Abstract
Background:
Genome-wide association studies (GWASs) of asthma have identified several risk alleles and loci, but most have been conducted in individuals with European-ancestry. Studies in Asians, especially children, are still lacking. We aimed to identify susceptibility loci by performing the first GWAS of asthma in Korean children with persistent asthma.
Methods:
We used a discovery set of 741 children with persistent asthma as cases and 589 healthy children and 551 healthy adults as controls to perform a GWAS. We validated our GWAS findings using UK Biobank data. We then used the Genotype-Tissue Expression database to identify expression quantitative trait loci of candidate variants. Finally, we quantified proteins of genes associated with asthma.
Results:
Variants at the 17q12–21 locus and SNPs in CYBRD1 and TNFSF15 genes were associated with persistent childhood asthma at genome-wide thresholds of significance. Four SNPs in the TNFSF15 gene were also associated with childhood-onset asthma in British white participants in the UK Biobank data. The asthma-associated rs7856856-C allele, the lead SNP, was associated with decreased TNFSF15 expression in whole blood and in arteries. Korean children with asthma had lower serum TNFSF15 levels than controls, and those with the asthma risk rs7856856-CC genotype exhibited the lowest serum TNFSF15 levels overall, especially asthmatic children.
Conclusions:
Our GWAS of persistent childhood asthma with allergic sensitization identified a new susceptibility gene, TNFSF15, and replicated associations at the 17q12–21 childhood-onset asthma locus. This novel association may be mediated by reduced expression of serum TNFSF15 and loss of suppression of angiogenesis.
Keywords: allergic sensitization, asthma, children, genome-wide association study, TNFSF15
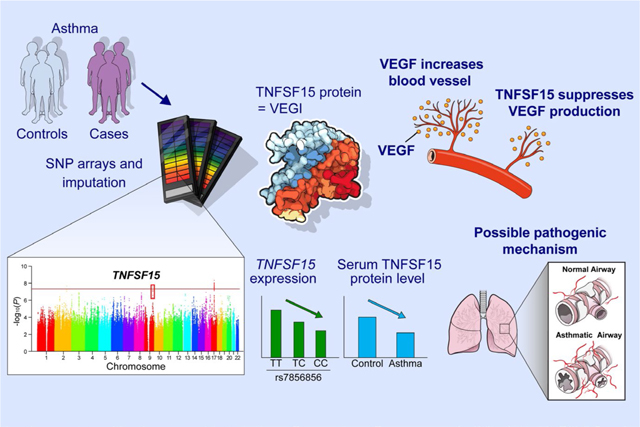
GRAPHICAL ABSTRACT
Our genome-wide association studies identify a new susceptibility gene, TNFSF15 for persistent asthma in children. Children with asthma have lower serum TNFSF15 levels than controls, and those with the asthma risk rs7856856-CC genotype exhibit the lowest serum TNFSF15 levels. This novel association may be mediated by loss of suppression of angiogenesis via lower expression of TNFSF15.
1 |. INTRODUCTION
Asthma is the most prevalent chronic respiratory disease in children worldwide.1 It is heterogeneous in nature and is caused by complex gene-gene and gene-environment interactions.2 Over the past decade, genome-wide association studies (GWASs) of asthma have identified many risk loci, including 17q12–21 (ORMDL3, GSDMB), 6p21 (HLA region), 2q12 (IL1RL1/IL18R1), 5q22 (TSLP), and 9p24 (IL33), which have provided an understanding of its genetic architecture and the molecular pathways involved in disease pathogenesis.3 Moreover, recent large-scale meta-analyses from the Trans-National Asthma Genetic Consortium (TAGC)4 and UK Biobank5 have identified novel asthma loci with trans-ethnic effects and further provided essential insights into the immunological relevance of asthma-associated risk variants.
Most GWASs and large meta-analyses of asthma have involved subjects of European-ancestry. Thus, inferences regarding the genetic architecture of asthma are based on observations in this population, whereas much less is known about the populations of Asian and African ancestry. As populations vary with respect to allele frequencies, patterns of linkage disequilibrium (LD), and effect sizes of variants that underlie disease risk,6,7 inferences based on Europeans may have limited utility in other groups. For example, a next-generation sequencing study of 10 asthma candidate genes revealed differences in allele frequencies and haplotype structures at the 17q12–21 asthma locus between Chinese and other ethnic groups.8 As a result, GWASs in European populations will most likely miss risk variants important in non-European populations.
Only two GWASs of asthma cases and controls in Asian populations have been reported in the GWAS catalog.9 Both were conducted in Japanese populations, one for childhood asthma (N = 938)10 and one for adult asthma (N = 7171).11 These studies identified HLA-DP as a childhood asthma locus and 5q22 (TSLP), 4q31(USP38), 10p14, and 6p21 (HLA region) as adult asthma loci. GWASs were also conducted in Koreans for toluene diisocyanate-induced asthma, an occupation-associated form of asthma, and for aspirin-exacerbated respiratory disease.12–16 There was a recent report on the first GWAS of asthma in Korean adults, but no genome-wide significant variants were identified.17 A GWAS of childhood asthma in the Korean population has not previously been reported.
In this study, we present the results of the first GWAS of asthma in Korean children. Our goal was to identify novel genetic susceptibilities predicting disease risk for persistent asthma in these children and to translate genetic findings into disease mechanisms via functional, bioinformatic, and enrichment analyses.
2 |. METHODS
2.1 |. Clinical evaluations
We recruited 741 children with both persistent asthma and allergic sensitization as cases. Asthma was confirmed based on persistent respiratory symptoms verified by physicians. Presence of either a bronchodilator (BD) response with ≥12% increase in forced expiratory volume in 1 s (FEV1) or bronchial hyper-responsiveness (BHR), defined as a decrease in FEV1 of ≥20% with inhalation of <16 mg/ml methacholine, was considered symptomatic of asthma. Persistent asthma was assessed based on the level of treatment required, with at least regular controller treatment, in compliance with the Global Initiative for Asthma.18 Allergic sensitization was defined by specific IgE levels >0.7 kU/L to at least one of the following foods or airborne allergens: egg white, milk, peanut, soybean, wheat, Dermatophagoides pteronyssinus (Der. p), Dermatophagoides farina (Der. f), Alternaria species, or Blattella germanica. Additionally, we recruited 589 children as controls during routine hospital visits. These children had a negative history of allergic diseases based on interviews with their parents, had negative serum specific IgE to six common allergens (egg white, milk, Der. p, Der. f, Alternaria species, or Blattella germanica) and had total serum IgE levels of less than 100 kU/L. We also confirmed that all control children had neither a BD response nor BHR. Lastly, we selected 551 adult control subjects with a negative history for both allergic diseases and allergic sensitization from among 1214 adults who had the results of BHR and allergic sensitization. Negative histories of allergic diseases, including asthma and atopic dermatitis, were based on self-administered questionnaires; lack of sensitization was based on a negative skin prick test response to 12 common allergens (Der. p, Der. f, two tree pollen mixtures, grass pollen mixture, ragweed, mugwort, cockroach, Alternaria species, Aspergillus species, cat dander, and dog dander). Blood eosinophils were determined using an NE-8000 hematology analyzer (Sysmex Corporation, Kobe, Japan), and total serum IgE, serum specific IgE, and eosinophil cationic protein were measured using the Pharmacia CAP assay (Uppsala, Sweden).
