Abstract
We report here the characterization of a bypass suppressor of pab1Δ which leads to a fourfold stabilization of the unstable MFA2 mRNA. Cloning of the wild-type gene for that suppressor reveals that it is identical to PAT1 (YCR077c), a gene whose product was reported to interact with Top2p. PAT1 is not an essential gene, but its deletion leads to a thermosensitive phenotype. Further analysis has shown that PAT1 is allelic with mrt1-3, a mutation previously reported to affect decapping and to bypass suppress pab1Δ, as is also the case for dcp1, spb8, and mrt3. Coimmunoprecipitation experiments show that Pat1p is associated with Spb8p. On sucrose gradients, the two proteins cosediment with fractions containing the polysomes. In the absence of Pat1p, however, Spb8p no longer cofractionates with the polysomes, while the removal of Spb8p leads to a sharp decrease in the level of Pat1p. Our results suggest that some of the factors involved in mRNA degradation could be associated with the mRNA that is still being translated, awaiting a specific signal to commit the mRNA to the degradation pathway.
A general pathway of mRNA degradation in the yeast Saccharomyces cerevisiae has been described in which mRNAs are deadenylated prior to decapping and then degraded in the 5′-to-3′ direction by the exonuclease Xrn1p (4, 12). The decapping enzyme has been identified in yeast and named Dcp1p (5). In most yeast strains, depletion of Dcp1p leads to a slow-growth phenotype and to stabilization of mRNAs that are accumulated in a capped and oligoadenylated form (5). However, to become functional, Dcp1p requires the activity of Dcp2p, a putative pyrophosphatase (14). Recently, a polypeptide (Mr 70,000) copurifying with Dcp1p has been identified as Ssa1p or Ssa2p, Hsp70 family members (50). Interestingly, two mutations that inhibit decapping, vps16 and mrt1, enhance the interaction of Ssa1p or Ssa2p with Dcp1p. This observation suggests that Ssa1p or Ssa2p could be involved in regulating the activity of Dcp1p.
In addition to Dcp1p and Dcp2p, other factors might regulate the decapping activity, since several mutations have been isolated that lead to stabilization of capped, oligoadenylated mRNAs. The best characterized of these factors is Spb8p (6), a protein containing an Sm-like domain that has also been referred to as Lsm1p (40). The Sm motif is found in a set of proteins that interact with the small nuclear RNAs involved in mRNA splicing. However, Spb8p is distinguished from other Sm or Lsm proteins by not being associated with any known small nuclear RNA (29, 38). Mutations within three other loci named mrt1, mrt3, and vps16 lead to an accumulation of capped, oligoadenylated mRNAs (21, 50). While VPS16 has been characterized (22, 50), the genes for mrt1 and mrt3 have not yet been cloned.
Several lines of evidence indicate the existence of a link between mRNA translation and degradation (23). It is now well established that the two structures present on the ends of an mRNA, the cap at the 5′ end and the poly(A) tail at the 3′ end, act synergistically to enhance mRNA translation (17, 41). The cap structure and the poly(A) tail also play a major role in mRNA stability, since they are the target of the first steps of mRNA degradation. The poly(A)-binding protein Pab1p establishes a bridge between the poly(A) tail and the cap due to its interaction with the initiation factor eIF4G. This interaction facilitates the recruitment of the 40S ribosomal subunit onto the mRNA, thus allowing translation initiation to proceed (41–43). Pab1p also plays a role in mRNA stability, and in its absence mRNA is decapped before being deadenylated (9). Relationships between translation and degradation are further supported by the observation that mutations in the 5′ region of PGK1 mRNA that inhibit translation also stimulate its decay (26, 30). Moreover, mutations in several of the genes coding for translation initiation factors lead to increased rates of deadenylation and decapping (39).
A search for suppressors of a deletion of the PAB1 gene (spb) in yeast has proven to be a powerful tool to isolate factors involved either in ribosome synthesis or in mRNA turnover (6, 35). Several mutations that alter mRNA decapping turned out to suppress a pab1Δ mutation, as is the case for dcp1, mrt1, and mrt3 (21). Recently, we reported the isolation of spb8-2 by using a transposon insertion mutagenesis of the yeast genome to isolate new spb mutants (6). During the course of this analysis, we found that some spb mutants were not linked to the transposon insertion but were linked to a secondary phenotype that could be used for their subsequent cloning. Here we report the characterization of an spb mutant that turned out to be identical to mrt1 and to the previously characterized gene PAT1. We show that Pat1p is associated with Spb8p and that the two proteins cofractionate with polysomes on sucrose gradients.
MATERIALS AND METHODS
Microbiological methods and recombinant DNA work.
The strains used in this study were handled by standard techniques (20). They were either constructed for this study (Table 1) from a derivative of W303 (3) or obtained from R. Parker: yRP1066 and yRP1067 (21), yRP1070 and yRP1062 (5), and yRP1070 transformed with pRP801 (26). 5-Fluoroorotic acid (5-FOA) was bought from Toronto Research Chemicals (Toronto, Ontario, Canada). 5-FOA was used to counterselect the URA3 marker, i.e., in our case, to select for the loss of the URA3 PAB1 plasmid. Yeast transformation was performed by using the lithium acetate method (18).
TABLE 1.
Yeast strains constructed for this study
| Strain | Relevant genotypea | Plasmid |
|---|---|---|
| YCB461 | MATa trp1Δ | pRP485 |
| YBL4145 | MATa spb10-1 pab1Δ::HIS3 | |
| YCB557 | MATα spb10-1 | |
| YCB451 | MATα spb10-1 | pFL44-A1 |
| YCB452 | MATα spb10-1 | pFL44-A1K |
| YCB571 | MATa/α pat1Δ::TRP1/PAT1 | |
| YCB499 | MATa pat1Δ::TRP1 | |
| YCB467 | MATa pat1Δ::TRP1 pab1Δ::HIS3 | pAS77 |
| YCB573 | MATa/α pat1Δ::TRP1/spb10-1 | |
| YCB572 | MATa/α pat1Δ::TRP1/pat1Δ::TRP1 | |
| YCB482 | MATα pat1Δ::TRP1 | pRP 485 |
| YCB574 | MATa/α mrt1-3 pat1Δ::TRP1 | |
| YCB575 | MATa/α mrt3-1 pat1Δ::TRP1 | |
| YCB562 | MATa dcp1Δ::URA3 pat1Δ::TRP1 | |
| YCB563 | MATα xrn1Δ::URA3 pat1Δ::TRP1 | |
| YBL4452 | MATa spb8Δ::LEU2 | |
| YCB568 | MATa spb8Δ::LEU2 pat1Δ::TRP1 | |
| YCB536 | MATα PAT1-protA::TRP1, pep4::URA3 |
All strains are ade2-1 his3-11 leu2-3,112 trp1-1 or trp1Δ ura3-1 can1-100, unless otherwise stated.
PCR was performed with Taq DNA polymerase (Gibco-BRL), and the products were purified on Sepharose CL6B spin columns (Pharmacia) prior to use. The yeast genomic DNA library prepared on a 2μm-based plasmid containing the URA3 marker (pFL44) (7) was a gift of F. Lacroute. Two clones were isolated that were able to complement the growth defect of the spb10 mutants at 37°C. Plasmids were extracted, analyzed, and reintroduced into two different alleles of spb10. Both plasmids were able to complement the thermosensitive phenotype of the two alleles. The smaller of the two plasmids (pFL44-A1) was further analyzed, and the sequence of the two extremities of the genomic fragment was determined. To destroy the PAT1 gene on the complementing plasmid, a frameshift was introduced by digesting plasmid pFL44-A1 with the restriction enzyme EcoNI, filling the ends with the Klenow enzyme, and religating to give plasmid pFL44-A1K. To construct the CEN-based plasmid pFL36-A1, a BamHI-SphI fragment containing the genomic insert was prepared from pFL44-A1 and cloned into pFL36 (7).
