Abstract
We present a patient with an acute kidney injury thought secondary to acute interstitial nephritis as a result of vedolizumab maintenance therapy for Crohn’s disease. This appears to be a rare but serious side effect in patients receiving this treatment which clinicians should consider in the event of renal dysfunction.
Keywords: Crohn's disease, renal system, acute renal failure, unwanted effects/adverse reactions
Background
Vedolizumab is a humanised immunoglobulin G1 monoclonal antibody to α4β7 integrin. By specifically inhibiting the gut tropic α4β7 integrin, vedolizumab is thought to selectively inhibit leucocyte trafficking to the gastrointestinal system.1 It has proven effective in the induction and maintenance of remission in inflammatory bowel disease.2 It is generally well tolerated and is considered to have a favourable safety profile compared with other ‘biologics’ used in Inflammatory bowel disease (IBD).3
However, in this case report, we present a patient with interstitial nephritis thought secondary to vedolizumab use, highlighting the importance of continued vigilance for rare adverse drug reactions even with otherwise well-tolerated treatments.
Case presentation
A 44-year-old woman was commenced on vedolizumab in June 2019 for control of her Crohn’s disease as stepwise progression following a failure to respond to multiple previous therapies.
The patient had a background of treatment resistant Crohn’s disease diagnosed in 2010 at the same time as Raynaud’s syndrome, congenital nystagmus, temporal lobe epilepsy and uterine fibroids.
The patient had a prior intolerance to azathioprine with deranged liver function, an infusion reaction to infliximab and had failed to respond to adalimumab and ustekinumab despite combination therapy with methotrexate.
Prior to commencing vedolizumab, the patient was receiving methotrexate 12.5 mg weekly and folic acid and ustekinumab 8 mg weekly. Despite treatment, she reported bowels moving 4–10 times a day associated with abdominal discomfort and nocturnal diarrhoea. There were also concerns that methotrexate was contributing to arthralgia. Colonoscopy in October 2018 had demonstrated mild-to-moderate colitis which was confirmed histologically.
In June 2019, the patient switched to intravenous vedolizumab infusion receiving induction doses at week 0, 2, 6, 10 and 14 followed by maintenance infusions every 8 weeks. There was an initial good response to induction doses but her symptoms flared in January 2020 requiring further corticosteroids.
In February 2020, while on 8 weekly infusions of vedolizumab, she reported recurrence of symptoms with bowels opening six times a day. She described worsening symptoms towards the end of each infusion cycle. Faecal calprotectin was mildly elevated at 302 but routine blood tests were normal. A decision was made to increase the frequency of vedolizumab infusions to six weekly.
In June 2020, 4 days following a vedolizumab infusion, she was admitted to hospital with an acute episode of fever, malaise and severe myalgia.
She was found to have acute kidney injury (AKI) stage I. Her serum creatine rose to 105 μmol/L compared with a baseline of 76 μmol/L in February 2020. Her blood urea was 4.8 mmol/L, white cell count was 14.3x109/L, eosinophil count was 0.16 and C reactive protein was 71. SARS-Cov-2 PCR was negative. She reported her bowels were moving six times a day which had persisted since February. She maintained a normal diet with no vomiting or reduced oral intake. Her abdomen was soft and non-tender on examination. She had one episode of fever at 38°C recorded during admission. Other observations including heart rate and blood pressure remained within the normal range with no evidence of hypovolaemia. A stool culture and blood cultures were also negative. No other focal source of infection was identified. Apart from vedolizumab the only other medication she reported taking were Accrete D3 and budesonide. There was no evidence of new onset hypertension or hyperglycaemia. She was discharged the following day on a reducing course of budesonide with a plan to increase the frequency of her vedolizumab infusions to four weekly if her symptoms did not improve. Her creatine was repeated in July prior to her last dose of vedolizumab and was found to remain elevated at 101 μmol/L.
