Abstract
The lymph nodes are major sites of cancer metastasis and immune activity, and thus represent important clinical targets. Although not as well-studied compared to subcutaneous administration, intravenous drug delivery is advantageous for lymph node delivery as it is commonly practiced in the clinic and has the potential to deliver therapeutics systemically to all lymph nodes. However, rapid clearance by the mononuclear phagocyte system, tight junctions of the blood vascular endothelium, and the collagenous matrix of the interstitium can limit the efficiency of lymph node drug delivery, which has prompted research into the design of nanoparticle-based drug delivery systems. In this mini review, we describe the physiological and biological barriers to lymph node targeting, how they inform nanoparticle design, and discuss the future outlook of lymph node targeting.
Keywords: Nanoparticles, drug delivery, barrier, lymph node, targeting, hitchhiking
Impact statement
The lymph nodes are clinical targets for drug delivery, due to their role in coordinating the adaptive immune response, as well as their involvement in a wide range of pathologies such as cancer, autoimmunity, and viral infection. This article describes the unique biological challenges to drug delivery to the lymph nodes upon intravenous administration and how nanoparticles can be designed to overcome such barriers. In sum, the information presented herein will inform nanoparticle development to improve drug delivery based on our understanding of lymph node physiology and lymph node-nanoparticle interactions.
Introduction
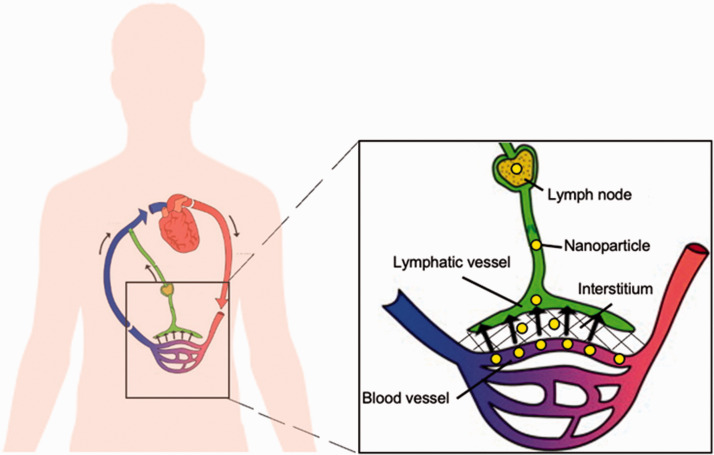
The lymphatic system is a network of organs, including the thymus, bone marrow, spleen, tonsils, and lymph nodes, interconnected by lymphatic vessels through which lymph flows. The primary functions of the lymphatic system are to maintain fluid homeostasis and regulate the adaptive immune response. Approximately 10% of blood plasma leaks out of the blood vessel capillaries and enters the interstitial space, with approximately 5–8 L of plasma pushed into the interstitium each day.1,2 As shown in Figure 1, this fluid enters the lymphatic system through highly permeable lymphatic capillaries, which feed the fluid, now called lymph, into the larger lymphatic vessels. Since there is no central pump in the lymphatic system, fluid flow in lymphatic vessels is generated by the contraction and relaxation of the surrounding tissues. One-way valves in lymphatic vessels ensure that lymph flows unidirectionally from the lymphatic capillaries to the subclavian veins, where lymph mixes with venous blood and re-enters the cardiovascular system. 3 Hence, one major function of the lymphatics is to maintain fluid balance in the circulatory system.4,5
Figure 1.
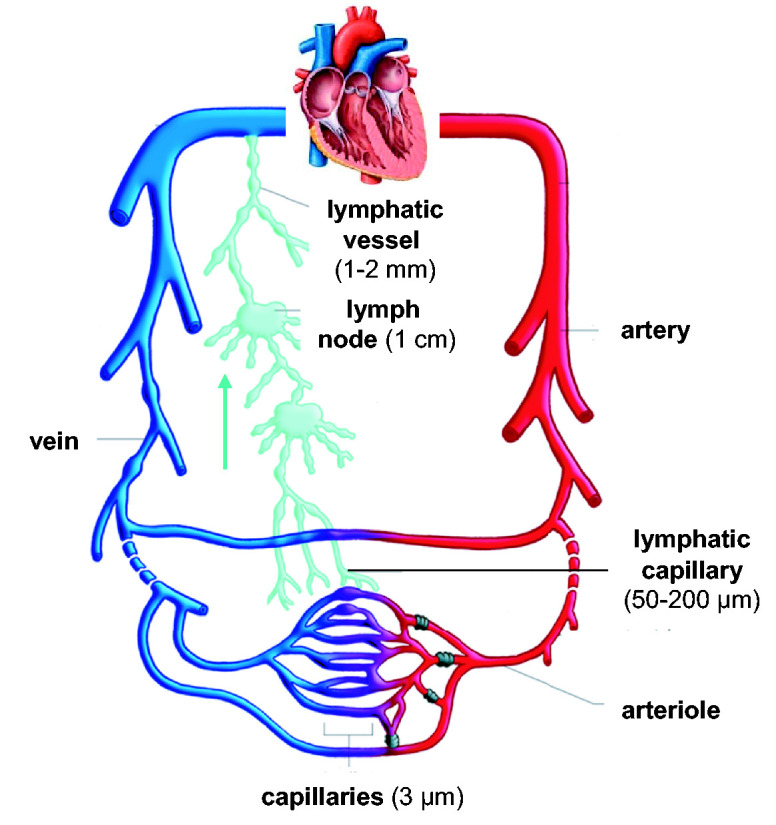
Lymphatic vessels drain interstitial fluid secreted by blood vessels and tissues. Lymph flows unidirectionally toward the subclavian veins, where it mixes with venous blood and re-enters circulation. (A color version of this figure is available in the online journal.)
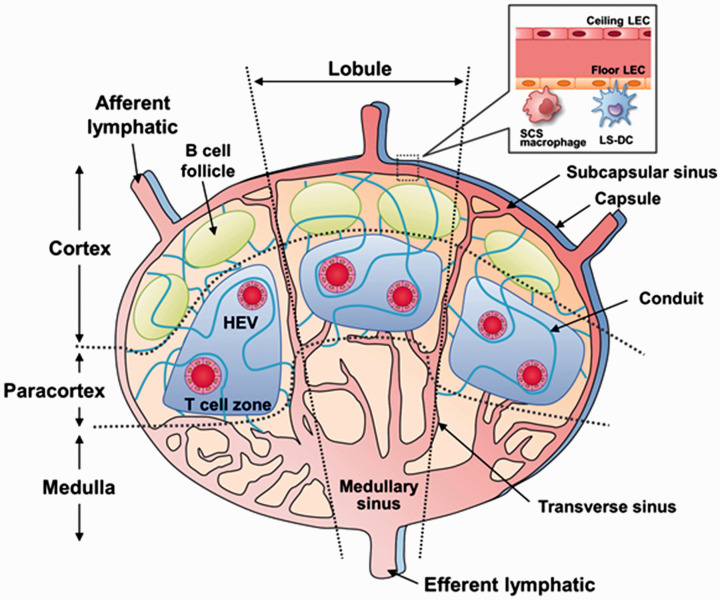
Another important function of the lymphatic system is immune surveillance.6,7 As lymph flows from the lymphatic capillaries toward the subclavian veins, it passes through several lymphoid organs called lymph nodes, which are densely populated with immune cells, such as macrophages, dendritic cells, B cells, and T cells. 8 As shown in Figure 2, lymph enters the lymph node through the afferent lymphatic vessels and flows through the node via the subcapsular sinus into the medullary sinus and out through the efferent lymphatic vessels. 9 The lymph node is home to several populations of resident immune cells, such as subcapsular sinus (SCS) macrophages, and dendritic cells (DCs) that line the subcapsular sinus or the conduits formed by the fibrous matrix inside the node. Their primary function is to sample incoming lymph for antigens to present to naïve B cells and T cells to initiate the adaptive immune response.10–12 Circulating B cells and T cells use an array of cell surface proteins such as CCR7 (C-C chemokine receptor 7), LFA-1 (lymphocyte function-associated antigen 1), and L-selectin to migrate into the lymph nodes through specialized blood vessels called high endothelial venules (HEVs) that innervate the lymph node, where they undergo clonal expansion in their respective zones following antigen exposure. 13 Because there is a rich population of immune cells within the lymph nodes, especially the effector cells of adaptive immunity (B cells and T cells), these lymphoid tissues have been fervently studied in the development of immunotherapeutic strategies.14–20
Figure 2.
Structure of the lymph node. Lymph enters via the afferent lymphatic vessels and exits via the efferent lymphatic vessels. Antigens from the lymph are sampled by node-resident immune cells such as lymphatic sinus associated dendritic cells (LS-DCs) and subcapsular sinus (SCS) macrophages and presented to B cells and T cells to generate an immune response. Antigen-naïve B cells and T cells enter the lymph node through specialized blood vessels called high endothelial venules (HEVs) and undergo expansion following antigen exposure in the B cell follicles and T cell zones, respectively. Reprinted with permission from Nakamura and Harashima. 9 (A color version of this figure is available in the online journal.)
