Abstract
Background:
The coronavirus disease 2019 (COVID-19) presents various phenotypes from asymptomatic involvement to death. Disseminated intravascular coagulopathy (DIC) is among the poor prognostic complications frequently observed in critical illness. To improve mortality, a timely diagnosis of DIC is essential. The International Society on Thrombosis and Hemostasis (ISTH) introduced a scoring system to detect overt DIC (score ≥5) and another category called sepsis-induced coagulopathy (SIC) to identify the initial stages of DIC (score ≥4). This study aimed to determine whether clinicians used these scoring systems while assessing COVID-19 patients and the role of relevant biomarkers in disease severity and outcome.
Materials and Methods:
An exhaustive search was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses, using Medline, Embase, Cochrane, CINAHL, and PubMed until August 2020. Studies considering disease severity or outcome with at least two relevant biomarkers were included. For all studies, the definite, maximum, and minimum ISTH/SIC scores were calculated.
Results:
A total of 37 papers and 12,463 cases were reviewed. Studies considering ISTH/SIC criteria to detect DIC suggested a higher rate of ISTH ≥5 and SIC ≥4 in severe cases and nonsurvivors compared with nonsevere cases and survivors. The calculated ISTH scores were dominantly higher in severe infections and nonsurvivors. Elevated D-dimer was the most consistent abnormality on admission.
Conclusion:
Higher ISTH and SIC scores positively correlate with disease severity and death. In addition, more patients with severe disease and nonsurvivors met the ISTH and SIC scores for DIC. Given the high prevalence of coagulopathy in COVID-19 infection, dynamic monitoring of relevant biomarkers in the form of ISTH and SIC scoring systems is of great importance to timely detect DIC in suspicious patients.
Keywords: Coagulopathy, coronavirus disease 2019, disseminated intravascular coagulation, International Society on Thrombosis and Hemostasis, sepsis-induced coagulopathy score
INTRODUCTION
The novel coronavirus disease 2019 (COVID-19) presents with various features ranging from asymptomatic and mild disease to life-threatening conditions and death.[1,2] Since the disease outbreak, many studies have focused on COVID-associated coagulopathy and its prognostic significance in disease severity and mortality rate.[3,4] Accumulated data revealed that in COVID-19 infection, the excessive inflammatory response, platelet aggregation, endothelial injury, and stasis cause systemic arterial, venous and capillary thrombosis. This results in laboratory changes such as lymphopenia, elevated lactate dehydrogenase, marked D-dimer, increased C-reactive protein, mild prothrombin time (PT) prolongation, and mild thrombocytopenia. Clinical manifestations include deep vein thrombosis, pulmonary embolism, disseminated intravascular coagulopathy (DIC), myocardial ischemia, acute kidney injury, acute mesenteric ischemia, and brain stroke.[5,6] DIC is one of the most poor-prognostic complications identified in COVID-19 patients. In a retrospective review of 183 COVID-19 cases, DIC appeared in over two-third of nonsurvivors and <1% of survivors, indicating a prognostic value of DIC in disease severity and outcome.[4]
Like other underlying etiologies, COVID-associated DIC is caused by dysregulation of the delicately balanced coagulation and fibrinolysis system, which leads to consumptive coagulopathy and presents with widespread clotting and concomitant bleeding.[7,8,9,10] However, unlike the classic pattern, COVID-associated DIC predominantly features thromboembolism rather than hyperfibrinolysis, resulting in adverse thromboembolic outcomes such as hypoperfusion and multi-organ damage.[10,11,12] It has been proposed that the early recognition and management of DIC in COVID-19 patients plays a fundamental role in reducing the death toll.[10]
There is no single biomarker of high certainty for the diagnosis of DIC. However, a combination of biomarkers changes, especially when repeated in a patient with clinical suspicion of DIC, can be used as a diagnostic tool with acceptable certainty. In 2001, the International Society on Thrombosis and Hemostasis (ISTH) launched a laboratory-based scoring system using platelet count, D-Dimer or fibrin degradation products (FDP), PT, and fibrinogen for diagnosis of DIC, with a score ≥5 being indicative of overt DIC.[11] To more accurately identify septic patients who were inclined to DIC, sepsis-induced coagulopathy (SIC) scoring system was introduced in 2017. It evaluates patients based on Sequential Organ Failure Assessment (SOFA) score; an indicator of four organs' function (respiratory, cardiovascular, hepatic, and renal); together with platelet count and international normalized ratio (INR) and suggests that patients with SIC score ≥4 are at an increased chance of developing overt DIC.[13]
Regarding the fact that DIC is a dynamic process,[14] regular monitoring of pertinent biomarkers in the disease course is pivotal to detect DIC in optimum time for planning treatments.[15] Previous reports of patients who died of an aggravated COVID-19 infection highlighted a noticeable rise in PT and D-dimer levels and a decrease in fibrinogen level in days 10–14 of disease onset.[4] Besides, the administration of anticoagulants in the initial stages of DIC (SIC ≥4) has offered significantly improved outcomes and mortality in COVID-19 patients, according to prior studies.[10]
Given the crucial role of diagnosis and treatment of DIC in preventing drastic complications and declining mortality,[10,16,17] the present systematic review aimed to explore whether clinicians considered ISTH/SIC criteria a valid scoring system for DIC detection and the value of these scores in predicting disease severity and mortality.
MATERIALS AND METHODS
Search strateg
A systematic literature search was conducted to assess the diagnostic value of ISTH criteria in patients with COVID-19, using Medline, Embase, Cochrane, and Cochrane Database of Systematic Reviews via Ovid, CINAHL through Ebsco, and PubMed until August 2020. Relevant articles were captured applying MeSH terms “ISTH,” “coagulopathy,” “DIC,” and “D-dimer” and “prothrombin time” and then added to the terms “COVID” OR “coronavirus” OR “SARS-Cov-2,” including possible adjacencies adjusted consistently for various databases. All studies were having the reports of relevant coagulation biomarkers (platelet count, D-dimer/FDP, PT/INR, Fibrinogen, and SOFA score) with regard to survival outcomes (survivors/nonsurvivors) and disease severity (severe/nonsevere disease) were included. The exclusion criteria were as follows: 1 - studies reporting <2 relevant coagulation biomarkers, 2 - case reports, reviews, conference abstracts, and studies published in a language other than English, 3 - studies conducted on children, pregnant population or patients with certain underlying conditions (chronic kidney disease, cardiovascular disease, respiratory disorders, etc.). Relevant abstracts and those needing further investigation were considered for full-text review, and eligible studies were included for data extraction. We also screened reference lists from included full-text articles. Publication review, screening, and data extraction were done by two investigators (HF and BRS) independently, and disagreements were discussed with the third author (MS).
