Abstract
Objectives: To assess the clinical outcomes of prostate cancer patients treated with salvage radiotherapy (SRT) for locoregional clinical recurrence (CR) after radical prostatectomy (RP). Methods: Records of 60 patients with macroscopic locoregional recurrence after prostatectomy and referrals for SRT were retrospectively investigated in the multi-institutional database. The median radiation dose was 70.2 Gy. Biochemical failure was defined as the prostate-specific antigen (PSA) ≥ nadir + 2 or initiation of androgen deprivation therapy (ADT) for increased PSA. Results: Median recurrent tumor size was 1.1 cm and pre-radiotherapy PSA level was 0.4 ng/ml. At a median follow-up of 83.1-month after SRT, 7-year biochemical failure-free survival (BCFFS), locoregional failure-free survival (LRFFS), distant metastasis-free survival (DMFS), and overall survival (OS) were 67.0%, 89.7%, 83.6%, and 91.2%, respectively. Higher Gleason's scores were associated with unfavorable BCFFS, DMFS, and OS. Pre-SRT PSA ≥0.5 ng/ml predicted worse BCFFS, LRFFS, and DMFS. In multivariate analyses, a Gleason's score of 8 to 10 was associated with decreased BCFFS (hazard ratio [HR] 3.12, 95% confidence interval [CI] 1.11-8.74, P = .031) and OS (HR 17.72, 95% CI 1.75-179.64, P = .015), and combined ADT decreased the risks of distant metastasis (HR 0.18, 95% CI 0.04-0.92, P = .039). Two patients (3.3%) experienced late grade 3 urinary toxicity. Conclusions: SRT for locoregional CR after RP achieved favorable outcomes with acceptable long-term toxicities. Higher Gleason's scores and pre-radiotherapy PSA level were unfavorable prognostic variables. Combined ADT may decrease the risks of metastases.
Keywords: prostatic neoplasms, prostatectomy, radiotherapy, recurrence
Introduction
Radical prostatectomy (RP) is a widely-used local treatment for localized prostate cancer. However, approximately one-third of patients who undergo RP will experience recurrence during long-term follow-up. 1 An increase of serum prostate-specific antigen (PSA), so-called biochemical failure (BCF), is usually preceded before overt clinical recurrence (CR) or metastasis. 2 Salvage radiotherapy (SRT) to the prostate bed at the time of BCF is a potentially curative treatment, and especially early implementation of SRT at PSA levels <0.5 ng/ml, which has shown better outcomes. 3 On the other hand, some patients are referred to radiation oncology following diagnosis of radiologically identifiable locoregional recurrence. The reasons for this could be due to disease progression during salvage androgen deprivation therapy (ADT) after postoperative BCF, or macroscopic locoregional recurrence that occurred without any preceding PSA elevation. Although SRT might be one of the most commonly considered treatments in these patients, previous studies have only reported the effectiveness of SRT in the BCF cases,4,5 and few studies have demonstrated the effectiveness of SRT in the locoregional CR. 6
The present study retrospectively evaluated the clinical outcomes of SRT in patients with locoregional CR after RP. Prognostic variables predicting outcomes associated with SRT were also assessed.
Methods
The KROG 18-01 protocol was designed to evaluate clinical outcomes of postoperative radiotherapy (RT) following RP in localized prostate cancer in a multi-institutional database. Inclusion/exclusion criteria for the protocol have been described previously. 3 In total, 1117 prostate cancer patients who were treated with postoperative RT between 2001 and 2012 at 19 institutions were identified. Among them, records of 60 patients with macroscopic locoregional recurrence after prostatectomy and referrals for SRT were retrospectively investigated. Forty-six patients (77%) had a history of BCF (serum PSA > 0.2 ng/ml) before the diagnosis of locoregional recurrence (interval ≥3months), but 14 patients (23%) were diagnosed with BCF and locoregional recurrence almost same time (interval <3months). In all participating institutions, postoperative biochemical recurrence was routinely assessed with whole-body imaging and pelvic magnetic resonance imaging (MRI). Whole-body imaging including bone scan or positron emission tomography (PET) was performed to evaluate metastatic disease before SRT. If there was no evidence of metastatic disease, we considered it as an isolated local recurrence. Locoregional CR was defined as newly developed nodules or mass-like lesions in the prostatic bed or the regional lymphatics, confirmed by pathologic specimens or detected by radiologic imaging, with pelvic multiparametric MRI with or without PET.7–9 According to European Association of Urology guidelines, a transrectal ultrasound-guided biopsy of the prostate bed is not routinely recommended for the diagnosis of locoregional recurrence because of relatively low predictive accuracy. 10 The routine follow-up assessments after SRT generally included a digital rectal examination and serum PSA measurements every 3 months during the first 2 years, every 6 months over the next 3 years, and annually thereafter.