2.2 |. Genotyping, imputation, and quality control in the GWAS
Figure S1 summarizes our study design. As the three-genotype arrays contained different genetic variants, we performed imputations for each platform using a standardized method. For details on genotyping, imputation, and quality control methods, see the Supporting Methods.
2.3 |. Replication studies
To further examine the association of 103 SNPs with p-values <1.0 × 10−7 in our GWAS, we validated p-values of those SNPs using GWAS data of Caucasian British individuals from the UK Biobank data released on July 10, 2017.19 We used summary statistics from a GWAS (onset younger than 12 years), which included 9433 cases with childhood-onset asthma and 318,237 control subjects without an asthma diagnosis (ie, no self-reports and no ICD-10 codes).20
2.4 |. Bioinformatics and functional enrichment analysis
Gene ontology (GO) of asthma-associated genes was analyzed using the Database for Annotation, Visualization, and Integrated Discovery (DAVID; david.abcc.ncifcrf.gov/knowledgebase).21 To select genes associated with both asthma and angiogenesis, we first searched for genes at asthma loci in the GWAS catalog.9 This yielded 340 genes, which were classified into 880 GO terms. Among them, we focused on the single term “cardiovascular system development,” which yielded 24 asthma-associated genes (Table S2). These 24 genes were further analyzed with TNFSF15; visualizing gene networks using GeneMANIA.22 For cis-expression quantitative trait loci (cis-eQTL) analysis of rs7856856 in human tissues, see the Supporting Methods.
2.5 |. Statistical analysis
We evaluated genome-wide associations between childhood asthma and imputed variants, adjusting for age and sex, using PLINK (v.1.07).23 Associations between asthma and variants were examined with logistic regression assuming an additive genetic model. Logistic regression was performed using Python and PLINK. A genomic inflation factor (λ) was calculated based on median chi-square statistics. Thereafter, a Manhattan plot and a quantile-quantile plot were obtained using R software. Regional association plots were generated with LocusZoom.24 We analyzed the serum TNFSF15 levels using the Mann–Whitney U test and Jonckheere–Terpstra test; the Mann-Whitney U tests whether the two samples are likely drawn from the same population and the Jonckheere-Terpstrat tests for determining statistically significant differences between two or more groups of an independent variable on a continuous variable. All statistical analyses were conducted using IBMSPSS Statistics software version 20.0 (IBM Inc, Armonk, NY).
3 |. RESULTS
3.1 |. Characteristics of study participants
Clinical details of childhood asthma cases and controls, and adult controls included in the main GWAS are summarized in Table 1. The children’s mean ages were 8.6 and 9.2 years in the asthma and control groups, respectively. There were more males among children with asthma (68.1%) than among control children (44.7%, p < .001). Total serum IgE levels and blood eosinophils were higher in children with asthma than in control children (p < .001 for both). As expected, all lung function phenotypes, including FEV1, FEF25–75 (forced expiratory flow at 25%–75% of forced vital capacity), FEV1/FVC, and maximal BD response, were significantly different between the asthma cases and control groups in children (p < .001). In adult controls from the Ansung population-based cohort, the mean age was 65.0 years, and the number of males was 211 (38.3%).
TABLE 1.
Subject characteristics in the discovery group
| Children cases (n = 741) |
Children controls (n = 589) |
Adult controls (n = 551) | |||||
|---|---|---|---|---|---|---|---|
| Study population | Cohort 1 | Cohort 2 | Totala | Cohort 1 | Cohort 2 | Totala | |
| Sample size | 498 | 243 | 741 | 481 | 108 | 589 | 551 |
| Age, year | 8.6 ±2.9 | 8.7 ±3.0 | 8.6 ±2.9* | 9.1 ± 3.1 | 9.5 ± 3.1 | 9.2 ±3.1 | 64.9 ± 8.4 |
| Male, sex | 337 (67.7) | 168 (69.1) | 505(68.1)** | 214 (45.5) | 49 (45.3) | 263 (44.7) | 211 (38.3) |
| Total serum IgE, kU/L | 827.6 ± 877.2 | 817.4 ± 771.6 | 824.1 ±844.7** | 41.1 ±32.2 | 40.2 ± 32.1 | 40.8 ± 32.1 | - |
| Blood eosinophils, /mm | 578.0 ±363.1 | 571.6 ±364.9 | 575.6 ±363.8** | 173.4 ± 135.0 | 175.0 ± 172.0 | 174.0 ± 149.1 | - |
| Asthma-related phenotypes | |||||||
| FEV1, % predicted | 92.2 ± 16.1 | 91.4 ± 15.9 | 91.9 ± 16.5** | 104.4 ± 13.8 | 101.1 ± 15.6 | 103.3 ± 14.5 | 114.5 ± 18.4 |
| FEF25–75, % predicted | 76.5 ±25.7 | 80.1 ±28.1 | 77.7 ±26.6** | 101.2 ± 22.6 | 103.8 ± 25.4 | 102.1 ±23.6 | 100.7 ± 31.3 |
| FEV1/FVC, % | 85.3 ± 9.3 | 87.9 ± 9.7 | 86.2 ± 9.5** | 90.8 ± 5.4 | 93.1 ± 6.1 | 91.7 ±5.8 | 78.8 ±8.3 |
| Maximal BD response, % change in FEV1 | 9.1 (3.9, 14.4) | 9.4 (4.2, 15.3) | 9.25** (4.0, 14.75) | 2.9 (0.9, 5.4) | 4.0 (1.4, 6.1) | 3.4 (1.1, 5.9) | - |
| PC20methacholine, mg/dl | 4.52 (1.9, 8.1) | 3.7 (1.6, 7.3) | 4.2 (1.8, 7.9) | - | - | - | - |
Note: Data expressed as number, number (%), mean ± standard deviation, or median (interquartile range).