Disruption of PAT1 was performed by using a PCR-based strategy with the diploid strain BMA64 (3) and with oligonucleotides Δpat1-U (5′-AAGGAAGC AAAGGTTTTAACCGGAAGTAAGAGCAGCAAGAAGCACTAGCACTG ATGCGGTATTTTCTCCT-3′) and Δpat1-L (5′-GGGAGAAAAAAAAATAC ATGCGTAAGTACATTAAAATTACAGGAAAAATCCGGGTGTTGGCG GGTGTC-3′) to amplify a TRP1 cassette. Disruption was confirmed by Southern hybridization. Sporulation of the Trp+ diploid cells led to four viable spores for each tetrad analyzed, with two slow-growing and two fast-growing spores. The two slow-growing spores failed to grow at 37°C, and the TRP1 marker segregated with the slow-growing and the thermosensitive phenotype in two successive backcrosses. Tagging of Pat1p with two immunoglobulin G-binding domains of Staphylococcus aureus protein A (Pat1-protAp) was performed by a PCR-based strategy with oligonucleotides PAT1-A-U (5′-TAAACGTTATGGGGTTGGTG TATCGCGATGGTGAAATATCAGAACTAAAGAAGCTGGAGCTCAAA AC-3′) and PAT1-A-L (5′-AGAAAAAAAAATACATGCGTAAGTACATTAAAATTACAGGAAAAATCTTATACGACTCACTATAGGG-3′) and plasmid pBS1173 as a template (32). A pep4Δ::URA3 mutation was introduced by genetic crossing to reduce the degradation of the tagged protein in native extracts.
To disrupt SPB8, a plasmid was constructed that contains the LEU2 gene flanked by the 5′ (450 bp) and the 3′ (245 bp) region of the SPB8 gene. These two regions were amplified by PCR with oligonucleotides oAS319 (6) plus oRB44 (5′-CGGGATCCCATATGTTTTGGTGAATTAATTCGATTCG-3′) and oRB45 (5′-CGGGATCCTAAGAATTCGAAAGAAAAACACAATACTAC- 3′) plus oAS320 (6), respectively, and cloned into the SalI-EcoRI sites of pBluescript-SK (Stratagene), creating plasmid pRB35. The LEU2 gene was amplified from YIplac128 (19) with oligonucleotides oRB49 (5′-GGAATTCCATATGAATAGGCGTATCACCAGCGG-3′) and oRB50 (5′-CGGAATTCCACCGAAACGCGCGAGACGAAAGGG-3′) and cloned as a NdeI-EcoRI fragment into pRB35 to yield pRB36. A SalI-XbaI fragment containing the LEU2 gene flanked by the upstream and downstream regions of SPB8 was prepared from that plasmid and used to transform the W303 haploid strain, since the gene is not essential for cell growth (6). To produce a recombinant Spb8 protein, the SPB8 open reading frame (ORF) was amplified by PCR from yeast genomic DNA with oRB46 (5′-CGGGATCCTCTGCAAATAGCAAGGACA-3′ and oRB47 (5′-GCGAATTCTTAGTACATGTCAGATTTATG-3′) and cloned as a BamHI-EcoRI fragment into the pGEX-2T expression vector (Pharmacia) to give plasmid pRB48.
RNA work.
RNA extraction was performed as previously described (11). Transcriptional shutoff experiments were done as described previously (13) with strains containing plasmid pRP485. RNA samples were separated by electrophoresis on 1.5% agarose slab gels. Capillary transfer to positively charged nylon was performed by using 40 mM NaOH as a transfer solution. Hybridization was done as described previously (10) with oRP140 (9) as a probe to reveal specifically MFA2pG species or with a PCR fragment corresponding to the scR1 RNA that served as an internal loading standard (15). This fragment was PCR amplified with scR1-U (5′-GGCTGTAATGGCTTTCTG-3′) and scR1-L (5′-CCTTTGCTGACGCTGGAT-3′) with genomic DNA as a template. Quantification was performed with a PhosphorImager (Molecular Dynamics).
Preparation of a rabbit antibody raised against recombinant Spb8p.
Plasmid pRB48 was transformed into the BL21 bacterial strain to express a fusion protein consisting of the glutathione S-transferase followed by a thrombin cleavage site and the Spb8p sequence. A bacterial lysate was obtained and incubated with glutathione-Sepharose 4B resin (Pharmacia). After two washes, the beads were treated with thrombin to release the recombinant Spb8p. A 1-liter volume of bacterial culture gave ca. 1.8 mg of recombinant protein. Rabbits were injected five times with 100 μg of recombinant protein per injection over a 4-month period before bleeding (Berkeley Antibody Co., Berkeley, Calif.).
Protein extraction from yeast cells and polysome analysis.
Protein extracts were obtained from mid-log-phase culture by trichloroacetic acid (TCA) extraction. TCA was directly added to the culture medium to reach a final concentration of 20%. Yeast cells were harvested by centrifugation, washed in 10% TCA, and frozen in liquid nitrogen. Cells were broken in 10% TCA with zirconium beads (Biospec Products, Bartlesville, Okla.) for 10 min on a multimixer (VWR, South Plainfield, N.J.). Extracts were collected and centrifuged, and the pellets were resuspended in Laemmli loading buffer (25).
Native protein extracts were prepared from mid-log-phase cells that were harvested by centrifugation, washed with ice-cold buffer (0.9% NaCl, 1 mM NaN3, 10 mM EDTA, 50 mM NaF), and frozen in liquid nitrogen. Zirconium beads were added with ice-cold breaking buffer (50 mM Tris-Cl [pH 7.5], 15 mM MgCl2, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1 mM dithiothreitol) supplemented with a complete protease inhibitor cocktail (Boehringer Mannheim) plus 0.1 mM phenylmethylsulfonyl fluoride, and cells were lysed by vortexing at 4°C for 5 min on a multimixer. Extracts were clarified three times by centrifugation at 4°C for 10 min at 16,000 × g.
Polysome analysis was performed as described previously (24), except that extracts were prepared in 20 mM HEPES (pH 7.4)–2 mM magnesium acetate–100 mM potassium acetate–0.5 mM dithiothreitol–100 μg of cycloheximide per ml. The cells were broken by alternate vortexing at top speed for 30 s and cooling on ice for 30 s for a total of 6 min.
Protein analysis, Western blotting, and coimmunoprecipitation.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described earlier (25). Gels were electroblotted onto a nitrocellulose membrane (Protran; Schleicher & Schuell) with a semidry blotter (Owl Scientific, Woburn, Mass.) for 2 h at 1.5 mA/cm2 in 30 mM glycine–48 mM Tris base–0.037% SDS–20% ethanol. The membranes were saturated in 25 mM Tris-Cl (pH 8)–2.6 mM KCl–13.8 mM NaCl–0.05% Tween–3% nonfat milk. Antibodies were incubated in the same medium for 1 h at room temperature. Primary antibodies were added at the following dilutions: anti-Pat1p (33), 1/1,000; anti-Pab1p (2), 1/10,000; rabbit anti-Spb8p (this work), 1/750; anti-Qsr1p (47), 1/1,000; and anti-Flag (Kodak), 1/1,000. Anti-mouse and anti-rabbit antibodies coupled to horseradish peroxidase were obtained from Sigma. Spb8p was immunoprecipitated in two steps. First, 2-mg samples of native protein extracts were incubated on ice for 1 h with 4 μl of a preimmune rabbit serum in the presence of 5 mg of bovine serum albumin (BSA) per ml. Then 20 μl of a 50% slurry of protein A-Sepharose (Pharmacia) was added, and the mixture was incubated for 1 h at 4°C on a wheel. Supernatant was recovered by centrifugation, treated with 4 μl of anti-Spb8p, and incubated as described above. Beads were recovered by centrifugation for 1 min at 400 × g and washed five times with 0.3 ml of breaking buffer. To analyze the proteins, Laemmli loading buffer was added before the samples were denatured at 90°C for 10 min. To precipitate Pat1-protAp, native extracts were incubated with 20 μl of a 50% slurry of immunoglobulin G-Sepharose beads (Pharmacia) for 2 h at 4°C. Then the beads were washed and processed as described above.