The lymph nodes also represent an important clinical target for chemotherapeutic drug delivery. In addition to being a primary site for lymphoid cancers such as lymphoma, the lymph nodes are the most common site of metastases for solid cancers, as an estimated 80% of cancer metastasis occurs through the lymphatics.21–24 Lymphatic vessels are prone to cancer cell invasion for several reasons. Firstly, lymphatic vessels are highly permeable especially in the initial lymphatics, which lack a complete basement membrane. Additionally, endothelial cells in the initial lymphatics are joined via discontinuous button-like junctions of vascular endothelial cadherins spaced 3 μm apart, which is in contrast to endothelial cells in downstream vessels that are joined via continuous zippers.25–30 Secondly, tumor cells secrete lymphangiogenic factors like VEGF-C and VEGF-D that stimulate the formation and dilation of lymphatic vessels near the tumor. 31 Thirdly, the lower rate of fluid flow in lymphatic vessels compared to blood vessels (0.4 dyne/cm2 vs. >30 dyne/cm2) results in less fluid shear stresses experienced by circulating cancer cells in lymphatic vessels, increasing their likelihood of survival.32–37
Early preclinical studies for lymph node drug delivery have focused on subcutaneous drug administration, as this route circumvents clearance by the mononuclear phagocyte system that patrols the blood vasculature.38–40 Comparatively, intravenous drug delivery to the lymph nodes has been less explored. However, intravenous drug delivery to the lymph nodes holds great clinical potential, because it can deliver drugs systemically to all lymph nodes, while subcutaneous injection concentrates drugs to lymph nodes downstream of the injection site and provides a local effect.41,42 Despite the clinical significance of the lymph nodes as a therapeutic target, intravenous drug delivery to the lymph nodes has challenges.39,43,44 This mini review will describe the physiological and biological barriers to lymphatic drug delivery, discuss the parameters for nanocarrier design to overcome such barriers, and highlight drug delivery systems that target the lymph nodes by “hitchhiking” with endogenous biomolecules and immune cells that regularly traffic through the lymph nodes.
Physiological barriers to lymph node drug delivery
Once encapsulated into a nanoparticle, the in vivo fate of therapeutic drugs is determined by the carrier’s interaction with plasma as well as the cellular and biological environment. Size and surface charge are two key physical parameters that influence a nanocarrier’s interaction with its environment as well as its biodistribution following intravenous injection.42,45–59 Notably, rational design of nanoparticle size and charge can be leveraged to bypass certain physiological barriers and instruct nanoparticle accumulation into specific tissues like the lymph nodes.60–62 As shown in Figure 3, nanocarriers designed for lymph node delivery must (1) avoid clearance from the bloodstream by the mononuclear phagocyte system (MPS), (2) extravasate out of fenestrated blood vessels, and (3) traverse the extracellular matrix of the interstitium. 63 Hence, a deep understanding of each of these physiological barriers and the size and charge constraints they place on nanoparticle design are critical to develop successful nanocarrier systems to the lymph nodes.
Figure 3.
To reach the lymph nodes, nanoparticles must avoid clearance from the blood stream by the mononuclear phagocyte system, extravasate out of the blood vessel endothelium, and diffuse past the extracellular matrix in the interstitium. Adapted with permission from Hong et al. 63 (A color version of this figure is available in the online journal.)
Mononuclear phagocyte system
The first challenge faced by nanocarriers following intravenous administration is to resist clearance from the bloodstream mediated by macrophages and monocytes of the mononuclear phagocyte system (MPS), as well as by neutrophils, which have been shown to have phagocytic capacity and affect nanoparticle clearance in vivo.54,64,65 Macrophages can be found in all major organs, such as the liver, kidneys, spleen, lungs, brain, and lymph nodes, while monocytes and neutrophils patrol the bloodstream. 66 The recognition of nanocarriers as foreign materials in the bloodstream is largely influenced by the adsorption of serum proteins called opsonins onto the particle surface, which are recognized by phagocytes through surface receptors. 54 This relationship between serum protein adsorption and subsequent phagocytosis and clearance has driven the investigation of antifouling polymer coatings like polyethylene glycol (PEG), as well as zwitterionic polymers like poly(carboxybetaine) (PCB), which have gained wider use due to potential PEG immunogenicity.67–71 In addition, the physical properties of nanoparticles, such as size and surface charge have been reported as important determinants of in vivo stability and clearance.
Nanoparticle size has been considered a major factor in phagocyte-mediated clearance that can be tuned to enhance nanoparticle circulation. Generally, nanoparticles that are <100 nm are associated with less protein adsorption and longer circulation half-lives.45,46 For example, Fang et al. incubated 80 nm, 171 nm, and 243 nm PEG-poly(cyanoacrylate-co-n-hexadecyl cyanoacrylate) (PEG-PHDCA) nanoparticles in 50% mouse serum found that protein adsorption increased with nanoparticle size (6%, 23%, and 34%, respectively), which was correlated to macrophage uptake, as the 80 nm particles were phagocytosed at a 40% lower rate than 243 nm particles when incubated with RAW 264.7 murine macrophages in vitro. 45 The same dependence of phagocytosis on nanoparticle size was also reported in a study by Bisso et al., who tested the uptake of 20 nm, 50 nm, 100 nm, and 200 nm polystyrene beads in human neutrophils in vitro. 72 To study the effects of nanoparticle size on in vivo half-life, Choi et al. injected PEG-coated gold nanoparticles of different sizes, ranging from 5 nm to 98 nm intravenously into BALB/c mice via the tail-vein while controlling particle concentration and surface charge and found that nanoparticle half-life decreased with size. 46 This trend was also found in studies by Perrault et al. who also found that increasing PEG weight prolonged the half-lives of PEGylated gold particles of similar core sizes. 73
The surface properties of nanoparticles like charge are also important design criteria to consider for avoiding clearance from circulation by phagocytes. Because most serum proteins are negatively charged, nanoparticles with strong positive surface charge (+30 mV) are generally phagocytosed and eliminated quicker than uncharged or negatively charged particles.47–49,74,75 For example, Feng et al. incubated 10 nm iron oxide nanoparticles coated with polyethylenimine (PEI, +29 mV) or PEG (−1 mV) with RAW264.7 macrophages in vitro and observed that the positively charged particles were phagocytosed at 3-fold the rate of the neutral particle. 74 When injected intravenously into mice, PEI-covered iron oxide particles (225 nm) were cleared from the bloodstream within 10 min, and had 78-fold lower AUC than negatively charged particles. 75 However, other studies report that negatively charged nanoparticles can also be prone to phagocytosis and clearance, due to greater interaction with the positively charged scavenger receptors of phagocytic cells.50–54,59 For example, Metz et al. reported 21 nm negatively charged iron oxide nanoparticles were internalized by phagocytic monocytes at a higher rate than similarly sized uncharged iron oxide particles. 50 Interestingly, this study also reported that 62 nm negatively charged iron oxide particles were phagocytosed at 3-fold the rate of 150 nm uncharged iron oxide particles, despite being much smaller, which suggests that particle surface charge may be more influential on clearance than nanoparticle size. These physical properties govern the ability of nanoparticles to resist phagocyte-mediated clearance from the bloodstream, which is a critical consideration for lymph node targeting strategies, as longer-circulating particles have more opportunities to extravasate from the blood endothelium and accumulate in lymphatic tissues.
Blood vessel endothelium
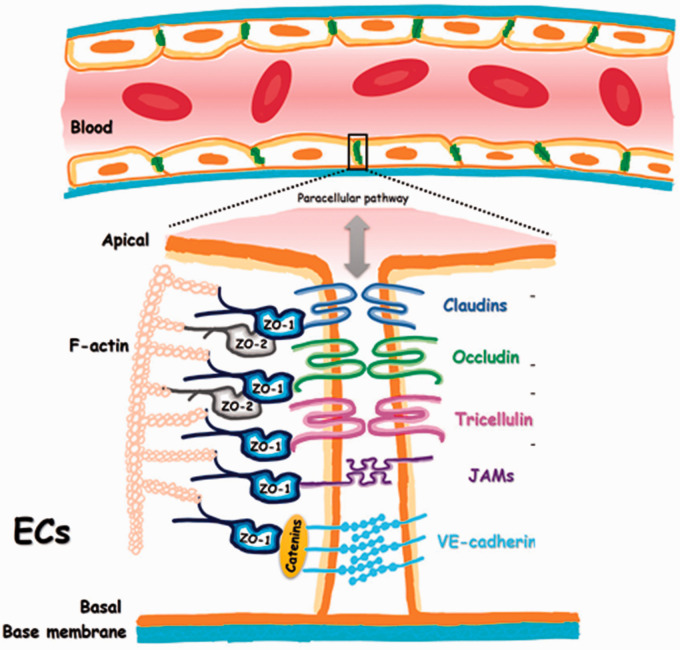
In addition to limiting phagocytic clearance, nanoparticles must extravasate out of the blood vasculature to reach the lymph nodes. As depicted in Figure 4, blood vessels are comprised of flat, quiescent endothelial cells joined laterally via tight junction proteins and attached to a basement membrane comprising collagen, fibronectin, laminin, and glycosaminoglycans.76,77 The blood vasculature functions primarily as a barrier restricting the movement of fluid, proteins, blood cells, and nanoparticles between the intravascular and interstitial compartments. 77 Before extravasation can occur, however, nanoparticles must first marginate towards the blood vessel walls, for which mathematical modeling and microfluidic studies have revealed that nanoparticles with higher aspect ratios like ellipsoid or rod-shaped particles can have superior margination to the vessel wall compared to spherical nanoparticles.78–80 Upon reaching the vessel wall, nanoparticles must extravasate out of the blood vessel through fenestrations in the endothelium or through transcellular transport.
Figure 4.
Junction proteins maintain tight cell–cell junctions between blood vessel endothelial cells. Adapted with permission from Cong and Kong. 76 (A color version of this figure is available in the online journal.)
