Abstract
Background:
Liposomal irinotecan (nal-IRI) plus 5-fluorouracil and leucovorin (5-FU/LV) is currently the standard second-line treatment for patients with pancreatic ductal adenocarcinoma (PDAC) after previous failed gemcitabine-based therapy. This population-based study aimed to evaluate the efficacy and safety of nal-IRI + 5-FU/LV and the association of pre-emptive nal-IRI dosing with treatment outcomes in patients with PDAC.
Methods:
We retrospectively enrolled a total of 667 consecutive patients with PDAC who received nal-IRI plus 5-FU/LV treatment between August 2018 and November 2020 at 9 medical centers in Taiwan. Patients were allocated into groups according to pre-emptive nal-IRI dosing (⩾75%, 50–74%, <50%) for comparison of treatment efficacy and safety.
Results:
The median overall survival (OS) and time to treatment failure (TTF) were 5.9 months [95% confidence interval (CI), 5.3–6.5] and 2.8 months (95% CI, 2.6–3.0), respectively. The median OS was 6.5 months (95% CI, 5.7–6.7), 5.0 months (95% CI, 3.4–6.5), and 4.1 months (95% CI, 2.7–5.6), respectively, among the ⩾75%, 50–74%, and <50% pre-emptive nal-IRI dosing groups, whereas the median TTF of the three groups was 3.0 months (95% CI, 2.6–3.4), 2.6 months (95% CI, 2.3–2.9), and 1.9 months (95% CI, 1.6–2.2), respectively. Pre-emptive nal-IRI dosing <50% was an independent negative prognostic factor for OS and TTF in multivariate analyses. The most common severe adverse events were neutropenia (22.9%), anemia (21.1%), and hypokalemia (15.4%). Patients in the <50% pre-emptive nal-IRI dosing group had a significantly lower incidence of neutropenia and non-neutropenic infection than those in the other groups.
Conclusion:
Our results support the use of nal-IRI + 5-FU/LV as standard clinical practice for treating patients with PDAC based on this large population-based study. Our findings encourage physicians to provide adequate doses of nal-IRI in order to achieve better outcomes without compromising safety profiles.
Keywords: dose reduction, liposomal irinotecan, outcome, pancreatic cancer, toxicity
Introduction
Pancreatic duct adenocarcinoma (PDAC) was the 14th most common cancer and the 7th leading cause of cancer-related death worldwide as of 2020. 1 More than 80% of diagnosed patients have unresectable disease at initial diagnosis; palliative chemotherapy is the standard of care for unresectable disease.2,3 Gemcitabine-based chemotherapy is a standard first-line (L1) treatment for patients with PDAC, with an associated median overall survival (OS) time and response rate of 6–11 months and 7–29%, respectively.4–7
With the improved efficacy and toxicity profile of L1 treatments, progression-free survival time has extended from 4.1 to 5.7 months within the past two decades.4–7 As a reflection of enhanced standardized L1 treatment, survival time following L1 chemotherapy failure has nearly doubled from 3 to 6 months.4–7 In addition, consequent to the aforementioned improved toxicity profile, more patients presented with good physical performance following L1 therapy and were eligible to receive second-line (L2) chemotherapy. 8 Our previous study reported that <30% of patients were able to receive L2 chemotherapy in 2010, while approximately 50% of patients received L2 chemotherapy in 2016.9,10 However, the survival benefit of L2 treatment in patients with PDAC remains controversial since previous phase III studies have reported a wide variation in tumor response rates (1–20.6%) and median OS (4.2–9.9 months).11–14
NAPOLI-1 was the most recent phase III study reporting a superior survival benefit in patients with PDAC who received liposomal irinotecan (nal-IRI) plus 5-fluorouracil and folic acid (5-FU/LV), as compared with those who received 5-FU/LV alone as subsequent treatment after gemcitabine-based therapy. 15 In this study cohort, 34.2% of the nal-IRI + 5-FU/LV arm needed a dose reduction due to toxicity, as opposed to 4.5% of the 5-FU/LV arm. 15 Several studies have reported real-world data on the clinical efficacy and toxicity of nal-IRI + 5-FU/LV in comparable settings.16–23 These published studies have reported the common practice of pre-emptive nal-IRI dose reduction to achieve better tolerability and safety profiles without compromising treatment efficacy.20,21 However, these studies were limited by a single institute experience and small numbers of patients.20,21 Furthermore, none of these studies explored the clinical impact of pre-emptive dose reduction on safety profiles. While standard dosage may have detrimental toxic effects, exploration of the effect of pre-emptive dose reduction on survival outcomes is warranted.
Based on the NAPOLI-1 study, 15 the combination regimen of nal-IRI + 5-FU/LV has been the standard L2 chemotherapy regimen in patients with PDAC in Taiwan following the reimbursement by the National Health Insurance in August 2018. This multicenter study aimed to (1) evaluate the efficacy and safety of nal-IRI + 5-FU/LV, and (2) explore the impact of pre-emptive nan-IRI dose reduction on efficacy and safety in patients with PDAC.
Methods
Patient selection
We retrospectively reviewed the medical records of patients who received nal-IRI plus 5-FU/LV for the treatment of PDAC between August 2018 and November 2020 at nine medical centers in Taiwan. A total of 667 patients were included in the current study.
All patients were either pathologically or cytologically confirmed as having PDAC. Patients received nal-IRI plus 5-FU/LV (nal-IRI 80 mg/m2 intravenously over 90 min, followed by LV 400 mg/m2 intravenously over 30 min and 5-FU 2400 mg/m2 over 46 h every 2 weeks) according to the NAPOLI-1 study. 15 Participants were allocated into three groups according to pre-emptive nal-IRI dosing: ⩾75% (no dose reduction), 50–74% (one dose reduction), and < 50% (two dose reduction), according to the NAPOLI-1 study protocol. 15 The pre-emptive nal-IRI dose was determined by primary care physicians. This study was approved by the institutional review boards of Chang Gung Memorial Hospital (202100783B0), China Medical University Hospital (CMUH109-REC2-176), Chung Shan Medical University Hospital (CS2-21095), National Cheng Kung University Hospital (A-ER-109-477), National Taiwan University Hospital (201911042RINC), Kaohsiung Medical University Hospital (KMUHIRB-E(I)-20210150), Taipei Veterans General Hospital (2021-08-001AC), and Tri-Service General Hospital (B202105057). Informed consent requirements were waived because of the retrospective nature of the analysis, and all data were de-identified as well as encrypted.
