Abstract
Background:
Insulinoma is the most common neuroendocrine neoplasm of the pancreas, characterised by hypoglycaemic symptoms. Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) and ethanol ablation (EUS-EA) are novel methods for treating insulinoma.
We aimed to perform a systematic review to assess the efficacy and safety of EUS-guided ablation techniques for pancreatic insulinomas.
Methods:
We systematically searched for articles detailing EUS-guided ablations of insulinomas. We performed a qualitative analysis and summarised data on the efficacy and safety of EUS-RFA and EUS-EA techniques.
Results:
In total, we identified 35 case reports and case series describing 75 patients with insulinomas treatment with EUS-guided ablation. Twenty-seven patients were treated with EUS-RFA, 47 patients with EUS-EA, and 1 patient received EUS-EA and EUS-RFA in the same session. In total, 84 insulinomas were ablated (EUS-RFA: 31, EUS-EA: 53). Most insulinomas were in the head of the pancreas (40%). The clinical success rate for EUS-guided ablation techniques was 98.5%. The median glucose level was 1.95 (Q1-Q3: 1.69–2.13) mmol/L before ablation compared to 6.20 (Q1-Q3: 5.30–7.05) mmol/L after treatment. The median insulin and C-peptide levels before and after RFA/EA were 230 (Q1–Q2: 120–257) pmol/L and 41 (Q1–Q2 35–42) pmol/L; 2077 (Q1–Q2 1644–2459) pmol/L and 819 (Q1–Q2 696–1072) pmol/L, respectively. There were eleven adverse events: seven abdominal pain, two mild acute pancreatitis, one necrotising acute pancreatitis and one local hematoma. All patients recovered, and there were no periprocedural deaths.
Conclusions:
EUS-guided ablation of insulinoma seems to be a safe and effective treatment and is an alternative to surgical resection in selected cases.
Keywords: endoscopic ultrasound, endoscopic ultrasound-guided ethanol ablation, endoscopic ultrasound-guided radiofrequency ablation, insulinoma
Introduction
Insulinomas are a rare type of pancreatic neuroendocrine tumours (pNET) that autonomously secrete insulin, 1 which can cause to inappropriate hypoglycaemic episodes during fasting, typically in the morning. 2
In addition, sympathetic activation and catecholamine secretion can lead to excessive perspiration, tachycardia, palpitation, generalised weakness, and food craving.3,4 Typically, all these symptoms promptly disappear following either oral or IV glucose administration. 5 Insulinomas are rare tumours; the prevalence of functioning insulinomas is estimated to be between one and four per million persons in the general population. 5 Most insulinomas are within the pancreas, where the majority (90%) are located in the pancreatic head and body, and the majority are benign. 6
Surgical resection is still the gold standard treatment for insulinomas. Even using minimally invasive laparoscopic techniques (i.e. surgical enucleation), complications such as pancreatic fistulas and sepsis can occur.7,8 Alternatively, non-surgical treatments such as high-carb foods, including night-time snacks, combined with medical therapy (e.g. diazoxide, somatostatin analogues or octreotide), which can control symptoms in some patients. 9
Image-guided ablative techniques are widely used as an alternative to major surgery for treating tumours in the lung, liver and kidney. The pancreas is readily accessible during EUS, and an EUS-guided ablation is, therefore, an option for delivering ablative treatments. EUS-guided ablation was first reported in 2006, 10 and there is a growing body of evidence on its safety and efficacy. These techniques have created new treatment pathways, especially for elderly or frail patients with multiple comorbidities where there are higher risks associated with major surgery. 10 There are two main techniques used, EUS-guided radiofrequency ablation (EUS-RFA) and EUS-guided ethanol ablation (EUS-EA). The concept behind RFA is to produce thermal energy within the tumour, which induces tissue necrosis by cell protein denaturation.11–13 Ethanol is also an effective cheap, and rapid-acting ablative agent. It has successfully stimulated tissue necrosis in various organs, e.g. thyroid gland, spleen, kidney and liver, with minimal side effects recorded.14–16 Previous studies showed that EUS-guided injection of alcohol was successfully performed to treat liver metastases, gastrointestinal stromal tumours (GISTs) and pancreatic cysts.17,18 Our systematic review aimed to perform a descriptive analysis of EUS ablation techniques with RFA and EA in insulinoma patients.
Material and methods
This study is reported following the Preferred Reporting Items for Systematic Review (PRISMA) Statement. 19 The protocol of the systematic review was registered in PROSPERO under the registration number CRD 42021234378.
Search
We conducted a systematic search (GES, LF) in six databases, including MEDLINE (via PubMed), EMBASE, Cochrane Controlled Register of Trials (CENTRAL), Web of Science, Scopus, and ClinicalTrials.gov. with the query (endoscopic ultrasound) AND (insulinoma). We searched all relevant reports from inception to 24/11/2020 without restrictions.
Selection, screening and data collection
Duplicates were removed using EndNote X7.4 (Clarivate Analytics, Philadelphia, PA, USA), then two independent review authors (GES, LF) screened all relevant titles, abstracts, and full-text publications against the eligibility criteria. Disagreements were resolved by consensus.
Eligibility criteria
We included all records on adults (>18 years) with insulinomas treated by EUS-guided RFA/EA. Regarding the study design, we narrowed the focus to case-control, case series studies and prospective and retrospective cohort studies (regardless of the publication type, i.e. conference abstract or full-text format). There was no language restriction. We excluded all articles where EUS-RFA or EUS–EA was carried out intraoperatively or used another type of EUS-guided ablation technique. All study designs were eligible, but we excluded reviews. If there were multiple publications on the same cohorts of patients, the larger and more recent study population was included.
Data extraction
All data were independently extracted by two co-authors (GES, LF) from relevant studies and added to a pre-defined Excel datasheet (Office 365, Microsoft, Redmond, WA, USA). The following data were extracted: study settings (study population, number of patients with insulinoma, indication for EUS-guided RFA/EA, geographical region, recruitment period, the total number of treated insulinoma, number of treatment sessions and passes, type of needle used in EUS-guided RFA/EA (Gr), median ethanol concentration in EA (%), the median time duration in RFA/EA (s), median energy setting (Watt), in RFA, and essential characteristics of the study population (age, gender, comorbidities, BMI, and laboratory parameters (glucose-, insulin-, and C-peptide levels), diagnostic criteria of insulinoma and EUS-guided ablation techniques. The patients’ data were retrospectively analysed.
Quality assessment
Two co-authors (GES, LF) used Murad et al 20 tool to assess case studies and case series to evaluate the included studies’ quality. This tool covered four main domains, including selection, ascertainment, causality, and reporting. We did not aggregate scores but discussed these findings as limitations of the evidence as per Murad et al 20 recommendations.
Results
Search and selection
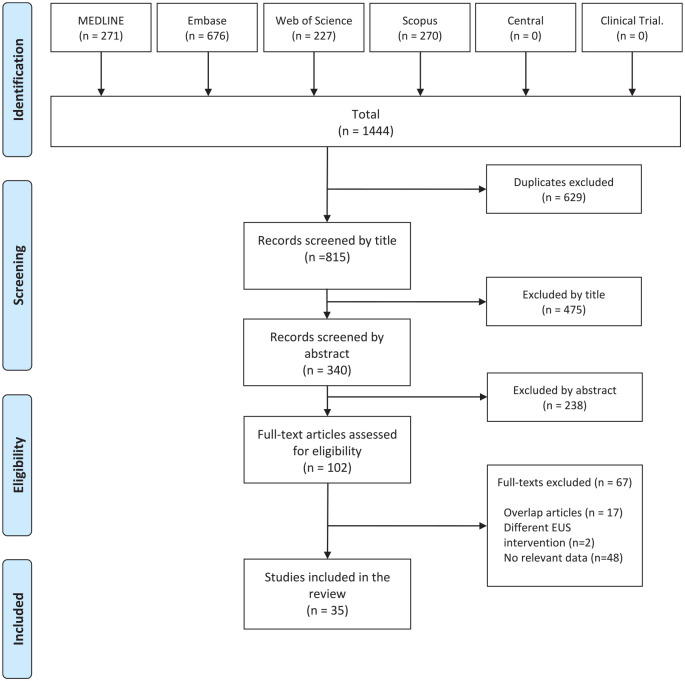
A total of 1444 records were identified in 6 databases. Out of the 102 publications screened by full text, we excluded 67 papers. The flow chart of the selection is detailed in Figure 1. The most common causes of exclusion on full-text assessment were lack of relevant data, overlapping case reports and case series, and the use of different EUS-ablation techniques. Finally, 35 non-overlapping case reports and case series were identified and included in the qualitative analysis. Eleven articles reported EUS-RFA and 23 articles EUS-EA technique, and 1 case report reported using both techniques.10,16,21–52
Figure 1.
