Abstract
Background:
Tuberculosis (TB) causes undernutrition, and it has a long recovery time after treatment. It is accompanied by adverse health outcomes, such as sarcopenia.
Objective:
We aimed to evaluate the prevalence of sarcopenia and its association with protein and total energy intakes among Korean TB survivors.
Methods:
Data of the population-based Korea National Health and Nutrition Examination Survey (2008–2011) were analyzed, including 9,203 participants aged ⩾ 40 years. We used three definitions for sarcopenia-appendicular skeletal muscle mass (ASM, kg) divided by body mass index (BMI, kg/m2), weight (kg), or height squared (m2). Daily protein and total energy intakes were estimated with a 24-h recall method. Multiple logistic regression was used to evaluate the association between dietary protein/total energy intake and sarcopenia among TB survivors.
Results:
The prevalence of sarcopenia was 11.2%, 10.7%, and 24.3% among TB survivors with sarcopenia defined by ASM divided by BMI, weight, and height squared, respectively. The prevalence of sarcopenia among TB survivors was higher than among those without TB. After adjusting for age, weight, sex, education level, employment status, smoking status, and drinking status, sufficient protein and total energy intakes were associated with a lower risk of sarcopenia in TB survivors.
Conclusion:
The prevalence of sarcopenia was higher in TB survivors than in those without TB. We suggest consuming sufficient protein intake along with increasing total energy intake in TB survivors.
Keywords: energy intake, KNHANES, protein intake, sarcopenia, tuberculosis
Introduction
Tuberculosis (TB) is one of the top 10 leading causes of death worldwide from a single infectious agent. In 2019, there were a total of 1.4 million TB-related deaths. 1 In addition, TB has been a significant public health problem in South Korea. Owing to improved living standards, advances in diagnostic and treatment techniques, and governmental and social efforts, TB incidence has steadily decreased in Korea since 1995. 2 Despite this, the prevalence of TB in South Korea is still relatively higher according to the Organization for Economic Cooperation and Development. 3
Simultaneously, the number of people who have survived treated TB has increased. According to Dodd et al., the number of TB survivors alive in 2020 is more than 10 times the estimated annual TB incidence. 4 However, even after TB treatment is completed, survivors experience adverse clinical outcomes, including substantial morbidity, and have higher all-cause mortality than those who have never had TB. 4 Recently, recognition of the effects of TB after treatment completion is increasing; however, there are still insufficient studies on TB survivors and the continuous impact of this disease on their lives.
TB is a wasting disease, and it leads to undernutrition. Undernutrition is associated with a significant impairment of cell-mediated immunity,5–8 making individuals vulnerable to TB infection. 9 According to the World Health Organization, proper TB treatment helps to restore average weight and nutritional status. However, the time to full nutritional recovery can be long, and many TB patients remain undernourished even after completing TB treatment.10–13 These results suggest that TB causes permanent loss of lean tissue, and this may have an adverse effect on survival and physical functions, resulting in future health risks, including sarcopenia.
Various factors including nutrition, physical activities, strength exercise, and smoking are influential factors of sarcopenia, but there are no clear reports on the effects of improving lifestyle factors. Several studies have recently reported the relationship between protein intake and sarcopenia according to sex or age.14–16 However, the association between sarcopenia and protein consumption in TB survivors has not been investigated. Therefore, we aimed to evaluate the prevalence and factors associated with sarcopenia in pulmonary TB survivors, focusing on protein and energy intake, using data from a representative Korean national population study.
Materials and methods
Study population and data collection
This study was based on the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHANES IV–V) from 2008 to 2011. The KNHANES is a national, cross-sectional, population-based survey designed to assess health-related behavior, health conditions, and the nutritional status of Koreans. 17 A nationally representative sample was chosen from the Korean population using household records developed by the 2005 Population and Housing Census in Korea. Twenty households from each district were selected using a stratified, multistage, probability cluster sampling method that considers the geographical area, age, and sex of each participant. 18
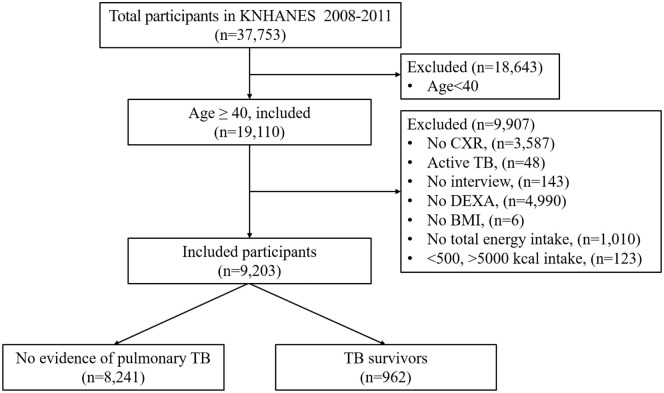
Among 37,753 participants in KNHANES IV–V, we included 19,110 participants aged 40 years or older. We excluded participants who had incomplete or invalid data for assessment of chest X-ray (CXR), sarcopenia, or nutritional intake; those who did not undergo dual-energy X-ray absorptiometry (DEXA) (n = 4990) and CXR (n = 3587); non-responders to health questionnaires (n = 143); non-responders to energy intake questions (n = 1010); those who had no data on body mass index (BMI) (n = 6); those whose energy intake was either under 500 kcal/day or over 5000 kcal/day (n = 123); and those being actively treated for TB during the survey (n = 48) (Figure 1).
Figure 1.
Flowchart of the study population.
BMI, body mass index; CXR, chest X-ray; DEXA, dual-energy X-ray absorptiometry; KNHANES, Korea National Health and Nutrition Examination Survey; TB, tuberculosis.
