Summary
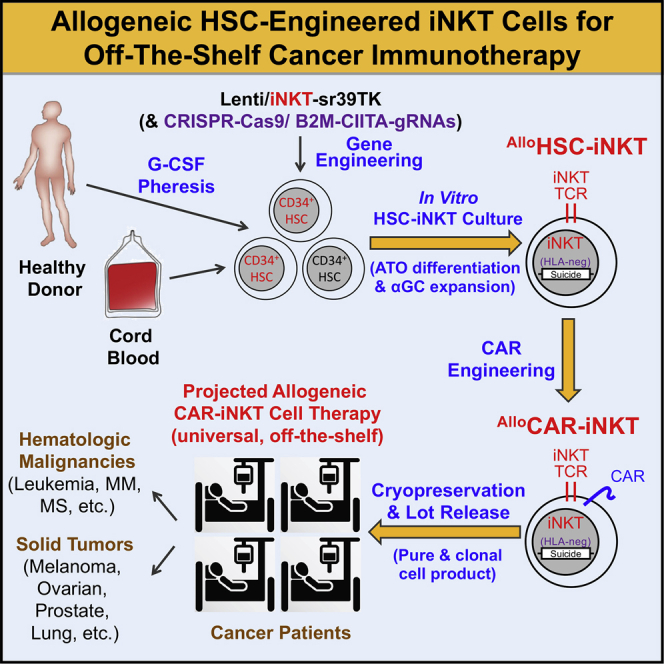
Cell-based immunotherapy has become the new-generation cancer medicine, and “off-the-shelf” cell products that can be manufactured at large scale and distributed readily to treat patients are necessary. Invariant natural killer T (iNKT) cells are ideal cell carriers for developing allogeneic cell therapy because they are powerful immune cells targeting cancers without graft-versus-host disease (GvHD) risk. However, healthy donor blood contains extremely low numbers of endogenous iNKT cells. Here, by combining hematopoietic stem cell (HSC) gene engineering and in vitro differentiation, we generate human allogeneic HSC-engineered iNKT (AlloHSC-iNKT) cells at high yield and purity; these cells closely resemble endogenous iNKT cells, effectively target tumor cells using multiple mechanisms, and exhibit high safety and low immunogenicity. These cells can be further engineered with chimeric antigen receptor (CAR) to enhance tumor targeting or/and gene edited to ablate surface human leukocyte antigen (HLA) molecules and further reduce immunogenicity. Collectively, these preclinical studies demonstrate the feasibility and cancer therapy potential of AlloHSC-iNKT cell products and lay a foundation for their translational and clinical development.
Keywords: hematopoietic stem cell, invariant natural killer T cells, cancer immunotherapy, allogeneic off-the-shelf cell therapy, chimeric antigen receptor, allogeneic HSC-engineered iNKT cells, HLA-ablated universal HSC-iNKT cells, CAR-engineered conventional αβ T cells, graft-versus-host disease, allorejection
Graphical abstract
Highlights
-
•
Allogeneic HSC-iNKT cells are generated in vitro at high yield and purity
-
•
Allogeneic HSC-iNKT cells effectively target tumor cells using multiple mechanisms
-
•
Allogeneic HSC-iNKT cells exhibit high safety and low immunogenicity
-
•
A preclinical study demonstrated feasibility, safety, and cancer therapy potential
Li et al. report the preclinical development of allogeneic-hematopoietic-stem-cell-engineered invariant natural killer T (HSC-iNKT) cells for off-the-shelf cancer therapy. Allogeneic HSC-iNKT cells demonstrate cancer therapy potential and a high safety profile.
Introduction
Over the past decade, immunotherapy has become the new-generation cancer medicine.1, 2, 3 In particular, T-cell-based cancer therapy has shown great promise.4 An outstanding example is the chimeric antigen receptor (CAR)-engineered T (CAR-T) cell adoptive therapy, which targets certain blood cancers at impressive efficacy and has been approved by the US Food and Drug Administration (FDA) to treat CD19+ B cell malignancies.4, 5, 6 Adoptive transfer of in vitro expanded tumor-infiltrating T lymphocytes (TILs) and T cell receptor (TCR)-engineered T cells also show promise in treating some blood cancers and solid tumors in the clinic.7,8 However, most of the current T cell therapies fall in the category of autologous cell therapy, wherein T cells collected from a patient are manufactured and used to treat that single patient. Such an approach is time consuming, logistically challenging, and costly; furthermore, for patients with heavily lymphopenic pretreatment or rapidly proliferative disease, it might not always be possible to produce autologous cell products.5,9 Allogenic cell products that can be manufactured at large scale and distributed readily to treat a broad range of cancer patients therefore are in great demand.
Conventional αβ T cells have been utilized for generating allogeneic cell products; however, these T cells risk inducing graft-versus-host disease (GvHD) in allogeneic hosts due to histocompatibility leukocyte antigen (HLA) incompatibility, thereby requiring additional gene editing to ablate their endogenous TCR expression that may potentially increase manufacture complexity.10, 11, 12, 13 Innate immune cells such as natural killer (NK) cells that have no GvHD risk have been investigated; however, NK cells may have limited in vivo clonal expansion and antitumor performance compared to T cells.14,15
Invariant NK T (iNKT) cells are a small population of αβ T lymphocytes.16 iNKT cells have several unique features, making them ideal for developing off-the-shelf cellular therapy for cancer. Compared to conventional T cells, iNKT cells can attack tumor cells using multiple mechanisms and at higher efficacy; can more effectively traffic to and infiltrate solid tumors; can alter solid tumor immunosuppressive microenvironment; and, most importantly, do not induce GvHD.17, 18, 19, 20, 21, 22, 23, 24, 25 However, human blood contains extremely low numbers of iNKT cells (0.001%–1%), making it very difficult to reliably grow large numbers of allogeneic iNKT cells for cell therapies.26 Moreover, allogeneic iNKT products expanded from blood may contain bystander allogeneic conventional αβ T cells and thus risk inducing GvHD. Technology breakthroughs are needed to exploit the allogeneic cell therapy potential of iNKT cells.
Previously, we have established a method to generate large numbers of iNKT cells through TCR gene engineering of hematopoietic stem cells (HSCs) followed by in vivo reconstitution; using this method, we have successfully generated both mouse and human HSC-engineered iNKT (HSC-iNKT) cells.27,28 However, such an in vivo approach cannot be used to produce off-the-shelf mature allogeneic iNKT cells.27,28 Here, we intended to build on the HSC-iNKT engineering approach and develop an in vitro culture method to produce large numbers of off-the-shelf human iNKT cells for allogeneic cell therapy applications. We report the preclinical development of the proposed allogeneic HSC-iNKT cell therapy, demonstrating its manufacture feasibility, cancer therapy potential, and high safety profile.
Results
Generation of allogeneic HSC-engineered iNKT (AlloHSC-iNKT) cells
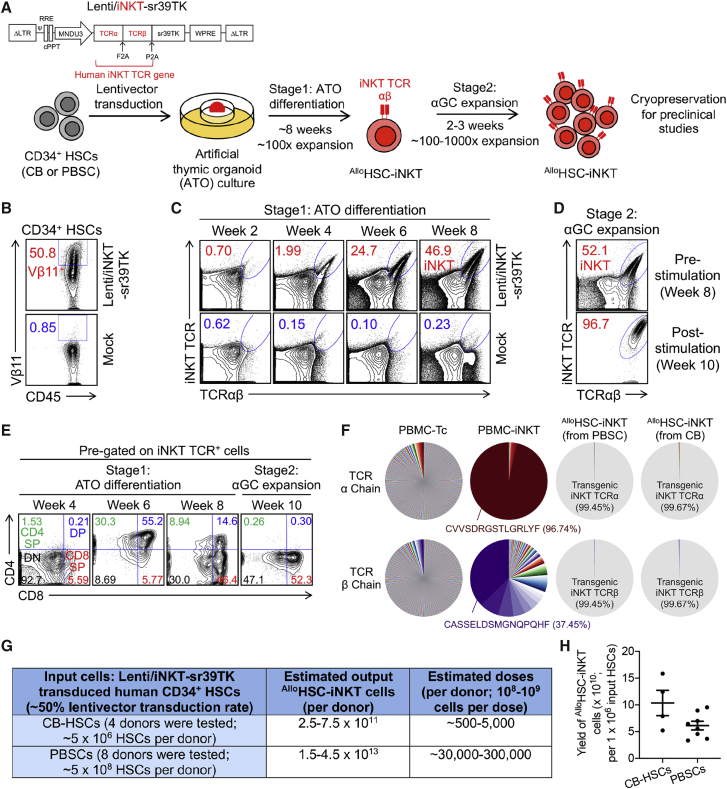
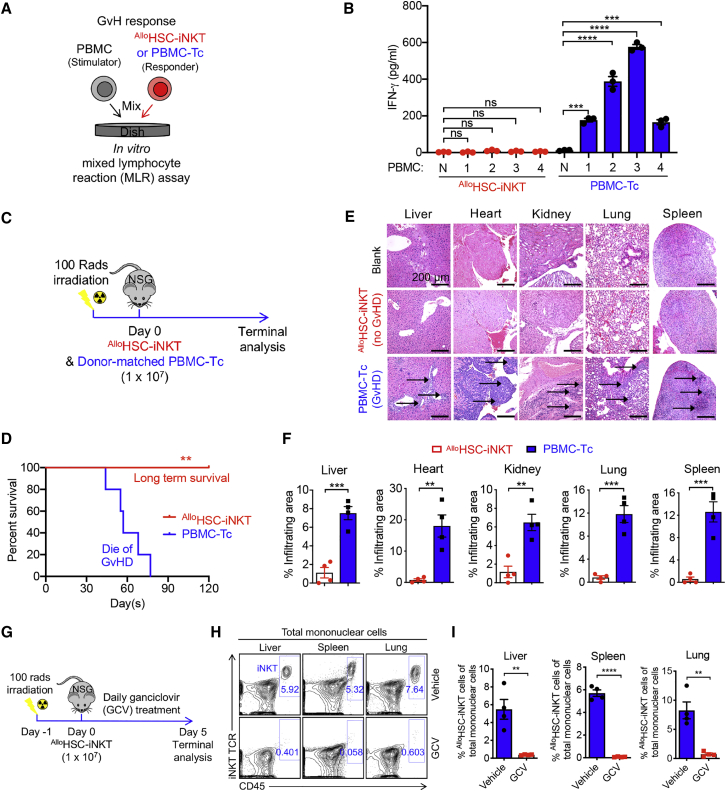
Human CD34+ cells collected from either cord blood (CB) or granulocyte-colony-stimulating factor (G-CSF)-mobilized human peripheral blood stem cells (PBSCs) were transduced with a Lenti/iNKT-sr39TK vector and then cultured in vitro in a two-stage artificial thymic organoid (ATO)/α-galactosylceramide (αGC) culture system (Figure 1A). Note CD34+ cells comprise both hematopoietic stem and progenitor cells; in this report we refer to CD34+ cells as HSCs. The Lenti/iNKT-sr39TK vector has been previously used to develop an autologous HSC-engineered iNKT cell therapy for cancer28; ATO is an in vitro 3D culture that supports the human HSC differentiation into T cells,29,30 while αGC is a synthetic agonist glycolipid ligand that specifically stimulates iNKT cells.16 We routinely achieved over 50% lentivector transduction rate of HSCs (Figure 1B). Transduced HSCs were then placed in the stage 1 ATO culture, where they differentiated into human iNKT cells over a course of 8 weeks with over 100-fold expansion (Figures 1A and 1C). At the end of stage 1 culture, ATOs were dissociated into single cells that were then placed in the stage 2 αGC expansion culture for another 2–3 weeks, resulting in another 100- to 1,000-fold expansion and an AlloHSC-iNKT cell product of high yield and purity (Figures 1A and 1D).
Figure 1.
In vitro generation of allogenic HSC-engineered iNKT (AlloHSC-iNKT) cells
(A) Experimental design to generate AlloHSC-iNKT cells in vitro. HSC, hematopoietic stem cell; CB, cord blood; PBSC, peripheral blood stem cell; αGC, α-galactosylceramide; Lenti/iNKT-sr39TK, lentiviral vector encoding an iNKT TCR gene and an sr39TK suicide/positron emission tomography (PET) imaging gene.
(B–E) Fluorescence-activated cell sorting (FACS) monitoring of AlloHSC-iNKT cell generation. (B) Intracellular expression of iNKT TCR (identified as Vβ11+) in CD34+ HSCs at 72 h after lentivector transduction. (C) Generation of iNKT cells (identified as iNKT TCR+TCRαβ+ cells) during stage 1 ATO differentiation culture. A 6B11 monoclonal antibody was used to stain iNKT TCR. (D) Expansion of iNKT cells during stage 2 αGC expansion culture. (E) Expression of CD4/CD8 co-receptors on AlloHSC-iNKT cells during stage 1 and stage 2 cultures. DN, CD4/CD8 double negative; CD4 SP, CD4 single positive; DP, CD4/CD8 double positive; CD8 SP, CD8 single positive.
(F) Single-cell TCR sequencing analysis of AlloHSC-iNKT cells. Healthy donor peripheral blood mononuclear cell (PBMC)-derived conventional αβ T (PBMC-Tc) and iNKT (PBMC-iNKT) cells were included as controls. The relative abundance of each unique T cell receptor (TCR) sequence among the total unique sequences identified for individual cells is represented by a pie slice.
(G) Table summarizing experiments that have successfully generated AlloHSC-iNKT cells.
(H) Yields of AlloHSC-iNKT cells generated from multiple HSC donors.
Representative of 1 (F) and >10 experiments (A–E).
In the ATO/αGC culture, AlloHSC-iNKT cells followed a typical iNKT cell development path defined by CD4/CD8 co-receptor expression.31 During stage 1, AlloHSC-iNKT cells transited from CD4−CD8− (DN) to CD4+CD8+ (DP), then toward CD4−CD8+/− (Figure 1E). At the end of stage 2, the majority (>99%) of AlloHSC-iNKT cells showed a CD4-CD8+/− (CD8 SP/DN) phenotype (Figure 1E). Note that the end AlloHSC-iNKT cell product did not contain a CD4+CD8− (CD4 SP) population that are present in the endogenous human iNKT cells (Figure 1E).31 In general, CD8 SP/DN human iNKT cells are considered to be proinflammatory and highly cytotoxic and thereby are desirable for cancer immunotherapy.24,31, 32, 33, 34, 35
Flow cytometry analysis showed that the AlloHSC-iNKT cell product comprised high-purity transgenic iNKT cells (Figure 1D); single-cell TCR sequencing analysis confirmed that these AlloHSC-iNKT cells uniformly expressed the transgenic iNKT TCRs while nearly undetectable randomly recombined endogenous αβ TCRs (Figure 1F). In sharp contrast to AlloHSC-iNKT cells, conventional αβ T cells isolated from health donor periphery blood (denoted as PBMC-Tc cells) expressed highly diverse endogenously recombined αβ TCRs, whereas iNKT cells isolated from health donor periphery blood (denoted as PBMC-iNKT cells) expressed a conserved invariant TCR α chain (Vα24-Jα18) and limited diverse TCR β chains (dominantly Vβ11) (Figure 1F).
The manufacture of AlloHSC-iNKT cells was highly robust; we generated AlloHSC-iNKT cell products of high purity and yield from all 12 donors tested (4 CB-HSCs and 8 PBSCs) (Figures 1G and 1H). Based on our results, it was estimated that from one CB donor (comprising ∼5 × 106 CB-HSCs), ∼5 × 1011 AlloHSC-iNKT cells could be generated that can potentially be formulated into ∼500–5,000 doses (∼108-109 cells per dose based on the approved CAR-T cell therapy doses)4; from one PBSC donor (comprising ∼5 × 108 PBSCs), ∼3 × 1013 AlloHSC-iNKT cells could be generated that can potentially be formulated into ∼30,000–300,000 doses (Figure 1G). The resulting AlloHSC-iNKT cell product contained pure transgenic iNKT cells and nearly undetectable bystander conventional αβ T cells, thereby lack of GvHD risk and suitable for “off-the-shelf” application.
Phenotype and functionality of AlloHSC-iNKT cells
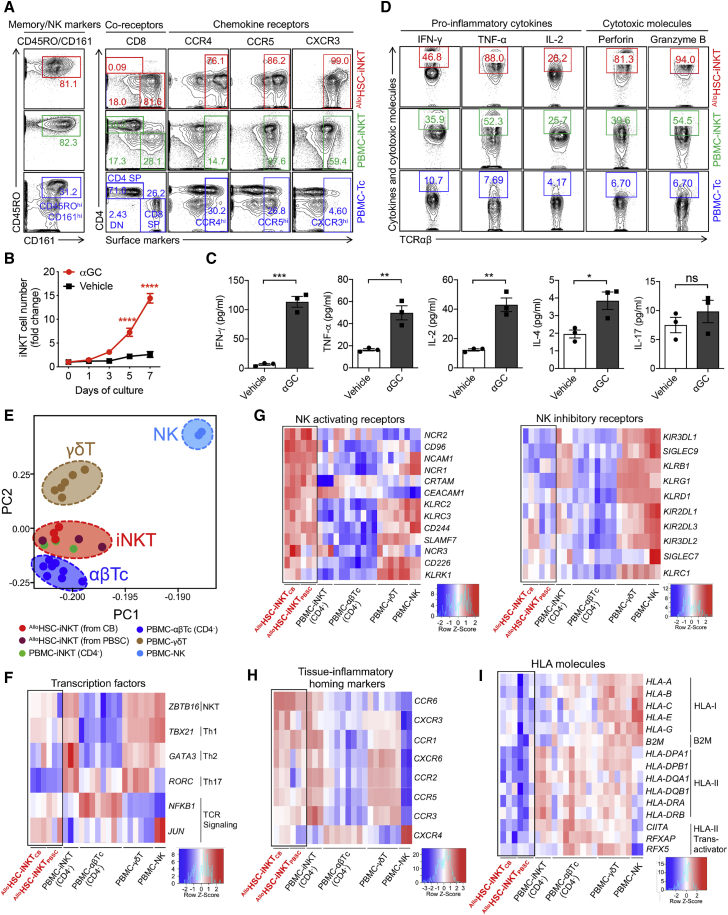
Next, we analyzed the phenotype and functionality of AlloHSC-iNKT cells in comparison with endogenous human PBMC-iNKT and PBMC-Tc cells. AlloHSC-iNKT cells displayed a phenotype closely resembling PBMC-iNKT cells but distinct from PBMC-Tc cells; they expressed high levels of memory T cell markers (i.e., CD45RO), NK cell markers (i.e., CD161), and peripheral tissue and inflammatory site homing markers (i.e., CCR4, CCR5 and CXCR3) (Figure 2A). When stimulated with αGC, AlloHSC-iNKT cells proliferated vigorously (Figure 2B) and secreted high levels of Th0/Th1 cytokines (i.e., interferon-γ [IFN-γ], tumor necrosis factor α [TNF-α], and interleukin-2 [IL-2]) but limited amounts of Th2 cytokines (i.e., IL-4) and Th17 cytokines (i.e., IL-17) (Figure 2C), indicating a Th0/Th1-prone functionality of AlloHSC-iNKT cells that agrees with their CD8 SP/DN phenotype (Figures 1E and 2A).24,31, 32, 33 Intracellular staining showed that at the single-cell level, AlloHSC-iNKT cells produced exceedingly high levels of effector cytokines (i.e., IFN-γ, TNF-α, and IL-2) and cytotoxic molecules (i.e., perforin and granzyme B) (Figure 2D). The ability to generate excess amounts of antitumor effector molecules is a promising signature of iNKT cells for cancer immunotherapy.26,36
Figure 2.
