Abstract
The extracellular region of the nerve growth factor (NGF) receptor, TrkA, contains two immunoglobulin (Ig)-like domains that are required for specific ligand binding. We have investigated the possible role of these two Ig-like domains in receptor dimerization and activation by using different mutants of the TrkA extracellular region. Deletions of each Ig-like domain, of both, and of the entire extracellular region were made. To probe the structural constraints on ligand-independent receptor dimerization, chimeric receptors were generated by swapping the Ig-like domains of the TrkA receptor for the third or fourth Ig-like domain of c-Kit. We also introduced single-amino-acid changes in conserved residues within the Ig-like domains of TrkA. Most of these TrkA variants did not bind NGF, and their expression in PC12nnr5 cells, which lack endogenous TrkA, promoted ligand-independent neurite outgrowth. Some TrkA mutant receptors induced malignant transformation of Rat-1 cells, as assessed by measuring proliferation in the absence of serum, anchorage-independent growth, and tumorigenesis in nude mice. These mutants exhibited constitutive phosphorylation and spontaneous dimerization consistent with their biological activities. Our data suggest that spontaneous dimerization of TrkA occurs when the structure of the Ig-like domains is altered, implying that the intact domains inhibit receptor dimerization in the absence of NGF.
trkA is the prototype of a family of genes which also includes trkB and trkC, encoding tyrosine kinase receptors for the neurotrophins of the nerve growth factor (NGF) family. Thus, NGF is the preferred ligand for TrkA, brain-derived neurotrophic factor and NT4/NT5 are ligands for TrkB, and NT3 is the only known ligand for TrkC (2). Neurotrophins are responsible for the survival, differentiation, and maintenance of specific populations of neurons in the developing and adult nervous system (7). NGF-triggered TrkA signaling is required for the survival of sensory and sympathetic neurons. In human neuroblastomas, expression of trkA is a good prognostic marker, suggesting that lack of trkA expression contributes to malignancy; perhaps because it results in the loss of signaling pathways important for growth arrest and/or differentiation of the neural crest-derived cells from which these tumors originate (4). On the other hand, in some tumors an autocrine loop, NGF-TrkA, is responsible for tumor progression, as is the case in prostatic carcinoma, in which tumor growth can be blocked by TrkA kinase inhibitors (8). Consequently, TrkA gain-of-function mutations can result in oncogenesis (9, 10).
The extracellular region of TrkA is characterized by a number of distinct structural motifs (22). The amino-terminal sequence consists of three tandem leucine repeats (LRM) flanked by two cysteine clusters. Following the cysteine-rich region there are two immunoglobulin (Ig)-like C2 type domains which contribute significantly to NGF binding (14, 24, 33). As for other receptor tyrosine kinases, ligand-induced homodimerization and conformational changes of TrkA have been proposed as mechanisms for activation of its intrinsic tyrosine kinase activity, followed by transphosphorylation of the two receptor molecules present in the dimer (16).
trkA was originally isolated from a human colon carcinoma as a transforming oncogene activated by a somatic rearrangement that fused a nonmuscle tropomyosin gene with the receptor tyrosine kinase-encoding trkA gene (19, 20). Similar mechanisms are responsible for the malignant activation of trkA in a significant fraction of papillary thyroid carcinomas (9, 10). Different oncogenic forms of trkA have been also identified by transformation of NIH 3T3 cells in culture (6). Among them, one had a partial deletion of the sequences encoding the second Ig-like domain (trk-5 oncogene), and another was mutated in a conserved cysteine residue within the second Ig-like domain. The rest of the oncogenic forms lacked the sequences encoding the extracellular and transmembrane domains (6). The mechanisms by which these receptors are activated by ligand-independent modes are unknown. The existence of TrkA oncoproteins lacking the transmembrane domain indicated that this region is not required for the activation of the tyrosine kinase domain (2). However, exchanging the TrkA transmembrane domain with the corresponding region of other receptors, such as that for tumor necrosis factor receptor 2, yields nonfunctional receptors (5), suggesting that the transmembrane region of TrkA might be required to properly transduce the signals leading to receptor dimerization and autophosphorylation.
In an attempt to determine the mechanisms that regulate TrkA activation and to investigate how the Ig-like domains are involved in TrkA dimerization, we have studied the differentiating and oncogenic potential of several deletion and chimeric TrkA mutants, as well as those of some single-amino-acid mutations in conserved TrkA residues considered crucial to maintain the structure of the Ig-like domains. Our results indicate that the Ig-like domains of TrkA serve not only to bind NGF but also, in the absence of ligand, to inhibit dimerization.
MATERIALS AND METHODS
Generation of trkA mutants.
DNA manipulations were carried out by established protocols (1, 27). Escherichia coli DH5α was used as the host for propagation of plasmids. Strains CJ236 and MV1190 were used for in vitro mutagenesis. Base substitutions were introduced according to the Muta-gene in vitro mutagenesis kit protocol (Bio-Rad).
Deletion of each of the Ig-like domains was facilitated by introducing appropriate restriction sites. Using as a template the rat trkA cDNA with unique sites reported in a previous work (24), we introduced an SphI site at the codons for amino acids V284 and S285, which mark the end of the first Ig-like domain. The first Ig domain was eliminated by cutting with SphI and religating the vector, creating mutant TrkA-ΔIg1. In a similar way, introducing an XhoI site at the codon for residue P390 allowed us to easily eliminate the second Ig-like domain in TrkA (TrkA-ΔIg2), or both domains when the XhoI site was introduced into TrkA-ΔIg1 and then the second domain was eliminated, generating the TrkA-ΔIg1,2 mutant. The entire extracellular domain was eliminated while maintaining the signal peptide by introducing an MluI site at the codon for residue C36, cutting with this enzyme, and religating afterwards. Restriction sites were introduced by using oligonucleotide 5′ C CAA GTC AGC GCATGC TTC CCA GC 3′ to generate TrkA-ΔIg1, 5′ GAG TTC AAC CTCGAG GAC CCC 3′ for TrkA-ΔIg2, and 5′ TGC GCC GCA TCC ACGCGT GAG GTC 3′ to generate TrkA-ΔECD. All the chimeric receptors were constructed by PCR amplification of the c-Kit domains using primers with the appropriate restriction sites to allow exchange with the corresponding TrkA domains. The oligonucleotides used were: T-KIT4.1 forward, 5′ ACA ACC TTG GCATGC GTA GAA AAA GGA TTC 3′; T-KIT4.1 backward, 5′ CAC GAG CCT CTCGAG AGT CAG GAT TTC TGG 3′; T-KIT4.2 forward, 5′ AAG AAC ACT CTCGAG TTT GTA ACC GAT GGA G 3′; T-KIT4.2 backward, 5′ CGT CAG CTCGAG TGG TTT TGT GTT CAC GTA 3′; T-KIT3.2 forward, 5′ AGT CAC CTCGAG AAG AAA GGG GAC ACA 3′; and T-KIT3.2 backward, 5′ AGT GTT CTCGAG AGG GGA AGA TGT TGA TGA 3′.
