Abstract
Objective
Insomnia is a highly prevalent and disturbing symptom in breast cancer patients under or post chemotherapy. If not appropriately treated, it can persist for years after the completion of cancer treatments. Acupuncture has been widely used for alleviating insomnia. The aim of this study is to examine the feasibility, efficacy and safety of acupuncture for chemotherapy-related insomnia among patients with breast cancer.
Materials and Methods
This is a trial protocol for a randomized, sham-controlled, subject- and assessor-blinded clinical trial. A total of 138 eligible participants will be assigned randomly to acupuncture or sham control group at a ratio of 1:1. Participants in acupuncture group will receive electroacupuncture (EA) plus auricular acupressure (AA) treatment, while subjects in sham acupuncture group will receive sham EA plus sham AA. Both acupuncture and sham treatments will be given twice weekly for 6 weeks, followed by maintenance treatments once every 4 weeks for 12 weeks (15 sessions totally). The primary outcome is the change of Insomnia Severity Index score between baseline and the end of 6-week treatment. Secondary outcome measurements include Actiwatch, sleep diary, Pittsburgh Sleep Quality Index, Functional Assessment of Cancer Therapy-Breast Cancer, Hospital Anxiety and Depression Scale, Brief Pain Inventory-Short Form, Brief Fatigue Inventory, Acupuncture Expectancy Scale, credibility, and adverse events. Participants will be followed up to 42 weeks.
Conclusions
This trial will expand our understanding of the feasibility, efficacy, and safety of acupuncture as a treatment for alleviating chemotherapy-related insomnia in patients with breast cancer. EA plus AA, if proven to be effective, can be implemented into routine settings to play a role in insomnia management for patients with breast cancer.
Keywords: insomnia, breast cancer, chemotherapy, electroacupuncture, auricular acupressure, sham control
Introduction
Insomnia is defined as difficulty initiating or maintaining sleep, or early morning awakenings with inability to fall back to sleep. 1 It is a disturbing and prevalent symptom in cancer patients.2,3 Among patients with breast cancer, the prevalence of insomnia is as high as 69.6%. 4 Though the causes of insomnia in cancer patients are multifaceted, it has been reported that cancer treatments, particularly chemotherapy, play a significant role in the development and aggravation of insomnia.5,6 More than 26.4% of the breast cancer patients undergoing chemotherapy developed new-onset clinical insomnia. 7 Breast cancer patients receive chemotherapy (29.1%) are more likely to develop persistent insomnia than those without chemotherapy (3.3%). 8 The percentage of patients with breast cancer that report sleep disturbances increases from 11.1% pre-chemotherapy to 36.1% post-chemotherapy. 9 The presence of insomnia places cancer patients at higher risks for psychological and physical comorbidity.10,11 It directly impacts cancer patients’ abilities to complete cancer treatments, recover, and ultimately survive with a satisfactory quality of life. If not appropriately treated, it can persist for years after completion of cancer treatments. 12
In conventional medicine, insomnia is commonly treated with hypnotic medications (eg, benzodiazepines and non-benzodiazepines). Although sleep medications are recommended for short-term use, some studies showed that 28% to 39% of cancer patients still used it 1-year following chemotherapy completion 13 or 9-years post cancer diagnosis. 12 As long-term use of sleep medications can lead to undesirable side effects such as addiction and tolerance, 14 it has been suggested that non-pharmacological therapies, such as cognitive behavioral therapy and acupuncture, are the treatment of choice for insomnia in cancer patients.15-17
Acupuncture has been used for thousands of years in China and other Asian countries to treat various diseases, including sleep disturbance. It is a non-pharmacological therapy that involves inserting needles into acupuncture points and sometimes applying mini-electrical current stimulation on acupuncture points via needles, or applying acupressure on the surface of points in different parts of body including ear and scalp. Acupuncture therapy is beneficial for improving sleep efficiency and decreasing hyperarousal level in patients with insomnia.18,19 Many studies have demonstrated its efficacy and safety for insomnia.20,21 Acupuncture is recommended for treating cancer-related symptoms, including gastrointestinal side effects, pain, hot flushes, fatigue, anxiety, and depression.22-25 Despite the popular use of acupuncture in symptoms management in cancer care, the evidence that supports the efficacy and safety of acupuncture in relieving chemotherapy-related insomnia is insufficient.
In the present protocol, we propose a randomized, sham-controlled, subject- and assessor-blinded trial, to examine the efficacy and safety of acupuncture for insomnia among breast cancer patients under or post chemotherapy. The aims of this study are 4-fold: (1) to determine whether the insomnia condition in the acupuncture group is significantly improved when compared to a sham control; (2) to determine whether other symptoms (eg, fatigue, pain, depression, and anxiety) in the acupuncture group improve more than those of the sham control; (3) to explore whether acupuncture is safe for treating chemotherapy-related insomnia in breast cancer patients; and (4) to determine whether a once every 4 weeks maintenance treatment protocol will prolong the effect of acupuncture for insomnia. Our hypothesis is that acupuncture is feasible, effective, and safe for alleviating chemotherapy-related insomnia in breast cancer patients as compared with a sham control.
