Abstract
Lead acetate associated tissue injury has been linked to altered antioxidant defenses, hyperuricemia and inflammation. We hypothesized that watermelon rind extract, would ameliorate lead acetate-induced hepato-renal injury.
Thirty Male Wistar rats received distilled water, lead acetate (Pb; 5 mg/kg) with or without watermelon rind extract (WM; 400 mg/kg; WM + Pb; 15 days of WM pretreatment); Pb + WM (15 days of WM post treatment) and simultaneous treatment (WM-Pb) for 30 days.
Lead toxicity led to elevated serum malondialdehyde, creatinine, urea, uric acid, lactate dehydrogenase, liver injury enzymes, as well as decreased body weight. Decreased serum levels of reduced glutathione, nitric oxide, total protein and glutathione peroxidase activity was also observed. However, these alterations were ameliorated by watermelon rind extract in lead acetate-treated rats.
Watermelon rind ethanol extract protects against lead acetate-induced hepato-renal injury through improved antioxidant defenses at least in part, via uric acid/nitric oxide-dependent pathway signifying the health benefits of this agricultural waste and a potential for waste recycling while limiting environmental pollution.
Keywords: ALP, Alkaline Phosphatase; ALT, Alanine Transferase; AST, Aspartate Transaminase; GPx, Glutathione Peroxidase; GSH, Reduced Glutathione; LDH, Lactate Dehydrogenase; MDA, Malondialdehyde; Pb, Lead Acetate; rpm, revolutions per minute; WM, Watermelon rind extract
Keywords: Watermelon, Uric acid, Nitric oxide, Oxidative stress, Lead acetate
Highlights
-
•
Lead acetate leads to oxidative stress in liver and kidney.
-
•
Watermelon rind extract improved the antioxidants status via uric acid/nitric oxide-dependent pathway.
-
•
Watermelon rind extract confers hepato-renal protection.
1. Introduction
Lead (Pb) a heavy metal widely known for its environmental intoxication effects remains a major health challenge affecting both humans and animals. Although several measures have been taken over the years to reduce the adverse impacts of this metal in the environment (European Food Safety AuthorityEFSA, 2013), about 674,000 deaths are still been recorded annually as a result of lead pollution across the globe (Lim et al., 2012). Moreover, experimental studies have demonstrated that lead poisoning is associated with hypertension (Hara et al., 2015) chronic renal failure (Kwon et al., 2015), liver injury (Fang et al., 2014) and impairment of male reproductive capabilities (Li et al., 2018). Consequently, lead may gain access into the body by ingestion, inhalation or through the skin and there is no known safe blood lead concentration; even concentration as low as 5 mg/dL, which was long regarded to be a safe level, can cause lower intellect, behavioral issues, and learning problems in children (Reuben et al., 2017). Even though the accurate mechanism of lead biotransformation is unknown, various experimental data have shown that lead toxicity involves basic biochemical processes such as metabolic inhibition, protein interaction, cell toxicity (including DNA damage) through lipid peroxidation and oxidative stress, and a reduction in antioxidant defenses (Dobrakowski et al., 2017).
Virtually all the substances absorbed by the intestine pass through the liver first and are filtered by the kidney via the detoxification process with high possibilities of the heavy metals accumulating and damaging the hepato-renal cytoarchitecture (Cheng et al., 2019). Thus, the damage to the kidneys and liver by lead exposure is mediated through alteration in the redox balance, reactive nitrogen specie generation and the body antioxidant system (Offor et al., 2017). Experimentally, increased lipid peroxidation and decreased glutathione (GSH) levels have been implicated as the contributory mechanisms involved in lead-induced hepato-renal dysfunctions (Zhang et al., 2017). In an attempt to combat the adverse effects of oxidative stress on the body system, there has been a renewed effort towards the development and the use of natural products with high antioxidant potentials and which can easily be incorporated into our daily diet.
Pathogenesis of cardiometabolic dysfunctions, chronic kidney and liver diseases has been associated to hyperuricemia (Kogure et al., 1997; Afzali et al., 2010; Yuan et al., 2015). It is important to note that elevated serum uric acid causes oxidative balance distortion including reduced bioavailability of nitric oxide followed by endothelial dysfunction consequently culminating in the development of hepato-renal tissue injury. Therefore, hyperuricemia may be a significant pathogenic link for the development of tissue damage (Babio et al., 2015). Although, the mechanistic links between serum uric acid and hepato-renal tissues injury have not been fully elucidated.
Watermelon also known as Citrullus lanatus is a fruit originating from the tropics that is mostly cultivated in the warm temperate zone and readily available in the market (Rahman et al., 2013). The watermelon's pink flesh is eaten raw or used to make juices and salads, but the rind is discarded as a waste product with no commercial value. However, watermelon rind pickles are also commonly consumed in the southern United States (Mandel et al., 2005). In order to promote health benefits and lower the glycemic index, watermelon rind has been stated as a potential substitute for wheat flour as a potential source of dietary fiber in making of cookie and cake (Naknaen et al., 2016), and for the control of diabetes control and alcoholic poisoning (Ahn et al., 2011).
Furthermore, watermelon is rich in both essential and unessential amino acids, and a number of antioxidant molecules especially L-citrulline which is a precursor for L-arginine in the kidney (Rahman et al., 2013). In the formation of nitric oxide, L-arginine is the substrate for nitric oxide synthase. Nitric oxide is an essential signaling molecule involved in the regulation of diverse range of physiological and cellular processes and act as a novel hydroxyl radical scavenger (Akashi et al., 2004).
Studies have revealed that watermelon possess anti-inflammatory, antioxidant, hepato-protective, antigiardial, anti-plasmodial, analgesic, antimicrobial, laxative, antidiabetic, and antiulcerogenic activities (Ahn et al., 2011; Mohammad et al., 2014; Oyenihi et al., 2016). The present study is therefore aimed at investigating the effects of the methanol extract of the water melon rind on lead-induced hepato-renal dysfunctions in male Wistar rats.