Definition of TB survivors
CXR images obtained with DigiRAD-PG (Sitec Medical; Kimpo-si, Korea) were used to assess abnormal lesions in the lungs. Two radiologists independently interpreted the CXR results for the presence of lung disease. Individual readings were compared weekly, and results showing TB-related lesions were re-interpreted by six radiology specialists to confirm the results.
TB survivors were defined as those with a self-reported previous history of physician-diagnosed TB or those with a healed TB lesion on CXR.
Demographic and anthropometric assessments
The participants’ demographic characteristics, including age, sex, education level, household income level, residence, employment status, smoking, alcohol consumption, physical activity, and other variables were collected via a questionnaire.
Smokers were defined as ex-smokers or current smokers, and alcohol drinking was defined as the intake of alcohol more than once every month in the last year. Physical activity level was assessed as moderate or vigorous exercise regularly (at least three times per week, 20 min each time). 19 The comorbidities assessed were as follows: diabetes mellitus, stroke, myocardial infarction, chronic obstructive pulmonary disease, chronic kidney disease, liver cirrhosis, and any cancer recorded in the survey. 20
The anthropometric assessments included height, weight, BMI, and other parameters. Body composition was measured via DEXA, using a Discovery fan-beam densitometer (Hologic, Bedford, MA). The appendicular skeletal muscle mass (ASM) was calculated from the sum of the skeletal muscles in the arms and legs. 19
Definition of sarcopenia
Sarcopenia was defined using the ASM in one of three ways: “sarcopenia_BMI = ASM/BMI,” “sarcopenia_height = ASM/height2,” and “sarcopenia_weight = ASM/weight * 100,” as mentioned in a previous study. 21 Sarcopenia was measured using the ASM/BMI, as recommended by the Foundation for the National Institutes of Health (FNIH) for men (<0.789) and women (<0.512); 22 using ASM/height2, as recommended by the Asian Working Group for Sarcopenia (AWGS) for men (<7.0) and women (<5.4); 23 and using ASM/weight × 100, which was calculated to be less than two standard deviations by the sex-specific mean for healthy young adults according to the 2008–2011 KNHANES data for men (<29.0) and women (<22.9).24–26
Dietary assessments
For dietary surveillance, well-trained dietitians conducted in-person interviews with participants, using 24-h recall methods and food frequency questionnaires (FFQs). The detailed nutrition survey protocol is presented on the KNHANES website. 17 Nutrients were calculated from daily consumption by the KNHANES nutrient database. 27
The participants accurately reported their daily consumption by recording details of the amount of food intake. Nutrient intakes were calculated from the daily consumption, including total energy, macronutrients, vitamins, and minerals. Protein consumption was estimated using the recommended serving size of the Recommended Nutrient Intake (RNI) according to Dietary Reference Intakes for Koreans (KDRIs). 28 The RNI for dietary protein was used with a cutoff of ⩾ 0.91 g/kg/day to categorize participants as those with low or sufficient protein consumption. 28 Total energy intake was estimated using the recommended serving size of Estimated Energy Requirements (EER) according to KDRIs. 28 The recommended total energy intake is 2400 kcal/day in men or 1900 kcal/day in women aged 40–49 years, 2200 kcal/day in men or 1800 kcal/day in women aged 50–64 years, and 2000 kcal/day in men or 1600 kcal/day in women aged ⩾65 years. 28 Total energy intake was categorized as low (EER) or sufficient (⩾EER).
Statistical analysis
All statistical analyses were conducted using the PROC SURVEY procedure in SAS software (version 9.4; SAS Institute, Cary, NC) for complex sampling design by multistage, stratified, clustered samples, and appropriate sampling weights of the national survey.
The chi-square test was used for categorical variables, and t-tests were used for continuous variables. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using multivariable logistic regression analysis to determine the association of the protein and total energy intake with sarcopenia. Multivariable logistic regression analysis was performed after adjusting for the covariates. p-values < 0.05 were considered significant.
Institutional review board statement
This research protocol was approved by the Institutional Review Board of Severance Hospital (IRB No. 44-2021-0386), and the study design was approved by the appropriate ethics review board. All methods were carried out in accordance with the approved guidelines and regulations. The Korea Centers for Disease Control and Prevention (KCDC) obtained written and informed consent from all survey participants.
Results
Participants and baseline characteristics
In this study, 9,203 participants aged 40 years or older were included, and 962 (9.7%) were TB survivors (Figure 1). Compared with the group without TB history, TB survivors were more likely to be older, male, and smokers. TB survivors also had lower educational backgrounds, lower incomes, and were more frequently unemployed than those without a history of TB. However, there were no significant differences between the two groups regarding residence, physical activity, alcohol consumption, or comorbidities (Table 1).
Table 1.
Baseline characteristics of the study participants.