In July 2020, she described 6 months of intermittent fever, tachycardia and lethargy lasting several days and occurring every couple of weeks. Suspecting a possible adverse drug reaction, her gastroenterologist recommended withholding the next infusion of vedolizumab (August 2020) to assess the effect on these systemic symptoms. Following this, the patient reported that the intermittent episodes of fever and myalgia had improved but her Crohn’s disease remained poorly controlled. She was keen to be rechallenged with another infusion of vedolizumab but was also referred to the combined medical/surgical IBD clinic for consideration of colectomy or enrolment in a clinical trial of a novel gut selective tyrosine kinase inhibitor.
Having elected to pursue a clinical trial, she entered screening for this in November 2020. However, screening blood tests revealed she had developed chronic kidney disease (CKD) stage 3b. She was found to have an elevated serum creatine of 160 μmol/L and a blood urea of 8.6 mmol/L. Six months before, her serum creatine had been 78 μmol/L, blood urea had been 4.9 mmol/L and estimated glomerular filtration rate (eGFR) had been >60 mL/min.
Investigations
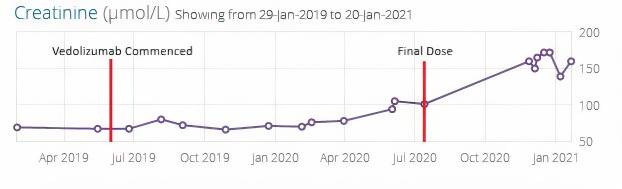
The patient had a baseline serum creatine of 76 μmol/L, blood urea of 4.9 mmol/L and eGFR >60 mL/min in February 2020. During her admission in June 2020, serum creatine was mildly raised at 105μmol/L giving a diagnosis of AKI stage I. In November, we can see progression to CKD stage 3b with an elevated serum creatine of 160 μmol/L (see figures 1 and 2).
Figure 1.
The patient’s serum creatine over time.
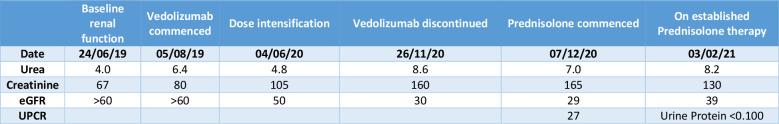
Figure 2.
Table of the patient’s renal function. Estimated Glomerular Filtration Rate (eGFR). Urine Protein Creatinine Ratio (UPCR).
At the time of detecting AKI, she had no rash or eosinophilia and had sterile pyuria. Urinalysis demonstrated mild (5-10 erythrocytes per μl) non-visible haematuria, proteinuria and leucocytes. Urine protein:creatine ratio was 27 mg/mmol (<20).
Ultrasound scan showed normal renal appearances with no evidence of ureteric or pelvicalyceal dilatation. Global renal perfusion, cortical thickness and bipolar diameter were normal.
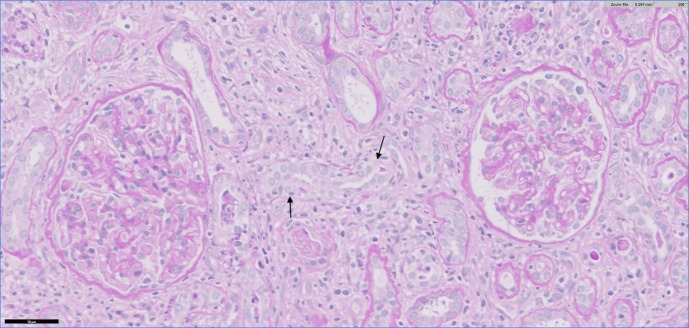
A renal biopsy showed acute interstitial nephritis (AIN) with lymphocytic, plasma cell and macrophage infiltrate in the interstitium and lymphocytic tubulitis (see figures 3 and 4). The renal interstitium demonstrated 20% fibrosis and tubular atrophy (IFTA). There was no evidence of deposition of immunoglobulin or complement in glomeruli, tubular basement membranes or blood vessels on immunofluorescence evaluation. On electron microscopy examination, no electron dense deposits were detected but mild changes consistent with a single tubuloreticular inclusion were present.
Figure 3.
