Abstract
Purpose:
This phase III study compared the efficacy and safety of proposed biosimilar MYL-1402O with reference bevacizumab (BEV), as first-line treatment for patients with stage IV non-squamous non-small-cell lung cancer.
Patients and methods:
Patients were randomly assigned (1:1) to receive MYL-1402O or bevacizumab with carboplatin-paclitaxel up to 18 weeks (6 cycles), followed by up to 24 weeks (8 cycles) of bevacizumab monotherapy. The primary objective was comparison of overall response rate (ORR), based on independently reviewed best tumor responses as assessed during the first 18 weeks. ORR was analyzed per US Food and Drug Administration (ratio of ORR) and European Medicines Agency (difference in ORRs) requirements for equivalence evaluation. Secondary end points included progression-free survival, disease control rate, duration of response, overall survival, safety, and immunogenicity over a period of 42 weeks, and pharmacokinetics (up to 18 weeks).
Results:
A total of 671 patients were included in the intent-to-treat population. The ratio of ORR was 0.96 [confidence interval (CI) 0.83, 1.12] and the difference in ORR was −1.6 (CI −9.0, 5.9) between treatment arms; CIs were within the predefined equivalence margins. Overall, the incidence of treatment-emergent adverse events and serious adverse events was comparable. Treatment-emergent anti-drug antibody (ADA) positivity was transient, with no notable differences between treatment arms (6.5% versus 4.8% ADA positivity rate in MYL-1402O versus BEV, respectively). The incidence of neutralizing antibody post-baseline was lower in the MYL-1402O arm (0.6%) compared to the bevacizumab arm (2.5%).
Conclusions:
MYL-1402O is therapeutically equivalent to bevacizumab, based on the ORR analyses, with comparable secondary endpoints.
Trial Registry Information
EU Clinical Trials Register, Registration # EudraCT no. 2015-005141-32https://www.clinicaltrialsregister.eu/ctr-search/search?query=2015-005141-32
Plain language summary
Previous studies established bioequivalence of the proposed bevacizumab biosimilar MYL-1402O to reference bevacizumab. In this randomized, double-blind, phase III trial, MYL-1402O (n = 337) demonstrated comparable efficacy to bevacizumab (n = 334) in treating advanced non-squamous non-small-cell lung cancer per Food and Drug Administration and European Medicines Agency requirements for equivalence; the ratio of objective response rate (ORR) was 0.96 [90% confidence interval (CI) 0.83, 1.12] and the difference in ORR (MYL-1402O:bevacizumab) was −1.6 (95% CI −9.0, 5.9). Median progression-free survival at 42 weeks was comparable: 7.6 (7.0, 9.5) with MYL-1402O versus 9.0 (7.2, 9.7) months (p = 0.0906) with bevacizumab, by independent review. Treatment-emergent adverse events leading to death (2.4% vs 1.5%), serious adverse events (17.6% vs 16.7%), and antidrug antibodies (6.5% vs 4.8%), were comparable in the MYL-1402O vs bevacizumab arms, respectively. The incidence of neutralizing antibody post-baseline was lower with MYL-1402O (0.6%) than with bevacizumab (2.5%). These findings confirm therapeutic equivalence of MYL-1402O to bevacizumab, providing opportunities for improving access to bevacizumab.
Keywords: bevacizumab, biosimilar, clinical trial, MYL-1402O, non-small-cell lung cancer
Introduction
Lung cancer is the most common cause of cancer-related mortality in the United States and worldwide, with 228,820 new cases and 135,720 deaths from lung and bronchial cancer estimated in the US in 2020.1,2 Significant therapeutic advances over the past decade, including the addition of targeted therapies, immunotherapy, and antiangiogenic agents, have altered the treatment paradigm of non-small-cell lung cancer (NSCLC), providing opportunities for inducing durable responses and prolonging survival.3–5 Antiangiogenic agents approved for NSCLC indications include the monoclonal antibodies bevacizumab (BEV) and ramucirumab, as well as the tyrosine kinase inhibitor nintedanib.3,6,7
Bevacizumab (Avastin; Genentech, South San Francisco, CA, USA), a vascular endothelial growth factor A-targeting monoclonal antibody with antiangiogenic properties across tumor types, was first approved 15 years ago by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for metastatic colorectal cancer. 8 Bevacizumab has since gained approval for multiple solid tumor indications, including for the first-line treatment of patients with advanced or recurrent non-squamous NSCLC (nsNSCLC), in combination with platinum-based chemotherapy, based on significant improvement in overall survival (OS).7–10
Despite improvements in OS and progression-free survival (PFS) with bevacizumab-based regimens in many cancers, patient access to this angiogenic therapy may be limited by lack of reimbursement and/or high out-of-pocket costs. 11 Bevacizumab biosimilars have been approved for all indications authorized for reference bevacizumab, except for indications covered by regulatory exclusivity.11–15 The development, regulatory approval, and availability of these and other proposed bevacizumab biosimilars may improve patient access to this antiangiogenic therapy that has become an integral, standard-of-care (SOC) component for many malignancies. 11
MYL-1402O, a proposed biosimilar to reference bevacizumab, has been developed and extensively characterized using state-of-the-art physicochemical and functional tests. These in vitro assays demonstrate that MYL-1402O is similar to bevacizumab in all critical quality attributes that could potentially affect the structure, safety, and efficacy. Subsequently, the bioequivalence with regard to pharmacokinetic (PK) parameters and comparability of most treatment-emergent adverse events (TEAEs) was confirmed in a single-center, randomized, double blind, three-arm, parallel-group phase I study (ClinicalTrials.gov Identifier: NCT02469987).16,17 The primary objective of the current confirmatory study was to demonstrate the equivalence of MYL-1402O to reference bevacizumab with regard to efficacy, safety, and immunogenicity, when used as a first-line treatment for stage IV nsNSCLC in combination with carboplatin and paclitaxel (CP).
Patients and methods
This study was conducted in compliance with the International Council for Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki. The study was reviewed and approved by an independent ethics committee or institutional review board for each of the 102 study sites. Written informed consent was obtained from all patients before randomization and before any study-related procedures were performed.
Patients
Eligible patients were adults ⩾18 years of age with a histological or cytological diagnosis of advanced nsNSCLC with negative or unknown sensitizing EGFR mutation, and negative or unknown EML4-ALK rearrangement; with a documented imaging diagnosis of stage IV unresectable, recurrent, or metastatic nsNSCLC per American Joint Committee on Cancer 8th edition classification; 18 with ⩾1 measurable lesions as defined by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1; with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, having at least 6 months of expected survival; who have not received any prior systemic therapy for first-line treatment of advanced lung cancer except adjuvant chemotherapy, and remained disease-free for at least 12 months from the time of surgery and at least 6 months from the last dose of chemotherapy; with adequate bone marrow, liver, and renal function. Key exclusion criteria included documented squamous NSCLC or small-cell type or large-cell neuroendocrine histology; active malignancy within the past 5 years; central tumors with proximity to large vessels; tumor with cavitation; and treatment with paclitaxel, bevacizumab, or carboplatin. A complete list of exclusion criteria can be found in the Supplemental Data.
