Abstract
Background:
Dyslipidemia (DL) is an important risk factor of coronary artery disease (CAD). We evaluated DL prevalence and its 5-year incidence rate in southeastern Iran, to assess the severity and growth rate of this CAD risk factor in the region.
Materials and Methods:
This study was a part of the Kerman CAD Risk Factors Study Phase 2 (2014–2018) among 9996 individuals aged 15–80 years, from whom 2820 individuals had also participated in Phase 1 (2009–2011). In mg/dl, cholesterol ≥240 and/or low-density lipoprotein cholesterol ≥160 and/or high-density lipoprotein cholesterol <40 for men and <50 for women and/or triglyceride >200 were defined as DL.
Results:
The lipid profile of 9911 persons was analyzed. Overall 19.6% had borderline cholesterol and 6.4% suffered from hypercholesterolemia. 56.6% of the population (62.5% of females vs. 48.5% of males) suffer from DL, from whom 73.4% were undiagnosed. Female gender, advanced age, obesity, hypertension, diabetes, anxiety, and depression predicted DL in the study population. The prevalence of DL was significantly lower in Phase 2 (56.6%) compared to Phase 1 (81.4%). The prevalence of undiagnosed DL (UDL) and diagnosed DL (DDL) was 40.7% and 16.2%, respectively. The 5-year incidence rate of DL was 2.58 persons/100 person-years (3.24 in females vs. 2.20 in males).
Conclusion:
Although there were promising signs of a reduction in DL and increase in DDL in the last 5 years, a high percentage of the population have DL yet, from whom mostly are undiagnosed. DL was significantly associated with other CAD risk factors. Therefore, the health-care management system should improve its strategies to reduce the health burden of DL.
Keywords: Coronary artery disease, dyslipidemia, incidence rate, prevalence
INTRODUCTION
There is a correlation between increased body fat and cardiovascular disease (CVD), dyslipidemia (DL), and other metabolic diseases incriminated as the leading causes of global mortality.[1] Several studies focusing on lipid derangements have confirmed the relationship between lipid profile abnormality and increased rate of mortality and morbidity.[2,3,4,5,6,7]
DL is defined as increased low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and triglycerides (TGs), or reduced high-density lipoprotein cholesterol (HDL-C) levels.[6,7,8,9,10] DL prevalence is different worldwide, yet about 50% of the world's adult population suffer from it.[2,3,5,11]
Although in recent years, CVD prevalence has had a downward trend in developed countries, it has been progressive in developing and low-income countries.[3,4] To reduce coronary artery disease (CAD) mortality, its risk factors (DL, diabetes mellitus, obesity, low physical activity, and hypertension) must be monitored and strictly managed.[5,6] Among these risk factors, DL is a multifactorial disease related to genetic susceptibilities, uncontrolled diet, and unhealthy lifestyle.[7,8,9,10]
The DL prevalence and its association with heart disease have been studied in different countries.[1,2,3,4] Opoku et al. reported that low HDL-C level was more prevalent in urban regions, while other types of DL were more common in rural regions in China.[6] Furthermore, they found that the prevalence was higher in women. Garcez et al. reported that reduced HDL-C is the most prevalent type of DL in Sao Paulo population.[1] Hedayatnia et al. reported that the prevalence of low HDL-C levels, high LDL-C, hypertriglyceridemia, and hypercholesterolemia in Mashhad city was 43.9, 35.5, 46, and 41.6, respectively.[11] Rinkūnienė et al. found DL the most prevalent risk factor in middle-age patients without CVD in Lithuania.[12] It has been shown that the prevalence of DL varies widely according to the ethnic, socioeconomic, and cultural characteristics of distinct population groups.[13] In our previous study between 2009 and 2011 on a population of 5900 adults in southeastern Iran (Kerman CAD Risk Factors Study [KERCADRS] Phase 1), DL was quite prevalent, and the prevalence of undiagnosed DL (UDL) (68.9%) was much higher than that of diagnosed DL (DDL) (12.5%); in addition, DL was found to be comorbid with obesity, advanced age, and low physical activity.[2]
To explore the trend of changes in the prevalence, and also the 5-year incidence rate of this important CAD risk factor in the region, the present study, namely the second phase of the KERCADRS (2014–2018), was performed on 9996 persons to determine the prevalence of DL, its components, and its predictors in the population aged 15–80 years. By comparing the results with the findings of the first phase, we also assessed the effectiveness of the health-care system in DL management in the last 5 years. The current prevalence of main CAD comorbidities accompanying DL is also reported in normal DL, DDL, and UDL subpopulations.