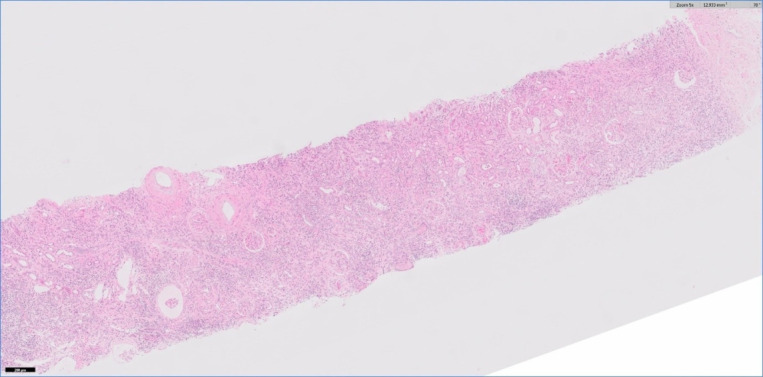
Low-power view showing a widespread inflammatory cell infiltrate in the interstitium. H&E stain.
Figure 4.
Medium-power view showing infiltrate of lymphocytes and macrophages in the interstitium and lymphocytic tubulitis (arrows). There is no significant abnormality in the two glomeruli. Periodic acid-Schiff stain.
Immunoperoxidase staining for CMV and SV40 large T antigen were negative. Ziehl-Neelsen and Grocott stains were negative for acid-alcohol-fast bacilli (AAFB) and fungi.
Autoantibody screen was taken which showed moderate positive antinuclear antibody (ANA) with a titre of 1/640 and homogeneous pattern. An extractable nuclear antigen panel was negative. Complement C3 and C4 levels were 1.26 and 0.35, respectively. Rheumatoid factor, antimyeloperoxidase antibody and antiproteinase 3 antibody were negative. DNA ab level was 1.0 (normal range 0–10). Screenings for current or previous HIV, hepatitis C and hepatitis B infection were also negative.
Faecal calprotein in March was 1531. Colonoscopy on 8 October 2020 showed mild-moderate inflammation from the upper rectum to ascending colon with no evidence of ulceration.
Differential diagnosis
The patient most likely developed drug-induced AIN secondary to vedolizumab. This diagnosis was supported by the patient’s symptoms reported after commencing vedolizumab. The episodes of myalgia and fever lead to her admission to hospital in June 2020 when renal function dysfunction was initially detected. A previous report of AIN secondary to vedolizumab similarly highlights myalgia and fever as initial symptoms reported by their patient.4 The delayed onset of AIN following initiation of vedolizumab may be explained by the frequent courses of steroids having a masking effect. Her decline in renal function also appears to coincide with dose intensification of vedolizumab.
AIN secondary to Crohn’s disease would be a potential alternative diagnosis with clinical, endoscopic, histological and biochemical evidence of active disease throughout. However, given this patient’s long history of refractory Crohn’s disease without previous AIN, the relatively low IFTA rate and the temporal relationship between commencing treatment with vedolizumab and AKI, we believe AIN secondary to vedolizumab to be a more likely diagnosis. The patient took ibuprofen 200 mg on a few occasions in November for a headache. Given that this was after the onset of her symptoms and detection of renal dysfunction, we feel this was not the main cause of her AKI or AIN. She took no other medication associated with AIN. Systemic lupus erythematous (SLE) is a rare cause of AIN. However, despite her positive ANA titre, there were a number of factors which make this diagnosis less likely. The negative extractable nuclear antigen (ENA) screen, lack of inclusion bodies on electron microscopy and the normal DNA ab level make SLE a much less likely cause. The isolated positive ANA therefore probably represents an immunological epiphenomenon.
Treatment
Following diagnosis, the patient was commenced on prednisolone 40 mg once daily. Famotidine 20 mg once daily and Calcichew D3 two times per day were prescribed for gastric and bone protection. Regular monitoring was arranged through the renal clinic.