Data collection
We retrospectively collected data on demographic, clinicopathological, radiologic, and laboratory [including hemogram, albumin, and carbohydrate antigen 19-9 (CA 19-9)] variables at the beginning of nal-IRI + 5-FU/LV treatment during the medical records review. Imaging studies were conducted during regular follow-up every 8–12 weeks or were clinically indicated during the period of chemotherapy. Tumor response was evaluated using imaging studies according to the Response Evaluation Criteria for Solid Tumors (RECIST) 1.1. Patients who required early termination of treatment or who died before imaging studies were conducted for response assessment were determined to have experienced disease progression. Adverse events were evaluated during every clinic visit and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 4.03. All adverse events were recorded from the initiation of nal-IRI + 5-FU/LV therapy until the end of treatment. All enrolled patients were followed up until 31 December 2020 or until death.
Statistical analysis
Basic patient demographic data were summarized as frequencies (%) for categorical variables and as medians with range for continuous variables. Differences between the different pre-emptive nal-IRI dosing groups were compared using the chi-square (χ 2 ) test or through Fisher’s exact test if the number in any cell was less than five. Time to treatment failure (TTF) was defined as the time from the initiation of nal-IRI + 5-FU/LV to the date of treatment discontinuation for any reason. OS was defined as the time between the initiation of nal-IRI + 5-FU/LV and death from any cause. TTF and OS were calculated using the Kaplan–Meier method. Log-rank tests were used to determine statistically significant differences among the survival curves. A pairwise comparison was performed for subgroup analysis among the three pre-emptive dose groups. All clinicopathological variables were evaluated using univariate Cox regression analysis to ascertain the impact of each variable on TTF and OS. All variables in the univariate analysis with p values < 0.10 were further analyzed using multivariate analysis. SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. All statistical assessments were two-sided and a p value of <0.05 was considered the threshold for statistical significance.
Results
The basic characteristics of the 667 patients are presented in Table 1. The median age was 63 years (range, 27–89 years), and 56% of the participants were men. The median prior treatment line for metastatic disease was 1 (range, 0–7). Thirteen patients (1.9%) received nal-IRI + 5-FU/LV as the first-line treatment, while 248 patients (36.9%) received nal-IRI + 5-FU/LV as the third line of treatment for metastatic PDAC. The most common frontline chemotherapeutic agents were gemcitabine (99.3%), TS-1 (58.9%), platinum (42.1%), 5-fluorouracil (17.8%), and irinotecan (12.9%). Pre-emptive nal-IRI dose reduction is a common practice and 69.7%, 17.2%, and 13.0% of the patients received ⩾75%, 50–74%, and <50% nal-IRI dosing, respectively, as the starting dose in our patient cohort. No statistical differences were observed among the different pre-emptive dose groups in terms of age, sex, primary tumor location, site of metastases, and time from first-line treatment to nal-IRI therapy. Patients in the pre-emptive dose <50% group had poorer ECOG performance status at baseline and were less likely to receive TS-1 or platinum treatment at the frontline than those in the other two groups.
Table 1.
Participant medical and demographic characteristics (n = 667).
| Variable | Category | Pre-emptive nal-IRI dose (80 mg/m2), n (%) | p a value | |||
|---|---|---|---|---|---|---|
| Overall (n = 667) | ⩾75% (n = 465) | 50–74% (n = 115) | <50% (n = 87) | |||
| Age, years | Median (range) | 63 (27–89) | 63 (27–89) | 64 (44–86) | 63 (43–82) | 0.61 |
| <65 | 397 (59.5) | 279 (60.0) | 64 (55.7) | 54 (62.1) | ||
| ⩾65 | 270 (40.5) | 186 (40.0) | 51 (44.3) | 33 (37.9) | ||
| Sex | Male | 376 (56.3) | 258 (55.5) | 72 (62.6) | 46 (52.9) | 0.30 |
| Female | 291 (43.7) | 207 (44.5) | 43 (37.4) | 41 (47.1) | ||
| Body mass index, kg/m2 | Median (range) | 21.1 (12.4–39.0) | 21.1 (12.4–39.0) | 21.5 (13.9–30.6) | 20.9 (13.1–33.8) | 0.42 |
| ECOG performance | 0–1 | 510 (76.5) | 379 (81.5) | 81 (70.4) | 50 (57.5) | 0.001 |
| 2–3 | 157 (23.5) | 86 (18.5) | 34 (29.6) | 37 (42.5) | ||
| Primary tumor location | Head | 359 (53.8) | 252 (54.2) | 61 (53.0) | 46 (52.9) | 0.65 |
| Body | 163 (24.4) | 116 (24.9) | 29 (25.2) | 18 (20.7) | ||
| Tail | 129 (19.3) | 89 (19.1) | 21 (18.3) | 19 (21.8) | ||
| Overlapping | 16 (2.4) | 8 (1.7) | 4 (3.5) | 4 (4.6) | ||
| Site of metastases prior to nal-IRI treatment | Liver | 439 (65.8) | 298 (64.1) | 78 (67.8) | 63 (72.4) | 0.29 |
| Lung | 133 (19.9) | 94 (20.2) | 24 (20.9) | 15 (17.2) | 0.47 | |
| Bone | 50 (7.5) | 28 (6.0) | 12 (10.4) | 10 (11.5) | 0.09 | |
| Peritoneum | 207 (31.0) | 141 (30.3) | 43 (37.4) | 23 (26.4) | 0.21 | |
| Distant lymph nodes | 192 (28.8) | 136 (29.2) | 35 (30.4) | 21 (24.1) | 0.61 | |
| Others | 52 (7.8) | 38 (8.2) | 8 (7.0) | 6 (6.8) | 0.23 | |
| Prior pancreatectomy | 228 (34.2) | 163 (35.1) | 34 (29.6) | 31 (35.6) | 0.52 | |
| CA 19-9 prior to nal-IRI treatment, µg/ml | Median (range) | 1860 (1–93,850) | 923 (1–93,850) | 1263 (2–66,900) | 1964 (3–12,677) | 0.36 |
| Unknown | 99 (14.8) | 68 (14.6) | 21 (18.3) | 11 (12.6) | 0.19 | |
| Prior treatment line for metastatic disease | Median (range) | 1 (0–7) | 1 (0–7) | 2 (0–5) | 1 (1–5) | 0.07 |
| 0 | 13 (1.9) | 7 (1.5) | 6 (5.2) | 0 | ||
| 1 | 407 (61.0) | 309 (66.5) | 48 (41.7) | 50 (57.5) | ||
| 2 | 192 (28.8) | 121 (26.0) | 43 (37.4) | 28 (32.2) | ||
| 3 | 39 (5.8) | 21 (3.5) | 13 (11.3) | 5 (5.7) | ||
| ⩾4 | 16 (2.4) | 7 (1.5) | 5 (4.4) | 4 (4.5) | ||
| Time from first-line treatment to nal-IRI therapy, months | Median (range) | 7.7 (0–93.8) | 7.7 (0–93.8) | 7.3 (0–66.6) | 8.5 (1.3–42.3) | 0.31 |
| Prior gemcitabine treatment | Yes | 662 (99.3) | 463 (99.6) | 113 (98.3) | 86 (98.8) | 0.87 |
| Prior TS-1 treatment | Yes | 393 (58.9) | 295 (63.4) | 61 (53.0) | 37 (42.5) | <0.001 |
| Prior platinum treatment | Yes | 281 (42.1) | 221 (47.5) | 51 (44.3) | 9 (10.3) | <0.001 |
| Prior 5-fluorouracil treatment | Yes | 119 (17.8) | 83 (17.8) | 24 (20.9) | 12 (13.8) | 0.43 |
| Prior irinotecan treatment | Yes | 86 (12.9) | 56 (12.0) | 20 (17.4) | 10 (11.5) | 0.28 |
CA 19-9, Carbohydrate antigen 19-9; ECOG, Eastern Cooperative Oncology Group Performance Status; nal-IRI, liposomal irinotecan.