Fenestrations in the blood vessel endothelium can span 60 nm, which can be up to 800 nm in tumor vasculature, and these fenestrations are believed to be a major route of extravasation for nanoparticles smaller than 200 nm out of the blood vasculature.42,55–58 As such, nanoparticle extravasation out of the blood vessel endothelium into the interstitium has been reported to increase as nanoparticle size is decreased. For example, Vu et al. used human umbilical vein endothelial cells (HUVECs) as an in vitro model of blood vasculature and reported an over 10-fold increase in the extravasation of 40 nm polystyrene nanoparticles out of the blood endothelium compared to that of 70 nm and 130 nm particles. 81 Kong et al. corroborated these results in vivo by using a mouse model of ovarian cancer to evaluate the extravasation of liposomes sized from 100 nm to 400 nm out of tumor blood vessels under hyperthermic conditions and reported that particle extravasation decreased with nanoparticle size. 82 However, none of the liposomes were observed to extravasate under physiological conditions, which highlights growing concerns with the potency of the enhanced permeability and retention (EPR) effect that many researchers have assumed lead to increased nanoparticle delivery to tumor tissue.83–85 Indeed, a study by Smith et al. reported that 25 nm quantum dots were able to extravasate out of the vasculature in only one of three murine tumor models. 86 These studies indicate that further research is necessary to characterize the complex interactions between nanoparticles and blood vasculature in vivo.
In addition to extravasation through gaps between endothelial cells, or paracellular transport, recent studies have suggested nanoparticles can take advantage of transcytosis through endothelial cells as an additional means of transport out of the blood vasculature (Figure 5).87,88 Positively charged nanoparticles have been reported to initiate transcytosis through increased interaction and adsorption to the negatively charged cell membranes of endothelial cells.89–91 Zhang et al. studied the transcytosis of neutral and positively charged polystyrene nanoparticles (22, 48, and 100 nm) through an in vitro model of blood vessel endothelium. While no size effects were reported to affect transcytosis in this study, positively charged particles entered the endothelium at 100-fold the rate of neutral particles, and a similar result was reported by Gil et al. comparing negatively charged (−12 mV) and positively charged (+14 mV) cyclodextrin nanoparticles.90,91
Figure 5.
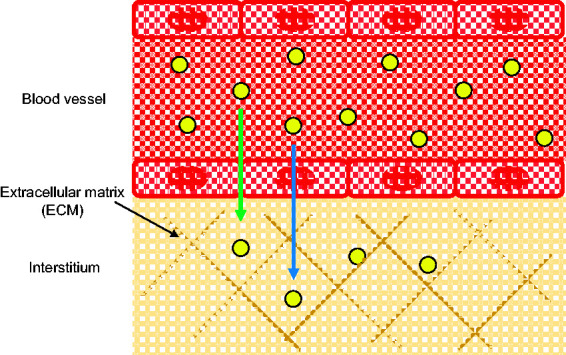
Nanoparticles can extravasate out of the blood vessel endothelium into the ECM-containing insterstitium through cell–cell junctions (green arrow) or transcytosis (blue arrow). (A color version of this figure is available in the online journal.)
Extracellular matrix of the interstitium
Upon extravasation out of the blood vasculature, nanoparticles can encounter another barrier in the form of the extracellular matrix (ECM) in the interstitium before reaching the lymph nodes. The ECM contains hundreds of different proteins including collagen, glycosaminoglycans, laminins, and fibronectin that are primarily produced by fibroblasts. 92 Collagen fibrils crosslink to form a mesh-like structure with pore sizes ranging from 20 nm up to 130 nm93,94 Given the variable nature of ECM pore sizes, smaller nanoparticles are believed to have increased diffusivity past the ECM.95–97 Wong et al. compared the diffusivity of 10 nm quantum dots with 100 nm quantum dot-coated silica nanoparticles in a cell-free collagen matrix. 95 The 10 nm particles had a diffusivity of 2.3 × 10−7 cm2/s, while the 100 nm particles were unable to diffuse in the matrix. While this study only compared nanoparticles of two sizes, Goodman et al. evaluated the ability of 20, 40, 100, and 200 nm polystyrene nanoparticles to penetrate the ECM of a tumor spheroid culture and found that diffusion past the ECM was inversely correlated with nanoparticle size, as 20 nm particles were most efficient in penetrating the ECM (57% of all particles), while 100 and 200 nm particles had <5% penetration. 96
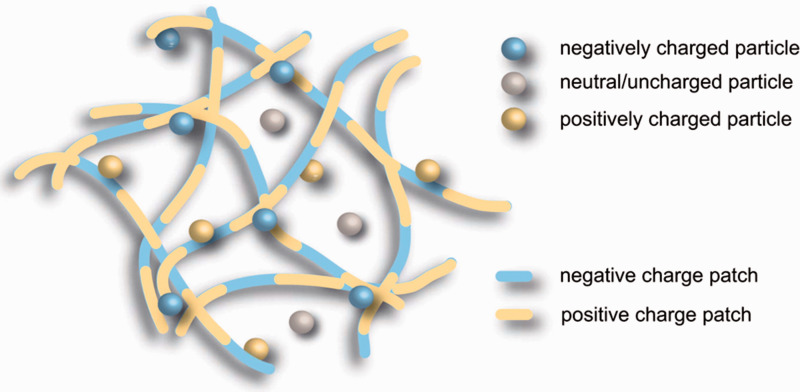
Along with positively charged collagen fibrils, the ECM also contains thin fibers of negatively charged glycosaminoglycans, namely hyaluronic acid, that effectively make the ECM a patchwork of positive and negative charges as shown in Figure 6.98,99 Le Goas et al. studied nanoparticle surface charge effects on the diffusion of 12–20 nm polymethacrylate-coated gold nanoparticles in Matrigel and found that negatively charged nanoparticles (<−20 mV) exhibited greater diffusivity, while positively charged particles (+10 mV) did not diffuse, a result also confirmed by Kim et al.100,101 However, others have reported that neutral particles have increased diffusion through the ECM compared to both positively and negatively charged nanoparticles.99,102 The observation of charged particles having hindered diffusion in ECM has been attributed to their interaction and binding with positively charged (collagen) and negatively charged (glycosaminoglycans) matrix proteins in the ECM.98,99 Interestingly, modeling studies performed by Stylianopoulos et al. using 1–10 nm quantum dots concluded that charge effects diminish with decreasing nanoparticle size, suggesting that smaller nanoparticles, regardless of surface charge, may have superior diffusivity based on their decreased interaction with the proteins of the ECM. 98 Once past the ECM and the other physiological barriers described in this section, nanoparticles can enter the lymphatic system via highly permeable lymphatic vessels and be carried by lymph into the lymph node to unload a therapeutic payload.
Figure 6.
The ECM in the interstitium forms a mesh with positively and negatively charged protein fibers. Reprinted with permission from Lieleg et al. 98 (A color version of this figure is available in the online journal.)
In vivo application of nanoparticles for lymph node targeting
The nanoparticle size and charge constraints imposed by the mononuclear phagocyte system, blood vessel endothelium, and extracellular matrix of the interstitium suggest that small, uncharged nanoparticles may target the lymph nodes more efficiently following intravenous injection. Nanoparticle delivery to the lymph nodes has been used in numerous clinical and pre-clinical applications, such as the imaging and treatment of lymph node cancer metastases, as well as for vaccine delivery.41,103–106 Although the majority of nanoparticle formulations are sequestered in the liver and spleen following intravenous administration, leading to lymph node concentrations of <0.1% of the injected dose (ID), engineering the physical characteristics of nanoparticles such as size and charge have been reported to boost lymph node accumulation.107–109 For example, dextran-coated ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles with hydrodynamic diameters of approximately 40 nm have been explored clinically for the identification of lymph node metastases in prostate cancer using MRI, with preclinical studies reporting up to 12% ID in the lymph nodes after intravenous administration in rats.42,110,111 The USPIO particles function as contrast agents by entering the lymph nodes and retention by node-resident macrophages. 42 The efficacy of USPIO particles for lymph node metastases detection was evaluated in a clinical study involving 80 patients and 334 lymph nodes; USPIO-aided MRI following intravenous injection improved the sensitivity of lymph node metastases detection from 35.4% to 90.5% compared to MRI alone. 110
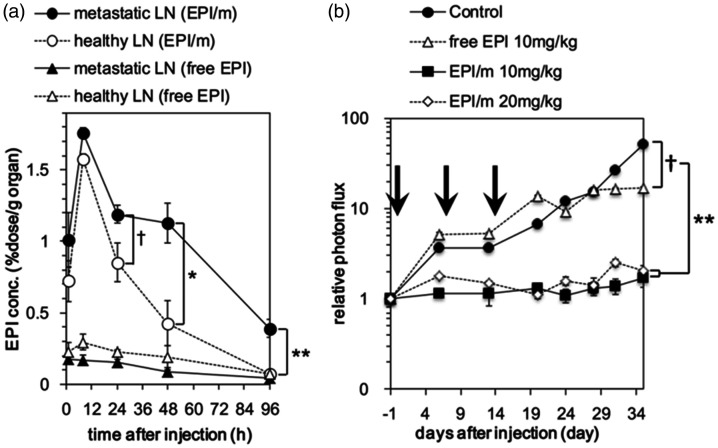
In addition to cancer imaging, nanoparticles targeted to the lymph nodes have also been investigated for cancer therapy. Cabral et al. investigated the ability of uncharged polymeric micelles administered intravenously to accumulate in the metastatic lymph nodes of B16F10 tumor-bearing mice and deliver a platinum-based drug. 41 While mice treated intravenously with 8 mg/kg of free oxaliplatin had similar tumor growth to an untreated control, mice treated with 3 mg/kg of the drug loaded into 30 nm polymeric micelles had significantly smaller tumors and lymph node metastases after nine days (three total injections given on days 0, 2, and 4). Moreover, the lymph node targeting of the micelles was reported to be size-dependent, as 30 nm micelles were reported to have approximately 4 times better accumulation in lymph node metastases than 70 nm micelles. Another study by this group investigated the ability of 55 nm micelles to deliver the chemotherapy drug epirubicin to the metastatic lymph nodes of a luciferase-expressing murine breast cancer model. 103 To limit off-target toxicity associated with drug release in healthy lymph nodes, the epirubicin was loaded into the micelles via pH-sensitive hydrazine linkers, to facilitate increased drug release in the acidic environment of the primary tumor and lymph node metastases. Eight hours after intravenous injection, the epirubicin concentration in the metastatic lymph nodes of micelle-treated mice was 10-fold higher than in the nodes of mice treated with free epirubicin, and the increased drug concentration resulted in significant reduction in the size of lymph node metastases (Figure 7). Moreover, the drug concentration in metastatic lymph nodes was almost 3-fold higher than the drug concentration in healthy nodes 48 h after injection.