Abbreviations: BD, bronchodilator; FEF25–75, forced expiratory flow at 25%−75% of forced vital capacity; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; PC20methacholine, provocation concentration of methacholine required to produce a 20% fall in FEV1
Information on cohort 1 and cohort 2 is available in Figure S1. The values of total case data were compared to total control data in children.
p < .001.; *p < .05.
3.2 |. Genome-wide associations with asthma
A total of 4.6 million SNPs passed imputation and quality control and were included in the asthma GWAS in the discovery group. The genomic inflation factor (λ) was 1.03, indicating that our findings were not affected by systemic bias in the underlying population substructure. This was also confirmed by the p-value deviation on the quantile-quantile plot (Figure S2A).
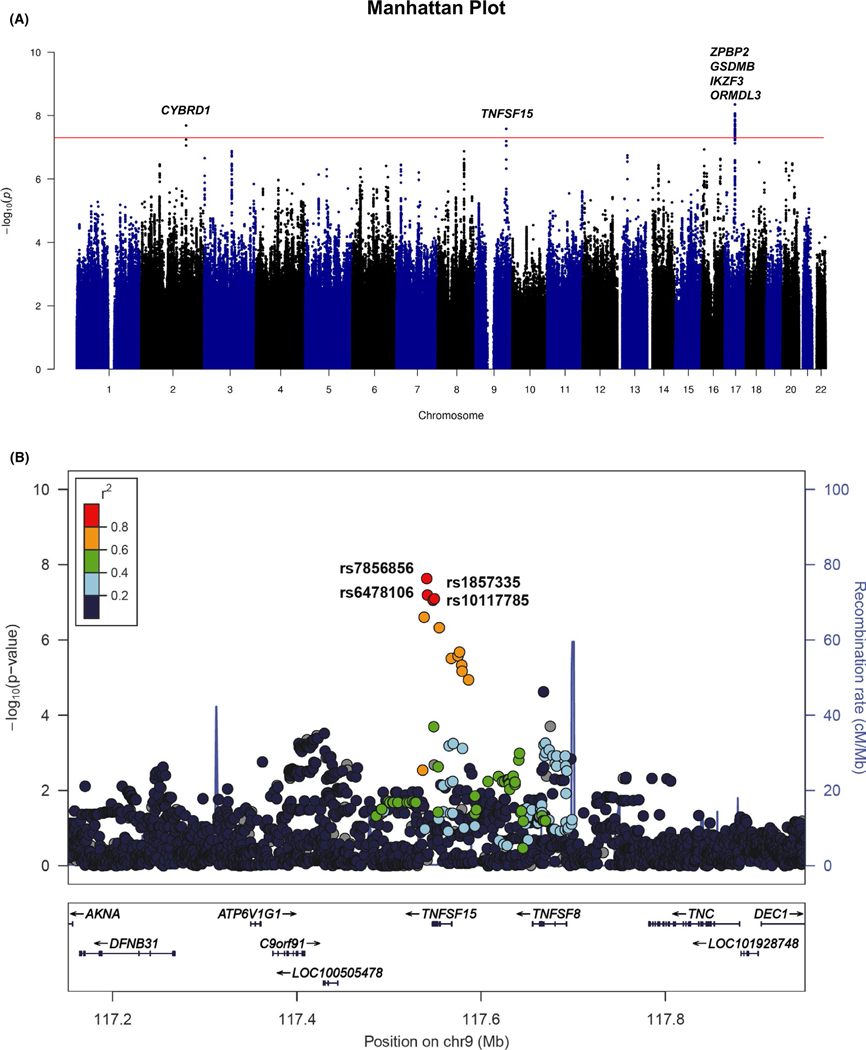
In the discovery GWAS (N = 1,881), at least one significant SNP at the three loci exhibited genome-wide significance (p < 5.0 × 10−8), with the most significant SNP with p-value = 4.45 × 10−9 at the chromosome 17q12–21 childhood-onset asthma locus (Figure 1A).25 Ninety-six SNPs at this locus had p < 1.0 × 10−7 (Table S1). The two other SNPs that exhibited genome-wide significance were in CYBRD1 on chromosome 2q31.1 and in TNFSF15 on chromosome 9q32; both of which are loci that have not been reported in previous asthma GWASs (Table 2). Two additional SNPs at the 2q31.1 locus and three additional SNPs at the 9q32 locus were significant at p < 1.0 × 10−7 (Table S1). To determine whether TNFSF15 variants were primarily associated with childhood asthma rather than allergic sensitization, total serum IgE levels were analyzed in both case and control groups by TNFSF15 genotype (Figure S5). There were no trends for the difference in total serum IgE levels between the genotypes, suggesting that TNFSF15 is primarily associated with childhood asthma but not with IgE levels (ie, allergic sensitization). We next sought to replicate the four most significant variants at the 17q12–21 locus in the discovery GWAS, and the seven variants at the two novel loci using summary statistics from a UK Biobank GWAS of childhood-onset asthma.20 The four variants at the 17q12–21 locus that were most associated with childhood asthma in Korean children were also highly associated with childhood-onset asthma in British white individuals (p < 10−96 for these variants). The four variants in TNFSF15 were also associated with childhood-onset asthma in the UK Biobank replication sample (p < 10−3) with the same direction of effect. However, the three variants in CYBRD1 were not associated with childhood-onset asthma in British white individuals (p > .299) (Table 2). Additionally, we investigated asthma loci that have been previously reported in multiple studies by looking at the results in our study for SNPs at previously reported at asthma GWAS loci. Our study identified SNPs at the 17q12–21 locus, the most widely replicated asthma region, with genome-wide significance, including rs7224129 (p = 4.45 × 10−9), rs34233420 (p = 1.01 × 10−8), and rs1008723 (p = 1.33 × 10−8). The widely replicated asthma loci including 2q12 (IL1RL1/IL18R1), 5q22 (TSLP), 5q31 (RAD50), 6p21 (HLA region), 12q13 (STAT6), and 15q22 (SMAD3) also showed nominal significance (p < .05) in our study, suggesting that these loci also contribute to asthma in Korean children but that our sample was not powered to detect these smaller effect loci compared to 17q12–21 (Table S4).
FIGURE 1.