The present study was conducted in accordance with the ethical principles of the Declaration of Helsinki and the International Conference on Harmonization guideline E6: Good Clinical Practice. As a principal institution of this multi-institutional study, National Cancer Center Institutional Review Board approved this protocol (NCC 2018-0116). And the other participant institutions were also approved by their institutional review boards, respectively. Collected data was managed by assigning case numbers to each hospital and anonymizing them. Data analysis was performed centrally in National cancer center, Korea. Written informed consent was not obtained due to the retrospective nature of the study.
The irradiated target volume of SRT was of the macroscopic recurrent lesion only (n = 40) or the extended field with prophylactic irradiation for regional pelvic lymphatics (n = 20). The total RT dose delivered to the planning target volume was 70.2 Gy (median value, interquartile range [IQR], 66.0-75.6]. The most common RT techniques for SRT were intensity-modulated RT (n = 48), followed by three-dimensional-conformal RT (n = 12). Before the diagnosis of locoregional CR, ADT for prior BCF had been administered in 28 patients (47%). Among them, 8 patients were considered as castration-resistant prostate cancer. Patients who received concurrent ADT were defined as having received ADT for at least 3 months after the start of SRT. Concurrent ADT with SRT was performed in 38 patients (63%) and among them, 11 patients received treatment for longer than 1 year.
Time to recurrence and survival rate were calculated from the first day of SRT. Although, there is no consensus on the definition of BCF after SRT, we defined BCF after SRT as a serum PSA level ≥ nadir + 2.0 ng/ml or the initiation of salvage ADT regardless of the PSA value, based on the results of a pooled analysis conducted for the radiation therapy oncology group (RTOG) 0534 protocol. 11 Locoregional failure was defined as the evidence of prostate cancer in the prostate bed or regional lymphatics with the following criteria in pelvic MRI: (1) At least a 20% increase in the sum of the longest diameter of the target lesions, or (2) appearance of 1 or more new lesions. Distant metastasis was diagnosed when disease progressions in distant organs or non-regional lymph nodes were confirmed by biopsy or found in radiologic studies including computed tomography, bone scan, or PET. Overall survival (OS) was defined as the interval between the first day of SRT and the day of death from any cause. The curves for biochemical failure-free survival (BCFFS), locoregional failure-free survival (LRFFS), distant metastasis-free survival (DMFS), and OS were generated using the Kaplan–Meier estimator. To evaluate the prognostic factor, the log-rank test was used for univariate analyses, and a Cox proportional hazards model was used for the multivariate analyses. The binary variables included to predict outcomes were: T-stage (T2-3a vs T3b-4), Gleason score (2-7 vs 8-10), pre-RT PSA level (<0.5 ng/mL vs ≥0.5 ng/mL), type of clinical failure (local vs regional or both), recurrent tumor size (<1 cm vs ≥1 cm), total RT dose (<70 Gy vs ≥70 Gy), and combined ADT (yes vs no). P-values <.05 were considered significant. Radiation-associated toxicity was evaluated according to the RTOG radiation toxicity criteria. Statistical analyses were performed using STATA software (version 14.0; STATA Corp., College Station, TX, USA).
Results
Patient and tumor characteristics are summarized in Table 1. The median age at diagnosis and at SRT was 66 years (IQR, 60-70 years) and 69 years (IQR, 62-73 years), respectively. In total, 41 patients (68.3%) were initially of pathologic stage T3 to 4 and 6 patients (10.0%) had pathologic N-positive disease. Approximately two-thirds of patients had a Gleason score of 7 and 13 patients (22%) had a Gleason score of 8 to 10. The location of the macroscopic recurrent lesion was mostly limited to the prostate bed (n = 50, 83.3%), however, 10 patients (16.7%) had a recurrent disease in the regional lymphatics. The largest diameter of a recurrent tumor measured 1.1 cm (median value, IQR 0.7-1.5 cm), and the serum PSA level before SRT was 0.4 ng/ml (median value, IQR 0.2-1.3). The majority of patients were diagnosed clinically by radiological methods (n = 58) and 2 patients were done with pathologic confirmation. One patient had undergone a trans-urethral resection of the tumor before being referred to the radiation oncology department. Among the 46 patients who had experienced prior BCF before SRT, the lag period from BCF to CR was 9.7 months (median value, IQR 6.1-25.0).
Table 1.