Figure 7.
(a) Epirubicin-loaded micelles deliver drugs to metastatic lymph nodes. (b) Epirubicin micelle treatment inhibits the growth of lymph node metastases in a luciferase expressing murine breast cancer model. *P < 0.05, **P < 0.01. Adapted with permission from Chida et al. 102
Because the majority of solid cancers metastasize via the lymphatics, lymph node-targeted nanoparticles have also been investigated for their ability to inhibit the formation of lymph node metastases. 112 Liu et al. characterized the distribution of nanoparticle clusters 100 nm in size assembled from 5 nm dendrimers loaded with a cisplatin prodrug and tested the nanoparticle’s ability to inhibit lymph node metastases. Particles injected intravenously were found to colocalize with FITC-labeled lymphatic vessels, confirming their ability to enter the lymphatics following systemic injection. Moreover, nanoparticle treatment inhibited the formation of lymph node metastases in a metastatic 4T1 breast cancer model and improved the median survival of mice from 45 days to 56 days relative to a PBS control, validating the efficacy of lymph node-targeted nanoparticles for cancer therapy.
In addition, nanoparticles have been used to deliver vaccines to the lymph nodes for immunization against cancer. Specifically, Baharom et al. explored intravenous delivery of 20–50 nm micelles loaded with MC38 tumor peptide neoantigens for anti-tumor vaccination of mice. 105 Mice challenged with MC38 colon cancer cells 28 days after vaccination were observed to have significantly reduced tumor growth compared to unvaccinated mice. Interestingly, intravenous vaccination of mice bearing established MC38 tumors was observed to control tumor growth, while subcutaneous vaccination was ineffective. These studies reported the success of small, uncharged nanoparticles for lymph node targeting, which coincides with the ideal nanoparticle physical properties described above. The rising number of studies regarding lymph node drug delivery for cancer therapy reflects the growing recognition of the lymphatic system’s role in mediating cancer metastasis and recurrence.
Hitchhiking strategies for lymph node drug delivery
Albumin-mediated drug delivery to the lymph nodes
An alternative approach to drug delivery to the lymph nodes is to design a delivery system that can bind to endogenous molecules or immune cells that are regularly trafficked to the lymph nodes. Albumin is the most abundant protein in blood, interstitial fluid, and lymph. 34 Albumin is produced in the liver and released into the bloodstream as a globular protein with 4 × 15 nm dimensions, with an average half-life of 19 days.113,114 The main functions of albumin are to maintain the osmotic pressure of blood, and to transport other biomolecules, such as fatty acids, hormones, ions, and steroids.115,116 About 5% of the albumin in circulation is pushed out of the blood vasculature every hour into the interstitial space, and nearly 100% of this albumin is absorbed into the lymphatics before re-entering circulation. 34
Given albumin’s excellent stability and half-life, as well as its frequent trafficking to the lymphatics, subcutaneous delivery systems leveraging albumin-hitchhiking for the delivery of vaccines and imaging molecules, such as the albumin-binding dye Evans Blue, to draining lymph nodes have been explored.34,117,118 Albumin has also been investigated as a nanocarrier for anticancer therapies, due to its ability to passively accumulate in primary tumors via leaky tumor vasculature, as well as its active transport into tumor cells via the Cav-1 protein.119,120
Additionally, albumin hitchhiking has been adopted for intravenous delivery systems targeted to the lymph nodes in preclinical and clinical studies.121–125 For example, Yu et al. synthesized combination nanoparticles by loading nanospheres comprised of crosslinked albumin with the chemotherapy drug gemcitabine and photodynamic therapy agent pheophorbide-a and evaluated therapeutic efficacy in the lymph node metastases of a murine BxPC-3 pancreatic cancer model. 125 Twenty-four hours after intravenous administration, the combination nanoparticle was observed to have increased accumulation in metastatic lymph nodes compared to free pheophorbide-a. Furthermore, in vivo efficacy studies demonstrated reduced volume and weight of metastatic lymph nodes of mice treated with nanoparticle.
Nab-paclitaxel (Abraxane) is a nanoformulation of the anti-cancer drug paclitaxel bound to albumin that is FDA approved for the treatment of metastatic breast cancer, non-small cell lung cancer, and pancreatic adenocarcinoma.126,127 Given its lymphatic targeting properties, it has also recently been examined in lymph node-resident cancers such as non-Hodgkin lymphoma.121–124 In two separate case reports of patients presenting diffuse large B-cell lymphoma, each achieving only partial responses after multiple lines of chemotherapy, the patients were prescribed combination therapies including nab-paclitaxel. The first patient received a weekly dose of nab-paclitaxel over six months after initial treatment with the chemotherapy drug azacitine, and PET-CT scans revealed complete disease remission after the third month of treatment. 122 Follow-up scans performed five years after the end of treatment showed no evidence of disease recurrence. The second patient was prescribed a combination of nab-paclitaxel and liposomal doxorubicin as a fourth-line chemotherapy regimen and achieved complete disease remission after four cycles of treatment, with PET-CT scans showing no disease recurrence after seven years. 121 Based on the success of these case reports, a phase II clinical trial involving 13 patients with relapsed or refractory diffuse large B cell lymphoma was performed using a combination therapy of rituximab, nab-paclitaxel, and liposomal doxorubicin. 123 Of the 13 patients in the study, 5 achieved complete response, 6 achieved partial response, and 2 patients had stable or progressive disease. Although clinical studies reported therapeutic success in patients unresponsive to other chemotherapies, they were limited by small sample sizes. Thus, the use of nab-paclitaxel as a monotherapy for diffuse large B cell lymphoma warrants further study.
Immune cell-mediated drug delivery to the lymph nodes
Another approach for delivering therapeutics to the lymph nodes is to hitch nanoparticles with immune cells that regularly traffic to the lymph nodes, such as T cells monocytes, and dendritic cells (DCs) which can enter the lymph node via the lymphatics, as well as through the blood vessels in the lymph node. Naïve T cells constantly travel between the blood circulation and lymphatics in search of antigen. Upon activation, T cells express chemokine receptors CCR5, CCR7, and CCR8 that facilitate their migration to the lymph nodes through the lymphatic vasculature and lymph node blood vasculature.128–130 Huang et al. exploited the lymph node homing ability of T cells by covalently linking cultured luciferase+ T cells to lipid nanocapsules (NCs) loaded with the anticancer drug SN-38. 131 After intravenous injection into a mouse model of lymphoma, T cells conjugated with the NCs accumulated in the lymph nodes within 20 h, reaching peak levels at 60 h post-injection, and were retained until the end of the study (80 h). Importantly, these kinetic properties were similar to unmodified T cells, confirming that conjugation to the NCs did not affect T cell trafficking. When drug concentrations in the tumor-bearing lymph nodes were evaluated, T cell-mediated NC delivery resulted in increased drug concentration, over 60-fold greater than the concentration achieved by the injection of unbound NCs. The increased drug concentration associated with T cell-NC treatment translated to an approximately 10-fold reduction in tumor burden compared to unbound NC treatment.
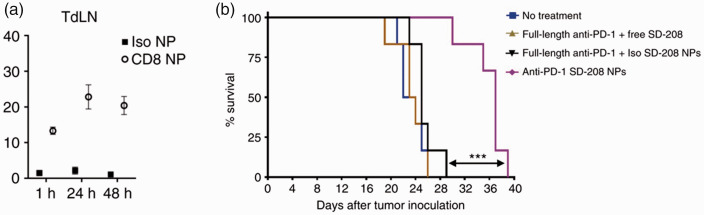
Additionally, T cell hitchhiking can also be achieved in situ. For example, Schmid et al. evaluated the ability of intravenously administered T cell-targeted PEG-PLGA nanoparticles to accumulate in the tumor-draining lymph nodes of a mouse colorectal cancer model and deliver the immunomodulatory drug SD-208. 132 SD-208 functions by inhibiting the TGF-BR1 kinase in cancer cells, which promotes immunosuppression. 133 T cell targeting was achieved by reacting maleimide-terminated nanoparticles with free thiol groups of PD1 or CD8 antibody fragments, as both PD1 and CD8 have been reported to be expressed in active T cells.132,134–136 Twenty-four hours following intravenous injection, T cell-targeted nanoparticles were observed to accumulate in the tumor-draining lymph nodes, with approximately 25% of T cells in the node bound to nanoparticles, while nanoparticles functionalized with non-targeting isotype control antibody fragments were not observed in the lymph nodes (Figure 8(a)). The increased lymph node accumulation of the targeted nanoparticles led to delayed tumor growth in a subcutaneous MC38 colorectal cancer model. While treatment with non-targeted nanoparticles had no effect on tumor growth, mice treated with T cell-targeted nanoparticles delayed tumor growth and prolonged mouse survival by approximately 12 days (Figure 8(b)).
Figure 8.
(a) T cell-targeted nanoparticles accumulate in the tumor-draining lymph nodes and (b) improve mouse survival (b). ***P < 0.001. Adapted with permission from Schmid et al. 131 (A color version of this figure is available in the online journal.)