Genome-wide association study (GWAS) reveals that TNFSF15 is associated with childhood asthma. (A) Manhattan plot of GWAS of persistent asthma with allergic sensitization in Korean children. Variants are plotted on the x-axis of the Manhattan plot according to their chromosomal position and their −log10 (p-value) is shown on the y-axis. The red line shows the genome-wide significance threshold (p < 5 × 10−8). (B) Regional association plot at the TNFSF15 locus for persistent childhood asthma. Genome build and linkage disequilibrium used in this plot is from hg19/1000 Genomes Asian (Nov 2014); the blue line indicates the recombination rate according to HapMap data. The four most significant SNPs in TNFSF15 are labeled. Colors correspond to LD (r2) with the lead SNP, rs7856856, as shown in the color scale in the upper left
TABLE 2.
Summary of significant SNPs for childhood asthma with p < 1.0 × 10−7 in a Korean population
| Chromosome | Position | rs number | Closestgenea | Allele | MAF | ORb (Discovery) | pc (Discovery) | ORb (Replication) | pc (Replication) |
|---|---|---|---|---|---|---|---|---|---|
| 2q31.1 | 172416992 | rs2542932 | CYBRD1 | C>G | 0.2095 | 0.517 (0.41–0.65) | 2.05 × 10−8 | 0.985 (0.956–1.014) | 3.12 × 10−1 |
| 172414462 | rs528 | CYBRD1 | G>A | 0.1953 | 0.52 (0.411–0.658) | 5.68 × 10−8 | 0.984 (0.956–1.013) | 3.02 × 10−1 | |
| 172412510 | rs2542940 | CYBRD1 | T>C | 0.1969 | 0.527(0.416–0.666) | 8.75 × 10−8 | 0.984 (0.956–1.013) | 2.99 × 10−1 | |
| 9q32 | 117540910 | rs7856856 | TNFSF15 | T>C | 0.349 | 1.819 (1.473–2.245) | 2.61 × 10−8 | 1.064(1.03–1.1) | 1.83 × 10−4 |
| 117545666 | rs6478106 | TNFSF15 | C>T | 0.347 | 1.798 (1.453–2.224) | 6.39 × 10−8 | 1.057(1.02–1.092) | 6.29 × 10−4 | |
| 117551718 | rs1857335 | TNFSF15 | C>T | 0.347 | 1.787(1.444–2.21) | 8.65 × 10−8 | 1.066(1.03–1.101) | 1.30 × 10−4 | |
| 117551603 | rs10117785 | TNFSF15 | T>A | 0.346 | 1.787(1.444–2.21) | 8.86 × 10−8 | 1.056(1.02–1.091) | 7.45 × 10−4 | |
| 17q12–21d | 38075426 | rs7224129 | GSDMB | G>A | 0.24 | 1.904(1.535–2.36) | 4.45 × 10−9 | 1.376 (1.337–1.417) | 7.25 × 10−102 |
| 38076137 | rs8074437 | ORMDL3 | T>G | 0.235 | 1.875 (1.513–2.323) | 9.01 × 10−9 | 1.378 (1.338–1.419) | 1.70 × 10−102 | |
| 38031857 | rs59716545 | ZPBP2 | T>G | 0.232 | 1.869 (1.502–2.324) | 1.91 × 10−8 | 1.388 (1.346–1.428) | 8.84 × 10−104 | |
| 38020419 | rs1453559 | IKZF3 | T>C | 0.234 | 1.851 (1.49–2.298) | 2.58 × 10−8 | 1.367(1.327–1.407) | 1.75 × 10−97 |
Abbreviations: MAF, minor allele frequency; NA, not applicable; OR, odds ratio.
The closest gene was annotated using the UCSC Genome Browser (GRCh37/hg19).
Odds ratio and 95% confidence interval (Cl) of minor allele.
p-values were determined by logistic regression analysis using an additive model.
The most significant SNPs at each gene are shown. All SNPs are listed in Table S1.
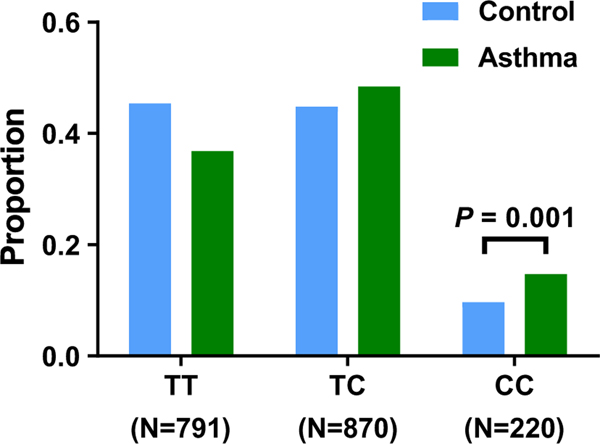
A regional association plot of the TNFSF15 locus is presented in Figure 1B. To further validate that the four imputed SNPs were associated with childhood asthma, we genotyped the lead SNP, rs7856856, which had high LD (r2 = 1.0, Figure S2B) with the other three SNPs in our discovery cohort. The proportion of CC genotypes in the asthma group (109/741, 14.7%) was significantly greater than in the control group (111/1140, 9.73%, p = .001) (Figure 2).
FIGURE 2.
