Abstract
Safety biomarkers are important drug development tools, both preclinically and clinically. It is a straightforward process to correlate the performance of nonclinical safety biomarkers with histopathology, and ideally, the biomarker is useful in all species commonly used in safety assessment. In clinical validation studies, where histopathology is not feasible, safety biomarkers are compared to the response of standard biomarkers and/or to clinical adjudication. Worldwide, regulatory agencies have put in place processes to qualify biomarkers to provide confidence in the manner of use and interpretation of biomarker data in drug development studies. This paper describes currently qualified safety biomarkers which can be utilized to monitor for nephrotoxicity and cardiotoxicity and ongoing projects to qualify safety biomarkers for liver, skeletal muscle, and vascular injury. In many cases, the development and use of these critical drug development tools is dependent upon partnerships and the precompetitive sharing of data to support qualification efforts.
Keywords: Biomarker qualification, safety biomarkers, novel methodologies, drug-induced tissue injury, translational biomarkers, drug development tools
Impact statement
Safety biomarkers are critical tools for drug development. Worldwide, regulatory agencies have put procedures in place to qualify biomarkers to provide confidence in the manner of use and interpretation of biomarkers data in drug development studies. To date, clinical safety biomarkers of nephrotoxicity and cardiotoxicity have been qualified for use. Ongoing projects include safety biomarkers for liver, skeletal muscle, and vascular injury. This manuscript aims to increase awareness about qualified safety biomarkers to increase their use as more specific and selective tools to address patient safety in drug development programs and to increase interest in participation in the qualification process especially through data sharing.
Introduction
Safety biomarkers are important drug development tools, both preclinically and clinically. The process of correlating nonclinical safety biomarker performance with histopathology is straightforward. Ideally, the biomarker is useful in all species commonly used in safety assessment (rat, dog, nonhuman primate). Although most translational safety biomarkers have focused on the relationship between the biomarker and histopathology (histopathology as the hard endpoint or truth), the response of a biomarker can be correlated with other hard endpoints such as functional measures or other imaging endpoints. In this situation, the measured regulatory accepted endpoint can be substituted for histopathology. In clinical studies, where histopathology is not feasible, safety biomarkers are compared to the current standard biomarker response and/or clinical adjudication.
A formal regulatory qualification process has been developed to ensure that safety biomarkers are reliable to support the drug development process and decision making in the regulatory setting, as well as patient safety in clinical trials. The process for safety biomarker qualification has been described in detail recently. 1 In the United States, the qualification process was initiated in response to FDA’s Critical Path Initiative, a strategy to allow for the introduction and use of innovative approaches in the development, evaluation, and manufacturing of medical products. 2 Like FDA, other regulatory agencies, EMA 3 and PMDA 4 have developed similar programs.
Recent efforts involving multiple stakeholders have focused on defining the level of evidence and the analytical requirements needed to qualify a biomarker. 5 In 2016, the Evidentiary Criteria Writing Group of the FNIH Biomarker Consortium produced a document which introduced a proposed framework for the evidentiary criteria to support the qualification process. The intention of this framework is broad applicability across all categories of biomarkers and context of use (COU) statements. 6 In 2018, the FDA released its “Biomarker Qualification: Evidentiary Framework Guidance for Industry and FDA staff.” 7 The document acts as a guide to addressing general considerations necessary for development of a biomarker for qualification under the 21st Century Cures Act. The 21st Century Cures Act added a new section (Section 507) to the Federal Food, Drug, and Cosmetic Act, entitled “Qualification of Drug Development Tools.” In addition, Critical Path Institute’s Biomarker Assay Collaborative Evidentiary Considerations Writing Group produced a Points to Consider document outlining the regulatory and scientific factors for analytical validation of assays used to support biomarker qualification in biological matrices. 8
Consortia are responsible for many of the currently qualified or ongoing safety biomarker projects. For example, the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium (PSTC) is a forum for pharmaceutical companies to communicate in a precompetitive space to validate groundbreaking safety testing methods under advisement of worldwide regulatory agencies including FDA, EMA, and PMDA. PSTC’s strategy is to use nonclinical tissue/organ-specific biomarker data, correlated with histopathology, in the translational qualification of clinical safety biomarkers. This strategy is comparable to the use of nonclinical information in the drug approval process, e.g. using nonclinical data to develop the relationship between tissue injury as evaluated by histopathology and the safety biomarker’s response. PSTC’s nonclinical safety biomarker projects have supported the qualification of novel nonclinical biomarkers of nephrotoxicity with regulatory agencies worldwide and the FDA’s qualification of clinical biomarkers of nephrotoxicity.
This paper describes the current state of nonclinical and clinical safety biomarkers that have been qualified with regulatory agencies including FDA, EMA, and PMDA as well as ongoing projects that have been accepted by FDA into the qualification process. These biomarker qualification projects utilize aggregated data from a number of sources to support the intended COU. It is hoped that the wide dissemination of information about qualified safety biomarkers will increase their use as more specific and selective tools to address patient safety in drug development programs and increase interest in participation in the qualification process, especially through data sharing.