2. Material and methods
2.1. Plant material and preparation of methanol extract
Watermelon (Citrullus lanatus) fruit were purchased from a local market in Iwo, Osun State, Nigeria. The plant was identified and authenticated by Dr. Ayanbamiji of the Department of Plant Biology, Faculty of Biological Sciences, Bowen University, Iwo, Osun State with a voucher # BUH-095. The whole fruit were thoroughly washed; the rinds were peeled off and air-dried at room temperature in the shade. Dried rinds were ground into a fine powder and stored in an airtight, light-protected container. The methanol extract was made by soaking 47 g of plant powder for 72 h in 100 ml of 98 percent methanol. After sieving with cheese cloth, the mixture was filtered with filter paper (Adesanya et al., 2011). To obtain a solid mass, the filtrate was concentrated by evaporation using a rotary evaporator (Hei-VAP, Heidolph, Germany). The paste-like extract yielded a mass of 24.5 g and a percentage yield of 52.1 percent. The extract was kept at 20 °C until it was needed (Janbaz et al., 2014).
2.2. Animals
The investigation followed the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and was approved by Bowen University's Ethical Review Committee. Thirty male Wistar rats, weighing 180–200 g were obtained from the animal house of the College of Health Sciences, Bowen University, Iwo, Nigeria. Rats had unrestricted access to standard rat chow and tap water. The animals were grouped into five groups (n = 6) after one week of acclimatization. The rats were kept in standard laboratory conditions of temperature, relative humidity and dark/light cycle.
2.3. Treatment
Control group received distilled water (vehicle) daily. Lead acetate (Pb; 5 mg/kg) with or without watermelon rind extract (WM; 400 mg/kg) designated as WM-pretreated group (WM + Pb); WM-post-treated group (Pb + WM) and simultaneous treatment (WM-Pb). The WM-pre-treated group received 400 mg/kg of watermelon rind extract daily for the first 15 days and 5 mg/kg of Pb daily for subsequent 15 days. Similarly, the WM-post-treated group received 5 mg/kg of Pb daily for the first 15 days followed by another 15 days of 400 mg/kg of watermelon rind extract treatment while the simultaneous group received both watermelon and Pb together daily for 30 days. All treatment was given by oral gavage.
2.4. Sample preparation
After the treatment, the rats were anesthetized with pentobarbital sodium (50 mg/kg) intraperitoneally. Blood sample was collected through cardiac puncture into plain sample bottle and allowed to stand for 30 min after which they were centrifuged for 5 min at 3000 rpm. The serum was frozen until it was time for the biochemical assay. The liver and kidneys were immediately removed, free of any adhering connective tissues, blotted, and weighed.
2.5. Biochemical assays
The levels of serum total protein, uric acid, urea, and creatinine were determined by a standardized enzymatic colorimetric approach using a Fortress diagnostics assay kit (Antrim, UK). Griess Reagent was used to determine the level of nitric oxide in the blood. Malondialdehyde (MDA) was determined using standardized enzymatic colorimetric methods and test kits from Oxford Biomedical Research, Inc. (Oxford, MI, USA). Serum lactate dehydrogenase (LDH), glutathione peroxidase (GPx), reduced glutathione (GSH), alkaline phosphatase (ALP), aspartate transaminase (AST), alanine transferase (ALT) were measured by standardized enzymatic colorimetric methods using reagents obtained from Randox Laboratory Ltd. (Antrim, UK).
2.6. Statistical analysis
All data were presented as means ± SEM. SPSS statistical software was used to conduct statistical group analysis (Chicago, IL). To compare the mean values of variables among the groups, one way analysis of variance was employed. The significance of pairwise comparisons of mean values among the groups was determined using Bonferroni's test. At p < 0.05, statistically significant differences were accepted.
3. Results
3.1. Effect of watermelon rind ethanol extract on body weight gain, heart, kidney and liver weights in lead acetate-exposed male Wistar rats
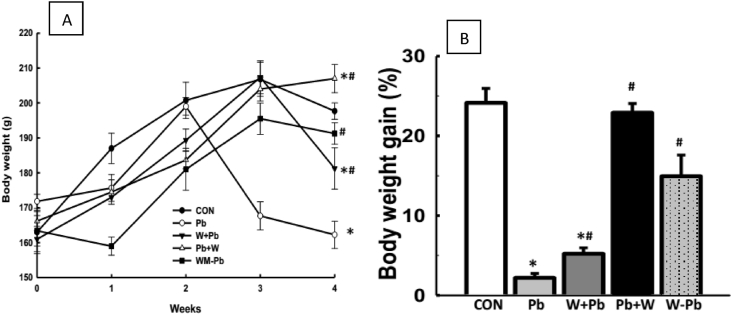
Lead acetate administration led to reduced body weight gain when compared with control. Moreover, watermelon rind ethanol extract improved body weight gain in Pb + WM- and WM-Pb-groups exception in WM + Pb-group when compared with Pb-exposed group (Fig. 1). Furthermore, the heart weight was increased by Pb-exposure which was reversed by treatment with the watermelon rind ethanol extract, but the kidney weight was however, increased across the experimental groups. However, the treatment did not affect the liver weight across the experimental groups (Table 1).
Fig. 1.
Experimental Design Scheme showing watermelon rind ethanol extract pre-treatment, post-treatment and simultaneous treatment in lead acetate treated rats. WM; watermelon rind extract, Pb; lead acetate.
Table 1.