Like T cells, dendritic cells (DCs) are immune cells that migrate to the lymph nodes and are critical for mediating adaptive immunity. As the primary antigen-presenting cell of the immune system, DCs are dispersed throughout the body and are typically found in higher concentrations in tissues exposed to the external environment, such as the skin, intestinal lining, and nasal passages.137,138 Although DCs have been reported to migrate to the lymph nodes to some degree during steady-state conditions, the majority of DC migration is reported in the context of post-antigen exposure, after which DCs begin to express surface receptors like CCR7 that are used to follow chemokines such as CCL19 and CCL21 to the lymphatics, where DCs initiate adaptive immune responses via antigen presentation to naïve T cells and B cells.139–141 Due to the role of DCs in adaptive immunity, a plethora of studies and reviews have been conducted with regards to targeting nanoparticles to DCs for immunotherapy and vaccination.142–145
Although many of the studies have used the subcutaneous or intradermal route for DC-targeting, intravenous injection of nanoparticles actively targeted to DCs has also shown success in immunotherapeutic and vaccination strategies. Sancho et al. utilized DC, NK lectin group receptor-1 (DNGR-1) for targeted antigen delivery to DCs for the generation of CD8 T cell responses against B16F10 melanoma tumors. 146 Specifically, anti-DNGR-1 antibodies conjugated to the immunogenic peptide SIINFEKL were injected intravenously into mice bearing B16F10-OVA tumors and were found to colocalize with DCs in the lymph nodes. This resulted in CD8 T cell responses that significantly mitigated tumor metastases in the lungs, confirming the potency of DC-targeting for immune stimulation. In addition, Stead et al. investigated DC-targeting in the context of generating immune tolerance to allogenic transplants in mice by using targeted silicon nanoparticles (160 nm) to deliver the immunosuppressive drug, rapamycin, to induce expansion and proliferation of regulatory T cells. 147 DC-targeting was achieved by functionalizing the particles with antibodies targeted to the DC marker CD11c. Mice received a total of three intravenous injections on days 0, 14, and 28 of the study and were euthanized after 40 days. When harvested spleens were evaluated for the presence of regulatory T cells, DC-targeted nanoparticle therapy showed a five-fold increase in the number of regulatory T cells compared to mice treated with nanoparticles functionalized with isotype control antibodies. Collectively, these studies demonstrate the feasibility of leveraging DC-targeted nanoparticles for the delivery of antigens and small molecule drugs to the lymph node to induce an immunomodulatory effect.
In addition to T cells and DCs, monocytes are immune cells that rapidly accumulate in the lymph nodes through blood vessels during and immediately following inflammation, with functions spanning antigen transport and presentation and differentiation into DCs, although they have also been reported to be present in steady-state conditions.12,148–153 The influx of monocytes during inflammatory states has been reported to be a result of the accumulation of lymph-borne chemokines such as monocyte chemoattractant protein-1 (MCP-1) in the lymph node, entering the lymph nodes via the afferent lymphatic vessels.149–152 The increased trafficking of monocytes to the lymph nodes during inflammatory states such as cancer makes monocyte targeting a useful means of drug delivery to the lymph nodes. Our group has explored this by synthesizing micelles functionalized with peptides derived from the CCR2-binding motif of the MCP-1 chemokine.154–158 When injected intravenously into B16F10 tumor-bearing mice, micelles containing the CCR2-binding motif were observed to accumulate in the lymph nodes at twice the rate of a non-targeted micelle 3 h post-injection, although further study is required to elucidated the mechanisms behind this. 154 The development of nanoparticle systems that can strongly associate with immune cells that migrate through the lymphatics offers a novel means of drug delivery to the lymph nodes.
Conclusions and future outlook
While early nanoparticle characterization and cancer efficacy studies focused on prolonging nanoparticle circulation in the blood vasculature and tumor accumulation, the prominent role of the lymphatic system, particularly the lymph nodes, in the progression of numerous pathologies, such as cancer, cardiovascular disease, autoimmunity, and viral infections, is now widely recognized.6,159,160 As a result, improving drug delivery to the lymph nodes has become a heavily researched topic.41,103--105,121--125,131,132,154 As reviewed here, intravenous drug delivery to the lymph nodes is challenged by numerous biological barriers, such as the MPS, blood vessel endothelium, and ECM, and overcoming these delivery barriers will be critical to their successful implementation in the clinic. 55--57,93,94,161--163
An area for further research is to design nanocarriers that incorporate ligands that actively target lymph node-specific biomarkers to increase nanoparticle retention in the lymph nodes. For example, targeting nanoparticles to the lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1) that is expressed by all lymphatic endothelial cells may promote nanoparticle accumulation deep into the cortex and paracortex of lymph nodes, similar to the use of peptides containing the integrin-binding RGD motif for tumor targeting and penetration.164–166 Another biomarker that can be used for lymph node targeting is cluster of differentiation 169 (CD169), which is expressed by macrophages lining the subcapsular sinus of lymph nodes, as well as macrophages in the bone marrow.167,168 Subcapsular sinus macrophages come into direct contact with lymph that flows into the node via the afferent lymphatic vessels and take up lymph-borne antigens that are presented to naïve B cells of the cortex.167,169 Thus, nanocarriers actively targeted to subcapsular sinus macrophages have the potential to improve antigen delivery for immunization purposes. Although CD169 ligands have been used to target liposomes to bone marrow macrophages, their use in targeting the CD169+ macrophages in the lymph nodes has yet to be explored. 170
An alternative route for lymph node entry is through extravasation out of the blood vessels that innervate the lymph nodes, called high endothelial venules (HEVs). HEVs are specialized venules whose primary function is to transport lymphocytes, such as T cells, in and out of the lymph nodes by producing lymphocyte-attracting chemokines like CCL21. 171 Compared to the flat, endothelial cells of normal blood vessels, HEV endothelial cells are cuboidal in shape and are supported by a thicker basement membrane. While several nanoparticle studies have reported HEVs as a primary means of lymph node accumulation, the mechanisms underlying HEV-mediated transport have not been well-characterized and require more rigorous study.42,110 Although intravenous drug delivery to the lymph nodes is challenged by several physiological barriers, studies characterizing these barriers have paved the way to rational nanocarrier design that can improve therapeutic outcomes. However, the design rules reviewed here apply to intravenous administration, and likely differ from other administration routes; hence, the intended route of administration must be taken into consideration when designing nanomedicine strategies for in vivo use.
Footnotes
AUTHORS’ CONTRIBUTIONS: NT and EJC contributed to the writing of the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors would like to acknowledge the financial support from the Women in Science and Engineering (WiSE) Gabilan Assistant Professorship, L. K. Whittier Foundation, the National Heart, Lung, and Blood Institute (NHLBI, R00HL124279) NIH New Innovator Award (DP2-DK121328), and the University of Southern California startup funds awarded to EJC.
ORCID iD: Eun Ji Chung https://orcid.org/0000-0002-7726-5555
References
- 1.Moore JE, Jr., Bertram CD. Lymphatic system flows. Annu Rev Fluid Mech 2018; 50:459–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von der Weid PY. Lymphatic vessel pumping. Adv Exp Med Biol 2019; 1124:357–77 [DOI] [PubMed] [Google Scholar]
- 3.Li H, Mei Y, Maimon N, Padera TP, Baish JW, Munn LL. The effects of valve leaflet mechanics on lymphatic pumping assessed using numerical simulations. Sci Rep 2019; 9:10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olszewski WL. The lymphatic system in body homeostasis: physiological conditions. Lymphat Res Biol 2003; 1:11–21; discussion 21–4 [DOI] [PubMed] [Google Scholar]
- 5.Huxley VH, Scallan J. Lymphatic fluid: exchange mechanisms and regulation. J Physiol 2011; 589:2935–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao S, Padera TP. Lymphatic function and immune regulation in health and disease. Lymphat Res Biol 2013; 11:136–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hampton HR, Chtanova T. Lymphatic migration of immune cells. Front Immunol 2019; 10:1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol 2006; 34:409–24 [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Harashima H. Dawn of lipid nanoparticles in lymph node targeting: potential in cancer immunotherapy. Adv Drug Deliv Rev 2020; 167:78–88 [DOI] [PubMed] [Google Scholar]
- 10.Jarjour M, Jorquera A, Mondor I, Wienert S, Narang P, Coles MC, Klauschen F, Bajenoff M. Fate mapping reveals origin and dynamics of lymph node follicular dendritic cells. J Exp Med 2014; 211:1109–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray EE, Cyster JG. Lymph node macrophages. J Innate Immun 2012; 4:424–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 1999; 11:753–61 [DOI] [PubMed] [Google Scholar]
