAO-WBC scintigraphy in the diagnosis of chronic osteomyelitis to be 94%, 91% and 92%, respectively. However, because the imaging procedures consist of drawing patients' venous blood first, separating and labeling WBCs, this examination is relatively environmental and technical demanding, and thus is limited in large-scale use.
FDG-PET
18F-FDG is a kind of imaging that reflects the glucose metabolism of cells and tissues. The imaging principle is that the glucose metabolism of infected tissues is higher than that of normal tissues. 18F cannot be metabolized by cells with deoxyglucose and thus remains in the cells, which therefore can be detected and imaged in vitro. It is a relatively fast whole body imaging method and can be used to detect multiple lesions. The disadvantage is that recent fractures and the presence of metal implants in the body may reduce the accuracy of FDG-PET, as FDG uptake also increases in the inflammatory response.53 For early diagnosis of non-bony infectious and inflammatory diseases,54 Mumme et al.55 compared the diagnostic effect between 18F-FDG-PET and TPBS for joint replacement related infections. It was found that the sensitivity and specificity of FDG-PET were 91% and 92%, respectively, and the accuracy was 91%, better than those for TPBS, the data of which were 78%, 70% and 74%, respectively. Goebel et al.56 reported that for 50 patients with suspected PTO, the sensitivity and specificity of FDG-PET for the whole study group were 92% and 69%, respectively, and the accuracy was 86%. The sensitivity, specificity and accuracy of using CT alone were 47%, 60% and 50%, respectively, while the data of using MRI alone were 82%, 43% and 67%, respectively. Wenter et al.57 reported 215 patients with chronic osteomyelitis collected from 2000 to 2013, whose results showed that 18F-FDG-PET can identify osteomyelitis with a high sensitivity in patients with non-specific clinical symptoms of infection in orthopedic surgery.
AGA scintigraphy
Immunonuclide imaging with 99mTc-labeled monoclonal anti-granulocyte antibody has a high sensitivity and specificity in the diagnosis of PTO.58,59 Horger et al.60 reported that the combined use of single PET and CT can improve the sensitivity and specificity and distinguish soft tissue infection, suppurative arthritis and osteomyelitis, as well as cortical, cortical and subperiosteal lesions. Glaudemans et al.61 reported that through a retrospective single center study, it was further proved that the sensitivity of osteomyelitis by AGA scintigraphy was 97.9%, specificity 91.8%, and diagnostic accuracy 93.1%.
MRI and CT
Although the technology and equipment of MRI and CT have been greatly developed,62 and the characteristics of low cost and fast scanning speed have made the two examinations commonly used in most hospitals, MRI and CT has limited accuracy in determining the specific infection location and extent of PTO. Kaim et al.63 reported that MRI is more sensitive to chronic osteomyelitis than to acute osteomyelitis, while PTO is more common chronic osteomyelitis. Goebel et al.56 reported 50 patients with suspected PTO, among whom the diagnostic sensitivity, specificity and accuracy by CT were 47%, 60% and 50%, respectively, and by MRI 82%, 43% and 67%, respectively. MRI and CT play a certain role in the diagnosis of PTO, but with the increased introduce of internal fixation implants following fractures, the diagnosis by MRI and CT in PTO has been largely affected.
To sum up, AGA scintigraphy combined with SPET/CT, 99mTc-HMPAO and 18F-FDG-PET is relatively accurate in the diagnosis of PTO.
Treatment of PTO
Timely and correct diagnosis & treatment of PTO are vital for its therapeutic effect. The main treatment principles of PTO are similar to osteomyelitis, i.e. infection control, thorough elimination of the dead space, postoperative irrigation, adequate drainage and delayed closure of the wound.
Antibiotic treatment
Early use of sensitive antibiotics is still the most important part of current treatment, especially for Staphylococcus aureus. In 1994, Patzakis et al.64 reported that 65%–70% of patients with chronic osteomyelitis were caused by Staphylococcus aureus. Yang et al.65 reported that 37.5% of Staphylococcus aureus and 5.8% of Staphylococcus epidermidis were found in patients with PTO. Peng et al.66 reported 84 PTO patients and 53.85% of them were infected by Gram-positive bacteria. So far, the drug resistance of bacteria is still a great challenge for clinicians.67 Although the detection rate of Gram-positive bacteria is lower than that at 20 years ago, they are still the dominant bacteria in PTO. At present, gentamicin bone cement and vancomycin bone cement are most widely used for PTO.