Effect of watermelon rind ethanol extract on organ weights and total protein in lead-exposed rats.
| CON | Pb | WM + Pb | Pb + WM | WM - Pb | |
|---|---|---|---|---|---|
| Heart (g/kg b.w) | 3.6 ± 0.4 | 4.5 ± 0.7* | 3.8 ± 0.3# | 4.0 ± 0.3*# | 3.9 ± 0.3*# |
| Kidney (g/kg b.w) | 2.7 ± 0.2 | 3.5 ± 0.6* | 3.4 ± 0.3* | 3.2 ± 0.3* | 3.3 ± 0.4* |
| Liver (g/kg b.w) | 24.6 ± 0.4 | 26.9 ± 9.9 | 25.0 ± 3.4 | 24.5 ± 3.4 | 26.9 ± 3.9 |
| Total protein (g/dl) | 6.5 ± 0.1 | 5.4 ± 0.2* | 6.3 ± 0.1# | 5.9 ± 0.2# | 6.2 ± 0.3# |
Values are expressed as mean ± SEM of 6 rats per group; *p < 0.05 vs CON, #p < 0.05 vs Pb group. Data were analyzed by one-way ANOVA and Bonferroni's Post hoc analysis; CON; control, Pb; lead, WM + Pb; watermelon rind pre-treated group, Pb + WM; watermelon rind post-treated group, WM – Pb; watermelon rind simultaneous treated group.
3.2. Effect of watermelon rind ethanol extract on oxidative stress, antioxidant biomarkers and total protein in lead acetate-exposed male Wistar rats
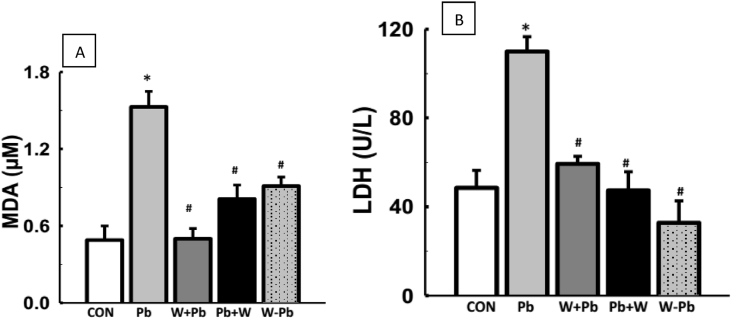
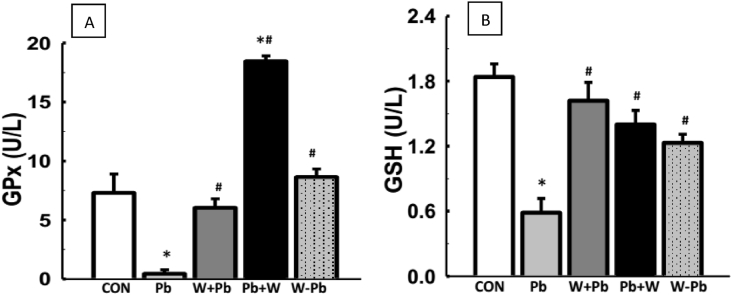
Serum LDH and MDA level were increased in Pb-exposed rats when compared with the control (Fig. 2). More so, WM-treatment did not alter serum LDH and MDA levels when compared with the control. However, WM-treatment on the other hand, led to reduced serum LDH and MDA levels in Pb-exposed rats when compared with Pb-exposed group (Fig. 2). Serum glutathione peroxidase and reduced glutathione were reduced in Pb-exposed rats when compared with control while WM-treatment led to elevated serum glutathione peroxidase and reduced glutathione concentration in Pb-exposed rats (Fig. 3). In addition, Pb-exposed rats had decreased serum total protein level when compared with control whereas WM-treatment reversed the alteration in serum total protein level when compared with Pb-exposed rats (Table 1).
Fig. 2.
Watermelon rind ethanol extract improved body weight gain in Pb-exposed male Wistar rats. Values are expressed as mean ± SEM of 6 rats per group; *p < 0.05 vs CON, #p < 0.05 vs Pb group. Data were analyzed by one-way ANOVA and Bonferroni's Post hoc analysis; CON; control, Pb; lead, WM + Pb; watermelon rind pre-treated group, Pb + WM; watermelon rind post-treated group, WM–Pb; watermelon rind simultaneous treated group.
Fig. 3.
WM reduced oxidative stress in Pb-exposed male Wistar rats. WM reversed elevated MDA and LDH levels in Pb-exposed male Wistar rats (A & B). Values are expressed as mean ± SEM of 6 rats per group; *p < 0.05 vs CON, #p < 0.05 vs Pb group. Data were analyzed by one-way ANOVA and Bonferroni's Post hoc analysis; CON; control, Pb; lead, WM + Pb; watermelon rind pre-treated group, Pb + WM; watermelon rind post-treated group, WM – Pb; watermelon rind simultaneous treated group.
3.3. Effect of watermelon rind ethanol extract on liver function enzymes and kidney biomarkers in lead acetate-exposed male Wistar rats
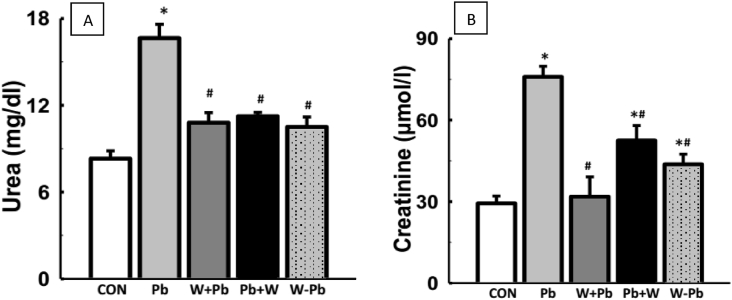
An increase in serum urea and creatinine concentration was seen in Pb-exposed rats when compared with the control. However, treatment of Pb-exposed rats with WM led to decreased serum urea and creatinine when compared with Pb-exposed rats only (Fig. 4).