To address the research question data regarding authors, year, study design, sample size, platelet count, D-dimer, FDP, PT, INR, fibrinogen, SOFA score, and the reports on ISTH/SIC, scores were extracted and arranged into a Microsoft Excel spreadsheet. Enrolled papers were categorized into two main groups based on disease severity (severe vs. nonsevere) and survival outcome (survivors vs. nonsurvivors). In terms of disease severity, in studies classifying patients into three or four groups (mild/moderate/severe/critical), data were manually integrated, considering mild to moderate involvement as nonsevere and severe to critical involvement as severe. Intensive care unit (ICU) admission was also accounted for a severe illness in contrast to non-ICU admission. Then, to further evaluate the role of ISTH and SIC in COVID-19 in studies not reporting these scores, the definite, maximum, and minimum ISTH and SIC scores were calculated. In studies having all relevant lab data, the definite ISTH and SIC scores were measured and reported. In studies with missing data, the minimum and maximum scores of ISTH (Min-ISTH and Max-ISTH) and SIC (Min-SIC and Max-SIC) were calculated considering the highest and the lowest possible points for the undocumented markers. The quality of the studies was evaluated using the Newcastle Ottawa Scale assessment form.[18]
Description of the method used for calculating the definite, minimum, and maximum scores of International Society on Thrombosis and Hemostasis and sepsis-induced coagulopathy
The distribution of points in the ISTH and SIC scoring systems is shown in Table 1. In studies with complete data for calculating ISTH and SIC, we reported the definite score by adding available points. We calculated the minimum and maximum scores by considering the lowest (0) and the highest possible points for the absent data in studies with missing data. For instance, in a study by He et al.,[19] the reported markers in severely ill patients were D-dimer = 9.89 μg/mL (reference range = 0–0.55), PT = 13 sec (reference range = 9–13 s), and fibrinogen = 3.3 g/L (reference range = 2–4) and the absent marker was platelet count. We summed the available points to calculate the min-ISTH: D-dimer >4 times ULN = 3 points, PT <3 s prolongation = 0 point, fibrinogen >1 = 0 point, and the Min-ISTH of 3 was obtained. To calculate the Max-ISTH, we considered the highest possible point for the absent marker (platelet <100,000 = 2 points) and added it to the minimum score (3); thus, the max-ISTH of 5 was achieved. We followed the same instruction for measuring the minimum and maximum SIC scores.
Table 1.
International Society on Thrombosis and hemostasis and sepsis-induced coagulopathy scoring systems for disseminated intravascular coagulopathy diagnosis
| Items | ISTH | SIC | Points |
|---|---|---|---|
| Platelet count (×109/L) | ≥100 | ≥150 | 0 |
| 50-100 | 100-150 | 1 | |
| <50 | <100 | 2 | |
| D-Dimer elevation or FDP elevation (mg/L) | <2 times ULN | - | 0 |
| 2-4 times ULN | - | 2 | |
| >4 times ULN | - | 3 | |
| <10 | - | 0 | |
| 10-25 | - | 2 | |
| >25 | - | 3 | |
| PT prolongation (s)/INR increase | <3 | <1.2 | 0 |
| 3-6 | 1.2-1.4 | 1 | |
| ≥3 | ≥1.4 | 2 | |
| Fibrinogen (g/L) | >1 | - | 0 |
| <1 | - | 1 | |
| SOFA score | - | 0 | 0 |
| - | 1 | 1 | |
| - | ≥2 | 2 |
Overt DIC: ISTH score ≥5, early stages of DIC: SIC score ≥4. DIC=Disseminated intravascular coagulopathy; ISTH=International Society on Thrombosis and hemostasis; SIC=Sepsis-induced coagulopathy; FDP=Fibrin degradation products; PT=Prothrombin time; INR=International normalized ratio; SOFA=Sequential organ failure assessment
RESULTS
A comprehensive search yielded 842 studies. After title and abstract screening and excluding duplicates (n = 739), 103 articles were selected for full-text screening. Of these, 37 were included in the review, following the exclusion of irrelevant articles. We also found 14 studies using backward citation searching (checking reference lists). Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart for the article selection process.[20]
Figure 1.
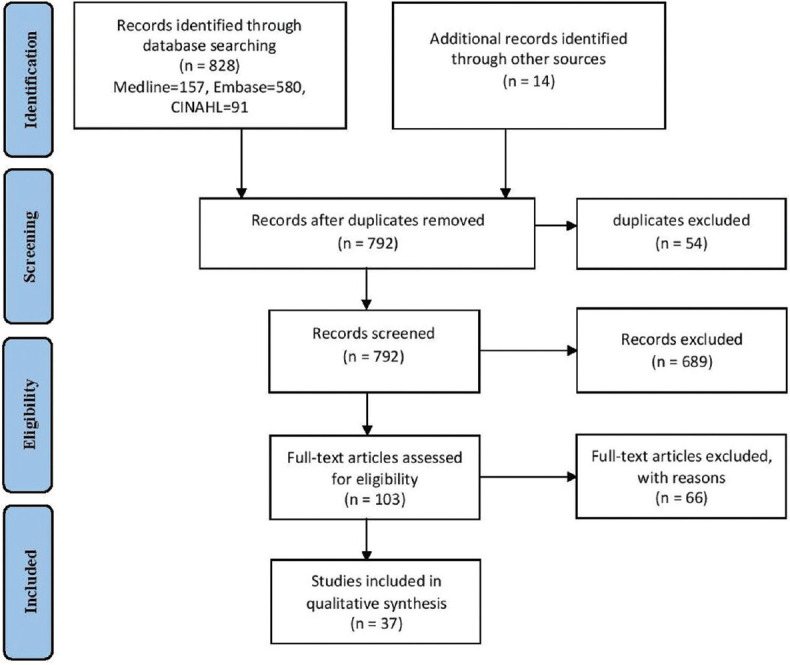
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram
A total number of 37 papers, including 12,463 cases of COVID-19, were systematically reviewed. All articles were published in 2020, among which 20 reported data about disease severity and 17 about disease outcome. Nearly all of the selected papers had a retrospective design, and most of them were assessed as high quality.