Patient and Tumor Characteristics (n = 60).
| Variables | Value | IQR or percentile | |
|---|---|---|---|
| Median age at diagnosis (IQR), year | 66 | 60-70 | |
| Median time to SRT from surgery (IQR), year | 2.5 | 1.1-3.8 | |
| Initial PSA level (ng/ml), median | 10.7 | 6.2-24.3 | |
| pT category | T2 | 16 | 27% |
| T3a | 23 | 38% | |
| T3b-4 | 18 | 30% | |
| Unknown | 3 | 5% | |
| pN category | N0-Nx | 54 | 90% |
| N1 | 6 | 10% | |
| Gleason score | 2-6 | 6 | 10% |
| 7 | 39 | 65% | |
| 8-10 | 13 | 22% | |
| Unknown | 2 | 3% | |
| Resection margin | Negative | 17 | 28% |
| Positive | 39 | 65% | |
| Unknown | 4 | 7% | |
| Type of clinical recurrence | Local | 50 | 83% |
| Regional | 3 | 5% | |
| Both | 7 | 12% | |
| Lag period from biochemical failure | Absent | 14 | 23% |
| Present a | 46 | 77% | |
| Prior salvage ADT for biochemical failure | No | 32 | 53% |
| Yes | 28 | 47% | |
| Combined ADT for clinical recurrence | No | 22 | 37% |
| Yes | 38 | 63% | |
Abbreviations: IQR, interquartile range; ADT, androgen deprivation therapy; SRT, salvage radiotherapy; PSA, prostate-specific antigen.
Lag period “present” means the interval between biochemical failure and locoregional recurrence was >3-month.
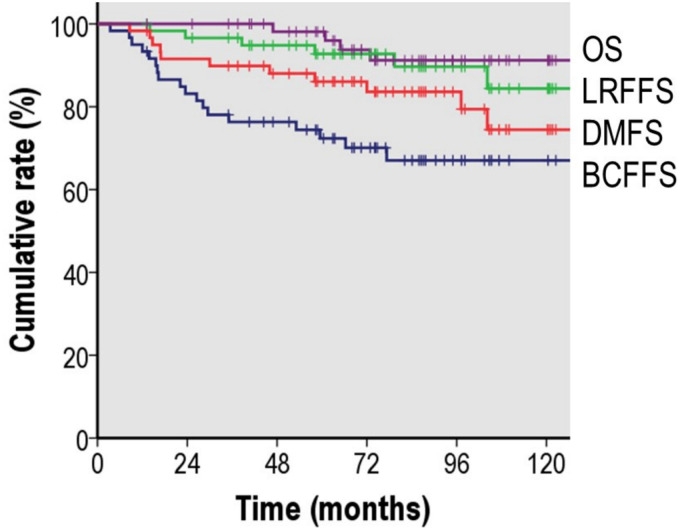
At a median value of 83.1 months (IQR 61.4-108.7) after SRT, the estimated 7-year BCFFS, LRFFS, DMFS, OS, and cancer-specific survival were 67.0%, 89.7%, 83.6%, 91.2%, and 95.2%, respectively (Figure 1). The median post-RT PSA nadir value was 0.002 (IQR 0.001-0.040) and the nadir of 45 patients (75.0%) was below 0.1 ng/ml. A total of 18 patients (30.0%) experienced BCF and 6 patients (18.3%) showed locoregional progression. Eleven patients developed distant metastases and the most common sites were non-regional lymph nodes, followed by bone and lung. Overall, total of thirteen patients experienced either locoregional failure or distant metastasis, or both (n = 4) after SRT. Among them, 5 patients had been co-administered ADT with SRT previously. As a salvage treatment for the SRT failure, most of the patients received ADT± chemotherapy except 2 patients who refused further treatment. Four patients (6.7%) died during the observation period and 3 (5.0%) died due to disease progression.
Figure 1.
Kaplan–Meier survival curve of biochemical failure-free survival, locoregional failure-free survival, distant metastasis-free survival, and overall survival after salvage radiotherapy for postoperative macroscopic locoregional recurrence.
The results of the univariate analysis of the prognostic factors associated with BCFFS, LRFFS, DMFS, and OS are listed in Table 2. Among the tumor-related variables, a higher Gleason score (≥8) and higher pre-RT PSA (≥0.5 ng/ml) predicted unfavorable BCFFS and DMFS. The higher Gleason score group also had decreased OS rates. A higher pre-RT PSA was the only predictor for worse LRFFS. Combined ADT with SRT was associated with improved BCFFS, DMFS, and with the increased OS. Pathologic T category, type of recurrence, recurrent tumor size, and total RT dose were not associated with any clinical endpoints. In a supplementary analysis, a decrease of serum PSA to an undetectable level (below 0.1 ng/ml) after SRT was evaluated its association with the prognosis (Supplemental Figure 1). Undetectable PSA after SRT was significantly associated with better outcomes in all clinical endpoints including biochemical failure-free survival, LRFFS, DMFS, and OS (P < .05).
