In December 2020, Her Majesty's Assistant Coroner for Inner South London, UK, Philip Barlow, ruled that traffic-related particulate matter and nitrogen dioxide were contributary factors in the death of a young girl from asthma whose house was on London's South Circular Road [1]. The coroner's subsequent Prevention of Future Deaths report highlights the need for all health professionals to communicate the risks of air pollution to parents and children with asthma. Generic advice is available for use in consultations.
Short abstract
Using an advice-based intervention in a cohort of children with asthma, designed to reduce personal pollution exposure, a mean reduction in black carbon exposure of 34% was found, the primary reduction happening within the home environment https://bit.ly/301Xemt
To the Editor:
In December 2020, Her Majesty's Assistant Coroner for Inner South London, UK, Philip Barlow, ruled that traffic-related particulate matter and nitrogen dioxide were contributary factors in the death of a young girl from asthma whose house was on London's South Circular Road [1]. The coroner's subsequent Prevention of Future Deaths report highlights the need for all health professionals to communicate the risks of air pollution to parents and children with asthma. Generic advice is available for use in consultations. For example, the British Lung Foundation recommends that on days of high (background) air pollution, “avoid main roads and busy streets on high pollution days”, and on days where levels of pollution are low, that “it is a good idea to avoid spending long periods of time in places where pollution levels build up, such as busy roads” [2]. The question for health professionals is, does air pollution mitigation advice reduce children's personal exposure? In this pilot study, we therefore aimed to assess whether individualised advice regarding reducing pollution exposure from 15–24-h personal monitoring of black carbon (a marker of diesel-exhaust particulate matter) reduces exposure of children with asthma to black carbon particulate matter.
Children and adolescents (7–16 years of age) with a diagnosis of asthma and on British Thoracic Society treatment step ≥2 were recruited from the Children's Asthma Clinic at the Royal London Hospital, Barts Health NHS Trust. After informed parental consent and child assent, each child underwent two 24-h periods of black carbon monitoring between September 2015 and December 2017. The baseline period was before mitigation advice and “post-advice” was after 1 week. 24-h black carbon was measured using a microAeth AE51 (AethLabs, San Francisco, CA, USA) set at a 30-s recording period. Children completed an activity diary to record the different exposure environments during the day (home, school, commute and “other”). The aethalometer records a value of black carbon each 30 s and data were expressed as mean (nanograms per cubic metre per 30 s) exposure and mean exposure for different environments.
An action plan was provided during the intervention visit that included generic actions within the home (use of extractor fan, opening windows at strategic timepoints, using environmentally safe cleaning products and dust removal strategies), on the commute to school (altering route to quieter areas, reduction in car use/combining trips and using public transport) and at school (avoiding standing near the busy road at pick-up time and using low-traffic exits), alongside an explanation of which ones were appropriate based on baseline black carbon exposure data, time activity patterns and preferred mode of travelling to school.
All statistical analyses were performed using GraphPad Prism 7.04 (GraphPad Software, La Jolla, CA, USA). Data were assessed for normality using the D'Agostino and Pearson normality test. Non-normally distributed data are summarised as median (interquartile range). Normally distributed data are expressed as mean±sem and compared by paired t-test. Data were excluded if there was <20 h of recordings for the same time period in both the baseline and post-advice periods. A secondary analysis limited to nonsmoking homes was planned to exclude the impact of secondary smoke exposure. The study was approved by the National Research Ethics Service Committee London – Harrow (ref. 15/LO/0799).
In total, 29 asthmatic children were recruited. 14 (48%) children had incomplete data (<20 h of time-matched recordings) because of battery failure. Complete paired data were obtained from 15 children (52%). The majority of children were from nonsmoking households (69%), 60% of families used a gas stove for cooking and 40% primarily walked to school.