With the increased incidence of traffic accidents and bone exposure after traumatic fractures, the ideal antibiotics for the treatment of PTO should meet the following requirements: wide antibacterial spectrum, high safety, strong thermal stability, good water solubility and hypoallergy.68 However, no single antibiotic can achieve a satisfactory result at present. Gentamicin belongs to aminoglycoside antibiotics, which has the advantages of wide antibacterial spectrum, good thermal stability and good absorption effect. Aminoglycosides play a role by specifically binding to the 16s rRNA decoding region A site of bacterial ribosomal 30s subunit, resulting in the formation of a stable inner ring structure of 16SrRNA.69 At the same time, ribosomes are active for a long time, so that non-complementary paired tRNA can also pass through site A, resulting in the formation of wrong proteins.70 At the same time, the conformational change of ribosome caused by the combination of aminoglycoside antibiotics and ribosomal 30s subunit can affect the migration of bacterial ribosomal subunit,71 which inhibits the binding of ribosomal recycling factor and slows down the process of ribosomal recycling, thus affecting the process of protein synthesis.72 The main factors of drug resistance of bacteria in aminoglycoside antibiotics are as follows: (1) change of the outer membrane permeability, decrease of the intimal transport and decrease of the intracellular drug accumulation caused by high expression of active efflux system73; (2) aminoglycoside antibiotics modified by aminoglycoside antibiotic inactivating enzyme (aminoglycoside modifying enzymes, AME)74; (3) the binding sites and targets of aminoglycoside antibiotics on 16s rRNA in bacterial ribosomal 30s subunit changed by mutation or methylation modification. The overexpression of aminoglycoside modifying enzyme in bacteria is the main mechanism of drug resistance of these antibiotics.75
Vancomycin belongs to glycopeptide antibiotics, which shows an excellent antibacterial effect on Staphylococcus aureus, but has hepatotoxicity and nephrotoxicity. Due to the routine use of antibiotics for a continuous 4–6 weeks for osteomyelitis, many patients will have liver & kidney damage as well as blood routine three-line decline, after a long-term use of vancomycin. Glycopeptide antibiotics are complex natural products synthesized by several Actinomycetes or Streptomyces. Their structures are linear peptides and are composed of heptapeptide skeletons highly modified by aromatic partial cross-linking. In addition, glycopeptide antibiotics are often modified by chlorination, glycosylation, methylation, acylation or sulfation. They inhibit bacterial growth by interfering with cell wall biosynthesis and target the bacterial cell wall component D-Ala-D-Ala, so as to prevent the cross-linking of the peptidoglycan layer. According to the difference of amino acids, it can be divided into four groups: vancomycin group, rivaletin group, avoparcin group and synmonic group. Because of its unique mode of action, glycopeptide antibiotics show excellent anti-Gram-positive bacteria activity, and thus are clinically used in the treatment of various diseases caused by Gram-positive bacteria such as MRSA and susceptible enterococci. The resistance of Staphylococcus aureus to vancomycin is caused by the thickening of cell wall with the accumulation of peptidoglycan, while the vancomycin-resistant Staphylococcus aureus is produced after the modification of VanA phenotype of vancomycin-resistant enterococci lipid Ⅱ.76 With the increase of drug resistance of Staphylococcus aureus, vancomycin may be losing the clinical role against MRSA infection.77
Surgical treatment
Surgical treatment is one of the most important parts in the treatment chain of PTO, which mainly includes adequate drainage, thorough debridement and dead space elimination.87 Debridement and lavage is one of the most important defensive lines against PTO. Complete debridement is essential to prevent the development of PTO after trauma: removing necrotic tissue, dead bones, sinus, and infected granulation tissue88 until massive bleeding can be seen on the bone segment and soft tissue. After debridement, the anatomical stability of the fracture site should be restored as much as possible. Early restoration of the anatomical stability of the fracture site is beneficial to reduce the risk of poor bone healing and joint contracture.89 If the wounded presents with bone exposure, iodophor solution, hydrogen peroxide solution and normal saline should be used for irrigation. Bone slices dropped outside the operating table should not be put back into the body in order to reduce the occurrence of PTO. After debridement, the patients should be treated with combined antibiotics and close monitor of the condition of the injured limb. Debridement treatment could be carried out again if necessary. At the same time, the principles of debridement and dressing change should be strictly followed to avoid PTO caused by contaminated wounds. Removal of the dead space helps to remove the pus and hematoma in the cavity and avoid the occurrence of PTO caused by bacterial reproductive infection.
Local antibiotic treatment with poly-methyl methacrylate (PMMA) cement combined with antibiotic preparation is another popular choice for the treatment of PTO, which has been going on for more than 40 years.78 In 1978, Wahlig et al.78 reported that the implantation of gentamicin-PMMA microspheres may prove to be a valuable new form of local antibiotic therapy. Calhoun et al.79 conducted a randomized controlled trial and reported that infective nonunion can be treated with local antibiotics instead of long-term systemic antibiotics. Ostermann et al.80 reported that the adjuvant use of PMMA microspheres with local antibiotics can reduce the incidence of infection in severe compound fractures. The topical use of antibiotics to treat osteomyelitis can reduce the side effects of long-term systemic medication, help patients to start walk exercises as soon as possible and reduce the cost of hospitalization. With the continuous development of new material technology, new biomaterials have been introduced as carriers for the treatment of osteomyelitis. Xie et al.81 used the biodegradable borate glass as the carrier of vancomycin in the treatment of osteomyelitis. Compared with traditional methods, this new material has better biocompatibility and can reduce the occurrence of rejection.
Ilizarov method has been widely applied in the treatment of osteomyelitis and bone defect. The Ilizarov annular external fixator, pierced by thin steel needle and fixed under tension, was invented in 1951. Ilizarov has been found to be able to give continuous, stable and slow pulling force to the broken end of the fracture, and the metaphysis of the long bone can stimulate the regeneration of bone tissue. Cattaneo et al.82 and Paley et al.83 reported their experience of treating the infectious nonunion and segmental defect of tibia by Ilizarov method. With the development of medical technology, a combined use of Ilizarov fixation and negative pressure closed drainage like vacuum sealing drainage has been adopted for clinical management of PTO.84 The advantage of this combined method is that the pus in the bone marrow cavity can be sucked in time, and the simultaneous antibiotic irrigation can improve the condition of PTO.