Table 2.
Univariate Analysis of Prognostic Factors Associated with BCFFS, LRFFS, DMFS, and OS.
| BCFFS | LRFFS | DMFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | n | 7-yr survival | P-value a | 7-yr survival | P-value | 7-yr survival | P-value | 7-yr survival | P-value | |
| T-stage | T2-3a | 39 | 72.2 | .154 | 88.2 | .578 | 89.4 | .267 | 93.7 | .792 |
| T3b-4 | 18 | 56.1 | 93.8 | 81.9 | 92.9 | |||||
| Gleason score | ≤7 | 45 | 75.6 | .003 | 92.3 | .178 | 90.4 | .001 | 97 | .001 |
| 8-10 | 13 | 38.5 | 77.1 | 62.2 | 67.5 | |||||
| Pre-RT PSA | <0.5 | 35 | 79.4 | .007 | 97.0 | .008 | 94.3 | .004 | 93.4 | .583 |
| ≥0.5 | 25 | 49.1 | 73.5 | 67.9 | 86.9 | |||||
| Type of clinical recurrence | Local | 50 | 66.3 | .994 | 91.7 | .193 | 84.2 | .820 | 89.5 | .360 |
| Regional or both | 10 | 70.0 | 80.0 | 80.0 | 100.0 | |||||
| Recurrent tumor size | <1.0 cm | 22 | 81.3 | .191 | 95.2 | .339 | 89.5 | .519 | 88.2 | .663 |
| ≥1.0 cm | 38 | 59.4 | 86.4 | 80.9 | 93.6 | |||||
| Total RT dose | <70 Gy | 17 | 60.5 | .389 | 94.1 | .761 | 63.7 | .071 | 76.9 | .139 |
| ≥70 Gy | 43 | 69.3 | 88.9 | 90.2 | 94.4 | |||||
| ADT for prior biochemical failure | No | 32 | 75.0 | .173 | 96.9 | .057 | 85.9 | .569 | 95.5 | .401 |
| Yes | 28 | 57.7 | 81.4 | 80.8 | 87.8 | |||||
| Combined ADT | No | 22 | 51.8 | .015 | 89.3 | .415 | 67.8 | .003 | 75.1 | .029 |
| Yes | 38 | 75.9 | 90.1 | 92.0 | 97.4 | |||||
Abbreviations: BCFFS, biochemical failure-free survival; LRFFS, locoregional failure-free survival; DMFS, distant metastasis-free survival; OS, overall survival; RT, radiotherapy; ADT, androgen deprivation therapy; PSA, prostate-specific antigen.
Log-rank test.
The multivariate analyses of BCFFS, DMFS, and OS were conducted using the variables that were statistically significant in the univariate analyses (Table 3). In terms of BCFFS, a Gleason score of 8 to 10 was associated with a significantly increased risk (hazard ratio [HR] 3.12, 95% confidence interval [CI] 1.11-8.74, P = .031). Meanwhile, combined ADT decreased the risk of BCF with borderline significance (HR 0.37, 95% CI 0.13-1.03, P = .057). In terms of DMFS, combined ADT significantly decreased the risk of distant metastasis compared to SRT alone (HR 0.18, 95% CI 0.04–0.92, P = .039). Multivariate analysis of OS showed a significantly increased risk of death in patients with a Gleason score of 8 to 10 (HR 17.72, 95% CI 1.75-179.64, P = .015), but pre-RT PSA and combined ADT were non-significant predictors.
Table 3.
Multivariate Analysis of Factors Associated With Biochemical Failure, Locoregional Failure, Distant Metastasis, and Overall Survival.
| Biochemical failure | Locoregional failure | Distant metastasis | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | HR (95% CI) | P-value a | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Gleason score | ≤7 | Ref. | .031 | Ref. | .238 | Ref. | .105 | Ref. | .015 |
| 8-10 | 3.12 (1.11-8.74) | 2.66 (0.52-13.52) | 3.23 (0.78-13.31) | 17.72 (1.75-179.64) | |||||
| Pre-RT PSA | <0.5 | Ref. | .126 | Ref. | .061 | Ref. | .219 | Ref. | .924 |
| ≥0.5 | 2.26 (0.8.-6.39) | 7.96 (0.91-69.82) | 2.81 (0.54-14.62) | 0.91 (0.12-6.81) | |||||
| Combined ADT | No | Ref. | .057 | Ref. | Ref. | .039 | Ref. | .126 | |
| Yes | 0.37 (0.13-1.03) | 0.72 (0.15-3.52) | .681 | 0.18 (0.04-0.92) | 0.17 (0.02-1.65) | ||||
Abbreviations: HR, hazard ratio; CI, confidence interval; RT, radiotherapy; PSA, prostate-specific antigen; ADT, androgen deprivation therapy.