Study design and treatment
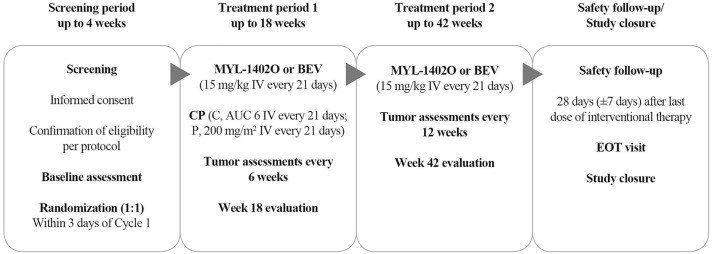
This was a multicenter, randomized, double-blind, two-arm, parallel-group, equivalence phase III study (EudraCT no.: 2015-005141-32; first entered 10 January 2017). The study was conducted at 102 sites in 17 countries, with 671 patients randomly assigned (first patient randomly assigned 21 January 2017): 456 (68.0%) patients from Europe, 191 (28.5%) from India, and 24 (3.6%) from Southeast Asia. Random assignment was stratified by sex (male or female), smoking status (smoker or <100 cigarettes in entire lifetime), and number of metastasis sites (one site or multiple sites). The study consisted of screening/baseline (up to 4 weeks), treatment period 1 (up to week 18) and period 2 (up to week 42), and safety follow-up. Patients were randomly assigned (1:1) to receive either MYL-1402O or bevacizumab with CP for up to 18 weeks (6 cycles) in period 1, with tumor assessments [computed tomography (CT) scan or magnetic resonance imaging (MRI)] performed every 6 weeks. This was followed by period 2, with up to 24 weeks (8 cycles) of monotherapy, if the patient had a response of stable disease (SD) or better [i.e. complete response (CR) or partial response (PR)] as assessed using RECIST 1.1 criteria at the end of period 1, with tumor assessments every 12 weeks (Figure 1).
Figure 1.
Study design.
AUC 6, area under the curve 6 (maximum carboplatin dose of 900 mg).
BEV, bevacizumab; C, carboplatin; EOT, end of treatment; IV, intravenous; P, paclitaxel.
Study drugs (MYL-1402O or bevacizumab; 15 mg/kg infusion) were administered intravenously on day 0 of each cycle, with each cycle lasting 21 days (±3 days) for up to 6 treatment cycles, in combination with CP and then alone as maintenance therapy. Chemotherapy consisted of carboplatin [dose, area under the curve (AUC) 6; maximum dose, 900 mg], followed by paclitaxel (200 mg/m2 or 175 mg/m2, as per institutional SOC), administered by continuous intravenous infusion, after infusion of study drugs was completed without infusion-related adverse events (AEs; Figure 1). The schedule of assessments and procedures are described in detail in the Supplemental Data.
The initial version of the protocol was finalized on 9 March 2016, and was amended twice; details of the changes can be found in the final version (dated 19 February 2019), available at Clinicaltrialsregister.eu.
Study endpoints
Efficacy
The primary efficacy endpoint was overall response rate (ORR), based on best tumor responses as assessed by an independent review (IR) at any time point during the first 18 weeks. Because the FDA and the EMA use different approaches to determine equivalence based on the ORR, two primary analyses for the ORR were conducted (see Statistical analyses). The subgroup analyses of ORR were performed based on the following subgroups: age, sex, race, smoking status, number of metastasis sites, prior radiation therapy, ECOG status, negative or unknown sensitizing EGFR mutation, negative or unknown EML4-ALK rearrangement, and geographical region. The secondary efficacy endpoints were disease control rate (DCR), PFS, OS, and duration of response (DOR). Time to first objective response was also calculated [(date of first objective response – date of randomization +1)/7] in weeks and categorized as 0–7, 13, and 19 weeks. Efficacy assessments consisted of independently reviewed tumor assessments (at least a CT scan or MRI of the thorax and abdomen), but treatment decisions were based on the investigator’s assessment (IA) of radiological data. Tumor response parameters – CR, PR, SD, or progressive disease (PD) – were evaluated using the RECIST 1.1 criteria. In period 1, tumor assessments were performed every 6 weeks after the first dose of bevacizumab (weeks 6, 12, and 18). In period 2, tumor assessments were performed every 12 weeks (weeks 30 and 42). PFS rates were calculated at 18, 30, and 42 weeks, and median PFS was determined at 42 weeks. OS rates were calculated at 18 and 42 weeks.
Safety
TEAEs were monitored throughout the study. Safety assessments included causality, incidence, nature, and severity of AEs (National Cancer Institute Common Terminology Criteria for Adverse Events; version 4.03 severity grading) including adverse drug reactions, ECOG performance status, clinical laboratory evaluations, detection of antibodies to bevacizumab, vital signs, and physical examinations. AEs of interest included hypertension, proteinuria, bleeding, gastrointestinal perforations, jaw osteonecrosis, venous and arterial thromboembolic events, and cardiac failure. Safety data were reported throughout the study, from the screening period, through bevacizumab treatment, and the end of treatment or safety follow-up visit (for patients who discontinued treatment).
Pharmacokinetics
Blood samples for PK analysis were collected from all patients at predose (if possible within 1 hour prior to bevacizumab dosing) in cycles 1 through 6 of period 1 and cycles 704 and 708 of period 2, and postdose (within 15 minutes after bevacizumab infusion) in cycles 1, 2, 4, and 6 of period 1, as well as two additional PK samples in all patients in any cycle in period 1 and at the safety follow-up visit. In addition, an unscheduled drug concentration blood sample was to be collected if a hypersensitivity reaction occurred, as quickly as possible after the event occurred. Serum samples were analyzed at a central laboratory for bevacizumab concentration using a fully validated enzyme-linked immunosorbent assay. The PK profiles of MYL-1402O and bevacizumab were compared using a population PK approach. The population PK set consisted of all randomly assigned patients who completed at least one dose of allocated study medication and who provided at least one evaluable postdose drug concentration for population PK analysis. The PK parameters and exposure estimates assessed included AUC, maximum concentration (Cmax), minimum concentration (Cmin), clearance (CL), volume, and terminal elimination half-life.
Immunogenicity
Immunogenicity testing was performed to detect antidrug antibodies (ADAs) and neutralizing antibodies against MYL-1402O and bevacizumab. Blood samples were collected before drug administration at baseline (predose) within 1 hour of drug administration in cycle 1, in cycles 2, 4, and 6 of period 1, in cycles 704 and 708 in period 2, and at the safety follow-up visit. An unscheduled blood sample was collected for immunogenicity analysis, concomitant with a drug concentration sample, if a hypersensitivity reaction occurred, as quickly/soon as possible after the event occurred. A single validated assay using MYL-1402O-labeled reagents was employed for detection of antibodies against MYL-1402O and bevacizumab. The method used a bridging immunoassay format combined with the Meso Scale Discovery detection technology. In keeping with the multitiered sample analysis recommendations for immunogenicity testing, the assay was structured into four tiers: screening, confirmation, titration, and neutralizing antibody (NAb). Only samples confirmed positive for ADAs were tested further for NAbs. A single validated competitive ligand-binding assay using MYL-1402O-labeled reagents was employed for the detection of NAbs against MYL-1402O and bevacizumab.