Materials and Methods
Design
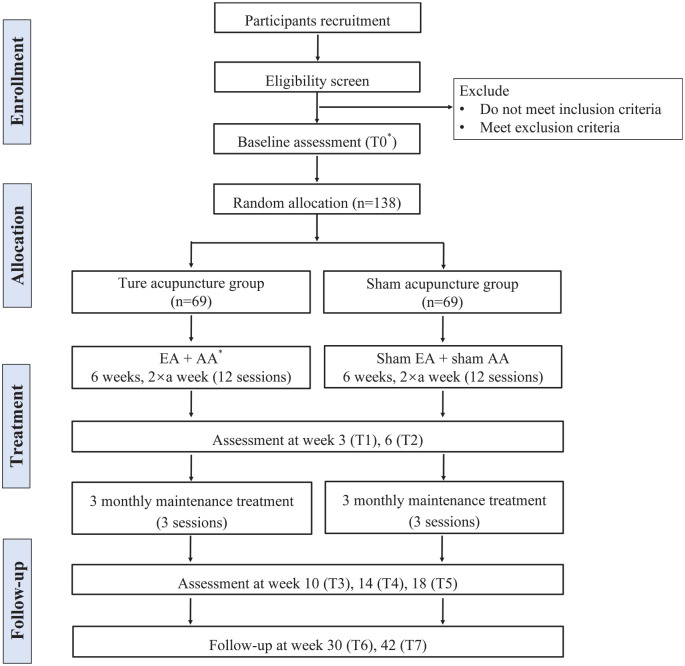
We present a protocol for a randomized, sham-controlled, subject- and assessor-blinded clinical trial. Eligible subjects (n = 138) will be randomly assigned to either an acupuncture group or a sham control group at 1:1 ratio. The study flow diagram is shown in Figure 1. This trial has been approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (Ref no: UW 19-045) and the Research Ethics Committee of the Hong Kong Sanatorium & Hospital Medical Group (REC-2019-14).
Figure 1.
Flow diagram of the electroacupuncture plus auricular acupressure on chemotherapy-related insomnia (EACRI) study.
Abbreviations: T, timepoint; EA, electroacupuncture; AA, auricular acupressure.
Study Procedure
Volunteer subjects will be asked to sign written informed consent forms. After screening, eligible subjects will be enrolled and complete baseline assessments. Thereafter, participants will be randomly assigned to either an acupuncture group or a sham control group. Acupuncture or sham treatments will be given twice weekly for 6 weeks, followed by maintenance treatments once every 4 weeks for 12 weeks, a total of 15 sessions. Participants in both groups will receive routine medical care for symptom management provided by their oncologists, which will include use of anti-histamines or hypnotics.
Participants will be assessed at following time points (see Table 1): baseline (T0), midpoint of main treatment (T1), end of 6-week main treatment (T2, primary outcome measure), once every 4 weeks during the maintenance treatment (T3, T4, T5), and follow-up at week 30 and 42 (T6, T7). Assessments include: Insomnia Severity Index (ISI), Actiwatch, sleep diary, Pittsburgh Sleep Quality Index (PSQI), Hospital Anxiety and Depression Scale (HADS), Brief Pain Inventory-Short Form (BPI-SF), Brief Fatigue Inventory (BFI), Functional Assessment of Cancer Therapy-Breast Cancer (FACT-B), Acupuncture Expectancy Scale (AES), credibility, sleeping pills consumption, and adverse events.
Table 1.
Schedule of Enrollment, Interventions, and Assessments.
| Time point | Enrollment | Baseline | Treatment | Maintenance treatment | Follow-up | ||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | ||
| Week | 0 | 3 | 6 | 10 | 14 | 18 | 30 | 42 | |
| Enrollment | |||||||||
| Eligibility screen | X | ||||||||
| Informed consent | X | ||||||||
| Allocation | X | ||||||||
| Groups | |||||||||
| Acupuncture |

|
||||||||
| Sham acupuncture | |||||||||
| Assessments | |||||||||
| ISI | X | X | X | X | X | X | X | X | X |
| Actiwatch | X | X | |||||||
| Sleep diary | X | X | |||||||
| PSQI | X | X | X | X | X | X | X | X | |
| HADS | X | X | X | X | X | X | X | X | |
| BPI-SF | X | X | X | X | X | X | X | X | |
| BFI | X | X | X | X | X | X | X | X | |
| FACT-B | X | X | X | X | X | X | X | X | |
| AES | X | X | X | ||||||
| Credibility | X | X | |||||||
| Sleeping pills | X | X | X | X | X | X | X | X | |
| Adverse events | X | X | X | X | X | X | X | X | |
Abbreviations: T, timepoint; ISI, Insomnia Severity Index; PSQI, Pittsburgh Sleep Quality Index; HADS, Hospital Anxiety and Depression Scale; BPI-SF, Brief Pain Inventory-Short Form; BFI, Brief Fatigue Inventory; FACT-B, Functional Assessment of Cancer Therapy-Breast Cancer; AES, Acupuncture Expectancy Scale.
Recruitment
Breast cancer patients with complaints of insomnia will be referred from Hong Kong Queen Mary Hospital and Hong Kong Sanatorium & Hospital by oncologists. To further facilitate recruitment, multiple promotions (eg, adding more sites, advertising on local newspaper) will be considered as needed.