Within-group difference.
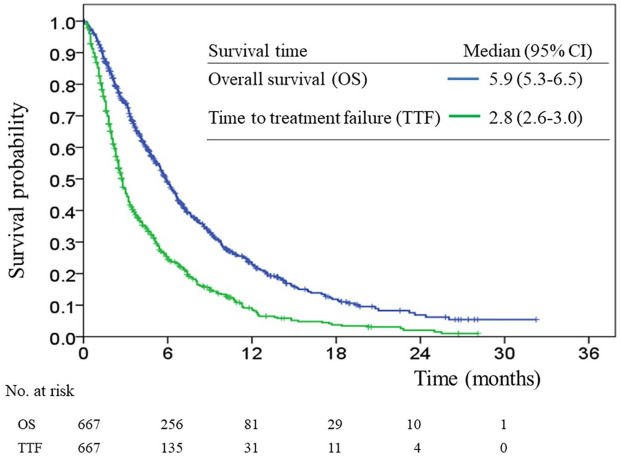
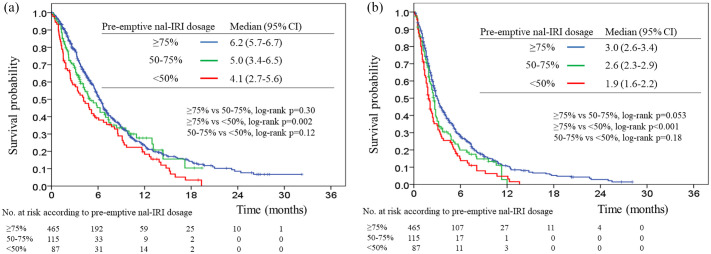
The median follow-up duration was 12.9 months (range, 2.1–28.2 months). At the end of our study, 475 (71.2%) of the 667 patients had died. The median OS and TTF were 5.9 months (95% CI, 5.3–6.5) and 2.8 months (95% CI, 2.6–3.0), respectively (Figure 1). The median OS among the nal-IRI pre-emptive dose ⩾75%, 50–74%, and <50% groups were 6.5 months (95% CI, 5.7–6.7), 5.0 months (95% CI, 3.4–6.5), and 4.1 months (95% CI, 2.7–5.6), respectively (Figure 2(a)). The median TTF was 3.0 months (95% CI, 2.6–3.4), 2.6 months (95% CI, 2.3–2.9), and 1.9 months (95% CI, 1.6–2.2), respectively (Figure 2(b)). There were statistically significant differences in OS and TTF between the ⩾75% and <50% pre-emptive dose groups (p = 0.002 and <0.001, respectively); there were no statistically significant differences in OS and TTF between the ⩾75% versus 50–74% and the 50–74% versus <50% pre-emptive dose groups.
Figure 1.
Overall survival and time to treatment failure curves.
Figure 2.
Kaplan–Meier estimates of overall survival (a) and time to treatment failure (b) among three pre-emptive nal-IRI dosing groups.
The Supplementary Table presents the results of univariate analyses examining overall survival and time to treatment failure. Univariate analysis showed that age ⩾65 years, no previous pancreatectomy, poor Eastern Cooperative Oncology Group (ECOG) performance, the presence of liver metastases, the presence of peritoneal metastases, the presence of lung metastases, primary tumor location at the pancreatic body or an overlapping site, CA 19-9 levels higher than the median, prior line chemotherapy for metastatic disease (⩾2), a neutrophil-to-lymphocyte ratio (NLR) >5, albumin levels <3.5 g/dl, and pre-emptive nal-IRI doses <50% were negative prognostic factors for OS. A pre-emptive nal-IRI dose <50% remained an independent negative factor in the multivariate analysis for OS (Table 2).
Table 2.
Multivariate analyses examining overall survival.