Fig. 4.
WM increased antioxidant biomarkers in Pb-exposed male Wistar rats. WM reversed reduced GPx and GSH levels in Pb-exposed male Wistar rats (A & B). Values are expressed as mean ± SEM of 6 rats per group; *p < 0.05 vs CON, #p < 0.05 vs Pb group. Data were analyzed by one-way ANOVA and Bonferroni's Post hoc analysis; CON; control, Pb; lead, WM + Pb; watermelon rind pre-treated group, Pb + WM; watermelon rind post-treated group, WM – Pb; watermelon rind simultaneous treated group.
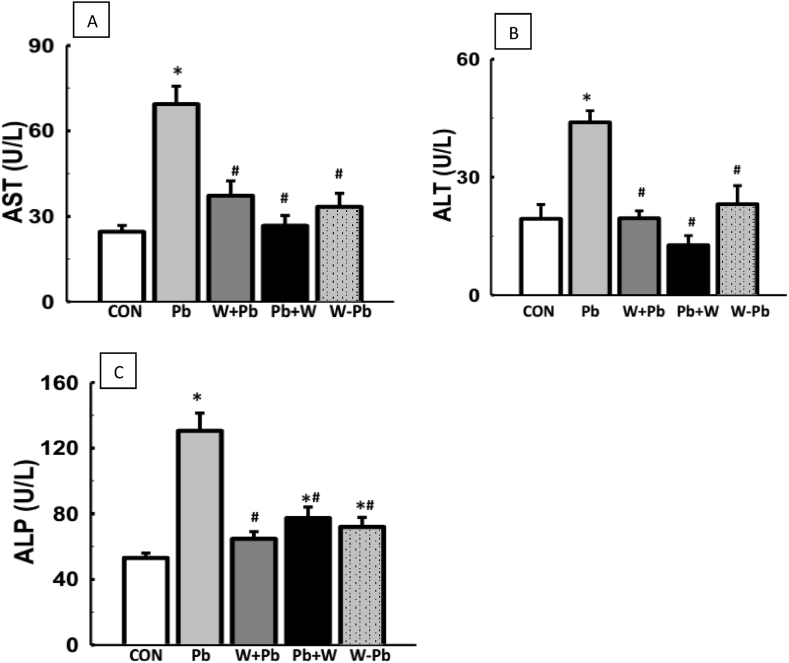
There was increased serum ALP, AST and ALT activities in Pb-exposed rats when compared with the control. However, WM-treatment in Pb-exposed rats led to reduced serum ALP, AST and ALT activities when compared with Pb-exposed rats (Fig. 5).
Fig. 5.
WM ameliorated impaired kidney function biomarkers in Pb-exposed male Wistar rats. WM reversed elevated serum creatinine and urea concentration in Pb-exposed rats (A&B). Values are expressed as mean ± SEM of 6 rats per group; *p < 0.05 vs CON, #p < 0.05 vs Pb group. Data were analyzed by one-way ANOVA and Bonferroni's Post hoc analysis; CON; control, Pb; lead, WM + Pb; watermelon rind pre-treated group, Pb + WM; watermelon rind post-treated group, WM – Pb; watermelon rind simultaneous treated group.
3.4. Effect of watermelon rind ethanol extract on serum uric acid and endothelial dysfunction biomarker in lead acetate-exposed male Wistar rats
Serum uric acid was increased whereas nitric oxide level was decreased in Pb-exposed rats when compared with the control whereas WM-treatment reduced serum uric acid and increased nitric oxide when compared with Pb-exposed group (Fig. 7).
Fig. 7.
WM reversed elevated serum uric acid (A) and reduced nitric oxide level (B) in Pb-exposed male Wistar rats. Values are expressed as mean ± SEM of 6 rats per group; *p < 0.05 vs CON, #p < 0.05 vs Pb group. Data were analyzed by one-way ANOVA and Bonferroni's Post hoc analysis; CON; control, Pb; lead, WM + Pb; watermelon rind pre-treated group, Pb + WM; watermelon rind post-treated group, WM – Pb; watermelon rind simultaneous treated group.
4. Discussion
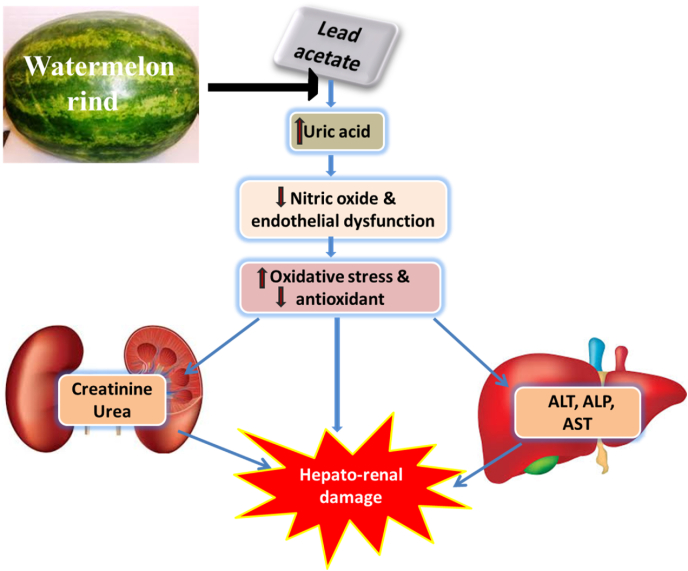
This study examined the potential of oral administration of watermelon rind ethanol extract against lead acetate-induced tissue damage. The experiment revealed that the extract improves the antioxidant defense system. In addition, the rats treated with watermelon rind extract before or after exposure to lead acetate had improved body weight gain. The ethanol extract of watermelon rind also caused a reduction in serum concentration of uric acid, urea and creatinine in lead acetate exposed rats indicating an improved kidney function. Similarly, liver function enzymes (ALT, AST & ALP) activities were decreased after treatment of Pb-exposed rats with watermelon rind extract. However, the ameliorative effect of watermelon rind extract against Pb-induced toxicity was accompanied by decreased serum MDA level and LDH activity while an increase in serum nitric oxide level, GSH concentration and GPx activity was noticed (Fig. 8).