To introduce single-amino-acid changes in the extracellular domain of TrkA, cDNA clones encoding single domains were used as the template. Once mutated and sequenced, they were used to replace the corresponding domains in the full-length wild-type rat trkA cDNA clone with unique sites, described previously (24). The mutagenic oligonucleotide primers, extending 10 bases on each side of the mismatch, were 5′ GAG GTG AGA AGC CAG GTG GA 3′ (L92V and L95V), 5′ C GTC TCC TTC GCA GCC AGT GTG 3′ (P287A), 5′ GAA GGG ATG GGA CCA GTG ATG 3′ (C302S), 5′ CG CAG GGA CGC TGC TGG CTG C 3′ (P313A), 5′ GCC GTT GAA GAA GAA GCG CAG GGA 3′ (W317A), 5′ CC GTT GAA GAA CGC GCG CAG GGA CGG 3′ (W317F), and 5′ CGG CAT GGC TCC CTT CGC CTC 3′ (C348S).
Cell culture and transfections.
The human embryonic epithelial kidney HEK293 and Rat-1 fibroblast cell lines were plated onto plastic tissue culture dishes in Dulbecco's modified Eagle's medium (DMEM) (Bio-Whittaker, Verviers, Belgium) supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, penicillin (50 U/ml), and streptomycin (50 μg/ml) (Bio-Whittaker). The rat adrenal pheochromocytoma PC12nnr5 cell line (11) was plated in DMEM supplemented with 6% heat-inactivated horse serum, 6% heat-inactivated fetal calf serum, penicillin (50 U/ml), streptomycin (50 μg/ml), and 2 mM l-glutamine. Cells were incubated at 37°C in a 95% air–5% CO2 atmosphere.
Plasmid DNA (15 μg) was transiently transfected into PC12nnr5 or HEK293 cells (5 × 106 cells/plate) following the calcium phosphate method, as described (27). Plasmid CMV-lacZ (3 μg), which contains the lacZ gene under the control of the cytomegalovirus (CMV) enhancer-promoter, was cotransfected and used as an internal control to normalize for transfection efficiency. For PC12nnr5 cell transfection, the DNA precipitate was left on the cells for 14 to 16 h before the glycerol shock was applied. trkA mutant constructs were stably transfected either in HEK293 cells, for biochemical studies, or in Rat-1 fibroblasts, for proliferation studies. Clones were selected with G418 (0.5 mg/ml) and analyzed for TrkA expression by immunoprecipitation and immunoblotting as described below.
Immunoprecipitation and immunoblot analysis.
Cells were lysed in a buffer containing 137 mM NaCl, 20 mM Tris-HCl (pH 8), 10% glycerol, 1% NP-40, 2 mM EDTA, and protease inhibitors (0.15 U of aprotinin per ml, 20 μM leupeptin, and 1 mM phenylmethylsulfonyl fluoride) at 4°C for 20 min. Immunoprecipitation was performed for 3 h at 4°C using 2 mg of total protein extract and the anti-203 pan-Trk antiserum (20) (1:1,000 dilution). After several washes, immunoprecipitates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blot with the pan-Trk antiserum (1:5,000) and horseradish peroxidase (HRP)-conjugated anti-rabbit Ig. Reactive protein bands were visualized by enhanced chemiluminescence detection (Amersham Corp.).
Constitutive phosphorylation of the mutant receptors was assayed by immunoprecipitation with the pan-Trk antiserum followed by Western blot using the monoclonal antiphosphotyrosine antibody 4G10 (1:40 dilution of the hybridoma culture supernatant).
Dimerization assay.
Spontaneous or NGF-induced dimerization of TrkA receptors was assayed by introducing two different epitopes in some of the receptor variants. Two hemagglutinin (HA) epitopes were introduced between amino acids 43 and 70, just after the signal peptide of the molecule. The sequence encoding the Myc epitope was introduced at the MluI site previously engineered into the rat trkA cDNA, after the second Ig-like domain (24).
Wild-type or mutant receptors carrying either two HA or Myc epitopes were cotransfected into HEK293 cells. At 36 h after transfection, dimerization of the receptors was analyzed by immunoprecipitation using the 12CA5 anti-HA antibody, followed by Western blotting with the 9E10 anti-Myc antibody (a generous gift from G. Evan).
[3H]thymidine incorporation.
Cells were seeded in 24-well plates at a density of 2 × 104 cells/well and incubated for 2 days in DMEM with 10% calf serum. Cells were starved for 22 h in medium with 0.2% serum and then treated with either NGF (100 ng/ml) or 10% calf serum or left in 0.2% serum for 12 h. At that time [3H]thymidine was added (0.5 μCi/ml), and incubation was continued for another 4 h at 37°C. Cells were washed with phosphate-buffered saline (PBS) containing 1 mM CaCl2 and 0.5 mM MgCl2, precipitated with 10% trichloroacetic acid, and resuspended in 0.1 ml of 0.2 N NaOH. The solution was neutralized by adding 0.1 ml of 0.2 N HCl. The amount of [3H]thymidine incorporated was quantitated by liquid scintillation counting.
Soft agar colony formation.
Rat-1 cell lines expressing mutant receptors were resuspended at a concentration of 103 cells in 2 ml of DMEM with 10% fetal calf serum and 0.5% agar at 45°C and overlaid onto plates containing 4 ml of solidified DMEM supplemented with 10% fetal calf serum and 1% agar. Plates were kept at 4°C for 5 min and incubated at 37°C for 21 days. Twice per week the cells were fed with 0.5 ml of DMEM plus 10% fetal calf serum.
Tumorigenesis in athymic nude mice.
Different Rat-1 cell lines expressing the mutant receptors were injected subcutaneously at 104 cells/mouse into 6-week-old female athymic Swiss nu/nu mice (two sites per mouse). Animals were housed under sterile conditions in a germ-free protected unit and fed ad libitum. Tumor growth was assessed weekly by caliper measurement of the tumor in three dimensions. When xenografts reached a mean diameter of 6 mm, the cell lines were scored as tumorigenic. Three mice were used for each cell line.
NGF binding studies.
Mouse submaxillary NGF (2.5S) was obtained from Harlan Scientific and radioiodinated by lactoperoxidase treatment as described previously (13). The specific activity ranged from 3,000 to 3,500 cpm/fmol. The [125I]NGF was used within 2 weeks of labeling. No proteolysis was detected by SDS-PAGE. [125I]NGF binding assays were performed in HEK293-derived cell lines expressing TrkA mutant receptors, and the dissociation constants (Kd) were determined by Scatchard plot analysis, as described (18, 24).
RESULTS
Generation of mutant and chimeric TrkA receptors.