| Variable | Category | Adjusted HR | 95% CI | p |
|---|---|---|---|---|
| Sex | Female versus Male | 0.83 | 0.69–1.02 | 0.08 |
| Age | <65 versus ⩾65 | 1.12 | 0.93–1.36 | 0.24 |
| Body mass index, kg/m2 | ⩽21.1 versus >21.1 | 0.77 | 0.64–0.94 | 0.008 |
| ECOG performance | 0–1 versus 2–3 | 2.44 | 1.70–2.69 | <0.001 |
| Primary tumor location | Head | reference | ||
| Body | 1.26 | 0.99–1.58 | 0.13 | |
| Tail | 1.14 | 0.88–1.47 | 0.31 | |
| Overlapping | 1.68 | 0.95–3.01 | 0.08 | |
| Presence of liver metastases | Yes versus No | 1.57 | 1.27–1.94 | <0.001 |
| Presence of peritoneum metastases | Yes versus No | 1.12 | 0.97–1.38 | 0.28 |
| Presence of lung metastases | Yes versus No | 1.23 | 0.97–1.55 | 0.09 |
| CA 19-9, µg/ml | <1860 (median) | reference | ||
| ⩾1860 | 1.62 | 1.31–2.01 | <0.001 | |
| Missing | 0.88 | 0.66–1.18 | 0.39 | |
| Albumin, g/dl | ⩾3.5 | reference | ||
| <3.5 | 1.68 | 1.31–2.14 | <0.001 | |
| Missing | 1.03 | 0.82–1.31 | 0.78 | |
| Neutrophil-to-lymphocyte ratio | <5 | reference | ||
| ⩾5 | 1.59 | 1.29–1.95 | <0.001 | |
| Missing | 1.48 | 0.94–2.34 | 0.09 | |
| Prior line of chemotherapy for metastatic disease | 0–1 versus 2–3 | 0.97 | 0.76–1.22 | 0.77 |
| Prior irinotecan treatment | Yes versus No | 1.10 | 0.74–1.63 | 0.64 |
| Prior TS-1 treatment | Yes versus No | 1.43 | 1.16–1.77 | 0.001 |
| Prior platinum treatment | Yes versus No | 1.12 | 0.90–1.39 | 0.32 |
| Prior 5-fluorouracil treatment | Yes versus No | 1.13 | 0.79–1.60 | 0.51 |
| Pre-emptive nal-IRI dose (80 mg/m2) | ⩾75% | reference | ||
| 50–74% | 1.14 | 0.86–1.49 | 0.36 | |
| <50% | 1.37 | 1.04–1.80 | 0.027 |
CA 19-9, Carbohydrate antigen 19-9; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group Performance Status; HR, hazard ratio; nal-IRI, liposomal irinotecan.
The univariate analysis showed that no previous pancreatectomy, poor ECOG performance, the presence of liver metastases, the presence of peritoneal metastases, the presence of lung metastases, the presence of other metastatic sites, a primary tumor location at an overlapping site, CA 19-9 levels higher than the median, NLR >5, albumin levels <3.5 g/dl, and pre-emptive nal-IRI doses <50% were negative prognostic factors for TTF. Similar to the analyses for OS, a pre-emptive nal-IRI dose <50% remained an independent negative factor in the multivariate analysis for TTF (Table 3).
Table 3.
Multivariate analyses examining time to treatment failure.
| Variable | Category | Adjusted HR | 95% CI | p |
|---|---|---|---|---|
| Sex | Female versus Male | 0.84 | 0.71–1.02 | 0.06 |
| Body mass index, kg/m2 | ⩽ 21.1 versus > 21.1 | 0.84 | 0.71–0.99 | 0.049 |
| ECOG performance | 0–1 versus 2–3 | 1.95 | 1.56–2.42 | <0.001 |
| Primary tumor location | Head | reference | ||
| Body | 0.97 | 0.77–1.18 | 0.77 | |
| Tail | 0.98 | 0.74–1.18 | 0.86 | |
| Overlapping | 1.65 | 0.97–2.79 | 0.07 | |
| Previous pancreatectomy | Yes versus No | 0.74 | 0.62–0.90 | 0.002 |
| Presence of liver metastases | Yes versus No | 1.23 | 1.12–1.49 | 0.032 |
| Presence of peritoneum metastases | Yes versus No | 1.09 | 0.90–1.32 | 0.40 |
| Presence of lung metastases | Yes versus No | 1.39 | 1.12–1.73 | 0.002 |
| Presence of other metastases | Yes versus No | 0.71 | 0.52–0.97 | 0.032 |
| CA 19-9, µg/ml | <1860 (median) | reference | ||
| ⩾1860 | 1.30 | 1.07–1.57 | 0.009 | |
| Missing | 1.02 | 0.79–1.32 | 0.86 | |
| Albumin, g/dl | ⩾3.5 | reference | ||
| <3.5 | 1.10 | 0.88–1.39 | 0.40 | |
| Missing | 0.85 | 0.69–1.05 | 0.13 | |
| Neutrophil-to-lymphocyte ratio | <5 | reference | ||
| ⩾5 | 1.28 | 1.06–1.55 | 0.012 | |
| Missing | 1.28 | 0.86–1.93 | 0.23 | |
| Prior line of chemotherapy for metastatic disease | 0–1 versus 2–3 | 0.95 | 0.77–1.17 | 0.61 |
| Prior irinotecan treatment | Yes versus No | 1.23 | 0.91–1.66 | 0.17 |
| Prior TS-1 treatment | Yes versus No | 1.33 | 1.10–1.60 | 0.003 |
| Pre-emptive nal-IRI dose (80 mg/m2) | ⩾75% | reference | ||
| 50–74% | 1.19 | 0.93–1.51 | 0.16 | |
| <50% | 1.43 | 1.11–1.86 | 0.006 |
CA 19-9, carbohydrate antigen 19-9; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group Performance Status; HR, hazard ratio; nal-IRI, liposomal irinotecan.
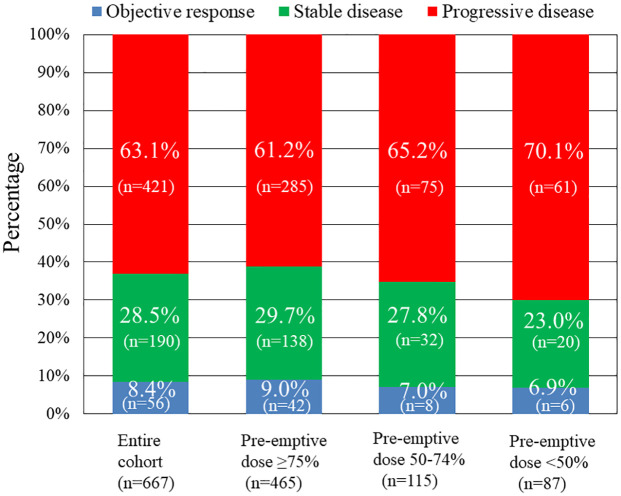
Regarding tumor response to nal-IRI + 5-FU/LV, 1 (0.1%), 55 (8.2%), 190 (28.5%), and 421 (63.1%) of the patients achieved complete response, partial response, stable disease, and progressive disease, respectively (Figure 3). Accordingly, the objective response and disease control rates were 8.4% and 36.9%, respectively. Patients in the pre-emptive nal-IRI ⩾75% group experienced a higher rate of objective response (9.0%) and stable disease (29.7%) than those in the pre-emptive nal-IRI 50–74% group (7.0% and 27.8%, respectively) and those in the pre-emptive nal-IRI <50% group (6.9% and 23.0%, respectively). There was a trend toward tumor response with progressive disease among patients who received lower nal-IRI dosage (p = 0.09).