RESULTS
Identification of spb10, a bypass suppressor of pab1Δ.
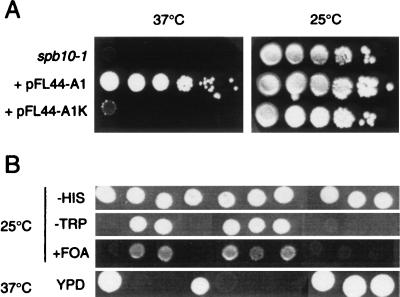
We previously reported the isolation of new bypass suppressors of a deletion of the PAB1 gene (spb) by using a transposon mutagenesis strategy (6). However, genetic analysis of the numerous spb mutations thus isolated revealed that several of them were not linked to the insertion of the transposon. Three of these spontaneous spb mutations were found to be thermosensitive and were thus further characterized. After genetic analysis, all three mutations were found to be recessive and allelic for the thermosensitive phenotype and the spb phenotype and were named spb10-1, spb10-2, and spb10-3 (Fig. 1A, first row, and data not shown). A yeast genomic DNA fragment borne on a URA3 2μm-based multicopy plasmid was isolated that was able to complement the growth defect of the spb10 mutants at 37°C (Fig. 1A, second row). The sequence of the insert reveals that it contains one characterized gene, PAT1, and two truncated ORFs. Inactivation of PAT1 by introducing a frameshift mutation (pFL44-A1K) results in the loss of the complementation activity (Fig. 1A, third row), providing strong support that PAT1 is responsible for the complementation of the spb10 mutation. When transferred to a CEN-based vector (pFL36-A1), PAT1 was still able to support the growth of an spb10-1 strain at 37°C and to suppress the spb phenotype (data not shown). This result demonstrates that PAT1 had not been isolated as a multicopy suppressor of spb10 and is capable of complementing the spb10 mutation.
FIG. 1.
Characterization of the spb10 mutation and cloning of the PAT1 gene. (A) Complementation testing for thermosensitivity of the spb10-1 allele with various plasmids. Yeast cultures were spotted as serial 10-fold dilution onto yeast-peptone-dextrose (YPD) plates and grown for 3 days either at the permissive (25°C, right panel) or at the restrictive (37°C, left panel) temperature. First row, spb10-1 mutant strain; second row, the same strain transformed with the pFL44-A1 plasmid that contains the PAT1 gene; third row, the spb10-1 strain transformed with the pFL44-A1K plasmid that contains the mutated PAT1 gene. (B) Deletion of PAT1 leads to thermosensitivity and to an spb phenotype. A diploid strain, pat1Δ::TRP1/PAT1 pab1Δ::HIS3/PAB1 (pAS77 [pPAB1 URA3 CEN]), was constructed by genetic crossing and sporulated, and tetrads were dissected and analyzed. His+ spores were selected, and their growth was tested under different conditions as indicated.
pat1Δ is allelic to spb10-1 and bypass suppresses pab1Δ.
PAT1 was previously identified as part of a yeast genome-sequencing project (33). Antibodies raised against an N-terminal peptide of the corresponding protein (anti-Ycr77c) detected a cytosolic protein (Mr 97,000). A partial deletion of this ORF led to a slightly reduced growth rate at 37°C. Later, the same gene was identified in a two-hybrid screen for proteins interacting with topoisomerase II and named PAT1 (for “protein associated with topoisomerase II”) (49). In that study, the authors found that deletion of about half of the gene was associated with a thermosensitive phenotype. We decided to replace the entire PAT1 gene with a TRP1 marker by using a PCR-based strategy (3) and found that deletion of PAT1 leads to a slow-growth and thermosensitive phenotype. Then, to determine whether the pat1Δ mutation was able to bypass the PAB1 gene, a diploid strain heterozygous for the two mutations pat1Δ::TRP1 and pab1Δ::HIS3 containing the pAS77 plasmid (pPAB1 URA3 CEN) (37) was constructed by standard genetic crossing. After sporulation and tetrad dissection, the growth of the His+ spores (i.e., with PAB1 deleted) was tested on various media (Fig. 1B). The Trp− clones (PAT1) were able to grow at 37°C, while the Trp+ (pat1Δ::TRP1) were unable to grow at the nonpermissive temperature, as expected. The pat1Δ::TRP1 clones were all able to form colonies on 5-FOA-containing plates, demonstrating their ability to lose the plasmid containing the PAB1 gene (Fig. 1B, third row). Finally, an spb10-1/pat1Δ diploid strain was constructed by genetic crossing and found to be thermosensitive. This strain was sporulated and dissected, and the four progeny spores of every tetrad were found to be thermosensitive (data not shown). This result demonstrates that pat1Δ::TRP1 is allelic with spb10-1. We conclude from these experiments that PAT1 is the wild-type gene corresponding to the spb10 mutants and that its deletion leads to thermosensitivity and an spb phenotype.
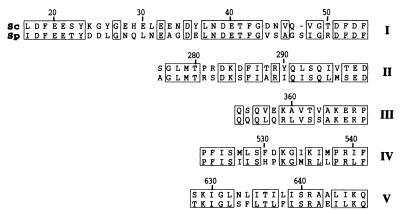
As previously reported, a search for typical patterns in Pat1p reveals the presence of only a single EF hand, a calcium-binding motif, between residues 648 and 660 (33). Another characteristic of Pat1p is the presence of a region enriched in proline and histidine between residues 114 and 200. Such regions are present in numerous proteins in the databases, although there is still no function assigned to these elements. Searching the current databases for potential homologues of Pat1p from S. cerevisiae revealed the existence of an ORF from Schizosaccharomyces pombe that could encode a 744-residue protein, compared to the 797-residue Pat1p from S. cerevisiae. The two proteins have about 25% similarity between the N and C termini. However, the similarity reaches about 45% when only a central domain of 440 residues is considered. Five motifs that are particularly well conserved between the two yeast proteins can be distinguished (Fig. 2).
FIG. 2.
Sequence comparison between Pat1p from budding and fission yeasts. The sequence of Pat1p from S. cerevisiae (Sc) and a translated ORF from S. pombe (Sp) (accession number CAA17064) were aligned (Clustal W [46]). Five regions of higher similarity were chosen and named motifs I through V (shown in Roman numerals on the right). Residues that are either identical or chemically equivalent are boxed. Numbers at the top refer to the Pat1p sequence from S. cerevisiae. The dash represents a gap inserted in motif I to optimize the alignment.
Deletion of PAT1 does not affect ribosome biogenesis but stabilizes MFA2 mRNA.