Statistical analyses
For the primary endpoint, ORR, two analyses were conducted based on the FDA and EMA requirements for assessment of equivalence. According to the FDA’s requirement, the ORRs for both MYL-1402O and bevacizumab were calculated and the ratio of the ORRs was used to determine if MYL-1402O was equivalent to bevacizumab. A two-sided 90% confidence interval (CI) for the ratio of the ORRs during the first 18 weeks was calculated, with no adjustment for covariates. According to the EMA’s requirement, an asymptotic two-sided 95% CI for the difference in ORRs at week 18 was calculated using the Wald CI without adjustment for covariates. Primary analysis was conducted in the intent-to-treat (ITT) population. Sensitivity analysis of the primary endpoint was performed using the following analysis populations: IR in per-protocol and best tumor response confirmed at another time point in the ITT population, and IA of tumor response [derived best overall response (BOR)] and best tumor response confirmed at another time point in the ITT population.
The DCR (rate of CR + PR + SD) was evaluated as a ratio, with the 90% CI estimated using logarithmic transformation with no adjustment for covariates. An asymptotic two-sided 95% CI for the difference in DCRs was also calculated. These analyses were based on the ITT population using BOR derived from IR data, with sensitivity analyses performed based on the per-protocol population and investigator data.
PFS and OS for the secondary efficacy endpoints were evaluated using Kaplan–Meier plots, with PFS rates calculated at 18, 30, and 42 weeks, and median PFS determined at 42 weeks. Survival rates at weeks 18 and 42 have been presented along with estimates of the relative risk and the associated 95% CI. DOR for the secondary efficacy endpoints was evaluated using the same statistical analysis methods outlined above. The study was not powered for these secondary endpoints to demonstrate equivalence, but comparability of the results was evaluated.
A mammillary, two-compartment model with zero-order input was utilized for the population PK analysis. Individual empiric Bayesian estimates were used to predict PK measures reflecting exposure to drug. The population PK analyses were conducted by grouping patients according to the treatment (MYL-1402O or bevacizumab) they actually received. All exploratory PK data analyses and presentations of data were performed using SAS 9.4 (Cary, NC, USA) and KIWI 4 (Cognigen; Buffalo, NY, USA). Population PK modeling was performed using the NONMEM version 7.3.0 (Icon Development Solutions, Ellicott City, MD, USA) non-linear mixed effects modeling software package. PK parameters and exposure estimates were compared between treatment arms.
The safety analysis was conducted with data for all randomized patients who completed at least one dose or partial dose of MYL-1402O or bevacizumab, with patients grouped according to the treatment they actually received. TEAEs and serious AEs were descriptively summarized by system organ class, preferred term, and treatment group, as well as by severity and relationship to MYL-1402O. Based on the known safety profile of bevacizumab, AEs of interest were searched against several key standardized MedDRA queries and analyzed fully to characterize and compare the safety profile of MYL-1402O with reference bevacizumab. Descriptive summaries of observed values and change from baseline were presented for clinical laboratory evaluations (hematology and serum biochemistry) by treatment group. For immunogenicity data, the number and percentage of ADA-positive patients were summarized for each treatment group.
Sample sizes of 588 patients for the FDA-recommended endpoint and 628 patients for the EMA-recommended endpoint were required to provide at least 80% power to declare MYL-1402O equivalent to bevacizumab in the analysis of ORR at week 18. These sample sizes assumed that both treatment groups would exhibit an ORR of 38% at week 18. Therefore, a sample size of 628 patients was chosen to satisfy the recommendations of both regulatory agencies for equivalence analysis. A planned blinded sample size re-estimation was conducted after at least 30% of the required patients had either discontinued early or completed week 18 and the sample size was increased to 670 patients based on a prespecified algorithm to maintain the power per EMA requirement.
Results
Patients and exposure
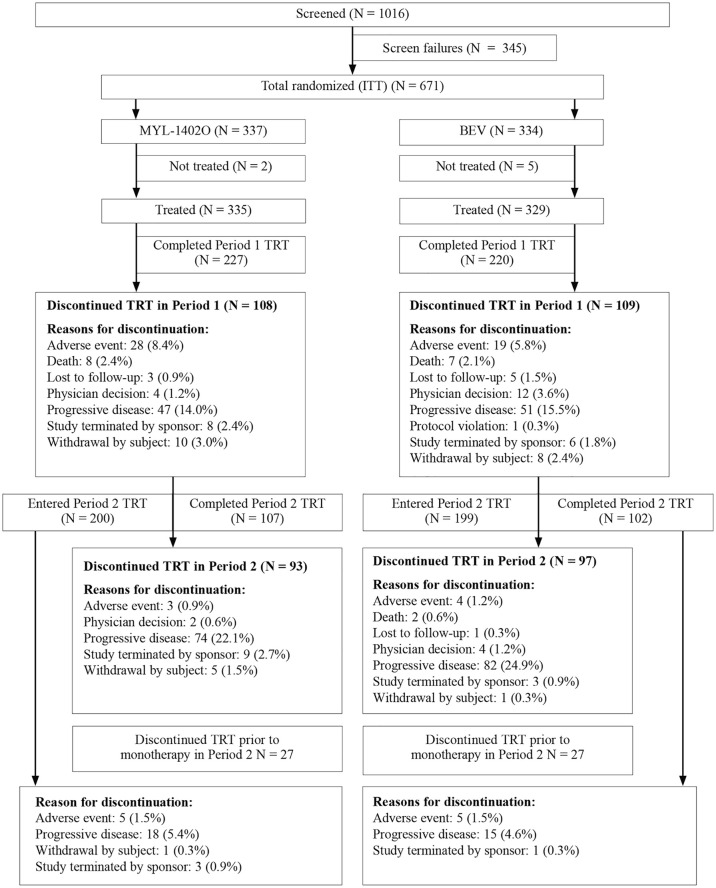
Between January 2017 and January 2019, 671 patients (MYL-1402O, 337; bevacizumab, 334) were enrolled at 102 sites in 17 countries and randomly assigned, with all randomly assigned patients included in the ITT population (Figure 2).
Figure 2.
Patient disposition: intent-to-treat set (42 weeks).
BEV, bevacizumab; ITT, intent-to-treat; TRT, treatment.
The safety population included 664 patients; 335 in the MYL-1402O arm and 329 in the bevacizumab arm, who completed at least one dose or partial dose of MYL-1402O or bevacizumab. A total of 199 (59.1%) patients in the MYL-1402O arm and 207 (62.0%) patients in the BEV arm completed all 42 weeks of the study (including those who did not enter treatment period 2, but completed follow-up until week 42).
Demographics and baseline disease characteristics were well balanced between treatment groups with respect to age, race, height, and ECOG status. Sex, smoking status, and number of metastasis sites were used for stratification, leading to balance between the treatment arms (Table 1) Additional patient characteristics and medical history were also balanced (Supplemental Tables 2 and 3). The mean age of patients was 59 years in both treatment arms; the majority (63.2%) of the patients were men and 53.5% were smokers. Most patients in both treatment groups were white (MYL-1402O, 67.1%; bevacizumab, 69.5%) and about 30% were Asian (MYL-1402O, 32.9%; bevacizumab, 30.5%). About 62% of the patients had multiple metastasis sites. The proportion of patients with M1c substage was slightly higher in the MYL-1402O arm [125 (37.1%) versus 117 (35.0%) in the bevacizumab arm]; however, all patients were in stage IV at the time of randomization, as per the study protocol. The number of patients with prior anticancer surgery was 86 (25.5%) in the MYL-1402O arm, compared with 100 (29.9%) in the bevacizumab arm; 44 (13.1%) and 33 (9.9%) patients had prior anticancer radiotherapy in the MYL-1402O and bevacizumab arms, respectively.