| KNHANES 2008–2011 at aged 40 years | p-value | |||
|---|---|---|---|---|
| Total |
No evidence of pulmonary TB |
TB survivors |
||
| n = 9,203 | n = 8,241 | n = 962 | ||
| Age, years | 55.5 ± 0.2 | 55.0 ± 0.2 | 60.0 ± 0.5 | <0.0001 |
| Sex, n (%) | ||||
| Men | 3780 (47.5) | 3243 (46.3) | 537 (58.1) | <0.0001 |
| Women | 5423 (52.5) | 4998 (53.7) | 425 (41.9) | |
| Education, n (%) | ||||
| < Elementary | 3675 (32.3) | 3262 (31.7) | 413 (38.6) | 0.002 |
| Middle school | 1449 (16.3) | 1287 (16.2) | 162 (6.9) | |
| High school | 2485 (30.8) | 2249 (31.2) | 236 (27.5) | |
| ⩾ College | 1556 (20.6) | 1412 (21.0) | 144 (17.1) | |
| Income, n (%) | ||||
| Lowest | 2515 (27.6) | 2199 (26.9) | 316 (33.4) | <0.0001 |
| Lower middle | 2217 (24.3) | 1973 (24.2) | 244 (25.8) | |
| Higher middle | 2138 (23.5) | 1920 (23.5) | 218 (23.0) | |
| Highest | 2239 (24.6) | 2070 (25.4) | 169 (17.9) | |
| Residence, n (%) | ||||
| Urban | 6476 (74.4) | 5787 (74.1) | 689 (76.9) | 0.16 |
| Rural | 2727 (25.6) | 2454 (25.9) | 273 (23.1) | |
| Employment status, n (%) | ||||
| Yes | 5251 (63.7) | 4799 (64.9) | 452 (52.1) | <0.0001 |
| No | 3900 (36.3) | 3398 (35.1) | 502 (47.9) | |
| Smoking, n (%) * | ||||
| No | 5627 (64.1) | 5152 (64.8) | 475 (56.9) | 0.0007 |
| Yes | 2466 (35.9) | 2150 (35.2) | 316 (43.1) | |
| Physical activity, n (%) ** | ||||
| No | 3531 (77.8) | 3201 (77.5) | 330 (81.7) | 0.11 |
| Yes | 1030 (22.2) | 953 (22.5) | 77 (18.3) | |
| Alcohol, n (%) *** | ||||
| No | 4971 (54.0) | 4472 (54.3) | 499 (51.9) | 1.00 |
| Yes | 4232 (46.0) | 3769 (45.7) | 463 (48.1) | |
| Number of chronic diseases, n (%) **** | ||||
| 0 | 8245 (91.6) | 7399 (91.8) | 846 (89.8) | 0.06 |
| 1 | 851 (7.6) | 750 (7.5) | 101 (8.6) | |
| ≧2 | 107 (0.8) | 92 (0.8) | 15 (1.5) | |
Data are presented as mean ± standard error or number of subjects and percentage (%). *Smokers were defined as ex-smokers or current. **Exercise was defined as engaging in moderate or vigorous exercise on a regular basis (at least three times per week, 20 min each time). ***Alcohol drinking was defined as the intake of alcohol more than once every month in the last year. ****Comorbidities include diabetes mellitus, stroke, myocardial infarction, chronic obstructive pulmonary disease, chronic kidney disease, liver cirrhosis, and any cancer recorded in this survey.
KNHANES, Korea National Health and Nutrition Examination Survey; TB, tuberculosis.
Body composition and nutritional values analyses are shown in Table 2. TB survivors were taller, underweight, and had a lower waist circumference than the group without TB history, and their mean BMI was 22.9 ± 0.1 kg/m2. There were differences in body composition variables between TB survivors and those without TB history: fat mass index and muscle mass index were significantly lower in TB survivors, but ASM was similar between the two groups. When ASM was divided by BMI, weight, and height squared, there was a difference between the two groups, with lower values observed in TB survivors. Total calorie intake, as well as energy components such as carbohydrate and fat intake, was not different between the two groups, but protein intake was lower in TB survivors than in the group without TB history. TB survivors tended to have a lower intake of vitamin B1, vitamin C, calcium, and iron than those without a history of TB.
Table 2.
Body composition and nutrition values of study participants.
| KNHANES 2008–2011 at aged 40 years | p-value | |||
|---|---|---|---|---|
| Total | No evidence of pulmonary TB |
TB survivors | ||
| n = 9,203 | n = 8,241 | n = 962 | ||
| Height, cm | 161.4 ± 0.1 | 161.3 ± 0.1 | 162.1 ± 0.4 | 0.031 |
| Weight, kg | 62.9 ± 0.2 | 63.2 ± 0.2 | 60.4 ± 0.4 | <0.0001 |
| WC, cm | 83.0 ± 0.2 | 83.2 ± 0.2 | 80.9 ± 0.4 | <0.0001 |
| BMI, kg/m2 | 24.1 ± 0.04 | 24.2 ± 0.04 | 22.9 ± 0.1 | <0.0001 |
| Total fat mass, kg | 17.7 ± 0.1 | 17.9 ± 0.1 | 15.9 ± 0.2 | <0.0001 |
| Total lean mass, kg | 44.7 ± 0.1 | 44.8 ± 0.1 | 43.8 ± 0.4 | 0.018 |
| FMI, kg/m2 | 6.9 ± 0.04 | 7.0 ± 0.04 | 6.1 ± 0.1 | <0.0001 |
| MMI, kg/m2 | 17.0 ± 0.04 | 17.0 ± 0.04 | 16.5 ± 0.1 | <0.0001 |
| % Total body fat | 28.5 ± 0.1 | 28.6 ± 0.1 | 26.6 ± 0.4 | <0.0001 |
| ASM, kg | 18.9 ± 0.07 | 19.0 ± 0.07 | 18.6 ± 0.2 | 0.071 |
| ASM(kg) / BMI (kg/m2) | 0.8 ± 0.003 | 0.79 ± 0.003 | 0.82 ± 0.009 | 0.001 |
| (ASM(kg) / weight(kg))×100, (%) | 29.9 ± 0.07 | 29.79 ± 0.07 | 30.66 ± 0.2 | <0.0001 |
| ASM(kg) /height2 (m2) | 7.2 ± 0.02 | 7.18 ± 0.02 | 6.97 ± 0.06 | 0.0002 |
| Total energy (kcal/day) | 1931.0 ± 12.7 | 1936.2 ± 12.7 | 1882.1 ± 37.5 | 0.143 |
| Carbohydrate (g/day) | 322.4 ± 2.2 | 322.6 ± 2.2 | 319.9 ± 6.1 | 0.635 |
| Protein (g/day) | 68.0 ± 0.6 | 68.4 ± 0.6 | 64.7 ± 1.5 | 0.013 |
| Protein (g/kg/day) | 1.09 ± 0.01 | 1.09 ± 0.01 | 1.08 ± 0.02 | 0.63 |
| Fat (g/day) | 34.2 ± 0.4 | 34.4 ± 0.5 | 32.3 ± 1.1 | 0.081 |
| Vitamin B1 (mg/day) | 1.3 ± 0.01 | 1.27 ± 0.01 | 1.19 ± 0.03 | 0.020 |
| Vitamin B2 (mg/day) | 1.1 ± 0.01 | 1.15 ± 0.01 | 1.10 ± 0.03 | 0.068 |
| Vitamin A (RE/day) | 812.6 ± 17.6 | 814.8 ± 16.8 | 791.4 ± 46.3 | 0.575 |
| Vitamin C (mg/day) | 107.3 ± 1.4 | 108.1 ± 1.5 | 99.9 ± 3.5 | 0.023 |
| Calcium (mg/day) | 514.0 ± 5.3 | 517.0 ± 5.5 | 486.2 ± 12.5 | 0.019 |
| Iron (mg/day) | 15.6 ± 0.2 | 15.7 ± 0.3 | 14.6 ± 0.4 | 0.010 |
| Fiber (g/day) | 8.0 ± 0.1 | 8.01 ± 0.1 | 7.81 ± 0.3 | 0.455 |
Data are presented as mean ± standard error or number of subjects and percentage (%). ASM was calculated as the sum of skeletal muscles in the arms and legs.