Cox proportional hazards model.
7 patients (11.7%) experienced grade 2 or more late radiation-related toxicity during the observation period. Among them, 5 patients experienced genitourinary toxicity, and 2 experienced gastrointestinal toxicity. Two patients (3.3%) reported late grade 3 urinary toxicity which required cystoscopic coagulation for gross hematuria. No ≥ grade 3 gastrointestinal toxicity was observed.
Discussion
This study reports the long-term clinical outcome of SRT for locoregional CR after RP. At a median of 83 months of follow-up, the majority of patients (67%) showed no evidence of BCF and approximately 90% of patients achieved locoregional control at 7-year. Over 90% of patients had long-term survival and only 3 (5%) patients died due to prostate cancer progression. The results of SRT for those patients in the present study are excellent and noteworthy, as locoregional clinical recurrences after RP are generally thought to be more advanced than BCF and may be expected to have unfavorable prognosis due to the lead-time difference between BCF and CR. The median lead times from RP to SRT in patients with BCF and CR were 10.5 and 29.4 months, respectively, in the KROG 18-01 data. 3
We were able to compare the efficacy of SRT in patients with BCF alone versus locoregional CR in the same cohort due to all the patients being part of the KROG 18-01 cohort (Table 4). In terms of the 7-year BCFFS, LRFFS, DMFS, and OS, statistically significant differences between 2 groups were not seen in any clinical endpoints. According to the previous report, pre-RT PSA level was an important prognostic factor after postoperative RT. 3 In this regard, although our study included patients with locoregional CR, the median pre-SRT PSA was low at 0.4 ng/ml. This is similar to the value of 0.3 ng/ml for that of BCF alone. 3 Similar pre-SRT PSA levels may be one of the reasons for those comparable results. Furthermore, the median total radiation dose to the planning target volume differed between BCF alone and locoregional CR (66.0 Gy vs 70.2 Gy, respectively), and this could account for the comparable level of disease control in the present study. When we compared BCF alone versus locoregional CR according to subgroups of Gleason score and pre-RT PSA (Table 4), the CR group showed significantly worse outcomes in the subgroups of Gleason 8 to 10 or pre-RT PSA ≥0.5 compared to the BCF group. For a patient who has an unfavorable prognosis, it seems more important to perform SRT before CR.
Table 4.
Comparing the Clinical Outcomes of SRT for Biochemical Failure Versus Clinical Recurrence in the KROG 18-01 Cohort.
| BCFFS | LRFFS | DMFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 7-yr survival | P-value a | 7-yr survival | P-value | 7-yr survival | P-value | 7-yr survival | P-value | |||
| All | BCF | 975 | 66.7 | .908 | 92.1 | .888 | 88.2 | .122 | 94.1 | .927 | |
| CR | 60 | 67.0 | 89.7 | 83.6 | 91.2 | ||||||
| Gleason score | ≤7 | BCF | 637 | 73.0 | .465 | 93.5 | .720 | 93.8 | .296 | 95.7 | .487 |
| CR | 45 | 77.0 | 92.0 | 90.2 | 96.9 | ||||||
| 8-10 | BCF | 418 | 60.1 | .028 | 88.1 | .561 | 81.4 | .046 | 92.4 | .046 | |
| CR | 13 | 35.7 | 81.5 | 68.5 | 73.3 | ||||||
| Pre-RT PSA | <0.5 | BCF | 725 | 73.6 | .439 | 92.2 | .087 | 92.0 | .513 | 95.5 | .872 |
| CR | 35 | 77.2 | 100.0 | 94.4 | 93.6 | ||||||
| ≥0.5 | BCF | 331 | 55.3 | .600 | 89.4 | .047 | 81.9 | .012 | 92.0 | .969 | |
| CR | 25 | 49.8 | 73.5 | 67.9 | 86.9 | ||||||
Abbreviations: BCF, biochemical failure; CR, clinical recurrence; BCFFS, biochemical failure-free survival; LRFFS, locoregional failure-free survival; DMFS, distant metastasis-free survival; OS, overall survival; RT, radiotherapy; PSA, prostate-specific antigen.