Inclusion criteria
Subjects meet the following criteria will be included:
(1) Female patients between 18 and 75 years of age.
(2) Diagnosis of stage (American Joint Committee on Cancer TNM) I to IV breast cancer.
(3) Currently undergoing chemotherapy, or have completed chemotherapy no more than 6 months previously.
(4) Insomnia occurs at least 3 nights/week, and presents for at least 1 month, with fulfilment of the diagnostic criteria for brief insomnia disorder of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).
(5) Insomnia severity as defined by an ISI score of no less than 10 in the past 2 weeks.
(6) Expected survival time of more than 6 months.
(7) Ability to understand the nature of the study and willingness to give informed consent.
(8) Ability to provide responses during outcome measurement.
Exclusion criteria
Participants who report any of the following conditions will be excluded:
(1) Other sleep disorders (eg, obstructive sleep apnea).
(2) Shift work or irregular sleep pattern.
(3) Severe visual, hearing, or language defects.
(4) Severe hematological dysfunction (platelet count <60 000/μL, hemoglobin <8 g/dL, or absolute neutrophil count <1000/μL).
(5) With pacemakers or other electronic implants that could interfere with electroacupuncture.
(6) History of acupuncture use in previous 3 months.
(7) Participation in other clinical trials with intervention in previous 3 months.
Randomization and Allocation Concealment
Block randomization with random block sizes (block sizes of 2, 4, and 6) is used. After completion of baseline assessments, participants will be randomly assigned to 2 groups in a 1:1 ratio according to the randomization sequence. This sequence is generated by an independent biostatistician with MS Excel prior to recruitment. Randomization information will be sealed in sequentially numbered opaque envelopes. Each envelope will be opened by acupuncturists after the participant completes baseline assessments.
Blinding
Except acupuncturists, participants and all other researchers, including those performing on-site screening, outcome assessments, data entry/re-entry, and data analysis, will be blinded to the group assignments. Participants are informed that they have a similar chance of allocation to either group and will be blinded to group assignments. Treatments for each participant are delivered in separate rooms to avoid communications regarding their treatment experience among participants. An eye mask is used to block the view from seen by the participant during treatment. Acupuncturists will be blinded to any other information of participants, such as hypnotic medications usage. To avoid accidental disclosure of group allocation by the acupuncturists, their interactions with the participants will be kept to a minimum.
Interventions
The acupuncture and sham acupuncture treatments will be performed in the out-patient clinic setting at School of Chinese Medicine, The University of Hong Kong. Registered Chinese medicine practitioners with at least 5 years’ acupuncture experience will be recruited and responsible for delivering treatment. The registered practitioners will receive a pre-job training from the principal investigators on the standard conversation with the participants and the standard procedures for conducing real and sham acupuncture treatment. The Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) 26 will be followed throughout the trial.
Acupuncture group
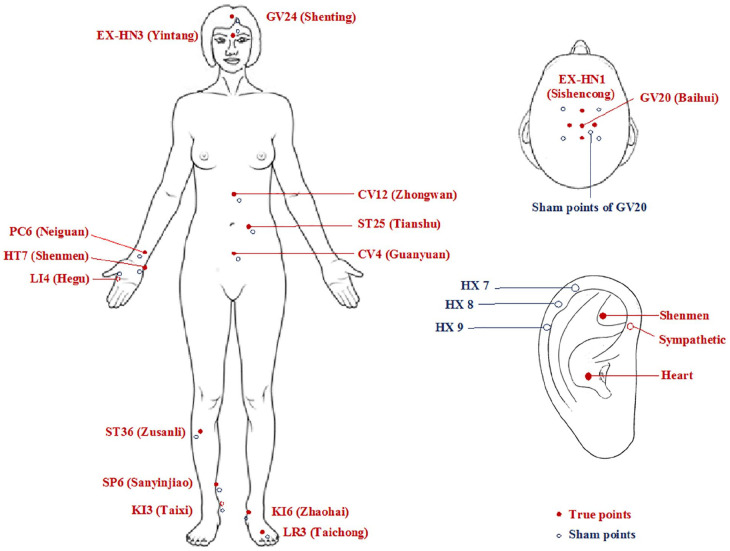
Subjects in acupuncture group will receive electroacupuncture (EA) and auricular acupressure (AA) treatment. Twelve sessions of treatment will be given twice weekly for 6 weeks, followed by 3 sessions once every 4 weeks for 12 weeks (total of 15 sessions). All points are selected based on clinical experience of acupuncture experts, previous clinical trials, 20 and acupuncture textbook. 27
For EA treatment, a semi-standardized acupoint prescription will be used. The prescription includes 10 points (see Figure 2), including 6 fixed points that are the frequently used to treat insomnia 28 and 4 additional points based on participants’ syndrome to address their particular constitutions. The 6 fixed points are: GV24 (Shenting), GV20 (Baihui), EX-HN1 (Sishencong), PC6 (Neiguan), SP6 (Sanyinjiao), and KI3 (Taixi). The 4 acupoints will be selected from the following list: EX-HN3 (Yintang), HT7 (Shenmen), LI4 (Hegu), CV4 (Guanyuan), CV12 (Zhongwan), ST25 (Tianshu), ST36 (Zusanli), KI6 (Zhaohai), LR3 (Taichong), and any other points if necessary. After cleansing of the skin on acupuncture points with alcohol swab, a sterilized disposable filiform needle with a guiding tube, 25 or 40 mm long and 0.2 mm in diameter, will be inserted into each point. Acupuncturists’ Deqi sensation will be achieved. 29 Four pairs of electrodes from the electric stimulator (AWQ-104L, USA; 2-5 Hz, continuous wave) will be connected to the end of the needles. The needles will remain in place for 25 minutes. Upon needle withdrawal, the points will be compressed with a clean cotton ball to prevent bleeding.