- 13.Ruddle NH. High endothelial venules and lymphatic vessels in tertiary lymphoid organs: characteristics, functions, and regulation. Front Immunol 2016; 7:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spurrell EL, Lockley M. Adaptive immunity in cancer immunology and therapeutics. Ecancermedicalscience 2014; 8:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019; 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wennhold K, Shimabukuro-Vornhagen A, von Bergwelt-Baildon M. B. Cell-based cancer immunotherapy. Transfus Med Hemother 2019; 46:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaplin DD. Overview of the immune response. J Allergy Clin Immunol 2010; 125:S3–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeanbart L, Ballester M, de Titta A, Corthesy P, Romero P, Hubbell JA, Swartz MA. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol Res 2014; 2:436–47 [DOI] [PubMed] [Google Scholar]
- 19.Yu X, Dai Y, Zhao Y, Qi S, Liu L, Lu L, Luo Q, Zhang Z. Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic anti-tumor immune response. Nat Commun 2020; 11:1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Zhao P, Dong S, Xu T, He X, Chen M. An albumin-binding polypeptide both targets cytotoxic T lymphocyte vaccines to lymph nodes and boosts vaccine presentation by dendritic cells. Theranostics 2018; 8:223–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones D, Pereira ER, Padera TP. Growth and immune evasion of lymph node metastasis. Front Oncol 2018; 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawada K, Taketo MM. Significance and mechanism of lymph node metastasis in cancer progression. Cancer Res 2011; 71:1214–8 [DOI] [PubMed] [Google Scholar]
- 23.Ulintz PJ, Greenson JK, Wu R, Fearon ER, Hardiman KM. Lymph node metastases in colon cancer are polyclonal. Clin Cancer Res 2018; 24:2214–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandrasekaran S, King MR. Microenvironment of tumor-draining lymph nodes: opportunities for liposome-based targeted therapy. Int J Mol Sci 2014; 15:20209–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trzewik J, Mallipattu SK, Artmann GM, Delano FA, Schmid-Schonbein GW. Evidence for a second valve system in lymphatics: endothelial microvalves. FASEB J 2001; 15:1711–7 [DOI] [PubMed] [Google Scholar]
- 26.Scallan J, Huxley VH, Korthuis RJ. Capillary fluid exchange: regulation, functions, and pathology. San Rafael, CA: Morgan & Claypool Life Sciences, 2010 [PubMed] [Google Scholar]
- 27.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 2007; 204:2349–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swartz MA, Skobe M. Lymphatic function, lymphangiogenesis, and cancer metastasis. Microsc Res Tech 2001; 55:92–9 [DOI] [PubMed] [Google Scholar]
- 29.Maisel K, Sasso MS, Potin L, Swartz MA. Exploiting lymphatic vessels for immunomodulation: rationale, opportunities, and challenges. Adv Drug Deliv Rev 2017; 114:43–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenlee JD, King MR. Engineered fluidic systems to understand lymphatic cancer metastasis. Biomicrofluidics 2020; 14:011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christiansen A, Detmar M. Lymphangiogenesis and cancer. Genes Cancer 2011; 2:1146–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 2006; 13:597–610 [DOI] [PubMed] [Google Scholar]
- 33.Papaioannou TG, Stefanadis C. Vascular wall shear stress: basic principles and methods. Hellenic J Cardiol 2005; 46:9–15 [PubMed] [Google Scholar]
- 34.Abdallah M, Mullertz OO, Styles IK, Morsdorf A, Quinn JF, Whittaker MR, Trevaskis NL. Lymphatic targeting by albumin-hitchhiking: applications and optimisation. J Control Release 2020; 327:117–28 [DOI] [PubMed] [Google Scholar]
- 35.Jafarnejad M, Woodruff MC, Zawieja DC, Carroll MC, Moore JE., Jr. Modeling lymph flow and fluid exchange with blood vessels in lymph nodes. Lymphat Res Biol 2015; 13:234–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blatter C, Meijer EF, Nam AS, Jones D, Bouma BE, Padera TP, Vakoc BJ. In vivo label-free measurement of lymph flow velocity and volumetric flow rates using Doppler optical coherence tomography. Sci Rep 2016; 6:29035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klarhofer M, Csapo B, Balassy C, Szeles JC, Moser E. High-resolution blood flow velocity measurements in the human finger. Magn Reson Med 2001; 45:716–9 [DOI] [PubMed] [Google Scholar]
- 38.Zhang XY, Lu WY. Recent advances in lymphatic targeted drug delivery system for tumor metastasis. Cancer Biol Med 2014; 11:247–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Y, Bagby TR, Cohen MS, Forrest ML. Drug delivery to the lymphatic system: importance in future cancer diagnosis and therapies. Expert Opin Drug Deliv 2009; 6:785–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oussoren C, Storm G. Liposomes to target the lymphatics by subcutaneous administration. Adv Drug Deliv Rev 2001; 50:143–56 [DOI] [PubMed] [Google Scholar]
- 41.Cabral H, Makino J, Matsumoto Y, Mi P, Wu H, Nomoto T, Toh K, Yamada N, Higuchi Y, Konishi S, Kano MR, Nishihara H, Miura Y, Nishiyama N, Kataoka K. Systemic targeting of lymph node metastasis through the blood vascular system by using size-controlled nanocarriers. ACS Nano 2015; 9:4957–67 [DOI] [PubMed] [Google Scholar]
- 42.Weissleder R, Elizondo G, Wittenberg J, Lee AS, Josephson L, Brady TJ. Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology 1990; 175:494–8 [DOI] [PubMed] [Google Scholar]
- 43.Worley DR, Hansen RJ, Wittenburg LA, Chubb LS, Gustafson DL. Docetaxel accumulates in lymphatic circulation following subcutaneous delivery compared to intravenous delivery in rats. Anticancer Res 2016; 36:5071–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J, Wang L, Yao Q, Ling R, Li K, Wang H. Drug concentrations in axillary lymph nodes after lymphatic chemotherapy on patients with breast cancer. Breast Cancer Res 2004; 6:R474–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang C, Shi B, Pei YY, Hong MH, Wu J, Chen HZ. In vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. Eur J Pharm Sci 2006; 27:27–36 [DOI] [PubMed] [Google Scholar]
- 46.Choi CH, Zuckerman JE, Webster P, Davis ME. Targeting kidney mesangium by nanoparticles of defined size. Proc Natl Acad Sci U S A 2011; 108:6656–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm 2008; 5:505–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arami H, Khandhar A, Liggitt D, Krishnan KM. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem Soc Rev 2015; 44:8576–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, Agarwal RG, Lam KS. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 2011; 32:3435–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metz S, Bonaterra G, Rudelius M, Settles M, Rummeny EJ, Daldrup-Link HE. Capacity of human monocytes to phagocytose approved iron oxide MR contrast agents in vitro. Eur Radiol 2004; 14:1851–8 [DOI] [PubMed] [Google Scholar]
- 51.Franca A, Aggarwal P, Barsov EV, Kozlov SV, Dobrovolskaia MA, Gonzalez-Fernandez A. Macrophage scavenger receptor a mediates the uptake of gold colloids by macrophages in vitro. Nanomedicine 2011; 6:1175–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patten DA, Shetty S. More than just a removal service: scavenger receptors in leukocyte trafficking. Front Immunol 2018; 9:2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chao Y, Karmali PP, Mukthavaram R, Kesari S, Kouznetsova VL, Tsigelny IF, Simberg D. Direct recognition of superparamagnetic nanocrystals by macrophage scavenger receptor SR-AI. ACS Nano 2013; 7:4289–98 [DOI] [PubMed] [Google Scholar]
- 54.Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano Today 2015; 10:487–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas OS, Weber W. Overcoming physiological barriers to nanoparticle delivery-are we there yet? Front Bioeng Biotechnol 2019; 7:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 2015; 33:941–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barua S, Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today 2014; 9:223–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tseng YC, Xu Z, Guley K, Yuan H, Huang L. Lipid-calcium phosphate nanoparticles for delivery to the lymphatic system and SPECT/CT imaging of lymph node metastases. Biomaterials 2014; 35:4688–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fromen CA, Rahhal TB, Robbins GR, Kai MP, Shen TW, Luft JC, DeSimone JM. Nanoparticle surface charge impacts distribution, uptake and lymph node trafficking by pulmonary antigen-presenting cells. Nanomedicine 2016; 12:677–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Masehi-Lano JJ, Chung EJ. Peptide and antibody ligands for renal targeting: nanomedicine strategies for kidney disease. Biomater Sci 2017; 5:1450–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan ZX, Shang Z, Gu J, He L. Renal targeting delivery systems. Future Med Chem 2019; 11:2237–40 [DOI] [PubMed] [Google Scholar]
- 62.Liang X, Wang H, Zhu Y, Zhang R, Cogger VC, Liu X, Xu ZP, Grice JE, Roberts MS. Short- and Long-Term tracking of anionic ultrasmall nanoparticles in kidney. ACS Nano 2016; 10:387–95 [DOI] [PubMed] [Google Scholar]
- 63.Hong YK, Shin JW, Detmar M. Development of the lymphatic vascular system: a mystery unravels. Dev Dyn 2004; 231:462–73 [DOI] [PubMed] [Google Scholar]
- 64.Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol 2010; 87:93–106 [DOI] [PubMed] [Google Scholar]
- 65.Silva MT, Correia-Neves M. Neutrophils and macrophages: the main partners of phagocyte cell systems. Front Immunol 2012; 3:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ 1972; 46:845–52 [PMC free article] [PubMed] [Google Scholar]
- 67.Fam SY, Chee CF, Yong CY, Ho KL, Mariatulqabtiah AR, Tan WS. Stealth coating of nanoparticles in drug-delivery systems. Nanomaterials 2020; 10: 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 2016; 99:28–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoang Thi TT, Pilkington EH, Nguyen DH, Lee JS, Park KD, Truong NP. The importance of poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers 2020; 12:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saptarshi SR, Duschl A, Lopata AL. Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. J Nanobiotechnology 2013; 11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sotnikov DV, Berlina AN, Ivanov VS, Zherdev AV, Dzantiev BB. Adsorption of proteins on gold nanoparticles: one or more layers? Colloids Surf B Biointerfaces 2019; 173:557–63 [DOI] [PubMed] [Google Scholar]