Log-rank test.
Previously, Shelan et al. retrospectively analyzed clinical outcomes of 69 cases who received SRT for locoregional CR after RP. 12 At a median follow-up of 38 months after SRT, the 5-year BCFFS and clinical progression-free survival were 44% and 76%, respectively. Bruni et al. also conducted a multicenter retrospective analysis to evaluate the role of SRT in patients with locoregional CR (n = 105). 6 At the median follow-up of 52 months, the 5-year estimates for those patients were 70%, 86%, and 86% for BCFFS, DMFS, and OS, respectively. 6 Above 2 studies used different biochemical definitions that the former used 0.2 ng/ml plus nadir and the latter used 0.5 ng/ml plus nadir, whereas we defined it as the serum PSA > 2.0 ng/ml plus nadir or initiation of salvage ADT after SRT. There is no consensus on the definition of BCF after SRT and it remains controversial. In patients with an identifiable gross tumor, nadir + 2 ng/ml or higher would be acceptable as a definition of BCF, as in patients with an intact prostate. Except for BCFFS, other clinical endpoints were relatively consistent with 5 to 7 year DMFS and clinical failure-free rates of 84% to 86% and 74% to 76%, respectively.
Analysis of the prognostic factors revealed that a lower Gleason score and lower pre-SRT PSA level were strongly associated with favorable outcomes, with 7-year BCFFS of 76% to 79% and DMFS of 90% to 94%. Patients with higher pre-SRT PSA levels (≥0.5 ng/ml) showed significantly decreased 7-year OS at 68%. In the multivariate analysis, a higher Gleason score (≥8) was significantly related with an increased risk of BCF (HR 3.12). These results are consistent with previous SRT studies for BCF.4,5 Stephenson et al. assessed the prognostic determinants after SRT for BCF in 1540 patients and showed that pre-SRT PSA level, Gleason score, PSA doubling time, surgical-margins status, and lymph node metastasis were significant determinants of progression-free survival. 4 Stish et al. also analyzed the clinical outcomes of 1106 patients following SRT for postoperative BCF and showed that 10-year BCFFS, DMFS, and cancer-specific survival differed significantly between the pre-SRT PSA level ≤0.5 and >0.5 ng/mL groups. 5 Although the disease conditions at the time of SRT differed, there was an overlap in the overall natural course between BCF and locoregional CR.
Concurrent ADT with SRT showed significantly improved BCFFS, DMFS, and OS compared to SRT alone in the univariate analysis. Consideration of other important variables in the multivariate analysis showed that concurrent ADT was consistently efficient in reducing the risk of distant metastasis (HR 0.18). The RTOG 9601 trial demonstrated that the addition of ADT to SRT in patients with BCF significantly decreased distant metastasis compared to SRT alone, and also prolonged patient survival. 13 The present study indicated that the major pattern of failure after SRT in patients with locoregional recurrence was distant metastasis rather than locoregional failure, hence more intensive systemic treatment would present a reasonable treatment option.
In our study, 10 patients (17%) underwent SRT to the regional lymphatics for nodal recurrence. There were no significant differences in outcomes between local recurrence and regional recurrence (Table 2); the 7-year BCFFS, LRFFS, DMFS, and OS were 66%, 92%, 84%, and 90% for local recurrence and 70%, 80%, 80%, and 100% for regional recurrence, respectively. Several studies have tested the feasibility of salvage lymph node dissection (LND) in nodal recurrence after RP. Rigatti et al. evaluated the feasibility of salvage LND, including the pelvic and retroperitoneal area in 72 patients with nodal recurrence after RP. 14 They excluded patients who had local recurrence and/or bone metastases. Approximately 20% of patients had received salvage RT prior to salvage LND. The 5-year BCFFS was 19% and preoperative PSA, time to BCF, and negative lymph nodes at initial RP were independent prognostic factors. 14 The 5-year CR-free and cancer-specific survivals were 34% and 75%, respectively. Karnes et al. also reported clinical outcomes of salvage LND for prostate cancer nodal recurrence after RP. 15 Median PSA at recurrence was 2.2 ng/ml. Fifty-two patients underwent salvage LND and the 3-year BCFFS, DMFS and cancer-specific survival were 46%, 47%, and 93%, respectively. 15 Direct comparisons of SRT in patients with regional failure may not be appropriate because the patient distribution is heterogeneous, but the outcome for SRT appears to be non-inferior to that for salvage LND, and SRT would be a useful treatment option for those patient groups. Recently, Ost et al. also demonstrated the efficacy of SRT for oligorecurrent nodal disease in the multicenter phase II trial. 16 At a median follow-up of 3 years, the median ADT-free survival was extended by SRT from 13 months for the surveillance group to 21 months (HR 0.60, P = .11).
