Learning objectives:
By reading this article, you should be able to:
-
•
Describe the normal physiological mechanism of human adaption to hypobaric hypoxia at high altitudes.
-
•
Name the most relevant measures to prevent high-altitude illnesses.
-
•
Discuss the symptoms and clinical signs of high-altitude cerebral and pulmonary oedema and be able to plan appropriate treatment.
Key points.
-
•
Hypobaric hypoxia is the primary trigger for altitude acclimatisation and high-altitude illnesses.
-
•
Hypobaric hypoxia at extreme altitudes is associated with substantial reductions in arterial oxygen saturation, maximal oxygen uptake and maximal aerobic capacity.
-
•
Acclimatisation improves hypoxaemia; metabolic compensation of respiratory alkalosis contributes to preserving respiratory drive.
-
•
Gradual ascent to high altitudes is the key to preventing high-altitude illness.
-
•
High-altitude cerebral and pulmonary oedema are life-threatening conditions that mandate immediate descent and pharmacological treatment.
Mountaineering activities at extreme altitudes are increasingly popular and a growing number of mountaineers seek summits of peaks higher than 5000 m. The Himalayan Database, which documents known attempts on peaks higher than 5000 m, reported 13,975 ascents for the period of 2015–2019 in Nepal, up from 8039 from 20 yrs prior.1
Barometric pressure and oxygen partial pressure (Po2) decrease roughly linearly with increasing altitude but also with lower temperature. Oxygen partial pressure decreases to 50% of the sea level value at 5500 m and to 30% of the sea level value at 8849 m, the summit altitude of Mount Everest.
Oxygen partial pressure is not a limiting factor for oxygen delivery at sea level. However, with increasing altitude the reduction in inspired Po2 reduces the driving pressure for gas exchange in the lungs resulting in progressive hypoxaemia. This form of hypoxia is termed hypobaric hypoxia, and it represents a state were ambient Po2 becomes the dominant limiting factor for oxygen delivery to the tissues and mitochondria.2
Hypobaric hypoxia triggers multiple acclimatisation processes. Hypoxia is the cause of all high-altitude illnesses (HAIs).3,4 Climbing at extreme altitudes is associated with a substantial risk of mortality. In one study of climbers attempting Mount Everest, the mortality rate among mountaineers above base camp was 1.3%, but was 3.4% in climbers who reached the summit.5
High-altitude acclimatisation
Acclimatisation refers to the normal compensatory responses to acute hypobaric hypoxia and consists of a complex series of physiologic adaptations involving multiple organ systems. Acclimatisation begins within minutes of reaching a new altitude and reaches a steady state after several weeks. Acclimatisation improves but does not normalise hypoxaemia. Complete adaptation is not possible at altitudes above 5500 m. Past acclimatisation (e.g. previous expeditions) does not shorten or enhance subsequent acclimatisation periods. Acclimatisation is lost several weeks after descending from high altitude.
Acclimatisation involves two main processes. Firstly, hypoxaemia improves because of increased alveolar ventilation, which increases the efficiency with which oxygen moves down the oxygen cascade from inspired gas to the mitochondria. However, increased alveolar ventilation results in respiratory alkalosis. Hence, secondary processes occur to normalise acid–base homeostasis.6
Ventilatory response and renal compensation
Within minutes of reaching an increased altitude and as a result of the concomitant decrease in arterial oxygen saturation (Sao2), hypoxic stimulation of chemoreceptors in the carotid and aortic bodies triggers an increase in minute ventilation. The extent of ventilatory increase is determined by the magnitude of the hypoxic ventilatory response (HVR). Hypoxic ventilatory response varies between individuals and is genetically determined. As alveolar ventilation increases, alveolar carbon dioxide tension (Paco2) decreases. It is important to appreciate that for the same ambient Po2, a lower alveolar Paco2 is associated with a higher alveolar Pao2, resulting in a higher arterial oxygen tension (Pao2). The decrease in alveolar Paco2 leads to a decrease in arterial Paco2 and an increase in arterial pH (respiratory alkalosis). The central chemoreceptors in the cerebral medulla respond to alkalaemia by inhibiting ventilation, thereby limiting the full HVR.
Within the renal tubular cells, carbonic anhydrase catalyses the interconversion of CO2 and H2O with H2CO3, which then dissociates into HCO3− and H+. Respiratory alkalosis inhibits carbonic anhydrase activity and leads to a reduction of H+ secretion and increased HCO3− excretion by the kidneys, resulting in a reduced plasma HCO3− concentration and decreased arterial pH. The (partial) correction of alkalaemia reduces the inhibition of the respiratory drive, allowing further hypoxia-induced increases in minute ventilation to occur. Renal compensation of respiratory alkalosis occurs after several days at the same altitude, which is when minute ventilation reaches a maximum.