Figure 3.
Best tumor responses to nal-IRI + 5-FU/LV treatment among the entire cohort and among the three pre-emptive nal-IRI dosing groups.
The median dose intensity of nal-IRI during the first six treatment cycles was 90% (range, 34–100%), 68% (range, 51–98%), and 43% (range 30–61%) among patients in the pre-emptive dose ⩾75%, 50–74%, and <50% groups, respectively (p < 0.001 for within-group comparison). A total of 105 (15.7%) patients—92 (19.8%), 11 (9.6%), and 2 (2.3%) patients in the pre-emptive dose ⩾75%, 50–74%, and <50% groups, respectively (p < 0.001 for all group comparisons)—required additional nal-IRI dose reduction due to adverse events. In total, 51 (7.6%) patients discontinued nal-IRI treatment because of intolerance to adverse events. The discontinuation rates due to adverse events were 8.8%, 5.2%, and 4.6% among patients in the pre-emptive dose ⩾75%, 50–74%, and <50% groups, respectively (p = 0.11 for all group comparisons).
The most common treatment-related adverse events of all grades were anemia (65.4%), fatigue (46.0%), and vomiting (42.9%) (Table 4). Neutropenia (22.9%) was the most common grade 3 or higher (severe) adverse event, followed by anemia (21.1%) and hypokalemia (15.4%). Patients in the <50% pre-emptive dose group had a statistically significantly lower incidence of all-grade neutropenia, anemia, hypokalemia, fatigue, vomiting, nausea, mucositis, and non-neutropenic infection than those in the other two groups. Regarding severe adverse events, no statistically significant differences in the adverse events were observed among these three patient groups, with the exception of patients in the <50% pre-emptive dose group; these patients had a statistically significantly lower incidence of neutropenia and non-neutropenic infection.
Table 4.
Treatment-related toxicity according to pre-emptive nal-IRI dosing.
| Variable | Entire cohort (n = 667) | Pre-emptive nal-IRI dose ⩾75% (n = 465) | Pre-emptive nal-IRI dose 50–74% (n = 115) | Pre-emptive nal-IRI dose <50% (n = 87) | p for all grades | p for grade 3 or higher | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| All grade | Grade 3 or higher | All grade | Grade 3 or higher | All grade | Grade 3 or higher | All grade | Grade 3 or higher | |||
| Anemia | 436 (65.4) | 141 (21.1) | 305 (65.6) | 97 (20.9) | 89 (77.4) | 30 (26.1) | 42 (48.3) | 14 (16.1) | <0.001 | 0.25 |
| Neutropenia | 275 (41.2) | 153 (22.9) | 213 (45.8) | 124 (26.7) | 30 (26.1) | 16 (13.9) | 32 (36.8) | 13 (14.9) | <0.001 | 0.003 |
| Thrombocytopenia | 184 (27.6) | 48 (7.2) | 128 (27.5) | 31 (6.7) | 39 (33.9) | 11 (9.6) | 17 (19.5) | 6 (6.9) | 0.09 | 0.54 |
| Febrile neutropenia | – | 24 (3.6) | – | 19 (4.1) | – | 4 (3.5) | – | 1 (1.1) | – | 0.38 |
| Fatigue | 307 (46.0) | 11 (1.6) | 228 (49.0) | 7 (1.5) | 50 (43.5) | 3 (2.6) | 29 (33.3) | 1 (1.1) | 0.029 | 0.64 |
| Vomiting | 286 (42.9) | 24 (3.6) | 221 (47.5) | 10 (2.2) | 41 (35.7) | 7 (6.1) | 24 (27.6) | 7 (8.0) | 0.001 | 0.009 |
| Nausea | 215 (32.2) | 3 (0.4) | 167 (35.9) | 1 (0.2) | 36 (31.3) | 1 (1.7) | 12 (13.8) | 0 | <0.001 | 0.07 |
| Diarrhea | 203 (30.4) | 18 (2.7) | 145 (31.2) | 12 (2.6) | 36 (31.3) | 4 (3.5) | 22 (25.3) | 2 (2.3) | 0.68 | 0.30 |
| Mucositis | 41 (6.1) | 4 (0.6) | 36 (7.7) | 2 (0.4) | 4 (3.5) | 1 (0.9) | 1 (1.1) | 1 (1.1) | 0.025 | 0.68 |
| Non-neutropenic infection | 20 (3.0) | 16 (2.4) | 12 (2.6) | 9 (1.9) | 8 (7.0) | 7 (6.1) | 0 | 0 | 0.009 | 0.009 |
| Hypokalemia | 236 (35.4) | 103 (15.4) | 179 (38.5) | 71 (15.3) | 40 (34.8) | 20 (17.4) | 17 (19.5) | 12 (13.8) | 0.002 | 0.72 |
| Elevation of AST | 215 (32.2) | 30 (4.5) | 150 (32.3) | 21 (4.5) | 41 (35.7) | 6 (5.2) | 24 (27.6) | 3 (3.4) | 0.39 | 0.81 |
| Elevation of ALT | 214 (32.0) | 23 (3.4) | 154 (33.1) | 19 (4.1) | 38 (33.0) | 1 (0.9) | 22 (25.3) | 3 (3.4) | 0.27 | 0.24 |
| Elevation of total bilirubin | 161 (24.1) | 55 (8.2) | 110 (23.7) | 36 (7.7) | 32 (27.8) | 14 (22.2) | 19 (21.8) | 5 (5.7) | 0.50 | 0.18 |
| Elevation of creatinine | 110 (16.5) | 9 (1.3) | 74 (15.9) | 5 (1.1) | 22 (19.1) | 4 (3.5) | 14 (16.1) | 0 | 0.68 | 0.07 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
To our knowledge, this study is the largest population-based series, including 667 patients from 9 institutes across Taiwan, to demonstrate the effect of nal-IRI + 5-FU/LV on survival outcomes and safety profiles in patients with PDAC after gemcitabine-based therapy. We demonstrated a median OS of 5.9 months and a manageable safety profile in Taiwanese patients receiving nal-IRI + 5-FU/LV. Comparable to NAPOLI’s median overall survival of 6.1 months, our results support the utility of nal-IRI + 5-FU/LV as a subsequent treatment for patients with PDAC after gemcitabine-based therapy. In addition, our study showed that among all the risk factors, a pre-emptive nal-IRI dose <50% was an independent poor prognostic factor for OS and TTF. Although a lower nal-IRI starting dosage may be more tolerable because of a better safety profile, our results demonstrate that this may occur at the cost of inferior tumor response and compromised survival outcomes in patients with PDAC. While our study revealed the safety and tolerability of the drug at a standard dosage, <50% of the standard dosage should be discouraged as it may compromise survival outcome.