Induced membrane technique is a simple, reliable and effective method for the treatment of PTO.85 This technique was first proposed by a French scholar Masquelet in 1986 to treat large segmental bone defects with induced membrane and autogenous bone transplantation. At present, Masquelet technology has been widely used in the treatment of infectious bone defect, aseptic bone defect and bone defect after tumor resection. Compared with Ilizarov method, Masquelet technique can overcome the limitations of needle track infection and long-term placement of external fixator. Tong et al.86 reported no significant difference between Masquelet technique and Ilizarov method regarding bone repair, but the Masquelet technique shows a better result in terms of function restoration of the affected limb.
A fresh skin flap with a good blood supply can provide a protective barrier and adequate blood supply to the wound, which further provides a good blood supply to the bone and thus promote bone regeneration. Studies have shown that the skin flap can provide growth factors and cytokines to promote bone regeneration.90,91 Covering the fracture area with the skin flap prevents direct contact with the air and avoids the possibility of recurrent infection. The flap can also protect the underlying bone from trauma or pressure ulcers for a long time.89 Flap repair not only avoid the growth of bacteria and formation of biofilm,91 but also reduce the chance of infection and shorten the duration of hospitalization.92 Gokalp et al.93 reported that in 30 cases of chronic osteomyelitis treated by skin flap transplantation, the combined use of antibiotics for 6 weeks after operation showed favorable outcome in 29 cases. Skin flap transplantation combined with antibiotics can well handle osteomyelitis and promote bone regeneration, but it still has the disadvantage of long time consuming, high cost, and not very high survival rate of the flap. The results of Schaverien et al.94 suggested that partial failure of the flap was related to smoking, diabetes and peripheral vascular disease. The significant increase in the number of smokers and patients with diabetes also brings new challenges and difficulties for skin flap repair. At present, skin flap mainly refers to myocutaneous flap, free myocutaneous flap and pedicled flap. The choice of each flap depends on the location of trauma and the degree of injury, and the bearing capacity of the patient. The vacuum sealing drainage device can be trimmed according to the size of the wound, which should be able to temporarily replace the skin flap, and simultaneously isolate the wound and air to avoid infection. Negative pressure can promote the growth of granulation tissue and crawl to cover the wound, which provides preparation conditions for later skin transplantation.
Other treatments
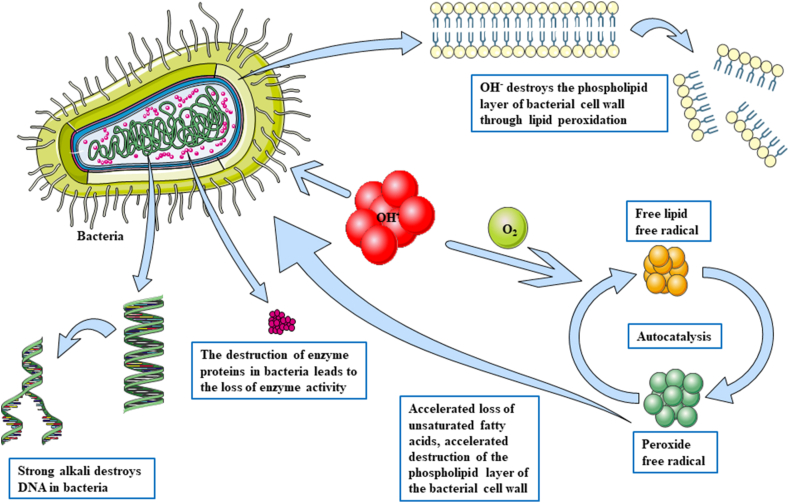
Bilge et al.95 reported that ozone treatment can enhance antioxidation and reduce oxidative stress to assist the treatment of osteomyelitis on an experimental model of rat osteomyelitis. Rose and Shields96 reported that hyperbaric oxygen was used to treat osteomyelitis. Hyperbaric oxygen may increase the partial pressure of oxygen in anoxic tissue to improve local hypoxia and induce oxidative stress. Liu et al.97 shared the external application of traditional Chinese medicine combined with surgery in the treatment of post-traumatic tibial osteomyelitis. Our team once investigated the effect of calcium hydroxide cement carrier system on chronic osteomyelitis, and found that OH− can by continuously released from calcium hydroxide, which could increase the bacterial cell membrane permeability, induce bacteria protein degeneration and cause bacteria DNA damage,98,99 resulting in bacterial death (Fig. 2). Meanwhile, the calcium hydroxide cement carrier system could dissolve the necrotic tissue in the medullary cavity, induce hard tissue deposition,100 inhibit the activity of osteoclasts, and promote healing of the infected bone in the end.
Fig. 2.
Mechanism of calcium hydroxide killing bacteria by producing OH−.
Future prospects
The treatment of PTO remains a major challenge in the field of trauma till now. The continuous development of new technologies provides more and more choices and new ideas for its management. Multidisciplinary research and personalized therapeutic strategy are the future research trends. In order to better study PTO, it is urgent to focus on its clinical classification. In addition, exploring a cheaper and more effective therapy to reduce the high burden and risk of amputation is crucial and remains unsolved.