Characterization and gene profiling of AlloHSC-iNKT cells
(A) FACS detection of surface markers on AlloHSC-iNKT cells. PBMC-iNKT and PBMC-Tc cells were included as controls.
(B and C) Antigen responses of AlloHSC-iNKT cells. AlloHSC-iNKT cells were cultured for 7 days, in the presence or absence of αGC (denoted as αGC or Vehicle, respectively). (B) Cell growth curve (n = 3). (C) ELISA analyses of cytokine (IFN-γ, TNF-α, IL-2, IL-4 and IL-17) production at day 7 post αGC stimulation (n = 3).
(D) FACS detection of intracellular cytokines and cytotoxic molecules in AlloHSC-iNKT cells. PBMC-iNKT and PBMC-Tc cells were included as controls.
(E–I) Deep RNA-seq analysis of AlloHSC-iNKT cells generated from CB- or PBSC-derived CD34+ HSCs (n = 3 for each). Healthy donor PBMC-derived conventional CD4− αβ T (PBMC-αβTc; n = 8), CD4− iNKT (PBMC-iNKT; n = 3), γδ T (PBMC- γδT; n = 6), and NK (PBMC-NK; n = 2) cells were included as controls. N indicates different donors. (E) Principal-component analysis (PCA) plot showing the ordination of all six cell types. (F–I) Heatmaps showing the expression of selected genes encoding transcription factors (F), NK activating and inhibitory receptors (G), tissue inflammatory homing markers (H), and HLA molecules (I) for all six cell types.
Representative of 1 (E–I) and 3 (A–D) experiments. Data are presented as the mean ± SEM ns, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, by Student’s t test.
Transcriptome profiling of AlloHSC-iNKT cells
To fully characterize AlloHSC-iNKT cells, we performed deep RNA sequencing (RNA-seq) analysis of these cells; AlloHSC-iNKT cells generated from both CB and PBSC CD34+ HSCs were studied. Healthy donor PBMC-derived endogenous iNKT (PBMC-iNKT), conventional αβ T (PBMC-αβTc), γδ T (PBMC-γδT), and NK (PBMC-NK) cells were included as controls. All cell types were prepared from multiple donors. Because AlloHSC-iNKT cells were dominantly CD4− (CD8 SP/DN), the CD4− subpopulations of PBMC-iNKT (CD8 SP/DN) and PBMC-αβTc (CD8+) cells were analyzed in this experiment.
Principal-component analysis (PCA) of the global gene expression profiles showed that AlloHSC-iNKT cells (both CB and PBSC-derived) were located the closest to PBMC-iNKT cells, next to PBMC-αβTc and PBMC-γδT cells, and the furthest from PBMC-NK cells, validating the iNKT cell nature of AlloHSC-iNKT cells (Figure 2E).37
“Master” transcription factor gene profiling analysis revealed a distinctive signature of AlloHSC-iNKT cells; they expressed high levels of ZBTB16 that encodes PLZF, a signature transcription factor of innate T (e.g., iNKT and γδT) cells and NK cells38; they expressed high levels of TBX21 that encodes T-bet, an essential transcription factor regulating Th1 polarization of T cells39; they expressed low levels of GATA3 and RORC that respectively encode GATA3 and RORγ, critical transcription factors regulating Th2 and Th17 polarization of T cells40; and they expressed high levels of NFKB1 and JUN that respectively encode NF-κB1 and c-Jun, important transcription factors for TCR signaling (Figure 2F).41,42 These transcription factors have been indicated to play important roles in regulating iNKT cell development and functionality.43, 44, 45 Of note, the TBX21highGATA3lowRORClow expression profile of AlloHSC-iNKT cells consists with their Th0/Th1-prone cytokine production profile (Figure 2C).
Further analysis of the various genes related to antitumor effector functions (e.g., genes encoding activation/homing markers, cytokines, and cytotoxic molecules) revealed AlloHSC-iNKT cells to be highly potent effector cells, in agreement with their in vitro phenotype and functionality characterization (Figures 2A–2D). In addition, several interesting gene signatures stood out, which are highlighted below.
Typical iNKT cells exert NK function besides T cell function, via surface expression of NK receptors.16,18,46 Interestingly, compared to PBMC-iNKT and even PBMC-NK cells, AlloHSC-iNKT cells expressed exceedingly high levels of NK activating receptor genes (e.g., NCAM1, NCR1, NCR2, KLR2, and KLR3) but low levels of NK inhibitory receptor genes (e.g., KIR3DL1, KIR3DL2, KIR2DL1, and KIR2DL2), suggesting that AlloHSC-iNKT cells might exhibit a superior antitumor NK function (Figure 2G).
Tissue inflammatory homing markers expressed on effector immune cells enable them to access inflammatory tissues including tumor sites.32,47 AlloHSC-iNKT cells expressed exceedingly high levels of multiple tissue inflammatory homing marker genes (e.g., CCR1, CCR2, CCR3, CCR5, CCR6, and CXCR3), comparable to those of endogenous innate T cells (i.e., PBMC-iNKT and PBMC-γδT cells) but significantly higher than those of endogenous conventional αβ T and NK cells (i.e., PBMC-αβTc and PBMC-NK cells), suggesting a strong capacity of AlloHSC-iNKT cells to home to and penetrate tumor sites (Figure 2H).
HLA incompatibility may trigger host T-cell-mediated allorejection of adoptively transferred allogeneic cellular products, thereby limiting their therapeutic efficacy.48,49 Interestingly, compared to all of the endogenous T cells (i.e., PBMC-iNKT, PBMC-αβTc, and PBMC-γδT cells) and NK cells (i.e., PBMC-NK cells) tested, AlloHSC-iNKT cells expressed much lower levels of HLA-expression-related genes (e.g., genes encoding HLA-I molecules, B2M, HLA-II molecules, and HLA-II transactivators), suggesting that AlloHSC-iNKT cells might naturally resist allorejection and thereby have certain advantages over many PBMC-derived allogeneic cell products for off-the-shelf cell therapy (Figure 2I).
Tumor targeting of AlloHSC-iNKT cells through intrinsic NK function
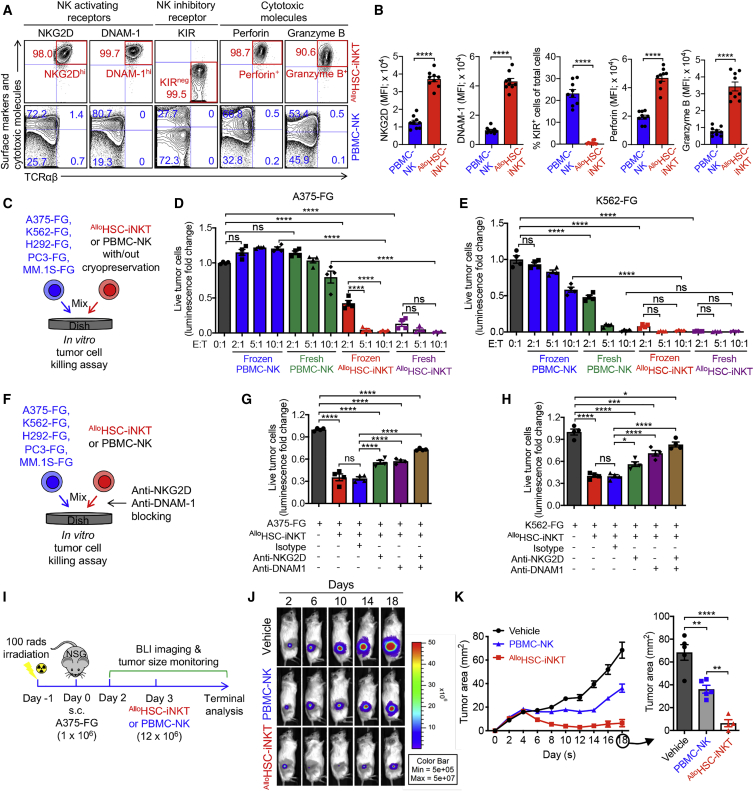
Following the NK lead of our RNA-seq study (Figure 2G), we investigated the NK phenotype and antitumor function of AlloHSC-iNKT cells in comparison with those of endogenous PBMC-NK cells. Flow cytometry analysis of cell surface markers showed that AlloHSC-iNKT cells expressed significantly higher levels of NK activating receptors (i.e., NKG2D and DNAM-1) while nearly undetectable NK inhibitory receptors (i.e., killer cell immunoglobulin-like receptors, KIRs) (Figures 3A and 3B). NK activating receptors recognize stress molecules (e.g., MIC-A/B and UL16-binding protein 1-4, ULBP1-4, recognized by NKG2D and CD112 and CD155 recognized by DNAM-1) upregulated on many tumor cells and trigger tumor targeting,50, 51, 52 while NK inhibitory receptors recognize matched “self” major histocompatibility complex (MHC) molecules and suppress tumor killing.53,54 Flow cytometry analysis of intracellular effector molecules showed that compared to PBMC-NK cells, AlloHSC-iNKT cells produced exceedingly higher levels of cytotoxic molecules (i.e., perforin and granzyme B) (Figures 3A and 3B). Collectively, these results confirmed a promising antitumor potential of AlloHSC-iNKT cells through their intrinsic NK function.
Figure 3.
Tumor targeting of AlloHSC-iNKT cells through intrinsic NK function
(A and B) FACS analyses of surface NK receptor expression and intracellular cytotoxic molecule production by AlloHSC-iNKT cells. PBMC-NK cells were included as a control. (A) Representative FACS plots. (B) Quantification of (A) (n = 9).
(C–E) In vitro direct killing of human tumor cells by AlloHSC-iNKT cells. PBMC-NK cells were included as a control. Both fresh and frozen-thawed cells were studied. Five human tumor cell lines were studied: A375 (melanoma), K562 (myelogenous leukemia), H292 (lung cancer), PC3 (prostate cancer), and MM.1S (multiple myeloma). All tumor cell lines were engineered to express firefly luciferase and green fluorescence protein (FG) dual reporters. (C) Experimental design. (D and E) Tumor killing data of A375-FG human melanoma cells (D) and K562-FG human myelogenous leukemia cells (E) at 24 h (n = 4).
(F–H) Tumor killing mechanisms of AlloHSC-iNKT cells. NKG2D- and DNAM-1-mediated pathways were studied. (F) Experimental design. (G) Tumor killing data of A375-FG human melanoma cells at 24 h (tumor/iNKT ratio 1:2; n = 4). (H) Tumor killing data of K562-FG human myelogenous leukemia cells at 24 h (tumor/iNKT ratio 1:1; n = 4).
(I–K) Studying the in vivo antitumor efficacy of AlloHSC-iNKT cells in an A375-FG human melanoma xenograft NSG mouse model. (I) Experimental design. BLI, live animal bioluminescence imaging. (J) BLI images showing tumor loads in experimental mice over time. (K) Tumor size measurements over time (n = 4–5).
Representative of three experiments. Data are presented as the mean ± SEM. ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001 by Student’s t test (B) or one-way ANOVA (D, E, G, H, and K). See also Figure S1.
An attractive feature of NK-cell-based cancer immunotherapy is its capacity to target a broad range of tumor cells independent of HLA and tumor antigen restrictions.50 However, NK-cell-based therapy also confronts several significant challenges, such as the limited in vivo efficacy of NK cells, as well as their intolerance to cryopreservation, that pose a significant technical hurdle to their clinical and commercial applications.55,56 Based on the “super-active” NK phenotype of AlloHSC-iNKT cells, we wondered whether these cells might exhibit improved antitumor NK function. Moreover, unlike NK cells, iNKT cells can resist cryopreservation57; we therefore wondered whether AlloHSC-iNKT cells might also improve on this aspect.
Using an in vitro tumor cell killing assay, we evaluated the tumor killing efficacy of AlloHSC-iNKT cells in comparison with PBMC-NK cells (Figure 3C). Five human tumor cell lines were used as targets, including a leukemia cell line (K562), a melanoma cell line (A375), a lung cancer cell line (H292), a prostate cancer cell line (PC3), and a multiple myeloma cell line (MM.1S). All five tumor cell lines were engineered to overexpress the firefly luciferase (Fluc) and enhanced green fluorescence protein (EGFP) dual reporters to enable the convenient monitoring of these tumor cells using either luciferase assay or flow cytometry (Figure S1A). Compared to PBMC-NK cells, AlloHSC-iNKT cells exhibited a significantly enhanced tumor killing efficacy across all five tumor cell lines (Figures 3D, 3E, and S1B–S1D). Interestingly, AlloHSC-iNKT cells sustained strong tumor killing efficacy after cryopreservation, whereas PBMC-NK cells were sensitive to freeze-thaw cycles and showed greatly reduced viability and antitumor capacity following cryopreservation (Figures 3D, 3E, and S1B–S1D). Blocking of NK activating receptors (i.e., NKG2D and DNAM-1) reduced tumor killing efficacy of AlloHSC-iNKT cells (Figures 3F–3H and S1E–S1G), confirming their NK-activating-receptor-mediated tumor-targeting function.
Next we evaluated the in vivo antitumor efficacy of AlloHSC-iNKT cells using a human melanoma xenograft NSG (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) mouse model. A375-FG tumor cells were subcutaneously inoculated into NSG mice to form solid tumors, followed by a paratumoral injection of AlloHSC-iNKT or PBMC-NK cells (Figure 3I). AlloHSC-iNKT cells effectively suppressed tumor growth at an efficacy higher than that of PBMC-NK cells, as evidenced by time-course live animal bioluminescence imaging (BLI) monitoring (Figures 3J and S1H), tumor size measurement (Figure 3K), and terminal tumor weight assessment (Figure S1I).
Taken together, these studies support a cancer therapy potential of AlloHSC-iNKT cells through their intrinsic NK function, allowing these cells to target a broad range of tumors independent of HLA and tumor antigen restrictions. Attractively, AlloHSC-iNKT cells may exhibit improved antitumor efficacy and cryopreservation resistance compared to NK-cell-based allogeneic cell therapy products.
Tumor targeting of AlloHSC-iNKT cells through engineered CARs
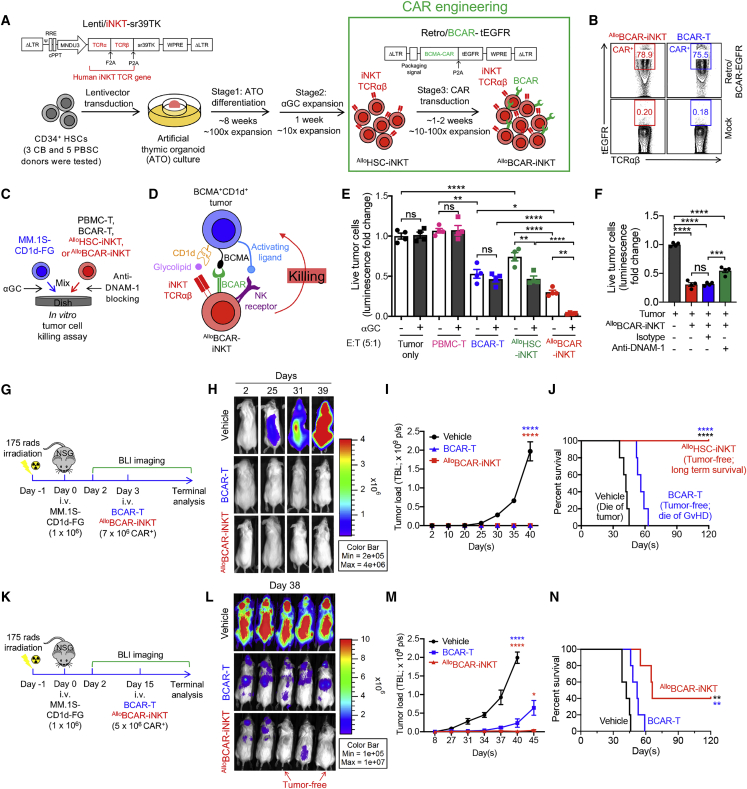
CAR-engineered cell therapy has great promise for treating cancer4, 5, 6,58; we therefore explored the potential of AlloHSC-iNKT cells as the allogeneic cell carriers for CAR-directed off-the-shelf cell therapy. A second-generation B cell maturation antigen (BCMA)-targeting CAR (BCAR) was used for this study (Figure S2A); this BCAR contains 4-1BB and CD3ζ signaling domains and has shown clinical efficacy in treating human multiple myeloma (MM).59
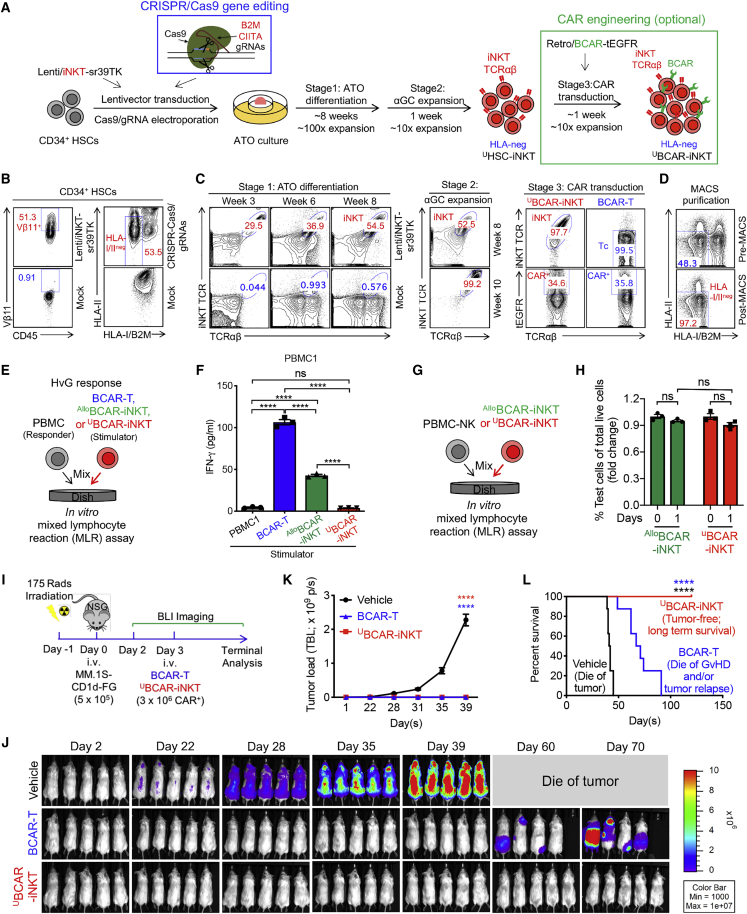
AlloHSC-iNKT cells were generated as previously described (Figure 1A); mature AlloHSC-iNKT cells were further transduced with a Retro/BCAR-tEGFR retroviral vector to produce the BCAR-engineered AlloHSC-iNKT cells (denoted as AlloBCAR-iNKT cells) (Figure 4A). The entire culture time (∼10–11 weeks) and cell yield (∼1011 per CB donor or ∼1012 per PBSC donor) were similar to those of generating non-CAR-engineered AlloHSC-iNKT cells (Figures 1A and 1G). The resulting AlloBCAR-iNKT cells were pure iNKT cells with a high BCAR expression rate (>98% iNKT TCR+ and up to 80% BCAR+; Figure 4B) and displayed a typical human iNKT cell phenotype and functionality similar to those of AlloHSC-iNKT cells (Figures 2A, 2D, S2C, and S2D). Therefore, AlloHSC-iNKT cells can be effectively engineered to express CARs without compromising cell yield and quality.