Table 1.
Patient demographic and baseline clinical characteristics according to treatment arm.
| Characteristic | MYL-1402O (N = 337) | Bevacizumab (N = 334) | Total (N = 671) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 59.3 (9.60) | 59.2 (9.73) | 59.3 (9.66) |
| Median | 60.0 | 59.0 | 60.0 |
| Min, max | 23, 86 | 35, 83 | 23, 86 |
| Age category (years), n (%) | |||
| <65 | 237 (70.3) | 236 (70.7) | 473 (70.5) |
| ⩾65 | 100 (29.7) | 98 (29.3) | 198 (29.5) |
| Sex, n (%) | |||
| Male | 213 (63.2) | 211 (63.2) | 424 (63.2) |
| Female | 124 (36.8) | 123 (36.8) | 247 (36.8) |
| Race, n (%) | |||
| White | 226 (67.1) | 232 (69.5) | 458 (68.3) |
| Asian | 111 (32.9) | 102 (30.5) | 213 (31.7) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 5 (1.5) | 4 (1.2) | 9 (1.3) |
| Not Hispanic or Latino | 332 (98.5) | 330 (98.8) | 662 (98.7) |
| ECOG performance status n (%) | |||
| 0 | 83 (24.6) | 76 (22.8) | 159 (23.7) |
| 1 | 254 (75.4) | 258 (77.2) | 512 (76.3) |
| Smoking status per CRF, n (%) | |||
| Smoker | 180 (53.4) | 179 (53.6) | 359 (53.5) |
| Non-smoker | 157 (46.6) | 155 (46.4) | 312 (46.5) |
| Number of metastasis sites per IWRS, n (%) | |||
| 1 | 126 (37.4) | 126 (37.7) | 252 (37.6) |
| Multiple | 211 (62.6) | 208 (62.3) | 419 (62.4) |
| Disease stage at initial diagnosis, n (%) | |||
| I | 15 (4.5) | 9 (2.7) | 24 (3.6) |
| II | 6 (1.8) | 10 (3.0) | 16 (2.4) |
| III | 16 (4.7) | 9 (2.7) | 25 (3.7) |
| IV | 298 (88.4) | 303 (90.7) | 601 (89.6) |
| Unknown | 2 (0.6) | 3 (0.9) | 5 (0.7) |
| Disease substage for stage IV patient a , n (%) | |||
| M1a | 109 (32.3) | 108 (32.3) | 217 (32.3) |
| M1b | 64 (19.0) | 78 (23.4) | 142 (21.2) |
| M1c | 125 (37.1) | 117 (35.0) | 242 (36.1) |
| Tumor histology, n (%) | |||
| Adenocarcinoma | 321 (95.3) | 320 (95.8) | 641 (95.5) |
| Large-cell carcinoma | 7 (2.1) | 6 (1.8) | 13 (1.9) |
| Bronchoalveolar | 1 (0.3) | 0 | 1 (0.1) |
| Not other specified | 8 (2.4) | 8 (2.4) | 16 (2.4) |
| Time since initial diagnosis of disease (months) | |||
| N | 333 | 333 | 666 |
| Mean (SD) | 3.69 (11.500) | 3.71 (11.003) | 3.70 (11.246) |
| Median | 1.15 | 1.12 | 1.13 |
| Min, max | 0.2, 152.8 | 0.2, 122.5 | 0.2, 152.8 |
| Time since initial diagnosis of metastatic or advanced disease (months) | |||
| N | 326 | 321 | 647 |
| Mean (SD) | 1.55 (1.434) | 1.73 (3.186) | 1.64 (2.464) |
| Median | 1.12 | 1.12 | 1.12 |
| Min, max | 0.1, 16.1 | 0.1, 45.6 | 0.1, 45.6 |
All patients were stage IV at the time of enrollment. Substage is not collected for 70 patients; MYL-1402O, 39 (11.5%) and bevacizumab, 31 (9.3%) who were not stage IV at the time of initial diagnosis.
CRF, case report form; ECOG, Eastern Cooperative Oncology Group; IWRS, interactive web-response system; SD, standard deviation.
Overall, study drug was administered to 664 patients (MYL-1402O, 335; bevacizumab, 329), who completed at least one dose or partial dose. Exposure to treatment agents was comparable between the arms; the mean duration of exposure was 27.2 [standard deviation (SD), 15.13] and 27.3 (14.65) weeks, and the mean number of doses was 8.7 (4.86) and 8.7 (4.74) in the MYL-1402O and bevacizumab arms, respectively. The mean dose intensity was 14.5 mg/kg per cycle in both treatment arms. Similar numbers of patients in the two treatment arms had at least one dose delay (period 1, 158 (47.2%) versus 147 (44.7%); period 2, 194 (57.9%) versus 186 (56.5%)] in the MYL-1402O and bevacizumab groups, respectively. There was no modification of bevacizumab dose during this study; however, the dose was recalculated if the patient’s weight changed by ⩾10% during the study. The extent of exposure for carboplatin and paclitaxel was comparable between the two treatment arms in terms of dose and duration of exposure up to week 18; the mean number of doses of carboplatin (4.9 versus 5.0) and paclitaxel (4.9 versus 5.0) were comparable between the treatment arms.
Efficacy
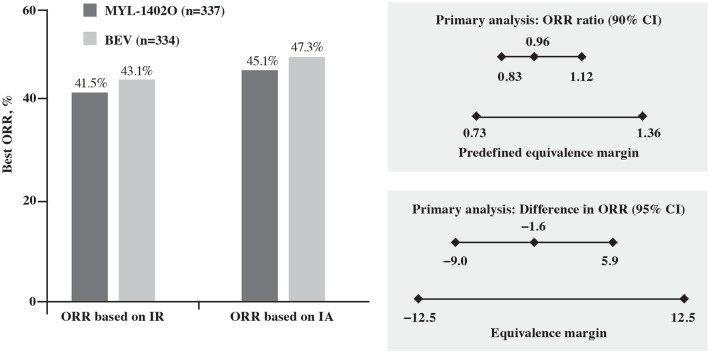
A total of 140 (41.5%) and 144 (43.1%) patients in the MYL-1402O and bevacizumab arms, respectively, had objective responses (CR or PR; BOR based on IR at 18 weeks). Efficacy was comparable between the MYL-1402O and bevacizumab arms; the ratio of ORR (90% CI) was 0.96 (0.83, 1.12) and the difference in ORR (95% CI) was −1.6 (−9.0, 5.9) between treatment arms. The CIs were within the predefined equivalence margin for ratio and difference in ORR (Table 2; Figure 3). These data demonstrate the similarity between MYL-1402O and bevacizumab for ORR, based on the prespecified criteria as per the FDA and EMA requirements.
Table 2.