ASM, appendicular skeletal muscle mass; BMI, body mass index; FMI, fat mass index; KNHANES, Korea National Health and Nutrition Examination Survey; MMI, muscle mass index; RE, retinol equivalent; TB, tuberculosis; WC, waist circumference.
Prevalence of sarcopenia in TB survivors
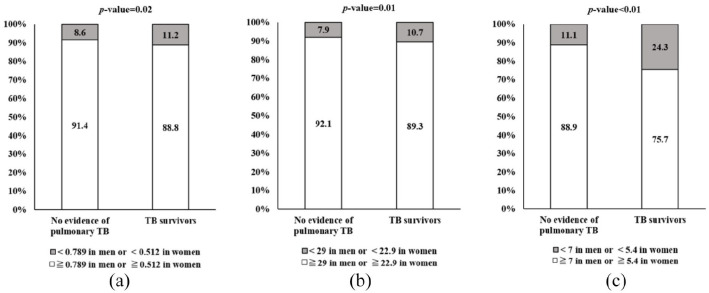
In this study, we used three definitions of sarcopenia: ASM (kg) divided by BMI (kg/m2), weight (kg), or height squared (m 2 ). The analysis of sarcopenia prevalence is shown in Figure 2. The frequency of sarcopenia was 126 (11.2%), 111 (10.7%), and 254 (24.3%) among TB survivors with sarcopenia defined by ASM divided by BMI, weight, and height squared, respectively. Sarcopenia prevalence was found to be higher among TB survivors than among those without TB history.
Figure 2.
Prevalence of sarcopenia in both TB survivors and those without TB history. (a) Sarcopenia_BMI, (b) Sarcopenia_weight, and (c) Sarcopenia_height.
BMI, body mass index; TB, tuberculosis.
Three definitions of sarcopenia were used. ASM divided by (a) BMI (kg/m2), (b) weight (kg)×100, and (c) height2 (m2). Chi-square tests were used to assess the significance of the difference of subject distribution in categorical variables. BMI; body mass index; TB; tuberculosis.
Demographic characteristics and nutritional status according to the presence of sarcopenia in TB survivors
Sociodemographic, clinical characteristics, and nutritional status according to the presence of sarcopenia are described in Table 3. TB survivors with sarcopenia were older and had low educational backgrounds, low incomes, and were more likely to have a smoking history. In terms of nutritional status, the level of protein consumption and total energy intake were lower in TB survivors with sarcopenia than in those without sarcopenia. Physical activity, number of chronic diseases, and residence were not different between the sarcopenia group and non-sarcopenia group among TB survivors.
Table 3.
Demographic characteristics and nutritional status according to sarcopenia status of tuberculosis survivors.
| KNHANES 2008–2011 at aged 40 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | TB survivors (n = 962) | |||||||||
| Sarcopenia_BMI | Sarcopenia_weight | Sarcopenia_height | ||||||||
| ≧0.789 in men or ≧0.512 in women |
< 0.789 in men or < 0.512in women |
p-value | ≧29 in men or ≧22.9 in women |
< 29 in men or < 22.9in women |
p-value | ≧7 in men or ≧5.4 in women |
< 7 in men or < 5.4in women |
p-value | ||
| n = 962 | n = 836 | n = 126 | n = 851 | n = 111 | n = 708 | n = 254 | ||||
| Age, year | 60.0 ± 0.5 | 59.3 ± 0.5 | 66.0 ± 1.5 | < .0001 | 59.6 ± .5 | 63.6 ± 1.5 | 0.01 | 58.9 ± 0.6 | 63.4 ± 1.1 | 0.0002 |
| Total energy intake (kcal/day) | 1882.1 ± 37.5 | 1903.7 ± 39.0 | 1711.2 ± 101.8 | 0.07 | 1913.3 ± 40.1 | 1621 ± 67.9 | 0.0001 | 1958.4 ± 41.8 | 1644.9 ± 53.2 | <0.0001 |
| Protein, g/day | 64.7 ± 1.5 | 65.5 ± 1.6 | 57.9 ± 4.0 | 0.08 | 65.7 ± 1.6 | 56.3 ± 3.0 | 0.005 | 67.6 ± 1.7 | 55.6 ± 2.2 | <0.0001 |
| Protein, g/kg/day | 1.08 ± 0.02 | 1.09 ± 0.03 | 0.96 ± 0.06 | 0.04 | 1.10 ± 0.03 | 0.90 ± 0.05 | 0.0002 | 1.09 ± 0.03 | 1.04 ± 0.04 | 0.29 |
| Height, cm | 162.1 ± 0.4 | 162.9 ± 0.4 | 156.2 ± 1.1 | < .0001 | 162.5 ± 0.4 | 159.0 ± 1.0 | 0.0006 | 162.4 ± 0.5 | 161.2 ± 0.6 | 0.11 |