Figure 4.
Tumor targeting of AlloHSC-iNKT cells through engineered chimeric antigen receptors (CARs)
(A) Experimental design to generate BCMA CAR-engineered AlloHSC-iNKT (AlloBCAR-iNKT) cells in vitro. BCMA, B cell maturation antigen; BCAR, BCMA CAR; Retro/BCAR-tEGFR, retroviral vector encoding a BCMA CAR gene as well as a truncated epidermal growth factor receptor (tEGFR) reporter gene. tEGFR was used as a staining marker indicating BCAR expression.
(B) FACS analysis of BCAR expression (identified as tEGFR+) on AlloBCAR-iNKT at 72 h after retrovector transduction. Healthy donor PBMC-T cells transduced with the same Retro/BCAR-tEGFR vector (denoted as BCAR-T cells) were included as a staining control.
(C–F) In vitro killing of human multiple myeloma cells by AlloBCAR-iNKT cells. MM.1S-CD1d-FG, human MM.1S cell line engineered to overexpress human CD1d as well as FG dual reporters. PBMC-T, BCAR-T, and AlloHSC-iNKT cells were included as effector cell controls. (C) Experimental design. (D) Diagram showing the tumor-targeting triple mechanisms of AlloBCAR-iNKT cells, mediated by NK activating receptors, iNKT TCR, and BCAR. (E) Tumor cell killing by the indicated effector cells with/out the addition of αGC (n = 4). (F) Tumor cell killing by AlloBCAR-iNKT cells with/out the blockade of DNAM-1 (n = 4). Tumor cell killing was analyzed at 8-h after co-culture (effector/tumor ratio 5:1).
(G–N) Studying the in vivo antitumor efficacy of AlloBCAR-iNKT cells in an MM.1S-CD1d-FG human multiple myeloma xenograft NSG mouse model. Tumor-bearing mice injected with BCAR-T cells or no cells (vehicle) were included as controls. (G–J) Low-tumor-load condition. (G) Experimental design. (H) BLI images showing tumor loads in experimental mice over time. (I) Quantification of (H) (n = 5). (J) Kaplan-Meier survival curves of experimental mice over a period of 4 months after tumor challenge (n = 5). (K–N) High-tumor-load condition. (K) Experimental design. (L) BLI images showing tumor loads in experimental mice at day 38. (M) Quantification of tumor load in experimental mice over time (n = 5). (N) Kaplan-Meier survival curves of experimental mice over a period of 4 months after tumor challenge (n = 5).
Representative of two experiments (K–N) and three experiments (A–J). Data are presented as the mean ± SEM. ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001 by one-way ANOVA (E, F, I, and M), or log rank (Mantel-Cox) test adjusted for multiple comparisons (J and N). See also Figure S2.
To assess the antitumor capacity of AlloBCAR-iNKT cells, we used an established in vitro MM.1S-CD1d-FG tumor cell killing assay28; non-CAR-engineered AlloHSC-iNKT cells, as well as healthy donor PBMC-derived conventional T cells with or without engineering with the same BCAR (denoted as PBMC-T or BCAR-T cells), were included as controls (Figure 4C). The MM.1S-CD1d-FG cell line was generated by engineering the parental BCMA-positive MM.1S human MM cell line to overexpress human CD1d and the Fluc-EGFP dual reporters,28 mimicking a large portion of primary patient MM samples that are BCMA+CD1d+ (Figure S2B).60 This in vitro tumor cell killing assay allowed us to evaluate the tumor killing capacity of AlloBCAR-iNKT cells, as well as investigate their possible NK/TCR/CAR triple mechanisms for targeting MM (Figure 4D).
AlloHSC-iNKT cells without BCAR engineering were able to kill MM.1S-CD1d-FG tumor cells, while PBMC-T cells could not, indicating an intrinsic antitumor NK function of AlloHSC-iNKT cells (Figures 4E, S1D, and S1G); this intrinsic antitumor NK function was inherited by AlloBCAR-iNKT cells, as confirmed by NK activating receptor (i.e., DNAM-1) blocking assay (Figure 4F). Meanwhile, the tumor cell killing efficacy of AlloHSC-iNKT cells was enhanced by the addition of αGC that did not happen to PBMC-T cells, indicating an iNKT TCR-mediated antitumor function of AlloHSC-iNKT cells; this TCR-mediated antitumor function was also inherited by AlloBCAR-iNKT cells (Figure 4E). Importantly, compared to AlloHSC-iNKT cells, AlloBCAR-iNKT cells showed stronger tumor cell killing, indicating a CAR-mediated antitumor function of AlloBCAR-iNKT cells (Figure 4E). Therefore, AlloBCAR-iNKT cells are able to target MM tumor cells using the NK/TCR/CAR triple mechanisms, which may grant AlloBCAR-iNKT cells a higher tumor-targeting efficacy and an enhanced capacity to counteract tumor antigen escape compared to conventional BCAR-T cells (Figures 4D–4F).25,61,62
The in vivo antitumor efficacy of AlloBCAR-iNKT cells was studied using an established MM.1S-CD1d-FG xenograft NSG mouse model28; conventional BCAR-T cells were included as a control. In low-tumor-load conditions, AlloBCAR-iNKT cells eliminated MM tumor cells as effectively as BCAR-T cells (Figures 4H and 4I); however, experimental mice treated with BCAR-T cells eventually died of graft-versus-host disease (GvHD) despite being tumor-free, while experimental mice treated with AlloBCAR-iNKT cells lived long-term with tumor-free and GvHD-free (Figures 4I and 4J). Impressively, in high-tumor-load conditions, AlloBCAR-iNKT cells still managed to suppress tumor growth effectively and achieved tumor clearance in a fraction of experimental mice (two out of five mice); conventional BCAR-T cells suppressed tumor growth less well and could not achieve tumor clearance (Figures 4K–4N and S2E).
Taken together, these studies support an attractive potential of AlloHSC-iNKT cells as off-the-shelf cell carriers for CAR-directed cancer immunotherapy. The high antitumor efficacy and multiple tumor-targeting mechanisms of CAR-engineered AlloHSC-iNKT cells may provide new opportunities to target hard-to-treat tumors and counteract tumor antigen escape.
Safety study of AlloHSC-iNKT cells
Graft-versus-host (GvH) response is the primary safety concern of an off-the-shelf allogeneic cell therapy.4 Since iNKT cells do not react to mismatched HLA molecules and protein alloantigens, these cells are not expected to mount GvH responses.17,19 To verify this safety feature of AlloHSC-iNKT cells, we performed both in vitro and in vivo studies. In an in vitro mixed lymphocyte reaction (MLR) assay, in sharp contrast to conventional PBMC-Tc cells, AlloHSC-iNKT cells did not react to all the mismatched healthy donor PBMCs tested, as evidence by their lack of IFN-γ production (Figures 5A and 5B). In an in vivo NSG mouse xenograft model, unlike PBMC-Tc cells that induced GvHD and killed experimental mice ∼2 months after PBMC-Tc cell transfer, AlloHSC-iNKT cells did not cause GvHD and sustained long-term survival of experimental mice (Figures 5C and 5D). The lack of GvHD in AlloHSC-iNKT cell engrafted experimental mice was confirmed by histology analysis showing healthy tissue structures without lymphocyte infiltrations; on the contrary, analyses of PBMC-Tc cell engrafted experimental mice showed severe tissue damages associated with heavy lymphocyte infiltrations (Figures 5E and 5F). To study the influence of CAR engineering, we analyzed the GvH response of AlloBCAR-iNKT cells; BCAR-T cells were included as a control. Distinct from BCAR-T cells, AlloBCAR-iNKT cells showed no response in the in vitro MLR assay (Figures S3A and S3B) and induced no GvHD in the human MM xenograft NSG mouse model (Figures S3C and S3D), indicating that CAR engineering and tumor encountering do not change the GvH-free safety feature of AlloHSC-iNKT cells.
Figure 5.
Safety study of AlloHSC-iNKT cells
(A and B) Studying the graft-versus-host (GvH) response of AlloHSC-iNKT cells using an in vitro mixed lymphocyte reaction (MLR) assay. PBMC-Tc cells were included as a responder cell control. (A) Experimental design. PBMCs from four different healthy donors were used as stimulator cells. (B) ELISA analyses of IFN-γ production at day 4 (n = 4). N, no stimulator cells.
(C–F) Studying the GvH response of AlloHSC-iNKT cells using an NSG mouse xenograft model. Donor-matched PBMC-Tc cells were included as a control. (C) Experimental design. (D) Kaplan-Meier survival curves of experimental mice over time (n = 5). (E) H&E-stained tissue sections. Blank indicates tissue sections collected from control NSG mice receiving no adoptive cell transfer. Arrows point to mononuclear cell infiltrates. Scale bar, 200 μm. (F) Quantification of (E) (n = 4).
(G–I) In vivo controlled depletion of AlloHSC-iNKT cells via GCV treatment. GCV, ganciclovir. (G) Experimental design. (H) FACS detection of AlloHSC-iNKT cells in the liver, spleen, and lung of NSG mice at day 5. (I) Quantification of (G) (n = 4).
Representative of two experiments. Data are presented as the mean ± SEM. ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001 by one-way ANOVA (B), Student’s t test (F and I), or log rank (Mantel-Cox) test adjusted for multiple comparisons (D). See also Figure S3.
Besides GvHD risk, allogeneic cell therapy may confer other safety risks, including those common to cell-based cancer immunotherapy, such as cytokine release syndrome (CRS) and neurotoxicity.63 Although we did not observe that AlloHSC-iNKT cells induced tissue toxicity in our NSG xenograft mouse models (Figures 5E and 5F), these safety studies may be limited by the utilized preclinical animal models.63 Additional safety controls may be necessary, especially for initial clinical development. We therefore have engineered a “safety switch” in AlloHSC-iNKT cell products by incorporating a suicide gene (i.e., sr39TK) in the human iNKT TCR gene delivery vector, resulting in AlloHSC-iNKT cells that are 100% labeled with the suicide gene (Figure 1A). In cell culture, addition of guanosine analog (ganciclovir [GCV]) effectively killed AlloHSC-iNKT cells (Figure S3E); in an NSG mouse xenograft model, administration of GCV effectively depleted AlloHSC-iNKT cells from all tissues examined (e.g., liver, spleen, and lung; Figures 5G–5I). Of note, GCV has been used clinically as a prodrug to induce sr39TK-mediated suicide effect in cellular products.64 Other alternative suicide switch systems (e.g., inducible Cas9 and truncated EGFR) can certainly be utilized.4,65, 66, 67
Taken together, our results show that AlloHSC-iNKT cells are free of GvHD risk and can be equipped with an additional safety switch, making them suitable for off-the-shelf allogeneic cell therapy.
Immunogenicity study of AlloHSC-iNKT cells
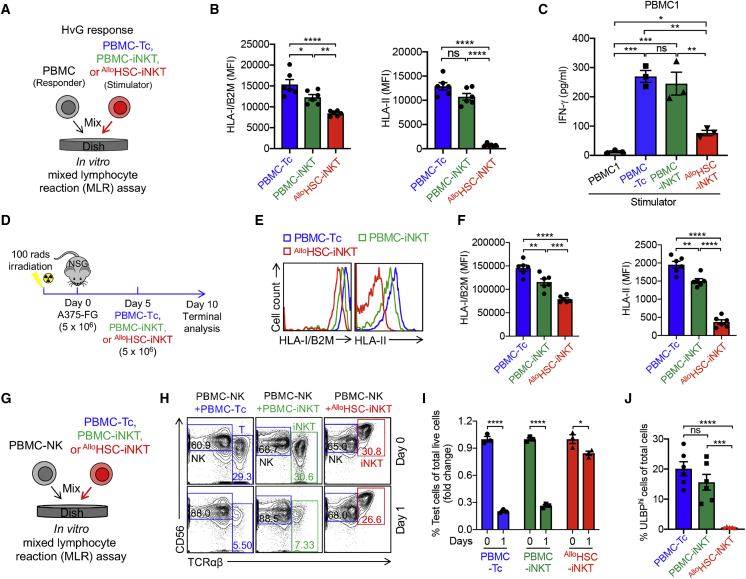
For allogeneic cell therapies, immunogenicity can be a concern, because allorejection by host T and NK cells can greatly limit the efficacy of therapeutic allogeneic cells.68 Host conventional CD8 and CD4 αβ T cells reject allogeneic cells by recognizing mismatched HLA-I and HLA-II molecules, respectively.69,70 In a classical in vitro MLR assay studying T-cell-mediated host-versus-graft (HvG) response via IFN-γ secretion reading, compared to endogenous conventional T and iNKT (i.e., PBMC-Tc and PBMC-iNKT) cells, AlloHSC-iNKT cells triggered a significantly reduced HvG response (Figures 6A, 6C, and S4A). Our previous RNA-seq study made the interesting observation that compared to endogenous immune cells (i.e., conventional αβT, iNKT, γδT, and NK cells), AlloHSC-iNKT cells globally downregulated the expression of many genes controlling the cell surface display of HLA-I and HLA-II molecules (Figure 2I). Flow cytometry analysis confirmed that compared to PBMC-Tc and PBMC-iNKT cells, AlloHSC-iNKT cells expressed significantly reduced levels of HLA-I molecules and nearly undetectable levels of HLA-II molecules (Figures 6B and S4B), which may account for their resistance to T-cell-mediated HvG response (Figures 6C and S4A). Because IFNs can upregulate HLA-I expression,71 we studied HLA-I expression on AlloHSC-iNKT cells under IFN-γ stimulation (Figure S5A). AlloHSC-iNKT cells slightly upregulated surface HLA-I expression after IFN-γ stimulation; however, their overall surface HLA-I level still remained significantly lower than those of PBMC-Tc and PBMC-iNKT cells (Figures S5B and S5C). Because an in vivo inflammatory tumor microenvironment may upregulate the expression of HLA molecules on tumor-infiltrating immune cells (e.g., via IFN-γ),72 we also assessed HLA expression on AlloHSC-iNKT cells in an A375-FG human melanoma xenograft NSG mouse model adapted from a previous study (Figures 3I). A375-FG melanoma cells were subcutaneously inoculated in NSG mice to form solid tumors, followed by injection of AlloHSC-iNKT cells; PBMC-Tc and PBMC-iNKT cells were included as controls (Figure 6D). Flow cytometry analysis of tumor-infiltrating AlloHSC-iNKT cells showed that these cells maintained low expression of HLA-I and HLA-II molecules at levels significantly lower than those of tumor-infiltrating PBMC-Tc and PBMC-iNKT cells (Figures 6E and 6F).
Figure 6.
Immunogenicity study of AlloHSC-iNKT cells
(A–C) Studying allogenic T cell response against AlloHSC-iNKT cells using an in vitro MLR assay. Irradiated AlloHSC-iNKT cells (as stimulators) were co-cultured with donor-mismatched PBMC cells (as responders). Irradiated PBMC-iNKT and PBMC-Tc cells were included as stimulator cell controls. (A) Experimental design. PBMCs from three different healthy donors were used as responders. (B) FACS analyses of HLA-I and HLA-II expression on the indicated stimulator cells (n = 6). (C) ELISA analyses of IFN-γ production at day 4 (n = 3).
(D–F) Studying HLA-I/II expression on AlloHSC-iNKT cells in vivo in an A375-FG human melanoma xenograft NSG mouse model. PBMC-iNKT and PBMC-Tc cells were included as effector cell controls. (D) Experimental design. (E) FACS analyses of HLA-I/II expression on the indicated effector cells isolated from A375-FG solid tumors. (F) Quantification of (E) (n = 5).