Summary of primary and secondary efficacy results (intent-to-treat population).
| Efficacy parameter | MYL-1402O (N = 337) n (%) | Bevacizumab (N = 334) n (%) | ||
|---|---|---|---|---|
| IR | IA | IR | IA | |
| ORR (CR+PR) (%) | 41.5 | 45.1 | 43.1 | 47.3 |
| CR | 2 (0.6) | 1 (0.3) | 3 (0.9) | 3 (0.9) |
| PR | 138 (40.9) | 151 (44.8) | 141 (42.2) | 155 (46.4) |
| SD | 134 (39.8) | 132 (39.2) | 144 (43.1) | 122 (36.5) |
| PD | 22 (6.5) | 15 (4.5) | 14 (4.2) | 21 (6.3) |
| Not evaluable | 0 | 0 | 0 | 2 (0.6) |
| Median PFS, a months (95% CI) | 7.6 (7.0, 9.5) | 7.8 (7.0, 9.5) | 9.0 (7.2, 9.7) | 7.3 (7.0, 8.9) |
| Median DOR, b months (95% CI) | 7.7 (6.2, 8.3) | 6.9 (5.8, 8.5) | ||
| DCR, % (95% CI) | 81.3 (77.1, 85.5) | 85.5 (81.9, 89.4) | ||
| Median OS, c months (95% CI) | NC (NC, NC) | NC (NC, NC) | ||
No statistically significant difference between treatment arms at 42 weeks; the log-rank test showed a p value of 0.0906 (IR) and 0.4748 (IA) between the treatment arms.
DOR is defined as the time from start of the first documentation of objective tumor response (CR or PR) to the first documentation of disease progression (per RECIST v1.1) or death due to any cause, whichever comes first. The p value median DOR for IR was 0.5698.
The median OS was not reached in the ITT population at week 42. According to the log-rank test, the difference between the survival curves for both treatment groups was not statistically significant (p = 0.1185) (Figure 3).
CR, complete response; DCR, disease control rate; DOR, duration of response; IA, investigator assessment; IR, independent review; NC, not calculable; ORR, overall/objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
Figure 3.
Response rate and objective response rate analyses.
BEV, bevacizumab; CI, confidence interval; IA, investigator assessment; IR, independent review; ORR, overall response rate.
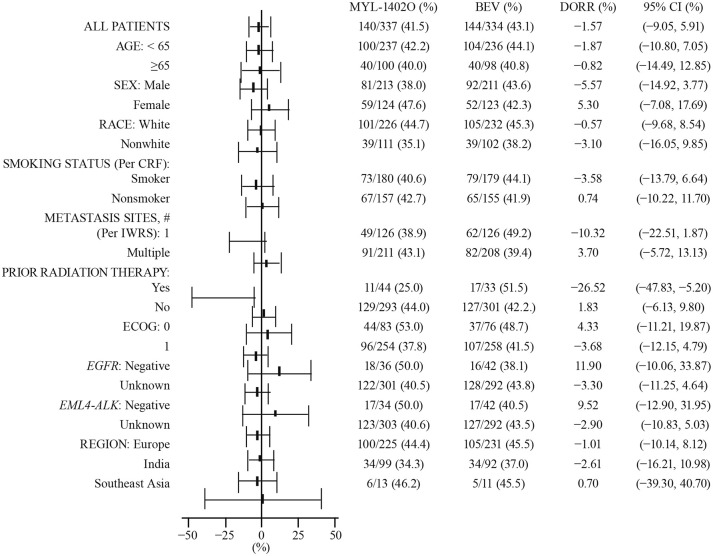
The ORRs based on IA at 18 weeks were comparable between treatment arms [152 (45.1%) versus 158 (47.3%)]. Results for sensitivity analyses for ratio and difference in ORR showed equivalence between the two treatment arms, thus confirming the robustness of the primary analyses (Supplemental Tables 4–12). The subgroup analyses of ORR and difference in ORR were performed based on the following subgroups: age, sex, race, smoking status, number of metastasis sites, prior radiation therapy, ECOG status, negative or unknown sensitizing EGFR mutation, negative or unknown sensitizing EML4-ALK rearrangement, and geographical region. Overall, the analyses of the ratio of ORR and the difference of ORR within each of these subgroups support equivalence between the treatment arms. However, for the number of metastasis sites (one; ORR ratio) and prior radiation therapy (yes; ORR ratio and difference in ORR), the MYL-1402O arm showed a slightly lower response compared with bevacizumab (Figure 4).
Figure 4.
Difference of objective response rate, forest plot, independent review, subgroup analysis: intent-to-treat set.
DORR (MYL-1402O; Avastin, DORR) and 95% CI. The asymptomatic two-sided 95% CI for the difference in ORRs is calculated based on the Wald CI.
Best overall response at any time point during the first 18 weeks is assessed according to Response Evaluation Criteria in Solid Tumors 1.1.
BEV, bevacizumab; CI, confidence interval; CRF, case report form; DORR, difference in objective response rates; ITT, intent-to-treat; IWRS, interactive web response system.
Due to relatively small numbers of patients in these subgroups, the results are not considered to be clinically relevant.
The secondary endpoints of PFS, DOR, DCR, and OS, analyzed at week 42, were also similar between the treatment arms. At week 42, the median PFS (95% CI) for IR was 7.6 (7.0, 9.5) months with MYL-1402O and 9.0 (7.2, 9.7) months with bevacizumab (p = 0.0906); PFS for IA was 7.8 (7.0, 9.5) months with MYL-1402O and 7.3 (7.0, 8.9) months with bevacizumab (p = 0.4748). The data for patients who received new anticancer therapy, two-fold in the bevacizumab arm [34 (10.2%) patients] compared with the MYL-1402O arm [17 (5.0%)], including those with reported radiological progression as per IA (bevacizumab, 27/34; MYL-1402O, 16/17), were not reported as PD during the IR and were therefore censored. The data censoring may explain the difference in median PFS between IR and IA. When new anticancer therapy was considered as an event (PD) as per the predefined sensitivity analyses, the resulting median PFS (95% CI) was comparable between the two arms, [MYL-1402O, 7.0 (6.9, 7.8) months; bevacizumab, 7.1 (7.0, 8.4); log-rank test p = 0.4691; Supplemental Table 13). The median DOR (95% CI) was 7.7 (6.2, 8.3) months in the MYL-1402O arm and 6.9 (5.8, 8.5) months in the BEV arm. The DCRs were comparable in the two treatment arms (81.3% versus 85.6%). The ratio of DCR (90% CI; based on IR through week 42) was 0.95 (0.90, 1.00) and the difference in DCR (95% CI) was −4.3 (−9.9, 1.3), indicating similar disease control in the treatment groups. The DOR and time to response analysis showed that, in the patients with BOR of CR/PR, the CR/PR rates were comparable at 6, 12, 18, and 30 weeks. Times to first objective response based on IR and IA in the ITT population were also comparable between the two treatment arms. Median OS was not reached at 42 weeks; OS rate was 70.0% and 75.4% for MYL-1402O and bevacizumab, respectively (p = 0.1185) at 42 weeks.