Out of 37 selected studies, only six used the ISTH/SIC scoring systems to detect DIC in COVID-19 infection [Table 2].[4,10,21,22,23,24] The findings confirmed that the incidence of overt DIC was notably higher in critically ill patients and those who died. Data for calculating the definite score were complete only in five studies for ISTH,[21,22,23,24,25] which showed no difference between groups in three studies (the score of 0 for severe and nonsevere/survivors and nonsurvivors) [Table 3].
Table 2.
The reported incidence of overt disseminated intravascular coagulopathy and early stages of disseminated intravascular coagulopathy
| Author | ISTH score ≥5 incidence | SIC score ≥4 incidence |
|---|---|---|
| Bao et al.[21] | 6.1% in severe cases versus 0% in nonsevere cases | - |
| Liao et al.[22] | 15% in severe cases | - |
| Liu et al.[23] | 57.1% in nonsurvivors versus 1.8% in survivors | - |
| Martin-Rojas et al.[24] | 22.2% in nonsurvivors versus 3.7% in survivors | - |
| Tang et al.[4] | 71.6% in nonsurvivors versus 0.6% in survivors | - |
| Tang et al.[10] | - | 41% in nonsurvivors vs 13.3% in survivors |
ISTH=International Society on Thrombosis and Hemostasis; SIC=Sepsis-induced coagulopathy
Table 3.
The calculated definite scores of International Society on Thrombosis and hemostasis in coronavirus disease 2019 cases
Table 4 describes the data related to the first group of studies that compares severe and nonsevere COVID-19 cases (n = 20). The total number of severe cases was 3,359, and nonsevere cases were 4469 patients. Regarding the components of the ISTH scoring system, the platelet count was reported in 12 out of 20 studies,[1,21,22,25,26,27,28,29,30,31,32,33] all of which were within the normal range but with lower values in the severe compared to the nonsevere group in nine studies. Only five studies noted FDP levels, with marked elevation in severe versus nonsevere illness.[19,22,34,35,36] When looking at SIC scoring system components, five studies recorded INR,[21,34,35,36,37] and only two studies reported the SOFA score,[27,29] with remarkably higher scores in severe involvement. According to Liu et al., the SOFA score in moderate, severe, and critical COVID-19 infection was 0, 1, and 5, respectively.[29] In another study by Li et al., the mean SOFA score was 4.3 ± 2.5 in severe cases versus 2.5 ± 2.3 in nonsevere.[27]
Table 4.
The most reported international society on thrombosis and hemostasis and sepsis-induced coagulopathy parameters in nonsevere and severe cases of coronavirus disease 2019
| Author/study design | Sample size | PLT × 1000 | D-dimer (µg/mL)/FDP (mg/L) | Fibrinogen (g/L) | PT (s)/INR | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Nonsevere | Severe | Nonsevere | Severe | Nonsevere | Severe | Nonsevere | Severe | ||
| Bao et al.[21]/prospective cohort | 178 | 251 | 186 | 0.42 | 1.05 | 3.23 | 3.62 | 12.7/1.06 | 14.5/1.21 |
| Gao et al.[39]/retrospective cohort | 43 | NR | NR | 0.21 | 0.49 | 3.11±0.83 | 3.84±1.00 | 11.26±1.42 | 12.03±1.21 |
| Han et al.[34]/retrospective cohort | 94 | NR | NR | 2.14/7.92 | 19.39/62.04 | 5.1 | 4.94 | 12.2/1.04 | 12.68/1.09 |
| He et al.[19]/retrospective case series | 53 | NR | NR | 0.38/0.9 | 9.89/32.7 | 3.6 | 3.3 | 12 | 13 |
| Huang et al.[1]/prospective cohort | 41 | 149 | 196 | 0.5 | 2.4 | NR | NR | 10.7 | 12.2 |
| Huang et al.[26]/retrospective cohort | 125 | 206.01±55.61 | 182.46±55.47 | 0.780 | 1.760 | NR | NR | 13.66±0.89 | 13.80±1.70 |
| Li et al.[27]/retrospective cohort | 312 | 233.7±92.6 | 157.4±95.3 | 0.5±0.3 | 4.8±2.1 | NR | NR | 12.7±5.4 | 14.8±4.5 |
| Li et al.[28]/retrospective cohort | 193 | 196 | 165 | 0.57 | 1.18 | NR | NR | 13.6 | 13.5 |
| Liao et al.[22]/retrospective cohort | 380 | 198 | 181.5 | 0.42/1.9 | 3.54/13.1 | 4.29 | 4.48 | 13 | 14.7 |
| Long et al.[38]/retrospective case series | 115 | NR | NR | 0.85 | 2.54 | 4.38 | 4.73 | 12.34 | 12.71 |
| Liu et al.[29]/retrospective cohort | 2044 | 229 | 208 | 0.47 | 1.80 | NR | NR | 13.6 | 14.26 |
| Liu et al.[37]/retrospective cohort | 122 | NR | NR | 0.59 | 1.06 | 4.2 | 4.7 | 10.9/0.93 | 11.7/0.99 |
| Qian et al.[30]/retrospective case series | 91 | 198 | 152 | 0.3 | 0.45 | 3.36 | 3.8 | NR | NR |
| Wang et al.[31]/retrospective case series | 138 | 165 | 142 | 0.166 | 0.414 | NR | NR | 12.9 | 13.2 |
| White et al.[25]/retrospective cross-sectional | 109 | 221 | 288 | 0.323 | 0.555 | 4.33 | 6.42 | 13.3 | 13.6 |
| Yu et al.[32]/retrospective case series | 1663 | 213 | 237 | 0.6 | 0.9 | NR | NR | 13.7 | 13.9 |
| Yu et al.[35]/retrospective cohort | 1561 | NR | NR | 0.5/4 | 1.8/6.5 | NR | NR | 13.35/1 | 14.4/1.1 |
| Zeng et al.[40]/retrospective cohort | 274 | NR | NR | 0.28 | 1.6 | NR | NR | 13.35 | 13.7 |
| Zhang et al.[33]/retrospective case series | 221 | 175 | 169 | 0.184 | 0.443 | NR | NR | 12.7 | 13.4 |
| Zhang et al.[36]/retrospective cohort | 71 | NR | NR | 0.44/2.4 | 5.95/13 | 3.96 | 3.84 | 11.5/1.02 | 12/1.07 |
PLT=Platelet count; FDP=Fibrin degradation product; PT=Prothrombin time; INR=International normalized ratio
Table 5 displays the minimum and maximum ISTH and SIC scores calculated by the authors in severe and nonsevere COVID-19 cases. In none of the included papers, Min-ISTH met the threshold either for overt DIC (ISTH ≥5) or early stages of DIC (SIC ≥4), while Max-ISTH reached the cutoff for overt DIC in five studies[19,34,35,36,38] in four of which only among the severe group and in one study in both groups. Except for six,[30,31,32,34,39,40] the rest 11 studies showed higher ISTH scores in severe than in nonsevere cases. SIC ≥4 was not met in any of the Min-ISTHs.