| Weight, kg | 60.4 ± 0.4 | 60.5 ± 0.5 | 59.3 ± 1.2 | 0.32 | 60.1 ± 0.5 | 62.6 ± .2 | 0.05 | 62.6 ± 0.5 | 53.6 ± 0.8 | <0.0001 |
| Gender, n (%) | ||||||||||
| Men | 537 (58.1) | 467 (58.9) | 70 (51.1) | 0.13 | 484 (60.1) | 53 (41.1) | 0.001 | 374 (56.8) | 163 (62.0) | 0.21 |
| Women | 425 (41.9) | 369 (41.1) | 56 (48.9) | 367 (39.9) | 58 (59.0) | 334 (43.2) | 91 (38.0) | |||
| Education, n (%) | ||||||||||
| < Elementary | 413 (38.6) | 337 (40.6) | 76 (61.3) | < .0001 | 351 (1.5) | 62 (56.4) | 0.09 | 285 (40.4) | 128 (51.2) | 0.04 |
| Middle school | 162 (16.9) | 142 (17.1) | 20 (16.1) | 147 (17.4) | 15 (13.6) | 121 (17.2) | 41 (16.4) | |||
| High school | 236 (27.5) | 218 (26.2) | 18 (4.5) | 212 (25.1) | 24 (21.8) | 184 (26.1) | 52 (20.8) | |||
| ⩾ College | 144 (17.1) | 134 (16.1) | 10 (8.1) | 135 (16.0) | 9 (8.2) | 115 (16.3) | 29 (11.6) | |||
| Income, n (%) | ||||||||||
| lowest | 316 (33.4) | 258 (31.3) | 58 (47.2) | 0.004 | 268 (32.0) | 48 (44.0) | 0.003 | 212 (30.5) | 104 (41.4) | <0.0001 |
| lower middle | 244 (25.8) | 207 (25.1) | 37 (30.1) | 210 (25.1) | 34 (31.19) | 166 (23.9) | 78 (31.1) | |||
| higher middle | 218 (23.0) | 201 (24.4) | 17 (13.8) | 204 (24.3) | 14 (12.84) | 184 (26.4) | 34 (13.6) | |||
| highest | 169 (17.9) | 158 (19.2) | 11 (8.9) | 156 (18.6) | 13 (11.93) | 134 (19.3) | 35 (13.9) | |||
| Residence, n (%) | ||||||||||
| Urban | 689 (76.9) | 601 (76.8) | 88 (77.6) | 0.87 | 601 (76.0) | 88 (84.5) | 0.08 | 511 (76.9) | 178 (77.1) | 0.96 |
| Rural | 273 (23.1) | 235 (23.2) | 38 (22.4) | 250 (24.0) | 23 (15.5) | 197 (23.1) | 76 (22.9) | |||
| Employment status, n (%) | ||||||||||
| Yes | 452 (52.1) | 408 (49.2) | 44 (35.5) | 0.0007 | 414 (49.1) | 38 (34.6) | 0.01 | 355 (55.3) | 97 (42.1) | 0.01 |
| No | 502 (47.9) | 422 (50.8) | 80 (64.5) | 430 (51.0) | 72 (65.5) | 349 (44.7) | 153 (57.9) | |||
| Smoking, n (%) * | ||||||||||
| No | 475 (56.9) | 413 (56.5) | 62 (60.0) | 0.55 | 414 (55.2) | 61 (69.9) | 0.03 | 371 (59.2) | 104 (49.7) | 0.04 |
| Yes | 316 (43.1) | 272 (43.5) | 44 (40.0) | 284 (44.8) | 32 (30.1) | 208 (40.8) | 108 (50.3) | |||
| Physical activity, n (%) ** | ||||||||||
| No | 330 (56.9) | 304 (82.6) | 26 (71.0) | 0.25 | 301 (81.7) | 29 (82.3) | 0.96 | 264 (82.3) | 66 (79.2) | 0.64 |
| Yes | 77 (43.1) | 67 (17.4) | 10 (29.0) | 71 (18.3) | 6 (7.7) | 61 (17.7) | 16 (20.8) | |||
| Alcohol, n (%) *** | ||||||||||
| No | 499 (51.9) | 424 (47.3) | 75 (59.4) | 0.04 | 430 (47.0) | 69 (62.4) | 0.01 | 354 (50.0) | 145 (57.1) | 0.13 |
| Yes | 463 (48.1) | 412 (52.7) | 51 (40.6) | 421 (53.0) | 42 (37.6) | 354 (50.0) | 109 (42.9) | |||
| Number of chronic diseases, n (%) | ||||||||||
| 0 | 846 (89.8) | 741 (90.0) | 105 (88.3) | 0.76 | 754 (90.0) | 92 (88.9) | 0.86 | 628 (90.4) | 218 (88.0) | 0.58 |
| 1 | 101 (8.6) | 83 (8.4) | 18 (10.3) | 86 (8.6) | 15 (9.1) | 71 (8.0) | 30 (10.5) | |||
| ≧2 | 15 (1.5) | 12 (1.5) | 3 (1.4) | 11.0 (1.5) | 4 (2.0) | 9 (1.5) | 6 (1.5) | |||
Data are presented as mean ± standard error or number of subjects and percentage (%). *Smokers were defined as ex-smokers or current. **Exercise was defined as engaging in moderate or vigorous exercise regularly (at least three times per week, 20 min each time). *** Alcohol drinking was defined as the intake of alcohol more than once every month in the last year.