We previously reported that the median OS was 4.2 months in patients with PDAC who received L2 therapy between 2010 and 2016 (before the availability of nal-IRI).24,25 In the NAPOL-1 study, the median OS and TTF in the nal-IRI + 5-FU/LV arm were 6.1 and 2.3 months, respectively. 15 The median OS ranged from 4.2 to 5.3 months in real-world studies among PDAC who received nal-IRI in the world.17,18,20,21 Using nal-IRI + 5-FU/LV as a L2 therapy, the OS benefit in our cohort was comparable to that of the NAPOLI-1 study and real-world data from the United States.15,17,18,20 However, the OS in our cohort was inferior to that of the subgroup analysis of Asian patients (OS, 8.9 months) in the NAPOLI-1 study. 26 The objective response and disease control rates (8.4% and 36.9%, respectively) in our cohort were lower than those in the NAPOLI-1 Asian subgroup (8.8% and 52.9%, respectively). 26 Similarly, Yoo et al. 19 reported a median OS of 9.4 months with a 10% objective response rate and a 55% disease control rate within real-world data in patients with PDAC receiving nal-IRI treatment in Korea. Differences in demographic characteristics among the three cohorts may potentially lead to variations in survival outcome.19,26 In our cohort, we had more cases with an ECOG performance of 2 or 3 (23.5% versus 0%), older median age, more liver metastases, and higher CA19-9, which are all well known as poor prognostic indicators for patients with PDAC receiving nal-IRI treatment 27 than patients in the NAPOLI-1 Asian subgroup series and Korean Cancer Study Group. 19 Furthermore, our cohort had more cases who received nal-IRI + 5-FU/LV as beyond second-line treatment for PDAC (37%) as compared with those in the NAPOLI-1 Asian subgroup (27%). Despite the innate demographic differences between our cohort and other studies, this study showed that survival outcomes of patients with PDAC in Taiwan have improved since the approval of nal-IRI + 5-FU/LV reimbursement.
Consistent with the NAPOLI-I study, our study showed that poor ECOG performance, the presence of liver metastases, higher CA 19-9 values, and NLR >5 were poor prognostic factors. 27 In addition, this study identified that initial nal-IRI dosing was an independent prognostic factor for patients with PDAC. Dose modifications, including dose reductions or dose delays, were used to manage adverse effects and prevent treatment discontinuation, thereby allowing patients to remain on treatment longer and achieve clinical benefit. 26 For instance, 33% of patients in the nal-IRI + 5-FU/LV arm in the NAPOLI-1 study experienced adverse events that resulted in a dose reduction. 15 The post hoc analysis of data from the NAPOLI-1 study demonstrated a similar outcome for OS and PFS between nal-IRI patients with and without early dose modifications. 28 The results of real-world studies suggest that appropriate dose modifications of nal-IRI + 5-FU/LV due to adverse events led to substantially better survival outcomes than those in patients without dose reduction.18,20 In contrast, the impact of pre-emptive nal-IRI dose reduction on patient survival has rarely been explored.
Our study showed that pre-emptive nal-IRI dose reduction is a common practice based on real-world data. Only 70% of our patient cohort received a pre-emptive nal-IRI dose of ⩾75% during the first treatment cycle. The proportion of patients with dose reductions was generally consistent with other real-world studies of nal-IRI + 5-FU/LV therapy in patients with pancreatic cancer.16,17,20,29 Glassman et al. 20 reported a real-world analysis based on 56 patients with PDAC in the United States; only 30% of the patients were treated with a starting nal-IRI dosage >70 mg/m2. The authors concluded that the starting dose of nal-IRI was not associated with survival. Su et al. 21 reported comparable survival outcomes between patients treated with a full dose and a 20% reduction in the starting dose among 32 patients with PDAC in Taiwan. Contrary to previous reports, our study showed that a pre-emptive nal-IRI dose <50% was an independent poor prognostic factor for OS and TTF. Furthermore, our data showed a trend toward lower objective tumor responses and disease control rates among patients who received lower pre-emptive nal-IRI dosing. As a result, a pre-emptive nal-IRI dose that is <50% of the standard dose is discouraged in clinical practice, as this would compromise survival outcomes.
While this study focuses on a subsequent line of treatment, different frontline chemotherapeutic agents may impact survival outcomes in patients with PDAC receiving subsequent nal-IRI + 5-FU/LV. In our study, prior TS-1 and irinotecan treatment were both poor prognostic factors for OS and TTF in univariate analyses, whereas only TS-1 remained an independent prognostic factor for OS and TTF after adjusting for other confounding factors. Nal-IRI comprised free irinotecan encapsulated in liposome nanoparticles. As a result, patients previously treated with irinotecan tended to be less responsive to nal-IRI. The subgroup analysis of the NAPOLI-1 study showed better survival time following 5-FU/LV treatment than following nal-IRI + 5-FU/LV treatment in patients previously treated with irinotecan. 15 Several real-world studies have reported similar results, demonstrating that previous irinotecan treatment was a poor prognostic variable for patients receiving nal-IRI treatment.17–20 The same concept applies to patients with previous exposure to 5-FU, which might also compromise the efficacy of nal-IRI + 5-FU/LV treatment. Because the two arms of the NAPOLI-1 study both contained 5-FU/LV, the influence of previous 5-FU use on subsequent treatment could not be demonstrated. In addition, since gemcitabine + nab-paclitaxel or gemcitabine monotherapy was the most common frontline regimen in Western countries prior to nal-IRI + 5-FU/LV being implemented, 6 real-world studies from Western countries have been unable to evaluate the impact of prior 5-FU exposure among patients with PDAC receiving nal-IRI plus 5-FU/LV. TS-1 is an oral 5-FU derivative widely used in Japan and Taiwan for treating patients with advanced PDAC and TS-1 in combination with gemcitabine was widely used as a L1 chemotherapy treatment for PDAC until nab-paclitaxel was reimbursed in Taiwan in November 2019. 7 This might explain why a high proportion of patients (59%) had been exposed to TS-1 in our patient cohort. The effect of prior irinotecan or TS-1 exposure on survival outcomes in patients with PDAC receiving subsequent nal-IRI + 5-FU/LV needs further study within prospective cohorts.