Fig. 8.
Schematic diagram illustrating the likely pathway through which WM protects the kidney and liver from damage in Pb-exposed rats. WM; watermelon rind extract; Pb; lead.
The finding of an improved antioxidant defense by watermelon rind extract in this study is line with previous reports that the watermelon rind is a rich source of numerous antioxidant and bioactive molecules such as betacarotene, lycopene, alkaloids, vitamin C, saponin, flavonoids, cardiac glycosides, moisture, phenol, protein, lipid, carbohydrates and fiber (Rahman et al., 2013). Likewise, watermelon rind extract caused an increase in serum nitric oxide and this is supported by the study that reported that the rind of watermelon is a rich source of citrulline, a known stimulator of nitric oxide (Mandel et al., 2005). Since, report has revealed that nitric oxide improves oxidative stress tolerance by acting as a hydroxyl radical scavenger (Akashi et al., 2004). Hence, the observed reduction in oxidative stress in the watermelon rind extract treated rats in the present study may be through nitric oxide-dependent mechanism (Fig. 7).
Consequently, the studies of Kolawole and coworkers also buttressed our finding that watermelon rind extract has a protective effect against lead-induced kidney and liver toxicity with their report of beneficial effects of methanol extract of watermelon rind on reproductive parameters, gastric ulcer and diabetes (Kolawole et al., 2016; Kolawole and Dapper, 2017). Likewise, previous studies have demonstrated the tissue protective, anti-urolithiatic, neuroprotective, antidiabetic, antioxidative and diuretic activities of watermelon juice in rodents (Akashi et al., 2004; Mohammad et al., 2014; Oseni et al., 2015; Oyenihi et al., 2016). Hence, the reduction in serum AST, ALT, ALP activities, urea, and creatinine levels by watermelon rind extract is noteworthy and further signify the hepato-renal protective potentials of the extract (Figs. 5 and 6).
The increase in the activities of ALT and AST in the lead acetate-treated rats implies damage was done to the cytoarchitectural hepatic integrity in the exposed rats. It is primarily due to the release of these enzymes from the hepatocytes into the blood stream (Concepcion et al., 1993). Also an increase in the ALT and AST activities are linked to elevation in hepatic microsomal membrane fluidity, free radical generation and alteration of the liver tissue (Ibrahim et al., 2012). Furthermore, elevated level of ALP is suggestive of biliary damage or blockage of the biliary tree, which impede hepatic blood flow (Farida et al., 2012). However, the observed decrease in the activities of these enzymes in serum by watermelon rind extract may be attributed to the extract's ability to prevent their leakage from the liver cytosol. Hence, watermelon rind improves liver function through reduced activities of the liver injury enzymes (Fig. 6).
Fig. 6.
WM reduced elevated liver injury markers in Pb-exposed male Wistar rats. WM reversed elevated serum ALT, AST and ALP in Pb-exposed rats (A–C). Values are expressed as mean ± SEM of 6 rats per group; *p < 0.05 vs CON, #p < 0.05 vs Pb group. Data were analyzed by one-way ANOVA and Bonferroni's Post hoc analysis; CON; control, Pb; lead, WM + Pb; watermelon rind pre-treated group, Pb + WM; watermelon rind post-treated group, WM – Pb; watermelon rind simultaneous treated group.
The results obtained in this study also showed increase in serum creatinine, urea and uric acid in the lead acetate-treated rats which were ameliorated in the watermelon rind extract-treated rats suggesting a positive impact of the extract on kidney function. Although serum creatinine concentration is an important biomarker of renal function, which is elevated in the blood due to faulty glomeruli resulting from renal damage (Miller et al., 2005). Urea another renal biomarker was examined in this study, interestingly the watermelon rind extract reversed the increase in urea level caused by lead acetate exposure. Urea is a toxic byproduct derived from ammonia metabolism in the body. In the present study, increased urea levels caused by lead acetate poisoning could be linked to renal ischemia, ammonia conversion to urea as a result of increased arginase synthesis, or other extra-renal disorders (El-Demerdash et al., 2004). Though the precise mechanism by which lead acetate causes renal damage is not fully know, but oxidative stress has been implicated.
Furthermore, watermelon rind extract in this study ameliorated elevation in serum uric acid induced by lead acetate. It is noteworthy that watermelon rind extract might have renoprotective effect. Also, serum uric acid accumulation has been associated with oxidative stress, endothelial dysfunction and impairment of nitric oxide biosynthesis which may play significant contributory role in the progression of chronic kidney disease. Uric acid is mainly cleared by the kidney; hence its level rises with regressing kidney functionality. More so, elevated serum uric acid levels have been linked with reduced glomerular filtration rate (Feig, 2009). Therefore, it has been suggested that uric acid itself plays a causative role in the pathogenesis of acute and chronic kidney injury. Studies have shown that the mechanism of action responsible for uric acid associated-kidney damage may include activation of the rennin-angiotensin system and inflammation (Kang and Chen, 2011).