The majority of the bypass suppressors of pab1Δ are impaired in 60S ribosomal subunit synthesis (31, 35, 36, 51). To determine whether Pat1p was involved in ribosome biogenesis, we monitored the accumulation of pre-rRNA processing intermediates in the spb10 mutant strain. Northern blot analysis was performed with oligonucleotide probes complementary to various spacer sequences of the pre-rRNA. No difference was observed in a pat1Δ strain compared to an isogenic wild-type strain (data not shown). As a different way to study ribosome biogenesis, ribosome subunit profiles were examined in the pat1Δ strain and found to be similar to those of a wild-type strain (data not shown). These observations led us to the conclusion that PAT1 is not required for ribosome biogenesis.
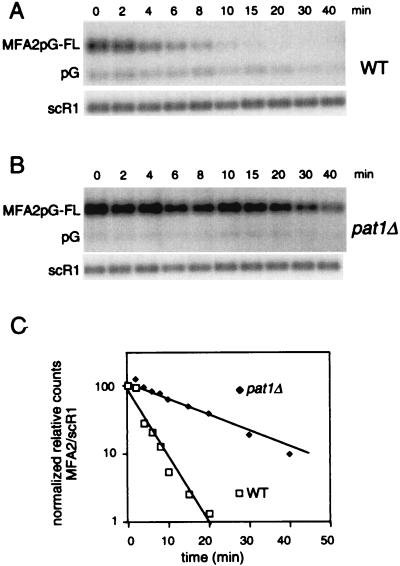
A second group of bypass suppressors of pab1Δ, which affect mRNA decay by stabilizing the cap structure, have been identified (6, 21). To determine whether the absence of Pat1p affects mRNA decay, we measured the half-life of the reporter MFA2pG mRNA in wild-type and pat1Δ strains. Insertion of a poly(G) tract in the 3′ untranslated region of MFA2 mRNA leads to the formation of a stable secondary structure that inhibits exonucleases, allowing the detection of an intermediate degradation product (called pG hereafter) (13). In these experiments, the MFA2pG reporter gene was under the control of the GAL1 promoter, which allows rapid transcriptional repression. Northern blot analysis shows that MFA2-pG mRNA has a rapid turnover in a wild-type strain with a half-life of about 3.5 min, while half-life is increased about fourfold in the pat1Δ strain (Fig. 3). In addition, the pG fragment was barely detected in the pat1Δ mutant. These observations demonstrate that, like DCP1, MRT1, MRT3, and SPB8, the PAT1 gene product is required for normal rates of mRNA turnover.
FIG. 3.
MFA2pG mRNA is stabilized in the pat1Δ strain. (A and B) Degradation of MFA2pG mRNA in wild-type (WT) (A) and pat1Δ (B) strains. Cells carrying the GAL::MFA2pG reporter gene were shifted from galactose- to glucose-containing medium to repress transcription. RNA was recovered from samples taken at the indicated times after repression and analyzed by Northern blotting. The top panel shows hybridization with an oligonucleotide (oRP140) recognizing the full-length MFA2pG mRNA and the pG degradation intermediate. The bottom panel shows the hybridization signal with the scR1 probe, which is used here as an internal control. (C) The level of full-length MFA2pG mRNA for each time point was quantitated by PhosphorImager (Molecular Dynamics) analysis, normalized to scR1, and then plotted on a log scale as a function of time.
PAT1 is identical to MRT1.
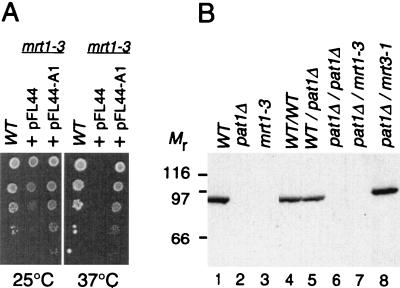
The pat1Δ mutant strain has certain similarities to the mrt1-3 mutant strain: unstable mRNAs are stabilized about fourfold in the two mutants, the two mutations can bypass a deletion of the PAB1 gene, and the two mutant strains are thermosensitive. These similarities suggested that MRT1 could be identical to PAT1. To test this hypothesis, the plasmid containing the wild-type PAT1 gene (pFL44-A1) was transformed into the mrt1-3 strain. This plasmid was able to support the growth of the mutant strain at high temperature, demonstrating that PAT1 can complement the thermosensitivity of the mrt1-3 mutation (Fig. 4A). To confirm this result, protein extracts were prepared from various mutant strains and analyzed by Western blotting with an antibody that recognizes the Pat1 protein (33). As expected, Pat1p could not be detected in the haploid pat1Δ strain (Fig. 4B, lane 2) or in the homozygous pat1Δ/pat1Δ diploid strain (lane 6). We then examined the mrt1-1, mrt1-2, and mrt1-3 haploid strains and the mrt1-3 pat1Δ double-mutant diploid strain and found that they do not contain any detectable Pat1p (Fig. 4B, lanes 3 and 7, and data not shown). In contrast, the mrt3-1 pat1Δ double-mutant diploid strain possesses normal levels of Pat1p (lane 8). Taken together, these data strongly suggest that PAT1 is identical to MRT1.
FIG. 4.
PAT1 is identical to MRT1. (A) A plasmid containing the wild-type (WT) PAT1 gene can support the growth of an mrt1-3 strain at 37°C. Tenfold serial dilutions of the indicated strains were spotted on yeast minimal medium plates lacking uracil and incubated at either 25 or 37°C as shown at the bottom. WT, a W303 derivative containing plasmid pRP485; +pFL44, yRP1066 (18) transformed with an empty pFL44 vector; +pFL44-A1, the same strain transformed with the plasmid containing the PAT1 gene. (B) Pat1p is not detected in an mrt1-3 haploid strain or in a diploid pat1Δ mrt1-3 double-mutant strain. Protein extracts (50 μg) were fractionated by SDS-PAGE (10% polyacrylamide), electroblotted onto nitrocellulose, and probed with antibodies raised against Pat1p. Lanes: 1 to 3, haploid strains; 4 to 8, diploid strains. Relevant genotypes are indicated above each lane. Mr, molecular weight markers in thousands.
Pat1p and Spb8p are associated in vivo as shown by coimmunoprecipitation experiments.
Mutations affecting the PAT1 gene lead to a phenotype that is reminiscent of an spb8-2 mutant strain (6), suggesting that Pat1p and Spb8p could be involved in the same pathway. To investigate this possibility, we first decided to search for potential interactions between Pat1p and Spb8p. A strain expressing a Pat1-protA fusion protein at the normal locus was constructed (see Materials and Methods). The growth rate of that strain was identical to that of an isogenic untagged strain, and the level of Pat1-protAp was similar to the level of the natural protein in a wild-type strain (data not shown).
To detect Spb8p, a polyclonal antibody was prepared by injecting rabbits with recombinant Spb8p. The specificity of this antibody was tested by Western blotting, with an extract from a wild-type yeast strain (Fig. 5A, lane 1). A unique band was detected that migrated as a Mr 20,000 polypeptide, a size in good agreement with the predicted size for Spb8p. The specificity of the reaction is illustrated by the absence of any signal when the extract was prepared from a strain in which the SPB8 gene had been deleted (see Materials and Methods) (lane 2). To assess the level of the protein tested, the same amounts of protein were loaded in each lane, as confirmed by staining the membrane with amido black and by probing the membrane with a control antibody (anti-Pab1p [data not shown]). The level of Spb8p was unchanged in a strain in which the PAT1 gene was deleted (lane 3). We then tested for the presence of Pat1p in various mutant strains. The level of Pat1p was unaffected in dcp1Δ, xrn1Δ, or mrt3-1 strains (Fig. 5B, lanes 4 to 6). In contrast, the level of Pat1p was reduced about 10-fold in the spb8Δ strain (lanes 1 to 3). This result suggests that Pat1p and Spb8p could interact, either physically or genetically. We constructed a series of double-mutant strains (spb8Δ pat1Δ, dcp1Δ pat1Δ, and xrn1Δ pat1Δ) and found that all of them were able to grow at the permissive temperature, demonstrating that spb8Δ, dcp1Δ, and xrn1Δ were not synthetically lethal with pat1Δ.