Funding
This review was supported by the National Natural Science Foundation of China (NSFC No. 82060347), Innovative Research Projects for Postgraduates in Higher Education Institutions in Hainan Province (Hys2020-342), Youth Incubation Fund of the First Affiliated Hospital of Hainan Medical University (HYYFYPY202005).
Ethical statement
Not applicable because this is a review.
Declaration of competing interest
The authors declared that no existing competing interests.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Govaert G.A., IJpma F.F., McNally M., et al. Accuracy of diagnostic imaging modalities for peripheral post-traumatic osteomyelitis - a systematic review of the recent literature. Eur J Nucl Med Mol Imag. 2017;44:1393–1407. doi: 10.1007/s00259-017-3683-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kishor C., Mishra R.R., Saraf S.K., et al. Phage therapy of staphylococcal chronic osteomyelitis in experimental animal model. Indian J Med Res. 2016;143:87–94. doi: 10.4103/0971-5916.178615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mauffrey C., Herbert B., Young H., et al. The role of biofilm on orthopaedic implants: the “Holy Grail” of post-traumatic infection management? Eur J Trauma Emerg Surg. 2016;42:411–416. doi: 10.1007/s00068-016-0694-1. [DOI] [PubMed] [Google Scholar]
- 4.Blaker C.L., Clarke E.C., Little C.B. Using mouse models to investigate the pathophysiology, treatment, and prevention of post-traumatic osteoarthritis. J Orthop Res. 2017;35:424–439. doi: 10.1002/jor.23343. [DOI] [PubMed] [Google Scholar]
- 5.Mandell J.C., Khurana B., Smith J.T., et al. Osteomyelitis of the lower extremity: pathophysiology, imaging, and classification, with an emphasis on diabetic foot infection. Emerg Radiol. 2018;25:175–188. doi: 10.1007/s10140-017-1564-9. [DOI] [PubMed] [Google Scholar]
- 6.Ertel W., Keel M., Marty D., et al. Significance of systemic inflammation in 1278 trauma patients. Unfallchirurg. 1998;101:520–526. doi: 10.1007/s001130050304. [DOI] [PubMed] [Google Scholar]
- 7.Worlock P., Slack R., Harvey L., et al. The prevention of infection in open fractures: an experimental study of the effect of fracture stability. Injury. 1994;25:31–38. doi: 10.1016/0020-1383(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 8.Nast-Kolb D., Betz A., Schweiberer L. The change in accident surgery in the last 10 years--a contribution to infection prevention. Chirurg. 1991;62:846–851. [PubMed] [Google Scholar]
- 9.Zhubrak M., Bar-David T. Systemic nickel allergy after internal fixation of a bunionectomy. J Foot Ankle Surg. 2014;53:466–467. doi: 10.1053/j.jfas.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Roberts T.T., Haines C.M., Uhl R.L. Allergic or hypersensitivity reactions to orthopaedic implants. J Am Acad Orthop Surg. 2017;25:693–702. doi: 10.5435/jaaos-d-16-00007. [DOI] [PubMed] [Google Scholar]
- 11.Swiontkowski M.F., Agel J., Schwappach J., et al. Cutaneous metal sensitivity in patients with orthopaedic injuries. J Orthop Trauma. 2001;15:86–89. doi: 10.1097/00005131-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Lew D.P., Waldvogel F.A. Osteomyelitis. Lancet. 2004;364:369–379. doi: 10.1016/s0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 13.Salcedo-Dueñas J.A., Algarín-Reyes J.A. The most frequent organisms in open fractures in Mexico. Acta Ortop Mex. 2011;25:276–281. [PubMed] [Google Scholar]
- 14.Lowy F.D. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/nejm199808203390806. [DOI] [PubMed] [Google Scholar]
- 15.Claro T., Widaa A., McDonnell C., et al. Staphylococcus aureus protein A binding to osteoblast tumour necrosis factor receptor 1 results in activation of nuclear factor kappa B and release of interleukin-6 in bone infection. Microbiology (Reading) 2013;159:147–154. doi: 10.1099/mic.0.063016-0. [DOI] [PubMed] [Google Scholar]
- 16.Marriott I., Gray D.L., Tranguch S.L., et al. Osteoblasts express the inflammatory cytokine interleukin-6 in a murine model of Staphylococcus aureus osteomyelitis and infected human bone tissue. Am J Pathol. 2004;164:1399–1406. doi: 10.1016/s0002-9440(10)63226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somayaji S.N., Ritchie S., Sahraei M., et al. Staphylococcus aureus induces expression of receptor activator of NF-kappaB ligand and prostaglandin E2 in infected murine osteoblasts. Infect Immun. 2008;76:5120–5126. doi: 10.1128/iai.00228-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander E.H., Rivera F.A., Marriott I., et al. Staphylococcus aureus - induced tumor necrosis factor - related apoptosis - inducing ligand expression mediates apoptosis and caspase-8 activation in infected osteoblasts. BMC Microbiol. 