Figure 2.
Acupuncture points and sham points.
For AA treatment, Vaccaria seeds (Wang Bu Liu Xing, 1-2 mm in diameter, black and solid sphere) will be embedded by acupuncturists on surface of 3 auricular points (Shenmen, Sympathetic, and Heart) and maintained between EA treatments. Subjects will be asked to gently press the seeds thrice daily.
Sham acupuncture group
The sham EA treatment procedure is the same as in acupuncture group, except that sham points will be located 1 to 2 cm exterior and inferior to real points, outside the meridians, 30 and no needle penetration or electric stimulation will be performed. Streitberger sham device, non-invasive retractable blunt tipped needles, will be applied. 31 The handles of these placebo needles will slide over the needle when it is compressed, giving the appearance of needle insertion. The needles will be held by surgical tape and connected to an electric stimulator, but no electric current will be delivered. The acupuncturists will avoid eliciting the Deqi sensation.
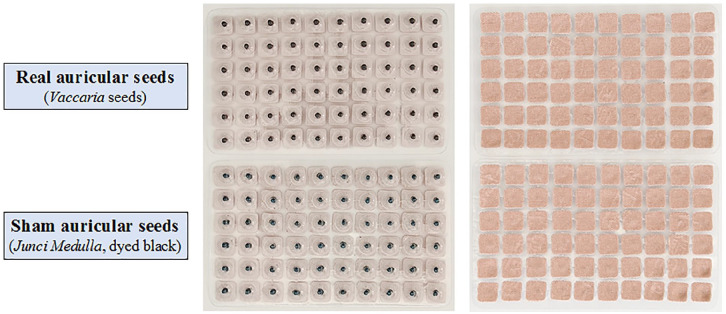
For sham AA treatment, 3 sham auricular points in helix region (HX7, HX8, HX9; see Figure 2) will be selected. These points are remote from the inner ear area, with no known effect on insomnia. 32 Instead of Vaccaria seeds, soft Junci Medulla (1-2 mm in length, dyed black) is used to mimic real AA with no pressure (see Figure 3). 33
Figure 3.
Real and sham auricular seeds.
Outcome Measures
Primary outcome
The primary outcome will be the mean change of ISI score between baseline and the end of 6-week treatment. ISI is a 7-item questionnaire devised to assess the severity of insomnia. 34 The total score ranges from 0 to 28. ISI score is interpreted as follows: no clinically significant insomnia (0-7), subthreshold insomnia (8-14), moderate insomnia (15-21), and severe insomnia (22-28). ISI has favorable internal consistency (Cronbach’s alpha of .9) and good construct validity for diagnosing insomnia in cancer patients. 35 A cut-off score of 10 has optimal sensitivity (86.1%) and specificity (87.7%) for detecting insomnia. 34 ISI score less than 8 at posttreatment, which has been found to indicate the absence of insomnia, is used as the insomnia remission criterion. 36
Secondary outcomes
The secondary outcomes include the response rate (the proportion of subjects with ≥50% reduction from the baseline ISI scores), the remission rate (the proportion of subjects with ISI scores <8), sleep quality measured by Actiwatch and sleep diary, and changes of PSQI, HADS, BPI-SF, BFI, and FACT-B scores from baseline.
(1) To objectively measure the sleep patterns of subjects, Actiwatch (Spectrum Plus, Philips Respironics; USA), a physical activity monitoring device, will be used. Subjects will be instructed to wear Actiwatch on their non-dominant wrists for 7 consecutive nights at baseline and the end of 6-week treatment. Actiware 6.0.9 (Philips Respironics; USA) will be used to analyze subjects’ sleep information recorded in Actiwatch.
(2) Sleep diary is a subjective measure of sleep quality. Sleep diary are considered a reliable and valid patient report of nightly insomnia symptoms. 37 Subjects will be given daily sleep diary for 1 week at baseline and the end of 6-week treatment. Subjects are instructed to record the bedtime and rising time, from which the total time in bed (TIB) is calculated. They are advised to estimate the sleep onset latency, wake time after sleep onset, and total sleep time (TST). Sleep efficiency is calculated as TST/TIB × 100%. Subjects are required to record their sleeping pills consumption in diary.