The most common severe adverse events among patients in the nal-IRI + 5-FU/LV arm of the NAPOLI-1 study were neutropenia (27%), fatigue (14%), and diarrhea (13%), whereas neutropenia (23%), anemia (21%), and hypokalemia (15%) were more common in our study. The lower starting dosage of nal-IRI in our study might be the main reason for the lower incidence of all grade and severe adverse events as compared with the NAPOLI-1 study. 15 Compared with nal-IRI dosing <50%, the only significant grade 3 or higher treatment-related toxicity among nal-IRI dosing of ⩾75% is neutropenia (26.7% versus 14.9%, p = 0.003) while no significant difference in neutropenic fever (4.1% versus 1.1%, p = 0.38) is noted. This suggests that even though standard dosage of nal-IRI does lead to neutropenia, it does not lead to notable consequential infection. Thus, >75% of standard dosage has a comparable toxicity and safety profile to <50% of standard dosage. In line with the NAPOLI-1 study, our study confirmed the safety profiles of nal-IRI in real-world practice.
The rationale for pre-emptive dose reduction often involves consideration of patient characteristics such as older age, poor performance, or malnutrition. In our study, patients in the pre-emptive dose <50% group had poorer performance than those in the other two groups. However, the decision for pre-emptive dose reduction at treatment initiation was multifactorial, and these factors varied with each individual and treating physician. We were unable to investigate all the possible reasons and components of the subjective decision to prescribe different initial treatment doses; this may have introduced a selection bias in our study. Our study revealed that the impact of pre-emptive dose reductions on survival outcomes remained after adjustment for these confounding factors in the multivariate analysis. Limited experience to a new regimen may also contribute to physician’s tendency toward dose reduction. In response to clinical concerns, our study has addressed the adequate safety profile and the proven efficacy of nal-IRI in the Asian population.
As the primary purpose of palliative chemotherapy centers around improving quality of life, survival improvement is also a desired expectation. As nal-IRI has manageable safety profile at a standard dosage, <50% of the standard dosage should be discouraged as it may compromise survival outcome.
Several limitations should be considered when interpreting the results of this study. First, selection and recall bias might have occurred due to biases inherent to retrospective observational studies. Second, patients might receive dose modification with either escalation or de-escalation in subsequent treatment cycles and we did not include variables representing subsequent dose modifications in the survival analysis because some patients who received only one cycle of nal-IRI + 5-FU/LV might have been excluded from the analysis. Third, the incidence of adverse events was calculated during nal-IRI treatment. The incidence of adverse events in patients with pre-emptive nal-IRI doses <50% may be underestimated because of the shorter treatment duration as compared with the other two groups. Further studies are necessary to address these issues.
Conclusion
This was the largest population-based study to examine the effectiveness and safety profile of nal-IRI + 5-FU/LV treatment in patients with PDAC. Our results supported the use of nal-IRI + 5-FU/LV as the standard clinical practice for treating patients with PDAC after treatment with gemcitabine-based therapy. Our study showed that pre-emptive nal-IRI dose reduction is a common practice based on real-world data. Although our study revealed the safety and tolerability of nal-IRI at a standard dose, an initial treatment dose reduction of <50% should be discouraged as it might compromise survival outcomes.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211058255 for Liposomal irinotecan pre-emptive dose reduction in patients with pancreatic ductal adenocarcinoma: 667 patients’ experience within a population-based study by Tai-Jan Chiu, Yung-Yeh Su, Shih-Hung Yang, Chung-Pin Li, Li-Yuan Bai, Nai-Jung Chiang, Shih-Chang Chuang, Yan-Shen Shan, De-Chuan Chan, Li-Tzong Chen, Chia-Jui Yen, Cheng-Ming Peng, Yen-Yang Chen, Jen-Shi Chen and Wen-Chi Chou in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors gratefully acknowledge the assistance of the patients who participated in this study.
Footnotes
Author contributions: Conception and design of study: CTJ, CWC, CNJ, SYY, CLT; Acquisition of data: HYS, PLC, YBL, CDC, YCJ, PCM, CYY, CJS; Analysis and interpretation of data: CWC, CTJ; Drafting of the manuscript: CTJ, SYY, YSH, LCP, BLY, CNJ, CSC, SYS, CDC, CLT, YCJ, PCM, CYY, CJS, CWC
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Wen-Chi Chou  https://orcid.org/0000-0002-0361-4548
https://orcid.org/0000-0002-0361-4548
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Tai-Jan Chiu, Division of Hematology-Oncology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University, Kaohsiung.
Yung-Yeh Su, National Institute of Cancer Research, National Health Research Institutes, Tainan; Division of Hematology and Oncology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan.
Shih-Hung Yang, Department of Oncology, National Taiwan University Hospital, National Taiwan University, Taipei.
Chung-Pin Li, Division of Gastroenterology and Hepatology, Department of Medicine and Division of Clinical Skills Training, Taipei; Veterans General Hospital, School of Medicine, National Yang-Ming Chiao Tung University, Taipei.
Li-Yuan Bai, Division of Hematology-Oncology, Department of Internal Medicine, China Medical University Hospital, China Medical University, Taichung.
Nai-Jung Chiang, National Institute of Cancer Research, National Health Research Institutes, Tainan; Division of Hematology and Oncology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan.
Shih-Chang Chuang, Division of General and Digestive Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung.
Yan-Shen Shan, Division of General Surgery, Department of Surgery, National Cheng Kung University Hospital, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan.
De-Chuan Chan, Division of General Surgery, Department of Surgery, Tri-Service General Hospital, National Defense Medical Center, Taipei.
Li-Tzong Chen, National Institute of Cancer Research, National Health Research Institutes, Tainan.
Chia-Jui Yen, Division of Hematology and Oncology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan.
Cheng-Ming Peng, Department of Surgery, Chung Shan Medical University Hospital, Chung Shan Medical University, Taichung.
Yen-Yang Chen, Division of Hematology-Oncology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University, Kaohsiung.
Jen-Shi Chen, Division of Hematology-Oncology, Department of Internal Medicine, Linkou Chang Gung Memorial Hospital and Chang Gung University, Taoyuan.