2003;3:5. doi: 10.1186/1471-2180-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young A.B., Cooley I.D., Chauhan V.S., et al. Causative agents of osteomyelitis induce death domain-containing TNF-related apoptosis-inducing ligand receptor expression on osteoblasts. Bone. 2011;48:857–863. doi: 10.1016/j.bone.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Cassat J.E., Hammer N.D., Campbell J.P., et al. A secreted bacterial protease tailors the Staphylococcus aureus virulence repertoire to modulate bone remodeling during osteomyelitis. Cell Host Microbe. 2013;13:759–772. doi: 10.1016/j.chom.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.König B., Prévost G., Piémont Y., et al. Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J Infect Dis. 1995;171:607–613. doi: 10.1093/infdis/171.3.607. [DOI] [PubMed] [Google Scholar]
- 22.Hofstee M.I., Muthukrishnan G., Atkins G.J., et al. Current concepts of osteomyelitis: from pathologic mechanisms to advanced research methods. Am J Pathol. 2020;190:1151–1163. doi: 10.1016/j.ajpath.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann S.R., Kapplusch F., Girschick H.J., et al. Chronic recurrent multifocal osteomyelitis (CRMO): presentation, pathogenesis, and treatment. Curr Osteoporos Rep. 2017;15:542–554. doi: 10.1007/s11914-017-0405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faist E., Kupper T.S., Baker C.C., et al. Depression of cellular immunity after major injury. Its association with posttraumatic complications and its reversal with immunomodulation. Arch Surg. 1986;121:1000–1005. doi: 10.1001/archsurg.1986.01400090026004. [DOI] [PubMed] [Google Scholar]
- 25.Hoch R.C., Rodriguez R., Manning T., et al. Effects of accidental trauma on cytokine and endotoxin production. Crit Care Med. 1993;21:839–845. doi: 10.1097/00003246-199306000-00010. [DOI] [PubMed] [Google Scholar]
- 26.O'Mahony J.B., Palder S.B., Wood J.J., et al. Depression of cellular immunity after multiple trauma in the absence of sepsis. J Trauma. 1984;24:869–875. doi: 10.1097/00005373-198410000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Wichmann M.W., Remmers D., Ayala A., et al. Contribution of soft tissue trauma and/or bone fracture to immune suppression after hemorrhagic shock in the animal experiment. Unfallchirurg. 1998;101:37–41. doi: 10.1007/s001130050230. [DOI] [PubMed] [Google Scholar]
- 28.Ertel W., Faist E. Immunologic monitoring after severe trauma. Unfallchirurg. 1993;96:200–212. [PubMed] [Google Scholar]
- 29.Deitch E.A. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillou P.J. Biological variation in the development of sepsis after surgery or trauma. Lancet. 1993;342:217–220. doi: 10.1016/0140-6736(93)92303-b. [DOI] [PubMed] [Google Scholar]
- 31.Romanò C.L., Romanò D., Logoluso N., et al. Bone and joint infections in adults: a comprehensive classification proposal. Eur Orthop Traumatol. 2011;1:207–217. doi: 10.1007/s12570-011-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ger R. Muscle transposition for treatment and prevention of chronic post-traumatic osteomyelitis of the tibia. J Bone Joint Surg Am. 1977;59:784–791. [PubMed] [Google Scholar]
- 33.Waldvogel F.A., Medoff G., Swartz M.N. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med. 1970;282:198–206. doi: 10.1056/nejm197001222820406. [DOI] [PubMed] [Google Scholar]
- 34.Kavanagh N., Ryan E.J., Widaa A., et al. Staphylococcal osteomyelitis: disease progression, treatment challenges, and future directions. Clin Microbiol Rev. 2018;31 doi: 10.1128/CMR.00084-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May J.W., Jupiter J.B., Weiland A.J., et al. Clinical classification of post-traumatic tibial osteomyelitis. J Bone Joint Surg Am. 1989;71:1422–1428. [PubMed] [Google Scholar]
- 36.Kelly P.J. Infected nonunion of the femur and tibia. Orthop Clin N Am. 1984;15:481–490. [PubMed] [Google Scholar]
- 37.Gordon L., Chiu E.J. Treatment of infected non-unions and segmental defects of the tibia with staged microvascular muscle transplantation and bone-grafting. J Bone Joint Surg Am. 1988;70:377–386. [PubMed] [Google Scholar]
- 38.May J.W., Jr., Gallico G.G., 3rd, Jupiter J., et al. Free latissimus dorsi muscle flap with skin graft for treatment of traumatic chronic bony wounds. Plast Reconstr Surg. 1984;73:641–651. doi: 10.1097/00006534-198404000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Cierny G., 3rd, Mader J.T., Penninck J.J. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003:7–24. doi: 10.1097/01.blo.0000088564.81746.62. [DOI] [PubMed] [Google Scholar]
- 40.Mader J.T., Shirtliff M., Calhoun J.H. Staging and staging application in osteomyelitis. Clin Infect Dis. 1997;25:1303–1309. doi: 10.1086/516149. [DOI] [PubMed] [Google Scholar]