PRISMA Flow chart of the selection process.
Characteristics of the studies included
We performed a descriptive analysis of all published EUS-guided RFA/EA of insulinomas. We used boxplots for all laboratory parameters pre-and post-EUS-guided RFA/EA techniques within the descriptive analysis. The included studies’ main characteristics are summarised in Tables 1–5. One article was in Hungarian. 23 The 34 remaining articles were written in English.10,16,21,22,24–52 Fifteen reports were from Europe,10,22–24,26–30,34,40,45,49–51 nine from Asia,25,32,33,35,37,41–43,53 eight from the United States of America,16,31,36,39,44,47,48,52 two from South America,38,46 and one from Egypt. 21 Our comprehensive search did not identify any comparative studies (controlled); all data emerged from descriptive studies (case reports or case series).
Table 1.
Main characteristics of the studies included. Patients treated with EUS-guided RFA technique. NA: not applicable, F: female, M: male, RFA: radifrequency ablation, p.: patient, EUS: endoscopic ultrasound.
| Study population | Country | No. of patients with insulinoma | Gender (M\F) | Age (y) | Indication for EUS-RFA | No of insulinomas | No of sessions | No of passes | Watts | RFA duration (s) | Glucose (mmol/L) | Insulin (pmol/L) | C-peptid (pmol/L) | Type of adverse event | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| before RFA | after RFA | before RFA | after RFA | before RFA | after RFA | ||||||||||||
| Bas-Cutrina et al. 22 | Spain | 1 | F | 63 | comorbidity | 1 | 1 | 3 | 10 | 360 | NA | NA | NA | NA | NA | NA | no |
| Choi et al. 25 | Korea | 1 | M | 34 | refuse and high surgical risk | 1 | 1 | 3 | 50 | 10 | NA | NA | NA | NA | NA | NA | no |
| De Nucci et al. 28 | Italy | 1 | F | 76 | comorbidity | 1 | 1 | 2 | 10 | 60 | 1.9 | NA | NA | NA | NA | NA | no |
| Godat et al. 30 | Switzerland | 1 | NA | NA | NA | 1 | 1 | 3 | 50 | 10 | NA | NA | NA | NA | NA | NA | abd. pain |
| Jonica and Wagh 31 | USA | 1 | F | 57 | comorbidity | 1 | 1 | 5 | 30 | 20 | 2.22 | 7.66 | 257 | NA | NA | NA | no |
| Kandula et al. 32 | India | 1 | F | 41 | refuse surgery | 2 | NA | NA | NA | NA | 2.22 | NA | NA | NA | NA | NA | abd. pain, acute pancreatitis |
| Kluz et al. 34 | Poland | 1 | M | 40 | refuse surgery | 1 | 3 | NA | 50 | 10 | NA | NA | NA | NA | NA | NA | acute necro. pancreatitis |
| Oleinikov et al. 40 | Israel | 7 | M | 44 | refuse surgery | 1 | 1 | NA | 10-50 | 5-12 | 3.38 ± 0.34 | 6.61 ± 0.13 | NA | NA | NA | NA | no |
| M | 65 | 1 | 1 | 50 | 5-12 | ||||||||||||
| M | 61 | 1 | 1 | 10-50 | 5-12 | ||||||||||||
| M | 73 | 1 | 1 | 10-50 | 5-12 | ||||||||||||
| F | 28 | 1 | 1 | 10-50 | 5-12 | ||||||||||||
| F | 56 | 1 | 1 | 10-50 | 5-12 | ||||||||||||
| F | 37 | 1 | 1 | 10-50 | 5-12 | ||||||||||||
| Sharma et al. 44 | USA | 2 | M | 44 | refuse surgery | 1 | 3 | NA | 10 | NA | 1.72 | NA | NA | NA | NA | NA | NA |
| F | 37 | 1 | 3 | 10 | NA | ||||||||||||
| Waung et al. 50 | UK | 1 | F | 70 | comorbidity | 1 | 3 | 3 | 10 | NA | 1.6 | NA | 120 | NA | 2418 | NA | NA |
| Lakhtakia et al. 53 | India | 10 | NA | NA | NA | 13 | 8 p./1 2 p./>1 |
NA | NA | NA | NA | normal | NA | normal | NA | normal | Abd.pain, acute panc. |
Table 2.
Main characteristics of the studies included. Patients treated with EUS-guided EA technique. NA: not applicable, F: female, M:male, EA: etanol ablation, EUS: endoscopic ultrasound.
| Study population | Country | No. of patients with insulinoma | Gender (M\F) | Age (years) | Indication for EUS-EA | No of insulinomas | No of sessions | No of passes | Ethanol concentration (%) | Ethanol volume (ml) | Glucose (mmol/L) | Insulin (pmol/L) | C-peptid (pmol/L) | Type of adverse event | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| before EA | after EA | before EA | after EA | before EA | after EA | ||||||||||||
| Altonbary et al. 21 | Egypt | 1 | M | 35 | refuse surgery | 1 | 2 | 2 | NA | 6 | 1.67 | NA | 1632 | NA | NA | NA | no |
| Bor et al. 23 | Hungary | 1 | F | 83 | comorbidity | 1 | 1 | 2 | 96 | 3 | 1.7 | 3.5 | 185 | 25.7 | NA | NA | no |
| Burghardt et al. 6 | Germany | 1 | F | 65 | comorbidity | 1 | 1 | NA | 96 | 1 | 2.1 | 7.5 | 271 | NA | NA | NA | no |
| Dabkowski et al. 26 | Poland | 1 | F | 81 | comorbidity | 1 | 2 | NA | 96 | 3 | 1.61 | 4.61 | 231 | 34 | 1586 | 728 | no |
| Sousa et al. 27 | Portugal | 1 | F | 89 | comorbidity | 1 | 1 | NA | 95 | 0.6 | 2.11 | NA | 83.3 | NA | NA | NA | no |
| Deprez et al. 29 | Belgium | 1 | F | 78 | comorbidity | 1 | NA | NA | 98 | 3.5 | 1.9 | 6.2 | 229.6 | 43.1 | 1970 | NA | duodenal perforation |
| Jürgensen et al. 10 | Germany | 1 | F | 78 | refuse surgery | 1 | 1 | 4 | 95 | 8 | 0.8 | 5.3 | 560 | 41 | 3880 | 1560 | abd.pain |
| Lee et al. 35 | Korea | 1 | F | 26 | tumour location (multiple ins.) | 4 | 1 | 3 | 95 | 3.2 | 2 | 5.3 | 106.3 | 61.8 | 1200 | 600 | no |
| Levy et al. 36 | USA | 5 | F | 72 | comorbidity | 1 | 2 | 5 | 98 | 0.37 | NA | 7.77 | NA | NA | NA | NA | no |
| M | 82 | comorbidity | 1 | 3 | 7 | 95 | 0.62 | NA | |||||||||
| F | 80 | comorbidity | 1 | 2 | 9 | 98 | 1.23 | NA | |||||||||
| F | 57 | refuse surgery | 1 | 2 | 7 | 98 | 4.5 | NA | |||||||||
| M | 34 | refuse surgery | 1 | 2 | 9 | 99 | 1.7 | NA | |||||||||
Table 3.
Main characteristics of the studies included. Patients treated with EUS-guided EA technique. NA: not applicable, EA: etanol ablation, EUS: endoscopic ultrasound.
| Study population | Country | No. of patients with insulinoma | Gender (M\F) | Age (y) | Indication for EUS-EA | No of insulinomas | No of sessions | No of passes | Ethanol concentration (%) | Ethanol volume (ml) | Glucose (mmol/L) | Insulin (pmol/L) | C-peptid (pmol/L) | Type of adverse event | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| before EA | after EA | before EA | after EA | before EA | after EA | ||||||||||||
| Luo et al. 37 | China | 1 | M | 32 | comorbidity | 1 | 1 | NA | 99 | 1.5 | NA | NA | NA | NA | NA | NA | no |
| Mittal et al. 16 | USA | 1 | M | 59 | unresectable | 1 | 2 | 2 | NA | 2.8 | NA | NA | NA | NA | NA | NA | no |
| Mosquera-Klinger et al. 38 | Colombia | 2 | F | 40 | refuse surgery | 1 | 1 | 2 | 96 | 2 | 2 | NA | NA | NA | NA | NA | no |
| F | 80 | comorbidity | 1 | 1 | 2 | 96 | 2 | NA | NA | NA | NA | NA | NA | no | |||
| Nelson et al. 39 | USA | 1 | F | 81 | comorbidity | 1 | 1 | NA | 98 | 1 | NA | NA | NA | NA | NA | NA | no |
| Paik et al. 41 | Korea | 3 | M | 99 | refuse surgery | 1 | 1 | NA | NA | 1.2 | NA | NA | NA | NA | NA | NA | no |
| M | 20 | refuse surgery | 1 | 1 | 2.5 | no | |||||||||||
| M | 32 | refuse surgery | 1 | 1 | 3 | abd.pain | |||||||||||
| Park et al. 42 | Korea | 2 | F | 66 | refuse surgery | 1 | 2 | 2 | 99 | 6.4 | NA | NA | NA | NA | NA | NA | no |
| F | 27 | comorbidity | 3 | 1 | 3 | 99 | 3.2 | no | |||||||||
Table 4.