rs7856856-C risk allele in TNFSF15 increases the susceptibility to asthma. Proportion of asthma by rs7856856 (T>C) by genotype. TNFSF15 rs7856856 genotypes on the x-axis indicate reference allele homozygotes (T/T), heterozygotes (T/C), and risk allele homozygotes (C/C). The proportion of CC genotypes in the asthma group (14.7%) was significantly higher than that in the control group (9.73%, p = .001)
3.3 |. Effect of rs7856856 on TNFSF15 expression
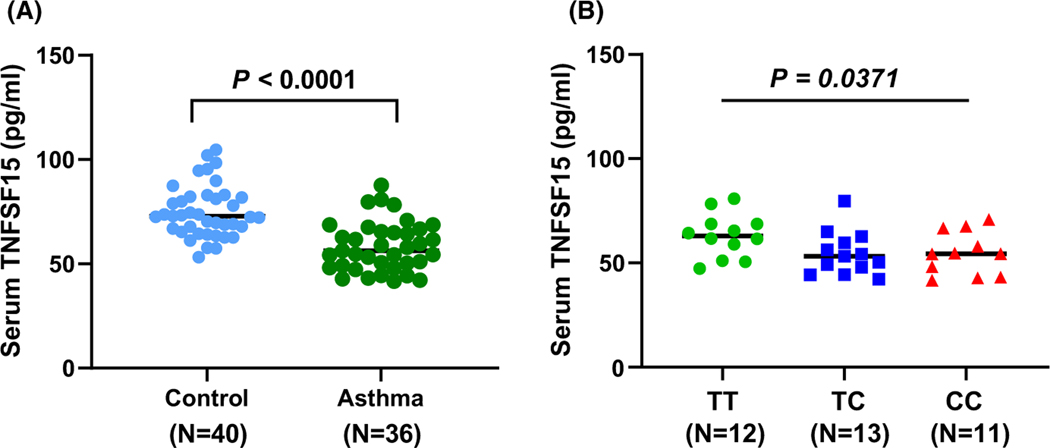
TNFSF15, which encodes the tumor necrosis factor super family-15 protein, is a modulator of vascular homeostasis and inflammation. It is also a cytokine that promotes the secretion of inflammatory factors that play important roles in immune regulation and inflammatory diseases.26–28 To further explore its function, we first examined the expression level of TNFSF15 in Genotype-Tissue Expression (GTEx).29 The asthma-associatedrs7856856-C allele, the lead SNP, was associated with decreased TNFSF15 expression in a dose-dependent (additive) manner in whole blood and in arteries (aorta and tibial), but not in the lung tissue (Figure S3). This suggested that TNFSF15 protein may be reduced in the blood of asthmatic children in our cohort compared to controls, and in children with the rs7856856-CC genotype. Indeed, in our sample, children with asthma had lower serum TNFSF15 levels (median [interquartile range], 56.30 [49.15–65.49] pg/ml) compared to controls (72.77 [60.50–81.88] pg/ml, p < .0001) (Figure 3A). Asthmatic children with the rs7856856-C risk allele had low TNFSF15 levels in serum (62.98 [57.00–68.66] pg/ml for TT, 53.17 [47.89–59.65] pg/ml for TC, 54.48 [45.72–62.34] pg/ml for CC, p for trend = .0371) (Figure 3B). There was no such trend in the controls (Figure S4), suggesting that both asthma status and the risk allele interact to affect TNFSF15 levels in the serum, possibly due to both transcriptional and post-transcriptional mechanisms. Taken together, our results indicate that the asthma-associated rs7856856 allele is correlated with decreased TNFSF15 transcript expression and that serum levels of TNFSF15 are decreased in asthmatics compared to controls, especially among asthmatic children with the rs7856856- C risk allele.
FIGURE 3.
rs7856856-C risk allele decreases serum levels of TNFSF15 in asthma group. (A) Serum TNFSF15 levels stratified by disease status. TNFSF15 levels in serum were lower in children with persistent asthma (median [interquartile range], 56.30 [49.15–65.49]) pg/ml than in controls (72.77 [60.50– 81.88] pg/ml, p < .0001; Mann-Whitney U test). (B) Serum TNFSF15 levels stratified by TNFSF15 rs7856856-C risk allele in children with asthma. Asthmatic children with the rs7856856-C risk allele had low TNFSF15 levels in the serum (62.98 [57.00–68.66] pg/ml for TT, 53.17 [47.89– 59.65] pg/ml for TC, 54.48 [45.72–62.34] pg/ml for CC, p for trend = .0371; Jonckheere–Terpstra test)
3.4 |. Effect of TNFSF15 on angiogenesis and its potential contribution to asthma
As serum levels of TNFSF15 were lower in asthmatic children compared to healthy controls, we investigated other known functions of TNFSF15. TNFSF15 also functions as an inhibitor of vascular endothelial cell growth factor, a well-known stimulator of angiogenesis. Angiogenesis has been regarded as an important event in the development of airway inflammation and tissue remodeling in asthma.30 Therefore, decreased TNFSF15 levels might contribute to increased angiogenesis in asthmatic patients.
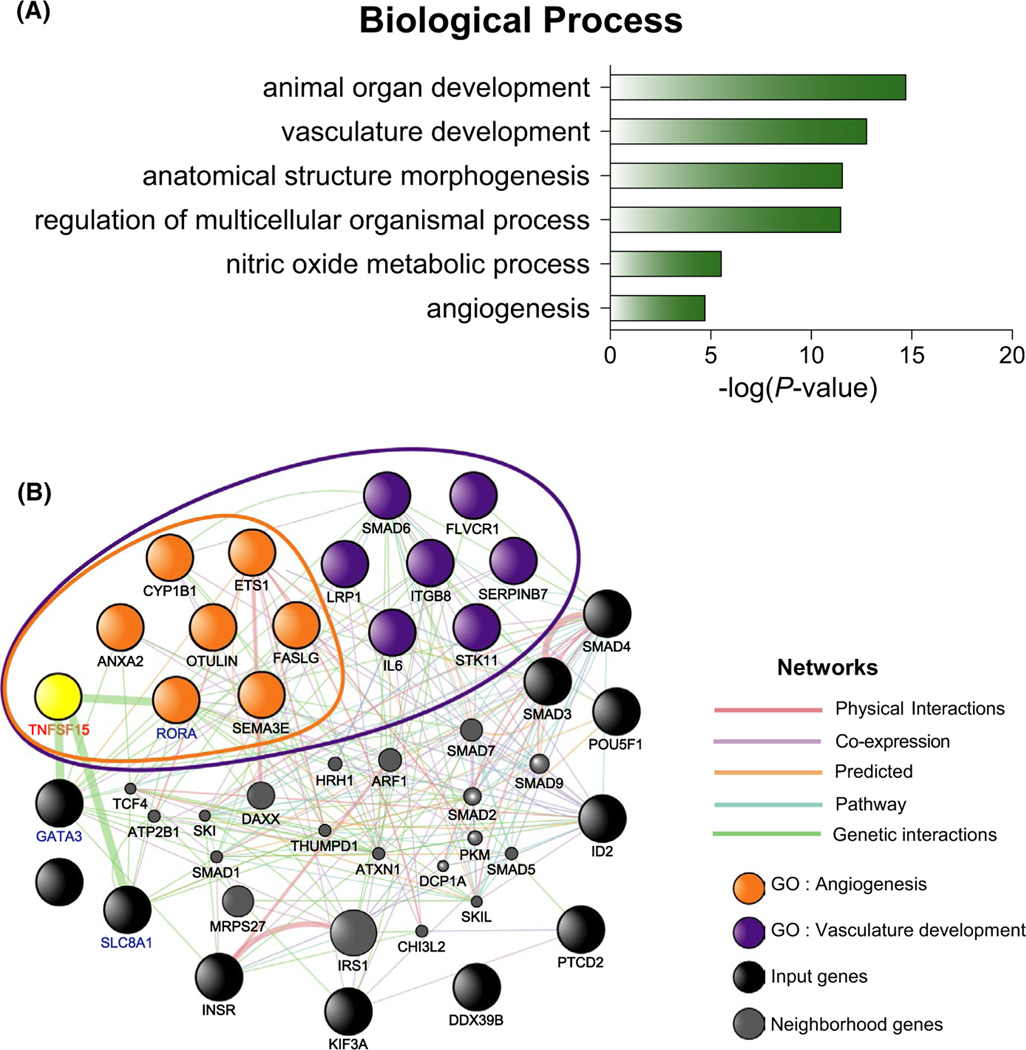
To further investigate this hypothesis, we examined asthma-associated genes for their involvement in angiogenesis. Among 340 genes reported in the GWAS Catalog for asthma, we focused on the 24 genes that were related to the GO term “cardiovascular system development” (Table S2). Further analysis of these 24 genes indicated that they are involved in six processes: animal organ development, vasculature development, anatomical structure morphogenesis, regulation of multicellular organismal development, nitric oxide metabolic process, and angiogenesis (Figure 4A and Table S3). Fourteen of the 24 genes were associated with the GO term “vasculature development,” and included all 7 genes associated with the GO term “angiogenesis” (Table S3). Finally, we examined the relationship between TNFSF15 and these 24 genes using GeneMANIA software. Genetic interactions were predicted between TNFSF15 with RORA, GATA3, and SLC8A1 (Figure 4B).