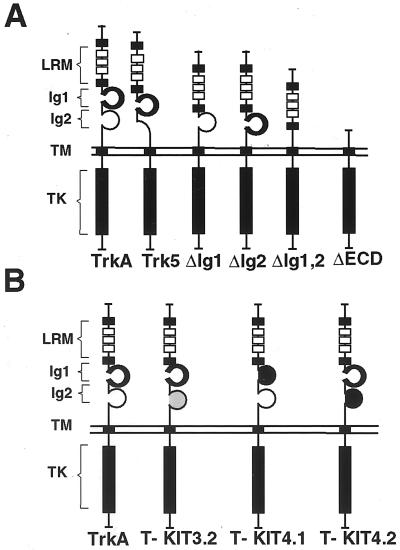
Like other receptor tyrosine kinases, TrkA undergoes dimerization and activation upon ligand binding. Thus, in the absence of NGF, some domains of the receptor, perhaps the same ones responsible for ligand binding, must be impeding its spontaneous dimerization at the cell surface. To investigate the contribution of the different domains within the extracellular region of TrkA to receptor function, each of the two Ig-like domains was individually deleted. Deletions of both Ig domains and of the entire extracellular domain (ECD) were also made (Fig. 1A).
FIG. 1.
Schematic representation of TrkA mutant receptors. (A) Mutants generated by deleting the Ig-like domains or the entire extracellular region. (B) TrkA/c-Kit chimeric receptors. TM, transmembrane domain; TK, tyrosine-kinase domain.
Since deletions of entire domains could alter the structure of the whole receptor, making it difficult to interpret their functional importance, we made some TrkA/c-Kit chimeric receptors (Fig. 1B) in which either of the two Ig-like domains of TrkA were replaced by the fourth Ig-like domain of the c-Kit receptor, which is involved in dimerization (3). As a control, we also made a chimeric receptor replacing the second Ig-like domain of TrkA with the third Ig domain of c-Kit, which is required for ligand binding but does not promote dimerization. [125I]NGF binding experiments demonstrated that neither the deletion nor the chimeric TrkA mutants were capable of binding to the ligand (data not shown).
Ligand-independent neurite formation by TrkA mutants in PC12nnr5 cells.
Ligand-activated TrkA promotes differentiation of the PC12 rat pheochromocytoma cell line. Addition of NGF to these cells causes them to stop proliferating and to acquire neurites in 2 to 3 days. We have used the PC12nnr5 mutant cell line, which does not express endogenous TrkA, to study the ability of TrkA mutants to induce differentiation in the absence of NGF.
Plasmids encoding mutant receptors were cotransfected with a plasmid carrying the lacZ gene. Three days after transfection, the percentage of transfected cells (β-galactosidase positive) bearing neurites at least twice the length of their cell bodies was scored in a blind fashion. Wild-type TrkA induced neurite formation in 7% of the transfected cells in the absence of NGF and in 60 to 70% of them when NGF was added to the medium. Surprisingly, the trk-5 oncogene induced ligand-independent neurite formation in only 38 to 40% of the transfected cells. The values obtained with the different mutant receptors were normalized to those obtained with the trk-5 oncogene, which was set at 100%, and are shown in Fig. 2. Deletion of the first Ig-like domain caused a slight but significant increase in neurite outgrowth in the absence of NGF. However, deletions including the second Ig domain (TrkA-ΔIg2, TrkA-ΔIg1,2, and TrkA-ΔECD) were considerably more active in this assay. These results suggest that the second Ig-like domain of TrkA plays a major role in preventing spontaneous dimerization of the receptor. Interestingly, receptors lacking the entire ECD were less active than those lacking only the Ig domains, suggesting a positive role for other sequences within the ECD in neurite outgrowth. It appears that deleting the two Ig-like domains eliminated a dimerization block and stimulated the differentiation activity of the receptor, whereas further deletions of the ECD reduce this activity. The activity of TrkA/c-Kit chimeric receptors confirmed the inhibitory role of the Ig-like domains of TrkA; thus, substitution of the first or second Ig domains of TrkA with an Ig-like domain that favors dimerization, such as the fourth domain of c-Kit (3), yielded a very active receptor, capable of strong ligand-independent differentiation activity. Again, replacing the second Ig-like domain of TrkA with the fourth Ig domain of c-Kit was more activating than replacing the first Ig domain. By contrast, replacing the second Ig-like domain of TrkA with the third Ig-like domain of the c-Kit receptor did not cause spontaneous differentiation activity (Fig. 2).
FIG. 2.
Ligand-independent neurite formation in PC12nnr5 cells transfected with TrkA mutant receptors. Neurite outgrowth was quantified 3 days after transfection by assessing the percentage of β-galactosidase-positive cells bearing neurites at least twice the length of their cell bodies. Results were normalized to the trk-5 oncogene response (set at 100%). Values were calculated from at least five independent experiments. Means and standard deviations (SD) are shown.
Dimerization of TrkA mutant receptors in the absence of NGF.
Dimerization is considered the mechanism responsible for initiating the activation of TrkA (16, 36). To determine whether the mutant receptors were capable of spontaneous dimerization, we tagged some of these mutants with either HA or Myc epitopes (Fig. 3A) and analyzed if they coimmunoprecipitated after transient cotransfection into HEK293 cells. Receptors were immunoprecipitated with anti-HA antibodies and detected by Western blotting using the 9E10 anti-Myc antibody. As a positive control, we cotransfected TrkA-HA and TrkA-Myc into HEK293 cells and treated them with NGF (100 ng/ml) for 5 min before lysis and immunoprecipitation. Neither the HA nor the Myc epitopes altered the affinity of wild-type TrkA for NGF (data not shown). The results obtained with wild-type TrkA and the deletion mutants TrkA-ΔIg1, TrkA-ΔIg2, and TrkA-ΔIg1,2 are shown in Fig. 3B. Anti-Myc immunoblot analysis of the anti-HA immunoprecipitates showed the presence of Myc-tagged TrkA proteins in the HA-TrkA immunoprecipitates of NGF-treated cells and in the HA-TrkA-ΔIg1, HA-TrkA-ΔIg2, and HA-TrkA-ΔIg1,2 immunoprecipitates in the absence of NGF. Therefore, significant spontaneous receptor dimerization occurs in those cells.
FIG. 3.
Ligand-independent dimerization of TrkA deletion mutants. (A) Schematic representation of HA- and Myc-tagged wild-type TrkA receptors. These epitopes were introduced in the same position within the different TrkA mutants. (B) Dimerization analysis in HEK293 cells transiently transfected with the following pairs of expression vectors: HA-TrkA and Myc-TrkA; HA-TrkA-ΔIg1 and Myc-TrkA-ΔIg1; HA-TrkA-ΔIg2 and Myc-TrkA-ΔIg2; and HA-TrkA-ΔIg1,2 and Myc-TrkA-ΔIg1,2. As a positive control, we used HA-TrkA- and Myc-TrkA-transfected cells treated with NGF (100 ng/ml) for 5 min. Two days after transfection, cells were lysed and immunoprecipitations (IP) were performed using the 12CA5 anti-HA antibody. Western blot was done with either 9E10 anti-Myc antibody (upper panel) or anti-203 pan-Trk antiserum (bottom panel). (C) Western blot of whole-cell extracts (40 μg of total protein) done with either 203 antiserum, 9E10, or 12CA5. Immunoreactive protein bands were detected by chemiluminescence. Sizes are shown in kilodaltons.