Main characteristics of the studies included. Patients treated with EUS-guided EA technique. NA: not applicable, EA: etanol ablation, EUS: endoscopic ultrasound.
| Study population | Country | No. of patients with insulinoma | Gender (M\F) | Age (years) | Indication for EUS-EA | No of insulinomas | No of sessions | No of passes | Ethanol concentration (%) | Ethanol volume (ml) | Glucose (mmol/L) | Insulin (pmol/L) | C-peptid (pmol/L) | Type of adverse event | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| before EA | after EA | before EA | after EA | before EA | after EA | ||||||||||||
| Qin et al. 43 | China | 17 | F | 48 | NA | 1 | 1 | 1 | NA | 0.5 | NA | NA | NA | NA | NA | NA | AMS level increase |
| F | 56 | 1 | 3 | 3 | 0.35 | no | |||||||||||
| M | 56 | 1 | 1 | 1 | 0.5 | no | |||||||||||
| M | 66 | 1 | 1 | 1 | 0.3 | no | |||||||||||
| M | 46 | 1 | 2 | 2 | 2 | no | |||||||||||
| M | 33 | 1 | 1 | 1 | 0.3 | no | |||||||||||
| F | 42 | 1 | 2 | 2 | 1.3 | no | |||||||||||
| F | 52 | 1 | 1 | 1 | 0.5 | no | |||||||||||
| M | 24 | 1 | 3 | 3 | 5.3 | no | |||||||||||
| M | 46 | 1 | 1 | 1 | 0.5 | no | |||||||||||
| M | 28 | 1 | 1 | 1 | 0.5 | no | |||||||||||
| M | 42 | 1 | 1 | 1 | 2 | no | |||||||||||
| F | 51 | 1 | 2 | 2 | 1.6 | no | |||||||||||
| F | 51 | 1 | 3 | 3 | 2.9 | no | |||||||||||
| F | 35 | 1 | 2 | 2 | 2 | no | |||||||||||
| F | 52 | 1 | 1 | 1 | 1.2 | no | |||||||||||
| F | 42 | 1 | 1 | 1 | 1 | no | |||||||||||
Table 5.
Main characteristics of the studies included. Patients treated with EUS-guided EA technique. NA: not applicable.
| Study population | Country | No. of patients with insulinoma | Gender (M\F) | Age (y) | Indication for EUS-EA | No of insulinomas | No of sessions | No of passes | Ethanol concentration (%) | Ethanol volume (ml) | Glucose (mmol/L) | Insulin (pmol/L) | C-peptid (pmol/L) | Type of adverse event | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| before EA | after EA | before EA | after EA | before EA | after EA | ||||||||||||
| Schnack et al. 45 | Denmark | 1 | M | 89 | comorbidity | 1 | NA | NA | 98 | 3.5 | NA | NA | NA | NA | NA | NA | mild AP |
| Silva et al. 46 | Brazil | 1 | F | 21 | comorbidity | 1 | NA | NA | 99 | 1.5 | NA | NA | NA | NA | NA | NA | no |
| Trevino et al. 47 | USA | 1 | F | 79 | tumour location (multiple ins.) | 2 | 1 | 2 | 98 | 12 | NA | NA | NA | NA | NA | NA | no |
| Trikudantha et al. 48 | USA | 1 | F | 66 | comorbidity | 1 | 1 | 4 | NA | 1 | 2.5 | NA | 228 | NA | 2473 | NA | no |
| Vleggaar et al. 49 | The Netherlands | 1 | F | 82 | comorbidity | 1 | NA | NA | 96 | 0.3 | 2.2 | 6.4 | 97.2 | 41.7 | 1820 | 910 | no |
| Zalewska et al. 51 | Poland | 1 | F | 72 | comorbidity | 1 | 2 | NA | 96 | 2 | 1.8 | NA | NA | NA | NA | NA | abd.pain |
| Zarug et al. 52 | USA | 1 | M | 56 | comorbidity | 1 | NA | NA | NA | 1.5 | 2.05 | NA | 252 | NA | NA | NA | no |
Quality assessment
The quality of the studies included is summarised in Supplementary Table 1.
Findings of the systematic review
Main epidemiology characteristics in studies included
Altogether we analysed 35 articles in our systematic review. Eleven studies reported on EUS-guided RFA:22,25,28,30–32,34,40,44,50 twenty-three on EUS-guided EA:10,16,21,23,24,26,27,29,35–39,41–43,45–49,51,52 and one on both techniques. 33 The total number of patients was seventy-five, of whom 26 were male, 38 female and 11 patients’ gender was not reported. 30 The male: female ratio in our study was 42:58%, respectively. The average age of all patients was 55 ± 19.91 years, of which the average male and female age were 51 ± 20.21 and 58 ± 19.36 years, respectively. For the detailed demographic characteristics, see Tables 6–8.
Table 6.
Basic characteristics across groups.
| Age | Total mean age | ||||||
|---|---|---|---|---|---|---|---|
| Gender | mean (y) | SD | Range | mean (y) | SD | Range | |
| Male | 26 | 51 | ±20.21 | 20–99 | 55 | ± 19.91 | 20–99 |
| Female | 38 | 58 | ±19.36 | 21–89 | |||
| N/A | 11 | ||||||
| Total | 75 | Total mean age | |||||
| mean (y) | SD | Range | |||||
| 55 | ± 19.91 | 20–99 | |||||
Table 7.
Age (y) distribution across EUS-guided radiofrequency and ethanol ablation groups.
| AGE-EA | AGE-RFA | ||||||
|---|---|---|---|---|---|---|---|
| MALE | FEMALE | MALE | FEMALE | Total age RFA | Total age EA | ||
| Mean | 49.73 | 60.21 | 51.57 | 51.66 | Mean total | 51.625 | 56.37 |
| Min | 20 | 21 | 34 | 28 | Min | 28 | 20 |
| Max | 99 | 89 | 73 | 76 | Max | 76 | 99 |
| SD | ± 22.58 | ± 19.99 | ± 14.63 | ± 16.61 | SD | ± 15.26 | ± 21.05 |
Table 8.
Main characteristics of included articles.
| Number of included articles | EUS-RFA: number of patients | EUS-EA: number of patients | |||
|---|---|---|---|---|---|
| EUS-RFA | 11 | MALE | FEMALE | MALE | FEMALE |
| EUS-EA | 23 | 7 | 9 | 19 | 28 |
| RFA + EA | 1 | TOTAL: 16 | TOTAL: 47 | ||
| TOTAL | 35 | 11 patient: unknown gender | |||
Main characteristics of RFA treated group
The EUS-RFA group included 27 patients; 11 patients had no gender and age reported. 30 The male and female ratio in this group was seven versus nine, respectively. The average age was 52 ± 14.63 and 52 ± 16.61 years, in males and females, respectively (Tables 6, 7).
Main characteristics of EA treated group
The EUS-EA group included 47 patients (male:19\female:28). The average age of male versus female was 50 ± 22.58 vs 60 ± 19.99 years, respectively (Tables 6, 7).
Main characteristics of insulinomas, ablation techniques, and location
Eighty-four insulinomas were ablated across all included patients (EUS-RFA: 31 insulinomas vs EA: 53 insulinomas). One patient received both EUS-guided EA and RFA in the same session. 33 Six patients had multiple insulinomas:33,35,38,40,42,47 two patients had three insulinomas located in the head, body and tail;35,42 one patient in the head and body; 40 two patients in the body and tail of the pancreas;38,47 one patients in the head and uncinate process of the pancreas. 40 All patients treated by RFA had solitary insulinoma. Sixty-five insulinomas were ablated in 47 patients by EA.