(3) PSQI is a valid self-reported questionnaire for assessing sleep dysfunction in cancer patients.38,39 This 19-item questionnaire comprise 7 components including subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleeping pills, and daytime dysfunction. The global score ranges from 0 to 21, with higher score indicating poorer sleep quality. Satisfactory factorial and concurrent validity, good internal consistency (Cronbach’s alpha of .75), and test-retest reliability have been demonstrated. 40
(4) HADS is a 14-item self-reported questionnaire with 2 subscales to evaluate severity of depressive (7 items) and anxiety (7 items) symptoms. Each item is scored on a 0 to 3 scale and the total score of each subscale ranges 0 to 21. 41 A score of 8 or higher on the depression or anxiety subscale indicates the presence of depressive or anxiety symptoms. HADS has good internal consistency (Cronbach’s alpha of .67-.90 for depression subscale, and .68-.93 for anxiety subscale), optimal sensitivity, and specificity assessing the depressive and anxiety symptom severity.42,43
(5) BPI-SF is a self-administered questionnaire developed to assess pain severity (4 items) and pain interference on daily function (7 items) for the past 24 hours. 44 Each item of pain severity and pain interference is scored by a 0 to 10 numerical rating scale. The total score is computed as the mean of responses to all items. BPI-SF has good internal reliability (Cronbach’s alpha of .89 for the pain severity scale, and .91 for the pain interference scale), high test-retest reliability (r value of .93), and good concurrent validity (r values in the range of .77-.86). 45
(6) BFI is a brief screening tool designed to assess the severity and impact of cancer-related fatigue on daily functioning. 46 It is a 9-item, 0 to 10 numerical rating scale. Higher scores on BFI correspond to greater self-reported levels of fatigue. BFI has good internal consistency (Cronbach’s alpha of .92 for fatigue severity items, and .90 for fatigue interference items) and external validity in measuring the severity and impact of cancer-related fatigue among Chinese patients. 46
(7) FACT-B (version 4) is a 37-item self-reported instrument devised to assess multidimensional health-related quality of life in patients with breast cancer. It consists of 5 subscales include physical, social/family, emotional, functional well-beings, and additional concerns for breast cancer called Breast Cancer Subscale. Each item is scored on a 0 to 4 scale and the sum of all 5 subscales ranges from 0 to 144. A higher score indicates a better quality of life. 47 The scale has acceptable internal consistency (Cronbach’s alpha of .59-.85 for 5 subscales) and test–retest reliability (r value of .82-.91). 48
Safety assessment
For safety assessment, participants will be asked whether they have experienced any adverse events on each visit. The severity of AEs will be assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 criteria. 49 Causality between acupuncture and AEs will be assessed. Serious adverse events will be reported to project investigators and Ethics Committee immediately, who will make a decision on whether the participant needs to temporarily interrupted or withdrawn from the study, or whether the study should be adjusted or terminated.
Credibility and expectancy test
The credibility and expectancy of the treatment are regarded as decisive variables in nonspecific treatment effects. 50 The credibility of the treatment is assessed with the 4-item Credibility Rating Scale. 51 Participants’ expectancy for clinical outcomes of treatment is assessed by the 4-item AES. 52
Blinding success assessment
The success of blinding to treatment is assessed after the third and last treatment session by asking participants the following question: “When you volunteered for the trial, you were informed that you had a similar chance of receiving traditional acupuncture or acupuncture-like simulation treatment. Which acupuncture treatment do you think you have received?” Three options are provided to choose from: acupuncture treatment, acupuncture-like simulation treatment, and uncertain. Those who choose acupuncture treatment or acupuncture-like simulation treatment will be asked to provide a reason. 53
Patient and Public Involvement
Patients will not be involved in study design including research question and outcome measures setting. The results will be disseminated to the participants in a short summary after the publication of the trial.
Data Management and Monitoring
An independent Data and Safety Monitoring Board (DSMB) is formed. 54 The Board includes independent experts of a statistician, an oncologist, and a researcher of insomnia. They are not part of the research team and will not be involved in the conduct of the trial. Regular meetings will be scheduled to review the progress of the trial.
Data will be collected by independent assessors who are blinded to group assignment. Data will be entered in password-protected computers using double-entry strategy. All collected data will be secured in compliance with Hong Kong Personal Data (Privacy) Ordinance (CAP 486). To enhance participant retention rate and prevent loss of follow-up, we will send text messages or make phone calls to participants the day before the scheduled visits. The travel allowance to each participant will be offered after the completion of the study. The whole process of the trial will be monitored regularly to ensure the quality. The research team will hold weekly meetings to troubleshoot issues pertaining to participant recruitment, data collection, and retention issues.
Sample Size Estimation
The sample is estimated based on the anticipated changes of the primary outcome (ISI score). Based on 2 previous trials,20,55 in which the reduction of ISI score ranges between 2.3 and 5.0, a middle ISI reduction of 2.5 with standard deviation of 4.7 in both groups is used for the sample size estimation. To detect a mean difference in ISI score reduction of 2.5 with a 95% level of significance (α) and 80% power (1 − β), the sample size for each group is 69 subjects, considering a 20% dropout. Therefore, this trial will include 138 subjects.
Statistical Analyses
Study hypothesis and primary outcome
The primary study hypothesis is that EA plus AA is more effective than sham EA plus sham AA in reducing ISI score at the end of week 6 in breast cancer patients with chemotherapy-related insomnia.
Analytic approach
All randomized participants will be included in the intent-to-treat population. The safety population is defined as randomized subjects who received at least 1 treatment. Summary tables will be provided for all variables. For continuous variables, means, and standard deviations (or medians, 25th, and 75th percentiles) will be presented. For categorical variables, numbers, and percentages will be presented.