By contrast, Myc-TrkA could not be visualized in the anti-HA immunoprecipitates of wild-type HA-TrkA in the absence of NGF, whereas immunoblot of the immunoprecipitates with anti-203 (Fig. 3B, bottom panel) revealed that the level of receptor immunoprecipitated was very similar in all the samples. Additionally, whole-extract immunoblot analysis with either anti-203, anti-Myc, or anti-HA antibodies of all the transfected cells (Fig. 3C) showed that the level of expression was similar for all the receptors. These data demonstrated spontaneous dimerization of those receptor mutants that were capable of stimulating neurite outgrowth in the absence of NGF.
Ligand-independent phosphorylation of TrkA mutant.
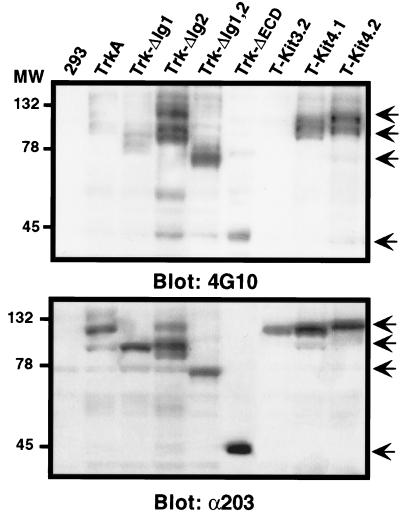
To confirm that the activated receptors were capable of spontaneous autophosphorylation, expression vectors for the wild-type and mutant receptors were transiently transfected into HEK293 cells. Two days after transfection, cells were lysed and extracts were analyzed by Western blot using the antiphosphotyrosine monoclonal antibody 4G10. As shown in Fig. 4 (upper panel), constitutive phosphorylation was observed for the TrkA deletion mutants except TrkA-ΔIg1 and for the chimeric mutants except T-Kit 3.2, which behaved like wild-type TrkA. These results were consistent with ligand-independent differentiating activity of the receptors, suggesting that this activity was due to their constitutive tyrosine autophosphorylation.
FIG. 4.
Tyrosine phosphorylation of TrkA mutant receptors in the absence of NGF. Expression vectors for the different mutants were transiently transfected into HEK293 cells. Two days after transfection, cells were lysed, and 50 μg of total protein extract was analyzed by Western blot using either 4G10 antiphosphotyrosine antibody (upper panel) or anti-203 pan-Trk antiserum (bottom panel). Sizes are shown in kilodaltons. Arrows indicate positions of the TrkA mutant receptors.
Proliferative potential of Rat-1 cell lines expressing TrkA mutants.
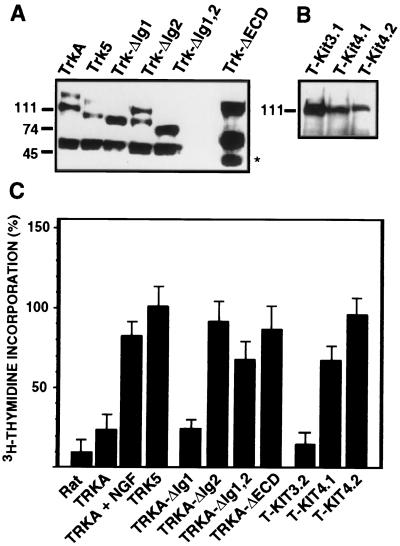
Wild-type and mutant trkA cDNAs were stably transfected in Rat-1 cells. Selected G418-resistant cell lines were isolated and analyzed for receptor expression by immunoprecipitation and Western blot using the 203 pan-Trk antiserum. A series of cell lines were used for further studies (Fig. 5A and B). To analyze the proliferative and oncogenic potentials of the TrkA mutants, we measured the rate of DNA synthesis as [3H]thymidine incorporation after serum starvation and the ability to grow in soft agar of Rat-1-derived cell lines expressing those mutant receptors. These experiments were also performed in the absence of NGF, since none of the deletion or chimeric receptors was capable of binding the ligand; therefore, we were analyzing the constitutive (i.e., ligand independent) activity of these receptor variants.
FIG. 5.
(A and B) Expression of mutant TrkA receptors in stably transfected Rat-1 cell lines. Immunoprecipitation and Western blot were performed on total cell extracts (2 mg of protein) using the 203 pan-Trk antiserum and HRP-labeled secondary anti-rabbit Ig antibody. Sizes are shown in kilodaltons. (C) [3H]thymidine incorporation by serum-starved Rat-1 cell lines stably expressing mutant TrkA receptors in the absence of NGF. Values are the means from at least five experiments performed with two independent clones of each mutant. Data are normalized to the [3H]thymidine incorporated by the cells in the presence of serum and expressed as a percentage of the incorporation measured in cells expressing the trk-5 oncogene (100%). Means and SD are shown.
The amount of [3H]thymidine incorporated was normalized to the incorporation reached by each clone in the presence of 10% serum. The results were expressed as percentage of the incorporation measured in cells expressing the trk-5 oncogene, which was set at 100% (Fig. 5C). All the mutants with deletions affecting the second Ig-like domain (TrkA-ΔIg2, TrkA-ΔIg1,2, and TrkA-ΔECD) stimulated cell proliferation to a level similar to that of the trk-5 oncogene, suggesting that the second Ig domain is required to prevent constitutive activation of the wild-type receptor. Deletion of the first Ig-like domain alone, TrkA-ΔIg1, did not have much proliferative effect (Fig. 5C).
Interestingly, receptors lacking the entire ECD were as proliferative as those lacking the second Ig domain, whereas they were not as active in the neuritogenesis assay. It appears that sequences in the ECD outside the Ig domains are required for full differentiating activity, but not for proliferation.
Cells expressing the chimeric T-Kit3.2 mutant receptor did not incorporate [3H]thymidine at levels above those of cells expressing wild-type TrkA. In contrast, cells expressing the T-Kit4.1 or T-Kit4.2 chimeric receptor, which contain the c-Kit dimerization domain, showed increased [3H]thymidine incorporation, indicating that those cells proliferate in the absence of serum. It seems that the fourth Ig-like dimerization domain of c-Kit has the opposite effect to that of the third Ig-like domain, which is a ligand-binding domain.