FDA biomarker qualification process and the 21st Century Cures Act
The Biomarkers, EndpointS and other Tools (BEST) glossary 9 labels a biomarker as “a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions.” The glossary also lists the categories of biomarkers as susceptibility/risk, diagnostic, monitoring, prognostic, predictive, pharmacodynamic/response and safety and provides clear definitions of each. A safety biomarker is defined as a “biomarker measured before or after an exposure to a medical product or an environmental agent to indicate the likelihood, presence, or extent of toxicity as an adverse effect.” 9 The FDA’s Biomarker Qualification Program (BQP) is a collaborative process in which requestors work together with FDA to develop a biomarker for qualification. The process depends on data and resource sharing in order to provide new tools to help speed the drug development process.
The 21st Century Cures Act became law in December 2016 and was designed to increase the speed and efficiency of medical product innovation and development. Biomarker qualification is a defined, three-stage procedure under Section 507 of the Federal Food, Drug, and Cosmetic Act added by the 21st Century Cures Act. It starts with a Letter of Intent (LOI) to initiate the qualification of a biomarker with a proposed COU. Stage 2 begins when the FDA accepts the LOI and gives the requestors the go ahead to develop a Qualification Plan (QP) which lays out the strategy to generate the data necessary to support the proposed COU. After discussion and acceptance of the QP by FDA, the requestor provides a Full Qualification Package (FQP) containing all data (including literature and prospective data) to support qualification of the biomarker for the proposed COU. Based on a comprehensive review of the FQP, FDA will determine whether the biomarker is qualified for its proposed COU. The FDA provides forms and other information that address the components of each of the stages in the qualification process. 10
EMA novel methodologies qualification process
The European Medicines Agency (EMA) also has a process in place to qualify novel methodologies to support research and development in pharmaceuticals. 11 EMA’s Committee for Medicinal Products for Human Use (CHMP) offers scientific advice to applicants based on recommendations provided by the Scientific Advice Working Party (SAWP). Opinions are issued on use of novel methodology, imaging method, or biomarker. The CHMP can also provide advice on methods and protocols which will be used to develop a novel method for qualification. The EMA process begins in a similar manner to the FDA process with submission of a LOI followed by a preparatory meeting where the applicant may get preliminary feedback on the sufficiency of the qualification plan. 3 The EMA procedure, which adheres to specific timelines, officially starts with submission of a draft report and SAWP review of the draft report. The qualification process ends with CHMP adoption of qualification advice and issuance of a qualification opinion.
PMDA biomarker qualification process
The PMDA process for qualification of drug development tools including pharmacogenomics and biomarkers supports their use as reliable tools that reflect a biological process, response, or event during drug development. 12 Although the PMDA process has not reached the same level of maturity as the FDA and EMA processes, it is most comparable to the EMA process. PMDA will provide consultation for study planning prior to application and document submission. PMDA will provide feedback during the process as necessary and will ultimately provide a draft record and final record to confirm qualification.
The processes put in place by each of these regulatory agencies have been used to qualify safety biomarkers of nephrotoxicity and cardiotoxicity. In addition, ongoing safety biomarker projects have been submitted for consideration to regulatory agencies including new safety biomarkers for liver toxicity, skeletal muscle injury, and vascular injury in order to provide new drug development tools to support clinical trials of new therapeutics.
Qualified nephrotoxicity biomarkers
Current standards to monitor for kidney toxicity in clinical studies and nonclinical studies are lagging indicators of kidney injury. Standard laboratory tests for serum creatinine (sCr) and blood urea nitrogen (BUN) increase only after significant kidney injury has occurred. Therefore, in order to monitor for kidney toxicity in drug development trials, new biomarkers are necessary, which reliably change in response to drug-induced kidney injury prior to irreversible kidney injury at lower exposures or earlier time points than increases in sCr and BUN.
Although the first nephrotoxicity biomarkers were nonclinical, the purpose of qualifying nonclinical safety biomarkers has changed in recent years. The gold standard for qualifying nonclinical safety biomarkers is comparison of their correlation with histopathological changes. Therefore, while it may not always necessary to qualify nonclinical biomarkers, the nonclinical data collected can be used to support clinical qualification projects much as nonclinical data is used to support new drug applications. The following sections describe nonclinical and clinical safety biomarkers of nephrotoxicity qualified with worldwide regulatory agencies.