In addition, increased serum uric acid levels and oxidative stress in lead-acetate treated rats in our findings is corroborated by the report that hyperuricemia strongly reveal and may even cause oxidative stress, metabolic syndrome and insulin resistance, which are risk factors for liver disease progression. Furthermore, hepatic vascular occlusion has been associated with uric acid elevation (Kogure et al., 1997). Hence high serum uric acid level has been connected with the development of cirrhosis and the occurrence of raised serum liver enzymes which are sensitive indicators of liver function and hepatocytes injury. Hence, the serum uric acid level might be a risk factor for the incidence of chronic liver injury (Afzali et al., 2010). In line with the finding of this study, that lead acetate caused elevated serum liver enzymes (AST, ALP & ALT) associated with hyperuricemia; studies have reported the association between elevated ALT and serum uric acid suggesting a relationship between serum uric acid level and liver function markers in hepatocytes injury (Zelber-Sagi et al., 2015). Therefore, this finding of uric acid-mediated tissue damage in lead acetate-treated rats is noteworthy because watermelon rind extract led to reduction in circulating uric acid levels and liver injury enzymes. This implies that watermelon rind extract confers hepatoprotective ability on lead acetate-treated rats through uric acid-mediated pathway.
Consequently, in this study, lead acetate induced hepato-renal injury is accompanied with impaired nitric oxide biosynthesis which has been associated with the development of endothelial dysfunction and oxidative stress. Furthermore, impaired nitric oxide bioavailability, oxidative stress and endothelial dysfunction may be due to the observed elevation in serum uric acid in the lead acetate-treated rats. This coincides with the report that elevated uric acid disrupts nitric oxide production and ultimately leads to tissue injury (Sánchez-Lozada et al., 2005, 2008). Nitric oxide is produced from L-arginine, which is catalytically degraded by arginase and activated by uric acid, resulting in a decrease in serum nitric oxide levels (Papežíková et al., 2013). Uric acid is a powerful predictor of macrovascular problems and can be utilized to identify the level of oxidative stress in a biological system (Bos et al., 2006; Sánchez-Lozada et al., 2008). As a result, the mechanism(s) through which uric acid might cause organ damage are not yet fully known, however there is mounting evidence that endothelial dysfunction is a key factor in uric acid-induced tissue damage (Papežíková et al., 2013). It is important to note that watermelon rind extract led to increased serum nitric oxide in lead acetate-treated rats. Therefore, our finding may be associated with the fact that watermelon rind extract is reach in citrulline a major precursor for L-arginine a nitric oxide synthase substrate in the production of nitric oxide. However, this implies that watermelon rind extract improved endothelial function through improved nitric oxide biosynthesis which may be through uric acid-mediated mechanism. Also, watermelon rind extract led to reduced serum uric acid level thereby mitigating against the impaired nitric oxide biosynthesis, endothelial dysfunction and tissue damage in lead acetate-treated rats. This aligns with the report that serum uric acid reduction attenuated renal damage, inflammation and macrophages M1/M2 ratio (Haryono et al., 2018).
Oxidative stress is defined as an imbalance between the creation of free radicals and the cell's ability to neutralize or repair the damage caused by these reactive products. Report exist that oxidative stress is the major mechanism of lead-induced toxicity (Flora et al., 2012). Lead acetate has been reported to generate reactive oxygen species and distort antioxidant action through the exhaustion of their intracellular reserves (Flora et al., 2012). Similarly, we observed in this study that serum GSH, SOD and GPx activities were reduced whereas serum MDA (lipid peroxidation) and LDH activity a biomarker of tissue damage was elevated in lead acetate-treated rats. However, GSH, SOD and GPx activities were increased while serum MDA and LDH were decreased by the protective effects of watermelon rind extract. This implies that watermelon rind extract protects against lead acetate-induced oxidative stress and tissue damage by improving the antioxidant defenses.
In conclusion, it can be established during this study that watermelon rind ethanol extract treatment which includes pre-, post- or simultaneous treatment result in significant hepato-renal protective potential of the extract against lead-induced toxicity. However, there is an exception of the reduced body weight gain noticed in the watermelon rind extract pre-treated group and elevated GPx activity in the watermelon rind extract post-treated group when compared with watermelon rind extract pre-treated and simultaneous treated groups respectively. Hence, watermelon rind (an agricultural waste) protects kidney and liver against lead toxicity through improved antioxidant defenses at least in part, via uric acid/nitric oxide-dependent pathway. Therefore, this study showed that agricultural wastes could be applied for mitigation against environmental and health hazards most especially lead containing toxic compounds or their related compounds. There is need to perform isolation, purification, and structural elucidation of the active constituents which are responsible for the protection of liver and kidney against lead toxicity.
Credit authors statement
O.S Michael and O. Bamidele: Conceptualization, Methodology, Supervision, O.S Michael, O. Writing - original draft. P. Ogheneovo and T.A Ariyo: Project administration, L.D Adedayo, C.O Adetunji and F.O Awobajo: Data curation and analysis. O.S Michael, O.I Oluranti, C.O. Adetunji, and E.O Soladoye: Review and editing. All authors have read and approved the final version of the manuscript.
Funding
There was no financial assistance received for the study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors appreciate Mr Adebowale of Bridge Biotech, Ilorin, Nigeria for his technical support.