It is hypothesised that a pronounced HVR is advantageous in accelerating acclimatisation, decreasing the risk of HAI and improving oxygenation and physical performance. However, this has not been consistently demonstrated in high-altitude climbers. Uncertainty regarding the benefits of a pronounced HVR might be explained by the fact that HVR only determines the magnitude of ventilatory response to acute hypoxaemia, which is only one of the factors contributing to the complex mechanisms of ventilatory acclimatisation at high altitudes.
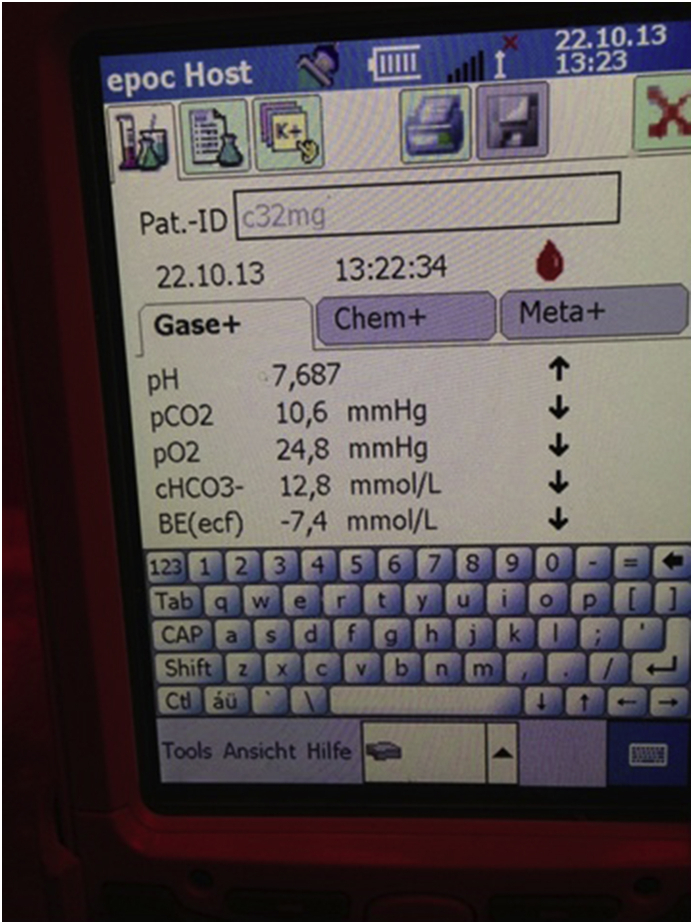
At extreme altitudes (above 5500 m), acclimatisation only improves hypoxaemia to a very limited extent and full compensation of alkalaemia does not occur. Climbers remain in a state of profound hypoxaemia and have severe acid–base imbalance (Fig. 1).
Fig 1.
Blood gas measurements at extreme altitude. The image shows the blood gas analysis of a climber after ascending and spending a night at 7020 m. The results indicate the presence of profound hypoxaemia and severe respiratory alkalosis with only partial renal compensation. The Spo2 measured with a pulse oximeter was 53%.
Circulatory adaptations
Acute exposure to hypobaric hypoxia leads to increased activity of the sympathetic nervous system, resulting in tachycardia, increased cardiac output and hypertension. Increased cardiac output helps maintain tissue oxygen delivery despite a decrease in Sao2. However, an increase in cardiac output may be attenuated by hypovolaemia, which can develop because of increased respiratory water loss associated with hyperventilation and increased renal fluid losses associated with renal compensation of alkalaemia. Hypovolaemia is more likely if climbers do not offset losses by increasing their intake of fluids. At moderate altitudes, systemic circulatory values return to baseline with progressive acclimatisation, whereas at extreme altitudes, the increased HR, cardiac output and arterial BP persist.
Pulmonary arteriolar smooth muscle cells respond to hypoxia by contracting, a process termed hypoxic pulmonary vasoconstriction (HPV). Hypoxic pulmonary vasoconstriction is an important mechanism for minimising ventilation–perfusion mismatching—and, therefore, for maintaining arterial oxygenation—in the context of regional abnormalities in ventilation. However, hypobaric hypoxia causes a marked increase in resistance across the whole pulmonary vascular bed, leading to increased pulmonary artery pressure. As with HVR, the magnitude of HPV is genetically determined and differs between individuals.