Insulinoma location and size
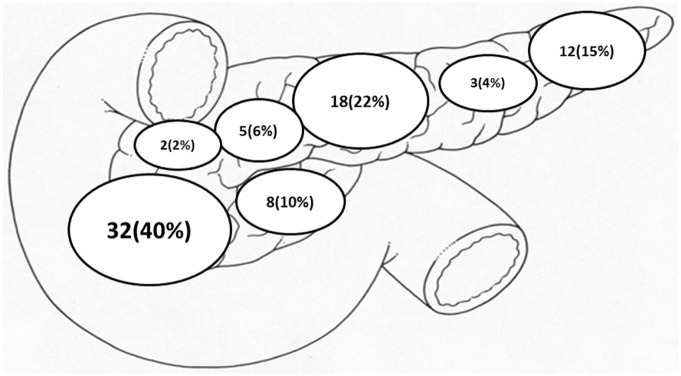
Thirty-two (40%) of the insulinomas were located in the head of the pancreas, 18 (22%) in the body, 12 (15%) in the tail, 8 (10%) in the uncinate process, 5 (6%) in the neck, 3 (4%) in the body/tail area, 2 (2%) in the head/body area and four insulinomas were unreported (Figure 2). The pre-treatment (EUS–guided ablation) median size of all insulinomas was 13 mm x 10 mm (range: 4-27 mm x 2–27 mm). The post-treatment median size of insulinomas was 3x5 mm (range: 3–7 mm x 5–7 mm).
Figure 2.
Location of insulinomas (n = 84).
Indications of EUS-guided ablation
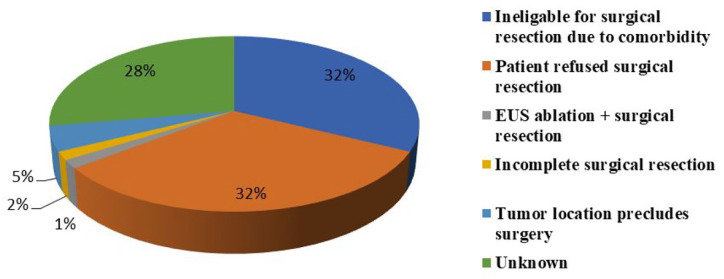
Twenty-one (32%) of patients had multiple co-morbidities precluding general anaesthesia and abdominal surgery (coronary heart diseases, arterial hypertension, cardiac valve replacement, severe aortic stenosis, cardio-vascular diseases, advanced kidney disease, morbid obesity, COPD, sleep apnoea and advanced age). Twenty-one (32%) refused surgery, and 3 (5%) had anatomically unfavourable insulinoma locations for surgical resection. Incomplete resections before EUS-guided ablation were identified in one patient. Surgical resection after EUS-guided ablation was noted in one of the cases. Five studies did not explain the indication (Figure 3).
Figure 3.
Indication for EUS-guided radiofrequency and ethanol ablation.
Descriptive analysis of EUS-guided EA
In total, 47 patients underwent EUS-guided EA (19 male, 28 female) with a median alcohol concentration of 98%, of whom 25 patients received one session (average volume injected 1.58 mL), 13 patients had two sessions (average volume 1.24 mL), four patients had three sessions (average volume 1 mL), and five patients had an unclear number of sessions and volume reported (Tables 9, 10). Volumes injected per session ranged from 0.3 mL to 6 mL (median volume 1.55 mL). The median number of sessions and passes were one and two, respectively. In all, 25-gauge needles were used for 4 patients, 22-gauge for 18 patients, and 19-gauge needles for two patients. The type of needle was not recorded in the other remaining cases.
Table 9.
Main characteristics of EUS-guided ethanol ablation technique.
| Alcohol concentration % | Number of passes | Number of pts | Number of session | Number of pts | |
|---|---|---|---|---|---|
| Median | 98 | 1. | 10 | 1. | 25 |
| Min | 95 | 2. | 11 | 2. | 13 |
| Max | 99 | 3. | 5 | 3. | 4 |
| 4. | 2 | NA: | 5 | ||
| 5. | 1 | Total: | 47 | ||
| 6. | 0 | ||||
| 7. | 2 | ||||
| 8. | 0 | ||||
| 9. | 2 | ||||
| NA: | 14 | ||||
| Total: | 47 | ||||
Table 10.
Main characteristics of EUS-guided radiofrequency and ethanol ablation techniques.
| Median numbers and range of RFA session | 2 (1–3) | Median numbers and range of EA session | 1 (1–3) |
| Median numbers and range of RFA passes | 3 (2–14) | Median numbers and range of EA passes | 2 (1–4) |
| Median type and range of needle (Gr) | 19 (18–22) | Median type and range of needle (Gr) | 22 (19–25) |
| Median energy and range (watts) | 10 (10–50) | Median and range of ethanol concetration (%) | 98 (95–99) |
| Median time duration and range (s) | 15 (10-360) | Median time duration and range (day) | 34.5 (3–93) |
| Median total alcohol volume and range (ml) | 1.55 (0.3–6) |
Descriptive analysis of EUS-guided RFA
Ten patients (4 male/5 female and one unknown gender) underwent EUS-RFA with a median energy setting at 10 Watts (range: 10–50 W) and a median ablation duration of 15 seconds. The energy setting was not recorded in the remaining cases. Twelve patients received one session, four patients had three sessions, and one patient had an unknown number of sessions. The median number of sessions and passes were 2 and 3, respectively (Table 1). In all, 19-gauge needles were used in 8 patients, and 18-gauge and 22-gauge in one patient each.22,33 (Tables 9, 10).
Laboratory parameters findings
Pre-ablation EUS-guided RFA and EA biochemical parameters revealed (average serum glucose- (data from 20 patients), insulin (13 patients), and C-peptide (10 patients) levels; 1.95 (Q1-Q3 1.69–2.13) mmol/L; 230 (Q1–Q3 120–257) pmol/L; 2077 (Q1-Q3 1644–2459) pmol/L, respectively). Post-ablation EUS-guided RFA and EA biochemical results showed 6.20 (Q1-Q3 5.30–7.05) mmol/L (11 patients); 41 (Q1-Q3 35–42) pmol/L (six patients); 819 (Q1-Q3 696–1072) pmol/L (four patients), respectively (Figure 4(a), (b), (c)).
Figure 4.
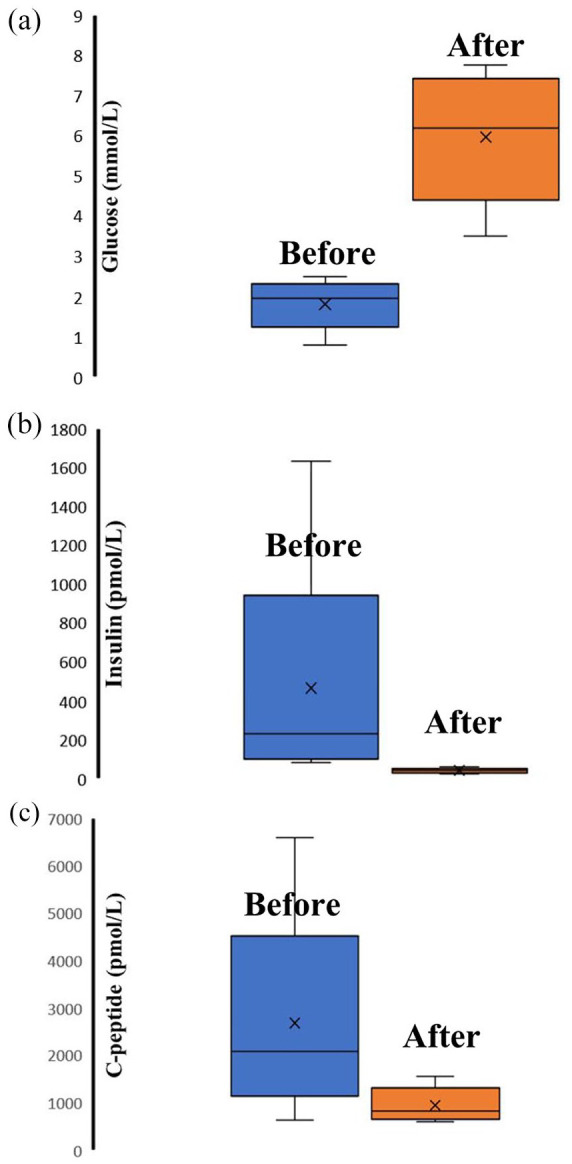
(a) Serum glucose level before and after EUS-guided radiofrequency and ethanol ablation. (b) Serum insulin level before and after EUS-guided radiofrequency and ethanol ablation. (c) Serum C-peptid level before and after EUS-guided radiofrequency and ethanol ablation.
Follow-up period of post-EUS ablation techniques and follow-up imaging techniques
Fifteen out of the 35 reviewed articles reported the imaging modality that was used for post-EUS ablation follow-up. Transabdominal US, MRI, and PET-CT follow-up were each reported in one article; seven reported the use of CT, and five articles reported the use of EUS. All these imaging techniques showed significant reduction or complete resolution of pancreatic insulinomas in the post-ablation follow-up period. Twenty-eight articles of 35 inconsistently reported the duration of the follow-up period. The average follow-up period post-ablation was 9 months (range: 5–1460 days).