Table 5.
The calculated minimum and maximum scores of International Society on Thrombosis and Hemostasis and sepsis-induced coagulopathy in severe and nonsevere coronavirus disease 2019 cases
| Author/study design | Minimum-ISTH | Maximum-ISTH | Minimum-SIC | Maximum-SIC | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Nonsevere | Severe | Nonsevere | Severe | Nonsevere | Severe | Nonsevere | Severe | |
| Bao et al.[21]/prospective cohort | - | - | - | - | 0 | 1 | 2 | 3 |
| Gao et al.[39]/retrospective cohort | 0 | 0 | 2 | 2 | 0 | 0 | 6 | 6 |
| Han et al.[34]/retrospective cohort | 3 | 3 | 5 | 5 | 0 | 0 | 4 | 4 |
| He et al.[19]/retrospective case series | 0 | 3 | 2 | 5 | 0 | 0 | 6 | 6 |
| Huang et al.[1]/prospective cohort | 0 | 2 | 1 | 3 | 1 | 0 | 5 | 4 |
| Huang et al.[26]/retrospective cohort | 0 | 2 | 1 | 3 | 0 | 0 | 4 | 4 |
| Li. T et al.[27]/retrospective cohort | 0 | 3 | 1 | 4 | 0 | 0 | 4 | 4 |
| Li et al.[28]/retrospective cohort | 0 | 2 | 1 | 3 | 0 | 0 | 4 | 4 |
| Liao et al.[22]/retrospective cohort | - | - | - | - | 0 | 0 | 4 | 4 |
| Long et al.[38]/retrospective case series | 0 | 3 | 2 | 5 | 0 | 0 | 6 | 6 |
| Liu et al.[29]/retrospective cohort | 0 | 2 | 1 | 3 | 0 | 2 | 2 | 4 |
| Liu et al.[37]/retrospective cohort | 0 | 2 | 2 | 4 | 0 | 0 | 4 | 4 |
| Qian et al.[30]/retrospective case series | 0 | 0 | 2 | 2 | 0 | 0 | 4 | 4 |
| Wang et al.[31]/retrospective case series | 0 | 0 | 1 | 1 | 0 | 3 | 4 | 5 |
| White et al.[25]/retrospective cross-sectional | - | - | - | - | 0 | 0 | 4 | 4 |
| Yu et al.[32]/retrospective case series | 0 | 0 | 1 | 1 | 0 | 0 | 4 | 4 |
| Yu et al.[35]/retrospective cohort | 0 | 2 | 3 | 5 | 0 | 0 | 4 | 4 |
| Zeng et al.[40]/retrospective cohort | 0 | 2 | 2 | 4 | 0 | 0 | 6 | 6 |
| Zhang et al.[33]/retrospective case series | 0 | 0 | 1 | 1 | 0 | 0 | 4 | 4 |
| Zhang et al.[36]/retrospective cohort | 0 | 3 | 2 | 5 | 0 | 0 | 4 | 4 |
ISTH=International Society on Thrombosis and Hemostasis; SIC=Sepsis-induced coagulopathy
Table 6 demonstrates the comparison of the ISTH and SIC scoring systems components between COVID-19 survivors and nonsurvivors (n = 17). The total number of patients who died due to COVID-19 and who recovered were 1280 and 3355, respectively. The lowest reported platelet count was 90,500 in nonsurvivors,[44] which reached the introduced platelet threshold for escalating the score in both ISTH and SIC (1 and 2 points, respectively). Fibrinogen level was only reported in three studies, which was 5 vs. 4.9,[23] 5.75 ± 1.83 vs. 5.26 ± 2.26,[24] and 4.51 vs. 5.16[4] in survivors and nonsurvivors, respectively. As for the SIC parameters, data on SOFA scores were present in six studies, all of which highlighted higher scores in the deceased versus the recovered.[23,44,49,51,52,53] Only two studies provided data on INR, which were higher among COVID-19 patients who failed to survive.[24,43]
Table 6.