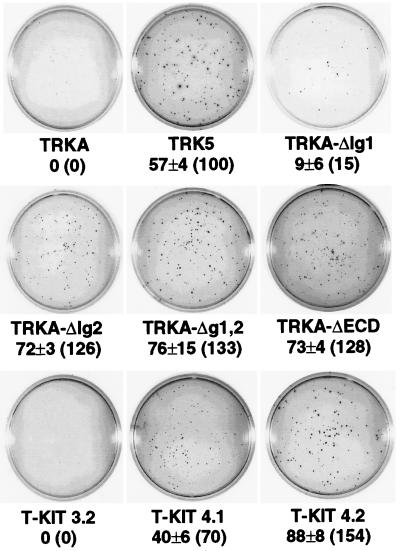
To further characterize the transforming ability of the different TrkA variants, we tested the ability of the Rat-1-derived lines to grow in soft agar. Cells (103) were cultured in soft agar for 21 days, and the number of growing colonies was scored as described in Materials and Methods. Data were normalized to the number of colonies formed by cells expressing the trk-5 oncogene (set at 100%), and the results obtained are shown in Fig. 6. Once more, cells expressing the TrkA-ΔIg1 mutant, carrying a deletion of the first Ig-like domain alone, were only slightly transforming (15% of the level with trk-5). Cells expressing the chimeric T-Kit3.2 mutant did not form any colonies. By contrast, cells expressing either mutants with deletions that include the second Ig-like domain or the T-Kit4.1 or T-Kit4.2 chimeric receptor induced a strong transforming activity, equivalent to or higher than that induced by trk-5. The results of the soft agar growth assay correlated well with those obtained in the thymidine incorporation assay and with the ability of the mutant receptors to undergo ligand-independent tyrosine phosphorylation (not shown).
FIG. 6.
Soft agar colony formation of Rat-1 cell lines expressing mutant TrkA receptors. Colonies were counted after 21 days in culture. Values are the means from at least five experiments performed with two independent clones of each mutant. The percentage of the foci formed by the trk-5-expressing clones (set at 100%) is shown in parentheses for each receptor.
In vivo tumorigenesis of TrkA mutant receptors.
We also assessed the ability of the different Rat-1 cell lines expressing TrkA mutant receptors to form tumors when injected into nude mice. Cells were injected subcutaneously, and animals were examined twice per week for tumor formation. A summary of the results is presented in Table 1. Neither normal Rat-1 cells nor cells expressing wild-type TrkA formed tumors. In contrast, all cells expressing deletion mutants formed progressively growing tumors, although there were differences in their aggressiveness. Thus, TrkA-ΔIg1 tumors developed very late (latency, 22 days), whereas TrkA-ΔIg2 and TrkA-ΔIg1,2 were extremely aggressive. Cells expressing the chimeric T-Kit3.2 mutant did not form tumors, whereas cells expressing either the T-Kit4.1 or T-Kit4.2 chimeric receptor caused tumors that were considerably more aggressive in the case of cells expressing T-Kit4.2. Overall, the tumorigenicity data correlated with those of the proliferation and soft agar growth assays and definitively demonstrated that altering the structure of the Ig-like domains leads to the constitutive, oncogenic activation of the TrkA receptor.
TABLE 1.
Tumor formation in nude mice caused by Rat-1 cells stably expressing mutant TrkA receptors
| Receptor | No. of mice with tumors/ no. of mice tested | Latency (days) |
|---|---|---|
| TrkA | 0/3 | |
| Trk-5 | 3/3 | 11 |
| TrkA-ΔIg1 | 3/3 | 22 |
| TrkA-ΔIg2 | 3/3 | 14 |
| TrkA-ΔIg1,2 | 3/3 | 16 |
| TrkA-ΔECD | 2/3 | 24 |
| T-Kit3.2 | 0/3 | |
| T-Kit4.1 | 3/3 | 22 |
| T-Kit4.2 | 3/3 | 14 |
Specific point mutations in the second Ig-like domain can cause spontaneous activation of TrkA.
From the results described above, it appears that the second Ig-like domain of TrkA plays a critical role in preventing spontaneous activation of the receptor. This is consistent with an important role of this domain in NGF binding. To gain insights on the role of specific residues within this domain, we generated several single-amino-acid mutations, aiming at altering its Ig-like structure. These mutations affected amino acids that are conserved in all members of the Trk family of receptors and that are considered important for maintaining the structure of the Ig-C2 type domains (29). Specifically, the cysteine residues that form the disulfide bridge (C302 and C348) were changed to serines, and the conserved P313 and W317 residues were changed to alanines (Fig. 7A). A tryptophan residue, separated approximately 15 amino acids from the first cysteine, is conserved in 80% of the Ig-C2 type domains, whereas P313 is conserved in only 30% of the Ig-C2 domains (29) but is present in all Trk receptors.
FIG. 7.
(A) Schematic representation of point mutations affecting individual amino acid residues within the extracellular region of TrkA. (B) Expression and ligand-independent phosphorylation of mutant TrkA receptors in stably transfected Rat-1 cell lines. Immunoprecipitation (IP) was performed on total cell extracts (2 mg of protein) using the anti-203 pan-Trk antiserum, followed by Western blot with either anti-203 antiserum (upper panel) or 4G10 antiphosphotyrosine monoclonal antibody (lower panel). Expression of TrkA in PC-12 cells (2 mg of protein) is shown for comparison. Sizes are shown in kilodaltons. (C) [3H]thymidine incorporation by Rat-1 cell lines stably expressing mutant TrkA receptors in the absence of serum. Values are the means from at least five experiments performed with two independent clones of each mutant. Data are normalized relative to the [3H]thymidine incorporated by the cells in the presence of serum and expressed as a percentage of the incorporation measured in cells expressing the trk-5 oncogene (100%). Means and SD are shown.
We also mutated residue P287, located at the beginning of the second Ig-like domain. Proline residues are present at the beginning of 30% of the Ig-like domains (29). As a control, we generated the L92V L95V double mutation that disrupts the first leucine repeat. This domain of the TrkA receptor is not required for ligand binding (24, 33).
We analyzed whether those mutations affected NGF binding to the receptor, since that could correlate with the alteration of the domain structure and the capacity to activate the receptor. Equilibrium [125I]NGF binding assays were performed using HEK293-derived clones, and the dissociation constants (Kd), calculated from Scatchard analysis, are shown in Table 2. As expected, the double mutation L92V L95V, altering the first leucine repeat, did not affect NGF binding. The mutation P287A, in the region between the two Ig-like domains, did not affect NGF binding either. Interestingly, mutations affecting amino acids within the Ig-like domain had different effects; mutation C302S completely abolished binding; by contrast, mutation C348S had no effect on NGF binding. According to the crystal structure of the NGF-bound domain (36), these two cysteines form a noncanonical disulfide bridge on the surface of the Ig domain, contributing to some hydrophobic interactions between residues I6 of NGF and V294 and L333 of TrkA. Our results suggest that C302 is important to maintain that interaction, whereas C348 and the disulfide bridge are not.
TABLE 2.