Adverse events
Eleven out of 75 (15%) patients had complications following EUS-guided ablation, five (18%) after EUS-RFA30,32,34,53 and six (13%) after EUS-EA.10,29,41,43,45,51 All five patients in the EUS-RFA group had experienced abdominal pain, two of them developing mild acute pancreatitis, and one had severe necrotising pancreatitis. In the case of EUS-guided EA, three patients complained of abdominal pain; one had duodenal perforation with bleeding. Technical and clinical success rates were 98.5% for both modalities, and no deaths occurred. One patient, following the failure of EUS-EA, needed surgical resection. 21
Body composition
Body mass index was only reported in six cases.10,22,29,31,37,46 In 5 female and 1 male patient, the average age was 55 (range: 21–78) years. Obesity (BMI > 40 kg/m2) was recorded in four cases.
Discussion
Efficacy of ablation techniques
Animal models have previously demonstrated the safety and efficacy of EUS-RFA and EUS-EA techniques. These results had prompted the use of these ablation techniques in different human conditions. 54 – 56 The published literature suggests that EUS-guided ablation techniques (RFA/EA) for insulinoma have a high technical success rate (64 out of 65; 98.5%); equally the clinical success rate was promising in this cohort of patients, as only 1 patient out of 65 patients required further surgical management. In our systematic review, the clinical success was measured with the normalisation of biochemical parameters, including serum glucose levels, C-peptide levels and insulin levels. The radiological response was also appreciated during the follow-up period, as most of these patients had complete resolution of their insulinomas after EUS-guided ablation (RFA/EA).10,16,22,23,25,27–32,37,42,47,49 In comparison, the French prospective multicentre study of Barthet et al 13 demonstrated 86% efficacy with the EUS-RFA technique of 14 solid pancreatic neuroendocrine tumours after 1-year follow-up and complete resolution of three insulinomas after a 2-year follow-up. 13 Jürgensen et al 10 reported the first case of EUS–EA of a 13 mm pancreatic insulinoma in 2006. Our review highlighted that multiple insulinomas were also described in our literature search that ethanol ablation was performed with excellent clinical outcome. Regardless of the number of insulinomas, EUS-EA proved to be effective and spared surgical intervention.
The first successful treatment of multiple insulinomas with EUS-EA in a multimorbid patient from the United States was published in 2011. It immediately improved the blood glucose levels; the treatment response was sustained at 4 months follow-up when no hypoglycaemic episodes occurred. 47 Both ablation techniques can be safely applied in the same patient with multiple insulinomas; this was demonstrated by Khoo et al. 33 where a patient had recurrent insulinoma following distal pancreatectomy. The insulinoma in the head was ablated with EUS-EA and the insulinoma in the body with EUS-RFA.
Ablation techniques
Based on our systematic review, the median volume and median alcohol concentration were used (1.55 mL and 98%, respectively), but due to the lack of data, we could not describe the exact volume of alcohol injected in each insulinoma depending on its distribution within the pancreatic gland. This finding can help to design a detailed protocol for this technique. There are no established guidelines on the volume of highly concentrated ethanol, the number of injections per session, the ideal tumour size, and the needle size.
EUS-guided ablation techniques have been trialled for different indications using variable techniques. Burghardt et al. 24 published the results of a multicentre, randomised, double-blind study comparing EUS-guided 80% ethanol lavage versus saline injection for cystic pancreatic lesions. They demonstrated that the ethanol group had a more significant decrease in the size of cystic pancreatic benign lesions than saline solution injection. EUS-guided alcohol injection is an established method of ablating the coeliac plexus (neurolysis) to achieve pain control; this is usually done with the same needle as FNA.57,58 We described that the alcohol could be delivered using variable needle sizes (19-gauge, 22-gauge and 25-gauge needle),21,36,49 and it does not influence its technical and clinical success. On the other hand, our data showed that the standard EUS-RFA probe needle used was a 19-gauge needle which allowed easy access to the lesions and successful ablations. Interestingly, the median energy setting of EUS-RFA was 10 Watt (range: 10–50 Watt). This is probably due to all cases reported had almost similar lesion size (median size: 13 mm x 10 mm).
EUS-guided RFA was first described in a porcine model. 54 Goldberg et al. in the year 1999 studied the effect of RFA on normal pancreatic tissue on Yorkshire pigs. His work included histological examination of the ablated healthy pancreatic tissues in his pig model, and this was carried out either immediately following RFA or 15 days later. This had acceptably shown a bleeding zone surrounding the central coagulated necrotic area, and after 2 weeks, creation of fibrotic scar tissue was noted. 54 Following Goldberg’s study, Carrara et al. described the feasibility of EUS-guided transluminal RFA of the pancreas on a living pig model.59,60 Gaidhane et al. also demonstrated the safety of EUS-guided RFA on the healthy pancreas in five Yucatan pigs showing no mortality and without significant complications reported.61,62 After 70 years from the first curative surgery of a benign pancreatic insulinoma, tumour localizations still remain challenging.59,63,64 According to available literature, surgical therapy is curative in 75% to 98% of patients.59,63 As concerns patients with inoperable solid pancreatic lesions or deemed non-surgical candidates, thermal ablations have become increasingly accepted. 65 In addition, RFA prevails the method of choice for biliary-, pancreatic- and peri-luminal lesions.66,67
Testoni et al. recently published a comprehensive literature review on EUS-guided ablation therapies. In addition, the author also elaborated on a novel RFA needle, which was designed with an internal cooling system to reduce the risk of over-heating of neighbouring healthy tissue knowing RFA can produce thermal tissue damage due to tissue overheating can be between 60°C and 100°C. Tissue overheating is a result of high frequency, alternating current and frictional heating, resulting in irreversible cellular damage, apoptosis, and coagulative tissue necrosis.68–70 The pancreas is a thermo-sensitive organ, and ablation of normal pancreas tissue can lead to an inflammatory reaction causing oedema, cystic transformation and later fibrosis.66,71 The first human pilot feasibility and safety study of EUS–guided RFA of the pancreatic lesion was published in 2015 using the Habib™ EUSRFA probe.68,69 This was a prospective multi-centre study in which eight patients were enrolled: six with pancreatic cystic neoplasms (four mucinous cystic neoplasms, one intraductal papillary mucinous neoplasm, and one micro-cystic adenoma) and two with pNETs. All these patients were non-surgical candidates.68,69,72
Waung et al. reported successful clinical and biochemical response in a sporadic case of 18 mm insulinoma by using the Habib™ RFA probe in a 70-year-old patient, who was not deemed fit for surgical resection due to comorbidities. Eventually, glucose replacement and octreotide therapy were both withdrawn. 50 Lakhtakia et al. similarly assessed the feasibility of the newest RFA probe with an internal cooling system (EUSRA) on symptomatic insulinoma as part of an observational study that included case series of three non-surgical patients. EUSRFA was technically successful in all the patients. 73 Wang et al. reported a 100% technical success rate of EUS–RFA using Habib tm catheter in Stage III pancreatic cancers in a series of three patients. During the first 2 weeks of the follow-up period, a mean reduction in tumour size of 13.49% on further transabdominal US imaging and no complications were observed up to 49-day follow-up period. 74 Pai et al. also demonstrated 100% successful completion of EUS-RFA in eight patients, two of which had neuroendocrine tumours of the pancreatic head. Complete resolution was observed in two out of six patients, and a 50% reduction in size was observed in three out of six patients with the pancreatic cystic lesion. Following EUS-RFA, pNET displayed a change in vascularity and central necrosis within the ablated tissue. No major complication was reported, and only two mild, self-limiting abdominal pain were recorded. 75
The efficacy of EUS-RFA of neuroendocrine tumours was further reported by Armellini et al. who successfully treated a 20 mm grade 2 endocrine tumour in an asymptomatic 76-year-old patient who had refused surgery. This lesion was completely ablated confirmed by an interval computed tomography (CT) scan, and no major complication was observed up to 1 month of follow-up. 76 Based on the published literature on safety and efficacy, 77 EUS-RFA is deemed safe in cases of locally advanced and metastatic pancreatic cancer. There is an excellent potential in researching the efficacy and safety of EUS-RFA in pancreatic cystic lesions using different endoscopic modalities aiming at effective cytoreductive treatment and, in some cases, a curative treatment. Treatment decisions should still be part of multidisciplinary discussions as this approach had always proven to be efficient in making complex decisions in patients diagnosed with pathological pancreatic lesions.
Imaging techniques
Our systematic review also highlighted an important clinical challenge. In some scenarios, pre-ablation imaging techniques (CT/MRI) did not show pancreatic insulinomas compared to EUS assessment in many reported cases (four out of 34 articles).21–23,49 This indicates that EUS assessment should be considered early to achieve a diagnosis when insulinomas are suspected. An interesting observation in our study is that four authors used the SonoVue contrast agent to demonstrate insulinomas pre- and post-ablations.25,41,42,50 Contrast-enhanced EUS can also be performed for endoscopic interval assessments of these lesions to decide on any further need for ablations.