FIGURE 4.
Interaction of TNFSF15 with genes related to angiogenesis. (A) Gene ontology (GO) analysis of the 24 asthma genes that belong to cardiovascular system development. GO enrichment was retrieved using DAVID. Top six representative GO terms (p < .005) in the biological process category are shown. (B) Functional prediction of TNFSF15 with the 24 asthma genes which belong to cardiovascular system development. Interactions and/or co-expression were predicted using GeneMANIA software. The black, yellow, orange, and purple nodes represent input genes, and gray nodes indicate neighborhood genes of the input genes. TNFSF15 is shown in yellow. Genes in the purple-lined circle belong to the GO term “vasculature development.” Genes in the orange-lined circle belong to the GO term “angiogenesis.” The names of genes that have a genetic association with TNFSF15 are shown in bold blue. Node size is inversely proportional to the rank of the gene in a list sorted by gene score
4 |. DISCUSSION
GWASs of asthma with large sample sizes are necessary to identify asthma-associated loci.3 Given that large-scale studies increase clinical heterogeneity,4,31 we hypothesized that focusing on more stringent inclusion criteria for subjects would identify additional genes. Hence, we focused on children with persistent asthma and allergic sensitization and included children without current or prior history of allergic disease and a lack of allergic sensitization based on laboratory tests as control subjects, and not only by questionnaires. Our stringent criteria for cases and controls in a homogeneous Asian population resulted in a sample with <2000 subjects, yet we identified new risk alleles at genome-wide levels of significance and replicated associations with variants in the TNFSF15gene on chromosome 9, as well as the 17q12–21 locus, the most frequently replicated and most statistically significant locus in childhood-onset asthma GWAS across different populations.3
The most significant association in our GWAS was with SNPs (N = 96, smallest p = 4.45 × 10−9) on 17q12–21, including ZPBP2, GSDMB, IKZF, and ORMDL3. Chromosome 17q12–21 has been robustly associated with childhood asthma in previous studies.25 Our GWAS confirmed that variants at the 17q12–21 locus were also associated with childhood asthma at genome-wide significance levels in an Asian population. Notably, our results confirm that variants at the 17q12–21 locus affect asthma risk across ethnicities,4 including Asians, despite differences in allele frequencies and haplotype structures at this locus.8,32 Taken together, these results suggest that variants at the 17q12–21 locus may improve the accuracy of trans-ethnic polygenic risk scores.33 There are two previous asthma GWAS (for childhood and adult asthma, respectively) in Asian populations, and both were conducted with Japanese subjects. The childhood asthma GWAS showed only nominally significant associations (p < .05),10 but the adult asthma GWAS did not report any association at this locus.11 Our data further support recent evidence suggesting that childhood asthma has greater genetic risk with more significant overall effects compared to adult-onset asthma.20
Our GWAS of childhood asthma in Korean children identified two novel loci. Among them, TNFSF15 at 9q32, has been reported in a GWAS of European individuals. Moreover, the asthma-associated variants (rs7856856 T>C, rs6478106 C>T, rs1857335 C>T, and rs10117785 T>A) in the TNFSF15 gene at the replicated locus were associated with lower transcript levels in blood and aorta (GTEx) and decreased serum TNFSF15 levels in children with asthma. This suggests that reduced TNFSF15 expression is associated with an increased risk of childhood asthma. In particular, the rs7856856-C allele was associated with persistent asthma and decreased serum TNFSF15 levels in children with asthma, but not in controls. This suggests that TNFSF15 expression may be influenced by the effect of thers7856856-C allele differently in children with or without asthma risk.
The more modest p-values for the TNFSF15 SNPs in the replication studies could be explained by several factors. First, although the replication cohort of British-white subjects was much larger, it was also more heterogeneous with respect to clinical features than our cohort, which consisted of Korean children with clinically diagnosed persistent asthma and allergic sensitization. In contrast, childhood-onset asthma in the replication cohort was defined retrospectively by self-reported questionnaires and medical records in adults. Thus, only a subset of subjects in the childhood-onset GWAS used for replication would have met our criteria for persistent asthma and allergic sensitization, and associations specific to this phenotype may have been diluted in the larger study. In this context, our study provides another example of how careful phenotyping in smaller samples can reveal novel loci for asthma.34–36 Second, more general differences between the discovery and replication cohorts could underlie differences in estimated effect sizes of alleles at this locus. In addition to geographic, environmental, and cultural differences, the subjects in the UK Biobank childhood-onset asthma GWAS were born 40–70 years prior to recruitment (approximately 1950s through 1980s), whereas the Korean children in the discovery GWAS were born within the last 15 years. Thus, numerous temporal factors that have influenced the increased asthma prevalence over the past decades37 may have also been enriched for specific subtypes of asthma. A GWAS of childhood-onset asthma in a more contemporaneous cohort would be needed to evaluate this hypothesis.