Hypobaric hypoxia and hypocapnia have opposing effects on the cerebral circulation. Hypoxia causes vasodilation, whereas hypocapnia causes vasoconstriction. Despite individual differences, vasodilation predominates over vasoconstriction, leading to increased cerebral blood flow.7 Cerebral oxygen delivery is determined by both blood flow and arterial oxygen content. Increased cerebral blood flow (combined with higher haemoglobin concentrations, see below) mitigates the effect of arterial hypoxaemia, helping to maintain cerebral oxygen delivery even at extreme altitudes.8 However, as a consequence, the volumes of the cerebral arterial and venous compartments are increased, as is total brain tissue volume.
Haematological response
Hypoxia stimulates the release of erythropoietin from renal cells, which stimulates erythropoiesis and leads to an increase in haemoglobin concentration within about 2 weeks. Higher haemoglobin concentration mitigates the decrease in Sao2 and helps maintain the oxygen content of arterial blood.
When starting an ascent from sea level, the initial reduction in Pao2 results in a relatively small decrease in Sao2 because of the sigmoid shape of the oxygen-haemoglobin dissociation curve. At higher altitudes, a further decrease in Pao2 of the same magnitude results in a much larger reduction in Sao2. This means that an ascent of 2500 m from sea level will only reduce Sao2 to ~92%, whereas a further 2500 m ascent to 5000 m will cause a much more pronounced decrease, to ~75–80%.
Alkalaemia causes a leftward shift of the oxygen–haemoglobin dissociation curve. However, alkalaemia is a potent stimulus for the formation of 2,3-diphosphoglycerate (2,3-DPG) by red blood cells, which shifts the dissociation curve to the right. Even at high altitudes, once acclimatisation has occurred, the opposing effects of alkalosis and increased 2,3-DPG production balance out. At extreme altitudes, the effect of alkalaemia predominates. The resulting leftward shift of the oxygen–haemoglobin dissociation curve facilitates binding of oxygen to haemoglobin in the lung, leading to an increase in Sao2.
Performance
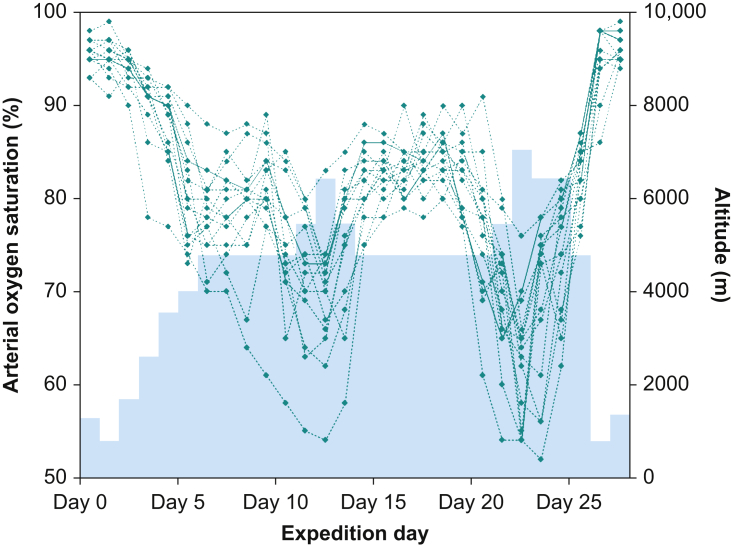
As a consequence of increased alveolar ventilation, values for maximal oxygen uptake (o2max) can be maintained up to an altitude of about 1500 m. Further ascent leads to a diffusion-limited reduction in arterial oxygen content. Figure 2 shows daily Spo2 measurements in 15 participants of a research expedition to 7000 m, illustrating the extent of hypoxaemia at extreme altitudes.
Fig 2.
Peripheral arterial oxygen saturation (Spo2) measurements at extreme altitude. Spo2 measurements from 15 participants of a high-altitude research expedition to Himlung Himal who reached a summit altitude of 7126 m during a 27-day expedition. The black rhombus shapes denote single daily pulse oximeter readings. The blue area indicates the corresponding sleeping altitude.
O2max is severely reduced above 4500 m. The underlying mechanisms are not fully understood, but we know from studies in high-altitude climbers that o2max decreases by ~1% for every 100 m at altitudes above 1500 m.9 Reduced o2max is a major determinant of performance at extreme altitudes. Climbers are severely limited in terms of ascent (and descent) speed and have almost no reserves in performance capacity. In addition, reduced o2max leads to a parallel decrease in body heat production even at maximal exertion and increases the risk of hypothermia.