Wen-Chi Chou, Division of Hematology-Oncology, Department of Internal Medicine, Linkou Chang Gung Memorial Hospital and College of Medicine, Chang Gung University, 5 Fu-Hsing Street, 333 Kwei-Shan Shiang, Taoyuan.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014; 371: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 3. Gurusamy KS, Kumar S, Davidson BR, et al. Resection versus other treatments for locally advanced pancreatic cancer. Cochrane Database Syst Rev 2014; 2: CD010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25: 1960–1966. [DOI] [PubMed] [Google Scholar]
- 5. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 6. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013; 31: 1640–1648. [DOI] [PubMed] [Google Scholar]
- 8. Walker EJ, Ko AH. Beyond first-line chemotherapy for advanced pancreatic cancer: an expanding array of therapeutic options? World J Gastroenterol 2014; 20: 2224–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chou WC, Chen YY, Hung CY, et al. Evolution of the chemotherapeutic landscape and survival outcome in patients with metastatic pancreatic cancer: a four-institute cohort study in Taiwan, 2010-2016. Cancer Manag Res 2019; 11: 2119–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lai HL, Chen YY, Lu CH, et al. Effect of S-1 on survival outcomes in 838 patients with advanced pancreatic cancer: a 7-year multicenter observational cohort study in Taiwan. Cancer Med 2019; 8: 2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 2011; 47: 1676–1681. [DOI] [PubMed] [Google Scholar]
- 12. Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 2014; 32: 2423–2429. [DOI] [PubMed] [Google Scholar]
- 13. Gill S, Ko YJ, Cripps C, et al. PANCREOX: a randomized phase III study of 5-fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol 2016; 34: 3914–3920. [DOI] [PubMed] [Google Scholar]
- 14. Ioka T, Ueno M, Ueno H, et al. TAS-118 (S-1 plus leucovorin) versus S-1 in patients with gemcitabine-refractory advanced pancreatic cancer: a randomised, open-label, phase 3 study (GRAPE trial). Eur J Cancer 2019; 106: 78–88. [DOI] [PubMed] [Google Scholar]
- 15. Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016; 387: 545–557. [DOI] [PubMed] [Google Scholar]
- 16. Kieler M, Unseld M, Bianconi D, et al. A real-world analysis of second-line treatment options in pancreatic cancer: liposomal-irinotecan plus 5-fluorouracil and folinic acid. Ther Adv Med Oncol 2019; 11: 1758835919853196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barzi A, Miksad R, Surinach A, et al. Real-world dosing patterns and outcomes of patients with metastatic pancreatic cancer treated with a liposomal irinotecan regimen in the United States. Pancreas 2020; 49: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kasi A, McGinnis T, Naik G, et al. Efficacy and tolerability of the combination of nano-liposomal irinotecan and 5-fluorouracil/leucovorin in advanced pancreatic adenocarcinoma: post-approval clinic experience. J Gastrointest Oncol 2021; 12: 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoo C, Im HS, Kim KP, et al. Real-world efficacy and safety of liposomal irinotecan plus fluorouracil/leucovorin in patients with metastatic pancreatic adenocarcinoma: a study by the Korean Cancer Study Group. Ther Adv Med Oncol 2019; 11: 1758835919871126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glassman DC, Palmaira RL, Covington CM, et al. Nanoliposomal irinotecan with fluorouracil for the treatment of advanced pancreatic cancer, a single institution experience. BMC Cancer 2018; 18: 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Su YY, Chiang NJ, Tsai HJ, et al. The impact of liposomal irinotecan on the treatment of advanced pancreatic adenocarcinoma: real-world experience in a Taiwanese cohort. Sci Rep 2020; 10: 7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park HS, Kang B, Chon HJ, et al. Liposomal irinotecan plus fluorouracil/leucovorin versus FOLFIRINOX as the second-line chemotherapy for patients with metastatic pancreatic cancer: a multicenter retrospective study of the Korean Cancer Study Group (KCSG). ESMO Open 2021; 6: 100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bang K, Cheon J, Jeong JH, et al. Clinical outcomes of liposomal irinotecan plus fluorouracil/leucovorin for metastatic pancreatic adenocarcinoma in patients previously treated with conventional irinotecan-containing chemotherapy. Ther Adv Med Oncol 2021; 13: 17588359211003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu SM, Lu CH, Liu KH, et al. External validation of the Besançon nomogram in Asian patients with advanced pancreatic cancer receiving second-line chemotherapy: a multi-institute experience in Taiwan. Pancreatology 2020; 20: 116–124. [DOI] [PubMed] [Google Scholar]
- 25. Hsu CC, Liu KH, Chang PH, et al. Development and validation of a prognostic nomogram to predict survival in patients with advanced pancreatic cancer receiving second-line palliative chemotherapy. J Gastroenterol Hepatol 2020; 35: 1694–1703. [DOI] [PubMed] [Google Scholar]
- 26. Bang YJ, Li CP, Lee KH, et al. Liposomal irinotecan in metastatic pancreatic adenocarcinoma in Asian patients: subgroup analysis of the NAPOLI-1 study. Cancer Sci 2020; 111: 513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen LT, Macarulla T, Blanc JF, et al. Nomogram for predicting survival in patients treated with liposomal irinotecan plus fluorouracil and leucovorin in metastatic pancreatic cancer. Cancers 2019; 11: 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen LT, Macarulla T, Blanc JF, et al. Early dose reduction/delay and the efficacy of liposomal irinotecan with fluorouracil and leucovorin in metastatic pancreatic ductal adenocarcinoma (mPDAC): a post hoc analysis of NAPOLI-1. Pancreatology 2021; 21: 192–199. [DOI] [PubMed] [Google Scholar]
- 29. Tossey JC, Reardon J, VanDeusen JB, et al. Comparison of conventional versus liposomal irinotecan in combination with fluorouracil for advanced pancreatic cancer: a single-institution experience. Med Oncol 2019; 36: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211058255 for Liposomal irinotecan pre-emptive dose reduction in patients with pancreatic ductal adenocarcinoma: 667 patients’ experience within a population-based study by Tai-Jan Chiu, Yung-Yeh Su, Shih-Hung Yang, Chung-Pin Li, Li-Yuan Bai, Nai-Jung Chiang, Shih-Chang Chuang, Yan-Shen Shan, De-Chuan Chan, Li-Tzong Chen, Chia-Jui Yen, Cheng-Ming Peng, Yen-Yang Chen, Jen-Shi Chen and Wen-Chi Chou in Therapeutic Advances in Medical Oncology