[125I]NGF binding to HEK293 cells stably expressing TrkA mutant receptorsa
| Receptor | Bindingb | Mean Kd (nM) ± SEM |
|---|---|---|
| TrkA | +++ | 0.9 ± 0.1 |
| L92V L95V | +++ | 1.0 ± 0.1 |
| P287A | +++ | 1.2 ± 0.1 |
| C302S | NB | NB |
| P313A | +++ | 1.0 ± 0.2 |
| W317A | + | 2.5 ± 0.3 |
| W317F | ++ | 1.7 ± 0.2 |
| C348S | +++ | 0.7 ± 0.1 |
Cells were washed and resuspended in PBS with bovine serum albumin and glucose at a concentration of 2 × 106 cells/ml. Binding under equilibrium conditions was carried out with different [125I]NGF concentrations for 2 h at 1°C. The values were calculated from triplicate determinations. Nonspecific ligand binding, which was determined in the presence of a 100-fold excess of unlabeled NGF, was subtracted from the total amount of cell-bound NGF.
+++, 80 to 100% TrkA binding; ++, 40 to 80% TrkA binding; +, 20 to 40% TrkA binding; NB, no binding.
Mutations W317A and W317F decreased NGF binding (Kd, 2.5 × 10−9 and 1.7 × 10−9 M, respectively), whereas the P313A mutation had no effect. According to the published crystal structure (36), these residues are not in contact with the NGF, but the tryptophan residue seems to be required to keep the Ig-like structure that allows NGF binding.
Stable Rat-1-derived cell lines expressing low levels of the single-amino-acid mutant receptors were generated, and the phosphorylation state of those receptors was assessed in the absence of NGF (Fig. 7B).
These cell lines displayed variable [3H]thymidine incorporation and proliferation responses (Fig. 7C). As expected, cells expressing the L92V L95V mutant did not show any [3H]thymidine incorporation in the absence of serum or NGF. Cells expressing the P287A mutant were highly proliferative. All the mutations in the second Ig-like domain stimulated proliferation except P313A and C348S, with the W317A mutant incorporating even more [3H]thymidine than the trk-5 oncogene mutant. This tryptophan residue seems to be required to maintain the Ig-like structure that appears to be necessary to block spontaneous activation; thus, the more conservative W317F mutation induced considerably less proliferation than W317A.
The number of colonies formed in soft agar by cell lines expressing single-amino-acid mutant receptors and the in vivo tumorigenicity of those cells are shown in Table 3. Cells expressing the L92V L95V double mutant did not grow in soft agar or form tumors in nude mice. The P287A mutant was highly transforming. As for the mutants within the second Ig-like domain, C302S and W317A were transforming, whereas P313A, C348S, and the conservative mutant W317F hardly formed any colonies in soft agar and cells expressing the C348S mutant were only mildly tumorigenic in nude mice.
TABLE 3.
Soft agar colony growth and tumor formation in nude mice caused by Rat-1 cells stably expressing mutant TrkA receptors
| Receptor | Mean no. of foci/ 103 cells ± SEM (% of control)a | No. of mice with tumors/ no. of mice tested | Latency (days) |
|---|---|---|---|
| TrkA | 0 (0) | 0/3 | |
| Trk-5 | 57 ± 4 (100) | 3/3 | 11 |
| L92V L95V | 0 (0) | 0/3 | |
| P287A | 40 ± 8 (70) | 3/3 | 11 |
| C302S | 24 ± 4 (42) | 2/3 | 24 |
| P313A | 4 ± 3 (7) | ND | ND |
| W317A | 45 ± 5 (79) | 3/3 | 18 |
| W317F | 6 ± 2 (11) | ND | ND |
| C348S | 6 ± 2 (11) | 3/3 | 32 |
Colonies were counted after 21 days in culture. Values are the means ± SD from at least five experiments performed with two independent clones of each mutant. Data normalized to the number of colonies formed by the trk-5-expressing clones (100%) are shown in parentheses. For tumor formation, 2 × 104 cells were injected per animal. ND, not determined.
In summary, the results obtained with the single-amino-acid TrkA variants were consistent with those obtained with the deletion and chimeric mutant receptors and indicated that the second Ig domain plays a critical role in preventing spontaneous receptor activation and that the Ig-like structure is required for that role.
DISCUSSION
Uncontrolled cell proliferation has commonly been associated with deregulated activity of oncogenes. Receptor tyrosine kinases, like many other signaling proteins, can function as oncoproteins. Upon ligand binding, wild-type receptor tyrosine kinases undergo dimerization or conformational changes or both (12, 15, 30), and those changes lead to the activation of the intrinsic protein tyrosine kinase activity that causes the trans-autophosphorylation of the receptor and the phosphorylation of other substrates.
Constitutive, ligand-independent activation of a tyrosine kinase receptor can occur by different mechanisms. In the present study, we have used a series of mutations in the extracellular region of TrkA in order to gain insights into the molecular basis of the activation of this receptor. In particular we have evaluated the hypothesis that the two Ig-like ligand-binding domains might be involved in blocking receptor homodimerization in the absence of ligand. To achieve this we have engineered several types of mutations and analyzed these TrkA mutant receptors in different functional assays. PC12nnr5 neurite outgrowth allowed us to analyze the ability of mutant receptors to induce differentiation in a neural environment, whereas their transforming potential was assessed by analyzing [3H]thymidine incorporation, soft agar growth, and in vivo tumorigenesis of Rat-1-derived cell lines expressing TrkA mutants. The results obtained from these different assays gave a consistent picture: elimination of the first or second Ig-like domain leads to oncogenic activation of the receptor. This suggests a role for both Ig-like domains in the stabilization of the monomeric form of the receptor, perhaps through repulsion that can be negated by ligand binding. The activation caused by deletion of the first Ig-like domain was weak in all the assays compared to that caused by deleting the second domain. The second Ig-like domain appears to be more critical in preventing spontaneous receptor dimerization, and this correlates with the more important role played by this domain in NGF binding (24, 33, 34, 36). In platelet-derived growth factor receptors, Ig-like domains 1 to 3 participate in ligand binding; however, only deletion of the Ig-like domain 3 caused oncogenic activation (32).
Chimeric receptors between TrkA and c-Kit demonstrated that dimerization could also be achieved by retaining the Ig-like structure but exchanging the TrkA Ig-like domains for a c-Kit domain responsible for dimerization. It has been postulated that in c-Kit (stem cell factor receptor), the fourth Ig-like domain favors receptor dimerization once the ligand is bound to Ig-like domains 1 to 3 (3, 23). Substitution of the second Ig domain of TrkA with the third Ig domain of c-Kit (T-Kit3.2) did not cause spontaneous activation, whereas its substitution with the fourth c-Kit domain (T-Kit4.2) renders the receptor highly oncogenic in the absence of NGF. The weaker activation of T-Kit4.1 than of T-Kit4.2 indicated again that the second Ig-like domain of TrkA provides a stronger barrier to spontaneous dimerization.