Outcome and follow-up
Following commencing prednisolone, there has been a gradual improvement in renal function; however, the patient’s renal function has not recovered to her previous baseline. In February 2021, the patient’s serum creatine was 130 μmol/L from a peak of 172 μmol/L (see figure 2). She has been diagnosed as CKD stage 3b. She is under regular review in renal clinic with an aim to slowly reduce her prednisolone dose. As of September 2021, there has been no further improvement and her renal function remains stable with a creatine of 133 μmol/L. A subtotal colectomy with stoma formation was performed in April 2021 to achieve remission of her Crohn’s disease.
Discussion
Vedolizumab has become established as an effective and well-tolerated treatment for IBD with reassuring long-term safety data.5 Its selective action on the gut is felt to be an advantage in terms of its potential side effects compared with anti-tumour necrosis factor (anti-TNF) agents.6 Although there is still uncertainty about the exact mechanism of action vedolizumab with recent studies showing it may modulate innate rather adaptive immunity.5 7 In the published meta-analysis of clinical trials of vedolizumab treatment in inflammatory bowel disease, we have found no mention of AIN as a reported adverse drug reaction.2 3
On review of published case reports, we have found one case of interstitial nephritis secondary to vedolizumab treatment in Crohn’s disease.4 In this case, the patient developed vomiting, asthenia, myalgia and fever 6 hours following vedolizumab administration. Six days following the initial infusion, the patient represented with an AKI. The patient developed biopsy proven AIN and responded to prednisolone with complete renal recovery in 1 month. Our case differs in the prolonged administration of vedolizumab over several months prior to diagnosis with concomitant courses of glucocorticoids.
On Vigibase, the WHO’s global database for adverse drug reactions, as of January 2021. we have found 49 cases of AKI reported as adverse drug reactions to vedolizumab with 18 cases of tubulointerstitial nephritis.8
On literature review, we found a case series with seven cases of T-cell dominant AIN as a result of vedolizumab administration. In four of the seven cases there was complete functional renal recovery. Electron microscopy was used to show characteristic lymphocytic infiltration into tubules as supportive evidence.9
In this case, a potential differential diagnosis was nephropathy secondary to Crohn’s disease. In a case–control study comparing the histological findings of 83 kidney biopsies in patients with known inflammatory bowel disease, the most common finding on biopsy was IgA nephropathy which was found in 24% of cases. The second most common finding in 19% of these cases was evidence of interstitial nephritis. Of the cases with interstitial nephritis 56% of the patients had known or past exposure to aminosalicylates. The authors theorised that the IgA nephropathy may represent a common pathogenic process in inflammatory bowel disease, while AIN may more likely be a result of drug therapy in inflammatory bowel disease.10 In this case, there were no histological features to suggest IgA nephropathy or other potential culprit drugs, hence vedolizumab AIN was considered likely.
Drug-induced interstitial nephritis typically causes tubulitis and interstitial inflammation. Interstitial infiltrate comprising predominantly of lymphocytes and monocytes or macrophages intermixed with eosinophils is characteristic. As with our case, small numbers of neutrophils may also be seen, but large numbers of neutrophils are more typical of pyelonephritis.11
The classic presentation of drug-induced interstitial nephritis is a triad of fever, eosinophilia and rash within a few days of commencing therapy; however, this only occurs in less than 10% of patients.12 As demonstrated in this case, onset of drug-induced interstitial nephritis may be delayed by weeks or even months following initiation of the offending drug.13
The mainstay of treatment is cessation of the causative drug followed by high-dose steroids if no improvement is seen in 3–5 days. Early recognition and treatment of drug-induced interstitial nephritis is associated with a lower likelihood of CKD.11 A definitive diagnosis of drug-induced interstitial nephritis requires a renal biopsy and should be performed promptly.13 Patients who had steroid therapy commenced within 2 weeks of withdrawal of the offending drug have been found more likely to recover to their baseline renal function than patients who had delayed steroid initiation to an average of 34 days.14
Early treatment with steroids is thought to be more effective as the characteristic early interstitial cellular infiltrates are steroid responsive but begin the transformation into areas of irreversible interstitial fibrosis within 7 days. The extent of interstitial fibrosis seen on biopsy is associated with a higher risk of chronic renal impairment and patients with established interstitial fibrosis on biopsy are less likely to respond to steroid treatment.15
Learning points.