- 41.Mader J.T., Shirtliff M., Calhoun J.H. The host and the skeletal infection: classification and pathogenesis of acute bacterial bone and joint sepsis. Baillieres Best Pract Res Clin Rheumatol. 1999;13:1–20. doi: 10.1053/berh.1999.0003. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt H.G., Diefenbeck M., Krenn V., et al. Classification of haematogenous and post-traumatic osteomyelitis. Z für Orthop Unfallchirurgie. 2014;152:334–342. doi: 10.1055/s-0034-1368620. [DOI] [PubMed] [Google Scholar]
- 43.Hake M.E., Oh J.K., Kim J.W., et al. Difficulties and challenges to diagnose and treat post-traumatic long bone osteomyelitis. Eur J Orthop Surg Traumatol. 2015;25:1–3. doi: 10.1007/s00590-014-1576-z. [DOI] [PubMed] [Google Scholar]
- 44.Vijayanathan S., Butt S., Gnanasegaran G., et al. Advantages and limitations of imaging the musculoskeletal system by conventional radiological, radionuclide, and hybrid modalities. Semin Nucl Med. 2009;39:357–368. doi: 10.1053/j.semnuclmed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Sanders J., Mauffrey C. Long bone osteomyelitis in adults: fundamental concepts and current techniques. Orthopedics. 2013;36:368–375. doi: 10.3928/01477447-20130426-07. [DOI] [PubMed] [Google Scholar]
- 46.Jutte P., Lazzeri E., Sconfienza L.M., et al. Diagnostic flowcharts in osteomyelitis, spondylodiscitis and prosthetic joint infection. Q J Nucl Med Mol Imaging. 2014;58:2–19. [PubMed] [Google Scholar]
- 47.Elgazzar A.H., Abdel-Dayem H.M., Clark J.D., et al. Multimodality imaging of osteomyelitis. Eur J Nucl Med. 1995;22:1043–1063. doi: 10.1007/bf00808418. [DOI] [PubMed] [Google Scholar]
- 48.Schauwecker D.S. The scintigraphic diagnosis of osteomyelitis. AJR Am J Roentgenol. 1992;158:9–18. doi: 10.2214/ajr.158.1.1727365. [DOI] [PubMed] [Google Scholar]
- 49.Devillers A., Garin E., Polard J.L., et al. Comparison of Tc-99m-labelled antileukocyte fragment Fab' and Tc-99m-HMPAO leukocyte scintigraphy in the diagnosis of bone and joint infections: a prospective study. Nucl Med Commun. 2000;21:747–753. doi: 10.1097/00006231-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Maugendre D., Poirier J.Y. Nuclear medicine in the diagnosis of diabetic foot osteomyelitis. Diabetes Metab. 2001;27:396–400. [PubMed] [Google Scholar]
- 51.de Lima Ramos P.A., Martin-Comin J., Bajén M.T., et al. Simultaneous administration of 99Tcm-HMPAO-labelled autologous leukocytes and 111In-labelled non-specific polyclonal human immunoglobulin G in bone and joint infections. Nucl Med Commun. 1996;17:749–757. doi: 10.1097/00006231-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Krznaric E., Roo M.D., Verbruggen A., et al. Chronic osteomyelitis: diagnosis with technetium-99m-d, l-hexamethylpropylene amine oxime labelled leucocytes. Eur J Nucl Med. 1996;23:792–797. doi: 10.1007/bf00843708. [DOI] [PubMed] [Google Scholar]
- 53.Glaudemans A.W., Israel O., Slart R.H. Pitfalls and limitations of radionuclide and hybrid imaging in infection and inflammation. Semin Nucl Med. 2015;45:500–512. doi: 10.1053/j.semnuclmed.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Vos F.J., Bleeker-Rovers C.P., Corstens F.H., et al. FDG-PET for imaging of non-osseous infection and inflammation. Q J Nucl Med Mol Imaging. 2006;50:121–130. [PubMed] [Google Scholar]
- 55.Mumme T., Reinartz P., Alfer J., et al. Diagnostic values of positron emission tomography versus triple-phase bone scan in hip arthroplasty loosening. Arch Orthop Trauma Surg. 2005;125:322–329. doi: 10.1007/s00402-005-0810-x. [DOI] [PubMed] [Google Scholar]
- 56.Goebel M., Rosa F., Tatsch K., et al. Diagnosis of chronic osteitis of the bones in the extremities. Relative value of F-18 FDG-PET. Unfallchirurg. 2007;110:859–866. doi: 10.1007/s00113-007-1302-y. [DOI] [PubMed] [Google Scholar]
- 57.Wenter V., Müller J.P., Albert N.L., et al. The diagnostic value of [(18)F]FDG PET for the detection of chronic osteomyelitis and implant-associated infection. Eur J Nucl Med Mol Imag. 2016;43:749–761. doi: 10.1007/s00259-015-3221-4. [DOI] [PubMed] [Google Scholar]
- 58.Palestro C.J., Kipper S.L., Weiland F.L., et al. Osteomyelitis: diagnosis with (99m)Tc-labeled antigranulocyte antibodies compared with diagnosis with (111)In-labeled leukocytes--initial experience. Radiology. 2002;223:758–764. doi: 10.1148/radiol.2233011072. [DOI] [PubMed] [Google Scholar]
- 59.Reuland P., Winker K.H., Heuchert T., et al. Detection of infection in postoperative orthopedic patients with technetium-99m-labeled monoclonal antibodies against granulocytes. J Nucl Med. 1991;32:2209–2214. [PubMed] [Google Scholar]
- 60.Horger M., Eschmann S.M., Pfannenberg C., et al. The value of SPET/CT in chronic osteomyelitis. Eur J Nucl Med Mol Imag. 2003;30:1665–1673. doi: 10.1007/s00259-003-1321-z. [DOI] [PubMed] [Google Scholar]