(G–J) Studying allogenic NK cell response against AlloHSC-iNKT cells using an in vitro MLR assay. AlloHSC-iNKT cells were co-cultured with donor-mismatched PBMC-NK cells. PBMC-iNKT and PBMC-Tc cells were included as controls. (G) Experimental design. (H) FACS analyses of the indicated cells at days 0 and 1. (I) Quantification of (H) (n = 3). (J) FACS analyses of ULBP expression on the indicated cells (n = 5–6).
Representative of two (D–F) and three (A–C and G–J) experiments. Data are presented as mean ± SEM. ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001 by Student’s t test (I) or one-way ANOVA (B, C, F, and G). See also Figures S4–S6.
Next, we studied NK-cell-mediated allorejection. Host NK cells reject allogeneic cells through a double-trigger mechanism: (1) “missing self” (i.e., missing of matching HLA-I molecules on allogeneic cells) triggers the release of KIR inhibition, and (2) a “stress signal” (i.e., upregulated stress molecules on allogeneic cells) triggers the activation of NK activating receptors such as NKG2D.52,73,74 Because AlloHSC-iNKT cells expressed low levels of HLA-I molecules, we wondered whether this might make AlloHSC-iNKT cells susceptible to host NK rejection. Surprisingly, in an in vitro MLR assay studying NK-cell-mediated allorejection, compared to endogenous conventional T and iNKT (i.e., PBMC-Tc and PBMC-iNKT) cells, AlloHSC-iNKT cells survived rejection by mismatched healthy donor PBMC-derived NK cells significantly better (Figures 6G–6I and S6); correspondingly, NK cells co-cultured with AlloHSC-iNKT cells exhibited less upregulation of NK activation markers (i.e., CD107a) (Figures S4D and S4E). Flow cytometry analysis revealed that compared to PBMC-Tc and PBMC-iNKT cells, AlloHSC-iNKT cells expressed much reduced and nearly undetectable levels of NKG2D ligands (i.e., ULBP; Figures 6J and S4C),75 which may be one of the possible mechanisms accounting for their resistance to NK-cell-mediated allorejection.
Collectively, these studies revealed a stable HLA-I/IIlow phenotype of AlloHSC-iNKT cells that may grant them advantage to resist host T-cell-mediated rejection compared to other healthy donor PBMC-derived allogeneic cell products; meanwhile, AlloHSC-iNKT cells also expressed low levels of stress molecules such as NKG2D ligands, making them also resistant to host NK-cell-mediated allorejection. These “low immunogenicity” features of AlloHSC-iNKT cells support their application for off-the-shelf cell therapy.
Development of HLA-ablated universal HSC-iNKT (UHSC-iNKT) cells and derivatives
Although AlloHSC-iNKT cells display a stable HLA-I/IIlow phenotype (Figures 2I, 6B, 6E, 6F, and S4B), their residual HLA-I/II molecules may still make them susceptible to certain levels of host T-cell-mediated allorejection. We therefore explored further engineering of the AlloHSC-iNKT cell products to achieve total ablation of their surface HLA-I/II molecules. Interestingly, this task can be accomplished by ablation of only two genes: (1) a B2M gene encoding the beta 2-microglobulin (B2M) that is required for the surface display of all types of HLA-I molecules,69 and (2) a CIITA gene encoding the class II transactivator (CIITA) that is required for the transcription of all types of HLA-II molecules.70 Ablation of B2M and CIITA genes can be achieved by using the powerful gene-editing tools like the CRISPR-Cas9/guide RNA (gRNA) system.76 We postulated that by combining iNKT TCR gene engineering and B2M/CIITA gene editing, we would produce HLA-ablated “universal” HSC-iNKT (UHSC-iNKT) cells totally resistant to host T-cell-mediated allorejection. We intended to perform both gene engineering and gene editing on the small numbers of starting HSCs upfront of the HSC-iNKT cell culture; this manufacturing design would save on the use of gene-engineering/editing materials (i.e., lentivector and CRISPR-Cas9/gRNA) that can be cost-limiting, and also enable the maximal gene engineering/editing efficiency that can be carried on into the final UHSC-iNKT cell products. Similar to the generation of AlloCAR-iNKT cell products (Figure 4A), a CAR engineering step can be incorporated after UHSC-iNKT cell differentiation, resulting in HLA-ablated universal CAR-engineered HSC-iNKT (UCAR-iNKT) cell products.
CB or PBSC-derived CD34+ HSCs were transduced with the Lenti/iNKT-sr39TK lentivector; 24 h later, these HSCs were electroporated with a CRISPR-Cas9/B2M-CIITA-gRNAs complex (Figure 7A). The gene-engineering/editing efficiency was high; we routinely achieved over 50% lentivector transduction rate and over 50% HLA-I/II double-ablation rate (Figures 7B and S7B). The engineered HSCs were then put into the HSC-iNKT differentiation ATO culture for 8 weeks followed by 1 week of αGC expansion (Figures 7A); CRISPR-Cas9 gene editing did not interfere with HSC differentiation into iNKT cells and we obtained high yield of differentiated UHSC-iNKT cells similar to that of AlloHSC-iNKT cells (Figures 1C and 7C). CRISPR-Cas9 gene editing also did not interfere with the follow-up CAR engineering; we obtained an efficient BCMA-targeting CAR (BCAR) engineering rate similar to that of engineering PBMC-derived conventional BCAR-T cells (Figure 7C). The high HLA-I/II double-ablation rate of the starting HSCs was inherited by the resulting UBCAR-iNKT cells (i.e., ∼50% of the resulting cells were HLA-I/II double negative); if needed, the HLA-I/II double-negative cells could be further enriched (to over 97%) using magnetic-activated cell sorting (MACS) via B2M and HLA-II magnetic beads labeling (Figures 7D).
Figure 7.
Development of HLA-ablated universal HSC-iNKT (UHSC-iNKT) cells and derivatives
(A) Experimental design to generate UHSC-iNKT and BCMA CAR-engineered UHSC-iNKT (UBCAR-iNKT) cells. CRISPR, clusters of regularly interspaced short palindromic repeats; Cas 9, CRISPR-associated protein 9; gRNA, guide RNA; B2M, beta-2-microglobulin; CIITA, class II major histocompatibility complex transactivator.
(B–D) FACS monitoring of UHSC-iNKT and UBCAR-iNKT cell generation. (B) Intracellular expression of iNKT TCR (identified as Vβ11+) and surface ablation of HLA-I/II (identified as HLA-I/B2M−HLA-II−) in CD34+ HSCs cells at day 5 (72 h after lentivector transduction and 48 h after CRISPR-Cas9 gene editing). (C) Generation of iNKT cells (identified as iNKT TCR+TCRαβ+ cells) during stage 1 ATO differentiation culture, stage 2 αGC expansion, and stage 3 CAR transduction. Healthy donor PBMC-T cells transduced with the same Retro/BCAR-tEGFR vector was included as a staining control (denoted as BCAR-T cells). (D) Purification of HLA-I/II-negative UHSC-iNKT cells using MACS.
(E and F) Studying allogenic T cell response against UBCAR-iNKT cells using an in vitro MLR assay. Irradiated UBCAR-iNKT cells (as stimulators) were co-cultured with donor-mismatched PBMCs (as responders). Irradiated AlloBCAR-iNKT and conventional BCAR-T cells were included as stimulator cell controls. (E) Experimental design. PBMCs from three different healthy donors were used as responders. (F) ELISA analyses of IFN-γ production at day 4 (n = 3).
(G and H) Studying allogenic NK cell response against UHSC-iNKT cells using an in vitro MLR assay. UHSC-iNKT cells were co-cultured with donor-mismatched PBMC-NK cells. AlloHSC-iNKT cells were included as a control. (G) Experimental design. (H) FACS quantification of the indicated cells (n = 3).
(I–L) Studying the in vivo antitumor efficacy of UBCAR-iNKT cells in an MM.1S-CD1d-FG human multiple myeloma xenograft NSG mouse model. (I) Experimental design. (J) BLI images showing tumor loads in experimental mice over time. (K) Quantification of (J) (n = 5). (L) Kaplan-Meier survival curves of experimental mice over a period of 4 months after tumor challenge (n = 8). Mice were combined from two independent experiments.
Representative of two (I–L) and three (B–H) experiments. Data are presented as mean ± SEM. ns, not significant; ∗∗∗∗p < 0.0001 by Student’s t test (H), one-way ANOVA (F and K), or log rank (Mantel-Cox) test adjusted for multiple comparisons (L). See also Figure S7.
Despite HLA-I/II ablation, UBCAR-iNKT cells displayed a typical iNKT phenotype and a highly cytotoxic functionality similar to those of AlloBCAR-iNKT cells (Figure S7A). As expected, UBCAR-iNKT cells induced nearly undetectable T-cell-mediated alloresponse when mixed with mismatched healthy donor PBMCs (Figures 7E, 7F, and S7F); meanwhile, UBCAR-iNKT cells maintained the resistance to NK-cell-mediated allorejection (Figures 7G, 7H, and S7G). Safety features of UBCAR-iNKT cells resembled those of AlloBCAR-iNKT cells: UBCAR-iNKT cells did not mount GvH response (Figures S7C and S7D), and they were sensitive to sr39TK/GCV-induced suicide control (Figure S7E).
To study whether HLA ablation may impact the antitumor efficacy of UBCAR-iNKT cells, we performed in vitro and in vivo antitumor assays. In an established in vitro MM tumor cell killing assay (Figure 4C), UBCAR-iNKT cells effectively killed tumor cells at an efficacy comparable to that of AlloBCAR-iNKT cells (Figures 4E, S7H, and S7I). Similar to AlloBCAR-iNKT cells, UBCAR-iNKT cells could also utilize an NK/TCR/CAR triple mechanisms targeting tumor cells (Figures 4E, 4F, S7I, and S7J), which may grant them an advantage over conventional BCAR-T cells to gain additional antitumor efficacy (Figure S7I), as well as to counteract the BCMA antigen escape that has been reported in conventional BCAR-T cell therapy clinical trials.25,61,62 In an established in vivo human MM xenograft NSG mouse model (Figure 4G), UBCAR-iNKT-cell-treated animals achieved total tumor clearance and long-term survival, while BCAR-T-cell-treated animals only achieved partial tumor suppression that was followed by tumor relapse and GvHD development, leading to limited survival benefit (Figures 7I–7L).
Collectively, these studies support the generation of HLA-ablated universal HSC-iNKT cell products and derivatives (e.g., CAR-iNKT cell products) that are fully resistant to host T-cell-mediated allorejection and thereby may have improved in vivo persistence and antitumor efficacy.
Discussion
Here, we report the generation and characterization of allogeneic HSC-engineered iNKT cells and derivatives. Using an in vitro HSC-iNKT differentiation cell culture, we generated AlloHSC-iNKT cells that were of high yield and purity and had high antitumor efficacy, high safety profile (GvH-free and suicide control), and low immunogenicity (largely resistant to T- and NK-cell-mediated allorejection). These AlloHSC-iNKT cells could be further engineered to express CAR, thereby enhancing their tumor-targeting capacity; these cells can also be further engineered to ablate their surface HLA molecules, thereby enhancing their resistance to host T-cell-mediated allorejection. Collectively, our studies have generated AlloHSC-iNKT cells and demonstrated them as promising cell carriers for developing off-the-shelf cancer immunotherapy.
Development of allogeneic off-the-shelf cell therapies, many equipped with CARs, is becoming a fast-evolving frontier of cancer immunotherapy. Two major categories of such allogeneic cell products are based on engineering healthy-donor-derived conventional αβ T cells or NK cells.14,68,69,77 Because conventional αβ T cells risk inducing GvHD in allogeneic hosts due to HLA incompatibility, these T cells need to be gene edited to ablate endogenous TCR expression, usually by disrupting the TRAC or/and TRBC gene loci, to make them suitable for allogeneic cell therapy but meanwhile may also potentially increase manufacture complexity.10, 11, 12, 13 On the other hand, NK-based allogeneic cell products are considered of low GvHD risk and therefore do not require additional gene editing, but their in vivo clonal expansion and antitumor performance may be limited compared to that of conventional αβ T cells.14 Two such allogeneic cell products, conventional αβ T-cell-based universal CD19-CAR-engineered T cells (UCART19) and NK-cell-based CB-derived CD19-CAR-engineered NK cells, were recently tested in phase 1 clinical trials treating CD19+ B cell malignancies.10,78 These studies reported the feasibility, certain antileukemic activity, and manageable safety profile of the two cell products, showing an encouraging step forward for the field of allogeneic cell therapy.10,78
Engineering unconventional innate-type T cells (e.g., iNKT, γδ T, and mucosal-associated invariant T cells) that have potent antitumor capacity while being free of GvHD risk represents another promising direction for developing allogeneic cell therapy for cancer, especially for solid tumors.25,79, 80, 81, 82, 83 In particular, iNKT-cell-based cancer immunotherapy has attracted considerable attention. A preclinical study reported the enhanced anti-lymphoma activity of CAR19-engineered iNKT cells compared to conventional CAR19-T cells.25 Another preclinical study reported the potent antitumor efficacy to neuroblastoma and no GvHD risk of CAR.GD2-engineered iNKT cells compared to conventional CAR.GD2-T cells.80 A recent clinical trial testing autologous GD2.CAR-engineered iNKT cells also showed safety and certain efficacy in patients with relapsed or refractory neuroblastoma.79 These studies suggest the therapeutical potential of iNKT-based cell products and support the development of such cell products for allogeneic cell therapy for cancer even solid tumors.
The in vitro HSC-iNKT differentiation cell culture was robust and of high yield (Figure 1G). The resulting AlloHSC-iNKT cell products were of high purity and nearly free of bystander conventional αβ T cells (Figure 1D), which may allow these cell products to be directly used for allogeneic cell therapy without an additional purification step; if necessary, commercially available human iNKT cell isolation reagents (e.g., human anti-iNKT microbeads from Miltenyi) can be used for further purification. Notably, we detected no expression of endogenous TCRs in AlloHSC-iNKT cells (Figure 1F), suggesting an induction of allelic exclusion by transgenic iNKT TCRs as previously reported.27,28 The robustness, high yield, and high purity of AlloHSC-iNKT cell products will facilitate their next-stage translational and clinical development.
The antitumor efficacy of AlloHSC-iNKT cells is promising. These cells display a typical iNKT phenotype and functionality; they co-express memory T cell and NK cell markers, express high levels of inflammatory tissue/tumor homing markers, and produce high levels of cytokines and cytotoxic molecules, outperforming T and NK cells (Figures 2 and 3). Interestingly, compared to endogenous cells (i.e., T, NK, and iNKT cells), AlloHSC-iNKT cells express exceedingly high levels of NK activating receptors and low levels of NK inhibitory receptors (Figure 2G), which is associated with their superior antitumor NK function in vitro and in vivo (Figure 3). In addition, the NK/TCR/CAR tumor-targeting triple mechanisms of AlloHSC-iNKT cells and their derivatives grant these cells a stronger antitumor efficacy (Figures 4D–4F, 7I–7J, and S6G–S6I) and may enable them to counteract tumor antigen escape, which has been observed in T-cell-based cancer therapies.4,25,61,62 Overall, in both the human blood cancer (i.e., MM) and human solid tumor (i.e., melanoma) preclinical mouse xenograft models utilized in this study, AlloHSC-iNKT cells or their CAR derivatives (i.e., AlloBCAR-iNKT and UBCAR-iNKT cells) showed an enhanced antitumor efficacy compared to healthy donor PBMC-derived NK or CAR-engineered conventional αβ T cells, highlighting their cancer therapy potential (Figures 3I–3K, S1H, S1I, 4G–4N, S2E, and 7I–7L). Notably, a synthetic iNKT cell antagonist, αGC, has been demonstrated to specifically stimulate and expand iNKT cells in both preclinical and clinical studies; αGC is clinically available and may be used as a “designer stimulator” to enhance the in vivo performance of AlloHSC-iNKT cells.18,23,84, 85, 86
The safety of AlloHSC-iNKT cells is appealing. In our studies, AlloHSC-iNKT cells showed no GvH responses against multiple random healthy donor PBMCs in an in vitro MLR assay (Figures 5A, 5B, S3A, and S3B) and no GvHD in vivo in multiple human tumor xenograft NSG mouse models (Figures 5C–5F, S3C, and S3D), consistent with their iNKT cell nature and high purity (Figure 1).16 A suicide switch (e.g., sr39TK/GCV) can also be incorporated into AlloHSC-iNKT cells to provide an additional safety control (Figure 5G, 5I, and S3E). The high safety of AlloHSC-iNKT cells strongly support their allogeneic application.
A serendipitous feature of AlloHSC-iNKT cells is their significantly lower immunogenicity compared to PBMC-derived endogenous immune cells (i.e., αβ T, iNKT, γδ T, and NK cells). AlloHSC-iNKT cells express reduced levels of HLA-I molecules and nearly undetectable levels of HLA-II molecules, which seems to be genomically programmed (Figure 2I) and stable through the in vitro culture and in vivo persistence even within the tumor microenvironment (Figures 6A–6F); this feature may allow these cells to resist allorejection by host T cells and thereby alleviate the need for additional HLA gene editing or intense host T cell depletion preconditioning treatment (e.g., CD52 antibody treatment).10,13 Meanwhile, AlloHSC-iNKT cells also express reduced levels of NK activating receptor ligands (e.g., ULBP) (Figure 6J); this feature may allow these cells to also resist allorejection by host NK cells and increase their suitability for allogeneic cell therapy (Figures 6G–6I). The biological regulations resulting in this low-immunogenicity feature of AlloHSC-iNKT cells remain to be illustrated; nonetheless, such biological regulations do not seem to interfere with the production or antitumor efficacy of AlloHSC-iNKT cells both in vitro and in vivo (Figures 3, 4, and 7).