The changes of ISI score (primary outcome) will be compared using a mixed-effect model adjusted for the baseline value, with time, group, and interaction between time and group as the fixed effects, and individual subject as the random effect. The same method will be used for analyses of all other continuous outcomes. Categorical outcomes will be compared between groups using a Wilcoxon rank-sum test or Fisher’s exact test. We will use a multiple imputation method under the missing at random (MAR) assumption for the primary outcome with missing data. To examine the sensitivity of MAR assumptions of missing data, we will perform a sensitivity analysis under the missing not at random (MNAR) assumption. 56 To explore the relationship between patients’ expectations and the primary outcome, a general linear model will be used. To assess whether the blinding of this randomized controlled trial is successful, the Bang’s 57 blinding index will be calculated. Fisher’s exact test will be used to compare the incidence of treatment related adverse events between groups. For all statistical analyses, SAS 9.4 software will be used. Hypothesis testing will be carried out at the 5% (2-sided) significance level.
Discussion
This study aims to explore the feasibility, efficacy and safety of using acupuncture to treat chemotherapy-related insomnia in patients with breast cancer. Insomnia is highly prevalent in cancer patients undergoing chemotherapy.58,59 Acupuncture has been widely used to treat insomnia and has been demonstrated as a safe and effective treatment for primary insomnia. 20 However, the long-term effect and safety of EA plus AA for insomnia in breast cancer patients under or post chemotherapy have not been reported in larger sample size trials. 60
In the present paper, we present a rigorously designed clinical trial to explore the feasibility, efficacy and safety of EA plus AA for treating chemotherapy-related insomnia in breast cancer patients. This protocol has a few unique and innovative features. Initially, we are recruiting participants from diverse backgrounds with no restrictions on cancer stage nor chemo agents, which can help us understand how acupuncture generalizes to various subsets of the population. We also use a semi-standardized acupuncture regimen to accommodate individual treatment needs. Furthermore, we adopt maintenance treatment (once every 4 weeks for 12 weeks) after 6-week treatment and long-term follow-up evaluation (12 and 24 weeks after the completion of acupuncture treatment). The maintenance treatment is a novel approach for prolonging the effect of acupuncture in managing cancer-related insomnia. Most studies on insomnia have had relatively short follow-up time (eg, 4 20 or 12 weeks 17 post-treatment), and the long-term effect of acupuncture has not been investigated and reported. The findings of this study will provide useful information on determining an additional benefit for the patients with insomnia which is cost-effective (the maintenance treatment only requires once every 4 weeks). Another unique feature of our design is that we will use a novel sham auricular pressure device that is a soft herbal material known as Junci Medulla which is dyed with same color and look as the real herbal Vaccaria seeds, but provides no non-specific pressure induced effect to the ear auricle. In order to ensure the data safety, high quality, and integrity of this trial, we will form an independent Data and Safety Monitoring Board to monitor the entire process of the trial. This rigorous design has not been widely reported by other acupuncture trials.
It is hoped that this study will provide strong evidence on the efficacy and safety of acupuncture for treating chemotherapy-related insomnia. EA and AA, if proven to be effective, can be implemented into routine settings to benefit breast cancer patient suffering from insomnia.
Acknowledgments
We acknowledge the DSMB members, Dr. Vincent Chi Ho Chung (The Jockey Club School of Public Health and Primary Care Faculty of Medicine, The Chinese University of Hong Kong), Dr. Lam Tai Chung (Department of Clinical Oncology, The University of Hong Kong), and Dr. Jihui Zhang (Department of Psychiatry, The Chinese University of Hong Kong) for reviewing the study design and monitoring the progress of this trial.
Footnotes
Author Contributions: JLZ drafted the manuscript. LXL, ZJZ, JLZ, MXY, HYC, KFC, WFY, WLL participated in the design of the study. FJ and ZSQ contributed to the statistical analysis plan. THS and TYC provided the source of participants. All authors approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This trial is funded by National Key R&D Program of China (Ref no: 2018YFC1705801), and Health and Medical Research Fund, Government of the Hong Kong Special Administrative Region of China Health and Medical Research Fund (Ref no: 16172761). The funding bodies are not involved in the study design and conduct, data collection, management, analysis, and interpretation.
Trial Status: This trial is now recruiting participants.