Pharmacokinetics
Population PK analyses indicated no differences between the PK profiles of patients in the MYL-1402O and bevacizumab arms. A number of demographic characteristics and clinical laboratory parameter covariates that had potential to influence the PK of the investigated drugs were also assessed. Following the development of the base structural model, the influence of covariates on selected parameters was evaluated using a stepwise forward selection followed by backward elimination approach. Treatment was not a significant covariate of CL (p = 0.453) or volume of the central compartment (p = 0.161) using the likelihood ratio χ2 test; model-based exposure measures such as AUC, half-life, Cmax, and Cmin values, predicted based on the final model for all patients in steady state, were also similar between treatment arms. These data are consistent with the pivotal three-way PK study that demonstrated bioequivalence between MYL-1402O and bevacizumab in normal, healthy volunteers.16,17
Safety
A summary of the TEAEs is included in Supplemental Table 14. Overall, 615 (92.6%) patients reported at least one TEAE through week 42 of the study. The incidence of TEAEs was similar between the two treatment arms, occurring in 311 (92.8%) patients in the MYL-1402O arm and 304 (92.4%) patients in the bevacizumab arm. The majority of the patients reported grade 1 or 2 TEAEs [MYL-1402O, 185 (55.2%); bevacizumab, 178 (54.1%)]; grade 3 or 4 TEAEs were reported by 101 (30.1%) patients in the MYL-1402O arm and 112 (34.0%) in the bevacizumab arm. A total of 64 (9.6%) patients discontinued treatment due to TEAEs; 36 (10.7%) and 28 (8.5%) in the MYL-1402O and bevacizumab arms, respectively. During period 1 (combination therapy), 31 (4.7%) grade 5 TEAEs were reported; 20 (6.0%) in the MYL 1402O arm and 11 (3.3%) in the bevacizumab arm] TEAEs leading to death in period 1 (combination therapy) were 32 (4.8%) patients; 20 (6.0%) and 12 (3.6%) in MYL-1402O and bevacizumab arms, respectively. In period 2 (monotherapy), two grade 5 TEAEs were reported; both in MYL-1402O (0.6%). The occurrence of most of the reported grade 5 TEAEs in the setting of advanced NSCLC and treatment with the chemotherapy–bevacizumab combination suggests that this higher incidence of death due to TEAE could be mostly attributed to chemotherapy-induced toxicity, rather than bevacizumab therapy alone.
The incidence of TEAEs of interest was similar between the arms [MYL-1402O, 71 (21.2%); bevacizumab, 81 (24.6%)], including the number of patients with TEAEs of interest of grade ⩾3 [MYL-1402O, 27 (8.1%) versus bevacizumab, 28 (8.5%)]. Among TEAEs of interest, epistaxis [6 (1.8%) versus 17 (5.2%)] and hemoptysis [4 (1.2%) versus 7 (2.1%)] had a numerically lower incidence in the MYL-1402O arm compared with the bevacizumab arm. Overall, the incidence of TEAEs in this study was comparable to that reported in phase III studies of other bevacizumab biosimilars in similar populations (e.g., 95% with ABP 215 and 93.5% with bevacizumab in the MAPLE study 14 and 96.6% with PF-06439535 and 96.9% with bevacizumab in NCT02364999). 19
Overall, 114 patients experienced serious AEs; 59 (17.6%) versus 55 (16.7%) in the MYL1402O and bevacizumab arms, respectively. The most commonly reported ⩾grade 3 AEs of interest occurring in >1% of patients were hypertension (2.1%), hemorrhage (2.0%) and venous thromboembolic events (VTEs; 1.8%), with similar incidence between the treatment arms. These events occurred at a rate similar to those reported for the originator product (across clinical studies, grade 3–4 hypertension, 5–18%; hemorrhage, 0.4–7%; and VTE, 11%). 20 No clinically significant differences between treatment groups were observed in hematology, serum chemistry, urinalysis results, vital signs, physical examination findings, or ECOG status from baseline through the end of the treatment.
Immunogenicity
The incidence of treatment-emergent ADAs (treatment-induced plus treatment-boosted) was similar for both treatment arms (MYL-1402O, 6.5%; bevacizumab, 4.8%). The post-baseline incidence of NAbs was numerically lower in the MYL-1402O arm (0.6%) than in the bevacizumab arm (2.5%). There were no hypersensitivity-associated AEs reported in patients with post-baseline ADA-positive status.
Discussion
This phase III study compared the efficacy, safety, PK, and immunogenicity of the proposed biosimilar MYL-1402O and the EU-reference bevacizumab. The study met its primary endpoint. The ratio of ORR (90% CI) between the MYL-1402O and bevacizumab groups was 0.96 (0.83, 1.12), with the CI falling within the predefined equivalence margin (0.73, 1.36). The difference in ORR (95% CI) between MYL-1402O and bevacizumab was −1.6 (−9.0, 5.9), falling within the predefined equivalence margin (−12.5, 12.5). All secondary efficacy endpoints and sensitivity analyses of the ORR further support similarity in clinical efficacy. These data provide statistical confirmation of the therapeutic equivalence of MYL-1402O and reference bevacizumab when administered in combination with CP, for both FDA and EMA-required prespecified equivalence considerations. Other efficacy endpoints, safety, and immunogenicity were also comparable. The frequency, type, and severity of TEAEs and AEs of interest were comparable between MYL-1402O and bevacizumab, falling within the expected range of the type and severity previously described in other bevacizumab biosimilar studies.14,16,21 Overall, TEAEs and TEAEs of interest occurred in similar proportions of patients (92.8% versus 92.4%) and (21.2% versus 24.6%), in the MYL-1402O and bevacizumab arms, respectively. Of the 39 patients who experienced TEAEs leading to death during the study, 13 [MYL-1402O, 8 (2.4%); bevacizumab, 5 (1.5%)] were considered to be bevacizumab-related. The incidence of grade 5 TEAEs was comparatively higher during bevacizumab–CP combination therapy (4.7% overall; MYL-1402O, 6.0%; bevacizumab, 3.3%) than during bevacizumab monotherapy [2, overall; both in MYL-1402O (0.6%)]. This higher incidence of severe (grade 5) TEAEs during the chemotherapy–bevacizumab combination treatment period than the monotherapy period suggests that chemotherapy-induced toxicity, rather than bevacizumab therapy, is the likely reason for the higher incidence of death due to TEAEs. No clinically meaningful differences were noted for the secondary efficacy endpoints. PK profiles and immunogenicity were also similar for the treatment groups.
The study was designed based on the Genentech-sponsored, randomized, phase II AVF0757g study, in which bevacizumab was added to the ECOG reference regimen established in Study ECOG 1594 with CP. 21 Additional design considerations included guidance from the FDA and EMA for establishing biosimilarity, and incorporation of advice/input from regulatory authorities.22,23 Systematic reviews and meta-analyses support the potential of improving ORR and prolonging OS and PFS with the addition of bevacizumab to chemotherapy, including the platinum-based CP regimen, in patients with advanced NSCLC.24–27 Patients in this study had similar efficacy and safety profiles to those in a pivotal phase III study (ECOG 4599) of reference bevacizumab in NSCLC. 9 The totality of these considerations supports advanced nsNSCLC as an appropriate setting to evaluate clinical equivalence of MYL-1402O, a bevacizumab biosimilar.
Other bevacizumab biosimilars, approved and proposed, have been evaluated in the setting of advanced nsNSCLC.12,14,15,18,28,29 Several of these studies also used ORR as the primary efficacy endpoint.14,28,29 ORR is a direct measure of drug antitumor activity and is recommended over survival endpoints for the comparison of biosimilars in oncology.22,23 Moreover, ORR as a surrogate endpoint, with a strong correlation to PFS, allows for a shorter study duration compared with OS as the endpoint. 23 Thus, for biosimilar studies, shorter study durations with usage of ORR as the primary efficacy endpoint would potentially result in less expensive studies, thereby accelerating opportunities for expanding access to treatments.