This case emphasises the need for clinicians to remain vigilant for adverse drug reactions in patients on treatments with favourable risk profiles.
This is important as early recognition and treatment of drug-induced interstitial nephritis is more likely to result in recovery of renal function.
In cases of suspected drug-induced acute interstitial nephritis, timely renal biopsy is required for a definitive diagnosis and can inform prognosis.
Footnotes
Contributors: JPS identified the case and proposed the manuscript. Following this, he was involved in the review and editing of the manuscript. He provided specialist input from the gastroenterology perspective of the patient case. RP provided specialist renal input directing the discussion of the case, interpreting the results of the patient’s investigations and the relevant differential diagnoses of the case. He was also involved in the review and editing of the manuscript following each draft prior to submission. DK reviewed the pathology and identified the most appropriate slides to include. Annotation of the slides and interpretation was also provided by him. NS was involved in writing the initial draft of the manuscript. He was then involved in editing the manuscript with input from JPS, RP and DK in their respective areas and submitting the case.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Cominelli F. Inhibition of leukocyte trafficking in inflammatory bowel disease. N Engl J Med 2013;369:775–6. 10.1056/NEJMe1307415 [DOI] [PubMed] [Google Scholar]
- 2.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2013;369:711–21. 10.1056/NEJMoa1215739 [DOI] [PubMed] [Google Scholar]
- 3.Colombel J-F, et al. The safety of Vedolizumab for ulcerative colitis and Crohn’s disease. BMJ 2017;66:839–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailly E, Von Tokarski F, Beau-Salinas F, et al. Interstitial nephritis secondary to Vedolizumab treatment in Crohn disease and safe rechallenge using steroids: a case report. Am J Kidney Dis 2018;71:142–5. 10.1053/j.ajkd.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 5.Lamb CA, Kennedy NA, Raine T, et al. British Society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1–106. 10.1136/gutjnl-2019-318484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen BL, Sachar DB. Update on anti-tumor necrosis factor agents and other new drugs for inflammatory bowel disease. BMJ 2017;357:j2505. 10.1136/bmj.j2505 [DOI] [PubMed] [Google Scholar]
- 7.Zeissig S, Rosati E, Dowds CM, et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut 2019;68:25–39. 10.1136/gutjnl-2018-316023 [DOI] [PubMed] [Google Scholar]
- 8.WHO . VigiBase®, the World Health Organization’s global database for ADRs, maintained by the Uppsala Monitoring Centre, 2021. VigiAccess Uppsala Monitoring Centre. Available: http://www.vigiaccess.org/ [Accessed February 2021]. [Google Scholar]
- 9.Zhang PL, Pancioli T, Li W, et al. Electron microscopic findings can support multiple etiologies of nephrotoxicity in renal tubules. Ultrastruct Pathol 2020;44:481–8. 10.1080/01913123.2020.1839152 [DOI] [PubMed] [Google Scholar]
- 10.Ambruzs JM, Walker PD, Larsen CP. The histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease. Clin J Am Soc Nephrol 2014;9:265–70. 10.2215/CJN.04660513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan N, Perazella MA. Drug-Induced acute interstitial nephritis: pathology, pathogenesis, and treatment. Iran J Kidney Dis 2015;9:3–13. [PubMed] [Google Scholar]
- 12.Moledina DG, Perazella MA. Drug-Induced acute interstitial nephritis. Clin J Am Soc Nephrol 2017;12:2046–9. 10.2215/CJN.07630717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perazella MA, Markowitz GS. Drug-Induced acute interstitial nephritis. Nat Rev Nephrol 2010;6:461–70. 10.1038/nrneph.2010.71 [DOI] [PubMed] [Google Scholar]
- 14.González E, Gutiérrez E, Galeano C, et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 2008;73:940–6. 10.1038/sj.ki.5002776 [DOI] [PubMed] [Google Scholar]
- 15.Praga M, González E. Acute interstitial nephritis. Kidney Int 2010;77:956–61. 10.1038/ki.2010.89 [DOI] [PubMed] [Google Scholar]