Prophylactic stents
This systematic review also alluded to the use of prophylactic pancreatic and bile duct stent before EUS-EA to prevent subsequent ductal stenosis and acute pancreatitis. Deprez et al. 29 reported a case with insulinoma who received EUS-EA after receiving prophylactic biliary and pancreatic stent with no clinical complication recorded post-procedure; however, there was a mild rise in amylase, which lasted for one day without clinical symptoms. Kandula et al. 32 reported EUS – RFA of insulinoma of a patient who received a prophylactic pancreatic stent 20 days prior ablation; post-procedure, this patient developed mild acute pancreatitis which was treated conservatively. In our opinion, this practice has not been adopted by many practitioners, and this is probably due to non-existing guidelines in prophylactic biliary and pancreatic stenting prior to EUS ablation techniques and further prospective studies required to show efficacy.
Intra-procedural technical limitations
The location of insulinomas can be a challenge during ablation, especially if it is close to the mesenteric vessels, the main pancreatic or common bile duct, and superficially located, which may risk a duodenal wall injury. Qin et al. 43 used a reduced dose of infused alcohol to a half or third when the tumour was very close to mesenteric vessels or the main pancreatic duct, as ethanol can cause consequent stricturing of these ducts after ablation. The median size of insulinomas shown in our study was 13 mm x 10 mm. It became apparent that there is no evidence-based consensus on insulinoma size to guide EUS-guided RFA/EA techniques, but based on expert opinion, 2–3 cm lesions can be safely ablated with this technique.
Comorbidities
Our results demonstrated that 21 out of 65 patients (32% of all cohort) had multiple co-morbidities, which precluded surgical intervention; however, EUS-guided RFA/EA was successful in them.6,16,22,23,25–29,31,36–39,41,42,45,46,48–52
Peri-procedural medical and dietetic management
Our current study reflected that prophylactic antibiotics were used; only four of the 35 included studies documented antibiotic use pre-ablations.22,23,25,42 Equally poor measures were taken for the prevention of acute pancreatitis, for example, the use of rectal NSAID. This may be explained by the lack of data to support agreed guidelines on EUS-guided ablation techniques.
In the case of inoperable disease or non-surgical candidates, medical treatment has been trialled, typically with Diazoxide which can be ineffective in many cases.6,10,23,26–28,33,36,38,46,48,50 In our current review, 8 out of 65 patients had developed side effects before EUS treatment (fluid retention); therefore, Diazoxide was discontinued in these patients. In our cohort, other medical therapy pre-EUS-guided ablations (somatostatin, elozimib, octreotide) proved to be less effective in general.24,28,29,36,48,50
Medical therapy has its limitations mainly due to a strict diet protocol, including overnight snacks to avoid hypoglycaemic episodes, resulting in weight gain and morbid obesity, and precluding surgical options. Our included cases had shown morbid obesity in six articles.10,22,29,31,37,46 Dietetic support for these patients is essential to manage their condition before EUS-guided RFA/EA. The psychological impact cannot be ignored due to the prevalence of depressive disorders, which usually leads to non-compliance with medical therapy. Despite improvement in surgical techniques, especially in tertiary centres, mortality has significantly dropped. 78 However, postoperative morbidity remained as high as 30% in the minimal laparoscopic approach 79 and can reach up to 40–50% in Vázquez Quintana. 80 Therefore, the use of a non-surgical approach (EUS-guided RFA and EUS-EA) should be discussed with patients when offering treatment options for insulinomas.
Strength and limitations
Our systematic review’s strength is its rigorous methodology (RFA/EA) and the extensive data from all published cases of EUS-guided RFA/EA. Our paper has several limitations (a) absence of international consensus around indications of EUS-guided ablation techniques, especially for RFA and EA, as this remains an option for patient’s selection. (b) There were no controlled trials; therefore, we could not make direct comparisons between different treatment methods. (c) We do not have detailed information about several key technical aspects of the RFA generators used for these cases. (d) Other limitations were noted in the lack of reported information about the biochemical assays utilised for the biochemical assessments. This systematic review reflected the lack of information on hospital stay duration, BMI, and the follow-up period required to ascertain final treatment outcomes. Finally, all the included articles were case studies and case series, indicating a low level of evidence. Based on Murad et al. quality assessment tool, the EUS-guided RFA/EA techniques were adequately performed in all included cases. On the other hand, 13 out of 34 articles did not contain relevant information about a detailed medical history, medical treatment and follow-up period.
Implications for clinical practice
In conclusion, this systematic review showed that EUS-guided RFA/EA techniques can be considered in the treatment algorithm of pancreatic insulinoma, especially for those patients where surgical intervention is contraindicated. The existing literature provides an overview of the possible complications from EUS-guided ablation and their frequency and severity, which will help discuss the procedure with patients and obtain informed consent. EUS-guided ablations of insulinoma allow patients to avoid the morbidity associated with surgical reductions. The advantage of the EUS-guided RFA/EA technique lies in its easiness, rapidity, low cost, and acceptable morbidities, even in high-risk patients with inadequate surgical conditions. Decisions in managing insulinomas should be part of a multidisciplinary discussion, including endocrinology opinion, surgical opinion, radiologist and an experienced EUS endoscopist.
Implications for research
Our results showed that a prospective (randomised controlled trial) study approach would have been more informative and recommended to be done as part of a multicentre EUS-guided ablation register to include all modalities of EUS-guided ablation techniques.
Conclusion
Our findings support that EUS-guided ablation techniques (RFA/EA) are feasible in treating insulinoma. This technique is safe in expert hands, and adverse events could be anticipated and managed. Therefore, non-surgical intervention with EUS-guided RFA/EA might become the future first-line option in selected cases.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848211042171 for Endoscopic ultrasound-guided ethanol and radiofrequency ablation of pancreatic insulinomas: a systematic literature review by Ghassan El Sayed, Levente Frim, Jamie Franklin, Raymond McCrudden, Charles Gordon, Safa Al-Shamma, Szabolcs Kiss, Péter Hegyi, Bálint Erőss and Péter Jenő Hegyi in Therapeutic Advances in Gastroenterology
Footnotes
Author contributions: Conceptualisation: Ghassan El-Sayed, Péter J. Hegyi, Bálint Erőss.
Data curation: Ghassan El Sayed, Levente Frim, Jamie Franklin, Raymond McCrudden, Charles Gordon, Safa Al-Shamma, Szabolcs Kiss, Péter Hegyi.
Formal analysis: Levente Frim.
Funding acquisition: Péter Hegyi.
Investigation: Jamie Franklin, Raymond McCrudden, Charles Gordon, Safa Al-Shamma, Péter Hegyi, Ghassan El-Sayed, Bálint Erőss, Péter J. Hegyi.
Methodology: Jamie Franklin, Raymond McCrudden, Charles Gordon, Safa Al-Shamma, Szabolcs Kiss, Péter Hegyi, Bálint Erőss, Péter J. Hegyi.
Supervision: Bálint Erőss, Péter J. Hegyi.
Validation: Péter J. Hegyi.
Writing-original draft: Ghassan El-Sayed, Levente Frim, Jamie Franklin, Raymond McCrudden, Charles Gordon, Safa Al-Shamma, Szabolcs Kiss, Péter Hegyi, Bálint Erőss, Péter J. Hegyi.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work will be funded by the Economic Development and Innovation Operational Programme Grant (GINOP-2.3.2-15-2016-80004 – STAY ALIVE and GINOP-2.3.4-15-2020-00001 Competence Centre for Health Data Analysis, Data Utilisation and Smart Device and Technology Development at the University of Pécs).
ORCID iD: Péter Jenő Hegyi  https://orcid.org/0000-0002-6443-0259
https://orcid.org/0000-0002-6443-0259
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ghassan El Sayed, The Royal Bournemouth Hospital, University Hospital Dorset, Bournemouth, UK.
Levente Frim, Institute for Translational Medicine, Medical School, University of Pécs, Pécs, Hungary.
Jamie Franklin, The Royal Bournemouth Hospital, University Hospital Dorset, Institute of Medical Imaging and Visualisation, Bournemouth, UKLevente Frim.
Raymond McCrudden, The Royal Bournemouth Hospital, University Hospital Dorset, Bournemouth, UK.
Charles Gordon, The Royal Bournemouth Hospital, University Hospital Dorset, Bournemouth, UK.
Safa Al-Shamma, The Royal Bournemouth Hospital, University Hospital Dorset, Bournemouth, UK.
Szabolcs Kiss, Institute for Translational Medicine, Medical School, University of Pécs, Pécs, Hungary; Szentágothai Research Centre, Medical School, University of Pécs, Pécs, Hungary.