FDA qualification of nonclinical biomarkers of drug-induced nephrotoxicity in rats
The first nonclinical biomarkers qualified, urinary kidney safety biomarkers include albumin (ALB), β2-microglobulin (β2 M), clusterin (CLU), cystatin C (CysC), kidney injury molecule 1 (KIM-1), total protein and trefoil factor-3 (TF-3), were submitted to FDA by C-Path’s PSTC in 2008. This project was part of the Pilot Process for Biomarker Qualification used by the FDA to develop/test the methods initially put in place. PSTC tested the performance of these seven urinary biomarkers in rats treated with model nephrotoxicants at three independent sites. The results were then compared by Receiver Operating Characteristic (ROC) curves, to sCr, BUN and the gold standard of histopathology. At the time of this qualification, the idea of a COU had not yet been clearly defined, but PSTC submitted “context claims” to FDA which they supported with data in their submissions. These context claims included that the biomarkers add information to sCr and BUN; correlate with either tubular alterations or glomerulopathy; are more accurate for acute kidney injury (AKI) than chronic kidney disease (CKD); can be used voluntarily in preclinical Good Laboratory Practice (GLP) studies; and can be used based on the specifics of the study in early preclinical studies when nephrotoxicity is seen in GLP studies. 13
In April 2008, the U.S. FDA stated that these biomarkers are “acceptable biomarkers for the detection of acute drug-induced nephrotoxicity in rats and can be included along with traditional clinical chemistry markers and histopathology in toxicology studies.” 14 Based on the data submitted and the context claims laid out by PSTC, FDA recommended an “application context” for voluntary use which states: “KIM-1, Albumin, Clusterin and Trefoil Factor-3 can be included as biomarkers of drug induced acute kidney tubular alterations in GLP rat studies used to support clinical trials. Total Protein, β2 Microglobulin and Cystatin C can be included as biomarkers of acute drug-induced glomerular alterations/damage and/or impairment of kidney tubular reabsorption in GLP rat studies used to support clinical trials.”
In addition, FDA stated that these biomarkers were not qualified for use in clinical studies of drug-induced kidney injury. However, they suggested that the biomarkers could be used in clinical trials after consultation with FDA. FDA also said that the clinical use of these nephrotoxicity biomarkers could be supported by measuring them in humans after exposure to know nephrotoxicants (i.e. aminoglycosides).
The International Life Sciences Institute (ILSI)/Health and Environmental Sciences Institute (HESI) qualified urinary CLU and urinary Renal Papillary Antigen (RPA-1) as nonclinical biomarkers of drug-induced nephrotoxicity in rats with FDA in 2010. 15 Urinary CLU was previously qualified in 2008. 14 In 2010, the FDA clarified the COU for urinary CLU as a “biomarker for voluntary use in the detection of acute drug-induced renal tubule alterations, particularly when regeneration is present, in male rats when used in conjunction with traditional clinical chemistry markers and histopathology in GLP toxicology studies for drugs for which there is previous preclinical evidence of drug induced nephrotoxicity or where it is likely given the experience with other members of the pharmacologic class.”
Urinary RPA-1 is qualified “for voluntary use in detecting acute drug-induced renal tubule alterations, particularly in the collecting duct, in male rats when used in conjunction with traditional clinical chemistry markers and histopathology in GLP toxicology studies for drugs for which there is previous preclinical evidence of drug induced nephrotoxicity or where it is likely given the experience with other members of the pharmacologic class.”
EMA qualification of nonclinical biomarkers of drug-induced nephrotoxicity in rats
In January 2009, EMA published “Final Conclusions on the Pilot Joint EMEA/FDA XVDS Experience on qualification of the Nephrotoxicity Biomarkers.” This qualified the same seven nonclinical safety biomarkers including ALB, β2 M, CLU, CysC, KIM-1, total protein and TF-3 with EMA, in a joint process with FDA. 16 Like FDA’s statement, EMA deemed the biomarkers “acceptable in the context of non-clinical drug development for the detection of acute drug-induced nephrotoxicity, either tubular or glomerular with associated tubular involvement.” EMA also stated that these biomarkers provide “additional and complementary information to BUN and serum creatinine to correlate with histopathological alterations considered to be the gold standard.” EMA was also interested in collection of data to determine if a correlation exists between these biomarkers and the development and resolution of acute kidney injury.
PMDA qualification of nonclinical biomarkers of drug-induced nephrotoxicity in rats
In May 2010, the Japanese PMDA also qualified these seven novel kidney safety biomarkers, ALB, β2 M, CLU, CysC, KIM-1, total protein, and TF-3, for use in nonclinical studies. 4 Three items were part of the consultation with PMDA: nonclinical results obtained for the seven biomarkers, agreement that the data supported qualification of the biomarkers, and the strategy for future studies designed to increase acceptance of the biomarkers and to increase understanding of the utility of the biomarkers. PMDA approved use of the kidney safety biomarkers, along with existing biomarkers such as sCr and BUN, to detect drug-induced acute urinary tubular changes or acute glomerular changes/injury in rat GLP studies.