FIG. 5.
The absence of Spb8p leads to a sharp decrease in the level of Pat1p. (A) Protein extracts (50 μg) prepared from the indicated strains were tested by Western blotting with the rabbit anti-Spb8p antibody. (B) Detection of Pat1p in various mutant strains. Protein extract (50 μg) prepared from each strain (5 μg for lane 3) was subjected to SDS-PAGE (10% polyacrylamide). The filters were probed with the anti-Pat1p antibody. Relevant genotypes are indicated above each lane. Mr, molecular weight markers in thousands. WT, wild type.
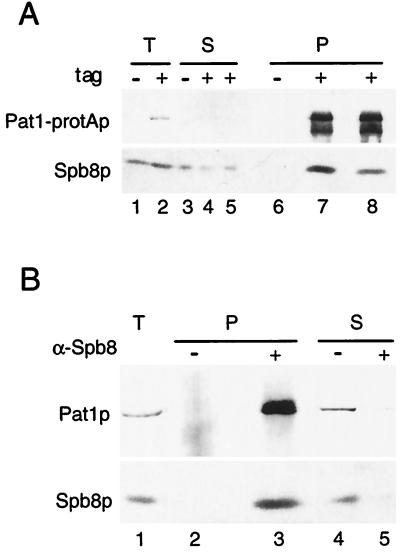
To test whether Pat1p interacts physically with Spb8p, extracts containing Pat1-protAp were incubated with immunoglobulin G-Sepharose beads to precipitate the protA-tagged protein and any associated proteins. Western blot analysis was performed with rabbit immunoglobulins coupled to horseradish peroxidase to reveal Pat1-protAp (Fig. 6A, upper panel) and with the anti-Spb8p antibody to reveal Spb8p (lower panel). Spb8p was detected in total-cell extracts (lanes 1 and 2) and in the pellet fraction when immunoprecipitation was performed with the strain expressing Pat1-protAp (lanes 7 and 8). No Spb8p was detected when the same experiment was performed with a strain expressing only the untagged Pat1p (lane 6). Interaction of Spb8p and Pat1p was unaffected by adding RNase A during the immunoprecipitation procedure (data not shown) or by washing the pellet with a buffer containing 0.5 M NaCl (lane 8). The reverse experiment was then carried out by first precipitating Spb8p with an anti-Spb8p antibody, by using a strain in which Pat1p was not tagged. Western blot analyses of the pellet with the anti-Pat1p antibody revealed that Pat1p was coimmunoprecipitated with Spb8p, confirming the physical association of the two proteins in vivo (Fig. 6B, lane 3). No Pat1p was precipitated in the control experiment when antibodies were omitted (lane 2).
FIG. 6.
Spb8p and Pat1p are coimmunoprecipitated. (A) Immunoprecipitation of Pat1-protAp. Native extracts prepared either from a wild-type strain or from a PAT1-protA tagged strain were incubated with immunoglobulin G-Sepharose beads. After extensive washes, proteins were eluted from the beads and separated by SDS-PAGE (10% polyacrylamide). Western blotting was performed either with rabbit immunoglobulin G coupled to horseradish peroxidase to detect the protA-tagged protein (upper panel) or with an anti-Spb8p antibody (lower panel). Lanes: 1, 3, and 6, wild-type strain (tag −); 2, 4, 5, 7, and 8, PAT1-protA strain (tag +). Lanes 1 to 5 contain 1/40 the amount of protein extract used in the immunoprecipitation experiment. T, total extract; S, supernatant; P, pellet. Lanes 5 and 8 contain the supernatant and pellet, respectively, of the immunoprecipitation, washed with a buffer containing 0.5 M NaCl. (B) Immunoprecipitation of Spb8p. Native extracts prepared from strain yRP1070 transformed with pRP801 (see Materials and Methods) were treated with a preimmune serum and incubated with protein A-Sepharose to remove any nonspecific complexes. Then the supernatants were treated with the anti-Spb8p antibody or left untreated; this was followed by precipitation with protein A-Sepharose. Proteins from the pellets and the supernatants were analyzed by Western blotting with either the anti-Pat1p (upper panel) or the anti-Spb8p (lower panel) antibodies. Lane 1 contains 1/40 the amount of protein used in the immunoprecipitation (T); lanes 2 and 3 contain pellets of the immunoprecipitation (P); lanes 4 and 5 contain 1/40 of the supernatants (S). Lanes 2 and 4, control immunoprecipitation performed without serum (−); lanes 3 and 5, immunoprecipitation done in the presence of the anti-Spb8p antibodies (+).
Pat1p and Spb8p cofractionate with polysomes.
Several lines of evidence have led to the proposal that mRNA degradation and mRNA translation are two interlaced processes in the cell (23). Recently, this view has received more support, with the finding that the 5′-to-3′ exoribonuclease Xrn1p, which is a major component of the mRNA degradation pathway, was associated with polysomes (28). Pab1p is also associated with polysomes and plays a role in mRNA stability. Since spb10 and spb8 are both suppressors of pab1Δ, we examined whether Pat1p and Spb8p could also be associated with polysomes. These experiments were performed with a strain in which Dcp1p was tagged with a Flag epitope that allows its detection (27).
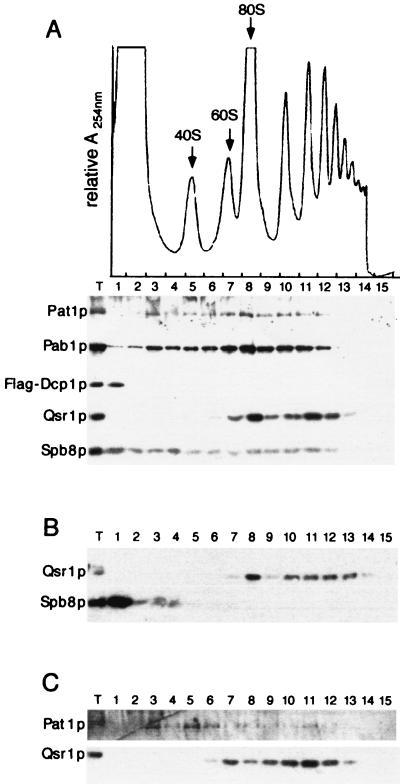
Polysomes were fractionated on sucrose gradients, and the fractions were analyzed by Western blotting with antibodies raised against Pat1p, Spb8p, Pab1p, Flag-Dcp1p, and Xrn1p. Qsr1p, a 60S ribosomal subunit protein, was used as a control for the integrity of the polysomes (47). Expression of the Flag-Dcp1p chimera does not alter the polysome profile, which is similar to that of the wild type (Fig. 7A). Pab1p is associated with the polysomes and with lighter fractions corresponding to the 60S, 40S, and even lighter structures. As expected, Western blot analysis with the anti-Qsr1p antibody detected the protein in fractions containing the 60S subunits and in fractions containing polysomes. Under the experimental conditions used here, Flag-Dcp1p was located exclusively in the upper part of the gradient (see Discussion). The antibodies raised against Spb8p detect the protein all along the gradient, as is also the case for Pab1p. In contrast, Pat1p was detected mostly in fractions containing mRNPs, monosomes, and polysomes but not with the lightest fractions. Therefore, even though a large fraction of Spb8p was associated with Pat1p, the distributions of the two proteins were slightly different. Interestingly, Xrn1p has the same distribution as Spb8p and can be found throughout the whole gradient (reference 28 and data not shown).