Abbreviations
- ALP
Alkaline phosphatase
- ALT
Alanine transferase
- AST
Aspartate transaminase
- GPx
Glutathione peroxidase
- GSH
Reduced glutathione
- LDH
Lactate dehydrogenase
- MDA
Malondialdhyde
- Pb
Lead acetate
- rpm
revolutions per minute
- WM
Watermelon rind extract
References
- Adesanya A.O., Olaseinde O.O., Oguntayo O.D., Otulana J.O., Adefule A.K. Effects of methanolic extract of Citrullus lanatus seed on experimentally induced prostatic hyperplasia. Eur. J. Med. Plants. 2011;1(4):171–179. [Google Scholar]
- Afzali A., Weiss N.S., Boyko E.J., Ioannou G.N. Association between serum uric acid level and chronic liver disease in the United States. Hepatology. 2010;52:578–589. doi: 10.1002/hep.23717. [DOI] [PubMed] [Google Scholar]
- Ahn J., Choi W., Kim S., Ha T. Anti-diabetic effect of watermelon (Citrullus vulgaris Schrad) on Streptozotocin-induced diabetic mice. Food Sci. Biotechnol. 2011;20(1):251–254. [Google Scholar]
- Akashi K., Nishimura N., Ishida Y., Yokota A. Potent hydroxylradical-scavenging activity of drought-induced type-2 metallothionein inwild watermelon. Biochem. Biophys. Res. Commun. 2004;323(1):72–78. doi: 10.1016/j.bbrc.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Babio N., Martínez-González M.A., Estruch R., Wärnberg J., Recondo J., Ortega-Calvo M., Serra-Majem L., et al. Associations between serum uric acid concentrations and metabolic syndrome and its components in the PREDIMED study. Nutr. Metabol. Cardiovasc. Dis. 2015;25:173–180. doi: 10.1016/j.numecd.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Bos M.J., Koudstaal P.J., Hofman A., Witteman J.C., Breteler M.M. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37(6):1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- Cheng D., Li H., Zhou J., Wang S. Chlorogenic acid relieves lead-induced cognitive impairments and hepato-renal damage via regulating the dysbiosis of the gut microbiota in mice. Food Funct. 2019;10(2):681–690. doi: 10.1039/c8fo01755g. [DOI] [PubMed] [Google Scholar]
- Concepcion N.M., Pilar M.M., Martín A., Jiménez J., Pilar U.M. Free radical scavenger and antihepatotoxic activity of Rosmarinus tomentosus. Planta Med. 1993;59:312–314. doi: 10.1055/s-2006-959688. [DOI] [PubMed] [Google Scholar]
- Dobrakowski M., Pawlas N., Kasperczyk A., Kozłowska A., Olewińska E., Machoń-Grecka A., Kasperczyk S. Oxidative DNA damage and oxidative stress in lead-exposed workers. Hum. Exp. Toxicol. 2017;36(7):744–754. doi: 10.1177/0960327116665674. [DOI] [PubMed] [Google Scholar]
- El-Demerdash F.M., Yousef M.I., Kedwany F.S., Baghdadi H.H. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and beta-carotene. Food Chem. Toxicol. 2004;42(10):1563–1571. doi: 10.1016/j.fct.2004.05.001. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority, EFSA . EFSA Parma Italy; 2013. Scientific Opinion on Lead in Food. EFSA Panel on Contaminants in the Food Chain (CONTAM) [Google Scholar]
- Fang J.Y., Wang P.W., Huang C.H., Hung Y.Y., Pan T.L. Evaluation of the hepatotoxic risk caused by lead acetate via skin exposure using a proteomic approach. Proteomics. 2014;14(21–22):2588–2599. doi: 10.1002/pmic.201400068. [DOI] [PubMed] [Google Scholar]
- Farida T., Salawu O.A., Tijani A.Y., Ejiofor J.I. Pharmacological evaluation of Ipomea asarifolia (Desr.) against carbon tetrachloride-induced hepatotoxicity in rats. J. Ethnopharmacol. 2012;142(3):642–646. doi: 10.1016/j.jep.2012.05.029. [DOI] [PubMed] [Google Scholar]
- Feig D.I. Uric acid: a novel mediator and marker of risk in chronic kidney disease? Curr. Opin. Nephrol. Hypertens. 2009;18(6):526–530. doi: 10.1097/MNH.0b013e328330d9d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora G., Gupta D., Tiwari A. Toxicity of lead: a review with recent updates. Interdiscipl. Toxicol. 2012;5:47–58. doi: 10.2478/v10102-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara A., Thijs L., Asayama K., Gu Y.M., Jacobs L., Zhang Z.Y., Liu Y.P., et al. Blood pressure in relation to environmental lead exposure in the national health and nutrition examination survey 2003 to 2010. Hypertension. 2015;65(1):62–69. doi: 10.1161/HYPERTENSIONAHA.114.04023. [DOI] [PubMed] [Google Scholar]
- Haryono A., Nugrahaningsih D.A.A., Sari D.C.R., Romi M.M., Arfian N. Reduction of serum uric acid associated with attenuation of renal injury, inflammation and macrophages M1/M2 ratio in hyperuricemic mice model. Kobe J. Med. Sci. 2018;64(3):E107–E114. [PMC free article] [PubMed] [Google Scholar]
- Ibrahim N.M., Eweis E.A., El-Beltagi H.S., Abdel-Mobdy Y.E. Effect of lead acetate toxicity on experimental male albino rat. Asian Pac. J. Trop. Biomed. 2012;2(1):41–46. doi: 10.1016/S2221-1691(11)60187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janbaz K.H., Shabbir A., Mehmood M.H., Gilani A.H. Pharmacological basis for the medicinal use of Rhus coriaria in hyperactive gut disorders. Bangladesh J. Pharmacol. 2014;9:636–644. [Google Scholar]
- Kang D.H., Chen W. Uric acid and chronic kidney disease: new understanding of an old problem. Semin. Nephrol. 2011;31(5):447–452. doi: 10.1016/j.semnephrol.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Kogure K., Tatemoto K., Maruyama Y., Ikarashi Y., Makuuchi M., Jamieson N.V. Uric acid changes in serum during different forms of hepatic vascular inflow occlusion. Life Sci. 1997;60(20):1781–1791. doi: 10.1016/s0024-3205(97)00138-0. [DOI] [PubMed] [Google Scholar]