BMI, body mass index; KNHANES, Korea National Health and Nutrition Examination Survey; TB, tuberculosis.
Analysis of sarcopenia risk according to protein and total energy intake
The associations between the influencing factors, such as protein and total energy intakes, and the risk of sarcopenia after adjusting for confounding factors are presented in Table 4. We categorized TB survivors into three groups (low protein intake, sufficient protein intake and low energy intake, and sufficient protein and sufficient energy intake) to analyze the association between nutritional intake and the risk of sarcopenia.
Table 4.
Combined effect of protein and energy intake on sarcopenia 1) in TB survivors.
| TB survivors | Low protein group (< RNI)
2)
& energy groups (≧EER) |
Sufficient protein group (≧RNI) & low energy group (<EER) |
Sufficient protein group (≧RNI) & Sufficient energy group (≧EER) |
|||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% C.I. 3) | p-value | OR | 95% C.I. | p-value | |||
| No. case/subjects (126/962) | 71 / 456 | 25 / 200 | 30 / 306 | |||||
| Sarcopenia_BMI | 1.00 | ref. | 1.10 | 0.57–2.13 | 0.88 | 1.09 | 0.59–2.01 | 0.90 |
| No. case/subjects (111/962) | 64 / 456 | 24 / 200 | 23 / 306 | |||||
| Sarcopenia_weight | 1.00 | ref. | 1.49 | 0.77–2.86 | 0.11 | 0.69 | 0.34–1.41 | 0.14 |
| No. case/subjects (254/962) | 130 / 456 | 69 / 200 | 55 / 306 | |||||
| Sarcopenia_height | 1.00 | ref. | 1.00 | 0.56–1.79 | 0.27 | 0.54 | 0.29–0.99 | 0.03 |
Three definitions of sarcopenia: Sarcopenia_BMI = ASM (kg) / BMI (kg/m2) was defined as < 0.789 in men or < 0.512 in women, according to the FNIH recommendation using ASM/BMI ratio; Sarcopenia_weight = ASM (kg) / weight (%) was defined as < 29.0 in men or < 22.9 in women, according to the 2008–2011 KNHANES data; Sarcopenia_height = ASM (kg) / height2 was defined as 7.0 kg/m2 in men or < 5.4 mg/m2 in women, according to the AWGS recommendation using height-adjusted skeletal muscle mass.
The RNI recommended grams per kilogram of body weight is 0.91 g/kg/d.
Multivariable logistic regression model adjusted for age (year), weight (kg), sex, education level, employment status, smoking status, and drinking status.
ASM, appendicular skeletal muscle mass; AWGS, Asian Working Group for Sarcopenia; BMI, body mass index; CI, confidence interval; EER, estimated energy requirements; FNIH, Foundation for the National Institutes of Health; OR, odds ratio; RNI, recommended nutrient intake; TB, tuberculosis.
After adjusting for age, weight, sex, education level, employment status, smoking status, and drinking status, the adjusted odds ratio for sarcopenia tended to decrease in TB survivors with adequate protein and total energy intake, especially according to the sarcopenia definition with ASM by height (OR = 1.09, 95% CI = 0.59–2.01; OR = 0.69, 95% CI = 0.34–1.41; OR = 0.54, 95% CI = 0.29–0.99 when defining sarcopenia with ASM by BMI, weight, or by height squared, respectively).
Discussion
In this study, we analyzed the prevalence of sarcopenia and its association with factors such as dietary protein intake in TB survivors aged 40 years or older, using nationally representative KNHANES data. The prevalence of sarcopenia was higher in TB survivors than in those without TB, and the risk of sarcopenia decreased with adequate protein intake as well as total energy intake, after adjusting for confounding factors. To our knowledge, this is the first study to examine the relationship between the prevalence of sarcopenia and protein consumption, including total energy intake in TB survivors, using a nationally representative sample.
TB is a wasting disease, and it can impact reductions in health-related quality of life even after treatment completion. One study reported that 363 million people in 190 countries developed TB between 1980 and 2019, of whom 155 million were alive in 2020. 4 This indicates that the number of TB survivors alive in 2020 is more than 10 times the estimated annual TB incidence. However, there are limited data on TB survivors and the impact of the disease on their lives. The association between malnutrition and infection, especially TB, is well established.29–31 Such nutritional depletion can lead to immune dysfunction that increases susceptibility to the disease and then increases the risk for TB-related mortality and treatment failure. In addition, some studies suggested that one cause of malnutrition in TB patients is that pro-inflammatory cytokines may impair the utilization of amino acids for protein synthesis, called the anabolic block phenomenon.31,32 This previous evidence implies that such a difference in metabolic response in TB patients may contribute to the severity of wasting, and improved energy intake is probably the significant factor for prognosis. Based on previous studies, many clinicians conducted studies on the relationship between macronutrient intake and body composition changes in TB patients, and their findings showed that better nutrition tended to show more clearance of bacteria in addition to greater weight gain.33–35 Such data suggested that chronic infections such as TB require a good supply of nutrition during the treatment and recovery phase for a better outcome. However, most studies have only been conducted on patients undergoing TB treatment. Our study focused on people who survived TB and analyzed the association between malnutrition status and sarcopenia as influencing factors on their life. The findings of our analysis showed that TB survivors had low energy intake than those without a history of TB.