Qualified clinical nephrotoxicity biomarkers
There have been five biomarker projects that have received qualification for use in clinical applications by the FDA. Among these projects is the qualification of a panel of novel kidney safety biomarkers in 2018. These same clinical nephrotoxicity biomarkers are in the process of qualification with EMA and PMDA. This groundbreaking clinical qualification with FDA represented a very important step forward in the use of novel safety biomarkers in drug development and regulatory decision making. Furthermore, this project fully utilized the translational qualification strategy for biomarkers discussed by Sauer and Porter. 1 This was also a major milestone for the FDA’s BQP, establishing the pathway and regulatory expectations for the clinical qualification of other safety biomarkers.
In 2018, this first FDA qualification of a clinical safety biomarker was based on a joint submission of data by the Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium (BC) and C-Path’s PSTC. The FDA determined that the Composite Measure (CM) composed of these six urinary biomarkers: CLU, CysC, KIM-1, N-acetyl-beta-D-glucosaminidase (NAG), Neutrophil Gelatinase-Associated Lipocalin (NGAL), and osteopontin (OPN) was qualified as a “safety composite biomarker panel to be used in conjunction with traditional measures to aid in the detection of kidney tubular injury in phase 1 trials in healthy volunteers when there is an a priori concern that a drug may cause renal tubular injury in humans. 17 ”
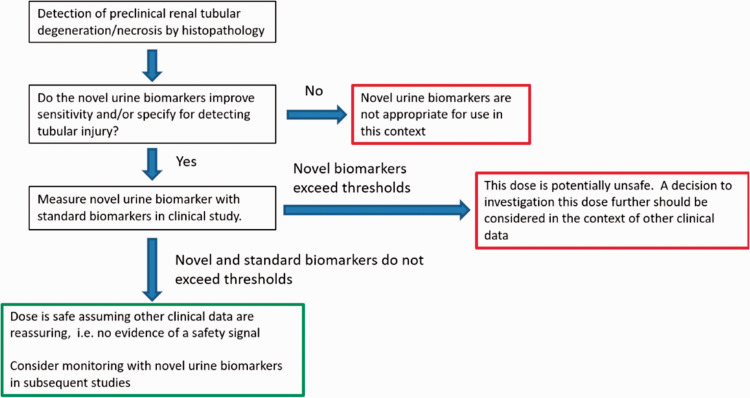
The CM is qualified as a safety biomarker for dose cohorts, not for individual patient/study subject monitoring. Use of the CM biomarker will support the development of safe and effective medicines where there is the potential that an investigational drug may cause kidney injury. To that end, a decision tree has been developed for use of the kidney safety CM in phase I trials (Figure 1).
Figure 1.
Proposed decision tree for clinical use of kidney safety CM in Phase 1 NHV trials.
In the clinical study, these six urinary biomarkers and creatinine were measured in all clinical samples and the result for each biomarker was normalized to urinary creatinine concentration. The CM was calculated for a cohort of subjects. Reliability of the measure is expected to increase with a larger number of subjects. The CM is not qualified for individual patient safety monitoring. However, if marked deviations from normal are measured in a single biomarker or a single subject, further evaluation should be considered. Baseline levels of the six biomarkers that make up the CM should be measured before study drug exposure, and also at a post-baseline time points. Sample collection times may rely on nonclinical animal toxicology data. The analytical validation for each individual biomarker assay should include the following performance characteristic testing: linearity, recovery (for accuracy), precision, limits of detection/quantitation, sample stability and handling, and interference.
Clinical validation of the CM was conducted using healthy volunteers to establish the mean values, normal range, and inter- and intra-subject variability of the six urinary biomarkers. Specimens from 39 patients with no evidence of kidney disease at baseline from the mesothelioma study (MS) were used in a retrospective analysis to establish the utility of the CM. Longitudinal sample collection preceded surgery or cisplatin treatment. This qualification does not intend to a set CM threshold to define kidney injury; instead, it provides information on the probability of obtaining a value greater than or equal to a particular value in a cohort of normal healthy volunteers of a specific sample size.
Additional information on the use of this CM can be found in the User’s Guide: Kidney Safety Composite Measure Biomarker for Use in Clinical Development. 18 Production of this User’s Guide is in keeping with PSTC’s goal to advance the use of qualified biomarkers through continued wide dissemination of information and education of drug development sponsors post-qualification.
Qualified cardiotoxicity biomarkers
Biomarkers of cardiotoxicity include creatine kinase and lactate dehydrogenase. 19 However, these biomarkers are not specific to cardiac tissue and therefore are of limited utility. In addition, measurement of left ventricular ejection fraction (LVEF) is limited by expense, technical issues, and the inability to detect early changes in cardiac function. 20 Improved biomarkers are critical to evaluating the safety of many oncology medications currently in use that have been shown to cause cardiotoxicity.20,21 In 2012, cardiac troponins were qualified with FDA as nonclinical biomarkers of cardiotoxicity. Work continues on more specific and selective clinical cardiotoxicity biomarkers including miRNAs and B-type natriuretic peptide (BNP) to support clinical drug development.