HAI
High-altitude illness refers to two different syndromes that occur after an ascent to high altitude. The cerebral forms of HAI include acute mountain sickness (AMS) and high-altitude cerebral (o)edema (HACE), whereas the pulmonary form consists of high-altitude pulmonary (o)edema (HAPE). Common risk factors for the development of HAI are a rapid ascent, lack of an adequate acclimatisation period and a past history of HAI under similar circumstances.10 Physical exertion in a non-acclimatised state can be a contributing factor.
Somewhat surprisingly, perhaps, the likelihood of developing HAI is not particularly associated with age, level of fitness or smoking history.
AMS and HACE
Epidemiology
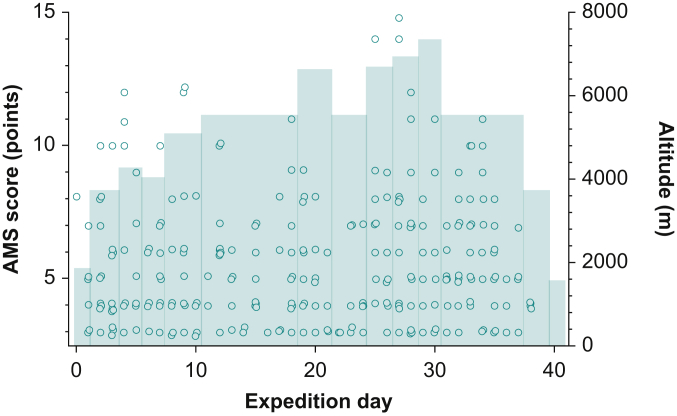
Acute mountain sickness is defined as the occurrence of headache plus additional symptoms such nausea, loss of appetite, vomiting, insomnia, dizziness and fatigue in a person who has recently ascended to high altitude. Acute mountain sickness is very common at high altitude. The incidence increases from ~25% of climbers at altitudes between 2000 and 3000 m to >50% of climbers acutely exposed to altitudes >4000 m. Acute mountain sickness poses an ever-present problem at extreme altitudes that is difficult to manage. Within a standard climbing team, on any given expedition day, a number of climbers will have various degrees of AMS (Fig. 3). The incidence of HACE is reported to be 0.3–4% at altitudes >5000 m.11,12 Our study group found cerebral microhaemorrhages indicative of substantial blood–brain barrier disruption and HACE in three of 15 climbers who reached altitudes >7000 m.13
Fig 3.
The incidence of AMS at extreme altitude. Lake Louise AMS ratings from 12 climbers during a 41-day research expedition to Mount Shishapangma (8046 m). Black circles denote each of the twice daily (morning/evening) reported scores. Lake Louise AMS scores can range from 0 (no symptoms) to 15 (severe symptoms); values of 3 or greater indicate presence of AMS. The blue area denotes the corresponding sleeping altitude. Note the high number of AMS episodes documented in this climbing team.
Pathophysiology
Acute mountain sickness and HACE are generally considered to represent different severities on a spectrum of a single disease with one underlying pathophysiology.14 However, the pathophysiology is incompletely understood and the following merely describes generally accepted concepts.
As previously discussed, hypoxia and hypocapnia lead to increased cerebral blood flow and to cerebral arterial and venous vasodilation. Increased capillary pressure and hypoxia-induced increase in the permeability of the blood–brain barrier causes fluid leakage from the cerebral capillaries into brain tissue, causing cerebral oedema. In addition, tissue hypoxia induces free radical formation, which reduces the activity of the Na+/K+ ATPase on the surface of astrocytes, further exacerbating cerebral oedema. Thirdly, the higher volumes of the venous and arterial cerebral compartments contribute to an increase of total brain volume. There is evidence that the above mechanisms occur in the majority, if not all individuals ascending to high altitudes, but the extent of swelling differs between individuals.
The ‘tight fit’ hypothesis suggests that not only the amount of swelling, but also the ability to tolerate increased brain volume determines whether an individual becomes symptomatic.15 Individuals with a greater brain-to-intracranial volume ratio will have less room to accommodate the same degree of cerebral oedema. Once all physiological reserve volumes (mainly CSF volumes in the subarachnoid space and the ventricular system) are exhausted, any further tissue volume expansion leads to an abrupt increase in ICP. Increased ICP causes a decrease in cerebral perfusion pressure and blood flow and, ultimately, to brain herniation and death. However, more commonly, death from HACE occurs from impaired cognition and ataxia leading to falls or an inability to descend to a safe altitude.