Our results showed similar but not identical requirements for cell transformation in fibroblasts and for differentiation in PC12 cells. Mutants lacking the entire ECD of TrkA were less active in neuritogenesis than mutants lacking only the Ig-like domains. This suggests that there are sequences in the ECD, perhaps the LRM, which play a positive role in differentiation. Consistent with this model, deleting the two Ig-like domains eliminated a dimerization block and stimulated the differentiation activity of the receptor, whereas further deletions of the ECD reduced this activity. This effect was not observed in proliferation assays, where deletion of the entire ECD had the same activating effect as the deletion of Ig domains 1 and 2. It appears that there are sequences in the ECD, outside the Ig domains, that play a positive role in differentiation activity but are not required to stimulate cell proliferation or transformation. Some observations suggest that the LRM leucine repeats may be responsible for this effect. Thus, mutant L92V L95V exhibits reduced ligand-induced neuritogenesis compared to the wild-type receptor in spite of having the same affinity for NGF (not shown). This is also consistent with the results reported previously (17).
The biological activities of the single-amino-acid mutants within the second Ig domain of TrkA corroborated the results obtained with the deletion and chimeric receptors and added information on the role of specific residues within this domain. The importance of W317 has been unveiled. The different activities of mutants C302S and C348S are difficult to interpret, since these residues form a disulfide bridge in the external part of the domain that is different from that found in canonical Ig domains (31, 36). Some activating mutations in the fibroblast growth factor receptor (FGFR) family result in the loss of a cysteine residue that disrupts disulfide bond formation between the two highly conserved cysteines of the third Ig-like domain (22). These mutations give rise to an unpaired cysteine residue that may form an intermolecular disulfide bond (26). The fact that mutation C302S in TrkA is considerably more activating than C348S could be explained by invoking a similar mechanism by which the first cysteine would be capable of forming intermolecular disulfide bonds more easily than C348. However, the double mutation C302S C348S is also activating (data not shown). This fact, together with the crystal structure of the NGF-bound domain, suggests that the unusual disulfide bridge formed by these two cysteine residues, in the external face of the domain, is not necessary to maintain the structure of the domain and that residue C302 is required to bind NGF and to keep the monomeric form of the receptor in the absence of ligand, whereas C348S is not.
It is intriguing that the point mutation P287A, affecting a proline conserved only in some Ig-like domains that allows binding of NGF with an affinity similar to that of the wild type receptor, can also induce ligand-independent TrkA-mediated transformation in Rat-1 fibroblasts. A mechanism other than alteration of the Ig-like structure must be responsible for the spontaneous dimerization of this mutant receptor. Some of the activating mutations in the colony stimulating factor-1 (CSF-1) receptor fall near the fourth Ig-like domain (35), and these mutant receptors are able to bind and respond to macrophage CSF M-CSF. Similarly, the only mutation identified in the human FGFR1 is a P252R substitution in the linker region between Ig-II and Ig-III domains that is associated with the Pfeiffer syndrome (21). Interestingly, this mutant receptor seems to bind slightly more radiolabeled FGF than the wild-type receptor (22). An analogous mutation has been identified in FGFR2 in individuals with Apert syndrome (37). Recent crystallization of the FGFR2 Ig-II and Ig-III domains bound to FGF2 has shown that the linker region between domains is important for the structure of the receptor (25). Only the second Ig-like domain of TrkA, bound to NGF, has been crystalized (36); therefore, the effect of mutations within this region cannot be interpreted.
In addition to ligand-receptor contacts, direct receptor- receptor interactions might contribute to dimerization. Our results indicate that the equilibrium between attractive and repulsive forces that is necessary to block TrkA receptor dimerization in the absence of ligand is achieved through the two Ig-like ligand-binding domains. As we have discussed above, indications exist pointing to a role of the extracellular domain in positively regulating TrkA receptor activity, since truncation of the entire ectodomain resulted in an activated receptor that was, however, less biologically active than other mutant receptors in its ability to stimulate neurite outgrowth.
In summary, the data presented here indicate that the ECD of TrkA exerts multiple regulatory effects on the intracellular catalytic domain by mechanisms involving ligand-induced dimerization and also by inhibiting spontaneous receptor dimerization and phosphorylation.
ACKNOWLEDGMENTS
We thank M. Sacristan for valuable discussions and for technical assistance and Annette Duwel for helping to generate the Myc-tagged TrkA. We also thank A. Pandiella for his comments on the manuscript.
J. C. Arevalo was the recipient of a predoctoral fellowship from the BIO4-CT96-0285 program. This work was supported by grants from the Spanish Ministry for Education, DGICYT PB94-1104, Fundacion Ramon Areces, European Union Program BIO4-CT96-0285, and NATO CRG 973118.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1995. [Google Scholar]
- 2.Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 3.Blechman J M, Lev S, Barg J, Eisenstein M, Vaks B, Vogel Z, Givol D, Yarden Y. The fourth immunoglobulin domain of the stem cell factor receptor couples ligand binding to signal transduction. Cell. 1995;80:103–113. doi: 10.1016/0092-8674(95)90455-7. [DOI] [PubMed] [Google Scholar]
- 4.Brodeur G M, Maris J M, Yamashiro D J, Hogarty M D, White P S. Biology and genetics of human neuroblastomas. J Pediatr Hematol Oncol. 1997;19:93–101. doi: 10.1097/00043426-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Canossa M, Rovelli G, Shooter E M. Transphosphorylation of the neurotrophin Trk receptors. J Biol Chem. 1996;271:5812–5818. doi: 10.1074/jbc.271.10.5812. [DOI] [PubMed] [Google Scholar]
- 6.Coulier F, Kumar R, Ernst M, Klein R, Martin-Zanca D, Barbacid M. Human trk oncogenes activated by point mutation, in-frame deletion, and duplication of the tyrosine kinase domain. Mol Cell Biol. 1990;10:4202–4210. doi: 10.1128/mcb.10.8.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies A M. The role of neurotrophins in the developing of nervous system. J Neurobiol. 1994;25:1334–1348. doi: 10.1002/neu.480251103. [DOI] [PubMed] [Google Scholar]