Limitations of our study may include its retrospective nature and the relatively small sample size (n = 60), which may have led to unidentified selection biases. However, designing a prospective trial or recruiting sufficient numbers of subjects is challenging in locoregional CR of prostate cancer, because SRT for those patients is a very rare clinical condition. Nevertheless, we were able to include a large number of patients by recruiting multi-institutional patients. Second, most patients (97%) were clinically diagnosed for locoregional recurrence by radiologic evaluation, without pathologic confirmation. The combination MRI technique of T2-weighted imaging and dynamic contrast enhancement with an endorectal coil was found to have a sensitivity of 84% to 97% and a specificity of 74% to 89% for the detection of locoregional recurrence. 7 In addition, PET using 68Ga prostate-specific membrane antigen (PSMA) or 11C-choline are complementary with MRI to localize recurrent lesions in a patient who presented BCF.7,8 However, they were not available during the period of study. Based on these aspects, the recent national survey in Swiss reported a similar practice pattern that a biopsy of the radiologically detected recurrence was not routinely performed. 17 If pathological confirmation was conducted in most cases, this would provide additional biological information on the tumor.
Even though SRT at the time of BCF is the standard care procedure, SRT for macroscopic recurrent disease also achieved favorable clinical outcomes, with a long-term OS of 91.2%. However, the delayed SRT until CR is not recommended especially for the patient who has an unfavorable prognosis such as a higher Gleason score or higher serum PSA level. These results should encourage medical professionals to perform SRT more actively in patients with locoregional recurrence after RP, and combined ADT may have additional benefits by decreasing distant metastasis.
Supplemental Material
Supplemental material, sj-docx-1-tct-10.1177_15330338211041212 for Clinical Outcome of Salvage Radiotherapy for Locoregional Clinical Recurrence After Radical Prostatectomy by Sung Uk Lee, Kwan Ho Cho, Jin Ho Kim, Young Seok Kim, Taek-Keun Nam, Jae-Sung Kim, Jaeho Cho, Seo Hee Choi, Su Jung Shim, Jin Hee Kim and Ah Ram Chang in Technology in Cancer Research & Treatment
Supplemental material, sj-tiff-2-tct-10.1177_15330338211041212 for Clinical Outcome of Salvage Radiotherapy for Locoregional Clinical Recurrence After Radical Prostatectomy by Sung Uk Lee, Kwan Ho Cho, Jin Ho Kim, Young Seok Kim, Taek-Keun Nam, Jae-Sung Kim, Jaeho Cho, Seo Hee Choi, Su Jung Shim, Jin Hee Kim and Ah Ram Chang in Technology in Cancer Research & Treatment
Abbreviations
- ADT
androgen deprivation therapy
- BCF
biochemical failure
- BCFFS
biochemical failure-free survival
- CI
confidence interval
- DMFS
distant metastasis-free survival
- HR
hazard ratio
- IQR
interquartile range
- LRFFS
locoregional failure-free survival
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- PSA
prostate-specific antigen
- RP
radical prostatectomy
- SRT
salvage radiotherapy.
Footnotes
Ethics Statement: The present study was conducted in accordance with the ethical principles of the Declaration of Helsinki and the International Conference on Harmonization guideline E6: Good Clinical Practice. As a principal institution of this multi-institutional study, NCC 2018-0116. And the other participant institutions were also approved by their institutional review boards, respectively. Collected data was managed by assigning case numbers to each hospital and anonymizing them. Data analysis was performed centrally in National Cancer Center, Korea. Written informed consent was not obtained due to the retrospective nature of the study.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a National Cancer Center Grant (NCC 1910300-3) and the Korean Radiation Oncology Group 18-01. The funding source had no role in the study design, data curation, or analysis and interpretation of data.