SUBJECTS AND METHODS
This population-based epidemiological research, as the second phase of KERCADRS, is a cross-sectional study conducted from 2014 to 2018 on 9996 individuals aged 15–80 years, from whom 2820 persons were individuals that participated in the first phase of the study (2009–2011) as well. These were residents of Kerman city, the largest city in southeastern Iran with a population of 750,000. The study protocol was approved by the Ethics Committee at Kerman University of Medical Sciences (Permission No.IR.KMU.REC.1392/405).
Measurements
Face-to-face individual interviews conducted by trained interviewers were used to collect demographic characteristics, high-risk behaviors, and medical history. People with no education were defined as illiterate, from 1 to 12 classes of education as primary to high school, and education at university level as above high school. The WHO Global Physical Activity Questionnaire was the tool used to measure daily physical activities at home, work, and recreation.[14] In order to evaluate the intensity of physical activity, metabolic equivalent of task which is the use of energy in an adult individual in sitting position (equivalent to 3.5 ml oxygen consumption/kg body weight in a minute) was used. Low, moderate, and high physical activities refer to consuming energy less than four times, four to eight times, and at least eight times in proportion to sitting, respectively.[15] The Beck Anxiety Inventory and Beck Depression Inventory rated the intensity of the mentioned disorders ranging from 0 to 63.[16] The score range for depression was considered: 0–30 as normal and 31–63 as abnormal. For anxiety, the score range was considered: 0–15 as normal and 16–63 as abnormal.
Height, weight, and blood pressure of participants were also measured according to standard protocols by a physician by means of clinical examination. Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, and/or taking any antihypertensive drug. Self-reported smoking status and opium use were also collected. The individuals were categorized into three groups: “never users” who had never consumed opium, “occasional users” who irregularly used opium (mostly for entertainment), and “dependent users” who regularly consumed opium. For cigarette smoking, participants were divided into nonsmokers (never smoked) and smokers (smoke at least one cigarette a day). Body mass index (BMI) was calculated by dividing weight (kg) by height (meter) squared. Participants with BMI 25–29.9 and ≥30 kg/m2 were categorized as overweight and obese, respectively. Furthermore, serum lipids and fasting blood glucose (FBG) were measured at a central laboratory using blood samples taken after at least 12 h of overnight fasting. Individuals with FBG below 100 mg/dl and those with FBG ≥126 mg/dl or under insulin or antidiabetes treatment were considered as normal and diabetic, respectively.[17] DL was defined as increased LDL-C, TC, and TG, and/or reduced HDL-C serum levels compared to normal values[6,7,8] and/or people who receive lipid-lowering medications [Table 1]. Enzymatical measurement and standard spectrophotometric technique were used to determine TC and TG, respectively. HDL-C was measured enzymatically in the serum. The Friedewald formula (LDL-C = TC − [HDL-C + TG/5]) was used to calculate LDL-C. Lipid profile cutoffs were categorized based on the current measurements, history of diagnosis, and taking drugs [Table 1].
Table 1.