The AlloHSC-iNKT cell production platform is robust and versatile, allowing the plug-in of additional engineering approaches. In our studies, we demonstrated the successful generation of CAR-engineered or/and HLA-I/II-ablated AlloHSC-iNKT cells by incorporating additional CAR gene engineering and CRISPR-Cas9/B2M-CIITA-gRNAs gene-editing steps (Figures 4 and 7). These additional engineering approaches did not interfere with the production and antitumor function of AlloHSC-iNKT cells, opening up the possibility of developing more advanced AlloHSC-iNKT cell products. For example, incorporation of multiple tumor-targeting molecules (e.g., CARs and TCRs) and functional enhancement factors (e.g., overexpression of immune enhancement genes like IL-15, and ablation of immune inhibitory genes like PD-1) may improve the cancer therapy potential of AlloHSC-iNKT cell products.87,88
Limitations of the study
Despite their promise, current AlloHSC-iNKT cell products confront certain limitations that may be further improved; the manufacturing process can benefit from switching to a feeder-free culture system that will greatly simplify and accelerate the clinical and commercial development, the sr39TK/GCV suicide switch can be replaced with an alternative suicide switch system (e.g., inducible Cas9 or truncated EGFR) that is less immunogenic and cell-cycle dependent,4,65, 66, 67,89 an HLA-E transgene can also be incorporated into the AlloHSC-iNKT cell products to further increase their resistance to host NK-cell-mediated allorejection,73,90 and pluripotent stem cells (i.e., embryonic stem cells and induced pluripotent stem cells [iPSCs]) may be utilized as an alternative “unlimited” cell source to derive HSCs for the generation of AlloHSC-iNKT cells.24 Further exploration of AlloHSC-iNKT cells as allogeneic cell carriers for developing off-the-shelf cell therapy for the treatment of cancer, especially solid tumors, will certainly be an interesting direction for future study.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-human IFN-γ (ELISA, capture) | BD Biosciences | CAT#551221, RRID: AB_394099 |
| Anti-human IFN-γ (ELISA, detection) | BD Biosciences | CAT#554550, RRID: AB_395472 |
| Anti-human TNFα (ELISA, capture) | BD Biosciences | CAT#551220, RRID: AB_394098 |
| Anti-human TNFα (ELISA, detection) | BD Biosciences | CAT#554511, RRID: AB_395442 |
| Anti-human IL-2 (ELISA, detection) | BD Biosciences | CAT#554563; RRID: AB_398570 |
| Anti-human IL-2 (ELISA, detection) | BD Biosciences | CAT#555040; RRID: AB_395666 |
| Anti-human IL-4 (ELISA, capture) | BD Biosciences | CAT#554515; RRID: AB_398567 |
| Anti-human IL-4 (ELISA, detection) | BD Biosciences | CAT#554483; RRID: AB_395422 |
| Anti-human CD45 (Clone H130) | Biolegend | CAT#304026, RFID: AB_893337 |
| Anti-human TCRαβ (Clone I26) | Biolegend | CAT#306716, RRID: AB_1953257 |
| Anti-human CD4 (Clone OKT4) | Biolegend | CAT#317414, RRID: AB_571959 |
| Anti-human CD8 (Clone SK1) | Biolegend | CAT#344714, RRID: AB_2044006 |
| Anti-human CD45RO (Clone UCHL1) | Biolegend | CAT#304216, RRID: AB_493659 |
| Anti-human CD161 (Clone HP-3G10) | Biolegend | CAT#339928, RRID: AB_2563967 |
| Anti-human CD69 (Clone FN50) | Biolegend | CAT#310914, RRID: AB_314849 |
| Anti-human CD56 (Clone HCD56) | Biolegend | CAT#318304, RRID: AB_604100 |
| Anti-human CD62L (Clone DREG-56) | Biolegend | CAT#304822, RRID: AB_830801 |
| Anti-human CD14 (Clone HCD14) | Biolegend | CAT#325608, RRID: AB_830681 |
| Anti-human CD11b (Clone ICRF44) | Biolegend | CAT#301330, RRID: AB_2561703 |
| Anti-human CD11c (Clone N418) | Biolegend | CAT#337234, RRID: AB_2566656 |
| Anti-human CD1d (Clone 51.1) | Biolegend | CAT#350308, RRID: AB_10642829 |
| Anti-human CCR4 (Clone L291H4) | Biolegend | CAT#359409, RRID: AB_2562430 |
| Anti-human CCR5 (Clone HEK/1/85a) | Biolegend | CAT#313705, RRID: AB_345305 |
| Anti-human CXCR3 (Clone G025H7) | Biolegend | CAT#306513, RRID: AB_2089652 |
| Anti-human NKG2D (Clone 1D11) | Biolegend | CAT#320812, RRID: AB_2234394 |
| Anti-human DNAM-1 (Clone 11A8) | Biolegend | CAT#338312, RRID: AB_2561952 |
| Anti-human CD158 (KIR2DL1/S1/S3/S5) (Clone HP-MA4) | Biolegend | CAT#339510, RRID: AB_2565577 |
| Anti-human IFN-γ (Clone B27) | Biolegend | CAT#506518, RRID: AB_2123321 |
| Anti-human granzyme B (Clone QA16A02) | Biolegend | CAT#372204, RRID: AB_2687028 |
| Anti-human perforin (Clone dG9) | Biolegend | CAT#308126, RRID: AB_2572049 |
| Anti-human TNFα (Clone Mab11) | Biolegend | CAT#502912, RRID: AB_315264 |
| Anti-human IL-2 (Clone MQ1-17H12) | Biolegend | CAT#500341, RRID: AB_2562854 |
| Anti-human HLAE (Clone 3D12) | Biolegend | CAT#342606, RRID: AB_342606 |
| Anti-human β2-microglobulin (B2M) (Clone 2M2) | Biolegend | CAT#316312, RRID: AB_10641281 |
| Anti-human HLA-DR (Clone L243) | Biolegend | CAT#307618, RRID: AB_493586 |
| Anti-human TCR Vδ2 (Clone B6) | Biolegend | CAT#331417, RRID: AB_2687323 |
| Anti-human CD107a (Clone H4A3) | Biolegend | CAT#328641, RRID: AB_2565965 |
| Anti-human CD34 (Clone 581) | BD Biosciences | CAT#555822, RRID: AB_396151 |
| Anti-human TCR Vα24-Jβ18 (Clone 6B11) | BD Biosciences | CAT#552825, RRID: AB_394478 |
| Anti-human ULBP-2,5,6 (Clone 165903) | R&D Systems | CAT#FAB1298A, RRID: AB_ 2257142 |
| Anti-human Vβ11 | Beckman-Coulter | CAT#A66905 |
| Human Fc Receptor Blocking Solution (TrueStain FcX) | Biolegend | CAT#422302 |
| Mouse Fc Block (anti-mouse CD16/32) | BD Biosciences | CAT#553142, RRID: AB_394657 |
| β-2-Microglobulin Antibody (Clone BBM.1) | Santa Cruz Biotechnology | CAT#sc-13565 |
| LEAF purified anti-human CD1d antibody (Clone 51.1) | Biolegend | CAT#350304 |
| LEAF purified Mouse IgG2b, k isotype ctrl (Clone MG2b-57) | Biolegend | CAT#401212 |
| LEAF purified anti-human NKG2D antibody (Clone 1D11) | Biolegend | CAT#320810, RRID: AB_2133276 |
| LEAF purified anti-human DNAM-1 antibody (Clone DX11) | BD Biosciences | CAT#559786, RRID: AB_397327 |
| Mouse IgG1, κ isotype control antibody (Clone MOPC-21) | Biolegend | CAT#400124 |
| Bacterial and virus strains | ||
| Lenti/iNKT-sr39TK | This paper | N/A |
| Lenti/BCAR-iNKT-sr39TK | This paper | N/A |
| Lenti/FG | This paper | N/A |
| Lenti/CD1d | This paper | N/A |
| Retro/BCMA-CAR-tEGFR | This paper | N/A |
| Biological samples | ||
| Human peripheral blood mononuclear cells (PBMCs) | UCLA | N/A |
| Human cord blood CD34+ hematopoietic stem and progenitor cells (HSCs) | UCLA | N/A |
| Human multiple myeloma patient bone marrow samples | UCLA | N/A |
| G-CSF-mobilized peripheral blood units | CCHMC | CAT#M001F-GCSF-3 |
| G-CSF-mobilized leukopak | HemaCare | CAT#M001CLPG-4-KIT |
| Cord Blood Cryo CD34 | HemaCare | CAT#CB34C-3 |
| Chemicals, peptides, and recombinant proteins | ||
| Streptavidin-HRP conjugate | Invitrogen | CAT#SA10001 |
| IFN-γ (ELISA, standard) | eBioscience | CAT#29-8319-65 |
| TNFα (ELISA, standard) | eBioscience | CAT#29-8329-65 |
| IL-2 (ELISA, standard) | eBioscience | CAT#29-8029-65 |
| IL-4 (ELISA, standard) | eBioscience | CAT#39-8049-65 |
| IL-17 (ELISA, standard) | eBioscience | CAT#29-8179-65 |
| Tetramethylbenzidine (TMB) | KPL | CAT#5120-0053 |
| Ganciclovir (GCV) | Sigma | CAT#ADV465749843 |
| α-Galactosylceramide (KRN7000) | Avanti Polar Lipids | SKU#867000P-1mg |
| Zoledronate | Sigma-Aldrich | CAT#SML0223 |
| Recombinant human IL-2 | Peprotech | CAT#200-02 |
| Recombinant human IL-3 | Peprotech | CAT#200-03 |
| Recombinant human IL-7 | Peprotech | CAT#200-07 |
| Recombinant human IL-15 | Peprotech | CAT#200-15 |
| Recombinant human Flt3-Ligand | Peprotech | CAT#300-19 |
| Recombinant human SCF | Peprotech | CAT#300-07 |
| Recombinant human TPO | Peprotech | CAT#300-18 |
| Recombinant human GM-CSF | Peprotech | CAT#300-03 |
| L-ascorbic acid 2-phosphate | Sigma | CAT#A8960-5G |
| B27™ Supplement (50X), serum free | ThermoFisher | CAT#17504044 |
| Cas9-NLS purified protein | UC Berkeley | N/A |
| X-VIVO 15 Serum-free Hematopoietic Cell Medium | Lonza | CAT#04-418Q |
| RPMI1640 cell culture medium | Corning Cellgro | CAT#10-040-CV |
| DMEM cell culture medium | Corning Cellgro | CAT#10-013-CV |
| Fetal Bovine Serum (FBS) | Sigma | CAT#F2442 |
| MACS BSA stock solution | Miltenyi | CAT#130-091-376 |
| 30% BSA | Gemini | CAT#50-753-3079 |
| Penicillin-Streptomycine-Glutamine (P/S/G) | GIBCO | CAT#10378016 |
| Penicillin: streptomycin (pen:strep) solution (P/S) | Gemini Bio-products | CAT#400-109 |
| MEM non-essential amino acids (NEAA) | GIBCO | CAT#11140050 |
| HEPES Buffer Solution | GIBCO | CAT#15630056 |
| Sodium Pyruvate | GIBCO | CAT#11360070 |
| Beta-Mercaptoethanol | Sigma | SKU#M6250 |
| Normocin | Invivogen | CAT#ant-nr-2 |
| Fixable Viability Dye eFluor506 | affymetrix eBioscience | CAT#65-0866-14 |
| Cell Fixation/Permeabilization Kit | BD Biosciences | CAT#554714 |
| RetroNectin recombination human fibronectin fragment, 2.5mg | Takara | CAT#T100B |
| 10% neutral-buffered formalin | Richard-Allan Scientific | CAT#5705 |
| D-Luciferin | Caliper LIfe Science | CAT#XR-1001 |
| Isoflurane | Zoetis | CAT#50019100 |
| Phosphate Buffered Saline (PBS) pH 7.4 (1X) | GIBCO | CAT#10010-023 |
| Formaldehyde | Sigma-Aldrich | CAT#F8775 |
| Golgistop Protein Transport Inhibitor | BD Biosciences | CAT#554724 |
| Phorbol-12-myristate-13-acetate (PMA) | Calbiochem | CAT#524400 |
| Ionomycin, Calcium salt, Streptomyces conglobatus | Calbiochem | CAT#407952 |
| Critical commercial assays | ||
| Human NK Cell Isolation Kit | Miltenyi Biotec | CAT#130-092-657 |
| Human CD34 MicroBeads Kit | Miltenyi Biotec | CAT#130-046-703 |
| Human CD14 MicroBeads Kit | Miltenyi Biotec | CAT#130-050-201 |
| Human Anti-iNKT MicroBeads | Miltenyi Biotec | CAT#130-094-842 |
| Human Anti-HLA-DR MicroBeads | Miltenyi Biotec | CAT#130-046-101 |
| Fixation/Permeabilization Solution Kit | BD Sciences | CAT#55474 |
| Amaxa™ P3 Primary Cell 4D-Nucleofector™ X Kit S | Lonza | CAT#V4XP-3032 |
| Dynabeads Human T-Activator CD3/CD28 | ThermoFisher | CAT#111.61D |
| miRNeasy Mini Kit | QIAGEN | CAT#217004 |
| Chromium single cell V(D)J enrichment kit, human T cell | 10 x Genomics | CAT#1000005 |
| Cryostor cell cryopreservation media | Sigma | CAT#C2874-100ML |
| Human IL-17A ELISA MAX Deluxe Kit | Biolegend | CAT#433915 |
| Deposited data | ||
| Deep RNA sequencing | This paper | Gene Expression Omnibus Database: GSE164425 |
| Single cell TCR sequencing | This paper | Gene Expression Omnibus Database: GSE164500 |
| Experimental models: Cell lines | ||
| Human multiple myeloma (MM) cell line MM.1S | ATCC | CRL-2974 |
| Human chronic myelogenous leukemia cancer cell line K562 | ATCC | CCL-243 |
| Human melanoma cell line A375 | ATCC | CRL-1619 |
| Human adenocarcinoma cell line PC3 | ATCC | CRL-1435 |
| Human mucoepidermoid pulmonary carcinoma H292 | ATCC | CRL-1848 |
| Mus musculus Leukemia packaging cell PG13 | ATCC | CRL-10686 |
| Human multiple myeloma (MM) cell line MM.1S-FG | This paper | N/A |
| Human multiple myeloma (MM) cell line MM.1S-CD1d-FG | This paper | N/A |
| Human chronic myelogenous leukemia cancer cell line K562-FG | This paper | N/A |
| Human adenocarcinoma cell line PC3-FG | This paper | N/A |
| Human mucoepidermoid pulmonary carcinoma H292-FG | This paper | N/A |
| Human melanoma cell line A375-FG | This paper | N/A |
| Mus musculus Leukemia packaging cell PG13-BCAR-tEGFR | This paper | N/A |
| Mouse bone marrow derived stromal cell line MS5-hDLL4 | Amelie et al., 2019 | N/A |
| Experimental models: Organisms/strains | ||
| NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) | The Jackson Laboratory | Stock #: 005557 |
| Oligonucleotides | ||
| gRNA (B2M): CGCGAGCACAGCUAA GGCCA |
Synthego | N/A |
| gRNA (CIITA): GAUAUUGGCAUAAGC CUCCC |
Synthego | N/A |
| Recombinant DNA | ||
| Vector: parental lentivector pMNDW | N/A | N/A |
| Vector: parental retrovector pMP71 | N/A | N/A |
| Software and algorithms | ||
| FlowJo Software | FlowJo | https://www.flowjo.com/solutions/flowjo/downloads |
| Living Imaging 2.50 software | Xenogen/PerkinElmer | https://www.perkinelmer.com/lab-products-and-services/resources/in-vivo-imaging-software-downloads.html |
| AURA imaging software | Spectral Instruments Imaging | https://spectralinvivo.com/software/ |
| I-control 1.7 Microplate Reader Software | Tecan | https://www.selectscience.net/tecan/i-control-microplate-reader-software/81307 |
| ImageJ | ImageJ | https://imagej.net/Downloads |
| Prism 6 | Graphpad | https://www.graphpad.com/scientific-software/prism/ |
| MATLAB | The MathWorks, Inc | https://www.mathworks.com/products/matlab.html |
| R | R | http://www.R-project.org/ |
Resource availability
Lead contact
Further information and requests for new reagents generated in this study may be directed to, and will be fulfilled by the Lead Contact, Lili Yang (liliyang@ucla.edu).
Materials availability
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Experimental model and subject details
Mice
NOD.Cg-PrkdcSCIDIl2rgtm1Wjl/SzJ (NOD/SCID/IL-2Rγ−/−, NSG) mice were maintained in the animal facilities of the University of California, Los Angeles (UCLA). 6-10 weeks old mice were used for all experiments unless otherwise indicated. All animal experiments were approved by the Institutional Animal Care and Use Committee of UCLA. All mice were bred and maintained under specific pathogen-free conditions, and all experiments were conducted in accordance with the animal care and use regulations of the Division of Laboratory Animal Medicine (DLAM) at the UCLA.
Cell Lines
The MS5-DLL4 murine bone marrow derived stromal cell line was obtained from Dr. Gay Crooks’ lab (UCLA). Human multiple myeloma cancer cell line MM.1S, chronic myelogenous leukemia cancer cell line K562, melanoma cell line A375, lung carcinoma cell line H292, and prostate cancer cell line PC3 were purchased from the American Type Culture Collection (ATCC). MM.1S cells were cultured in R10 medium. K562 cells were cultured in C10 medium. A375, H292, and PC3 were cultured in D10 medium.
To make stable tumor cell lines overexpressing human CD1d, and/or firefly luciferase and enhanced green fluorescence protein (Fluc-EGFP) dual-reporters, the parental tumor cell lines were transduced with lentiviral vectors encoding the intended gene(s)28. 72h post lentivector transduction, cells were subjected to flow cytometry sorting to isolate gene-engineered cells for making stable cell lines. Six stable tumor cell lines were generated for this study, including MM.1S-FG, MM.1S-CD1d-FG, A375-FG, PC3-FG, H292-FG, and K562-FG.
Human CD34+ Hematopoietic Stem Cells (HSCs), Periphery Blood Mononuclear Cells (PBMCs), and Patient Bone Marrow Samples
Cord blood cells were purchased from HemaCare. G-CSF-mobilized healthy donor peripheral blood cells were purchased from HemaCare or Cincinnati Children’s Hospital Medical Center (CCHMC). Human CD34+ HSCs were isolated through magnetic-activated cell sorting using ClinMACs CD34+ microbeads (Miltenyi Biotech). Cells were cryopreserved in Cryostor CS10 (Sigma) using CoolCell (BioCision), and were frozen in liquid nitrogen for all experiments and long-term storage. Healthy donor human PBMCs were obtained from the UCLA/CFAR Virology Core Laboratory, without identification information under federal and state regulations. Patient bone marrow samples were collected following UCLA IRB approval (IRB#15-000062).