Signs and symptoms
Headache is the primary symptom of AMS. Pain perception occurs via afferent fibres from pain receptors located in the large intracranial blood vessels and meninges. Potential mechanisms of pain receptor activation include vascular distension, increased intravascular pressure, brain swelling and the release of nociceptive substances.16 Connections of afferent fibres to vegetative centres can cause other symptoms, such as nausea and vomiting.
The Lake Louise AMS score is the most widely used scoring system for AMS. The scoring system rates four symptoms (headache; gastrointestinal symptoms; fatigue, weakness, or both; dizziness/light-headedness) on a four-point scale from 0 (not present) to 3 (severe). The presence of AMS is defined as a total score of ≥3 from the four rated symptoms, with at least one point from headache in the setting of a recent ascent or gain in altitude.17
Severity rankings are not fully established, but it has been suggested to define mild AMS as 3–5 points, moderate AMS as 6–9 points and severe AMS as 10–12 points. HACE is characterised by the development of encephalopathic symptoms and signs, including ataxia, severe lassitude and progressive decline of cognitive function and consciousness (drowsiness and confusion, stupor and coma). High-altitude cerebral oedema can occur rapidly and without an obvious prodromal phase of AMS of increasing severity (see Clinical scenario 1).
Clinical scenario 1.
A 28-year-old high-altitude porter began an ascent carrying supplies from a camp at 6400 m to the next camp at 6900 m during an expedition to Muztagh Ata in western China. He did not report any symptoms of acute mountain sickness or show any signs of ataxia while getting ready. After approximately 40 min and 100 m of ascent he collapsed and had a brief generalised seizure. He regained consciousness but was unable to stand up. The expedition doctor found the young man in a confused and agitated state with severe ataxia. Several prolonged tonic-clonic seizures followed. The patient was treated with i.v. dexamethasone and also i.v. midazolam, as an anticonvulsant. Supplemental oxygen was applied by a non-rebreather mask. A rescue team transported the man to the base camp, at an altitude of 4200 m, which was reached after 5 hours. By the time he arrived at base camp, he had regained consciousness and was neurologically normal.
Prophylaxis and treatment
Prophylaxis of AMS and HACE consists of adequate acclimatisation by avoiding an abrupt ascent to sleeping altitudes above 3000 m. When planning further ascent, subsequent nights should be spent at elevations of <500 m higher than the previous night.18 An additional rest day should ideally occur every 1000 m of further ascent.
Pharmacological prophylaxis with acetazolamide is possible but should be restricted to individuals with a history of altitude intolerance and where an adequate acclimatisation is difficult or not possible. Acetazolamide inhibits renal carbonic anhydrase, thereby reducing the reabsorption of bicarbonate and causing a bicarbonate diuresis equal to the renal compensation of respiratory alkalosis.19 Acetazolamide stimulates ventilation and improves oxygenation by disinhibiting central chemoreceptors.
Climbers with severe AMS should avoid further ascent and limit strenuous physical activity. Symptomatic therapy for headache and nausea consists of paracetamol and NSAIDs along with antiemetics such as ondansetron. Severe AMS that does not respond to the above treatment should be regarded as potential HACE and requires immediate intervention.
Severe AMS/HACE at extreme altitudes is a life-threatening condition and immediate descent by at least 1000 m is warranted. In addition, and to facilitate descent and to temporise the illness, dexamethasone, supplemental oxygen, hyperbaric therapy in a portable hyperbaric chamber, or all three should be used.
Dexamethasone is given at an initial dose of 8 mg p.o., i.m., or i.v., followed by 4 mg every 6 h until descent is completed and neurological symptoms have resolved. The mechanism of action of dexamethasone for control of vasogenic cerebral oedema is not fully understood.20 In patients with brain tumours, dexamethasone reduces the permeability of the blood–brain barrier by upregulating angiopoietin 1, a barrier-stabilising factor, and downregulating vascular endothelial growth factor, which induces an abnormal permeability of the endothelium.21 The effectiveness of dexamethasone in the treatment of severe AMS and HACE is based on broad clinical experience, but has not been extensively studied.
Dexamethasone is effective in preventing AMS/HACE but does not aid acclimatisation. If HACE occurs despite using dexamethasone for prevention, further doses will only have a limited effect. Experts therefore recommend using prophylactic dexamethasone only after careful risk–benefit evaluation in circumstances where a rapid ascent of inadequately acclimatised climbers is unavoidable, for example, in high-altitude rescue operations. Dexamethasone should not be used to facilitate a more rapid ascent in non-emergency situations.