Péter Hegyi, Institute for Translational Medicine, Medical School, University of Pécs, Pécs, Hungary; Szentágothai Research Centre, Medical School, University of Pécs, Pécs, Hungary; Doctoral School of Clinical Medicine, University of Szeged, Szeged, Hungary; Centre for Translational Medicine, Semmelweis University, Budapest, Hungary.
Bálint Erőss, Institute for Translational Medicine, Medical School, University of Pécs, Pécs, Hungary.
Péter Jenő Hegyi, Institute for Translational Medicine, Medical School, University of Pécs, Szigeti Street 12, Pecs H-7624, Hungary.
References
- 1. Halfdanarson TR, Rabe KG, Rubin J. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008; 19: 1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kar P, Price P, Sawers S. Insulinomas may present with normoglycemia after prolonged fasting but glucose-stimulated hypoglycemia. J Clin Endocrinol Metab 2006; 91: 4733–4736. [DOI] [PubMed] [Google Scholar]
- 3. Whipple AO, Frantz VK. Adenoma of islet cells with hyperinsulinism: a review. Ann Surg 1935; 101: 1299–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shin JJ, Gorden P, Libutti SK. Insulinoma: pathophysiology, localization and management. Future Oncol 2010; 6: 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okabayashi T, Shima Y, Sumiyoshi T. Diagnosis and management of insulinoma. World J Gastroenterol 2013; 19: 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burghardt K, Kaemmerer D, Michael A. Successful endoscopic ultrasound-guided ethanol ablation of a symptomatic sporadic insulinoma in a patient with severe comorbidities not suitable for pancreatic surgery. Diabetes Metabolism 2018; 44: 84–86. [DOI] [PubMed] [Google Scholar]
- 7. Grant CS. Surgical aspects of hyperinsulinemic hypoglycemia. Endocrinol Metab Clin North Am 1999; 28: 533–554. [DOI] [PubMed] [Google Scholar]
- 8. Ho V, Heslin MJ. Effect of hospital volume and experience on in-hospital mortality for pancreaticoduodenectomy. Ann Surg 2003; 237: 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fajans SS, Vinik AI. Insulin-producing islet cell tumors. Endocrinol Metabol Clin North Am 1989; 18: 45–74. [PubMed] [Google Scholar]
- 10. Jürgensen C, Schuppan D, Neser F, et al. EUS-guided alcohol ablation of an insulinoma. Gastrointest Endosc 2006; 63: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 11. Armellini E, Crinò SF, Ballarè M. Endoscopic ultrasound-guided ethanol ablation of pancreatic neuroendocrine tumours: a case study and literature review. World J Gastrointest Endosc 2016; 8: 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chaudhary S, Sun S-Y. Endoscopic ultrasound-guided radiofrequency ablation in gastroenterology: new horizons in search. World J Gastroenterol 2017; 23: 4892–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barthet M, Giovannini M, Lesavre N. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy 2019; 51: 836–842. [DOI] [PubMed] [Google Scholar]
- 14. Gan SI, Thompson CC, Lauwers GY. Ethanol lavage of pancreatic cystic lesions: initial pilot study. Gastrointest Endosc 2005; 61: 746–752. [DOI] [PubMed] [Google Scholar]
- 15. Omerović S, Zerem E. Alcohol sclerotherapy in the treatment of symptomatic simple renal cysts. Bosn J Basic Med Sci 2008; 8: 337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mittal M, Yip B, Lee JG. Endoscopic ultrasound-guided ethanol ablation for control of local-regional metastatic insulinoma. Pancreas 2017; 46: e17–e18. [DOI] [PubMed] [Google Scholar]
- 17. Fu C, Liu N, Deng Q. Successful treatment of gastrointestinal stromal tumor with multiple liver metastases with radiofrequency ablation and imatinib: a case report. Oncol Lett 2015; 10: 875–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang W-Y, Li Z-S, Jin Z-D. Endoscopic ultrasound-guided ethanol ablation therapy for tumors. World J Gastroenterol 2013; 19: 3397–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liberati A, Altman DG, Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical Research Ed) 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murad MH, Sultan S, Haffar S. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018; 23: 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Altonbary A, Hakim H, Elkashef W. Endoscopic ultrasound-guided ethanol ablation for functioning insulinoma. Endoscopic Ultrasound 2017; 6: S45. [Google Scholar]
- 22. Bas-Cutrina F, Bargalló D, Gornals JB. Small pancreatic insulinoma: successful endoscopic ultrasound-guided radiofrequency ablation in a single session using a 22-G fine needle. Dig Endosc 2017; 29: 636–638. [DOI] [PubMed] [Google Scholar]
- 23. Bor R, Farkas K, Bálint A. Endoscopic ultrasound-guided ethanol ablation: an alternative option for the treatment of pancreatic insulinoma. Orvosi Hetilap 2014; 155: 1647–1651. [DOI] [PubMed] [Google Scholar]
- 24. Burghardt K, Kaemmerer D, Michael A, et al. Successful endoscopic ultrasound-guided ethanol ablation of a symptomatic sporadic insulinoma in a patient with severe comorbidities not suitable for pancreatic surgery. Diabetes Metabolism 2018; 44: 84–86. [DOI] [PubMed] [Google Scholar]
- 25. Choi JH, Seo DW, Song TJ. Endoscopic ultrasound-guided radiofrequency ablation for management of benign solid pancreatic tumors. Endoscopy 2018; 50: 1099–1104. [DOI] [PubMed] [Google Scholar]
- 26. Dabkowski K, Gajewska P, Walter K, et al. Successful EUS-guided ethanol ablation of insulinoma, four-year follow-up. Endokrynol Pol 2017; 68: 472–479. [DOI] [PubMed] [Google Scholar]
- 27. de Sousa Lages A, Paiva I, Oliveira P, et al. Endoscopic ultrasound-guided ethanol ablation therapy for pancreatic insulinoma: an unusual strategy. Endocrinol Diabetes Metab Case Rep 2017; 2017: 160145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Nucci G, Mandelli ED, Morganti D. Endoscopy ultrasound guided radiofrequency ablation of functioning insulinoma in symptomatic patients unfit for surgery. Digest Liver Disease 2018; 50: e159–e160. [Google Scholar]
- 29. Deprez PH, Claessens A, Borbath I. Successful endoscopic ultrasound-guided ethanol ablation of a sporadic insulinoma. Acta Gastro-Enterologica Belgica 2008; 71: 333–337. [PubMed] [Google Scholar]
- 30. Godat S, Lamine F, David G. Video sessions: endoscopic ultrasound (EUS) guided pancreas radiofrequency ablation of a benign insulinoma. Swiss Medical Weekly 2018; 148: 12S. [Google Scholar]
- 31. Jonica ER, Wagh MS. Endoscopic treatment of symptomatic insulinoma with a new EUS-guided radiofrequency ablation device. Videogie 2020; 5: 483–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kandula S, Aurangabadker G, Modi V. Management of a difficult case of insulinoma with radiofrequency ablation. Indian J Endocrinol Metabol 2019; 23: S46–1S7. [Google Scholar]
- 33. Khoo S, Hilmi I, Dhir V. An emerging indication for endoscopic ultrasound: case report of endoscopic ultrasound -guided ablation of a multifocal insulinoma. Endoscopic Ultrasound 2017; 6: S25. [Google Scholar]
- 34. Kluz M, Staroń R, Krupa Ł. Successful endoscopic ultrasound–guided radiofrequency ablation of a pancreatic insulinoma. Polish Arch Intern Med 2020; 130: 145–146. [DOI] [PubMed] [Google Scholar]
- 35. Lee MJ, Jung CH, Jang JE. Successful endoscopic ultrasound-guided ethanol ablation of multiple insulinomas accompanied with multiple endocrine neoplasia type 1. Intern Med J 2013; 43: 948–950. [DOI] [PubMed] [Google Scholar]
- 36. Levy MJ, Thompson GB, Topazian MD. US-guided ethanol ablation of insulinomas: a new treatment option. Gastrointest Endosc 2012; 75: 200–206. [DOI] [PubMed] [Google Scholar]
- 37. Luo YP, Li J, Yang AM. Ga-68-Exendin-4PET/CT in evaluation of endoscopic ultrasound-guided ethanol ablation of an insulinoma. Clin Nucl Med 2017; 42: 310–311. [DOI] [PubMed] [Google Scholar]
- 38. Mosquera-Klinger G, Carvajal JJ. Endoscopic ultrasound-guided ethanol ablation for the management of a symptomatic pancreatic insulinoma. Rev Esp Enferm Dig 2020; 113: 48–51. [DOI] [PubMed] [Google Scholar]
- 39. Nelson K, Lakha A, Malafa M. Symptomatic pancreatic insulinoma successfully treated with alcohol ablation. Am J Gastroenterol 2014; 109: S456. [Google Scholar]