ORCID iDs: Jialing Zhang  https://orcid.org/0000-0003-0900-4617
https://orcid.org/0000-0003-0900-4617
Haiyong Chen  https://orcid.org/0000-0002-4889-2752
https://orcid.org/0000-0002-4889-2752
References
- 1. American Psychiatric Pub. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub; 2013. [Google Scholar]
- 2. Delgado-Guay M, Yennurajalingam S, Parsons H, Palmer JL, Bruera E. Association between self-reported sleep disturbance and other symptoms in patients with advanced cancer. J Pain Symptom Manag. 2011;41:819-827. [DOI] [PubMed] [Google Scholar]
- 3. Cheng KK, Yeung RM. Impact of mood disturbance, sleep disturbance, fatigue and pain among patients receiving cancer therapy. Eur J Cancer Care. 2013;22:70-78. [DOI] [PubMed] [Google Scholar]
- 4. Savard J, Ivers H, Villa J, Caplette-Gingras A, Morin CM. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol. 2011;29:3580-3586. [DOI] [PubMed] [Google Scholar]
- 5. Savard J, Ivers H, Savard MH, Morin CM. Cancer treatments and their side effects are associated with aggravation of insomnia: results of a longitudinal study. Cancer. 2015;121:1703-1711. [DOI] [PubMed] [Google Scholar]
- 6. Palesh O, Peppone L, Innominato PF, et al. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep. 2012;4:151-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoang HTX, Molassiotis A, Chan CW, Nguyen TH, Liep Nguyen V. New-onset insomnia among cancer patients undergoing chemotherapy: prevalence, risk factors, and its correlation with other symptoms. Sleep Breath. 2020;24:241-251. [DOI] [PubMed] [Google Scholar]
- 8. Fleming L, Randell K, Stewart E, et al. Insomnia in breast cancer: a prospective observational study. Sleep. 2019;42:zsy245. [DOI] [PubMed] [Google Scholar]
- 9. Fakih R, Rahal M, Hilal L, et al. Prevalence and severity of sleep disturbances among patients with early breast cancer. Indian J Palliat Care. 2018;24:35-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haque R, Hsu JW, Avila C, Olmstead R, Carroll JE, Irwin MR. Insomnia and susceptibility to depressive symptoms and fatigue in diverse breast cancer survivors. J Womens Health. Published online October 9, 2020. doi: 10.1089/jwh.2019.8135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleming L, Gillespie S, Espie CA. The development and impact of insomnia on cancer survivors: a qualitative analysis. Psychooncology. 2010;19:991-996. [DOI] [PubMed] [Google Scholar]
- 12. Purnell JQ, Mustian K, Jean-Pierre P, et al. The psychosocial and functional impact of radiation therapy. In: ALERT-Adverse Late Effects of Cancer Treatment (Rubin P., Constine L. S., Marks L. B., eds.), Springer; 2014:257-272. [Google Scholar]
- 13. Moore TA, Berger AM, Dizona P. Sleep aid use during and following breast cancer adjuvant chemotherapy. Psychooncology. 2011;20:321-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895-908. [DOI] [PubMed] [Google Scholar]
- 15. Howell D, Oliver TK, Keller-Olaman S, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol. 2014;25:791-800. [DOI] [PubMed] [Google Scholar]
- 16. Ma Y, Hall DL, Ngo LH, Liu Q, Bain PA, Yeh GY. Efficacy of cognitive behavioral therapy for insomnia in breast cancer: a meta-analysis. Sleep Med Rev. 2021;55:101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garland SN, Xie SX, DuHamel K, et al. Acupuncture versus cognitive behavioral therapy for insomnia in cancer survivors: a randomized clinical trial. J Natl Cancer Inst. 2019;111:1323-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fu C, Zhao N, Liu Z, et al. Acupuncture improves peri-menopausal insomnia: a randomized controlled trial. Sleep. 2017;40:5. [DOI] [PubMed] [Google Scholar]
- 19. Liu Y, Feng H, Liu W, et al. [Regulation action and nerve electrophysiology mechanism of acupuncture on arousal state in patients of primary insomnia]. Zhongguo Zhen Jiu. 2017;37:19-23. [DOI] [PubMed] [Google Scholar]
- 20. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193-200. [DOI] [PubMed] [Google Scholar]
- 21. Bergdahl L, Broman JE, Berman AH, Haglund K, von Knorring L, Markström A. Sleep patterns in a randomized controlled trial of auricular acupuncture and cognitive behavioral therapy for insomnia. Complement Ther Clin Pract. 2017;28:220-226. [DOI] [PubMed] [Google Scholar]
- 22. Greenlee H, DuPont-Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. 2017;67:194-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hershman DL, Unger JM, Greenlee H, et al. Effect of acupuncture vs sham acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early-stage breast cancer: a randomized clinical trial. JAMA. 2018;320:167-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lesi G, Razzini G, Musti MA, et al. Acupuncture as an integrative approach for the treatment of hot flashes in women with breast cancer: a prospective multicenter randomized controlled trial (AcCliMaT). J Clin Oncol. 2016;34:1795-1802. [DOI] [PubMed] [Google Scholar]
- 25. Molassiotis A, Bardy J, Finnegan-John J, et al. Acupuncture for cancer-related fatigue in patients with breast cancer: a pragmatic randomized controlled trial. J Clin Oncol. 2012;30:4470-4476. [DOI] [PubMed] [Google Scholar]
- 26. MacPherson H, Altman DG, Hammerschlag R, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLoS Med. 2010;7:e1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang J, Zhao B, Lao L. Acupuncture and Moxibustion (International Standard Library of Chinese Medicine). People’s Medical Publishing House; 2014. [Google Scholar]