Although cross-trial comparisons should be made with caution, the ORR data reported here are within the range of ORR reported in published data for the reference product.9,21,30,31 The response in the arm of ECOG 4599 treated with reference bevacizumab in combination with CP, for instance, was similar to that observed in this study (ORR in ECOG 4599 bevacizumab+CP arm, 35%; ORR for IR in this study, MYL-1402O+CP, 41.5%; bevacizumab+CP, 43.1%). 21 More importantly, the ORR observed with MYL-1402O is comparable to that reported in recent studies with approved14,19 and proposed bevacizumab biosimilars.28,29 It is also consistent with the anticipated ORR based on the meta-analyses conducted as part of the study design and sample size calculation for this study.
In this study, the IR of the best tumor responses in the ITT population, confirmed at a second time point, was lower (29.8%) than the IR-BOR at week 18 (42.3%). This discrepancy could be partly attributable to the limitations of study design, wherein radiological assessments were conducted every 6 weeks until week 18 and every 12 weeks thereafter. As BOR could not be confirmed at the second time point for patients with first objective response (CR or PR) at week 18, they were assessed as non-responders for sensitivity analysis. However, these data are still comparable with the published ORR results in a similar population treated with the bevacizumab plus CP combination; 35% in ECOG 4599, 9 31.5% in the 2004 phase II study, 21 and 41.7% in the US-bevacizumab arm of the MAPLE study, with a confirmed response of 33%.14,32 It is important to note the slight differences in study population, timing of confirmed response analyses, and analysis criteria in the historical studies compared with the current study. Whereas this study enrolled patients with stage IV nsNSCLC and used RECIST 1.1 criteria, the historical studies also included stage IIIb nsNSCLC with a better prognosis and used RECIST 1.0.9,21 DOR and OS were included as secondary efficacy endpoints in this study, allowing analysis of long-term response and survival outcomes; however, median OS was not reached at the time of analysis. As the primary aim of clinical studies of biosimilars is to establish therapeutic equivalence and not clinical benefit per se, as the clinical benefit for the reference product is already established prior to biosimilar development, the use of ORR as the primary efficacy endpoint for comparison is a reasonable and appropriate choice.
In conclusion, based on the totality of evidence of analytical similarity, PK, comparability, and clinical safety and efficacy comparability, biosimilar MYL-1402O demonstrates therapeutic equivalence to bevacizumab, statistically confirmed by both FDA and EMA requirements, when given in combination with CP, as measured by ORR. Other efficacy endpoints, safety, and immunogenicity were also comparable. Demonstration of therapeutic equivalence in nsNSCLC is expected to be applicable to other approved indications for bevacizumab, because it has the same mechanism of action across all indications and the dose used for NSCLC is highest among all approved indications. Therefore, the therapeutic equivalence of MYL-1402O to bevacizumab supports extrapolation to all applicable indications for bevacizumab.
Bevacizumab in combination with platinum-doublet chemotherapy continues to be a key first-line option in the treatment of advanced or metastatic nsNSCLC, especially in those lacking actionable mutations amenable to targeted therapies and in combination with other targeted therapies, such as erlotinib and atezolizumab.3,8,33,34 The development and availability of MYL-1402O and other biosimilars is an important step in improving patient access and alleviating healthcare costs in NSCLC and other malignancies, where bevacizumab is a significant and integral component in the cancer management paradigm.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211045845 for Phase III double-blind study comparing the efficacy and safety of proposed biosimilar MYL-1402O and reference bevacizumab in stage IV non-small-cell lung cancer by Mark A. Socinski, Cornelius F. Waller, Tazeen Idris, Igor Bondarenko, Alexander Luft, Katrin Beckmann, Ashwini Vishweswaramurthy, Subramanian Loganathan, Charles Donnelly, Matthew A. Hummel, Roxann Shapiro, Melody Woods, Anita Rao, Vivek G Nayak, Gopinath Ranganna and Abhijit Barve in Therapeutic Advances in Medical Oncology
Acknowledgments
Technical, editorial, and medical writing assistance were provided under the direction of the authors by Krithika Subramanian, and Strategix, an affiliate of The Lynx Group LLC; funding for this support, including article publication charges, was provided by Viatris. The authors received no financial support or other form of compensation related to the development of this manuscript. All authors had full access to the full data in the study and accept responsibility to submit for publication.
The authors would like to thank the following Biocon team members: Nilanjan Sengupta, Sanjukta Chatterjee Yogesh Bhai Patel, Indresh Kumar R, Suma Krishna Reddy, Sumit Jha, and Thangamma KS for their contributions in the development of this manuscript
Footnotes
Author contributions: Manuscript writing, final approval and accountable for all aspects of the work: MS, CW, TI, IB, AL, KB, AV, SL, CD, MH, RS, MW, AR, VN, GR, AB.
Conflict of interest statement: M. Socinski: Research/grant funding from Spectrum, Novartis, Lilly, Genentech, and AstraZeneca; speaker bureau for Genentech, AstraZeneca, Guardant, Bristol Myers Squibb, Novartis, and Bayer. C. Waller: Member of advisory board for Viatris (formally Mylan Inc.). I. Bondarenko, A. Luft: Nothing to disclose. T. Idris, K. Beckmann, G. Ranganna, A. Barve, C. Donnelly, M. Hummel, R. Shapiro, M. Woods: Full-time employees of Viatris (formally Mylan Inc.) and may hold stock of Viatris (formally Mylan Inc.). A. Vishweswaramurthy, S. Loganathan, V. Nayak, A. Rao: Full-time employees of Biocon.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mark A. Socinski  https://orcid.org/0000-0003-2915-9325
https://orcid.org/0000-0003-2915-9325
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mark A. Socinski, AdventHealth Cancer Institute, 2501 North Orange Avenue, Suite 289, Orlando, FL 32803, USA.
Cornelius F. Waller, Department of Hematology, Oncology and Stem Cell Transplantation, University Medical Centre Freiburg, Freiburg, Germany
Tazeen Idris, Viatris, Hyderabad, Telangana, India.
Igor Bondarenko, Dnipropetrovsk Medical Academy, Dnipropetrovsk Oblast, Ukraine.
Alexander Luft, Leningrad Regional Clinical Hospital, St. Petersburg, Russian Federation.
Katrin Beckmann, Mylan Healthcare GmbH (A Viatris Company), Hannover, Germany.
Ashwini Vishweswaramurthy, Biocon Research Ltd., Bangalore, India.
Subramanian Loganathan, Biocon Research Ltd., Bangalore, India.
Charles Donnelly, Viatris, Morgantown, WV, USA.
Matthew A. Hummel, Viatris, Morgantown, WV, USA
Roxann Shapiro, Viatris, Morgantown, WV, USA.
Melody Woods, Viatris, Morgantown, WV, USA.
Anita Rao, Biocon Research Ltd., Bangalore, India.
Vivek G Nayak, Biocon Research Ltd., Bangalore, India.
Gopinath Ranganna, Viatris, Bengaluru, KA, India.
Abhijit Barve, Viatris, Canonsburg, PA, USA.