The most reported International Society on Thrombosis and Hemostasis and sepsis-induced coagulopathy parameters in coronavirus disease 2019 survivors and nonsurvivors
| Author/study design | Sample size | PLT ×1000 | D-dimer (µg/mL)/FDP (mg/L) | SOFA score | PT (sec)/INR | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Survivors | Nonsurvivors | Survivors | Nonsurvivors | Survivors | Nonsurvivors | Survivors | Nonsurvivors | ||
| Ronderos Botero et al.[41]/retrospective cohort | 157 | 218.4±96.2 | 219.3±111 | 1.682±4.759 | 8.705±17.608 | NR | NR | 13.3±1.8 | 14±1.9 |
| Chen et al.[42]/retrospective case series | 274 | 198 | 156 | 1.1 | 4.6 | NR | NR | 13.9 | 15.5 |
| Giusti et al.[43]/prospective cohort | 209 | 189 | 174 | 0.739 | 1.149 | NR | NR | 13.8±2.4/1.22±0.21 | 15.2±3.1/1.31±0.21 |
| Liu et al.[44]/retrospective cohort | 1190 | 201 | 90.5 | 0.8 | 17.8 | 2 | 10 | 11.4 | 14 |
| Liu et al.[23]/retrospective cohort | 147 | 221 | 155 | 1.3/4.8 | 7.8/70.8 | 1 | 5 | 14.3 | 15.9 |
| Luo et al.[45]/retrospective cohort | 403 | 205 | 169 | 0.5 | 5.38 | NR | NR | NR | NR |
| Martin-Rojas et al.[24]/retrospective cohort | 206 | 262 | 203 | 0.385 | 1.472 | NR | NR | 12.7/1.13 | 14/1.25 |
| Shang et al.[46]/retrospective cohort | 113 | 160 | 142 | 0.23 | 3.4 | NR | NR | 13.1 | 13.3 |
| Tang et al.[4]/retrospective cohort | 183 | NR | NR | 0.61/4 | 2.12/7.6 | NR | NR | 13.6 | 15.5 |
| Tang et al.[10]/retrospective cohort | 449 | 231±99 | 178±92 | 1.47 | 4.70 | NR | NR | 14.6±2.1 | 16.5±8.4 |
| Wang et al.[47]/training cohort | 296 | NR | NR | 0.2 | 0.5 | NR | NR | 13.3±1.9 | 13.7±1.9 |
| Wang et al.[47]/validation cohort | 44 | NR | NR | 0.6 | 1.1 | NR | NR | 13.2±0.8 | 14.1±1.2 |
| Wang et al.[48]/retrospective cohort | 339 | 211 | 172 | 1.08 | 4.38 | NR | NR | 12 | 12.9 |
| Wang et al.[49]/retrospective case series | 59 | 192.2±85.5 | 145.8±71.1 | 6.1±8.0/36.6±56 | 12.0±9.7/76.8±63.9 | 3.4±3.3 | 8.9±9.5 | 15.3±1.5 | 16.7±4.3 |
| Wu et al.[50]/retrospective cohort | 84 | 204 | 162 | 0.49 | 3.95 | NR | NR | 11.75 | 11.6 |
| Xu et al.[51]/retrospective cohort | 239 | 186 | 160 | NR | NR | 5 | 6 | 11.6±1.4 | 12.0±1. |
| Yang et al.[52]/retrospective cohort | 52 | 164 | 191 | NR | NR | 4 | 6 | 10.9 | 12.9 |
| Zhou et al.[53]/retrospective cohort[53] | 191 | 220 | 165.5 | 0.6 | 5.2 | 1 | 4.5 | 11.4 | 12.1 |
PLT=Platelet count; FDP=Fibrin degradation product; SOFA=Sequential organ failure assessment; PT=Prothrombin time; INR=International normalized ratio
Table 7 reflects the calculated minimum and maximum ISTH and SIC scores in COVID-19 survivors and nonsurvivors. The highest Min-ISTH was 4, which was obtained among nonsurvivors in two studies.[10,44] ISTH ≥5 appeared in Max-ISTH for the nonsurvivors of four studies,[10,44,45,47] and SIC ≥4 was only fulfilled in Min-ISTH of one study for nonsurvivors.[44] In 80% of the calculated ISTH scores, the score was higher among the deceased, mainly as a result of a sharper rise in D-dimer level in this population.
Table 7.
The calculated minimum and maximum scores of International Society on Thrombosis and Hemostasis and sepsis-induced coagulopathy in coronavirus disease 2019 survivors and nonsurvivors
| Author/study design | Minimum-ISTH | Maximum-ISTH | Minimum-SIC | Maximum-SIC | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Survivors | Nonsurvivors | Survivors | Nonsurvivors | Survivors | Nonsurvivors | Survivors | Nonsurvivors | |
| Ronderos Botero et al.[41]/retrospective cohort | 2 | 3 | 3 | 4 | 0 | 0 | 4 | 4 |
| Chen et al.[42]/retrospective case series | 2 | 3 | 3 | 4 | 0 | 0 | 2 | 2 |
| Giusti et al.[43]/prospective cohort | 1 | 2 | 2 | 3 | 1 | 1 | 3 | 3 |
| Liu et al.[44]/retrospective cohort | 0 | 4 | 1 | 5 | 2 | 4 | 4 | 4 |
| Liu et al.[23]/retrospective cohort | - | - | - | - | 0 | 1 | 2 | 3 |
| Luo et al.[45]/retrospective cohort | 0 | 3 | 3 | 6 | 0 | 0 | 4 | 4 |
| Martin-Rojas et al.[24]/retrospective cohort | - | - | - | - | 0 | 1 | 2 | 3 |
| Shang et al.[46]/retrospective cohort | 0 | 3 | 1 | 4 | 0 | 1 | 4 | 4 |
| Tang et al.[4]/retrospective cohort | 0 | 2 | 2 | 4 | 0 | 0 | 6 | 6 |
| Tang et al.[10]/retrospective cohort | 2 | 4 | 3 | 5 | 0 | 0 | 4 | 4 |
| Wang et al.[47]/training cohort | 0 | 0 | 2 | 2 | 0 | 0 | 6 | 6 |
| Wang et al.[47]/validation cohort | 0 | 2 | 3 | 5 | 0 | 0 | 6 | 6 |
| Wang et al.[48]/retrospective cohort | 0 | 3 | 3 | 4 | 0 | 0 | 4 | 4 |
| Wang et al.[49]/retrospective case series | 3 | 3 | 4 | 4 | 2 | 3 | 4 | 5 |
| Wu et al.[50]/retrospective cohort | 0 | 2 | 1 | 3 | 0 | 0 | 4 | 4 |
| Xu et al.[51]/retrospective cohort | 0 | 0 | 4 | 4 | 2 | 2 | 4 | 4 |
| Yang et al.[52]/retrospective cohort | 0 | 0 | 4 | 4 | 2 | 2 | 4 | 4 |
| Zhou et al.[53]/retrospective cohort | 0 | 3 | 1 | 4 | 1 | 2 | 3 | 4 |
ISTH=International Society on Thrombosis and Hemostasis; SIC=Sepsis-induced coagulopathy
DISCUSSION
Considering the poor prognosis of DIC, there is little doubt that DIC's diagnosis and risk classification plays a pivotal role in the triage, prognosis, and management of COVID-19 patients, especially in critical cases. ISTH proposes applicable, highly valid, and laboratory-based scoring systems for identifying patients with overt DIC and septic patients inclined to DIC regardless of their clinical symptoms.[54,55] This systematic review revealed that although many studies did not systematically evaluate ISTH parameters in COVID-19 patients, based on available data, patients with higher ISTH/SIC scores had a higher likelihood of disease severity, death, and the need for special treatment strategies such as ICU admission and mechanical ventilation. Additionally, it was shown that when calculating the maximum scores for missing ISTH parameters in COVID-19 patients enrolled in the reviewed studies, patients with more severe disease and nonsurvivors received higher scores compared to patients with the nonsevere disease and those who survived, which signified the importance of timely and precise DIC risk stratification to adjust treatment and achieve better outcomes.