Serum lipid profile categories based on the normal values
| Lipid profile categories | TC | LDL-C | HDL-C | TG | Previously diagnosed dyslipidemia | Taking anti-dyslipidemia drug |
|---|---|---|---|---|---|---|
| Normal (mg/dl) | <200 | <100 optimal (100-129 near-optimal) |
≥60 | <150 | Negative | Negative |
| Borderline (mg/dl) | 200-239 | 130-159 | 40-59 (men) 50-59 (women) |
150-199 | Negative | Negative |
| Undiagnosed High risk/very high risk |
≥240 | 160-189 high ≥190 very high | <40 men <50 women | 200-499 high ≥500 very high |
Negative | Negative |
| Diagnosed controlled | <200 | <130 | >40 (men) >50 (women) |
<150 | Positive | Positive or negative |
| Diagnosed uncontrolled | >200 | >130 | <40 (men) <50 (women) |
>150 | Positive | Positive or negative |
People having the characteristics of the lower three rows are defined as individuals with dyslipidemia. TC=Total cholesterol; HDL-C=High-density lipoprotein-cholesterol; LDL-C=Low-density lipoprotein-cholesterol; TG=Triglyceride
Incidence rate of dyslipidemia
The data obtained from those who had normal serum lipids in Phase 1, and therefore, were at risk of DL during follow-up, were used to calculate the incidence rate of DL [Figure 1]. Therefore, 81.4% of the 5558 participants in Phase 1 (n = 4524) who already had DL were excluded from the incidence calculation. Out of the remaining participants (n = 1034), 642 persons (18.6% of the participants) were lost to follow-up or died during the 5-year follow-up. As a result, the numerator equaled the number of new cases (among the 1034 cases) diagnosed with DL during the follow-up period. For those who had normal lipid in Phase 1, the time difference (year) between the visits in Phases 1 and 2 was calculated as person-years at risk. Therefore, the denominator was equal to the sum of the time each of the 1034 persons, who were at risk of DL, was followed (person-year). Then, the incidence rate (persons/100 person-years) was calculated using the following formula.[18]
Figure 1.
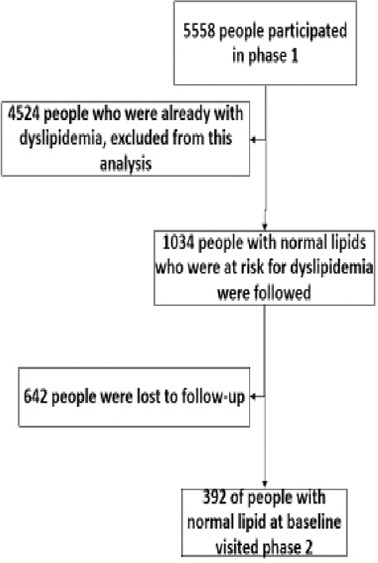
Flowchart of people who participated in both phases of the study

Statistical analysis
All statistical analyses were performed in STATA version 15 (Stata Corp. 2015 College Station, Texas USA). To provide age–sex standardized prevalence estimates, we used Kerman census 2016 population data as the reference population. Survey logistic regression model was applied to identify the variables associated with the outcome. All prevalence rates were weighted according to the sampling weight (reciprocal of the probability of selection) and the individual response rate. Data are presented as absolute and relative frequencies as well as 95% confidence intervals (95% CIs). All models were adjusted for potential confounders such as gender, age, education level, smoking status, and BMI. Given the high sample size in our data set, we made full adjustment and included all variables in the multivariate model. Z-test and the Chi-square test were used to compare the prevalence between Phases 1 and 2 [Figures 2 and 3]. P =0.05 was used as a significance level.
Figure 2.
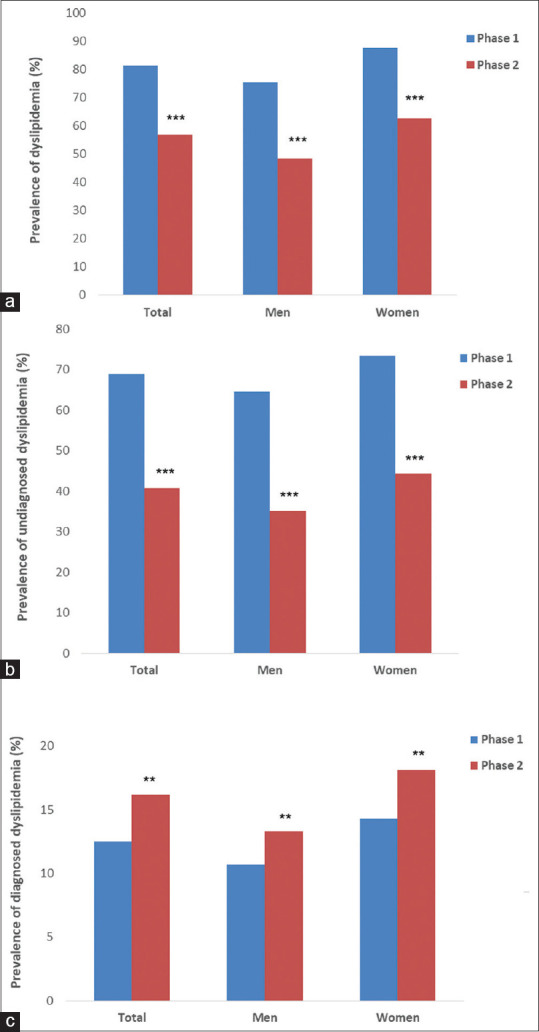
Prevalence of dyslipidemia (a), undiagnosed dyslipidemia (b), and diagnosed dyslipidemia (c) of the participants in the study by sex groups. Community-Based Cohort Study (KERCADRS), Kerman, Iran, Phase 1, 2009–2011 and Phase 2, 2014–2018. Total sample size = 9911 in Phase 2 and 5855 in Phase 1. **P < 0.01, ***P < 0.001 compared to Phase 1. The data of Phase 1 are extracted from reference[2] for comparison
Figure 3.