In 2012, circulating cardiac cTnT and cTnl were qualified by a group of pharmaceutical companies and academic institutes for use in nonclinical drug development studies in nonclinical species including rats, dogs, and monkeys. This was the first literature-based qualification submission that was reviewed by the FDA and was cited as a “situation of reverse translation” in that the cardiac troponins were widely used and accepted in both veterinary and clinical medicine. 22 This qualification was based on the evaluation of 90 references reviewed collectively. FDA qualified the cardiac troponins for three specific COU statements. 23 The first qualified use of circulating cardiac troponins is “when there is previous indication of cardiac structural damage with a particular drug.” In this case, nonclinical studies can help choose doses for human testing to support a no observed effect level (NOEL) identified by histology. Cardiac troponins are also approved for use “when there is known cardiac structural damage with a particular pharmacologic class of a drug and histopathologic analyses do not reveal structural damage.” In this case, cardiac troponins can be used to “support or refute the inference of low cardiotoxic potential.” And the third COU statement in the decision letter states that the troponins may be used “when unexpected cardiac structural toxicity is found in a nonclinical study, the retroactive ("reflex") examination of serum or plasma from that study for cardiac troponins can be used to help determine a no observed adverse effect level (NOAEL) or lowest observed adverse effect level (LOAEL).”
Ongoing safety biomarker qualification programs
Liver toxicity biomarker: glutamate dehydrogenase
Drug-induced liver injury (DILI) is still the single greatest cause for ending development of drug candidates and market withdrawal of approved drugs. 24 Although alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are sensitive markers of hepatocellular injury, both enzymes are also expressed in other tissues such as muscle. This clearly limits the utility of ALT and AST as markers of the onset of liver injury in those with underlying muscle impairments, such as Duchenne muscular dystrophy (DMD) or other neuromuscular diseases or even in subjects engaging in strenuous exercise.
Glutamate dehydrogenase (GLDH) is an enzyme found in the mitochondria. GLDH plays a role in amino acid oxidation and urea production. GLDH is found primarily in the liver with only a trace amount in skeletal muscle.25,26 Therefore, PSTC has initiated a qualification project for GLDH is as a biomarker of DILI with FDA and EMA supported by strong nonclinical studies characterizing GLDH response to prototype toxicants. In November 2017, the EMA issued a Letter of Support to “encourage the further study of serum GLDH for monitoring for hepatocellular liver injury.” 27 The qualification of GLDH will also be initiated with PMDA shortly. The proposed COU for serum GLDH as a “safety biomarker capable of detecting drug-induced hepatocellular injury that can be used in clinical trials for patients with elevated serum transaminases due to muscle injury or degeneration and with no pre-existing hepatic disease.” The proposed COU also states that GLDH should be used with standard hepatic injury monitoring biomarkers (e.g. total bilirubin, ALT, and alkaline phosphatase).
In November 2019, PSTC submitted a QP to the FDA for review, 28 and in May 2020, the FDA’s BQP accepted the QP for GLDH 29 and asked the team to prepare a Full Qualification Package (FQP) for submission. The letter from FDA contained specific scientific issues and recommendations that need to be addressed in the FQP. This was the first QP accepted by the FDA’s BQP under Section 507 of the Federal Food, Drug, and Cosmetic Act added by the 21st Century Cures Act. The FQP is expected to be submitted to FDA in early 2021 along with a simultaneous submission to EMA. PSTC is currently collaborating with the Japan’s National Institute of Health Sciences (NIHS) on the clinical qualification of GLDH with the PMDA.
Skeletal muscle injury biomarkers
Skeletal muscle (SKM) injury, characterized as myocyte degeneration/necrosis, is currently monitored in clinical drug development trials and in muscular and neuromuscular diseases using circulating concentrations of the standard biomarkers creatine kinase (CK) and aspartate amino transferase (AST). However, these biomarkers lack the desired sensitivity and tissue specificity. Furthermore, clinical symptoms of SKM injury are difficult to interpret, due to the subjective nature of self-reporting. As a result, drug-induced SKM injury remains a poorly understood and difficult to predict side-effect of new and existing medications.
Therefore, PSTC has submitted a LOI to FDA, to qualify four novel molecular plasma biomarkers of skeletal muscle injury including skeletal troponin I fast-twitch (Type II) a component of myofilaments with expression restricted to SKM; myosin light chain 3 (MYL3) a component of myofilaments found principally in slow-twitch SKM and cardiac muscle; fatty-acid binding protein 3 (FABP3) a cytosolic lipid transport protein abundant in SKM and cardiac muscle, also found in brain, liver, and small intestine; and creatine kinase muscle type (CKM), a cytosolic enzyme that uses and generates adenosine triphosphate (ATP), highly abundant in skeletal muscle. The COU for the four biomarkers is as a “safety biomarker panel to aid in the detection of acute drug induced skeletal muscle injury in phase 1 trials in healthy volunteers in conjunction with AST and total CK enzymatic activity when there is an a priori concern that a drug may cause skeletal muscle injury in humans.”