- 72.Bisso PW, Gaglione S, Guimaraes PPG, Mitchell MJ, Langer R. Nanomaterial interactions with human neutrophils. ACS Biomater Sci Eng 2018; 4:4255–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett 2009; 9:1909–15 [DOI] [PubMed] [Google Scholar]
- 74.Feng Q, Liu Y, Huang J, Chen K, Huang J, Xiao K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci Rep 2018; 8:2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chertok B, David AE, Yang VC. Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intra-carotid administration. Biomaterials 2010; 31:6317–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cong X, Kong W. Endothelial tight junctions and their regulatory signaling pathways in vascular homeostasis and disease. Cell Signal 2020; 66:109485. [DOI] [PubMed] [Google Scholar]
- 77.Rodrigues SF, Granger DN. Blood cells and endothelial barrier function. Tissue Barriers 2015; 3:e978720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toy R, Hayden E, Shoup C, Baskaran H, Karathanasis E. The effects of particle size, density and shape on margination of nanoparticles in microcirculation. Nanotechnology 2011; 22:115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Decuzzi P, Lee S, Bhushan B, Ferrari M. A theoretical model for the margination of particles within blood vessels. Ann Biomed Eng 2005; 33:179–90 [DOI] [PubMed] [Google Scholar]
- 80.Thompson AJ, Mastria EM, Eniola-Adefeso O. The margination propensity of ellipsoidal micro/nanoparticles to the endothelium in human blood flow. Biomaterials 2013; 34:5863–71 [DOI] [PubMed] [Google Scholar]
- 81.Vu MN, Rajasekhar P, Poole DP, Khor SY, Truong NP, Nowell CJ, Quinn JF, Whittaker M, Veldhuis NA, Davis TP. Rapid assessment of nanoparticle extravasation in a microfluidic tumor model. ACS Appl Nano Mater 2019; 2:1844–56 [Google Scholar]
- 82.Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res 2000; 60:4440–5 [PubMed] [Google Scholar]
- 83.Smith BR, Cheng Z, De A, Rosenberg J, Gambhir SS. Dynamic visualization of RGD-quantum dot binding to tumor neovasculature and extravasation in multiple living mouse models using intravital microscopy. Small 2010; 6:2222–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hansen AE, Petersen AL, Henriksen JR, Boerresen B, Rasmussen P, Elema DR, af Rosenschold PM, Kristensen AT, Kjaer A, Andresen TL. Positron emission tomography based elucidation of the enhanced permeability and retention effect in dogs with cancer using copper-64 liposomes. ACS Nano 2015; 9:6985–95 [DOI] [PubMed] [Google Scholar]
- 85.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nat Rev Mater 2016; 1:16014 [Google Scholar]
- 86.Smith BR, Kempen P, Bouley D, Xu A, Liu Z, Melosh N, Dai H, Sinclair R, Gambhir SS. Shape Matters: intravital microscopy reveals surprising geometrical dependence for nanoparticles in tumor models of extravasation. Nano Lett 2012; 12:3369–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pandit S, Dutta D, Nie S. Active transcytosis and new opportunities for cancer nanomedicine. Nat Mater 2020; 19:478–80 [DOI] [PubMed] [Google Scholar]
- 88.Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, MacMillan P, Zhang Y, Rajesh NU, Hoang T, Wu JLY, Wilhelm S, Zilman A, Gadde S, Sulaiman A, Ouyang B, Lin Z, Wang L, Egeblad M, Chan WCW. The entry of nanoparticles into solid tumours. Nat Mater 2020; 19:566–75 [DOI] [PubMed] [Google Scholar]
- 89.Pulgar VM. Transcytosis to cross the blood brain barrier, new advancements and challenges. Front Neurosci 2018; 12:1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang L, Fan J, Li G, Yin Z, Fu BM. Transcellular model for neutral and charged nanoparticles across an in vitro blood-brain barrier. Cardiovasc Eng Technol 2020; 11:607–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gil ES, Li J, Xiao H, Lowe TL. Quaternary ammonium beta-cyclodextrin nanoparticles for enhancing doxorubicin permeability across the in vitro blood-brain barrier. Biomacromolecules 2009; 10:505–16 [DOI] [PubMed] [Google Scholar]
- 92.Yue B. Biology of the extracellular matrix: an overview. J Glaucoma 2014; 23:S20–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Engin AB, Nikitovic D, Neagu M, Henrich-Noack P, Docea AO, Shtilman MI, Golokhvast K, Tsatsakis AM. Mechanistic understanding of nanoparticles' interactions with extracellular matrix: the cell and immune system. Part Fibre Toxicol 2017; 14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Willis AL, Sabeh F, Li XY, Weiss SJ. Extracellular matrix determinants and the regulation of cancer cell invasion stratagems. J Microsc 2013; 251:250–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A 2011; 108:2426–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goodman TT, Olive PL, Pun SH. Increased nanoparticle penetration in collagenase-treated multicellular spheroids. Int J Nanomed 2007; 2:265–74 [PMC free article] [PubMed] [Google Scholar]
- 97.Yohan D, Cruje C, Lu X, Chithrani DB. Size-dependent gold nanoparticle interaction at nano-micro interface using both monolayer and multilayer (tissue-like) cell models. Nanomicro Lett 2016; 8:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stylianopoulos T, Poh MZ, Insin N, Bawendi MG, Fukumura D, Munn LL, Jain RK. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions. Biophys J 2010; 99:1342–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lieleg O, Baumgartel RM, Bausch AR. Selective filtering of particles by the extracellular matrix: an electrostatic bandpass. Biophys J 2009; 97:1569–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Le Goas M, Testard F, Tache O, Debou N, Cambien B, Carrot G, Renault JP. How do surface properties of nanoparticles influence their diffusion in the extracellular matrix? A model study in matrigel using polymer-grafted nanoparticles. Langmuir 2020; 36:10460–70 [DOI] [PubMed] [Google Scholar]
- 101.Kim B, Han G, Toley BJ, Kim CK, Rotello VM, Forbes NS. Tuning payload delivery in tumour cylindroids using gold nanoparticles. Nat Nanotechnol 2010; 5:465–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tchoryk A, Taresco V, Argent RH, Ashford M, Gellert PR, Stolnik S, Grabowska A, Garnett MC. Penetration and uptake of nanoparticles in 3D tumor spheroids. Bioconjug Chem 2019; 30:1371–84 [DOI] [PubMed] [Google Scholar]
- 103.Chida T, Miura Y, Cabral H, Nomoto T, Kataoka K, Nishiyama N. Epirubicin-loaded polymeric micelles effectively treat axillary lymph nodes metastasis of breast cancer through selective accumulation and pH-triggered drug release. J Control Release 2018; 292:130–40 [DOI] [PubMed] [Google Scholar]
- 104.Hu H, Wang J, Wang H, Tan T, Li J, Wang Z, Sun K, Li Y, Zhang Z. Cell-penetrating peptide-based nanovehicles potentiate lymph metastasis targeting and deep penetration for anti-metastasis therapy. Theranostics 2018; 8:3597–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baharom F, Ramirez-Valdez RA, Tobin KKS, Yamane H, Dutertre CA, Khalilnezhad A, Reynoso GV, Coble VL, Lynn GM, Mule MP, Martins AJ, Finnigan JP, Zhang XM, Hamerman JA, Bhardwaj N, Tsang JS, Hickman HD, Ginhoux F, Ishizuka AS, Seder RA. Intravenous nanoparticle vaccination generates stem-like TCF1(+) neoantigen-specific CD8(+) T cells. Nat Immunol 2021; 22:41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin L, Zhang F, Lu X, Wei X, Wang J, Fang X, Si D, Wang Y, Zhang C, Yang R, Liu C, Liang W. Polymeric micelles for enhanced lymphatic drug delivery to treat metastatic tumors. J Control Release 2013; 171:133–42 [DOI] [PubMed] [Google Scholar]
- 107.Kaminskas LM, Kota J, McLeod VM, Kelly BD, Karellas P, Porter CJ. PEGylation of polylysine dendrimers improves absorption and lymphatic targeting following SC administration in rats. J Control Release 2009; 140:108–16 [DOI] [PubMed] [Google Scholar]
- 108.Wilson KD, Raney SG, Sekirov L, Chikh G, deJong SD, Cullis PR, Tam YK. Effects of intravenous and subcutaneous administration on the pharmacokinetics, biodistribution, cellular uptake and immunostimulatory activity of CpG ODN encapsulated in liposomal nanoparticles. Int Immunopharmacol 2007; 7:1064–75 [DOI] [PubMed] [Google Scholar]
- 109.Elgrabli D, Beaudouin R, Jbilou N, Floriani M, Pery A, Rogerieux F, Lacroix G. Biodistribution and clearance of TiO2 nanoparticles in rats after intravenous injection. PLoS One 2015; 10:e0124490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med 2003; 348:2491–9 [DOI] [PubMed] [Google Scholar]
- 111.Rety F, Clement O, Siauve N, Cuenod CA, Carnot F, Sich M, Buisine A, Frija G. MR lymphography using iron oxide nanoparticles in rats: pharmacokinetics in the lymphatic system after intravenous injection. J Magn Reson Imaging 2000; 12:734–9 [DOI] [PubMed] [Google Scholar]
- 112.Liu J, Li HJ, Luo YL, Xu CF, Du XJ, Du JZ, Wang J. Enhanced primary tumor penetration facilitates nanoparticle draining into lymph nodes after systemic injection for tumor metastasis inhibition. ACS Nano 2019; 13:8648–58 [DOI] [PubMed] [Google Scholar]
- 113.Tojo A, Kinugasa S. Mechanisms of glomerular albumin filtration and tubular reabsorption. Int J Nephrol 2012; 2012:481520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin-more than just a serum protein. Front Physiol 2014; 5:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peters T., Jr., Serum albumin. Adv Protein Chem 1985; 37:161–245 [DOI] [PubMed] [Google Scholar]
- 116.Chien SC, Chen CY, Lin CF, Yeh HI. Critical appraisal of the role of serum albumin in cardiovascular disease. Biomark Res 2017; 5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yao L, Xue X, Yu P, Ni Y, Chen F. Evans blue dye: a revisit of its applications in biomedicine. Contrast Media Mol Imaging 2018; 2018:7628037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 2014; 507:519–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoogenboezem EN, Duvall CL. Harnessing albumin as a carrier for cancer therapies. Adv Drug Deliv Rev 2018; 130:73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chatterjee M, Ben-Josef E, Robb R, Vedaie M, Seum S, Thirumoorthy K, Palanichamy K, Harbrecht M, Chakravarti A, Williams TM. Caveolae-mediated endocytosis is critical for albumin cellular uptake and response to albumin-bound chemotherapy. Cancer Res 2017; 77:5925–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang L, Xia ZJ. Combination of albumin-bound paclitaxel and pegylated liposomal doxorubicin is effective in treatment of heavily treated relapsed/refractory diffuse large B-cell lymphoma: a case report. Leuk Lymphoma 2013; 54:2553–5 [DOI] [PubMed] [Google Scholar]