- 61.Glaudemans A.W., de Vries E.F., Vermeulen L.E., et al. A large retrospective single-centre study to define the best image acquisition protocols and interpretation criteria for white blood cell scintigraphy with ⁹⁹mTc-HMPAO-labelled leucocytes in musculoskeletal infections. Eur J Nucl Med Mol Imag. 2013;40:1760–1769. doi: 10.1007/s00259-013-2481-0. [DOI] [PubMed] [Google Scholar]
- 62.Gupta A., Subhas N., Primak A.N., et al. Metal artifact reduction: standard and advanced magnetic resonance and computed tomography techniques. Radiol Clin. 2015;53:531–547. doi: 10.1016/j.rcl.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Kaim A., Ledermann H.P., Bongartz G., et al. Chronic post-traumatic osteomyelitis of the lower extremity: comparison of magnetic resonance imaging and combined bone scintigraphy/immunoscintigraphy with radiolabelled monoclonal antigranulocyte antibodies. Skeletal Radiol. 2000;29:378–386. doi: 10.1007/s002560000228. [DOI] [PubMed] [Google Scholar]
- 64.Patzakis M.J., Wilkins J., Kumar J., et al. Comparison of the results of bacterial cultures from multiple sites in chronic osteomyelitis of long bones. A prospective study. J Bone Joint Surg Am. 1994;76:664–666. doi: 10.2106/00004623-199405000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Yang L., Feng J., Liu J., et al. Pathogen identification in 84 Patients with post-traumatic osteomyelitis after limb fractures. Ann Palliat Med. 2020;9:451–458. doi: 10.21037/apm.2020.03.29. [DOI] [PubMed] [Google Scholar]
- 66.Peng J., Ren Y., He W., et al. Epidemiological, clinical and microbiological characteristics of patients with post-traumatic osteomyelitis of limb fractures in southwest China: a hospital-based study. J Bone Jt Infect. 2017;2:149–153. doi: 10.7150/jbji.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stengel D., Bauwens K., Sehouli J., et al. Systematic review and meta-analysis of antibiotic therapy for bone and joint infections. Lancet Infect Dis. 2001;1:175–188. doi: 10.1016/s1473-3099(01)00094-9. [DOI] [PubMed] [Google Scholar]
- 68.Kuehn K.D., Ege W., Gopp U. Acrylic bone cements: composition and properties. Orthop Clin N Am. 2005;36:17–28. doi: 10.1016/j.ocl.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 69.Shandrick S., Zhao Q., Han Q., et al. Monitoring molecular recognition of the ribosomal decoding site. Angew Chem Int Ed Engl. 2004;43:3177–3182. doi: 10.1002/anie.200454217. [DOI] [PubMed] [Google Scholar]
- 70.Kaul M., Barbieri C.M., Pilch D.S. Fluorescence-based approach for detecting and characterizing antibiotic-induced conformational changes in ribosomal RNA: comparing aminoglycoside binding to prokaryotic and eukaryotic ribosomal RNA sequences. J Am Chem Soc. 2004;126:3447–3453. doi: 10.1021/ja030568i. [DOI] [PubMed] [Google Scholar]
- 71.Borovinskaya M.A., Pai R.D., Zhang W., et al. Structural basis for aminoglycoside inhibition of bacterial ribosome recycling. Nat Struct Mol Biol. 2007;14:727–732. doi: 10.1038/nsmb1271. [DOI] [PubMed] [Google Scholar]
- 72.Wang L., Pulk A., Wasserman M.R., et al. Allosteric control of the ribosome by small-molecule antibiotics. Nat Struct Mol Biol. 2012;19:957–963. doi: 10.1038/nsmb.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Magalhães M.L., Vetting M.W., Gao F., et al. Kinetic and structural analysis of bisubstrate inhibition of the Salmonella enterica aminoglycoside 6'-N-acetyltransferase. Biochemistry. 2008;47:579–584. doi: 10.1021/bi701957c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Azucena E., Mobashery S. Aminoglycoside-modifying enzymes: mechanisms of catalytic processes and inhibition. Drug Resist Updates. 2001;4:106–117. doi: 10.1054/drup.2001.0197. [DOI] [PubMed] [Google Scholar]
- 75.Wright G.D. Bacterial resistance to antibiotics: enzymatic degradation and modification. Adv Drug Deliv Rev. 2005;57:1451–1470. doi: 10.1016/j.addr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Peacock S.J., Paterson G.K. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu Rev Biochem. 2015;84:577–601. doi: 10.1146/annurev-biochem-060614-034516. [DOI] [PubMed] [Google Scholar]
- 77.Beach J.E., Perrott J., Turgeon R.D., et al. Penetration of Vancomycin into the cerebrospinal fluid: a systematic review. Clin Pharmacokinet. 2017;56:1479–1490. doi: 10.1007/s40262-017-0548-y. [DOI] [PubMed] [Google Scholar]
- 78.Wahlig H., Dingeldein E., Bergmann R., et al. The release of gentamicin from polymethylmethacrylate beads. An experimental and pharmacokinetic study. J Bone Joint Surg. 1978;60-B:270–275. doi: 10.1302/0301-620x.60b2.659478. [DOI] [PubMed] [Google Scholar]
- 79.Calhoun J.H., Henry S.L., Anger D.M., et al. The treatment of infected nonunions with gentamicin-polymethylmethacrylate antibiotic beads. Clin Orthop Relat Res. 1993:23–27. [PubMed] [Google Scholar]