- 40. Oleinikov K, Dancour A, Epshtein J. Endoscopic ultrasound-guided radiofrequency ablation: a new therapeutic approach for pancreatic neuroendocrine tumors. J Clin Endocrinol Metabol 2019; 104: 2637–2647. [DOI] [PubMed] [Google Scholar]
- 41. Paik WH, Seo DW, Dhir V. Safety and efficacy of EUS-guided ethanol ablation for treating small solid pancreatic neoplasm. Medicine (Baltimore) 2016; 95: e2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Park DH, Choi JH, Oh D. Endoscopic ultrasonography-guided ethanol ablation for small pancreatic neuroendocrine tumors: results of a pilot study. Clin Endosc 2015; 48: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qin S, Jiang H, Luo W. Efficacy of EUS-guided ethanol injection in the treatment of benign and symptomatic insulinomas. Gastroenterology 2016; 150: S510. [Google Scholar]
- 44. Sharma NR, Sharma A, Perelman A. Spare the Whipple: novel endoscopic radiofrequency ablation treatment for insulin secreting tumors. Am J Gastroenterol 2017; 112: S1133. [Google Scholar]
- 45. Schnack C, Hansen C, Nielsen HO. Successfull endoscopic ultrasound-guided alcoholic ablation of insulinoma. Neuroendocrinology 2011; 94: 43. [Google Scholar]
- 46. Silva F, Colaiacovo R, Araki O. Endoscopic ultrasound-guided fine needle injection of alcohol for ablation of an insulinoma: a well documented successful procedure. Endoscopy 2019; 51: E57–E58. [DOI] [PubMed] [Google Scholar]
- 47. Trevino J, Calloway J, Eloubeidi M. EUS-guided ablation of pancreatic insulinomas. Gastrointest Endosc 2011; 73: AB110. [Google Scholar]
- 48. Trikudanathan G, Mallery SJ, Amateau SK. Successful endoscopic ultrasound-guided alcohol ablation of sporadic insulinoma using three-dimensional targeting (with video). Clin Endosc 2016; 49: 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vleggaar FP, de Vaate EAB, Valk GD. Endoscopic ultrasound-guided ethanol ablation of a symptomatic sporadic insulinoma. Endoscopy 2011; 43: E328–E329. [DOI] [PubMed] [Google Scholar]
- 50. Waung JA, Todd JF, Keane MG. Successful management of a sporadic pancreatic insulinoma by endoscopic ultrasound-guided radiofrequency ablation. Endoscopy 2016; 48(Suppl. 1): E144–E145. [DOI] [PubMed] [Google Scholar]
- 51. Zalewska E, Kłosowski P, Dubowik M. Endoscopic ultrasound-guided ethanol ablation of insulinoma. Endokrynologia Polska 2020; 71: 585–586. [DOI] [PubMed] [Google Scholar]
- 52. Zarug KR, Ronshaugen N, Mehendiratta V. EUS-guided ethanol ablation as a treatment option for insulinoma. Endocrine Reviews 2015; 36, https://endo.confex.com/endo/2015endo/webprogram/Paper20051.html [Google Scholar]
- 53. Lakhtakia S, Memon SFF, Medarapalem JB. 99 sustained clinical response with eus guided rfa of pancreatic insulinoma. Gastrointest Endosc 2020; 91: AB14. [Google Scholar]
- 54. Goldberg SN, Mallery S, Gazelle GS. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc 1999; 50: 392–401. [DOI] [PubMed] [Google Scholar]
- 55. Kim HJ, Seo DW, Hassanuddin A. EUS-guided radiofrequency ablation of the porcine pancreas. Gastrointest Endosc 2012; 76: 1039–1043. [DOI] [PubMed] [Google Scholar]
- 56. Matthes K, Mino-Kenudson M, Sahani DV. Concentration-dependent ablation of pancreatic tissue by EUS-guided ethanol injection. Gastrointest Endosc 2007; 65: 272–277. [DOI] [PubMed] [Google Scholar]
- 57. Luz LP, Al-Haddad MA, DeWitt JA. EUS-guided celiac plexus interventions in pancreatic cancer pain: an update and controversies for the endosonographer. Endosc Ultrasound 2014; 3: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee KH, Lee JK. Interventional endoscopic ultrasonography: present and future. Clin Endosc 2011; 44: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Limmer S, Huppert PE, Juette V. Radiofrequency ablation of solitary pancreatic insulinoma in a patient with episodes of severe hypoglycemia. Eur J Gastroenterol Hepatol 2009; 21: 1097–1101. [DOI] [PubMed] [Google Scholar]
- 60. Carrara S, Arcidiacono PG, Albarello L. Endoscopic ultrasound-guided application of a new hybrid cryotherm probe in porcine pancreas: a preliminary study. Endoscopy 2008; 40: 321–326. [DOI] [PubMed] [Google Scholar]
- 61. Signoretti M, Valente R, Repici A. Endoscopy-guided ablation of pancreatic lesions: technical possibilities and clinical outlook. World J Gastrointest Endosc 2017; 9: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gaidhane M, Smith I, Ellen K. Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) of the pancreas in a porcine model. Gastroenterol Res Pract 2012; 2012: 431451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tucker ON, Crotty PL, Conlon KC. The management of insulinoma. Br J Surg 2006; 93: 264–275. [DOI] [PubMed] [Google Scholar]
- 64. Finlayson E, Clark OH. Surgical treatment of insulinomas. Surg Clinics North Am 2004; 84: 775–785. [DOI] [PubMed] [Google Scholar]
- 65. Larghi A, Rizzatti G, Rimbaş M, et al. EUS-guided radiofrequency ablation as an alternative to surgery for pancreatic neuroendocrine neoplasms: who should we treat. Endosc Ultrasound 2019; 8: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lakhtakia S. Therapy of pancreatic neuroendocrine tumors: fine needle intervention including ethanol and radiofrequency ablation. Clin Endosc 2017; 50: 546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Decadt B, Siriwardena AK. Radiofrequency ablation of liver tumours: systematic review. Lancet Oncol 2004; 5: 550–560. [DOI] [PubMed] [Google Scholar]
- 68. Testoni SGG, Healey AJ, Dietrich CF. Systematic review of endoscopy ultrasound-guided thermal ablation treatment for pancreatic cancer. Endosc Ultrasound 2020; 9: 83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Paiella S, Salvia R, Ramera M. Local ablative strategies for ductal pancreatic cancer (radiofrequency ablation, irreversible electroporation): a review. Gastroenterol Res Pract 2016; 2016: 4508376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 2014; 14: 199–208. [DOI] [PubMed] [Google Scholar]
- 71. Egorov AV, Vasilyev IA, Musayev GH. The role of microwave ablation in management of functioning pancreatic neuroendocrine tumors. Gland Surg 2019; 8: 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Öberg K. Management of functional neuroendocrine tumors of the pancreas. Gland Surg 2018; 7: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lakhtakia S, Ramchandani M, Galasso D. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos). Gastrointest Endosc 2016; 83: 234–239. [DOI] [PubMed] [Google Scholar]
- 74. Wang D, Jin Z, Lei W. Mo1524 endoscopic ultrasound guided radiofrequency ablation for the treatment of advanced pancreatic carcinoma. Gastrointest Endosc 2013; 77: AB414. [Google Scholar]
- 75. Pai M, Habib N, Senturk H. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg 2015; 7: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Armellini E, Crinò SF, Ballarè M. Endoscopic ultrasound-guided radiofrequency ablation of a pancreatic neuroendocrine tumor. Endoscopy 2015; 47: 600–601. [DOI] [PubMed] [Google Scholar]
- 77. Rossi S, Viera FT, Ghittoni G. Radiofrequency ablation of pancreatic neuroendocrine tumors: a pilot study of feasibility, efficacy, and safety. Pancreas 2014; 43: 938–945. [DOI] [PubMed] [Google Scholar]
- 78. Shirota T, Nagakawa Y, Sahara Y. Surgical resection of neuroendocrine tumors of the pancreas (pNETs) by minimally invasive surgery: the laparoscopic approach. Gland Surg 2018; 7: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Al-Kurd A, Chapchay K, Grozinsky-Glasberg S. Laparoscopic resection of pancreatic neuroendocrine tumors. World J Gastroenterol 2014; 20: 4908–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vázquez Quintana E. The surgical management of insulinoma. Boletin de la Asociacion Medica de Puerto Rico 2004; 96: 33–38. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848211042171 for Endoscopic ultrasound-guided ethanol and radiofrequency ablation of pancreatic insulinomas: a systematic literature review by Ghassan El Sayed, Levente Frim, Jamie Franklin, Raymond McCrudden, Charles Gordon, Safa Al-Shamma, Szabolcs Kiss, Péter Hegyi, Bálint Erőss and Péter Jenő Hegyi in Therapeutic Advances in Gastroenterology