Sarcopenia is associated with poor health quality and functional dependence, muscle mass, loss of muscle strength, and physical performance are factors considered for evaluation. The pathophysiology for sarcopenia is complex and involves a multifactorial process including neurodegenerative processes, reduction in anabolic hormone synthesis, dysregulation of cytokine secretion, and changes of the inflammatory state. 36 However, the pathogenesis of sarcopenia in TB patients is clearly unknown. Several lung diseases such as TB, chronic obstructive pulmonary disease (COPD), and asthma can result in systemic inflammation, which can cause muscle loss and further exacerbate skeletal muscle detriment. 21 In addition, several studies showed that TB is also a risk factor for COPD and can increase the risk for restriction dysfunction due to residual lung damage.37–42 These factors may contribute to sarcopenia in TB patients. TB patients are at risk for multiple aspects of sarcopenia. A major feature of TB is weight loss and nutritional recovery; however, the full nutritional recovery time can be long, and many TB patients remain undernourished after treatment completion. From this point of view, the findings of our study showed an association between dietary protein and energy intake and sarcopenia in TB survivors and suggested that sufficient protein and energy consumption may prevent sarcopenia.
The definitions and diagnoses of sarcopenia are still evolving as new findings challenge our current understanding. In our study, we adopted three different definitions of sarcopenia to reflect various understandings of sarcopenia; sarcopenia_BMI based on FNIH, 22 sarcopenia_weight based on the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), 26 and sarcopenia_height from AWGS. 43 In our analysis, a significant relationship between adequate protein/energy intake and sarcopenia was shown only in the group defined by sarcopenia_height. We need to interpret this cautiously because the association of protein/energy intake and sarcopenia was not universal for all definitions of sarcopenia. However, diagnosing sarcopenia in Asian people requires some special considerations because of anthropometric and cultural or lifestyle-related differences compared with their Western counterparts; for example, Asian people tend to have a relatively smaller body size, higher adiposity, and less mechanized and more physically active lifestyles. 23
Limitations
Our study has several limitations. First, this was a cross-sectional study, and it was impossible to determine a causal relationship. Some participants were excluded due to incomplete information for CXR and DEXA. Second, sarcopenia was defined only by low skeletal muscle mass, and its evaluation did not consider muscle strength or physical performance. Third, we used a 24-h recall method to assess dietary variables, which can under- and overestimate the actual intake and might be too short to characterize the usual food intake patterns. However, the nutritional survey of the KNHANES is currently the best available data to estimate the dietary intake of the Korean population. Fourth, there are heterogeneities within the groups, such as different prevalence timepoints of TB and disease severity of TB, which might influence data analysis; however, we did not consider these variables. Finally, inactivity is widely known as a contributor to sarcopenia.26,44 When adjusting for the effect of physical activity in our study, we could not differentiate between the types of exercise, such as resistance exercise.
Conclusions
In conclusion, the prevalence of sarcopenia was higher in TB survivors with inadequate protein consumption and total energy intake in this large population study based on national data. Our study suggests that both sufficient energy and protein intake are related to the prevention of sarcopenia.
Footnotes
Author contributions: MKS and JYC designed the report and wrote the paper. SYK, EYK, SHL, KSC, JYJ, MSP, and YSK drafted and revised the manuscript. YAK designed the concept and finally approved the paper. All authors have taken due care to ensure the integrity of this work, and this final manuscript has been seen and approved by all authors.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea [grant number: HI19C1235].
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Young Ae Kang  https://orcid.org/0000-0002-7783-5271
https://orcid.org/0000-0002-7783-5271
Data availability statement: This study was based on data obtained from KNHANES between 2008 and 2011. The datasets are available from the official KNHANES website: https://knhanes.kdca.go.kr/knhanes/main.do
Contributor Information
Moon-Kyung Shin, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea.
Ji Yeon Choi, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea.
Song Yee Kim, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea.
Eun Young Kim, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea.
Sang Hoon Lee, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea.
Kyung Soo Chung, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea.
Ji Ye Jung, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea.
Moo Suk Park, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea.
Young Sam Kim, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea.
Young Ae Kang, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul 03722, Republic of Korea Institute for Immunology and Immunological Disease, Yonsei University College of Medicine, 50-1, Yonsei-Ro, Seodaemun-gu, Seoul 03722, Republic of Korea.
References
- 1. Annabel B, Anna D, Hannah M. Global tuberculosis report 2019. Geneva: World Health Organization, 2019, pp. 7–9. [Google Scholar]
- 2. Song J-H, Huh K, Chung DR. Modern history of tuberculosis in Korea. Infect Chemother 2019; 51: 414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Global tuberculosis report 2020, https://www.who.int/publications/i/item/9789240013131
- 4. Dodd PJ, Yuen CM, Jayasooriya SM, et al. Quantifying the global number of tuberculosis survivors: a modelling study. Lancet Infect Dis 2021; 21: 984–992. [DOI] [PubMed] [Google Scholar]
- 5. Chandra RK, Gupta S, Singh H. Inducer and suppressor T cell subsets in protein-energy malnutrition: analysis by monoclonal antibodies. Nutr Res 1982; 2: 21–26. [Google Scholar]
- 6. Crevel RW. Hygiene and the immune system. J Infect 2001; 43: 65–69. [DOI] [PubMed] [Google Scholar]
- 7. Kuvibidila S, Yu L, Ode D, et al. The immune response in protein-energy malnutrition and single nutrient deficiencies. In: Nutrition and immunology. Springer, 1993, pp. 121–155, https://link.springer.com/chapter/10.1007/978-1-4615-2900-2_6 [Google Scholar]