Only a few studies among the reviewed articles have reported ISTH scores or systematically assessed all biomarkers required for calculating ISTH and SIC scores among patients with moderate, severe, and critical COVID-19. However, these few studies confirmed that the DIC scoring system is a strong independent predictor of a fatal outcome in COVID-19 patients. Tang et al. research has revealed that 71.4% of nonsurvivor COVID-19 patients fulfilled the ISTH criteria for overt DIC (≥5 points), compared with only 0.6% survivors.[4] Moreover, in another study by Tang et al., in severely ill patients with SIC ≥ 4, those who received heparin had a significantly lower 28-day mortality rate compared with those who did not (40% vs. 64.2%, P = 0.029). It appears that anticoagulant treatment in this population might postpone or even reverse the harmful pro-coagulant pattern.[10] consistent with the results of Tang et al., a retrospective cohort study by Luan et al. illustrated that patients with ISTH score ≥2 (COVID-19 associated coagulopathy patients) have higher rates of admission in ICU (42.6% vs. 16.1%), and higher incidence of acute hepatic injury, acute respiratory distress syndrome, and invasive ventilation requirement. These results confirmed that ISTH score ≥2 is an essential index in COVID-19 patients' triage.[56]
Only the nonsurvivor population of one study passed the SIC threshold, and none fulfilled the criteria for DIC when the minimum possible scores were calculated. However, the max-ISTH reached the overt DIC threshold in four and five patient populations in nonsurvivors and severe groups, compared to only one nonsevere patient population. Hence, it appears that using ISTH scores assists physicians to better predict prognosis and adjust management. Considering the extent of missing data for calculating the SIC score, according to the max-SICs, almost all severe and nonsevere patients in these studies were prone to DIC.
ISTH and SIC offer that decreased platelet and fibrinogen, elevated D-dimer/FDP, prolonged PT/increased INR, and a higher level of SOFA score increase the likelihood of DIC.[57] Reviewing these parameters in the enrolled studies revealed that although most parameters did not meet the scoring threshold, the changes from the normal range in these parameters in severe cases and nonsurvivors were consistent with biomarker changes offered by ISTH and SIC, which showed a significant difference when compared to that of nonsevere and survivor patients. One major reason for not meeting the ISTH DIC/SIC threshold can be that all studies only evaluated coagulation parameters at the point of admission and failed to monitor the changes throughout the disease course. The importance of dynamic monitoring of hematological and coagulation parameters in optimizing the management of COVID-19 patients was evident in Liao et al. article. In their study, D-dimer, SIC, and overt DIC scores increased over time in the nonsurvivors. The median SIC score reached the diagnostic threshold (≥4) on day 10, whereas the median ISTH score reached the overt DIC threshold (≥5) after day 19.[22] However, it is worth mentioning that despite the predictive value of ISTH/SIC scores, they are best used when interpreted in the clinical context and during regular monitoring. Accordingly, if the clinical feature is not suggestive of DIC, using these scoring systems is not beneficial even if the defined scores are met.[58]
Among all introduced parameters, D-dimer was the most reported data and the most consistent coagulation abnormality that reached the scoring threshold on admission in the included studies, which raised the patients' ISTH score. Pooled data congruently confirmed a significant D-dimer/FDP elevation, PT/INR prolongation, and a higher SOFA score in patients who were severely ill or died from COVID-19. The SOFA score, as a highly sensitive and specific score for tracking the extent of organ failure to predict mortality, especially in ICU patients, significantly added to the odds of DIC in severe illness. Although most studies highlighted notably lower platelet counts in aggravated illness and deaths, in two studies, the baseline platelet count contradictorily showed a statistically significant rise in severe infection,[22,29] which can be explained by the role of platelets as an inflammatory mediator.[59] As for fibrinogen, most results were counter-intuitively in line with a remarkable increase in baseline fibrinogen in critically ill patients and nonsurvivors. This discrepancy with what is expected in DIC patients can be justified by the multifaceted role of fibrinogen and the pro-inflammatory and prothrombotic nature of COVID-19 infection.[60,61] It seems that the rise in fibrinogen concentration is responsive to the initial cytokine release, followed by a gradual fall in the disease course. The studies that monitored fibrinogen dynamically during the disease corroborated this hypothesis as the higher fibrinogen on admission in severe cases remarkably dropped in the late phase.[62]
Strength and limitations
The present study provides a comprehensive and relatively large-scale review of all available data on the diagnostic and prognostic utility of the ISTH/SIC scoring system in COVID-associated coagulopathy. However, some potential limitations should be taken into account. First, there was some level of heterogeneity. Except for two studies, the rest included studies with retrospective and nonrandomized design, with most of them not considering the control group. Second, most studies lacked the components needed for accurate ISTH and SIC score calculation. Finally, there was a discrepancy between studies in choosing guidelines for classifying COVID-19 illness as severe or nonsevere.
CONCLUSION
This systematic review implies that according to reported and calculated ISTH and SIC scores, there is a positive correlation between higher scores and disease severity and mortality rate. Despite the high prevalence of coagulation abnormalities and DIC in critically ill COVID-19 patients and its prognostic value, physicians relied mainly on their clinical suspicion and routine lab tests instead of using a specific scoring system like ISTH and SIC for diagnosing DIC. Given that timely management of DIC improves outcomes and reduces costs, detection in the initial stages is essential. Therefore, health-care providers should use highly valued criteria that encompass all relevant markers such as ISTH and SIC while assessing suspicious patients for DIC and requesting lab tests on admission and during the disease course. The full picture of the ISTH and SIC scoring system in the management of COVID-19 coagulopathy is still incomplete, and additional research needs to be done to establish its effectiveness. We recommend more prospective research with further emphasis on ISTH and SIC criteria and serial monitoring of relevant markers in detecting COVID-associated DIC, which provides an accurate guide for evaluating COVID-19 patients and distinguishing those prone to more severe disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker BM, Heart VJ, Rattan RJ. Coagulopathy in COVID-19: Review and Recommendations. 2020. Available from: https://www.facs.org/-/media/files/covid19/umiami_study_uses_of_coagulopathy.ashx .