- 8.Delsite R, Djakiew D. Antiproliferative effect of the kinase inhibitor K252a on human prostatic carcinoma cell lines. J Androl. 1996;17:481–490. [PubMed] [Google Scholar]
- 9.Greco A, Miranda C, Pagliardini S, Fusetti L, Bongarzone I, Pierotti M A. Chromosome 1 rearrangements involving the genes TPR and NTRK1 produce structurally different thyroid specific TRK oncogenes. Genes Chromosomes Cancer. 1997;19:112–123. [PubMed] [Google Scholar]
- 10.Greco A, Fusetti L, Miranda C, Villa R, Zanotti S, Pagliardini S, Pierotti M A. Role of the TGF N-terminus and coiled-coil domain in the transforming activity of the thyroid TRK-T3 oncogen. Oncogene. 1998;16:809–816. doi: 10.1038/sj.onc.1201596. [DOI] [PubMed] [Google Scholar]
- 11.Green S H, Rydel R E, Connolly J L, Greene L A. PC12 cell mutants that possess low but not high affinity nerve growth factor receptors neither respond to nor internalize nerve growth factor. J Cell Biol. 1986;102:830–843. doi: 10.1083/jcb.102.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heldin C H. Dimerization of cell surface receptor in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- 13.Hempstead B L, Schleifer L, Chao M. Expression of functional nerve growth factor receptors after gene transfer. Science. 1989;243:373–375. doi: 10.1126/science.2536190. [DOI] [PubMed] [Google Scholar]
- 14.Holden P H, Asopa V, Robertson A G S, Clarke A R, Tyler S, Bennett G S, Brain S D, Wilcock G K, Allen S J, Smith S K F, Dawbarn D. Immunoglobulin-like domains define the nerve growth factor binding site of the TrkA receptor. Nat Biotechnol. 1997;15:668–672. doi: 10.1038/nbt0797-668. [DOI] [PubMed] [Google Scholar]
- 15.Jiang G, Hunter T. Receptor signaling: when dimerization is not enough. Curr Biol. 1999;9:R568–R571. doi: 10.1016/s0960-9822(99)80357-1. [DOI] [PubMed] [Google Scholar]
- 16.Jing S, Tapley P, Barbacid M. Nerve growth factor mediates signal transduction through trk homodimer receptors. Neuron. 1992;9:1067–1079. doi: 10.1016/0896-6273(92)90066-m. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald J I S, Meakin S O. Deletions in the extracellular domain of rat TrkA lead to an altered differentiative phenotype in neurotrophin responsive cells. Mol Cell Neurosci. 1996;7:371–390. doi: 10.1006/mcne.1996.0027. [DOI] [PubMed] [Google Scholar]
- 18.Mahadeo D, Kaplan L, Chao M V, Hempstead B L. High affinity nerve growth factor binding displays a faster rate of association than p140trk binding. J Biol Chem. 1994;269:6884–6891. [PubMed] [Google Scholar]
- 19.Martin-Zanca D, Hughes S, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature. 1986;319:743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- 20.Martín-Zanca D, Oskam R, Mitra G, Copeland T, Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989;9:24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muenke M, Schell U, Hehr A, Robin N H, Losken H W, Schinzel A, Pulleyn L J, Rutland P, Reardon W, Malcolm S, Winter R M. A common mutation in the fibroblast growth receptor 1 gene in Pfeiffer syndrome. Nat Genet. 1994;8:269–274. doi: 10.1038/ng1194-269. [DOI] [PubMed] [Google Scholar]
- 22.Neilson K M, Friesel R. Ligand-independent activation of fibroblast growth factor receptors by point mutations in the extracellular, transmembrane and kinase domains. J Biol Chem. 1996;271:25049–25057. doi: 10.1074/jbc.271.40.25049. [DOI] [PubMed] [Google Scholar]
- 23.Omura T, Heldin C H, Ostman A. Immunoglobulin-like domain 4-mediated receptor-receptor interactions contribute to platelet-derived growth factor-induced receptor dimerization. J Biol Chem. 1997;272:12676–12682. doi: 10.1074/jbc.272.19.12676. [DOI] [PubMed] [Google Scholar]
- 24.Perez P, Coll P M, Hempstead B L, Martin-Zanca D, Chao M V. NGF binding to the trk tyrosine kinase receptor requires the extracellular immunoglobulin-like domains. Mol Cell Neurosci. 1995;6:97–105. doi: 10.1006/mcne.1995.1010. [DOI] [PubMed] [Google Scholar]
- 25.Plotnikov A N, Schlessinger J, Hubbard S R, Mohammadi M. Structural basis for FGF receptor dimerization and activation. Cell. 1999;98:641–650. doi: 10.1016/s0092-8674(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 26.Robertson S C, Meyer A N, Hart K C, Galvin B D, Webster M K, Donoghue D J. Activating mutations in the extracellular domain of the fibroblast growth factor receptor 2 function by disruption of the disulfide bond in the third immunoglobulin-like domain. Proc Natl Acad Sci USA. 1998;95:4567–4572. doi: 10.1073/pnas.95.8.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Schneider R, Schweiger M. A novel modular mosaic of cell adhesion motifs in the extracellular domains of the neurogenic trk and trk B tyrosine kinase receptors. Oncogene. 1991;6:1807–1811. [PubMed] [Google Scholar]
- 29.Smith D K, Xue H. Sequence profiles of immunoglobuline and immunoglobulin-like domains. J Mol Biol. 1997;274:530–545. doi: 10.1006/jmbi.1997.1432. [DOI] [PubMed] [Google Scholar]
- 30.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 31.Ultsch M H, Wiesmann C, Simmons L C, Henrich J, Yang M, Reilly D, Bass S H, de Vos A. Crystal structures of the neurotrophin-binding domain of TrkA, TrkB and TrkC. J Mol Biol. 1999;290:149–159. doi: 10.1006/jmbi.1999.2816. [DOI] [PubMed] [Google Scholar]
- 32.Uren A, Yu J C, Karcaaltincaba M, Pierce J H, Heidaran M A. Oncogenic activation of the αPDGFR defines a domain that negatively regulates receptor dimerization. Oncogene. 1997;14:157–162. doi: 10.1038/sj.onc.1200810. [DOI] [PubMed] [Google Scholar]
- 33.Urfer R, Tsoulofas P, O'Connell L, Shelton D L, Parada L F, Presta L G. An immunoglobulin-like domain determines the specificity of neurotrophin receptors. EMBO J. 1995;14:2795–2805. doi: 10.1002/j.1460-2075.1995.tb07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urfer R, Tsoulfas P, O'Connell L, Hongo J A, Zhao W, Presta L G. High resolution mapping of the binding site of TrkA for nerve growth factor and TrkC for neurotrophin-3 on the second immunoglobulin-like domain of the Trk receptors. J Biol Chem. 1998;273:5829–5840. doi: 10.1074/jbc.273.10.5829. [DOI] [PubMed] [Google Scholar]
- 35.Van Daalen Wetters T, Hawkins S A, Roussel M F, Sherr C J. Random mutagenesis of CSF-1 receptor (FMS) reveals multiple sites for activating mutations within the extracellular domain. EMBO J. 1992;11:551–557. doi: 10.1002/j.1460-2075.1992.tb05086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiesmann C, Ultsch M H, Bass S H, de Vos A M. Crystal structures of the nerve growth factor in complex with the ligand-binding domain of the TrkA receptor. Nature. 1999;401:184–188. doi: 10.1038/43705. [DOI] [PubMed] [Google Scholar]
- 37.Wilkie A O M, Slaney S F, Oldrigde M, Poole M D, Ashworth G J, Hockley A D, Hayward R D, David D J, Pulleyn L, Rutland P. Apert syndrome results from localized mutations of IGFR2 and is allelic with Crouzon syndrome. Nat Genet. 1995;9:165–172. doi: 10.1038/ng0295-165. [DOI] [PubMed] [Google Scholar]