At baseline, the median proportion of black carbon exposure at home was 41.0 (29–54)%, for commute to school was 9.7 (1.8–22.1)%, 34.5 (23.5–45.5)% for school and 4.4 (0–17)% for other. Black carbon concentrations at baseline were: overall, 1752 (1232–2231) ng·m−3; home, 1164 (658–2231) ng·m−3; commute, 4204 (2522–7534) ng·m−3; and school, 1863 (876–3887) ng·m−3.
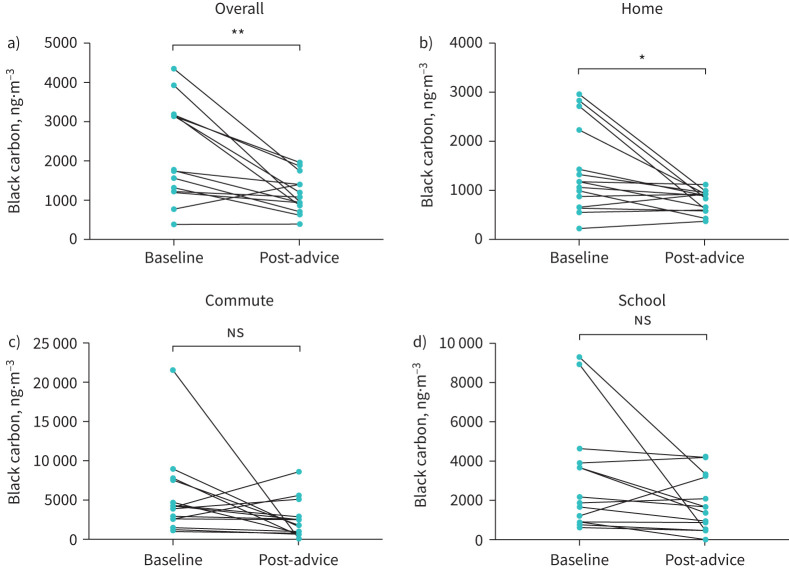
Since differences between baseline and post-advice black carbon were normally distributed, paired t-test analysis was used. Compared with baseline, post-advice black carbon was reduced (change −1038±265 ng·m−3, n = 15; p<0.01) (figure 1a) with a mean reduction in black carbon exposure of 34.4±10.05%. There was no difference in the time spent in the different microenvironments between baseline and post-advice. For the different microenvironments, there was a significant reduction in black carbon at home (change −603.5±212 ng·m−3, p=0.013) (figure 1b). By contrast, there was no reduction in mean black carbon for either the commute (change −2857±1599 ng·m−3, p=0.095) (figure 1c) or at school (change −1279±680 ng·m−3, p=0.081) (figure 1d).
FIGURE 1.
Change in black carbon between baseline and post-advice for a) mean overall 24 h, b) home, c) commute and d) school. ns: not significant. *: p<0.05; **: p<0.01.
An analysis limited to nonsmoking homes also demonstrated a significant reduction. In this subgroup, compared with baseline, post-advice black carbon was reduced (change −1349±312 ng·m−3 per 30 s, n = 11; p<0.01) and there was a significant reduction in black carbon at home (change −778.8±268.4 ng·m−3 per 30 s, p=0.013).
We found that the MicroAeth generated high-quality individual exposure data, with expected black carbon peaks during children's commute to school. However, the limited battery life of the device resulted in significant loss of paired data. Despite this, paired data were available for 15 children. In this subgroup, the proportion of black carbon exposure in the different exposure environments was broadly compatible with our small study of six healthy children that informed a UNICEF report [3]. This is not the first time that microenvironment exposures have been reported in children with asthma, such as those reported by Rabinovitch et al. [4] in 2016, and Hwang and Lee [5] in 2018. However, it is the first time personal black carbon exposure profiles have been used to guide advice on exposure-mitigation options in children with asthma across multiple microenvironments.