- 122.Bowen RC, Hahn AW, Butler TW, Khong HT. Complete response to azacitidine priming and nab-paclitaxel in non-Hodgkin lymphoma resistant to biochemotherapy. Mol Clin Oncol 2017; 6:122–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang L, Chen XQ, Xia ZJ. New tricks for old drugs: combination of rituximab and two nanoparticle-delivered chemotherapy drugs, albumin-bound paclitaxel and pegylated liposomal doxorubicin, in the treatment of relapsed/refractory diffuse large B cell lymphoma. Leuk Lymphoma 2020; 61:2502–6 [DOI] [PubMed] [Google Scholar]
- 124.Murphy EA, Majeti BK, Mukthavaram R, Acevedo LM, Barnes LA, Cheresh DA. Targeted nanogels: a versatile platform for drug delivery to tumors. Mol Cancer Ther 2011; 10:972–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yu X, Zhu W, Di Y, Gu J, Guo Z, Li H, Fu D, Jin C. Triple-functional albumin-based nanoparticles for combined chemotherapy and photodynamic therapy of pancreatic cancer with lymphatic metastases. Int J Nanomed 2017; 12:6771–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (abraxane ABI-007) in the treatment of breast cancer. Int J Nanomed 2009; 4:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kundranda MN, Niu J. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Des Devel Ther 2015; 9:3767–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martens R, Permanyer M, Werth K, Yu K, Braun A, Halle O, Halle S, Patzer GE, Bosnjak B, Kiefer F, Janssen A, Friedrichsen M, Poetzsch J, Kohli K, Lueder Y, Gutierrez Jauregui R, Eckert N, Worbs T, Galla M, Forster R. Efficient homing of T cells via afferent lymphatics requires mechanical arrest and integrin-supported chemokine guidance. Nat Commun 2020; 11:1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stein JV, Nombela-Arrieta C. Chemokine control of lymphocyte trafficking: a general overview. Immunology 2005; 116:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hunter MC, Teijeira A, Halin C. T cell trafficking through lymphatic vessels. Front Immunol 2016; 7:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang B, Abraham WD, Zheng Y, Bustamante Lopez SC, Luo SS, Irvine DJ. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci Transl Med 2015; 7:291ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schmid D, Park CG, Hartl CA, Subedi N, Cartwright AN, Puerto RB, Zheng Y, Maiarana J, Freeman GJ, Wucherpfennig KW, Irvine DJ, Goldberg MS. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat Commun 2017; 8:1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Uhl M, Aulwurm S, Wischhusen J, Weiler M, Ma JY, Almirez R, Mangadu R, Liu YW, Platten M, Herrlinger U, Murphy A, Wong DH, Wick W, Higgins LS, Weller M. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res 2004; 64:7954–61 [DOI] [PubMed] [Google Scholar]
- 134.Simon S, Labarriere N. PD-1 expression on tumor-specific T cells: friend or foe for immunotherapy? Oncoimmunology 2017; 7:e1364828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ahn E, Araki K, Hashimoto M, Li W, Riley JL, Cheung J, Sharpe AH, Freeman GJ, Irving BA, Ahmed R. Role of PD-1 during effector CD8 T cell differentiation. Proc Natl Acad Sci U S A 2018; 115:4749–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sauce D, Almeida JR, Larsen M, Haro L, Autran B, Freeman GJ, Appay V. PD-1 expression on human CD8 T cells depends on both state of differentiation and activation status. Aids 2007; 21:2005–13 [DOI] [PubMed] [Google Scholar]
- 137.Egawa G, Kabashima K. Skin as a peripheral lymphoid organ: revisiting the concept of skin-associated lymphoid tissues. J Invest Dermatol 2011; 131:2178–85 [DOI] [PubMed] [Google Scholar]
- 138.Balan S, Saxena M, Bhardwaj N. Dendritic cell subsets and locations. Int Rev Cell Mol Biol 2019; 348:1–68 [DOI] [PubMed] [Google Scholar]
- 139.Brand CU, Hunziker T, Braathen LR. Studies on human skin lymph containing langerhans cells from sodium lauryl sulphate contact dermatitis. J Invest Dermatol 1992; 99:109S–10S [DOI] [PubMed] [Google Scholar]
- 140.Granucci F, Ferrero E, Foti M, Aggujaro D, Vettoretto K, Ricciardi-Castagnoli P. Early events in dendritic cell maturation induced by LPS. Microbes Infect 1999; 1:1079–84 [DOI] [PubMed] [Google Scholar]
- 141.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity 2008; 29:325–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sehgal K, Dhodapkar KM, Dhodapkar MV. Targeting human dendritic cells in situ to improve vaccines. Immunol Lett 2014; 162:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol 2007; 7:790–802 [DOI] [PubMed] [Google Scholar]
- 144.Cruz LJ, Tacken PJ, Rueda F, Domingo JC, Albericio F, Figdor CG. Targeting nanoparticles to dendritic cells for immunotherapy. Methods Enzymol 2012; 509:143–63 [DOI] [PubMed] [Google Scholar]
- 145.Jia J, Zhang Y, Xin Y, Jiang C, Yan B, Zhai S. Interactions between nanoparticles and dendritic cells: from the perspective of cancer immunotherapy. Front Oncol 2018; 8:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, Carlyle JR. Reis e sousa C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest 2008; 118:2098–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stead SO, Kireta S, McInnes SJP, Kette FD, Sivanathan KN, Kim J, Cueto-Diaz EJ, Cunin F, Durand JO, Drogemuller CJ, Carroll RP, Voelcker NH, Coates PT. Murine and non-human primate dendritic cell targeting nanoparticles for in vivo generation of regulatory T-cells. ACS Nano 2018; 12:6637–47 [DOI] [PubMed] [Google Scholar]
- 148.Muller WA. New mechanisms and pathways for monocyte recruitment. J Exp Med 2001; 194:F47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med 2001; 194:1361–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Janatpour MJ, Hudak S, Sathe M, Sedgwick JD, McEvoy LM. Tumor necrosis factor-dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment. J Exp Med 2001; 194:1375–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med 2000; 192:1425–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Teh YC, Ding JL, Ng LG, Chong SZ. Capturing the fantastic voyage of monocytes through time and space. Front Immunol 2019; 10:834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma'ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 2013; 39:599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Trac N, Chen LY, Zhang A, Liao CP, Poon C, Wang J, Ando Y, Joo J, Garri C, Shen K, Kani K, Gross ME, Chung EJ. CCR2-targeted micelles for anti-cancer peptide delivery and immune stimulation. J Control Release 2021; 329:614–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chung EJ, Mlinar LB, Nord K, Sugimoto MJ, Wonder E, Alenghat FJ, Fang Y, Tirrell M. Monocyte-targeting supramolecular micellar assemblies: a molecular diagnostic tool for atherosclerosis. Adv Healthc Mater 2015; 4:367–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Poon C, Chowdhuri S, Kuo CH, Fang Y, Alenghat FJ, Hyatt D, Kani K, Gross ME, Chung EJ. Protein mimetic and anticancer properties of monocyte-targeting peptide amphiphile micelles. ACS Biomater Sci Eng 2017; 3:3273–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chin DD, Poon C, Trac N, Wang J, Joo J, Jiang ZJY, Maria NSS, Jacobs RE, Chung EJ, Cook J. Collagenase-cleavable peptide amphiphile micelles as a novel theranostic strategy in atherosclerosis. Adv Ther 2020; 3:1900196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Joo J, Poon C, Yoo SP, Chung EJ. Shape effects of peptide amphiphile micelles for targeting monocytes. Molecules 2018; 23:2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jones D, Min W. An overview of lymphatic vessels and their emerging role in cardiovascular disease. J Cardiovasc Dis Res 2011; 2:141–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schwartz N, Chalasani MLS, Li TM, Feng Z, Shipman WD, Lu TT. Lymphatic function in autoimmune diseases. Front Immunol 2019; 10:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tang Y, Wang X, Li J, Nie Y, Liao G, Yu Y, Li C. Overcoming the reticuloendothelial system barrier to drug delivery with a “Don't-Eat-Us” strategy. ACS Nano 2019; 13:13015–26 [DOI] [PubMed] [Google Scholar]
- 162.Singh A. Eliciting B cell immunity against infectious diseases using nanovaccines. Nat Nanotechnol 2021; 16:16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schudel A, Francis DM, Thomas SN. Material design for lymph node drug delivery. Nat Rev Mater 2019; 4:415–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang F, Li Y, Shen Y, Wang A, Wang S, Xie T. The functions and applications of RGD in tumor therapy and tissue engineering. Int J Mol Sci 2013; 14:13447–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ruoslahti E. Tumor penetrating peptides for improved drug delivery. Adv Drug Deliv Rev 2017; 110-111:3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guo Q, Liu Y, Xu K, Ren K, Sun W. Mouse lymphatic endothelial cell targeted probes: anti-LYVE-1 antibody-based magnetic nanoparticles. Int J Nanomed 2013; 8:2273–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Louie DAP, Liao S. Lymph node subcapsular sinus macrophages as the frontline of lymphatic immune defense. Front Immunol 2019; 10:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 2011; 208:261–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Harwood NE, Batista FD. Antigen presentation to B cells. F1000. Biol Rep 2010; 2:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen WC, Kawasaki N, Nycholat CM, Han S, Pilotte J, Crocker PR, Paulson JC. Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169. PLoS One 2012; 7:e39039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ager A. High endothelial venules and other blood vessels: critical regulators of lymphoid organ development and function. Front Immunol 2017; 8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]