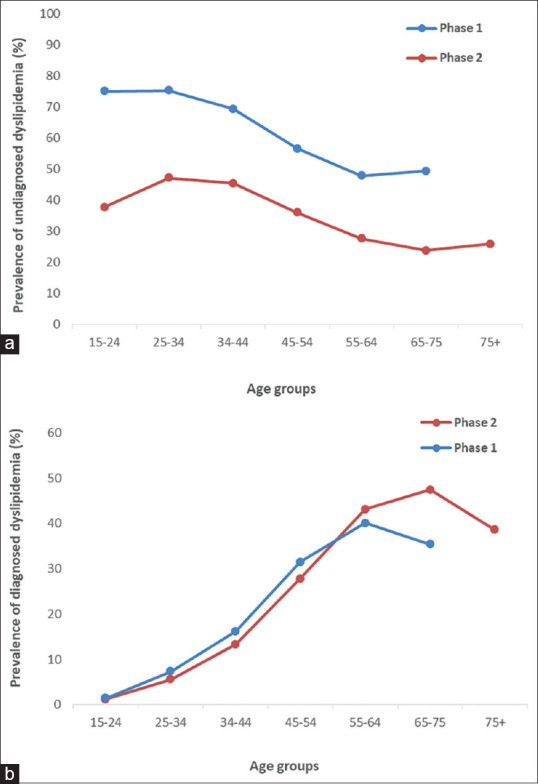
The prevalence of undiagnosed dyslipidemia (a) and diagnosed dyslipidemia (b) in the participants by age groups. Community-Based Cohort Study (KERCADRS), Kerman, Iran, Phase 1, 2009–2011 and Phase 2, 2014–2018 (total sample size = 9911 in Phase 2 and 5855 in Phase 1). The data of Phase 1 are extracted from reference[2] for comparison
RESULTS
For this analysis, we used the data for 9911 individuals (out of 9996 participants) who had complete laboratory lipid measurements. In this population, 59.6% were female. Overall 6.4% and 11.8% of the population had hypercholesterolemia and hypertriglyceridemia, respectively [Table 2]. Low HDL-C level was the most common type of DL (42.9%).
Table 2.
The standardized prevalence, percentage (confidence interval) of abnormal lipid profiles (dyslipidemia), community-based cohort study - Kerman Coronary Artery Disease Risk Factors Study 2nd Phase, Kerman, Iran, n=9911)
| Subgroups | TC (%) (95% CI) | TG (%) (95% CI) | LDL-C (%) (95%CI) | HDL-C (%) (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Borderline (n=1942) | High (n=634) | Borderline (n=1427) | High (n=1169) | Borderline (n=1456) | High (n=525) | Borderline (n=4133) | Low (n=4251) | |
| Total | 19.6 (18.8-20.4) | 6.4 (5.9-6.9) | 14.4 (13.7-5.1) | 11.8 (11.2-12.5) | 14.7 (14.0-5.4) | 5.3 (4.9-5.8) | 41.7 (40.7-42.7) | 42.9 (42.0-43.9) |
| Sex | ||||||||
| Male | 19.5 (18.2-20.8) | 5.7 (4.9-6.5) | 15.8 (14.5-17.3) | 15.5 (14.3-16.8) | 15.0 (13.7-16.3) | 5.1 (4.4-5.9) | 59.5 (57.5-61.5) | 32.1 (30.0-34.3) |
| Female | 19.7 (18.6-20.8) | 6.9 (6.3-7.6) | 13.4 (12.4-14.4) | 9.3 (8.5-10.2) | 14.5 (13.5-15.6) | 5.5 (5.0-6.1) | 29.6 (28.2-31.1) | 50.3 (48.4-52.2) |
| P | 0.06 | <0.001 | 0.58 | <0.001 | ||||
| Age group | ||||||||
| 15-24 | 8.6 (6.9-10.7) | 1.3 (0.7-2.4) | 7.1 (5.6-9.0) | 4.4 (3.2-6.0) | 6.1 (4.6-8.0) | 1.2 (0.6-2.1) | 49.6 (46.3-52.9) | 35.7 (32.3-39.2) |
| Male | 8.2 (5.8-11.4) | 1.2 (0.5-2.9) | 8.7 (6.4-11.7) | 5.7 (3.8-8.4) | 5.2 (3.3-8.2) | 1.2 (0.5-3.0) | 62.5 (57.6-67.2) | 26.1 (21.7-31.0) |
| Female | 9.1 (6.8-12.2) | 1.3 (0.6-2.8) | 5.3 (3.5-8.1) | 3.0 (1.7-5.1) | 7.0 (4.9-9.9) | 1.1 (0.4-2.5) | 34.9 (30.8-39.3) | 46.6 (42.0-51.2) |