- 80.Ostermann P.A., Seligson D., Henry S.L. Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. J Bone Joint Surg. 1995;77:93–97. [PubMed] [Google Scholar]
- 81.Xie Z., Liu X., Jia W., et al. Treatment of osteomyelitis and repair of bone defect by degradable bioactive borate glass releasing vancomycin. J Contr Release. 2009;139:118–126. doi: 10.1016/j.jconrel.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 82.Cattaneo R., Catagni M., Johnson E.E. The treatment of infected nonunions and segmental defects of the tibia by the methods of Ilizarov. Clin Orthop Relat Res. 1992;280:143–152. [PubMed] [Google Scholar]
- 83.Paley D., Catagni M.A., Argnani F., et al. Ilizarov treatment of tibial nonunions with bone loss. Clin Orthop Relat Res. 1989;241:146–165. [PubMed] [Google Scholar]
- 84.Yikemu X., Tuxun A., Nuermaimaiti M., et al. Effects of vacuum sealing drainage combined with Ilizarov bone transport technique in the treatment of tibial traumatic osteomyelitis. Med Sci Monit. 2019;25:6864–6871. doi: 10.12659/msm.915450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X., Luo F., Huang K., et al. Induced membrane technique for the treatment of bone defects due to post-traumatic osteomyelitis. Bone Joint Res. 2016;5:101–105. doi: 10.1302/2046-3758.53.2000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tong K., Zhong Z., Peng Y., et al. Masquelet technique versus Ilizarov bone transport for reconstruction of lower extremity bone defects following posttraumatic osteomyelitis. Injury. 2017;48:1616–1622. doi: 10.1016/j.injury.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 87.Karger C., Kishi T., Schneider L., et al. Treatment of posttraumatic bone defects by the induced membrane technique. Orthop Traumatol Surg Res. 2012;98:97–102. doi: 10.1016/j.otsr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 88.Thaddeus Chika A., Emeka O.M. Whole clavicle sequestration from chronic osteomyelitis in a 10 year old boy: a case report and review of the literature. Ann Med Surg (Lond). 2016;6:92–95. doi: 10.1016/j.amsu.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chan J.K.K., Ferguson J.Y., Scarborough M., et al. Management of post-traumatic osteomyelitis in the lower limb: current state of the art. Indian J Plast Surg. 2019;52:62–72. doi: 10.1055/s-0039-1687920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chan J.K., Harry L., Williams G., et al. Soft-tissue reconstruction of open fractures of the lower limb: muscle versus fasciocutaneous flaps. Plast Reconstr Surg. 2012;130:284e–295e. doi: 10.1097/PRS.0b013e3182589e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cierny G., 3rd, Byrd H.S., Jones R.E. Primary versus delayed soft tissue coverage for severe open tibial fractures. A comparison of results. Clin Orthop Relat Res. 1983;178:54–63. [PubMed] [Google Scholar]
- 92.Mouës C.M., Vos M.C., van den Bemd G.J., et al. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11–17. doi: 10.1111/j.1067-1927.2004.12105.x. [DOI] [PubMed] [Google Scholar]
- 93.Gokalp M.A., Guner S., Ceylan M.F., et al. Results of treatment of chronic osteomyelitis by “gutter procedure and muscle flap transposition operation”. Eur J Orthop Surg Traumatol. 2014;24:415–419. doi: 10.1007/s00590-013-1196-z. [DOI] [PubMed] [Google Scholar]
- 94.Schaverien M.V., Hamilton S.A., Fairburn N., et al. Lower limb reconstruction using the islanded posterior tibial artery perforator flap. Plast Reconstr Surg. 2010;125:1735–1743. doi: 10.1097/PRS.0b013e3181ccdc08. [DOI] [PubMed] [Google Scholar]
- 95.Bilge A., Öztürk Ö., Adali Y., et al. Could ozone treatment be a promising alternative for osteomyelitis? An experimental study. Acta Ortopédica Bras. 2018;26:67–71. doi: 10.1590/1413-785220182601179926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rose D. Hyperbaric oxygen therapy for chronic refractory osteomyelitis. Am Fam Physician. 2012;86:888. [PubMed] [Google Scholar]
- 97.Liu X.T., Zhang C.J., Li Z., et al. Chinese herbs application combined with operation for treating post-traumatic tibial osteomyelitis complicated with bone-skin defects. Zhongguo Gu Shang = China J Orthop Traumatol. 2008;21:218–220. [PubMed] [Google Scholar]
- 98.Wu Z.Q., Zeng D.L., Yao J.L., et al. Research progress on diagnosis and treatment of chronic osteomyelitis. Chin Med Sci J. 2019;34:211–220. doi: 10.24920/003493. [DOI] [PubMed] [Google Scholar]
- 99.Imlay J.A., Linn S. Damage and oxygen radical toxicity. Science. 1988;240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- 100.Mohammadi Z., Dummer P.M. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44:697–730. doi: 10.1111/j.1365-2591.2011.01886.x. [DOI] [PubMed] [Google Scholar]