HAPE
Epidemiology
The risk of HAPE depends on individual susceptibility, the ascent rate and the altitude that is reached. High-altitude pulmonary oedema is uncommon below 4500 m but increases to 0.2–6% above 4500 m and to 2–15% at altitudes of higher than 5500 m.22
Pathophysiology
High-altitude pulmonary oedema is a form of non-cardiogenic pulmonary oedema triggered by hypobaric hypoxia. The generally accepted mechanism of HAPE suggests that HPV at the level of the pulmonary arterioles is extensive but not uniform. In areas where there is limited arteriolar vasoconstriction, pulmonary hypertension causes overperfusion of capillaries, leading to a breakdown in the pulmonary blood-gas barrier and to the accumulation of plasma and red blood cells in the alveolar space. Secondly, hypoxia-induced vasoconstriction also occurs in pulmonary veins, increasing the resistance downstream of the region of fluid filtration. The genetically determined variability of the extent and homogeneity of HPV plays an important role in the risk of HAPE and may explain the marked differences in individual susceptibility. A low HVR possibly increases susceptibility to HAPE because more pronounced hypoxia causes more severe HPV. However, the considerable overlap in extent of HVR in HAPE-susceptible and HAPE-resistant individuals suggests that low HVR is not a major contributor to HAPE.
Active removal of alveolar fluid is driven by an osmotic Na+ gradient created by transepithelial Na+ transport from the alveolar space into the interstitium, generating an osmotic gradient for the reabsorption of water. Hypoxia inhibits the activity and expression of various Na+ transporters of the alveolar epithelial cells; the most important being apical membrane epithelial Na+ channels and basolateral Na+/K+ ATPase, and thereby decreases transepithelial Na+ transport and the reabsorption of alveolar fluid.23 The accumulation of extravascular fluid in the alveolar spaces further impairs gas exchange, leading to profound and potentially lethal hypoxaemia.
Signs and symptoms
Symptoms of HAPE often start with affected climbers experiencing sudden shortness of breath that is out of proportion with the level of exertion. Later, dyspnoea with minimal exertion and a non-productive cough develop (see Clinical scenario 2). Symptoms can rapidly progress to severe HAPE characterised by cough, productive of pink, frothy sputum or even frank haemoptysis. Profound hypoxia develops, debilitating the climber by severely restricting his or her exercise tolerance. HAPE is associated with the development of AMS in >50% of cases and the risk of concomitant HACE is markedly increased.
Clinical scenario 2.
A 23-year-old male climber ascended from camp I at 6400 m toward camp II at 6900 m during a skiing expedition to Shishapangma in Tibet. He felt well at the start of the ascent. Around 2 h into the ascent he reported the of sudden onset of fatigue and breathlessness that was out of proportion with the physical work of climbing. Within 15 min he developed dyspnoea even when standing still and developed a cough that was productive of pink, frothy sputum. His Sao2 measured on a portable pulse oximeter was 54%. He was treated with 20 mg of slow release nifedipine. The small group of five climbers decided that immediate descent was necessary but were unable to carry the patient. Supplemental oxygen was applied by a non-rebreather mask. As an excellent skier, he was able to ski to the snow line at 6000 m despite severe dyspnoea. The following 8 km descent on foot to the basecamp at 5600 m took 6 hours because of the patient's severe shortness of breath and difficulty in walking. Upon arrival at basecamp late in the evening the patient was placed into a hyperbaric chamber. His condition slowly improved overnight with his Sao2 increasing to 75%.
Prophylaxis and treatment
As with AMS, gradual ascent is the most important strategy for preventing HAPE. High-altitude pulmonary oedema is a life-threatening condition. The two principles of treatment are to improve oxygenation and to reduce pulmonary arterial pressure. Immediate descent and supplemental oxygen, hyperbaric therapy in a portable hyperbaric chamber, or both are warranted. Pharmacologic treatment consists of pulmonary vasodilators.
Nifedipine is a non-specific calcium channel blocker that reduces pulmonary vascular resistance and pulmonary arterial pressure.24 The phosphodiesterase-5 inhibitors tadalafil and sildenafil increase the pulmonary vasodilatory effects of nitric oxide.25,26 Standard (oral) doses are nifedipine 30 mg of a slow-release formulation (12-hourly), tadalafil 10 mg (12-hourly) or sildenafil 50 mg (8-hourly).