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–7. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Society of Hematology. COVID-19 and Coagulopathy: Frequently Asked Questions 2020. [[Last accessed on 2020 Nov 15]]. Available from: https://www.hematology.org/covid-19/covid-19-and-coagulopathy .
- 6.Deng Y, Liu W, Liu K, Fang YY, Shang J, Zhou L, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: A retrospective study. Chin Med J (Engl) 2020;133:1261–7. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt FCF, Manolov V, Morgenstern J, Fleming T, Heitmeier S, Uhle F, et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: Results of an observational pilot study. Ann Intensive Care. 2019;9:19. doi: 10.1186/s13613-019-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–9. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH).Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–30. [PubMed] [Google Scholar]
- 12.Tal S, Spectre G, Kornowski R, Perl L. Venous thromboembolism complicated with COVID-19: What do we know so far? Acta Haematol. 2020;143:417–24. doi: 10.1159/000508233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: A retrospective analysis of a nationwide survey. BMJ Open. 2017;7:e017046. doi: 10.1136/bmjopen-2017-017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toh CH, Dennis M. Disseminated intravascular coagulation: Old disease, new hope. BMJ. 2003;327:974–7. doi: 10.1136/bmj.327.7421.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fourrier FJ. Coagulations intra-vasculaires disséminées [En: Disseminated Intravascular Coagulation].French. Sang Thrombose Vaisseaux. 2003;15:333–9. [Google Scholar]
- 16.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock: For the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:775–87. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iba T, Levy JH, Warkentin TE, Thachil J, van der Poll T, Levi M, et al. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17:1989–94. doi: 10.1111/jth.14578. [DOI] [PubMed] [Google Scholar]
- 18.Peterson J, Welch V, Losos M, Tugwell PJ. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. [[Last accessed on 2020 Nov 15]]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 19.He B, Wang J, Wang Y, Zhao J, Huang J, Tian Y, et al. The metabolic changes and immune profiles in patients With COVID-19. Front Immunol. 2020;11:2075. doi: 10.3389/fimmu.2020.02075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Bao C, Tao X, Cui W, Yi B, Pan T, Young KH, et al. SARS-CoV-2 induced thrombocytopenia as an important biomarker significantly correlated with abnormal coagulation function, increased intravascular blood clot risk and mortality in COVID-19 patients. Exp Hematol Oncol. 2020;9:16. doi: 10.1186/s40164-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao D, Zhou F, Luo L, Xu M, Wang H, Xia J, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: A retrospective cohort study. Lancet Haematol. 2020;7:e671–8. doi: 10.1016/S2352-3026(20)30217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Gao W, Guo W, Guo Y, Shi M, Dong G, et al. Prominent coagulation disorder is closely related to inflammatory response and could be as a prognostic indicator for ICU patients with COVID-19. J Thromb Thrombolysis. 2020;50:825–32. doi: 10.1007/s11239-020-02174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martín-Rojas RM, Pérez-Rus G, Delgado-Pinos VE, Domingo-González A, Regalado-Artamendi I, Alba-Urdiales N, et al. COVID-19 coagulopathy: An in-depth analysis of the coagulation system. Eur J Haematol. 2020;105:741–50. doi: 10.1111/ejh.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White D, MacDonald S, Edwards T, Bridgeman C, Hayman M, Sharp M, et al. Evaluation of COVID-19 coagulopathy; laboratory characterization using thrombin generation and nonconventional haemostasis assays. Int J Lab Hematol. 2021;43:123–30. doi: 10.1111/ijlh.13329. [DOI] [PubMed] [Google Scholar]
- 26.Huang H, Cai S, Li Y, Li Y, Fan Y, Li L, et al. Prognostic factors for COVID-19 pneumonia progression to severe symptom based on the earlier clinical features: A retrospective analysis. [[Last accessed on 2020 Nov 15]];Front Med (Lausanne) [internet] 2020 7:557453. doi: 10.3389/fmed.2020.557453. Doi: 10.3389/fmed.2020.557453. Available from: https://pubmed.ncbi.nlm.nih.gov/33123541/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li T, Lu L, Zhang W, Tao Y, Wang L, Bao J, et al. Clinical characteristics of 312 hospitalized older patients with COVID-19 in Wuhan, China. [[Last accessed on 2020 Nov 15]];Arch Gerontol Geriatr [Internet] 2020 91:104185. doi: 10.1016/j.archger.2020.104185. doi: 10.1016/j.archger.2020.104185. Available from: https://pubmed.ncbi.nlm.nih.gov/32688107/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Wu M, Yao J, Guo J, Liao X, Song S, et al. Caution on kidney dysfunctions of COVID-19 patients. medRevix [preprint] 2020. Available from: https://www.medrxiv.org/content/10.1101/2020.02.08.20021212v2 .
- 29.Liu D, Cui P, Zeng S, Wang S, Feng X, Xu S, et al. Risk factors for developing into critical COVID-19 patients in Wuhan, China: A multicenter, retrospective, cohort study. [[Last accessed on 2020 Nov 15]];EClinicalMedicine [Internet] 2020 25:100471. doi: 10.1016/j.eclinm.2020.100471. doi: 10.1016/j.eclinm.2020.100471. Available from: https://pubmed.ncbi.nlm.nih.gov/32840491/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian GQ, Yang NB, Ding F, Ma AH, Wang ZY, Shen YF, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: A retrospective, multi-centre case series. QJM. 2020;113:474–81. doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu C, Lei Q, Li W, Wang X, Li W, Liu W. Epidemiological and clinical characteristics of 1663 hospitalized patients infected with COVID-19 in Wuhan, China: A single-center experience. J Infect Public Health. 2020;13:1202–9. doi: 10.1016/j.jiph.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J, et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. [[Last accessed on 2020 Nov 15]];J Clinical Virol [Internet] 2020 127:104364. doi: 10.1016/j.jcv.2020.104364. doi: 10.1016/j.jcv.2020.104364. Available from: https://pubmed.ncbi.nlm.nih.gov/32311650/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han H, Yang L, Liu R, Liu F, Wu KL, Li J, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–20. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 35.Yu HH, Qin C, Chen M, Wang W, Tian DS. D-dimer level is associated with the severity of COVID-19. Thromb Res. 2020;195:219–25. doi: 10.1016/j.thromres.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, He L, Chen H, Lu S, Xiong Y, Liu J, et al. Manifestations of blood coagulation and its relation to clinical outcomes in severe COVID-19 patients. Retrospect Anal. 2020;42:766–72. doi: 10.1111/ijlh.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Tu C, Zhu M, Wang J, Yang C, Liu W, et al. Exploring the Law of Development and Prognostic Factors of Common and Severe COVID-19: A Retrospective Case-Control Study in 122 Patients with Complete Course of Disease. SSRN [preprint] 2020. SSRN 3555209. Available from: https://dx.doi.org/10.2139/ssrn.3555209 .