Media and Reagents
α-Galactosylceramide (αGC, KRN7000) was purchased from Avanti Polar Lipids. Recombinant human IL-2, IL-3, IL-4, IL-7, IL-15, Flt3-Ligand, Stem Cell Factor (SCF), Thrombopoietin (TPO), and Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) were purchased from Peprotech. Ganciclovir (GCV) was purchased from Sigma.
X-VIVO 15 Serum-free Hematopoietic Cell Medium was purchased from Lonza. RPMI 1640 and DMEM cell culture medium were purchased from Corning Cellgro. Fetal bovine serum (FBS) was purchased from Sigma. Medium supplements, including Penicillin-Streptomycine-Glutamine (P/S/G), MEM non-essential amino acids (NEAA), HEPES Buffer Solution, and Sodium Pyruvate, were purchased from GIBCO. Beta-Mercaptoethanol (β-ME) was purchased from Sigma. Normocin was purchased from InvivoGen. Complete lymphocyte culture medium (denoted as C10 medium) was made of RPMI 1640 supplemented with FBS (10% vol/vol), P/S/G (1% vol/vol), MEM NEAA (1% vol/vol), HEPES (10 mM), Sodium Pyruvate (1 mM), β-ME (50 mM), and Normocin (100 mg/ml). Medium for culturing human MM.1S tumor cell line (denoted as R10 medium) was made of RPMI 1640 supplemented with FBS (10% vol/vol) and P/S/G (1% vol/vol). Adherent cell culture medium (denoted as D10 medium) was made of DMEM supplemented with FBS (10% vol/vol) and P/S/G (1% vol/vol).
Method details
Lentiviral and Retroviral Vectors
Lentiviral vectors used in this study were all constructed from a parental lentivector pMNDW as previously described28. The Lenti/iNKT-sr39TK vector was constructed by inserting into pMNDW vector a synthetic tricistronic gene encoding human iNKT TCRα-F2A-TCRβ-P2A-sr39TK; the Lenti/FG vector was constructed by inserting into pMNDW a synthetic bicistronic gene encoding Fluc-P2A-EGFP; the Lenti/CD1d vector was constructed by inserting into pMNDW a synthetic gene encoding human CD1d. The synthetic gene fragments were obtained from GenScript and IDT. Lentiviruses were produced using HEK293T cells, following a standard calcium precipitation protocol and an ultracentrifigation concentration protocol as previously described27,28. Lentivector titers were measured by transducing HT29 cells with serial dilutions and performing digital qPCR, following established protocols27,28.
The Retro/BCAR-tEGFR vector was constructed by inserting into the parental MP71 vector a synthetic gene encoding human BCMA scFV-41BB-CD3ζ-P2A-tEGFR. The synthetic gene fragments were obtained from IDT. Vsv-g-pseudotyped Retro/BCAR-tEGFR retroviruses were generated by transfecting HEK293T cells following a standard calcium precipitation protocol27,28; the viruses were then used to transduce PG13 cells to generate a stable retroviral packaging cell line producing Retro/BCAR-tEGFR retroviruses (denoted as PG13-BCAR-tEGFR cell line). For retrovirus production, the PG13-BCAR-tEGFR cells were seeded at a density of 8 × 105 cells per ml in D10 medium and cultured in a 15 cm-dish (30 mL per dish) for 2 days; virus supernatants were then harvested and stored at −80°C for future use.
Antibodies and Flow Cytometry
All flow cytometry stains were performed in PBS for 15 min at 4°C. The samples were stained with Fixable Viability Dye eFluor506 (e506) mixed with Mouse Fc Block (anti-mouse CD16/32) or Human Fc Receptor Blocking Solution (TrueStain FcX) prior to antibody staining. Antibody staining was performed at a dilution according to the manufacturer’s instructions. Fluorochrome-conjugated antibodies specific for human CD45 (Clone H130), TCRαβ (Clone I26), CD4 (Clone OKT4), CD8 (Clone SK1), CD45RO (Clone UCHL1), CD161 (Clone HP-3G10), CD69 (Clone FN50), CD56 (Clone HCD56), CD62L (Clone DREG-56), CD14 (Clone HCD14), CD11b (Clone ICRF44), CD11c (Clone N418), CD1d (Clone 51.1), CCR4 (Clone L291H4), CCR5 (Clone HEK/1/85a), CXCR3 (Clone G025H7), NKG2D (Clone 1D11), DNAM-1 (Clone 11A8), CD158 (KIR2DL1/S1/S3/S5) (Clone HP-MA4), IFN-γ (Clone B27), granzyme B (Clone QA16A02), perforin (Clone dG9), TNF-α (Clone Mab11), IL-2 (Clone MQ1-17H12), HLAE (Clone 3D12), β2-microglobulin (B2M) (Clone 2M2), HLA-DR (Clone L243), TCR Vδ2 (Clone B6) were purchased from BioLegend; Fluorochrome-conjugated antibodies specific for human CD34 (Clone 581) and TCR Vɑ24-Jβ18 (Clone 6B11) were purchased from BD Biosciences; Fluorochrome-conjugated antibodies specific for human Vβ11 was purchased from Beckman-Coulter; Fluorochrome-conjugated antibodies specific for human ULBP-2,5,6 (Clone 165903) was purchased from R&D Systems. Human Fc Receptor Blocking Solution (TrueStain FcX) was purchased from Biolegend, and Mouse Fc Block (anti-mouse CD16/32) was purchased from BD Biosciences. Fixable Viability Dye e506 were purchased from Affymetrix eBioscience. Intracellular cytokines were stained using a Cell Fixation/Permeabilization Kit (BD Biosciences). Stained cells were analyzed using a MACSQuant Analyzer 10 flow cytometer (Miltenyi Biotech). FlowJo software was utilized to analyze the data.
Enzyme-Linked Immunosorbent Cytokine Assays (ELISA)
The ELISAs for detecting human cytokines were performed following a standard protocol from BD Biosciences. Supernatants from co-culture assays were collected and assayed to quantify IFN-γ, TNF-α, IL-2, IL-4, and IL-17. The capture and biotinylated pairs for detecting cytokines were purchased from BD Biosciences. The streptavidin-HRP conjugate was purchased from Invitrogen. Human cytokine standards were purchased from eBioscience. Tetramethylbenzidine (TMB) substrate was purchased from KPL. The samples were analyzed for absorbance at 450 nm using an Infinite M1000 microplate reader (Tecan).
In Vitro Generation of Allogeneic HSC-Engineered iNKT (AlloHSC-iNKT) Cells
Frozen-thawed human CD34+ HSCs were revived in HSC-culture medium composed of X-VIVO 15 Serum-free Hematopoietic Cell Medium supplemented with SCF (50 ng/ml), FLT3-L (50 ng/ml), TPO (50 ng/ml), and IL-3 (10 ng/ml) for 24 h; the cells were then transduced with Lenti/iNKT-sr39TK viruses for another 24 h following an established protocol28. The transduced HSCs were then collected and put into a 2-Stage in vitro HSC-iNKT culture.
At Stage 1, gene-engineered HSCs were differentiated into iNKT cells in an artificial thymic organoid (ATO) culture over 8 weeks. ATO was generated following a previously established protocol, with certain modifications29,30. Briefly, MS5-DLL4 cells were harvested by trypsinization and resuspended in serum free ATO culture medium (‘‘RB27’’) composed of RPMI 1640 (Corning), 4% B27 supplement (ThermoFisher Scientific), 30 mM L-ascorbic acid 2-phosphate sesquimagnesium salt hydrate (Sigma-Aldrich) reconstituted in PBS, 1% penicillin/streptomycin (Gemini Bio-Products), 1% Glutamax (ThermoFisher Scientific), 5 ng/ml rhFLT3L and 5 ng/ml rhIL-7 (Peprotech). RB27 was made fresh weekly. 1.5-6 × 105 MS5-DLL4 cells were combined with 0.3-10 × 104 transduced HSCs per ATO in 1.5 mL Eppendorf tubes (up to 12 ATOs per tube) and centrifuged at 300 g for 5 min at 4°C in a swinging bucket centrifuge. Supernatants were carefully removed, and the cell pellet was resuspended in 6 mL RB27 per ATO and mixed by brief vortexing. ATOs were plated on a 0.4 mm Millicell transwell insert (EMD Millipore; Cat. PICM0RG50) placed in a 6-well plate containing 1 mL RB27 per well. Medium was changed completely every 3-4 days by aspiration from around the cell insert followed by replacement with 1 mL fresh RB27/cytokines. ATO cells were harvested by adding FACS buffer (PBS/0.5% bovine serum album/2mM EDTA) to each well and briefly disaggregating the ATO by pipetting with a 1 mL ‘‘P1000’’ pipet, followed by passage through a 50 mm nylon strainer.
At Stage 2, isolated ATO cells comprising AlloHSC-iNKT cells were expanded with αGC-loaded PBMCs (αGC-PBMCs). αGC-PBMCs were prepared by incubating 107-108 PBMCs in 5 mL C10 medium containing 5 μg/ml αGC for 1 h, followed by irradiation at 6,000 rads. ATO cells were mixed with irradiated αGC-PBMCs at ratio 1:1, followed by culturing in C10 medium supplemented with human IL-7 (10 ng/ml) and IL-15 (10 ng/ml) for 2 weeks; cell cultures were split and fresh media/cytokines were added if needed. The Stage 2 expansion culture could be extended to 3 weeks by adding additional αGC-PBMCs at ratio 1:1 at the end of week 2. At the end of Stage 2 culture, the resulting AlloHSC-iNKT cell products were collected and cryopreserved for future use.
In Vitro Generation of BCMA CAR-Engineered AlloHSC-iNKT (AlloBCAR-iNKT) Cells
AlloHSC-iNKT cells were generated in the 2-Stage HSC-iNKT culture as described above, followed by an additional Stage 3 CAR engineering culture. At one week into Stage 2 culture, AlloHSC-iNKT cells were collected and stimulated with CD3/CD28 T-activator beads (ThermoFisher Scientific) in the presence of recombinant human IL-15 (10 ng/ml) and human IL-7 (10 ng/ml) for two days; the cells were then spin-infected with frozen-thawed Retro/BCAR-tEGFR retroviral supernatants supplemented with polybrene (10 μg/ml, Sigma-Aldrich) at 660 g at 30°C for 90 min. Retronectin (Takara) could be pre-coated on plate to increase transduction efficiency. After transduction, the resulting AlloBCAR-iNKT cells were expanded for another 1-2 weeks in C10 medium supplemented with recombinant human IL-15 (10 ng/ml) and IL-7 (10 ng/ml), and then were collected and cryopreserved for future use.
In Vitro Generation of HLA-Ablated Universal HSC-iNKT (UHSC-iNKT) Cells
UHSC-iNKT cells were generated following the protocol of generating AlloHSC-iNKT cells, with one additional gene-editing step. CD34+ HSCs were revived in HSC-culture medium on day 1, transduced with Lenti/iNKT-sr39TK viruses on day 2, and then were electroporated with a CRISPR-Cas9/B2M-CIITA-gRNAs complex on day 3, followed by entering the 2-Stage HSC-iNKT culture to generate UHSC-iNKT cells. A small portion of engineered CD34+ HSCs were cultured in HSC-culture medium for 5 days, and flow cytometry was performed to evaluate the genome-editing efficiencies.
UBCAR-iNKT cells were generated following the protocol of generating AlloBCAR-iNKT cells, with the same additional gene-editing step. CD34+ HSCs were revived in HSC-culture medium on day 1, transduced with Lenti/iNKT-sr39TK viruses on day 2, and then were electroporated with a CRISPR-Cas9/B2M-CIITA-gRNAs complex on day 3, followed by entering the 3-Stage BCAR-iNKT culture to generate UBCAR-iNKT cells.
For electroporation, 2 × 105 HSCs per condition were pelleted at 90 x g for 10 min at room temperature (RT), resuspended in 20 μL P3 solution (Lonza), mixed with pre-aliquoted B2M and CIITA gRNAs (1 μL of each gRNA at 100 μM) and Cas9 (4 μL at 6.5 mg/ml), and pulsed once at 250 V for 5 ms in an Amaxa 4D Nucleofector X Unit (Lonza). After electroporation, HSCs were rested at RT for 10 min, and then transferred to a 24-well tissue culture treated plate overnight before entering the 2-Stage HSC-iNKT or 3-Stage BCAR-iNKT culture.
If necessary, the resulting UHSC-iNKT or UBCAR-iNKT cell products collected at the end of the in vitro culture could be purified using Magnetic-Activate Cell Sorting (MACS) via B2M (Santa Cruz Biotechnology) and HLA-II magnetic beads labeling (Miltenyi Biotec), to enrich the HLA-I/II double negative cells in the final UHSC-iNKT or UBCAR-iNKT cell products.
Generation of PBMC-Derived Conventional αβT, iNKT, γδT, and NK Cells
Healthy donor PBMCs were obtained from the UCLA/CFAR Virology Core Laboratory, and were used to generate the PBMC-Tc, PBMC-iNKT, PBMC-γδT, and PBMC-NK cells.
To generate PBMC-Tc cells, PBMCs were stimulated with CD3/CD28 T-activator beads (ThermoFisher Scientific) and cultured in C10 medium supplemented with human IL-2 (20 ng/mL) for 2-3 weeks, following the manufacturer’s instructions.
To generate PBMC-iNKT cells, PBMCs were MACS-sorted via anti-iNKT microbeads (Miltenyi Biotech) labeling to enrich iNKT cells, which were then stimulated with donor-matched irradiated αGC-PBMCs at the ratio of 1:1, and cultured in C10 medium supplemented with human IL-7 (10 ng/ml) and IL-15 (10 ng/ml) for 2-3 weeks. If needed, the resulting PBMC-iNKT cells could be further purified using Fluorescence-Activated Cell Sorting (FACS) via human iNKT TCR antibody (Clone 6B11; BD Biosciences) staining.
To generate PBMC-γδT cells, PBMCs were stimulated with Zoledronate (5 μM; Sigma-Aldrich) and cultured in C10 medium supplemented with human IL-2 (20 ng/ml) for 2 weeks. If needed, the resulting PBMC-γδT cells could be further purified using FACS via human TCR Vδ2 antibody (Clone B6; Biolegend) staining or via MACS using a human TCRγ/δ T Cell Isolation Kit (Miltenyi Biotech).
To generate PBMC-NK cells, PBMCs were FACS-sorted via human CD56 antibody (Clone HCD56; Biolegend) labeling or MACS-sorted using a human NK Cell Isolation Kit (Miltenyi Biotech).
Generation of BCMA CAR-Engineered PBMC T (BCAR-T) cells
Healthy donor PBMCs were stimulated with CD3/CD28 T-activator beads (ThermoFisher Scientific) in the presence of recombinant human IL-2 (30 ng/ml), following the manufacturer’s instructions. On day 2, cells were spin-infected with frozen-thawed Retro/BCAR-tEGFR retroviral supernatants supplemented with polybrene (10 μg/ml, Sigma-Aldrich) at 660 g at 30°C for 90 min. Retronectin (Takara) could be pre-coated on plate to increase transduction efficiency. The resulting BCAR-T cells were expanded for another 7-10 days, and then were cryopreserved for future use.
Single Cell TCR Sequencing
AlloHSC-iNKT (6B11+TCRαβ+), PBMC-iNKT (6B11+TCRαβ+), and PBMC-Tc (6B11-TCRαβ+) cells were sorted using a FACSAria II flow cytometer. Sorted cells were immediately delivered to the UCLA TCGB (Technology Center for Genomics and Bioinformatics) Core to perform single cell TCR sequencing using a 10X Genomics Chromium™ Controller Single Cell Sequencing System (10X Genomics), following the manufacturer’s instructions and the TCGB Core’s standard protocol. Libraries were constructed using an Illumina TruSeq RNA Sample Prep Kit (Cat#FC-122-1001) and sequenced with 150 bp paired end reads (5,000 reads/cell) on an Illumina NovaSeq. The reads were mapped to the human T cell receptor reference genome (hg38) using Cell Ranger VDJ. The frequencies of the α or β chain recombination were plotted.
Deep RNA Sequencing (Deep RNaseq) and Data Analysis
A total of 25 cell samples were analyzed, as described in the following table:
| Sample name | Number of replicates (from different donors) | FACS sorting markers | Description |
|---|---|---|---|
| AlloHSC-iNKT (from PBSC) | 3 | 6B11+TCRαβ+ | AlloHSC-iNKT cells derived from G-CSF mobilized peripheral blood CD34+ HSCs |
| AlloHSC-iNKT (from CB) | 3 | 6B11+TCRαβ+ | AlloHSC-iNKT cells derived from cord blood CD34+ HSCs |
| PBMC-iNKT (CD4-) | 3 | 6B11+TCRαβ+CD4- | PBMC-iNKT cells derived from healthy donor PBMCs (CD4- cells were analyzed) |
| PBMC-αβTc (CD4-) | 8 | 6B11-TCRαβ+CD4- | PBMC-αβTc cells derived from healthy donor PBMCs (CD4- cells were analyzed) |
| PBMC-NK | 2 | CD56+TCRαβ- | PBMC-NK cells derived from healthy donor PBMCs |
| PBMC-γδT | 6 | TCRγδ+TCRαβ- | PBMC-γδT cells derived from healthy donor PBMCs |
Both AlloHSC-iNKT and PBMC-iNKT cells were activated in vitro with αGC, PBMC-αβTc cells were stimulated with CD3/CD28 T-activator beads, and PBMC-γδT cells were stimulated with Zoledronate. Cell samples were sorted using a FACSAria II flow cytometer. Total RNAs were isolated from each cell sample using an miRNeasy Mini Kit (QIAGEN) and delivered to the UCLA TCGB Core to perform Deep RNA sequencing using an Illumina HiSeq3000, following the manufacturer’s instructions and the TCGB Core’s standard protocol. cDNAs were synthesized using an iScript cDNA Synthesis Kit (1708890, BioRad). Libraries were constructed using an Illumina TruSeq Stranded Total RNA Sample Prep kit and sequenced with 50 bp single end reads (20 M reads/sample) on an Illumina HiSeq3000. The reads were mapped with STAR 2.5.3a to the human genome (hg38). The counts for each gene were obtained using–quantMode GeneCounts in STAR commands, and the other parameters during alignment were set to default. Data quality was checked using Illumina’s proprietary software. Sequencing depth normalized counts were obtained from the differential expression analysis and were used for principal component analysis.