| P | 0.90 | 0.02 | 0.56 | <0.001 | ||||
| 25-34 | 15.7 (14.0-17.6) | 4.0 (3.1-5.2) | 12.4 (10.7-14.2) | 10.9 (9.4-12.6) | 11.8 (10.3-13.5) | 3.4 (2.5-4.5) | 43.2 (40.9-45.5) | 43.5 (40.9-46.1) |
| Male | 17.6 (14.8-20.8) | 5.0 (3.5-7.1) | 15.0 (12.3-18.1) | 15.5 (13.0-18.3) | 14.5 (12.0-17.5) | 4.2 (2.8-6.3) | 59.1 (55.2-62.8) | 33.6 (30.0-37.4) |
| Female | 13.8 (11.7-16.1) | 2.9 (2.1-4.2) | 9.7 (8.0-11.6) | 6.3 (4.9-8.0) | 9.0 (7.5-10.90 | 2.5 (1.7-3.6) | 26.9 (24.4-29.5) | 53.6 (50.4-56.7) |
| P | 0.01 | <0.001 | <0.001 | <0.001 | ||||
| 35-44 | 24.4 (22.4-26.4) | 7.2 (6.0-8.6) | 17.3 (15.5-19.2) | 16.5 (14.7-18.5) | 19.2 (17.4-21.2) | 6.0 (4.9-7.3) | 41.9 (39.5-44.2) | 46.0 (43.4-48.6) |
| Male | 26.2 (23.2-29.3) | 7.9 (6.1-10.2) | 19.8 (17.0-22.9) | 22.4 (19.4-25.7) | 20.2 (17.4-23.3) | 7.5 (5.7-9.7) | 56.2 (52.4-60.0) | 38.1 (34.4-41.9) |
| Female | 22.6 (20.3-25.1) | 6.5 (5.2-8.1) | 14.7 (12.8-16.9) | 10.5 (8.9-12.4) | 18.2 (16.1-20.5) | 4.6 (3.6-5.9) | 27.3 (24.7-30.0) | 54.0 (50.8-57.2) |
| P | 0.06 | <0.001 | 0.62 | <0.001 | ||||
| 45-54 | 28.3 (26.3-30.4) | 11.4 (10.1-12.9) | 21.1 (19.3-23.0) | 17.2 (15.5-19.0) | 22.0 (20.1-24.0) | 9.1 (7.8-10.5) | 45.4 (43.0-47.7) | 40.1 (37.6-42.7) |
| Male | 26.6 (23.5-30.0) | 9.4 (7.5-11.7) | 21.9 (19.0-25.1) | 20.4 (17.6-23.6) | 20.2 (17.4-23.2) | 7.9 (6.2-10.1) | 59.7 (55.9-63.4) | 32.5 (28.9-36.4) |
| Female | 29.9 (27.4-32.6) | 13.4 (11.7-15.4) | 20.3 (18.2-22.6) | 13.9 (12.2-15.8) | 23.8 (21.5-26.3) | 10.2 (8.6-12.1) | 31.0 (28.5-33.5) | 47.8 (44.8-50.8) |
| P | 0.002 | <0.001 | 0.02 | <0.001 | ||||
| 55-64 | 28.9 (26.9-30.9) | 12.0 (10.7-13.5) | 18.8 (17.1-20.6) | 18.3 (16.5-20.3) | 20.8 (19.0-22.7) | 10.7 (9.5-12.1) | 44.5 (42.3-46.7) | 39.8 (37.4-42.2) |
| Male | 24.3 (21.4-27.5) | 8.1 (6.3-10.2) | 16.9 (14.5-19.6) | 18.1 (15.6-21.0) | 19.2 (16.5-22.2) | 7.3 (5.7-9.3) | 58.8 (55.5-62.1) | 33.0 (29.8-36.4) |
| Female | 33.3 (30.8-35.9) | 15.9 (14.0-18.0) | 20.6 (18.3-23.2) | 18.6 (16.3-21.1) | 22.3 (19.9-24.9) | 14.1 (12.3-16.1) | 30.4 (27.7-33.3) | 46.4 (43.4-49.5) |
| P | <0.001 | 0.1 | <0.001 | <0.001 | ||||