- Kolawole T.A., Dapper D.V. Gastro-protective effects of the methanolic extract of the rind of Citrullus lanatus on indomethacin induced gastric ulceration in male wistar rats. Acta Pharmaceut. Sci. 2017;55:4. doi: 10.23893/1307-2080.APS.05526. [DOI] [Google Scholar]
- Kolawole T.A., Ojeka S.O., Dapper D.V. Anti-diabetic effects of the methanolic extract of the rind of Citrullus lanatus (watermelon) in alloxan induced diabetes in male albino wistar rats. J. Med. Med. Sci. 2016;7(2):23–29. [Google Scholar]
- Kwon S.Y., Bae O.N., Noh J.Y., Kim K., Kang S., Shin Y.J., Lim K.M., et al. Erythrophagocytosis of lead-exposed erythrocytes by renal tubular cells: possible role in lead-induced nephrotoxicity. Environ. Health Perspect. 2015;123(2):120–127. doi: 10.1289/ehp.1408094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Zhao K., Zhang H., Liu L., Xiong F., Wang K., Chen B. Lead exposure reduces sperm quality and DNA integrity in mice. Environ. Toxicol. 2018;33(5):594–602. doi: 10.1002/tox.22545. [DOI] [PubMed] [Google Scholar]
- Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H., Amann M., et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel H., Levy N., Izkovitch S., Korman S.H. Elevated plasma citrulline and arginine due to consumption of Citrullus vulgaris (watermelon) J. Inherit. Metab. Dis. 2005;28(4):467–472. doi: 10.1007/s10545-005-0467-1. [DOI] [PubMed] [Google Scholar]
- Miller W.G., Myers G.L., Ashwood E.R., Killeen A.A., Wang E., Thienpont L.M., Siekmann L. Creatinine measurement: state of the art in accuracy and interlaboratory harmonization. Arch. Pathol. Lab Med. 2005;129(3):297–304. doi: 10.5858/2005-129-297-CMSOTA. [DOI] [PubMed] [Google Scholar]
- Mohammad M.K., Mohamed M.I., Zakaria A.M., Abdul Razak H.R., Saad W.M. Watermelon (Citrullus lanatus (Thunb.) Matsum. and Nakai) juice modulates oxidative damage induced by low dose X-ray in mice. BioMed. Res. Int. 2014;2014:512834. doi: 10.1155/2014/512834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naknaen P., Itthisoponkul T., Sondee A., Angsombat N. Utilization of watermelon rind waste as a potential source of dietary fiber to improve health promoting properties and reduce glycemic index for cookie making. Food Sci. Biotechnol. 2016;25(2):415–424. doi: 10.1007/s10068-016-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offor S.J., Mbagwu H.O., Orisakwe O.E. Lead Induced hepato-renal damage in male albin rats and effects of activated charcoal. Front. Pharmacol. 2017;8:107. doi: 10.3389/fphar.2017.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseni O.A., Odesanmi O.E., Oladele F.C. Antioxidative and antidiabetic activities of watermelon (Citrullus lanatus) juice on oxidative stress in alloxan-induced diabetic male Wistar albino rats. Niger. Med. J. 2015;56(4):272–277. doi: 10.4103/0300-1652.169707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyenihi O.R., Afolabi B.A., Oyenihi A.B., Ogunmokun O.J., Oguntibeju O.O. Hepato- and neuro-protective effects of watermelon juice on acute ethanol-induced oxidative stress in rats. Toxicol. Rep. 2016;12(3):288–294. doi: 10.1016/j.toxrep.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papežíková I., Pekarová M., Kolářová H., Klinke A., Lau D., Baldus S., Lojek A., et al. Uric acid modulates vascular endothelial function through the down regulation of nitric oxide production. Free Radic. Res. 2013;47(2):82–88. doi: 10.3109/10715762.2012.747677. [DOI] [PubMed] [Google Scholar]
- Rahman H., Priyanka P., Lavanya T., Srilakshmi N., Kumar P.R. A review on ethnobotany, phytochemistry and pharmacology of Citrullus lanatus L. Int. Res. J. Pharmaceut. Appl. Sci. 2013;3(2):77–81. [Google Scholar]
- Reuben A., Caspi A., Belsky D.W., Broadbent J., Harrington H., Sugden K., Houts R.M., et al. Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood. J. Am. Med. Assoc. 2017;317(12):1244–1251. doi: 10.1001/jama.2017.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Lozada L.G., Soto V., Tapia E., Avila-Casado C., Sautin Y.Y., Nakagawa T., Franco M., et al. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am. J. Physiol. Ren. Physiol. 2008;295(4):F1134–F1141. doi: 10.1152/ajprenal.00104.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Lozada L.G., Tapia E., Santamaría J., Avila-Casado C., Soto V., Nepomuceno T., Rodríguez-Iturbe B., et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67(1):237–247. doi: 10.1111/j.1523-1755.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- Yuan H., Yu C., Li X., Sun L., Zhu X., Zhao C., Zhang Z., Yang Z. Serum uric acid levels and risk of metabolic syndrome: a dose-response meta-analysis of prospective studies. J. Clin. Endocrinol. Metab. 2015;100(11):4198–4207. doi: 10.1210/jc.2015-2527. [DOI] [PubMed] [Google Scholar]
- Zelber-Sagi S., Ben-Assuli O., Rabinowich L., Goldstein A., Magid A., Shalev V., Shibolet O., et al. The association between the serum levels of uric acid and alanine aminotransferase in a population-based cohort. Liver Int. 2015;35(11):2408–2415. doi: 10.1111/liv.12842. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Gao X., Guo M., Jiang H., Cao Y., Zhang N. The protective effect of baicalin against lead-induced renal oxidative damage in mice. Biol. Trace Elem. Res. 2017;175(1):129–135. doi: 10.1007/s12011-016-0731-2. [DOI] [PubMed] [Google Scholar]