ORCID iD: Kwan Ho Cho https://orcid.org/0000-0003-3860-6959
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3478 consecutive patients: long-term results. J Urol. 2004;172(3):910-914. doi: 10.1097/01.ju.0000134888.22332.bb [DOI] [PubMed] [Google Scholar]
- 2.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591-1597. doi: 10.1001/jama.281.17.1591 [DOI] [PubMed] [Google Scholar]
- 3.Lee SU, Cho KH, Park W, et al. Clinical outcomes of postoperative radiotherapy following radical prostatectomy in patients with localized prostate cancer: a multicenter retrospective study (KROG 18-01) of a Korean population. Cancer Res Treat. 2020;52(1):167-180. doi: 10.4143/crt.2019.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25(15):2035-2041. doi: 10.1200/jco.2006.08.9607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stish BJ, Pisansky TM, Harmsen WS, et al. Improved metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J Clin Oncol. 2016;34(32):3864-3871. doi: 10.1200/jco.2016.68.3425 [DOI] [PubMed] [Google Scholar]
- 6.Bruni A, Ingrosso G, Trippa F, et al. Macroscopic locoregional relapse from prostate cancer: which role for salvage radiotherapy? Clin Transl Oncol. 2019;21(11):1532-1537. doi: 10.1007/s12094-019-02084-0 [DOI] [PubMed] [Google Scholar]
- 7.Kitajima K, Murphy RC, Nathan MA, et al. Detection of recurrent prostate cancer after radical prostatectomy: comparison of 11C-choline PET/CT with pelvic multiparametric MR imaging with endorectal coil. J Nucl Med. 2014;55(2):223-232. [DOI] [PubMed] [Google Scholar]
- 8.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56(5):668-674. [DOI] [PubMed] [Google Scholar]
- 9.Sandgren K, Westerlinck P, Jonsson JH, et al. Imaging for the detection of locoregional recurrences in biochemical progression after radical prostatectomy—a systematic review. Eur Urol Focus. 2019;5(4):550-560. doi: 10.1016/j.euf.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 10.European Assocation of Urology (EAU) Guidelines on prostate cancer. European Assocation of Urology (EAU). Arnhem. 2012. https://uroweb.org/wp-content/uploads/08-Prostate-Cancer_LR-March-13th-20122.pdf
- 11.Pollack A, Low D, Watkins-Bruner D, et al. Radiation Therapy Oncology Group RTOG 0534: a phase III trial of short term androgen deprivation with pelvic lymph node or prostate bed only radiotherapy (SPPORT) in prostate cancer patients with a rising PSA after radical prostatectomy. Protoc Broadcast. 2011;11:2-3. [Google Scholar]
- 12.Shelan M, Odermatt S, Bojaxhiu B, et al. Disease control with delayed salvage radiotherapy for macroscopic local recurrence following radical prostatectomy. Front Oncol. 2019 Feb 28;9:12. doi: 10.3389/fonc.2019.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376(5):417-428. doi: 10.1056/NEJMoa1607529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigatti P, Suardi N, Briganti A, et al. Pelvic/retroperitoneal salvage lymph node dissection for patients treated with radical prostatectomy with biochemical recurrence and nodal recurrence detected by [11C]choline positron emission tomography/computed tomography. Eur Urol. 2011;60(5):935-943. doi: 10.1016/j.eururo.2011.07.060 [DOI] [PubMed] [Google Scholar]
- 15.Karnes RJ, Murphy CR, Bergstralh EJ, et al. Salvage lymph node dissection for prostate cancer nodal recurrence detected by 11C-choline positron emission tomography/computerized tomography. J Urol. 2015;193(1):111-116. doi: 10.1016/j.juro.2014.08.082 [DOI] [PubMed] [Google Scholar]
- 16.Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453. doi: 10.1200/jco.2017.75.4853 [DOI] [PubMed] [Google Scholar]
- 17.Dal Pra A, Panje C, Zilli T, et al. Salvage radiotherapy for macroscopic local recurrences after radical prostatectomy: a national survey on patterns of practice. Strahlenther Onkol. 2018;194(1):9-16. doi: 10.1007/s00066-017-1172-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tct-10.1177_15330338211041212 for Clinical Outcome of Salvage Radiotherapy for Locoregional Clinical Recurrence After Radical Prostatectomy by Sung Uk Lee, Kwan Ho Cho, Jin Ho Kim, Young Seok Kim, Taek-Keun Nam, Jae-Sung Kim, Jaeho Cho, Seo Hee Choi, Su Jung Shim, Jin Hee Kim and Ah Ram Chang in Technology in Cancer Research & Treatment
Supplemental material, sj-tiff-2-tct-10.1177_15330338211041212 for Clinical Outcome of Salvage Radiotherapy for Locoregional Clinical Recurrence After Radical Prostatectomy by Sung Uk Lee, Kwan Ho Cho, Jin Ho Kim, Young Seok Kim, Taek-Keun Nam, Jae-Sung Kim, Jaeho Cho, Seo Hee Choi, Su Jung Shim, Jin Hee Kim and Ah Ram Chang in Technology in Cancer Research & Treatment