- 38.Long H, Nie L, Xiang X, Li H, Zhang X, Fu X, et al. D-dimer and prothrombin time are the significant indicators of severe COVID-19 and poor prognosis. Biomed Res Int [Internet] 2020. [[Last accessed on 2020 Nov 15]]. p. 6159720. doi: 10.1155/2020/6159720. Available from: https://pubmed.ncbi.nlm.nih.gov/32596339/ [DOI] [PMC free article] [PubMed]
- 39.Gao Y, Li T, Han M, Li X, Wu D, Xu Y, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92:791–6. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng DX, Xu JL, Mao QX, Liu R, Zhang WY, Qian HY, et al. Association of Padua prediction score with in-hospital prognosis in COVID-19 patients. QJM. 2020;113:789–93. doi: 10.1093/qjmed/hcaa224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ronderos Botero DM, Omar AM, Sun HK, Mantri N, Fortuzi K, Choi Y, et al. COVID-19 in the healthy patient population: Demographic and clinical phenotypic characterization and predictors of in-hospital outcomes. Arterioscler Thromb Vasc Biol. 2020;40:2764–75. doi: 10.1161/ATVBAHA.120.314845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giusti B, Gori AM, Alessi M, Rogolino A, Lotti E, Poli D, et al. Sars-CoV-2 induced coagulopathy and prognosis in hospitalized patients: A snapshot from Italy. Thromb Haemost. 2020;120:1233–6. doi: 10.1055/s-0040-1712918. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Zhang S, Wu Z, Shang Y, Dong X, Li G, et al. Clinical outcomes of COVID-19 in Wuhan, China: A large cohort study. Ann Intensive Care. 2020;10:99. doi: 10.1186/s13613-020-00706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo X, Xia H, Yang W, Wang B, Guo T, Xiong J, et al. Characteristics of patients with COVID-19 during epidemic ongoing outbreak in Wuhan, China. medRvix [preprint] 2020. [[Last accessed on 2020 Nov 15]]. Available from: https://doi.org/10.1101/2020.03.19.20033175 .
- 46.Shang Y, Liu T, Wei Y, Li J, Shao L, Liu M, et al. Scoring systems for predicting mortality for severe patients with COVID-19. [[Last accessed 2020 Nov 15]];EClinicalMedicine [Internet] 2020 24:100426. doi: 10.1016/j.eclinm.2020.100426. doi: 10.1016/j.eclinm.2020.100426. Available from: https://pubmed.ncbi.nlm.nih.gov/32766541/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang K, Zuo P, Liu Y, Zhang M, Zhao X, Xie S, et al. Clinical and laboratory predictors of in-hospital mortality in patients with coronavirus disease-2019: A cohort study in Wuhan, China. Clin Infect Dis. 2020;71:2079–88. doi: 10.1093/cid/ciaa538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639–45. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang ZH, Shu C, Ran X, Xie CH, Zhang L. Critically Ill patients with coronavirus disease 2019 in a designated ICU: Clinical features and predictors for mortality. Risk Manag Healthc Policy. 2020;13:833–45. doi: 10.2147/RMHP.S263095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–43. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J, Yang X, Yang L, Zou X, Wang Y, Wu Y, et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: A multicenter retrospective study from Wuhan, China. Crit Care. 2020;24:394. doi: 10.1186/s13054-020-03098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bakhtiari K, Meijers JC, de Jonge E, Levi M. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32:2416–21. doi: 10.1097/01.ccm.0000147769.07699.e3. [DOI] [PubMed] [Google Scholar]
- 55.Levi M, Meijers JC. DIC: Which laboratory tests are most useful. Blood Rev. 2011;25:33–7. doi: 10.1016/j.blre.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Luan YY, Liu Y, Liu XY, Yu BJ, Chen RL, Peng M, et al. Coronavirus disease 2019 (COVID-19) associated coagulopathy and its impact on outcomes in Shenzhen, China: A retrospective cohort study. Thromb Res. 2020;195:62–8. doi: 10.1016/j.thromres.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toh C, Hoots W SSC on Disseminated Intravascular Coagulation of the ISTH. The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: A 5-year overview. J Thromb Haemost. 2007;5:604–6. doi: 10.1111/j.1538-7836.2007.02313.x. [DOI] [PubMed] [Google Scholar]
- 58.Görlinger K, Dirkmann D, Gandhi A, Simioni P. COVID-19-associated coagulopathy and inflammatory response: What do we know already and what are the knowledge gaps? Anesth Analg. 2020;131:1324–33. doi: 10.1213/ANE.0000000000005147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. J Thromb Haemost. 2014;12:1764–75. doi: 10.1111/jth.12730. [DOI] [PubMed] [Google Scholar]
- 60.Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133:511–20. doi: 10.1182/blood-2018-07-818211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayıroğlu Mİ, Çınar T, Tekkeşin Aİ. Fibrinogen and D-dimer variances and anticoagulation recommendations in Covid-19: Current literature review. Rev Assoc Med Bras (1992) 2020;66:842–8. doi: 10.1590/1806-9282.66.6.842. [DOI] [PubMed] [Google Scholar]
- 62.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. [[Last accessed on 2020 Nov 15]];BMJ [Internet] 2020 368:m1091. doi: 10.1136/bmj.m1091. doi: 10.1136/bmj.m1091. Available from: https://pubmed.ncbi.nlm.nih.gov/32217556/ [DOI] [PMC free article] [PubMed] [Google Scholar]