FIG. 7.
Spb8p and Pat1p cosediment with polysomes on sucrose gradients. (A) A total-cell extract prepared from strain yRP1070 transformed with pRP801, which expresses a tagged version of Dcp1p, was fractionated on a 7 to 50% linear sucrose gradient. (Top) A continuous record of the absorbance at 254 nm (A254nm) for the gradient is presented, with the top of the gradient on the left. The arrows indicate the peaks for the 40S, 60S, and 80S subunits. (Bottom) Western blot analysis of the 15 fractions collected from the sucrose gradient was performed with antibodies raised against the indicated proteins. T, total-cell extract. (B) A total-cell extract was prepared from a strain with PAT1 deleted and fractionated on a sucrose gradient as described for panel A. The 15 fractions were analyzed by Western blotting with the anti-Qsr1p (upper row) or the anti-Spb8p (bottom row) antibody. (C) As in panel B, except that extracts were prepared from a strain with SPB8 deleted and tested with the anti-Pat1p (upper row) or the anti-Qsr1p (bottom row) antibody.
Since Pat1p interacts physically with Spb8p, we then searched whether the presence of one of these proteins in the fractions containing polysomes could depend on the presence of the other. Cellular extracts were prepared from different mutant strains and fractionated on sucrose gradients. The fractions were then analyzed by Western blotting as described above. In a pat1Δ strain, Spb8p was detected mostly at the top of the gradient (Fig. 7B). In contrast, in an spb8Δ strain, the level of Pat1p was sharply reduced (Fig. 5B) but the rest of Pat1p was still detected in the fractions containing polysomes (Fig. 7C).
To assess whether cofractionation of Spb8p and Pat1p with polysomes was fortuitous or was due to physical interactions of these proteins with polysomes, we performed sucrose gradient analysis under conditions that disrupt either free monosomes or polysome structure. We already knew that the presence of these proteins within fractions containing high-molecular-weight structures was rather labile, since they were shifted to the top of the gradient when polysomes were prepared and analyzed by standard methods (reference 16 and data not shown). Therefore, when gradients were run in high-salt containing buffer (28), Spb8p and Pat1p were detected only in the upper part of the gradient, as we expected (data not shown). We then assayed the association of these factors in the presence of EDTA, which disrupts polysomes and leads to an accumulation of free 40S and 60S subunits. This shift in ribosomal subunits was accompanied by a shift in the sedimentation pattern of Spb8p and Pat1p (Fig. 8). This result suggests that these two proteins are associated with the translated mRNAs.
FIG. 8.
Addition of EDTA results in a shift of Spb8p and Pat1p to the upper part of the sucrose gradient. Extracts were prepared and analyzed as described in the legend to Fig. 7, except that 40 mM EDTA was added to the breaking buffer and to the sucrose gradient solutions. (Top) Relative profile of the absorbance at 254 nm (A254nm) with the top of the gradient on the left. Arrows indicate the positions of the 40S and 60S subunits. (Bottom) Western blot analysis of the fractions using either the anti-Pat1p or the anti-Spb8p antibodies.
DISCUSSION
We report here the characterization of spb10, a suppressor of the lethality of pab1Δ that lies within the PAT1 gene. Sequence alignment of Pat1p from S. cerevisiae with a putative homologue from S. pombe reveals five motifs that are conserved in several putative proteins from metazoans. It is worth noticing that a single EF-hand motif that was detected in the S. cerevisiae sequence is not conserved in the S. pombe sequence, thus weakening its potential role. Interestingly, a putative homologue from Xenopus laevis, the P100 protein, which has been localized exclusively to the cytoplasm of the oocyte, is able to bind single-stranded DNA in vitro (see below) (34).
Pat1p was previously found to interact with topoisomerase II (49). A strain with PAT1 partially deleted exhibits alterations of chromosome segregation and rDNA recombination (48, 49). Our data and those of previous workers support another role for this protein. The first report on Ycr77c had shown that the protein is mostly associated with the cytosolic fraction (33), and we found that Pat1p cosediments on sucrose gradients with polysomes, two observations that favor the idea that Pat1p is located mostly within the cytoplasm. We cannot exclude that a subset of the protein is localized within the nucleus and interacts there with Top2p. Alternatively, Pat1p could be associated with a subset of Top2p in the cytoplasm, where it could regulate its function. It is also conceivable that chromosome segregation alterations seen in a pat1Δ strain are indirect consequences of an inhibition of mRNA decay, which may preferentially affect certain mRNAs whose changed expression would lead to this phenotype.
We also found that PAT1 is identical to MRT1, for which mutations had been reported to affect mRNA decay by stabilizing the cap structure (21). The mrt1 mutations were isolated from a collection of temperature-sensitive strains that stabilize a reporter MFA2pG mRNA. Other mutations were isolated in XRN1, DCP1, and MRT3 genes, but none of the mutants were actually thermosensitive per se. In contrast, the decapping defect associated with the mrt1 mutations was linked to the thermosensitive phenotype. This result is in agreement with our observations that the pat1Δ mutant is thermosensitive and leads to mRNA stabilization. The mrt1 mutations have now been directly linked to the PAT1 gene by sequencing the DNA corresponding to the PAT1 ORF in the three mrt1 mutants (44).
Decapping can be activated by two distinct mechanisms. In the general mRNA decay pathway, decapping is activated after deadenylation of the mRNA. Mutations in either MRT1 (PAT1), SPB8, or MRT3 prevent this deadenylation-dependent decapping activity in vivo (6, 21). Extensive mutagenesis of the DCP1 gene has revealed mutations that abolish the deadenylation-dependent decapping activity in vivo without changing the activity in vitro (45). These results suggest that the deadenylation-dependent decapping activity in vivo requires a particular domain of Dcp1p that might interact with other factors such as Pat1p, Spb8p, and Mrt3p. In contrast, in the nonsense-mediated decay (NMD) pathway, decapping occurs prior to deadenylation and does not require Pat1p, Spb8p, or Mrt3p. Also, mutations of DCP1 that alter deadenylation-dependent decapping do not affect NMD, suggesting that there are two different ways to activate Dcp1p: one that requires Pat1p, Spb8p, or Mrt3p and is responding to the activation by deadenylation and another that is activated directly by the NMD pathway.
Another mutation, vps16, inhibits decapping of normal mRNAs but also prevents NMD (50), as do mutations in DCP1 or DCP2. Ssa1p or Ssa2p, an Hsp70 family member, copurifies with Dcp1p. Interestingly, this interaction is enhanced in mrt1 or in vps16 strains, an observation that could explain the reduced decapping activity of these mutants (50). Moreover, Sis1p, which is required for translation initiation, belongs to the DnaJ protein family, whose members modulate the activity of the Hsp70 proteins. SIS1 has been genetically linked to PAB1, establishing another link between heat shock proteins, initiation of translation, and the Pab1 protein (51).