- 28. Yeung W-F. Acupuncture for Insomnia: A Systematic Review and Randomized Placebo-Controlled Trials. University of Hong Kong; 2010. [Google Scholar]
- 29. Yang XY, Shi GX, Li QQ, Zhang ZH, Xu Q, Liu CZ. Characterization of deqi sensation and acupuncture effect. Evid Based Complement Alternat Med. 2013;2013:319734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang XG, Ying L, Tian XP, Liang FR. Comments on selection of non-acupoints beyond meridians in studies of acupuncture and moxibustion. J Tradit Chin Med. 2010;30:309-313. [DOI] [PubMed] [Google Scholar]
- 31. Streitberger K, Kleinhenz J. Introducing a placebo needle into acupuncture research. Lancet. 1998;352:364-365. [DOI] [PubMed] [Google Scholar]
- 32. Zou C, Yang L, Wu Y, et al. Auricular acupressure on specific points for hemodialysis patients with insomnia: a pilot randomized controlled trial. PLoS One. 2015;10:e0122724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suen LK, Wong TK, Leung AW. Effectiveness of auricular therapy on sleep promotion in the elderly. Am J Chin Med. 2002;30:429-449. [DOI] [PubMed] [Google Scholar]
- 34. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the insomnia severity index in cancer patients. Psychooncology. 2005;14:429-441. [DOI] [PubMed] [Google Scholar]
- 36. Savard J, Savard MH, Ivers H. Moderators of treatment effects of a video-based cognitive-behavioral therapy for insomnia comorbid with cancer. Behav Sleep Med. 2018;16:294-309. [DOI] [PubMed] [Google Scholar]
- 37. Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. [DOI] [PubMed] [Google Scholar]
- 39. Ho RT, Fong TC. Factor structure of the Chinese version of the Pittsburgh sleep quality index in breast cancer patients. Sleep Med. 2014;15:565-569. [DOI] [PubMed] [Google Scholar]
- 40. Chong AML, Cheung CK. Factor structure of a Cantonese-version Pittsburgh sleep quality index. Sleep Biol Rhythms. 2012;10:118-125. [Google Scholar]
- 41. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361-370. [DOI] [PubMed] [Google Scholar]
- 42. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale: an updated literature review. J Psychosom Res. 2002;52:69-77. [DOI] [PubMed] [Google Scholar]
- 43. Leung CM, Wing YK, Kwong PK, Lo A, Shum K. Validation of the Chinese-Cantonese version of the hospital anxiety and depression scale and comparison with the Hamilton rating scale of depression. Acta Psychiatr Scand. 1999;100:456-461. [DOI] [PubMed] [Google Scholar]
- 44. Mendoza T, Mayne T, Rublee D, Cleeland C. Reliability and validity of a modified brief pain inventory short form in patients with osteoarthritis. Eur J Pain. 2006;10:353-361. [DOI] [PubMed] [Google Scholar]
- 45. Dhingra L, Lam K, Homel P, et al. Pain in underserved community-dwelling Chinese American cancer patients: demographic and medical correlates. Oncologist. 2011;16:523-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang XS, Hao X-S, Wang Y, et al. Validation study of the Chinese version of the brief fatigue inventory (BFI-C). J Pain Symptom Manag. 2004;27:322-332. [DOI] [PubMed] [Google Scholar]
- 47. Ng R, Lee CF, Wong NS, et al. Measurement properties of the English and Chinese versions of the functional assessment of cancer therapy-breast (FACT-B) in Asian breast cancer patients. Breast Cancer Res Treat. 2012;131:619-625. [DOI] [PubMed] [Google Scholar]
- 48. Wan C, Zhang D, Yang Z, et al. Validation of the simplified Chinese version of the FACT-B for measuring quality of life for patients with breast cancer. Breast Cancer Res Treat. 2007;106:413-418. [DOI] [PubMed] [Google Scholar]
- 49. U.S. Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) v5. 0. Published November 27, 2017. [Google Scholar]
- 50. Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31:73-86. [DOI] [PubMed] [Google Scholar]
- 51. Vincent C. Credibility assessment in trials of acupuncture. Complement Med Res. 1990;4:8. [Google Scholar]
- 52. Mao JJ, Armstrong K, Farrar JT, Bowman MA. Acupuncture expectancy scale: development and preliminary validation in China. Explore. 2007;3:372-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lao L, Bergman S, Hamilton GR, Langenberg P, Berman B. Evaluation of acupuncture for pain control after oral surgery: a placebo-controlled trial. Arch Otolaryngol Head Neck Surg. 1999;125:567-572. [DOI] [PubMed] [Google Scholar]
- 54. National Institutes of Health. Data and Safety Monitoring Board (DSMB) Guidelines. National Institutes of Health; 2018. [Google Scholar]
- 55. Yeung WF, Chung KF, Zhang SP, Yap TG, Law AC. Electroacupuncture for primary insomnia: a randomized controlled trial. Sleep. 2009;32:1039-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Little RJ, D'Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367:1355-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bang H. Random guess and wishful thinking are the best blinding scenarios. Contemp Clin Trials Commun. 2016;3:117-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology program. J Clin Oncol. 2010;28:292-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Savard J, Liu L, Natarajan L, et al. Breast cancer patients have progressively impaired sleep-wake activity rhythms during chemotherapy. Sleep. 2009;32:1155-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang JL, Qin ZS, So TH, et al. Electroacupuncture Plus Auricular Acupressure for Chemotherapy-Associated Insomnia in Breast Cancer Patients: A Pilot Randomized Controlled Trial. Integr Cancer Ther. 2021;20:15347354211019103. [DOI] [PMC free article] [PubMed] [Google Scholar]