| 65-74 | 25.4 (22.8-28.3) | 9.6 (7.8-11.8) | 22.0 (19.4-24.9) | 15.6 (13.4-18.1) | 17.8 (15.3-20.5) | 8.9 (7.2-11.0) | 44.0 (41.1-47.0) | 38.1 (35.0-41.4) |
| Male | 23.7 (19.9-27.9) | 5.3 (3.6-7.7) | 19.0 (15.6-23.0) | 15.5 (12.4-19.2) | 18.1 (14.8-22.1) | 4.9 (3.2-7.3) | 60.0 (55.5-64.3) | 28.5 (24.7-32.7) |
| Female | 27.2 (23.4-31.3) | 13.9 (11.0-17.5) | 24.9 (21.0-29.3) | 15.7 (12.6-19.5) | 17.4 (14.3-20.9) | 12.9 (10.2-16.2) | 28.2 (24.4-32.4) | 47.6 (43.0-52.4) |
| P | <0.001 | 0.09 | <0.001 | <0.001 | ||||
| 75+ | 21.7 (17.6-26.4) | 10.0 (7.0-14.0) | 16.4 (12.5-21.1) | 11.1 (8.2-14.8) | 15.8 (12.2-20.2) | 8.3 (5.5-12.3) | 46.1 (40.6-51.7) | 35.3 (30.3-40.5) |
| Male | 22.3 (16.8-28.9) | 5.9 (3.1-10.9) | 13.4 (9.4-18.6) | 10.9 (7.2-16.1) | 14.4 (10.1-20.0) | 6.9 (4.2-11.3) | 63.9 (56.5-70.7) | 23.3 (17.6-30.1) |
| Female | 21.1 (15.0-28.7) | 14.3 (9.1-21.7) | 19.5 (13.6-27.4) | 11.3 (7.0-17.6) | 17.3 (12.0-24.3) | 9.8 (5.5-16.7) | 27.1 (19.5-36.3) | 48.1 (39.2-57.2) |
| P | 0.06 | <0.001 | 0.62 | <0.001 | ||||
TC=Total cholesterol; HDL-C=High-density cholesterol; LDL-C=Low-density cholesterol; TG=Triglyceride; CI=Confidence interval
Hypertriglyceridemia and borderline TG levels were more frequent in women than they were in men, and women had significantly lower low HDL-C than men did (32.1% vs. 50.3%). The prevalence of hypercholesterolemia, hypertriglyceridemia, and high LDL-C levels gradually increased with age, peaking in the 55–64-year-old age group [Table 2]. On the other hand, the peak of low HDL-C prevalence was found in the 35–44-year-old group.
Our data showed that 56.9% (n = 5639) of the patients had DL. The standardized prevalence of DL (UDL and DDL) in different genders and age subcategories are shown in Table 3. The overall prevalence of UDL was 40.7% (n = 4034), and that of DDL was 16.2% (n = 1605). Among patients with DL, the prevalence of UDL was 73.4%, and that of DDL was 14.3%, both of which were more prevalent in women. The prevalence of UDL was higher in illiterate individuals than it was in those with higher educational level, in the obese compared with those with normal weight, and in opium nonusers compared with opium users [Table 3].