The use of the four biomarkers will be evaluated as a composite biomarker panel and compared to the performance of the individual biomarkers in monitoring SKM injury. The biomarkers are measured using sandwich ELISA procedures on an electrochemiluminescent platform (Meso Scale Discovery platform, Meso Scale Diagnostics, LLC) which are Research Use Only assays. Nonclinical performance of these biomarkers has been correlated with histopathology, not just toxicity mechanism or disease pathogenesis and some clinical data has been generated. Therefore, it is likely that the four biomarkers will translate from preclinical to clinical. Clinical validation of the assay platform will be performed under the QP. The four biomarkers will be measured in several populations to establish a reference range in healthy subjects; evaluate the effect of age, gender, and ethnicity for each biomarker and characterize inter- and intra-subject variability in healthy subjects; establish the performance of each biomarker in the presence of SKM injury and determine the correlation of each biomarker with AST and total enzymatic CK; establish thresholds (cut-points) in plasma that indicate medically important SKM injury for each biomarker in a population with SKM injury associated with myocytes degeneration/necrosis; and assess the capability of each biomarker to differentiate liver, kidney and/or heart injury from SKM injury in human subjects with myocyte degeneration/necrosis and in rats with various drug-induced end organ injuries confirmed by histopathology.
In May 2020, the FDA accepted the SKM project into the BQP 30 stating that “We support and encourage your ongoing study and expanding the use of this promising safety biomarker panel to aid in the detection of acute drug induced skeletal muscle injury in phase 1 trials.” The stated COU is as a “safety biomarker panel to aid in the detection of acute drug induced skeletal muscle injury in phase 1 trials in healthy volunteers in conjunction with aspartate transaminase (AST) and total creatine kinase (CK) enzymatic activity when there is an a priori concern that a drug may cause skeletal muscle injury in humans.”
Per Section 507 of the Federal Food, Drug, and Cosmetic Act added by the 21st Century Cures Act, the FDA has requested that the team prepare a Qualification Plan (QP) for submission.
The FDA’s LOI Determination Letter contained specific scientific issues and recommendations that need to be addressed in the QP. Analytical validation suggestions include utilizing clinical samples to determine the limit of detection (LoD) and the limit of quantitation (LoQ) to support the stated COU; determine validation study acceptance criteria based on the necessary performance to support the COU; and that sample stability studies should demonstrate that no conditions the samples may be exposed to could negatively impact the interpretation of results obtained. Clinical considerations addressed in the FDA’s letter include consideration of pursuing the qualification of the biomarker panel in the nonclinical setting first in other nonclinical species include mice, dogs and macaques by correlating histopathology with biomarker response; prospectively collecting samples from drug development programs where drug-related myopathy/rhabdomyolysis occurs with some subjects that experience recovery from injury; and several questions of clinical utility.
Additional ongoing FDA safety biomarker qualification projects
Other safety biomarker projects currently being considered for qualification by FDA are listed on the website (https://www.fda.gov/drugs/biomarker-qualification-program/biomarker-qualification-submissions). 28 These projects include a transcriptomic biomarker panel to be used as a nonclinical safety biomarker to “identify in vitro mammalian cell structural chromosome damage” submitted by ILSI/HESI.
C-Path’s PSTC and the FNIH BC-KSP are also working on the QP after acceptance of the LOI for a panel of 8 urinary protein safety biomarkers (CLU, CysC, KIM-1, NAG, NGAL, OPN, ALB, total protein) to be used in conjunction with three conventional kidney safety biomarkers (sCr, BUN, and sCysC) to indicate potential drug-induced injury to the renal tubule in individuals with normal renal function enrolled in early phase drug development clinical trials. FDA’s acceptance letter requested analytical validation information to support the panel’s use in individual study subjects; sample storage conditions; more information on the statistical analysis plan (SAP); and specific information on the individual biomarker assays. The results of the clinical studies will determine whether all of the biomarkers, a subset of the biomarkers, or a single biomarker can be qualified for the specific COU.
The Innovative Medicines Initiative’s Safer and Faster Evidence-based Translation (IMI SAFE-T) Consortium has three qualification projects listed with FDA including urine and serum biomarkers of drug-induced kidney injury, and serum biomarkers of drug induced hepatic injury which are legacy projects in transition to the new 507 process. The third project, qualification of serum biomarkers of drug-induced vascular injury has had the LOI accepted into the BQP with preparation of the QP as the next step.
The AnaBios Corporation is working on an algorithm-derived biomarker assessed by myocardial cell assay as a safety biomarker to compare pro-arrhythmic drugs based on the likelihood that they will cause arrhythmia. They have received a 507 Summary Response Letter and have been invited by FDA to submit a QP for this project.