Our results demonstrate that Pat1p and Spb8p share several properties, supporting the view that they operate in the same metabolic pathway. Coimmunoprecipitation experiments indicate that they interact with each other in vivo. This association does not depend on the presence of an RNA, since it is not sensitive to RNase treatment. Moreover, these two proteins have a strong affinity for each other, since coimmunoprecipitation is not affected by high-salt washes. In contrast, their presence in the fractions containing polysomes is rather labile (data not shown), suggesting that they are not intrinsic constituents of the polysomes. However, part of Spb8p is detected in the upper part of the gradient, in fractions in which Pat1p is either absent or present in very small amounts. This observation suggests that Spb8p is likely to be involved in different structures, some that contain Pat1p and some that do not. Functional interactions between Spb8p and Pat1p are further supported by the observation that the level of Pat1p is sharply reduced in the absence of Spb8p. Moreover, in the absence of Pat1p, Spb8p is detected mostly in the upper part of the gradient. This suggests either that Pat1p could bind to the polysomes to establish a link with Spb8p or that Spb8p could bind directly to the polysomes but would require Pat1p to interact steadily. It is worth noticing that there is a protein in X. laevis that presents a certain degree of similarity to Pat1p. This protein, P100, was shown to bind preferentially in vitro to single-stranded DNA-cellulose over double-stranded DNA-cellulose (34). However, this protein has been localized to the cytoplasm of the oocyte, a result that opens the possibility that P100 is in fact an RNA-binding protein. Following this view, it is conceivable that one function of Pat1p would be to bind mRNAs, thus bringing to the polysomes a complex containing Spb8p.
Spb8p possesses an Sm-like motif, which is the signature of a group of proteins involved in snRNA metabolism (40). Two recent reports have demonstrated that Spb8p (or Lsm1p) is associated with a group of six other Lsm proteins (Lsm2 to Lsm7) (8, 44) that are likely to form a doughnut-shaped structure (1). Mutations in several of these LSM genes also alter mRNA decapping. Two-hybrid analyses have shown that Lsm proteins interact with Dcp1p, Xrn1p, Dcp2p, Pat1p, and a number of other yet uncharacterized ORF products (M. Fromont-Racine and P. Legrain, personal communication). Pat1p was also found to interact physically with Xrn1p (8) and Dcp1p (44). Our data indicate that the Spb8p-Pat1p complex cosediments with polysomes, like the exonuclease Xrn1p. Using our experimental conditions, we detected Dcp1p only at the top of the gradient, suggesting that most of Dcp1p is not associated with polysomes. However, for these experiments we used a Flag-Dcp1p construction that is overexpressed 50- to 100-fold compared to the natural levels (R. Parker, personal communication). It is therefore possible that the Dcp1p seen at the top of the gradient reflects the extra Dcp1p while the functional Dcp1p that associates with polysomes is below the detection level in these experiments. Alternatively, the Spb8p-Pat1p complex, which is associated with polysomes, could await a specific signal, probably mediated by Pab1p, to recruit the Dcp1p-Dcp2p complex onto the mRNA to activate decapping. Future work will focus on characterizing all the factors involved in decapping and their associated components such as Dcp2p, Ssa1p, or Ssa2p, and the factors recently discovered by two-hybrid analysis. This should serve as a basis to describe the mechanisms that control the switch from mRNA translation to degradation.
ACKNOWLEDGMENTS
We are indebted to A. Sachs, who initiated this work. We thank F. Lacroute for the gift of the yeast genomic library, and we thank R. Lill, M. Swanson, and B. Trumpower for providing antibodies used in this study. We are grateful to J. Morrissey and D. Tollervey for comments on the manuscript and to F. Martin for the chromatography experiments and M. Doère for technical assistance with polysome analysis. We thank L. Pintard and M. Brengues for their help and for stimulating discussions, and we thank E. Schwob, G. Lutfalla, and G. Uzé for generously sharing equipment and supplies.
This work was supported by the Centre National de la Recherche Scientifique and by grants from the Ligue contre le Cancer and from the Fondation pour la Recherche Médicale. R.B. was supported by a grant from the Ernst et Lucie Schmidheiny foundation, and C.B. is part of the Institut National de la Santé et de la Recherche Médicale.
REFERENCES
- 1.Achsel T, Brahms H, Kastner B, Bachi A, Wilm M, Luhrmann R. A doughnut-shaped heteromer of human Sm-like proteins binds to the 3′-end of U6 snRNA, thereby facilitating U4/U6 duplex formation in vitro. EMBO J. 1999;18:5789–5802. doi: 10.1093/emboj/18.20.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam S A, Nakagawa T, Swanson M S, Woodruff T K, Dreyfuss G. mRNA polyadenylate-binding protein: gene isolation and sequencing and identification of a ribonucleoprotein consensus sequence. Mol Cell Biol. 1986;6:2932–2943. doi: 10.1128/mcb.6.8.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudin A, Ozier-Kalogeropoulos O, Denouel A, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beelman C A, Parker R. Degradation of mRNA in eukaryotes. Cell. 1995;81:179–183. doi: 10.1016/0092-8674(95)90326-7. [DOI] [PubMed] [Google Scholar]
- 5.Beelman C A, Stevens A, Caponigro G, LaGrandeur T E, Hatfield L, Fortner D M, Parker R. An essential component of the decapping enzyme required for normal rates of mRNA turnover. Nature. 1996;382:642–646. doi: 10.1038/382642a0. [DOI] [PubMed] [Google Scholar]
- 6.Boeck R, Lapeyre B, Brown C E, Sachs A B. Capped mRNA degradation intermediates accumulate in the yeast spb8-2 mutant. Mol Cell Biol. 1998;18:5062–5072. doi: 10.1128/mcb.18.9.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonneaud N, Ozier-Kalogeropoulos O, Li G Y, Labouesse M, Minvielle-Sebastia L, Lacroute F. A family of low and high copy replicative, integrative and single-stranded S. cerevisiae/E. coli shuttle vectors. Yeast. 1991;7:609–615. doi: 10.1002/yea.320070609. [DOI] [PubMed] [Google Scholar]
- 8.Bouveret E, Rigaut G, Shevchenko A, Wilm M, Séraphin B. A Sm-like protein complex that participates in mRNA degradation. EMBO J. 2000;19:1661–1671. doi: 10.1093/emboj/19.7.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caponigro G, Parker R. Multiple functions for the poly(A)-binding protein in mRNA decapping and deadenylation in yeast. Genes Dev. 1995;9:2421–2432. doi: 10.1101/gad.9.19.2421. [DOI] [PubMed] [Google Scholar]
- 10.Church G M, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross F R, Tinkelenberg A H. A potential positive feedback loop controlling CLN1 and CLN2 gene expression at the start of the yeast cell cycle. Cell. 1991;65:875–883. doi: 10.1016/0092-8674(91)90394-e. [DOI] [PubMed] [Google Scholar]
- 12.Decker C J, Parker R. Mechanisms of mRNA degradation in eukaryotes. Trends Biochem Sci. 1994;19:336–340. doi: 10.1016/0968-0004(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 13.Decker C J, Parker R. A turnover pathway for both stable and unstable mRNAs in yeast: evidence for a requirement for deadenylation. Genes Dev. 1993;7:1632–1643. doi: 10.1101/gad.7.8.1632. [DOI] [PubMed] [Google Scholar]