A role of TNFSF15 in allergic asthma is indirectly supported by existing data. One of the functions of TNFSF15 is mediating the inflammatory pathway through activation of NF-kappa-B.38 Consistent with this role, variants at the TNFSF15 locus have been associated with increased susceptibility for inflammatory autoimmune diseases, such as Crohn’s disease,39 ulcerative colitis,40 ankylosing spondylitis,41 and psoriasis.41 TNFSF15 is also expressed in the lungs (Figure S3). Specifically, TNFSF15 is predominantly expressed in type II pneumocytes of both proximal and distal airway stromal cells (Figure S6). However, previous studies suggested that increased expression of TNFSF15 was associated with disease risk, whereas our study indicated the asthma-associated SNPs at this locus were associated with decreased expression levels of TNFSF15 in whole blood and arteries (GTEx), and lower serum levels of TNFSF15 in Korean children with asthma. The opposite direction of the allele effect on TNFSF15 expression is consistent with the hypothesis that many variants associated with both asthma and autoimmune diseases have opposite directions of effects42,43 even though they are both considered chronic inflammatory diseases.
In this regard, our findings are consistent with the view that suppression of angiogenesis may promote the development of childhood-onset asthma. Through GO and network analysis, we identified potential genetic interactions between TNFSF15 and genes at three asthma-associated GWAS loci: RORA, GATA3, and SLC8A1. Interestingly, these three genes have been implicated in angiogenesis,44–46 as well as in asthma.3,47 For example, an SNP in the RORA gene increased the incidence of neovascularization in age-related macular degeneration.44 SNPs in the SLC8A1 gene were associated with higher rates of coronary artery abnormalities via increased intracellular calcium mobilization,46 and GATA3 has been shown to mediate angiopoietin-induced AKT signaling and Tie2 expression in endothelial cells.45 All three of these genes are at reproducible loci in asthma GWASs. Further research is needed to elucidate the nature of the interactions between these genes and TNFSF15 in asthma, especially through angiogenic mechanisms. Taken together, our study not only identifies a novel locus for childhood asthma but also highlights the role of angiogenesis in the pathogenesis of asthma. In fact, targeting angiogenesis has been considered as a potential therapeutic option for the treatment of asthma.48,49
Despite the strengths of our study with respect to the careful clinical evaluations leading to novel GWAS discoveries, there were limitations. First, our data show that reduced TNFSF15 gene expression and reduced TNFSF15 levels are associated with the asthma risk rs7856856 variant (T>C), suggesting that loss of angiogenesis inhibition is a possible mechanism. However, functional studies directly evaluating this hypothesis have not yet been conducted. The fact that the effect of rs7856856 on TNFSF15 expression differs between tissues indicates that TNFSF15 expression in other tissues that have not been included in GTEx may be more applicable to asthma, such as airway epithelial or smooth muscle cells, or specific immune cell types. Thus, it remains possible that increased TNFSF15 expression in other tissues induces asthma via the TNFSF15 inflammatory pathway. Second, while the replication of our findings in a GWAS of European-ancestry subjects suggests that the associations at the TNFSF15 locus with childhood asthma is common across populations, this question still needs to be more broadly explored in cohorts of other ancestries and ethnicities.
In conclusion, we report an association between variations in the TNFSF15 gene and the development of persistent asthma in children of Korean descent. We suggest that this association is mediated by loss of suppression of angiogenesis via lower expression of TNFSF15. Further studies are needed to elucidate the mechanism linking altered TNFSF15 expression and angiogenesis to childhood asthma. Furthermore, these studies can also prospectively explore whether reduced serum levels of TNFSF15 can serve as an early marker for the development of persistent asthma and allergy in childhood.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge all participants and thank Medical Illustration & Design, part of the Medical Research Support Services of Yonsei University College of Medicine, for all artistic support related to this work. This work was supported by the National Research Foundation Grant funded by the Korean Government (NRF-2019R1F1A1058910, NRF-2018R1A5A2025079, NRF-2013R1A1A2062110, and NRF-2020R1A2B5B02001713), and funded by Research of Korea Centers for Disease Control and Prevention (2017-NG67003-00, 2017-NG67003-01, 2017-NG67003-02). This study was conducted with bioresources from National Biobank of Korea, the Centers for Disease Control and Prevention, Republic of Korea (KBN-2017-026). C.O. was supported by NIH grants R01 HL129735 and UG3 OD 023282; N.S. was supported by NIH grant K08 HL153955.
Funding information
Korean Government, Grant/Award Number: NRF-2019R1F1A1058910, NRF-, 2018R1A5A2025079, NRF-2013R1A1A2062110 and NRF-2020R1A2B5B02001713; Research of Korea Centers for Disease Control and Prevention, Grant/Award Number: 2017-NG67003-00, 2017-NG67003-01 and 2017-NG67003-02
Dr. Kim has nothing to disclose. Dr. Yoon has nothing to disclose. Dr. Jang has nothing to disclose. Dr. SCHOETTLER has nothing to disclose. Dr. Hong has nothing to disclose. Dr. Lee has nothing to disclose. Dr. Ober has nothing to disclose. Dr. Gee has nothing to disclose. Dr. Sohn has nothing to disclose.