Inhaled beta-adrenergic agonists, such as salbutamol, increase sodium transport across the alveolar endothelium, thereby enhancing the clearance of alveolar fluid. Evidence suggests that salbutamol is effective in preventing HAPE in susceptible individuals.23 The role of salbutamol as a treatment of HAPE is less clear.
Treatment should be continued until the descent is completed and the symptoms of HAPE have resolved. Pharmacological prevention of HAPE with pulmonary vasodilators and dexamethasone is possible.26,27 However, if HAPE occurs despite pharmacological prophylaxis, further treatment options are limited. Again, the prophylactic use of pulmonary vasodilators and dexamethasone should only be considered after careful risk–benefit evaluation in circumstances where a rapid ascent of non-adequately acclimatised climbers is unavoidable. We strongly advise against the use of pharmacological HAPE prophylaxis to enable HAPE-prone individuals to ascend faster.
Conclusion
Acclimatisation to a high-altitude environment improves arterial oxygen content and increases and maintains respiratory drive mainly by correcting respiratory alkalosis. Acclimatisation begins within minutes of ascent, but requires days to weeks to complete. Gradual ascent to high altitude is the most important factor in preventing HAI. Severe AMS, HACE and HAPE occur not infrequently at extreme altitudes and are associated with a high mortality. Patients should receive adequate pharmacological treatment and supplemental oxygen and should be transported to lower altitudes as soon as possible.
Declaration of interests
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Tobias MerzMD FCICM is an intensive care specialist at Auckland City Hospital with a research background in various aspects of intensive care and high-altitude medicine.
Jacqueline Pichler HeftiMD is a consultant respiratory physician with a special interest in pulmonary hypertension and high-altitude medicine. Both authors have a long-standing research collaboration in high-altitude field studies to altitudes above 7000 m.
Matrix codes: 1A01, 2A12, 3I00
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjae.2021.07.005.
Supplementary Material
The following is the Supplementary data to this article:
The video associated with this article can be viewed from the article in BJA Education online.
References
- 1.The Himalayan Database. Available from: http://www.himalayandatabase.com (Accessed 10/03/2021).
- 2.West J.B., Hackett P.H., Maret K.H., et al. Pulmonary gas exchange on the summit of Mount Everest. J Appl Physiol. 1983;55:678–687. doi: 10.1152/jappl.1983.55.3.678. [DOI] [PubMed] [Google Scholar]
- 3.Schoene R.B. Illnesses at high altitude. Chest. 2008;134:402–416. doi: 10.1378/chest.07-0561. [DOI] [PubMed] [Google Scholar]
- 4.Hackett P.H., Roach R.C. High-altitude illness. N Engl J Med. 2001;345:107–114. doi: 10.1056/NEJM200107123450206. [DOI] [PubMed] [Google Scholar]
- 5.Firth P.G., Zheng H., Windsor J.S., et al. Mortality on Mount Everest, 1921-2006: descriptive study. BMJ. 2008;337:a2654. doi: 10.1136/bmj.a2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West J.B. The physiologic basis of high-altitude diseases. Ann Intern Med. 2004;141:789–800. doi: 10.7326/0003-4819-141-10-200411160-00010. [DOI] [PubMed] [Google Scholar]
- 7.Hoiland R.L., Howe C.A., Coombs G.B., Ainslie P.N. Ventilatory and cerebrovascular regulation and integration at high altitude. Clin Auton Res. 2018;28:423–435. doi: 10.1007/s10286-018-0522-2. [DOI] [PubMed] [Google Scholar]
- 8.Bosch M.M., Merz T.M., Barthelmes D., et al. New insights into ocular blood flow at very high altitudes. J Appl Physiol. 2009;106:454–460. doi: 10.1152/japplphysiol.90904.2008. 1985. [DOI] [PubMed] [Google Scholar]
- 9.Cymerman A., Reeves J.T., Sutton J.R., et al. Operation Everest II: maximal oxygen uptake at extreme altitude. J Appl Physiol. 1989;66:2446–2453. doi: 10.1152/jappl.1989.66.5.2446. 1985. [DOI] [PubMed] [Google Scholar]