- 14.Dunckley T, Parker R. The Dcp2 protein is required for mRNA decapping in Saccharomyces cerevisiae and contains a functional MutT motif. EMBO J. 1999;18:5411–5422. doi: 10.1093/emboj/18.19.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felici F, Cesareni G, Hughes J M. The most abundant small cytoplasmic RNA of Saccharomyces cerevisiae has an important function required for normal cell growth. Mol Cell Biol. 1989;9:3260–3268. doi: 10.1128/mcb.9.8.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foiani M, Cigan A M, Paddon C J, Harashima S, Hinnebusch A G. GCD2, a translational repressor of the GCN4 gene, has a general function in the initiation of protein synthesis in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:3203–3216. doi: 10.1128/mcb.11.6.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallie D R. The cap and poly(A) tail function synergistically to regulate mRNA translational efficiency. Genes Dev. 1991;5:2108–2116. doi: 10.1101/gad.5.11.2108. [DOI] [PubMed] [Google Scholar]
- 18.Gietz D, St. Jean A, Woods R A, Schiestl R H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gietz R D, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 20.Guthrie C, Fink R G. Guide to yeast genetics and molecular biology. Methods Enzymol. 1991;194:1–863. [PubMed] [Google Scholar]
- 21.Hatfield L, Beelman C A, Stevens A, Parker R. Mutations in trans-acting factors affecting mRNA decapping in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:5830–5838. doi: 10.1128/mcb.16.10.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horazdovsky B F, Emr S D. The VPS16 gene product associates with a sedimentable protein complex and is essential for vacuolar protein sorting in yeast. J Biol Chem. 1993;268:4953–4962. [PubMed] [Google Scholar]
- 23.Jacobson A, Peltz S W. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu Rev Biochem. 1996;65:693–739. doi: 10.1146/annurev.bi.65.070196.003401. [DOI] [PubMed] [Google Scholar]
- 24.Kressler D, Doère M, Rojo M, Linder P. Synthetic lethality with conditional dbp6 alleles identifies Rsa1p, a nucleoplasmic protein involved in the assembly of 60S ribosomal subunits. Mol Cell Biol. 1999;19:8633–8645. doi: 10.1128/mcb.19.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.LaGrandeur T, Parker R. The cis acting sequences responsible for the differential decay of the unstable MFA2 and stable PGK1 transcripts in yeast include the context of the translational start codon. RNA. 1999;5:420–433. doi: 10.1017/s1355838299981748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaGrandeur T E, Parker R. Isolation and characterization of Dcp1p, the yeast mRNA decapping enzyme. EMBO J. 1998;17:1487–1496. doi: 10.1093/emboj/17.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangus D A, Jacobson A. Linking mRNA turnover and translation: assessing the polyribosomal association of mRNA decay factors and degradative intermediates. Methods. 1999;17:28–37. doi: 10.1006/meth.1998.0704. [DOI] [PubMed] [Google Scholar]
- 29.Mayes A E, Verdone L, Legrain P, Beggs J D. Characterization of Sm-like proteins in yeast and their association with U6 snRNA. EMBO J. 1999;18:4321–4331. doi: 10.1093/emboj/18.15.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muhlrad D, Decker C J, Parker R. Turnover mechanisms of the stable yeast PGK1 mRNA. Mol Cell Biol. 1995;15:2145–2156. doi: 10.1128/mcb.15.4.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pintard L, Kressler D, Lapeyre B. Spb1p is a yeast nucleolar protein associated with Nop1p and Nop58p that is able to bind S-adenosyl-l-methionine in vitro. Mol Cell Biol. 2000;20:1370–1381. doi: 10.1128/mcb.20.4.1370-1381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puig O, Rutz B, Luukkonen B G, Kandels-Lewis S, Bragado-Nilsson E, Séraphin B. New constructs and strategies for efficient PCR-based gene manipulations in yeast. Yeast. 1998;14:1139–1146. doi: 10.1002/(SICI)1097-0061(19980915)14:12<1139::AID-YEA306>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Cousino N, Lill R, Neupert W, Court D A. Identification and initial characterization of the cytosolic protein Ycr77p. Yeast. 1995;11:581–585. doi: 10.1002/yea.320110608. [DOI] [PubMed] [Google Scholar]
- 34.Rother R P, Frank M B, Thomas P S. Purification, primary structure, bacterial expression and subcellular distribution of an oocyte-specific protein in Xenopus. Eur J Biochem. 1992;206:673–683. doi: 10.1111/j.1432-1033.1992.tb16973.x. [DOI] [PubMed] [Google Scholar]
- 35.Sachs A B, Davis R W. The poly(A) binding protein is required for poly(A) shortening and 60S ribosomal subunit-dependent translation initiation. Cell. 1989;58:857–867. doi: 10.1016/0092-8674(89)90938-0. [DOI] [PubMed] [Google Scholar]
- 36.Sachs A B, Davis R W. Translation initiation and ribosomal biogenesis: involvement of a putative rRNA helicase and RPL46. Science. 1990;247:1077–1079. doi: 10.1126/science.2408148. [DOI] [PubMed] [Google Scholar]
- 37.Sachs A B, Davis R W, Kornberg R D. A single domain of yeast poly(A)-binding protein is necessary and sufficient for RNA binding and cell viability. Mol Cell Biol. 1987;7:3268–3276. doi: 10.1128/mcb.7.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salgado-Garrido J, Bragado-Nilsson E, Kandels-Lewis S, Séraphin B. Sm and Sm-like proteins assemble in two related complexes of deep evolutionary origin. EMBO J. 1999;18:3451–3462. doi: 10.1093/emboj/18.12.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz D C, Parker R. Mutations in translation initiation factors lead to increased rates of deadenylation and decapping of mRNAs in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:5247–5256. doi: 10.1128/mcb.19.8.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Séraphin B. Sm and Sm-like proteins belong to a large family: identification of proteins of the U6 as well as the U1, U2, U4 and U5 snRNPs. EMBO J. 1995;14:2089–2098. doi: 10.1002/j.1460-2075.1995.tb07200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarun S, Sachs A B. A common function for mRNA 5′ and 3′ ends in translation initiation in yeast. Genes Dev. 1995;9:2997–3007. doi: 10.1101/gad.9.23.2997. [DOI] [PubMed] [Google Scholar]
- 42.Tarun S Z, Jr, Wells S E, Deardorff J A, Sachs A B. Translation initiation factor eIF4G mediates in vitro poly(A) tail-dependent translation. Proc Natl Acad Sci USA. 1997;94:9046–9051. doi: 10.1073/pnas.94.17.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarun S Z J, Sachs A B. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 1996;15:7168–7177. [PMC free article] [PubMed] [Google Scholar]
- 44.Tharun S, He W, Mayes A E, Lennertz P, Beggs J D, Parker R. Yeast Sm-like proteins function in mRNA decapping and decay. Nature. 2000;404:515–518. doi: 10.1038/35006676. [DOI] [PubMed] [Google Scholar]
- 45.Tharun S, Parker R. Analysis of mutations in the yeast mRNA decapping enzyme. Genetics. 1999;151:1273–1285. doi: 10.1093/genetics/151.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tron T, Yang M, Dick F A, Schmitt M E, Trumpower B L. QSR1, an essential yeast gene with a genetic relationship to a subunit of the mitochondrial cytochrome bc1 complex, is homologous to a gene implicated in eukaryotic cell differentiation. J Biol Chem. 1995;270:9961–9970. doi: 10.1074/jbc.270.17.9961. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Watt P M, Borts R H, Louis E J, Hickson I D. The topoisomerase II-associated protein, Pat1p, is required for maintenance of rDNA locus stability in Saccharomyces cerevisiae. Mol Gen Genet. 1999;261:831–840. doi: 10.1007/s004380050027. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Watt P M, Louis E J, Borts R H, Hickson I D. Pat1: a topoisomerase II-associated protein required for faithful chromosome transmission in Saccharomyces cerevisiae. Nucleic Acids Res. 1996;24:4791–4797. doi: 10.1093/nar/24.23.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang S, Williams C J, Hagan K, Peltz S W. Mutations in VPS16 and MRT1 stabilize mRNAs by activating an inhibitor of the decapping enzyme. Mol Cell Biol. 1999;19:7568–7576. doi: 10.1128/mcb.19.11.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong T, Arndt K T. The yeast SIS1 protein, a DnaJ homolog, is required for the initiation of translation. Cell. 1993;73:1175–1186. doi: 10.1016/0092-8674(93)90646-8. [DOI] [PubMed] [Google Scholar]