AlloHSC-iNKT Cell Phenotype and Functional Study
AlloHSC-iNKT cells and their derivatives (i.e., AlloBCAR-iNKT, UHSC-iNKT, and UBCAR-iNKT cells) were analyzed in comparison with PBMC-Tc, PBMC-NK, PBMC-iNKT, or/and BCAR-T cells. Phenotype of these cells was studied using flow cytometry, by analyzing cell surface markers including co-receptors (i.e., CD4 and CD8), NK cell receptors (i.e., CD161, NKG2D, DNAM-1, and KIR), memory T cell markers (i.e., CD45RO), and inflammatory tissue/tumor homing markers (i.e., CCR4, CCR5, and CXCR3). Capacity of these cells to produce cytokines (i.e., IFN-γ, TNF-α, and IL-2) and cytotoxic molecules (i.e., perforin and granzyme B) were studied using flow cytometry via intracellular staining.
Response of AlloHSC-iNKT cells to antigen stimulation was studied by culturing AlloHSC-iNKT cells in vitro in C10 medium for 7 days, in the presence or absence of αGC (100 ng/ml). Proliferation of AlloHSC-iNKT cells was measured by cell counting and flow cytometry (identified as 6B11+TCRαβ+) over time. Cytokine production was assessed by ELISA analysis of cell culture supernatants collected on day 7 (for human IFN-γ. TNF-α, IL-2, IL-4, and IL-17).
In Vitro Tumor Cell Killing Assay
Tumor cells (1 × 104 cells per well) were co-cultured with effector cells (at ratios indicated in figure legends) in Corning 96-well clear bottom black plates for 8-24 h, in C10 medium with or without the addition of αGC (100 ng/ml). At the end of culture, live tumor cells were quantified by adding D-luciferin (150 μg/ml; Caliper Life Science) to cell cultures and reading out luciferase activities using an Infinite M1000 microplate reader (Tecan).
In some experiments, 10 μg/ml of LEAF™ purified anti-human NKG2D (Clone 1D11, Biolegend), anti-human DNAM-1 antibody (Clone 11A8, Biolegend), or LEAF™ purified mouse lgG2bk isotype control antibody (Clone MG2B-57, Biolegend) was added to co-cultures, to study NK activating receptor-mediated tumor cell killing mechanism.
All the PBMC-derived cells and HSC-derived iNKT cells were cryopreserved before use. The cells for comparison (e.g., AlloHSC-iNKT and PBMC-NK cells in Figure 3) were cryopreserved and thawed for testing side-by-side using Mr. Frosty Freezing Container (Thermo Scientific Nalgene) following the manufacture’s instructions.
In Vitro Mixed Lymphocyte Reaction (MLR) Assay: Studying Graft-Versus-Host (GvH) Response
PBMCs of multiple healthy donors were irradiated at 2,500 rads and used as stimulators, to study the GvH response of AlloHSC-iNKT cells and their derivatives (i.e., AlloBCAR-iNKT and UBCAR-iNKT cells) as responders. PBMC-Tc or BCAR-T cells were included as responder controls. Stimulators (5 × 105 cells/well) and responders (2 × 104 cells/well) were co-cultured in 96-well round bottom plates in C10 medium for 4 days; the cell culture supernatants were then collected to measure IFN-γ production using ELISA.
In Vitro MLR Assay: Studying Host-Versus-Graft (HvG) Response
PBMCs of multiple healthy donors were used as responders, to study the HvG response of AlloHSC-iNKT cells and their derivatives (i.e., AlloBCAR-iNKT and UBCAR-iNKT cells) as stimulators (irradiated at 2,500 rads). PBMC-Tc, PBMC-iNKT or BCAR-T cells were included as stimulator controls. Stimulators (5 × 105 cells/well) and responders (2 × 104 cells/well) were co-cultured in 96-well round bottom plates in C10 medium for 4 days; the cell culture supernatants were then collected to measure IFN-γ production using ELISA.
In Vitro MLR Assay: Studying NK Cell-Mediated Allorejection
PBMC-NK cells isolated from PBMCs of multiple healthy donors were used to study the NK cell-mediated allorejection of AlloHSC-iNKT cells and their derivatives (i.e., AlloBCAR-iNKT and UBCAR-iNKT cells). Allogeneic PBMC-Tc or PBMC-iNKT cells were included as controls. PBMC-NK cells (2 × 104 cells/well) and the corresponding allogeneic cells (2 × 104 cells/well) were co-cultured in 96-well round bottom plates in C10 medium for 24 h; the cell cultures were then collected to quantify live cells using flow cytometry.
Bioluminescence Live Animal Imaging (BLI)
BLI was performed using an IVIS 100 imaging system (Xenogen/PerkinElmer) or a Spectral Advanced Molecular Imaging (AMI) HTX imaging system (Spectral instrument Imaging). Live animal imaging was acquired 5 min after intraperitoneal (i.p.) injection of D-Luciferin (1 mg per mouse). Imaging results were analyzed using a Living Imaging 2.50 software (Xenogen/ PerkinElmer) or an AURA imaging software (Spectral Instrument Imaging).
AlloHSC-iNKT Cell In Vivo Antitumor Efficacy Study: A375 Human Melanoma Xenograft NSG Mouse Model
NSG mice were pre-conditioned with 100 rads of total body irradiation (day −1), followed by subcutaneous inoculation with 1 × 106 A375-FG cells (day 0). On day 3, the tumor-bearing experimental mice received intravenous (i.v.) injection of vehicle (PBS), 1.2 × 107 AlloHSC-iNKT cells, or 1.2 × 107 PBMC-NK cells. Over time, tumor loads were monitored by measuring total body luminescence using BLI and by measuring tumor size using a Fisherbrand™ Traceable™ digital caliper (Thermo Fisher Scientific). The tumor size was calculated as W x L mm2. At around week 3, mice were terminated, and solid tumors were retrieved and weighted using a PA84 precision balance (Ohaus).
AlloBCAR-iNKT Cell In Vivo Antitumor Efficacy Study: MM.1S Human MM Xenograft NSG Mouse Model
NSG mice were pre-conditioned with 175 rads of total body irradiation (day −1), followed by intravenous inoculation with 1 × 106 MM-CD1d-FGFP cells (day 0). To study antitumor efficacy under low tumor load condition, on day 3, the tumor-bearing experimental mice received i.v. injection of vehicle (PBS), AlloBCAR-iNKT cells (7 × 106 CAR+ cells), or conventional BCAR-T cells (7 × 106 CAR+ cells). To study antitumor efficacy under high tumor load condition, on day 15, the tumor-bearing experimental mice received i.v. injection of vehicle (PBS), AlloBCAR-iNKT cells (5 × 106 CAR+ cells), or conventional BCAR-T cells (5 × 106 CAR+ cells). Over time, experimental mice were monitored for survival, and their tumor loads were measured using BLI.
UBCAR-iNKT Cell In Vivo Antitumor Efficacy Study: MM.1S Human MM Xenograft NSG Mouse Model
NSG mice were pre-conditioned with 175 rads of total body irradiation (day −1), followed by intravenous inoculation with 5 × 105 MM-CD1d-FGFP cells (day 0). On day 3 the tumor-bearing experimental mice received i.v. injection of vehicle (PBS), AlloBCAR-iNKT cells (3 × 106 CAR+ cells), or conventional BCAR-T cells (3 × 106 CAR+ cells). Over time, experimental mice were monitored for survival, and their tumor loads were measured using BLI.
Ganciclovir (GCV) In Vitro and In Vivo Killing Assay
For GCV in vitro killing assay, AlloHSC-iNKT cells were cultured in C10 medium in the presence of titrated amount of GCV (0-50 μM) for 4 days; live AlloHSC-iNKT cells were then counted using a hematocytometer (VWR) via Trypan Blue staining (Fisher Scientific).
GCV in vivo killing assay was performed using an NSG xenograft mouse model. NSG mice received i.v. injection of 1 × 107 AlloHSC-iNKT cells on day 0, followed by i.p. injection of GCV for 5 consecutive days (50 mg/kg per injection per day). On day 5, mice were terminated. Multiple tissues (i.e., spleen, liver, and lung) were collected and processed for flow cytometry analysis to detect tissue-infiltrating AlloHSC-iNKT cells (identified as iNKT TCR+CD45+), following established protocols.28
Histological Analysis
Tissues (i.e., heart, liver, kidney, lung, and spleen) were collected from the experimental mice, fixed in 10% Neutral Buffered Formalin for up to 36 h, then embedded in paraffin for sectioning (5 μm thickness). Tissue sections were prepared and stained with Hematoxylin and Eosin (H&E) by the UCLA Translational Pathology Core Laboratory, following the Core’s standard protocols. Stained sections were imaged using an Olympus BX51 upright microscope equipped with an Optronics Macrofire CCD camera (AU Optronics) at 20 x and 40 x magnifications. The images were analyzed using Optronics PictureFrame software (AU Optronics).
Statistical Analysis
GraphPad Prism 6 (Graphpad Software) was used for statistical data analysis. Student’s two-tailed t test was used for pairwise comparisons. Ordinary 1-way ANOVA followed by Tukey’s or Dunnett’s multiple comparisons test was used for multiple comparisons. Log rank (Mantel-Cox) test adjusted for multiple comparisons was used for Meier survival curves analysis. Data are presented as the mean ± SEM, unless otherwise indicated. In all figures and figure legends, “n” represents the number of samples or animals utilized in the indicated experiments. A P value of less than 0.05 was considered significant. ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Acknowledgments
We thank the University of California, Los Angeles (UCLA) animal facility for providing animal support; the UCLA Translational Pathology Core Laboratory (TPCL) for providing histology support; the UCLA Technology Center for Genomics & Bioinformatics (TCGB) facility for providing RNA-seq services; the UCLA CFAR Virology Core for providing human cells; and the UCLA BSCRC Flow Cytometry Core Facility for cell sorting support. This work was supported by a Director’s New Innovator Award from the NIH (DP2 CA196335, to L.Y.), a Partnering Opportunity for Translational Research Projects Award and a Partnering Opportunity for Discovery Stage Award from the California Institute for Regenerative Medicine (CIRM TRAN1-08533 and DISC2-11157, to L.Y.), a Stem Cell Research Award from the Concern Foundation (to L.Y.), a Research Career Development Award from the STOP CANCER Foundation (to L.Y.), a BSCRC-RHF Research Award from the Rose Hills Research Foundation (to L.Y.), and an Ablon Scholars Award (to L.Y.). Y.-R.L. is a predoctoral fellow supported by the UCLA Whitcome predoctoral fellowship in molecular biology. J.Y. is a predoctoral fellow supported by the UCLA Broad Stem Cell Research Center (BSCRC) predoctoral fellowship. D.L. is a predoctoral fellow supported by T32 Microbial Pathogenesis Training Grant (Ruth L. Kirschstein National Research Service Award, T32-AI007323). Z.L. is a postdoctoral fellow supported by the UCLA Tumor Immunology Training Grant (USHHS Ruth L. Kirschstein Institutional National Research Service Award, T32-CA009120). S.Z. is a predoctoral fellow supported by the UCLA Medical Scientist Training Program Grant (T32-GM008042).
Author contributions
Y.-R.L., Y.Zhou, and L.Y. designed the experiments, analyzed the data, and wrote the manuscript. L.Y. conceived and oversaw the study, with assistance from Y.-R.L. and Y.Zhou and suggestions from G.M.C., S.M.L., M.P., A.R., D.B.K., O.N.W., and P.W. Y.-R.L. and Y.Z. performed all experiments, with assistance from Y.J.K., Y. Zhu, F.M., J.Y., Y.-C.W., X.C., Z.L., Z.S., XiWang, D.L., J.K., T.T., C.H., and J.H. D.C. performed cell sorting. A.M.-H. and C.S.S. helped with ATO culture. J.S. provided MM patient BM samples. F.M. helped with analysis of RNA-seq data. Xiaoyan Wang helped with the statistical analysis of data.
Declaration of interests
Y.-R.L., Y.J.K., J.Y., P.W., Y. Zhu, G.M.C., A.M.-H., C.S.S., and L.Y. are inventors on patents relating to this study filed by UCLA. Y.J.K. is currently an employee of Nkarta. J.Y. and X.W. are currently employees of Appia Bio. X.C. is currently an employee of Atara Bio. Z.L. is currently an employee of Allogene. S.M.L. is a stockholder of 1200 Pharma and TORL BioTherapeutics. A.R. is a consultant for Amgen, Bristol-Meyers Squibb, Chugai, Genentech-Roche, Merck-MSD, Novartis, and Sanofi; a scientific advisory board member of and stockholder with Advaxis, Apricity, Arcus, Bioncotech, Compugen, CytomX, Five Prime, FLX-Bio, ImaginAb, Isoplexis, Kite-Gilead, Merus, Rgenix, and Appia Bio; and a co-founder and scientific advisory board member of Lutris, PACT Pharma, and Tango Therapeutics. J.S. is a consultant for Kite and on the speaker bureau for Kite and BMS. D.B.K. is a stockholder and scientific advisory board member of Allogene Therapeutics, Myogene Bio, ImmunoVec and Pluto Therapeutics. O.N.W. currently has consulting, equity, and/or board relationships with Trethera Corporation, Kronos Biosciences, Sofie Biosciences, Breakthrough Properties, Vida Ventures, Nammi Therapeutics, Two River, Iconovir, Appia BioSciences, Neogene Therapeutics, and Allogene Therapeutics. P.W. is a co-founder, stockholder, consultant, and advisory board member of HRain Biotechnology, TCRCure Biopharma, and Appia Bio. G.M.C., C.S.S., and A.M.-H. are cofounders and stockholders of Pluto Immunotherapeutics. L.Y. is a scientific advisor to AlzChem and Amberstone Biosciensec, and a co-founder, stockholder, and advisory board member of Appia Bio. None of the declared companies contributed to or directed any of the research reported in this article. The remaining authors declare no competing interests.
Published: November 16, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2021.100449.
Supplemental information
Data and code availability
-
•
The genomics data generated during this study are available from the public repository Gene Expression Omnibus Database: GSE164425, GSE164500.
-
•
All data reported in this manuscript are available from the Lead Contact without restriction
-
•
No custom computer code was reported in this manuscript.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request.