References
- 1. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016; 893: 1–19. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Assoun S, Brosseau S, Steinmetz C. et al. Bevacizumab in advanced lung cancer: state of the art. Future Oncol 2017; 13: 2515–2535. [DOI] [PubMed] [Google Scholar]
- 4. Shroff GS, de Groot PM, Papadimitrakopoulou VA. et al. Targeted therapy and immunotherapy in the treatment of non-small cell lung cancer. Radiol Clin North Am 2018; 56: 485–495. [DOI] [PubMed] [Google Scholar]
- 5. Chen R, Manochakian R, James L. et al. Emerging therapeutic agents for advanced non-small cell lung cancer. J Hematol Oncol 2020; 13: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Janning M, Loges S. Anti-angiogenics: their value in lung cancer therapy. Oncol Res Treat 2018; 41: 172–180. [DOI] [PubMed] [Google Scholar]
- 7. Shukla NA, Yan MN, Hanna N. The story of angiogenesis inhibitors in non-small-cell lung cancer: the past, present, and future. Clin Lung Cancer 2020; 21: 308–313. [DOI] [PubMed] [Google Scholar]
- 8. Garcia J, Hurwitz HI, Sandler AB, et al. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev 2020; 86: 102017. [DOI] [PubMed] [Google Scholar]
- 9. Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355: 2542–2550. [DOI] [PubMed] [Google Scholar]
- 10. Cohen MH, Gootenberg J, Keegan P, et al. FDA drug approval summary: bevacizumab (Avastin) plus carboplatin and paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist 2007; 12: 713–718. [DOI] [PubMed] [Google Scholar]
- 11. Monk BJ, Lammers PE, Cartwright T, et al. Barriers to the access of bevacizumab in patients with solid tumors and the potential impact of biosimilars: a physician survey. Pharmaceuticals (Basel) 2017; 10: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Melosky B, Reardon DA, Nixon AB, et al. Bevacizumab biosimilars: scientific justification for extrapolation of indications. Future Oncol 2018; 14: 2507–2520. [DOI] [PubMed] [Google Scholar]
- 13. MVASITM (bevacizumab-awwb) injection [package insert]. Thousand Oaks, CA: Amgen. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/mvasi/mvasi_pi_hcp_english.pdf (2020, accessed 26 September 2020). [Google Scholar]
- 14. Thomas M, Thatcher N, Goldschmidt J, et al. Totality of evidence in the development of ABP 215, an approved bevacizumab biosimilar. Immunotherapy 2019; 11: 1337–1351. [DOI] [PubMed] [Google Scholar]
- 15. Thatcher N, Goldschmidt JH, Thomas M, et al. Efficacy and safety of the biosimilar ABP 215 compared with bevacizumab in patients with advanced nonsquamous non-small cell lung cancer (MAPLE): a randomized, double-blind, phase III study. Clin Cancer Res 2019; 25: 2088–2095. [DOI] [PubMed] [Google Scholar]
- 16. Hummel M, Bosje T, Shaw A, et al. A pharmacokinetics study of proposed bevacizumab biosimilar MYL-1402O vs EU-bevacizumab and US-bevacizumab. J Cancer Res Clin Oncol. Epub ahead of print 17 April 2021. DOI: 10.1007/s00432-021-03628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. ZIRABEV, bevacizumab-bvzr injection, solution [package insert]. New York, NY: Pfizer Labs. http://labeling.pfizer.com/ShowLabeling.aspx?id=11860 (2020, accessed 26 September 2020). [Google Scholar]
- 18. Detterbeck FC, Boffa DJ, Kim AW, et al. The eighth edition lung cancer stage classification. Chest 2017; 151: 193–203. [DOI] [PubMed] [Google Scholar]
- 19. Reinmuth N, Bryl M, Bondarenko I, et al. PF-06439535 (a bevacizumab biosimilar) compared with reference bevacizumab (Avastin®), both plus paclitaxel and carboplatin, as first-line treatment for advanced non-squamous non-small-cell lung cancer: a randomized, double-blind study. BioDrugs 2019; 33: 555–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. AVASTIN® (bevacizumab) injection, for intravenous use [package insert]. South San Francisco, CA: Genentech Inc. www.gene.com/download/pdf/avastin_prescribing.pdf (2020, accessed 26 September 2020). [Google Scholar]
- 21. Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004; 22: 2184–2191. [DOI] [PubMed] [Google Scholar]
- 22. European Medicines Agency. Guideline on similar biological medicinal products containing monoclonal antibodies – non-clinical and clinical issues, May 30, 2012. www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-monoclonal-antibodies-non-clinical_en.pdf (2012, accessed 26 September 2020).
- 23. US Food and Drug Administration. Clinical trial endpoints for the approval of cancer drugs and biologics, Guidance for Industry. www.fda.gov/media/71195/download. (2018, accessed 26 September 2020).
- 24. Botrel TEA, Clark O, Clark L, et al. Efficacy of bevacizumab (BEV) plus chemotherapy (CT) compared to CT alone in previously untreated locally advanced or metastatic non-small cell lung cancer (NSCLC): systematic review and meta-analysis. Lung Cancer 2011; 74: 89–97. [DOI] [PubMed] [Google Scholar]
- 25. Lima ABC, Macedo LT, Sasse AD. Addition of bevacizumab to chemotherapy in advanced non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2011; 6: e22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soria J-C, Mauguen A, Reck M, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol 2013; 24: 20–30. [DOI] [PubMed] [Google Scholar]
- 27. Han S, Hong Y, Liu T, et al. The efficacy and safety of paclitaxel and carboplatin with versus without bevacizumab in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Oncotarget 2018; 9: 14619–14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reck M, Luft A, Bondarenko I, et al. A phase III, randomized, double-blind, multicenter study to compare the efficacy, safety, pharmacokinetics, and immunogenicity between SB8 (proposed bevacizumab biosimilar) and reference bevacizumab in patients with metastatic or recurrent nonsquamous non-small cell lung cancer. Lung Cancer 2020; 146: 12–18. [DOI] [PubMed] [Google Scholar]
- 29. Yang Y, Wu B, Huang L, et al. Biosimilar candidate IBI305 plus paclitaxel/carboplatin for the treatment of non-squamous non-small cell lung cancer. Transl Lung Cancer Res 2019; 8: 989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin–gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol 2010; 21: 1804–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Niho S, Kunitoh H, Nokihara H, et al. Randomized phase II study of first-line carboplatin–paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer 2012; 76: 362–367. [DOI] [PubMed] [Google Scholar]
- 32. US Food and Drug Administration. FDA Briefing Document, Oncologic Drugs Advisory Committee, BLA 761028, ABP215, a proposed biosimilar to Avastin (bevacizumab). www.fda.gov/media/106528/download (2017, accessed 26 September 2020).
- 33. Planchard D, Popat S, Kerr K, et al. ESMO Guidelines Committee. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29 (Suppl. 4): iv192–iv237. [DOI] [PubMed] [Google Scholar]
- 34. National Comprehensive Cancer Network. Non-small cell lung cancer (version 6.2020). www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (2020, accessed 26 September 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211045845 for Phase III double-blind study comparing the efficacy and safety of proposed biosimilar MYL-1402O and reference bevacizumab in stage IV non-small-cell lung cancer by Mark A. Socinski, Cornelius F. Waller, Tazeen Idris, Igor Bondarenko, Alexander Luft, Katrin Beckmann, Ashwini Vishweswaramurthy, Subramanian Loganathan, Charles Donnelly, Matthew A. Hummel, Roxann Shapiro, Melody Woods, Anita Rao, Vivek G Nayak, Gopinath Ranganna and Abhijit Barve in Therapeutic Advances in Medical Oncology