The reduction in mean 24-h black carbon after advice provides preliminary evidence of the effectiveness of black carbon exposure mitigation advice to children and parents. However, analysis of black carbon change by microenvironment suggests that the overall reduction in black carbon was due to reduced indoor exposure and not reduced exposure during the commute. Although we did not record specific indoor changes, parents reported that they tried to keep children away from the kitchen during cooking. Indeed, previous studies have investigated the use of home monitoring to reduce smoking within the home [6], but in the present study, changes in smoking behaviour is unlikely to be a major factor, since a significant reduction in mean 24-h black carbon exposure and black carbon home exposure was present in the analysis limited to the 11 nonsmoking households. The absence of change in black carbon post-advice on commute exposure may either be due to lack of study power or that children had little option in the way they travelled to school. To determine effects on commute will need larger studies.
The study had its limitations. Firstly, we used black carbon monitors rather than particulate matter (for which health guidelines are written). This was due to effectiveness and usability of monitors, with the MicroAeth known as a well validated monitor for personal monitoring [7, 8]. Black carbon is known to correlate strongly with traffic-related air pollutant and household particulate matter [9]. Secondly, while we asked for feedback on the interventions, this was not related back to individual datasets. This would be important in future studies intending to do this on a wider scale. Finally, the datasets usable for analysis were strongly impacted by the battery life of the monitors. We have now developed good, structured advice that ensures that the monitors last the full monitoring period, which can be implemented in future studies.
In summary, using personal black carbon monitoring and feedback, this pilot study suggests that it is possible to reduce black carbon exposure of asthmatic children. Our data suggest that exposure indoors may be more amenable to this intervention than outdoor exposure.
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: J. Grigg reports personal fees from GSK, Vifor Pharma, AstraZeneca, Novartis and Omron outside the submitted work. A. Whitehouse and L. Koh have no disclosures.
Support statement: This study was supported by an Asthma UK PhD studentship. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.London Inner South Coroner's Court . Inquest touching the death of Ella Roberta Adoo Kissi-Debrah. https://www.innersouthlondoncoroner.org.uk/news/2020/nov/inquest-touching-the-death-of-ella-roberta-adoo-kissi-debrah. Date last updated: 18 November 2020. Date last accessed: 16 December 2020.
- 2.British Lung Foundation . How can I protect myself from air pollution? https://www.blf.org.uk/support-for-you/air-pollution/tips. Date last updated April 2020. Date last accessed: 16 December 2015.
- 3.UNICEF UK . The toxic school run. London, UNICEF UK, 2019. [Google Scholar]
- 4.Rabinovitch N, Adams CD, Strand M, et al. Within-microenvironment exposure to particulate matter and health effects in children with asthma: a pilot study utilizing real-time personal monitoring with GPS interface. Environ Health 2016; 15: 96. doi: 10.1186/s12940-016-0181-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang Y, Lee K. Contribution of microenvironments to personal exposures to PM. AtmEn 2018; 175: 192–198. [Google Scholar]
- 6.Vardavas C, Semple SRD, O'Donnell R, et al. Measuring for change: a multi-centre pre-post trial of an air quality feedback intervention to promote smoke-free homes. Environ Int 2020; 140; 105738. doi: 10.1016/j.envint.2020.105738 [DOI] [PubMed] [Google Scholar]
- 7.Aethlabs . microAeth®/AE51. https://aethlabs.com/microaeth/ae51/overview. Date last updated: 2021. Date last accessed: 9 February 2021.
- 8.Cai J, Yan B, Ross J, et al. Validation of MicroAeth® as a black carbon monitor for fixed-site measurement and optimization for personal exposure characterization. Aerosol Air Qual Res 2014; 14: 1–9. doi: 10.4209/aaqr.2013.03.0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitehouse A, Barratt B, Grigg J. Does modelled exposure of children to particulate matter air pollution for the school address reflect personal exposure to black carbon? Eur Respir J 2016; 48: Suppl. 60, PA1320. [Google Scholar]