Abbreviations:
- SNP
single nucleotide polymorphism
- TNFSF15
tumor necrosis factor superfamily 15
- VEGF
vascular endothelial growth factor
- VEGI
vascular endothelial growth inhibitor
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
REFERENCES
- 1.Soriano JB, Kendrick PJ, Paulson KR, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respirat Med. 2020;8(6):585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–56. [DOI] [PubMed] [Google Scholar]
- 3.Kim KW, Ober C. Lessons learned from GWAS of asthma. Allergy Asthma Immunol Res. 2019;11(2):170–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demenais F, Margaritte-Jeannin P, Barnes KC, et al. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat Genet. 2018;50(1):42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han Y, Jia Q, Jahani PS, et al. Genome-wide analysis highlights contribution of immune system pathways to the genetic architecture of asthma. Nat Commun. 2020;11(1):1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shriner D, Adeyemo A, Gerry NP, et al. Transferability and fine-mapping of genome-wide associated loci for adult height across human populations. PLoS One. 2009;4(12):e8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baye TM, Kovacic MB, Myers JMB, et al. Differences in candidate gene association between European ancestry and African American asthmatic children. PLoS One. 2011;6(2):e16522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung TF, Ko FWS, Sy HY, Tsui SKW, Wong GWK. Differences in asthma genetics between Chinese and other populations. J Allergy Clin Immunol. 2014;133(1):42–48. [DOI] [PubMed] [Google Scholar]
- 9.Buniello A, MacArthur JAL, Cerezo M, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47(D1):D 1005–D1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noguchi E, Sakamoto H, Hirota T, et al. Genome-wide association study identifies HLA-DP as a susceptibility gene for pediatric asthma in Asian populations. PLoS Genet. 2011;7(7):e1002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirota T, Takahashi A, Kubo M, et al. Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat Genet. 2011;43(9):893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SH, Cho BY, Park CS, et al. Alpha-T-c atenin (CTNNA3) gene was identified as a risk variant for toluene diisocyanate-induced asthma by genome-wide association analysis. Clin Exp Allergy. 2009;39(2):203–212. [DOI] [PubMed] [Google Scholar]
- 13.Kim J-H, Park B-L, Cheong HS, et al. Genome-wide and follow-up studies identify CEP68 gene variants associated with risk of aspirin-intolerant asthma. PLoS One. 2010;5(11):e13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin SW, Park J, Kim YJ, et al. A highly sensitive and specific genetic marker to diagnose aspirin-exacerbated respiratory disease using a genome-wide association study. DNA Cell Biol. 2012;31(11):1604–1609. [DOI] [PubMed] [Google Scholar]
- 15.Park BL, Kim T-H, Kim J-H, et al. Genome-wide association study of aspirin-exacerbated respiratory disease in a Korean population. Hum Genet. 2013;132(3):313–321. [DOI] [PubMed] [Google Scholar]
- 16.Kim S-H, Cho B-Y, Choi H, et al. The SNP rs3128965 of HLA-DPB1 as a genetic marker of the AERD phenotype. PLoS One. 2014;9(12):e111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SY, Jung H, Kim JH, et al. Longitudinal analysis to better characterize Asthma-COPD overlap syndrome: findings from an adult asthma cohort in Korea (COREA). Clin Exp Allergy. 2019;49(5):603–614. [DOI] [PubMed] [Google Scholar]
- 18.Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respirat J. 2015;46(3):622–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pividori M, Schoettler N, Nicolae DL, Ober C, Im HK. Shared and distinct genetic risk factors for childhood-onset and adult-onset asthma: genome-wide and transcriptome-wide studies. Lancet Respirat Med. 2019;7(6):509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warde-Farley D, Donaldson SL, Comes O, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38(suppl_2):W214–W220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pruim RJ, Welch RP, Sanna S, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26(18):2336–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein MM, Thompson EE, Schoettler N, et al. A decade of research on the 17q12–21 asthma locus: piecing together the puzzle. J Allergy Clin Immunol. 2018;142(3):749–764.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Yu D, Lu J, et al. Functional genetic variants of TNFSF15 and their association with gastric adenocarcinoma: a case-control study. PLoS One. 2014;9(9):e108321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aiba Y, Nakamura M. The role of TL1A and DR3 in autoimmune and inflammatory diseases. Mediators Inflamm. 2013;2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meylan F, Richard AC, Siegel RM. TL1A and DR3, a TNF family ligand-receptor pair that promotes lymphocyte costimulation, mucosal hyperplasia, and autoimmune inflammation. Immunol Rev. 2011;244(1):188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Consortium G. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walters EH, Soltani A, Reid DW, Ward C. Vascular remodelling in asthma. Curr Opin Allergy Clin Immunol. 2008;8(1):39–43. [DOI] [PubMed] [Google Scholar]
- 31.Pickrell JK, Berisa T, Liu JZ, Ségurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet. 2016;48(7):709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ober C, McKennan CG, Magnaye KM, et al. Expression quantitative trait locus fine mapping of the 17q12–21 asthma locus in African American children: a genetic association and gene expression study. Lancet Respirat Med. 2020;8(5):482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Guo J, Ni G, Yang J, Visscher PM, Yengo L. Theoretical and empirical quantification of the accuracy of polygenic scores in ancestry divergent populations. Nat Commun. 2020;11(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bønnelykke K, Ober C. Leveraging gene-environment interactions and endotypes for asthma gene discovery. J Allergy Clin Immunol. 2016;137(3):667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bønnelykke K, Sleiman P, Nielsen K, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46(1):51. [DOI] [PubMed] [Google Scholar]
- 36.Schoettler N, Rodríguez E, Weidinger S, Ober C. Advances in asthma and allergic disease genetics: is bigger always better? J Allergy Clin Immunol. 2019;144(6):1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bach J-F. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911–920. [DOI] [PubMed] [Google Scholar]
- 38.Migone TS, Zhang J, Luo X, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16(3):479–492. [DOI] [PubMed] [Google Scholar]
- 39.Barrett JC, Hansoul S, Nicolae DL, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40(8):955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson CA, Boucher G, Lees CW, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43(3):246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellinghaus D, Jostins L, Spain SL, et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat Genet. 2016;48(5):510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Ampleford EJ, Howard TD, et al. Genome-wide association studies of asthma indicate opposite immunopathogenesis direction from autoimmune diseases. J Allergy Clin Immunol. 2012;130(4):861–868.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreiner E, Waage J, Standl M, et al. Shared genetic variants suggest common pathways in allergy and autoimmune diseases. J Allergy Clin Immunol. 2017;140(3):771–781. [DOI] [PubMed] [Google Scholar]
- 44.Schaumberg DA, Chasman D, Morrison MA, et al. Prospective study of common variants in the retinoic acid receptor-related orphan receptor α gene and risk of neovascular age-related macular degeneration. Arch Ophthalmol. 2010;128(11):1462–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song H, Suehiro J-I, Kanki Y, et al. Critical role for GATA3 in mediating Tie2 expression and function in large vessel endothelial cells. J Biol Chem. 2009;284(42):29109–29124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimizu C, Eleftherohorinou H, Wright VJ, et al. Genetic variation in the SLC8A1 calcium signaling pathway is associated with susceptibility to Kawasaki disease and coronary artery abnormalities. Circ: Cardiovasc Genet. 2016;9(6):559–568. [DOI] [PubMed] [Google Scholar]
- 47.Almoguera B, Vazquez L, Mentch F, et al. Identification of four novel loci in asthma in European American and African American populations. Am J Respir Crit Care Med. 2017;195(4):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanazawa H. VEGF, angiopoietin-1 and-2 in bronchial asthma: new molecular targets in airway angiogenesis and microvascular remodeling. Recent Pat Inflamm Allergy Drug Discov. 2007;1(1):1–8. [DOI] [PubMed] [Google Scholar]
- 49.Lanza GM, Jenkins J, Schmieder AH, et al. Anti-angiogenic nanotherapy inhibits airway remodeling and hyper-responsiveness of dust mite triggered asthma in the Brown Norway rat. Theranostics. 2017;7(2):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.