Drs. Benesic and Gerbes are working on a cell-based assay designed to measure a proteomic biomarker panel using blood monocytes as a safety biomarker of idiopathic drug-induced liver injury. This is another legacy project in transition to the 507 process.
The IMI Translational Safety Biomarker Pipeline (TransBioLine) also has an accepted LOI with FDA for biomarkers of DIVI. 31 Their mission, similar to that of PSTC, is to develop biomarkers “that will reliably indicate injury of the liver, kidneys, pancreas, blood vessels, and central nervous system.” In the FDA accepted LOI, TransBioLine proposes the evaluation of protein-based molecular biomarkers across three categories (endothelial biomarkers, smooth muscle biomarkers and inflammation biomarkers) and circulating miRNA biomarkers. The top candidates, based on this learning phase and nonclinical data, will then enter the confirmatory phase in which a biomarker panel of at least one biomarker from each category will be tested. The COU for this panel states that it is expected to “aid in the detection of acute drug-induced vascular injury (DIVI) in early clinical trials in healthy volunteers when there is an a priori concern that a drug may cause DIVI in humans.”
Conclusions
Nonclinical biomarker data is being used in the translational qualification of clinical biomarkers, because nonclinical biomarkers can be compared to the gold standard of histopathology. Ultimately, this process is very similar to the way in which nonclinical information is used in the approval of drug development candidates.
The regulatory qualification of biomarkers results in certainty in how the biomarkers can be used and interpreted in drug development studies. The FDA’s qualification process has evolved significantly over the last 10 years, especially with respect to Section 507 of the Federal Food, Drug, and Cosmetic Act added by of the 21st Century Cures Act passed in December 2016. Efforts over the past couple of years have better defined the expectations, both scientific and regulatory, for the successful qualification of biomarkers across all stakeholders. In many cases consortia, with years of experience in biomarker qualification, are using novel approaches to increase the speed of the process, lower the resource utilization, and increase accessibility of biomarker qualification data to drug development sponsors and those pursing new biomarker programs. This description of many of the qualified safety biomarkers and the ongoing safety biomarker projects is meant to increase the use of these drug development tools for their qualified COU and encourage those with novel safety biomarker data to make it available for the development of new, more specific and sensitive safety biomarkers. Future work will also evaluate these organ/tissue safety biomarkers for use as efficacy biomarkers in the evaluation of new candidates for treatment of disease.
Footnotes
AUTHORS’ CONTRIBUTIONS: All authors participated in the drafting, review, and finalization of this manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: Critical Path Institute is supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) with 62% funded by FDA/HHS totaling $14,448,917 (Grant# 2U18FD005320-06 Critical Path to Public-Private Partnerships) and 38% percent funded by non-government source(s) totaling $8,669,646. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA/HHS, or the U.S. Government.
ORCID iD: John-Michael Sauer https://orcid.org/0000-0003-4815-4802
References
- 1.Sauer J-M, Porter AC. The regulatory acceptance of translational safety biomarkers. Curr Opin Toxicol 2020; 23–24:80–6 [Google Scholar]
- 2.US Food and Drug Administration. Critical Path Initiative – FDA’s Critical Path Initiative, www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/ucm076689.htm (2004, accessed 27 July 2016)
- 3.European Medicines Agency. Qualification of novel methodologies for drug development: guidance to applicants, www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004201.pdf (2014, accessed 9 May 2016)
- 4.Japan Pharmaceuticals and Medical Devices Agency. PMDA Record of the Consultation on Pharmacogenomics/Biomarkers, www.pmda.go.jp/files/000160006.pdf (2010, accessed 19 May 2017)
- 5.Leptak C, Menetski JP, Wagner JA.Aubrecht J, Brady L, Brumfield M, Chin, WW, Hoffman S, Kelloff G, Lavessari G, Ranganthan R, Sauer J-M, Sistare FD, Zabka T, Wholley D. What evidence do we need for biomarker qualification? Sci Transl Med November 2017; 9:22. [DOI] [PubMed] [Google Scholar]
- 6.Evidentiary Criteria Writing Group. Framework for Defining Evidentiary Criteria for Biomarker Qualification, https://fnih.org/sites/default/files/final/pdf/Evidentiary%20Criteria%20Framework%20Final%20Version%20Oct%2020%202016.pdf (2016, accessed 20 October 2020)
- 7.US Food and Drug Administration. Biomarker Qualification: Evidentiary Framework Guidance for Industry and FDA Staff DRAFT GUIDANCE, www.fda.gov/media/119271/download (2018, accessed 2 March 2020).