- 8. Cegielski JP, Arab L, Cornoni-Huntley J. Nutritional risk factors for tuberculosis among adults in the United States, 1971–1992. Am J Epidemiol 2012; 176: 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cegielski J, McMurray D. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 2004; 8: 286–298. [PubMed] [Google Scholar]
- 10. Onwubalili J. Malnutrition among tuberculosis patients in Harrow, England. Eur J Clin Nutr 1988; 42: 363–366. [PubMed] [Google Scholar]
- 11. Harries A, Nkhoma W, Thompson P, et al. Nutritional status in Malawian patients with pulmonary tuberculosis and response to chemotherapy. Eur J Clin Nutr 1988; 42: 445–450. [PubMed] [Google Scholar]
- 12. Khan A, Sterling TR, Reves R, et al. Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am J Respir Crit Care Med 2006; 174: 344–348. [DOI] [PubMed] [Google Scholar]
- 13. Mupere E, Malone L, Zalwango S, et al. Wasting among Uganda men with pulmonary tuberculosis is associated with linear regain in lean tissue mass during and after treatment in contrast to women with wasting who regain fat tissue mass: prospective cohort study. BMC Infect Dis 2014; 14: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Granic A, Mendonça N, Sayer AA, et al. Effects of dietary patterns and low protein intake on sarcopenia risk in the very old: the Newcastle 85+ study. Clin Nutr 2020; 39: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris T. Muscle mass and strength: relation to function in population studies. J Nutr 1997; 127(Suppl. 5): 1004S–1006S. [DOI] [PubMed] [Google Scholar]
- 16. Ju HJ, Bae WK, Jung SY, et al. Association between dietary protein intake and sarcopenia in Korean elderly. Korean J Fam Pract 2017; 7: 258–263. [Google Scholar]
- 17. Korea National Health and Nutrition Examination Survey (KNHANES), https://knhanes.kdca.go.kr/knhanes/eng/index.do
- 18. Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014; 43: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi C-J, Choi W-S, Kim C-M, et al. Risk of sarcopenia and osteoporosis in male tuberculosis survivors: Korea National Health and Nutrition Examination Survey. Sci Rep 2017; 7: 13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee J-H, Lee HS, Kim H, et al. Association of milk consumption frequency on muscle mass and strength: an analysis of three representative Korean population studies. Eur J Nutr 2020; 59: 3257–3267. [DOI] [PubMed] [Google Scholar]
- 21. Hong KS, Kim MC, Ahn JH. Sarcopenia is an independent risk factor for NAFLD in COPD: a nationwide survey (KNHANES 2008–2011). Int J Chron Obstruct Pulmon Dis 2020; 15: 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014; 69: 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen L-K, Liu L-K, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 24. Koo BK, Kim D, Joo SK, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol 2017; 66: 123–131. [DOI] [PubMed] [Google Scholar]
- 25. Lee Y-H, Kim JE, Roh YH, et al. The combination of vitamin D deficiency and mild to moderate chronic kidney disease is associated with low bone mineral density and deteriorated femoral microarchitecture: results from the KNHANES 2008–2011. J Clin Endocrinol Metab 2014; 99: 3879–3888. [DOI] [PubMed] [Google Scholar]
- 26. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Korea Disease Control and Prevention Agency. Korea National Health and Nutrition Examination Survey, https://knhanes.kdca.go.kr/knhanes/sub03/sub03_02_05.do
- 28. Shin S, Kim S, Joung H. Evidence-based approaches for establishing the 2015 Dietary Reference Intakes for Koreans. Nutr Res Pract 2018; 12: 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med 2007; 4: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mupere E, Parraga IM, Tisch DJ, et al. Low nutrient intake among adult women and patients with severe tuberculosis disease in Uganda: a cross-sectional study. BMC Public Health 2012; 12: 1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Macallan DC. Malnutrition in tuberculosis. Diagn Microbiol Infect Dis 1999; 34: 153–157. [DOI] [PubMed] [Google Scholar]
- 32. Macallan DC, McNurlan MA, Kurpad AV, et al. Whole body protein metabolism in human pulmonary tuberculosis and undernutrition: evidence for anabolic block in tuberculosis. Clin Sci 1998; 94: 321–331. [DOI] [PubMed] [Google Scholar]
- 33. Frediani JK, Sanikidze E, Kipiani M, et al. Macronutrient intake and body composition changes during anti-tuberculosis therapy in adults. Clin Nutr 2016; 35: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwenk A, Hodgson L, Wright A, et al. Nutrient partitioning during treatment of tuberculosis: gain in body fat mass but not in protein mass. Am J Clin Nutr 2004; 79: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 35. Sanchez A, Azen C, Jones B, et al. Relationship of acute phase reactants and fat accumulation during treatment for tuberculosis. Tuberc Res Treat 2011; 2011: 346295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab 2013; 20: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi C-J, Choi W-S, Lee S-Y, et al. The definition of past tuberculosis affects the magnitude of association between pulmonary tuberculosis and respiratory dysfunction: Korea National Health and Nutrition Examination Survey, 2008–2012. J Korean Med Sci 2017; 32: 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee K-Y, Ito K, Maneechotesuwan K. Inflammation to pulmonary diseases. Hindawi 2016; 2016: 7401245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qaisar R, Karim A, Muhammad T, et al. Circulating biomarkers of accelerated sarcopenia in respiratory diseases. Biology 2020; 9: 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee SE, Park J-H, Kim K-A, et al. Association between sarcopenic obesity and pulmonary function in Korean elderly: results from the Korean National Health and Nutrition Examination Survey. Calcif Tissue Int 2020; 106: 124–130. [DOI] [PubMed] [Google Scholar]
- 41. Meghji J, Lesosky M, Joekes E, et al. Patient outcomes associated with post-tuberculosis lung damage in Malawi: a prospective cohort study. Thorax 2020; 75: 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Kampen SC, Wanner A, Edwards M, et al. International research and guidelines on post-tuberculosis chronic lung disorders: a systematic scoping review. BMJ Glob Health 2018; 3: e000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020; 21: 300–307.e2. [DOI] [PubMed] [Google Scholar]
- 44. Arai H, Wakabayashi H, Yoshimura Y, et al. Chapter 4 treatment of sarcopenia. Geriatr Gerontol Int 2018; 18(Suppl. 1): 28–44. [DOI] [PubMed] [Google Scholar]