References
- 1.Restifo N.P., Dudley M.E., Rosenberg S.A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalos M., June C.H. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maus M.V., Fraietta J.A., Levine B.L., Kalos M., Zhao Y., June C.H. Adoptive immunotherapy for cancer or viruses. Annu. Rev. Immunol. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labanieh L., Majzner R.G., Mackall C.L. Programming CAR-T cells to kill cancer. Nat. Biomed. Eng. 2018;2:377–391. doi: 10.1038/s41551-018-0235-9. [DOI] [PubMed] [Google Scholar]
- 5.Mikkilineni L., Kochenderfer J.N. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood. 2017;130:2594–2602. doi: 10.1182/blood-2017-06-793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.June C.H., Sadelain M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leko V., Rosenberg S.A. Identifying and Targeting Human Tumor Antigens for T Cell-Based Immunotherapy of Solid Tumors. Cancer Cell. 2020;38:454–472. doi: 10.1016/j.ccell.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg S.A., Restifo N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim W.A., June C.H. The Principles of Engineering Immune Cells to Treat Cancer. Cell. 2017;168:724–740. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin R., Graham C., Yallop D., Jozwik A., Mirci-Danicar O.C., Lucchini G., Pinner D., Jain N., Kantarjian H., Boissel N., et al. UCART19 Group Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet. 2020;396:1885–1894. doi: 10.1016/S0140-6736(20)32334-5. [DOI] [PubMed] [Google Scholar]
- 11.Qasim W., Zhan H., Samarasinghe S., Adams S., Amrolia P., Stafford S., Butler K., Rivat C., Wright G., Somana K., et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 2017;9:1–9. doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 12.Stadtmauer E.A., Fraietta J.A., Davis M.M., Cohen A.D., Weber K.L., Lancaster E., et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367:1–20. doi: 10.1126/science.aba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamin R., Graham C., Yallop D., Jozwik A., Ciocarlie O., Jain N., et al. Preliminary Data on Safety, Cellular Kinetics and Anti-Leukemic Activity of UCART19, an Allogeneic Anti-CD19 CAR T-Cell Product, in a Pool of Adult and Pediatric Patients with High-Risk CD19+ Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia. Blood. 2018;132(Supplement 1):896. [Google Scholar]
- 14.Basar R., Daher M., Rezvani K. Next-generation cell therapies: the emerging role of CAR-NK cells. Blood Adv. 2020;4:5868–5876. doi: 10.1182/bloodadvances.2020002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker P.S.A., Suck G., Nowakowska P., Ullrich E., Seifried E., Bader P., Tonn T., Seidl C. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunol. Immunother. 2016;65:477–484. doi: 10.1007/s00262-016-1792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bendelac A., Savage P.B., Teyton L. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 17.Haraguchi K., Takahashi T., Hiruma K., Kanda Y., Tanaka Y., Ogawa S., Chiba S., Miura O., Sakamaki H., Hirai H. Recovery of Valpha24+ NKT cells after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;34:595–602. doi: 10.1038/sj.bmt.1704582. [DOI] [PubMed] [Google Scholar]
- 18.Fujii S., Shimizu K., Okamoto Y., Kunii N., Nakayama T., Motohashi S., et al. NKT cells as an ideal anti-tumor immunotherapeutic. Front. Immunol. 2013;4:1–7. doi: 10.3389/fimmu.2013.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lalla C., Rinaldi A., Montagna D., Azzimonti L., Bernardo M.E., Sangalli L.M., Paganoni A.M., Maccario R., Di Cesare-Merlone A., Zecca M., et al. Invariant NKT cell reconstitution in pediatric leukemia patients given HLA-haploidentical stem cell transplantation defines distinct CD4+ and CD4- subset dynamics and correlates with remission state. J. Immunol. 2011;186:4490–4499. doi: 10.4049/jimmunol.1003748. [DOI] [PubMed] [Google Scholar]
- 20.Chaidos A., Patterson S., Szydlo R., Chaudhry M.S., Dazzi F., Kanfer E., McDonald D., Marin D., Milojkovic D., Pavlu J., et al. Graft invariant natural killer T-cell dose predicts risk of acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Blood. 2012;119:5030–5036. doi: 10.1182/blood-2011-11-389304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubio M.T., Bouillié M., Bouazza N., Coman T., Trebeden-Nègre H., Gomez A., Suarez F., Sibon D., Brignier A., Paubelle E., et al. Pre-transplant donor CD4- invariant NKT cell expansion capacity predicts the occurrence of acute graft-versus-host disease. Leukemia. 2017;31:903–912. doi: 10.1038/leu.2016.281. [DOI] [PubMed] [Google Scholar]
- 22.Bae E.A., Seo H., Kim I.K., Jeon I., Kang C.Y. Roles of NKT cells in cancer immunotherapy. Arch. Pharm. Res. 2019;42:543–548. doi: 10.1007/s12272-019-01139-8. [DOI] [PubMed] [Google Scholar]
- 23.Exley M.A., Friedlander P., Alatrakchi N., Vriend L., Yue S., Sasada T., Zeng W., Mizukami Y., Clark J., Nemer D., et al. Adoptive transfer of invariant NKT cells as immunotherapy for advanced melanoma: A phase I clinical trial. Clin. Cancer Res. 2017;23:3510–3519. doi: 10.1158/1078-0432.CCR-16-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitayama S., Zhang R., Liu T.Y., Ueda N., Iriguchi S., Yasui Y., Kawai Y., Tatsumi M., Hirai N., Mizoro Y., et al. Cellular Adjuvant Properties, Direct Cytotoxicity of Re-differentiated Vα24 Invariant NKT-like Cells from Human Induced Pluripotent Stem Cells. Stem Cell Reports. 2016;6:213–227. doi: 10.1016/j.stemcr.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rotolo A., Caputo V.S., Holubova M., Baxan N., Dubois O., Chaudhry M.S., Xiao X., Goudevenou K., Pitcher D.S., Petevi K., et al. Enhanced Anti-lymphoma Activity of CAR19-iNKT Cells Underpinned by Dual CD19 and CD1d Targeting. Cancer Cell. 2018;34:596–610.e11. doi: 10.1016/j.ccell.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krijgsman D., Hokland M., Kuppen P.J.K. The role of natural killer T cells in cancer-A phenotypical and functional approach. Front. Immunol. 2018;9:367. doi: 10.3389/fimmu.2018.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith D.J., Liu S., Ji S., Li B., McLaughlin J., Cheng D., Witte O.N., Yang L. Genetic engineering of hematopoietic stem cells to generate invariant natural killer T cells. Proc. Natl. Acad. Sci. USA. 2015;112:1523–1528. doi: 10.1073/pnas.1424877112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Y., Smith D.J., Zhou Y., Li Y.R., Yu J., Lee D., Wang Y.C., Di Biase S., Wang X., Hardoy C., et al. Development of Hematopoietic Stem Cell-Engineered Invariant Natural Killer T Cell Therapy for Cancer. Cell Stem Cell. 2019;25:542–557.e9. doi: 10.1016/j.stem.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seet C.S., He C., Bethune M.T., Li S., Chick B., Gschweng E.H., Zhu Y., Kim K., Kohn D.B., Baltimore D., et al. Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nat. Methods. 2017;14:521–530. doi: 10.1038/nmeth.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montel-Hagen A., Seet C.S., Li S., Chick B., Zhu Y., Chang P., Tsai S., Sun V., Lopez S., Chen H.C., et al. Organoid-Induced Differentiation of Conventional T Cells from Human Pluripotent Stem Cells. Cell Stem Cell. 2019;24:376–389.e8. doi: 10.1016/j.stem.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godfrey D.I., Berzins S.P. Control points in NKT-cell development. Nat. Rev. Immunol. 2007;7:505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 32.Lee P.T., Benlagha K., Teyton L., Bendelac A. Distinct functional lineages of human V(α)24 natural killer T cells. J. Exp. Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gumperz J.E., Miyake S., Yamamura T., Brenner M.B. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujii S., Liu K., Smith C., Bonito A.J., Steinman R.M. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujii S., Shimizu K., Hemmi H., Steinman R.M. Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol. Rev. 2007;220:183–198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 36.Lam P.Y., Nissen M.D., Mattarollo S.R. Invariant natural killer T cells in immune regulation of blood cancers: Harnessing their potential in immunotherapies. Front. Immunol. 2017;8:1355. doi: 10.3389/fimmu.2017.01355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menon V. Clustering single cells: a review of approaches on high-and low-depth single-cell RNA-seq data. Brief. Funct. Genomics. 2018;17:240–245. doi: 10.1093/bfgp/elx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alonzo E.S., Sant’Angelo D.B. Development of PLZF-expressing innate T cells. Curr. Opin. Immunol. 2011;23:220–227. doi: 10.1016/j.coi.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., Zhang Y., Gu W., Sun B. TH1/TH2 cell differentiation and molecular signals. Adv. Exp. Med. Biol. 2014;841:15–44. doi: 10.1007/978-94-017-9487-9_2. [DOI] [PubMed] [Google Scholar]
- 40.Gagliani N., Huber S. Basic aspects of T helper cell differentiation. Methods Mol. Biol. 2017;1514:19–30. doi: 10.1007/978-1-4939-6548-9_2. [DOI] [PubMed] [Google Scholar]
- 41.Cartwright T., Perkins N.D., L Wilson C. NFKB1: a suppressor of inflammation, ageing and cancer. FEBS J. 2016;283:1812–1822. doi: 10.1111/febs.13627. [DOI] [PubMed] [Google Scholar]
- 42.Riera-Sans L., Behrens A. Regulation of alphabeta/gammadelta T cell development by the activator protein 1 transcription factor c-Jun. J. Immunol. 2007;178:5690–5700. doi: 10.4049/jimmunol.178.9.5690. [DOI] [PubMed] [Google Scholar]
- 43.Park J.Y., DiPalma D.T., Kwon J., Fink J., Park J.H. Quantitative Difference in PLZF Protein Expression Determines iNKT Lineage Fate and Controls Innate CD8 T Cell Generation. Cell Rep. 2019;27:2548–2557.e4. doi: 10.1016/j.celrep.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovalovsky D., Uche O.U., Eladad S., Hobbs R.M., Yi W., Alonzo E., Chua K., Eidson M., Kim H.J., Im J.S., et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat. Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuda J.L., Zhang Q., Ndonye R., Richardson S.K., Howell A.R., Gapin L. T-bet concomitantly controls migration, survival, and effector functions during the development of Valpha14i NKT cells. Blood. 2006;107:2797–2805. doi: 10.1182/blood-2005-08-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vivier E., Ugolini S., Blaise D., Chabannon C., Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012;12:239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas S.Y., Hou R., Boyson J.E., Means T.K., Hess C., Olson D.P., Strominger J.L., Brenner M.B., Gumperz J.E., Wilson S.B., Luster A.D. CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J. Immunol. 2003;171:2571–2580. doi: 10.4049/jimmunol.171.5.2571. [DOI] [PubMed] [Google Scholar]
- 48.Fürst D., Neuchel C., Tsamadou C., Schrezenmeier H., Mytilineos J. HLA Matching in Unrelated Stem Cell Transplantation up to Date. Transfus. Med. Hemother. 2019;46:326–336. doi: 10.1159/000502263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozdemir Z.N., Civriz Bozdağ S. Graft failure after allogeneic hematopoietic stem cell transplantation. Transfus. Apheresis Sci. 2018;57:163–167. doi: 10.1016/j.transci.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Lee H.H., Kang H., Cho H. Natural killer cells and tumor metastasis. Arch. Pharm. Res. 2017;40:1037–1049. doi: 10.1007/s12272-017-0951-9. [DOI] [PubMed] [Google Scholar]
- 51.Liu H., Wang S., Xin J., Wang J., Yao C., Zhang Z. Role of NKG2D and its ligands in cancer immunotherapy. Am. J. Cancer Res. 2019;9:2064–2078. [PMC free article] [PubMed] [Google Scholar]
- 52.Paul S., Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017;8:1124. doi: 10.3389/fimmu.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Del Zotto G., Marcenaro E., Vacca P., Sivori S., Pende D., Della Chiesa M., Moretta F., Ingegnere T., Mingari M.C., Moretta A., Moretta L. Markers and function of human NK cells in normal and pathological conditions. Cytometry B Clin. Cytom. 2017;92:100–114. doi: 10.1002/cyto.b.21508. [DOI] [PubMed] [Google Scholar]
- 54.Ewen E.M., Pahl J.H.W., Miller M., Watzl C., Cerwenka A. KIR downregulation by IL-12/15/18 unleashes human NK cells from KIR/HLA-I inhibition and enhances killing of tumor cells. Eur. J. Immunol. 2018;48:355–365. doi: 10.1002/eji.201747128. [DOI] [PubMed] [Google Scholar]
- 55.Dominguez E., Lowdell M.W., Perez-Cruz I., Madrigal A., Cohen S.B.A. Natural killer cell function is altered by freezing in DMSO. Biochem. Soc. Trans. 1997;25:175S. doi: 10.1042/bst025175s. [DOI] [PubMed] [Google Scholar]
- 56.Li R., Johnson R., Yu G., McKenna D.H., Hubel A., LI RUI Preservation of cell-based immunotherapies for clinical trials. Cytotherapy. 2019;21:943–957. doi: 10.1016/j.jcyt.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo Y., Wang P., Liu H., Zhu Z., Li C., Gao Y. The state of T cells before cryopreservation: Effects on post-thaw proliferation and function. Cryobiology. 2017;79:65–70. doi: 10.1016/j.cryobiol.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Timmers M., Roex G., Wang Y., Campillo-Davo D., Van Tendeloo V.F.I., Chu Y., Berneman Z.N., Luo F., Van Acker H.H., Anguille S. Chimeric antigen receptor-modified T cell therapy in multiple myeloma: Beyond B cell maturation antigen. Front. Immunol. 2019;10:1613. doi: 10.3389/fimmu.2019.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y., Chen Z., Fang H., Wei R., Yu K., Jiang S., et al. Durable Remission Achieved from Bcma-Directed CAR-T Therapy Against Relapsed or Refractory Multiple Myeloma. Blood. 2018;132(Supplement 1):956. [Google Scholar]
- 60.Cohen A.D. CAR T Cells and Other Cellular Therapies for Multiple Myeloma: 2018 Update. Am. Soc. Clin. Oncol. Educ. Book. 2018;38:e6–e15. doi: 10.1200/EDBK_200889. [DOI] [PubMed] [Google Scholar]
- 61.Brudno J.N., Maric I., Hartman S.D., Rose J.J., Wang M., Lam N., Stetler-Stevenson M., Salem D., Yuan C., Pavletic S., et al. T cells genetically modified to express an anti–B-Cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J. Clin. Oncol. 2018;36:2267–2280. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen A.D., Garfall A.L., Stadtmauer E.A., Melenhorst J.J., Lacey S.F., Lancaster E., Vogl D.T., Weiss B.M., Dengel K., Nelson A., et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Invest. 2019;129:2210–2221. doi: 10.1172/JCI126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neelapu S.S., Tummala S., Kebriaei P., Wierda W., Gutierrez C., Locke F.L., Komanduri K.V., Lin Y., Jain N., Daver N., et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Candolfi M., Kroeger K.M., Muhammad A.K., Yagiz K., Farrokhi C., Pechnick R.N., Lowenstein P.R., Castro M.G. Gene therapy for brain cancer: combination therapies provide enhanced efficacy and safety. Curr. Gene Ther. 2009;9:409–421. doi: 10.2174/156652309789753301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Straathof K.C., Pulè M.A., Yotnda P., Dotti G., Vanin E.F., Brenner M.K., Heslop H.E., Spencer D.M., Rooney C.M. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomis D.C., Marktel S., Bonini C., Traversari C., Gilman M., Bordignon C., Clackson T. A Fas-based suicide switch in human T cells for the treatment of graft-versus-host disease. Blood. 2001;97:1249–1257. doi: 10.1182/blood.v97.5.1249. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe K., Kuramitsu S., Posey A.D., Jr., June C.H. Expanding the therapeutic window for CAR T cell therapy in solid tumors: The knowns and unknowns of CAR T cell biology. Front. Immunol. 2018;9:2486. doi: 10.3389/fimmu.2018.02486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lanza R., Russell D.W., Nagy A. Engineering universal cells that evade immune detection. Nat. Rev. Immunol. 2019;19:723–733. doi: 10.1038/s41577-019-0200-1. [DOI] [PubMed] [Google Scholar]
- 69.Ren J., Liu X., Fang C., Jiang S., June C.H., Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 2017;23:2255–2266. doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steimle V., Siegrist C.A., Mottet A., Lisowska-Grospierre B., Mach B. Regulation of MHC class II expression by interferon-γ mediated by the transactivator gene CIITA. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 71.Axelrod M.L., Cook R.S., Johnson D.B., Balko J.M. Biological consequences of MHC-II expression by tumor cells in cancer. Clin. Cancer Res. 2019;25:2392–2402. doi: 10.1158/1078-0432.CCR-18-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castro F., Cardoso A.P., Gonçalves R.M., Serre K., Oliveira M.J. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 2018;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Torikai H., Reik A., Soldner F., Warren E.H., Yuen C., Zhou Y., Crossland D.L., Huls H., Littman N., Zhang Z., et al. Toward eliminating HLA class I expression to generate universal cells from allogeneic donors. Blood. 2013;122:1341–1349. doi: 10.1182/blood-2013-03-478255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Braud V.M., Allan D.S., O’Callaghan C.A., Söderström K., D’Andrea A., Ogg G.S., Lazetic S., Young N.T., Bell J.I., Phillips J.H., et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 75.Cosman D., Müllberg J., Sutherland C.L., Chin W., Armitage R., Fanslow W., Kubin M., Chalupny N.J. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 76.Abrahimi P., Chang W.G., Kluger M.S., Qyang Y., Tellides G., Saltzman W.M., Pober J.S. Efficient gene disruption in cultured primary human endothelial cells by CRISPR/Cas9. Circ. Res. 2015;117:121–128. doi: 10.1161/CIRCRESAHA.117.306290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aftab B.T., Sasu B., Krishnamurthy J., Gschweng E., Alcazer V., Depil S. Toward “off-the-shelf” allogeneic CAR T cells. Adv. Cell Gene Ther. 2020;3:1–11. [Google Scholar]
- 78.Liu E., Marin D., Banerjee P., Macapinlac H.A., Thompson P., Basar R., Nassif Kerbauy L., Overman B., Thall P., Kaplan M., et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020;382:545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heczey A., Courtney A.N., Montalbano A., Robinson S., Liu K., Li M., Ghatwai N., Dakhova O., Liu B., Raveh-Sadka T., et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat. Med. 2020;26:1686–1690. doi: 10.1038/s41591-020-1074-2. [DOI] [PubMed] [Google Scholar]
- 80.Heczey A., Liu D., Tian G., Courtney A.N., Wei J., Marinova E., Gao X., Guo L., Yvon E., Hicks J., et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood. 2014;124:2824–2833. doi: 10.1182/blood-2013-11-541235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deniger D.C., Moyes J.S., Cooper L.J.N. Clinical applications of gamma delta T cells with multivalent immunity. Front. Immunol. 2014;5:636. doi: 10.3389/fimmu.2014.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y.-R., Zhou Y., Kramer A., Yang L. Engineering stem cells for cancer immunotherapy. Trends Cancer. 2021 doi: 10.1016/j.trecan.2021.08.004. S2405-8033(21)00171-0. [DOI] [PubMed] [Google Scholar]
- 83.Zhou Y., Li Y.-R., Zeng S., Yang L. Methods for Studying Mouse and Human Invariant Natural Killer T Cells. Methods Mol. Biol. 2021;2388:35–57. doi: 10.1007/978-1-0716-1775-5_4. [DOI] [PubMed] [Google Scholar]
- 84.Kunii N., Horiguchi S., Motohashi S., Yamamoto H., Ueno N., Yamamoto S., Sakurai D., Taniguchi M., Nakayama T., Okamoto Y. Combination therapy of in vitro-expanded natural killer T cells and α-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009;100:1092–1098. doi: 10.1111/j.1349-7006.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Motohashi S., Nagato K., Kunii N., Yamamoto H., Yamasaki K., Okita K., Hanaoka H., Shimizu N., Suzuki M., Yoshino I., et al. A phase I-II study of α-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J. Immunol. 2009;182:2492–2501. doi: 10.4049/jimmunol.0800126. [DOI] [PubMed] [Google Scholar]
- 86.Yamasaki K., Horiguchi S., Kurosaki M., Kunii N., Nagato K., Hanaoka H., Shimizu N., Ueno N., Yamamoto S., Taniguchi M., et al. Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin. Immunol. 2011;138:255–265. doi: 10.1016/j.clim.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 87.Gordy L.E., Bezbradica J.S., Flyak A.I., Spencer C.T., Dunkle A., Sun J., Stanic A.K., Boothby M.R., He Y.W., Zhao Z., et al. IL-15 regulates homeostasis and terminal maturation of NKT cells. J. Immunol. 2011;187:6335–6345. doi: 10.4049/jimmunol.1003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bae E.A., Seo H., Kim B.S., Choi J., Jeon I., Shin K.S., Koh C.H., Song B., Kim I.K., Min B.S., et al. Activation of NKT cells in an anti-PD-1–resistant tumor model enhances antitumor immunity by reinvigorating exhausted CD8 T cells. Cancer Res. 2018;78:5315–5326. doi: 10.1158/0008-5472.CAN-18-0734. [DOI] [PubMed] [Google Scholar]
- 89.Verzeletti S., Bonini C., Marktel S., Nobili N., Ciceri F., Traversari C., et al. Herpes Simplex Virus Thymidine Kinase Gene Transfer for Controlled Graft-versus-Host Disease and Leukemia: Clinical Follow-up and Improved New Vectors. Hum. Gene Ther. 1998;9:2243–2251. doi: 10.1089/hum.1998.9.15-2243. [DOI] [PubMed] [Google Scholar]
- 90.Lee N., Llano M., Carretero M., Ishitani A., Navarro F., López-Botet M., Geraghty D.E. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl. Acad. Sci. USA. 1998;95:5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The genomics data generated during this study are available from the public repository Gene Expression Omnibus Database: GSE164425, GSE164500.
-
•
All data reported in this manuscript are available from the Lead Contact without restriction
-
•
No custom computer code was reported in this manuscript.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request.