- 8.Biomarker Assay Collaborative Evidentiary Considerations, Writing Group, Critical Path Institute (C-Path). Scientific and Regulatory Considerations for the Analytical Validation of Assays Used in the Qualification of Biomarkers in Biological Matrices, https://healthpolicy.duke.edu/events/scientific-and-regulatory-considerations-analytical-validation-assays-used-qualification (2016, accessed 12 September 2017).
- 9.FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource, http://www.ncbi.nlm.nih.gov/books/NBK338448/ (2016, accessed 4 May 2016) [PubMed]
- 10.U.S. Food and Drug Administration. Resources for biomarker requestors, www.fda.gov/drugs/cder-biomarker-qualification-program/resources-biomarker-requestors (2020, accessed 7 May 2020)
- 11.European Medicines Agency. Qualification of novel methodologies for medicine development, https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-advice-protocol-assistance/qualification-novel-methodologies-medicine-development-0 (2020, accessed 17 August 2020)
- 12.Otsubo Y. Use of pharmacogenomics and biomarkers in the development of new drugs for Alzheimer disease in Japan. Clin Ther 2015; 37:1627–31 [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration. Review of qualification data for biomarkers of nephrotoxicity submitted by the predictive safety testing consortium, www.fda.gov/media/87781/download (2009, accessed 17 August 2020)
- 14.US Food and Drug Administration. FDA Qualification of Seven Biomarkers of Drug-Induced Nephrotoxicity in rats, www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/UCM285031.pdf (2008, accessed 19 May 2017)
- 15.U.S. Food and Drug Administration. Biomarker Qualification Decision: Urinary Clusterin and Renal Papillary Antigen (RPA-1), www.fda.gov/media/82521/download (2010, accessed 3 March 2020)
- 16.European Medicines Agency. EMA final conclusions on the pilot joint EMEA/FDA VXDS experience on qualification of nephrotoxicity biomarkers, www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004205.pdf (2009, accessed 19 May 2017)
- 17.U.S. Food and Drug AdministrationC for DE and FDA Qualification of Composite Measure of 6 urinary biomarkers to aid in detection of kidney tubular injury in Phase 1 trials of healthy volunteers, www.fda.gov/media/115690/download (2018, accessed 21 January 2020)
- 18.PSTC Nephrotoxicity Working Group. User’s guide for kidney safety composite measure biomarker for use in clinical development, https://c-path.org/wp-content/uploads/2019/02/ksp-compositecou-userguide-v1-01-11-2019_final.pdf (2019, accessed 21 January 2020)
- 19.Yamaura Y, Kanki M, Sasaki D.Nakajima M, Unami A. Serum miR-206 as a biomarker for drug-induced skeletal muscle injury in rats. J Toxicol Sci 2020; 45:503–13 [DOI] [PubMed] [Google Scholar]
- 20.Riddell E, Lenihan D. The role of cardiac biomarkers in cardio-oncology. Curr Probl Cancer 2018; 42:375–85 [DOI] [PubMed] [Google Scholar]
- 21.Upshaw JN. The role of biomarkers to evaluate cardiotoxicity. Curr Treat Options Oncol 2020; 21:79. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration. Review of qualification data for cardiac troponins, www.fda.gov/media/87774/download (2011, accessed 20 August 2020).
- 23.US Food and Drug Administration. Biomarker Qualification Decision: serum/plasma cTnT and cTnl, www.fda.gov/media/83152/download (2012, accessed 3 March 2020).
- 24.Yuan L, Kaplowitz N. Mechanisms of drug induced liver injury. Clin Liver Dis 2013; 17:507–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mastorodemos V, Zaganas I, Spanaki C.Bessa M, Plaitakis A. Molecular basis of human glutamate dehydrogenase regulation under changing energy demands. J Neurosci Res 2005; 79:65–73 [DOI] [PubMed] [Google Scholar]
- 26.McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res 2013; 30:2174–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.EMA. Letter of support for glutamate dehydrogenase, a biomarker of hepatocellular liver injury, www.ema.europa.eu/en/documents/other/letter-support-glutamate-dehydrogenase-biomarker-hepatocellular-liver-injury_en.pdf (2017, accessed 14 November 2019)
- 28.US Food and Drug Administration. Biomarker qualification submissions, https://www.fda.gov/drugs/biomarker-qualification-program/biomarker-qualification-submissions (2020, accessed 29 July 2020)
- 29.U.S. Food and Drug Administration. Qualification Plan Determination: DDTBMQ000050 (GLDH), www.fda.gov/media/138320/download (2020, accessed 29 July 2020)
- 30.U.S. Food and Drug Administration. LOI determination Letter: DDTBMQ000081 – Amendment 1 (SKM plasma biomarkers), www.fda.gov/media/139662/download (2020, accessed 29 July 2020)
- 31.US Food and Drug Administration. FDA LOI Decision Letter for TransBioLine Biomarkers of Drug-Induced Vascular Injury (DIVI), https://www.fda.gov/media/135116/download (2020, accessed 15 April 2020).