- 10.Richalet J.-P., Larmignat P., Poitrine E., Letournel M., Canouï-Poitrine F. Physiological risk factors for severe high-altitude illness. Am J Respir Crit Care Med. 2012;185:192–198. doi: 10.1164/rccm.201108-1396OC. [DOI] [PubMed] [Google Scholar]
- 11.Wu T.-Y., Ding S.Q., Liu J.L., et al. Who should not go high: chronic disease and work at altitude during construction of the Qinghai-Tibet railroad. High Alt Med Biol. 2007;8:88–107. doi: 10.1089/ham.2007.1015. [DOI] [PubMed] [Google Scholar]
- 12.Basnyat B., Murdoch D.R. High-altitude illness. Lancet. 2003;361:1967–1974. doi: 10.1016/S0140-6736(03)13591-X. [DOI] [PubMed] [Google Scholar]
- 13.Kottke R., Pichler Hefti J., Rummel C., Hauf M., Hefti U., Merz T.M. Morphological brain changes after climbing to extreme altitudes – a prospective cohort study. PloS One. 2015;10 doi: 10.1371/journal.pone.0141097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson M.H., Newman S., Imray C.H. The cerebral effects of ascent to high altitudes. Lancet Neurol. 2009;8:175–191. doi: 10.1016/S1474-4422(09)70014-6. [DOI] [PubMed] [Google Scholar]
- 15.Imray C., Booth A., Wright A., Bradwell A. Acute altitude illnesses. BMJ. 2011;343:d4943. doi: 10.1136/bmj.d4943. [DOI] [PubMed] [Google Scholar]
- 16.Bailey D.M., Bärtsch P., Knauth M., Baumgartner R.W. Emerging concepts in acute mountain sickness and high-altitude cerebral edema: from the molecular to the morphological. Cell Mol Life Sci. 2009;66:3583–3594. doi: 10.1007/s00018-009-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roach R.C., Hackett P.H., Oelz O., et al. The 2018 Lake Louise acute mountain sickness score. High Alt Med Biol. 2018;19:4–6. doi: 10.1089/ham.2017.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luks A.M., Auerbach P.S., Freer L., et al. Wilderness Medical Society clinical practice guidelines for the prevention and treatment of acute altitude illness: 2019 Update. Wilderness Environ Med. 2019;30:S3–S18. doi: 10.1016/j.wem.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Nieto Estrada V.H., Molano Franco D., Medina R.D., Gonzalez Garay A.G., Martí-Carvajal A.J., Arevalo-Rodriguez I. Interventions for preventing high altitude illness: Part 1. Commonly-used classes of drugs. Cochrane Database Syst Rev. 2017;6:CD009761. doi: 10.1002/14651858.CD009761.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subudhi A.W., Dimmen A.C., Julian C.G., Wilson M.J., Panerai R.B., Roach R.C. Effects of acetazolamide and dexamethasone on cerebral hemodynamics in hypoxia. J Appl Physiol. 2011;110:1219–1225. doi: 10.1152/japplphysiol.01393.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H., Lee J.M., Park J.S., et al. Dexamethasone coordinately regulates angiopoietin-1 and VEGF: a mechanism of glucocorticoid-induced stabilization of blood-brain barrier. Biochem Biophys Res Commun. 2008;372:243–248. doi: 10.1016/j.bbrc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 22.Jensen J.D., Vincent A.L. StatPearls. StatPearls Publishing; Treasure island, FL: 2021. High altitude pulmonary edema. [Google Scholar]
- 23.Sartori C., Allemann Y., Duplain H., et al. Salmeterol for the prevention of high-altitude pulmonary edema. N Engl J Med. 2002;346:1631–1636. doi: 10.1056/NEJMoa013183. [DOI] [PubMed] [Google Scholar]
- 24.Oelz O., Maggiorini M., Ritter M., et al. Nifedipine for high altitude pulmonary oedema. Lancet. 1989;2:1241–1244. doi: 10.1016/s0140-6736(89)91851-5. [DOI] [PubMed] [Google Scholar]
- 25.Richalet J.-P., Gratadour P., Robach P., et al. Sildenafil inhibits altitude-induced hypoxemia and pulmonary hypertension. Am J Respir Crit Care Med. 2005;171:275–281. doi: 10.1164/rccm.200406-804OC. [DOI] [PubMed] [Google Scholar]
- 26.Maggiorini M., Brunner-La Rocca H.-P., Peth S., et al. Both tadalafil and dexamethasone may reduce the incidence of high-altitude pulmonary edema. Ann Intern Med. 2006;145:497–506. doi: 10.7326/0003-4819-145-7-200610030-00007. [DOI] [PubMed] [Google Scholar]
- 27.Bärtsch P., Maggiorini M., Ritter M., Noti C., Vock P., Oelz O. Prevention of high-altitude pulmonary edema by nifedipine. N Engl J Med. 1991;325:1284–1289. doi: 10.1056/NEJM199110313251805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.