Table 3.
The standardized prevalence of dyslipidemia, and adjusted odds ratio for different predictors of dyslipidemia, community-based cohort study (Kerman Coronary Artery Disease Risk Factors Study 2nd Phase, Kerman, Iran, n=9911)
| Subgroups | n (%) | Normal (%) (CI) | Undiagnosed dyslipidemia (%) (CI) | Diagnosed dyslipidemia (%) (CI) | AOR | P |
|---|---|---|---|---|---|---|
| Overall | 9911 | 43.16 (42.2-44.1) | 40.7 (39.7-41.7) | 16.2 (15.4-16.9) | - | - |
| Sex | ||||||
| Male | 4007 (40.4) | 51.5 (49.5-53.4) | 35.2 (33.2-37.2) | 13.3 (12.3-14.4) | 1 | <0.001 |
| Female | 5904 (59.6) | 37.5 (35.8-39.3) | 44.4 (42.7-46.2) | 18.1 (17.3-18.9) | 1.78 (1.61-1.98) | |
| Age groups | ||||||
| 15-24 | 873 (8.8) | 61.1 (57.6-64.4) | 37.7 (34.4-41.1) | 1.2 (0.6-2.2) | 1 | |
| 25-34 | 1713 (17.3) | 47.3 (44.7-49.9) | 47.1 (44.4-49.7) | 5.6 (4.6-7.0) | 1.35 (1.12-1.62) | <0.01 |
| 35-44 | 2000 (20.2) | 41.3 (38.8-43.7) | 45.4 (43.1-47.8) | 13.3 (11.8-15.0) | 1.56 (1.31-1.85) | <0.001 |
| 45-54 | 2033 (20.5) | 36.3 (34.1-38.6) | 36.0 (33.6-38.4) | 27.8 (25.8-29.8) | 1.85 (1.54-2.22) | <0.001 |
| 55-64 | 2012 (20.3) | 29.3 (27.3-31.3) | 27.6 (25.4-29.9) | 43.1 (40.9-45.4) | 2.65 (2.18-3.22) | <0.001 |
| 65-74 | 941 (9.5) | 28.8 (26.3-31.4) | 23.8 (21.1-26.8) | 47.4 (44.4-50.3) | 2.79 (2.26-3.44) | <0.001 |
| 75+ | 335 (3.4) | 35.5 (30.7-40.5) | 25.9 (21.3-31.0) | 38.6 (33.8-43.7) | 2.25 (1.70-2.97) | <0.001 |
| Education | ||||||
| Illiterate | 929 (9.6) | 29.3 (19.6-41.3) | 49.1 (38.4-59.8) | 21.7 (20.3-23.1) | 1 | |
| Primary to high school | 3678 (37.9) | 44.5 (42.2-46.8) | 40.4 (38.0-42.8) | 15.1 (14.0-16.3) | 0.95 (0.80-1.13) | 0.56 |
| Above high school | 5073 (52.4) | 44.6 (42.8-46.4) | 38.9 (37.2-40.7) | 16.5 (15.5-17.5) | 0.91 (0.76-1.08) | 0.28 |
| Cigarette smoker | ||||||
| No | 9011 (91) | 44.5 (43.0-45.9) | 39.6 (38.1-41.1) | 15.9 (15.2-16.7) | 1 | 0.08 |
| Yes | 891 (9) | 48.9 (38.9-59.1) | 39.6 (30.0-50.1) | 11.5 (9.3-14.1) | 1.16 (0.98-1.38) | |
| Opium use | ||||||
| No | 8360 (87.6) | 44.1 (42.6-45.7) | 39.7 (38.1-41.3) | 16.2 (15.4-17.0) | 1 | |
| Occasional | 486 (5.1) | 44.5 (39.4-49.8) | 42.2 (37.1-47.5) | 13.3 (10.8-16.3) | 0.92 (0.75-1.13) | 0.45 |
| Dependent | 697 (7.3) | 58.1 (53.6-62.5) | 27.3 (22.8-32.4) | 14.6 (12.1-17.4) | 0.97 (0.76-1.23) | 0.79 |
| Depression | ||||||
| No | 8304 (83.9) | 44.9 (43.4-46.4) | 40.0 (38.4-41.6) | 15.1 (14.4-15.9) | 1 | 0.01 |
| Yes | 1593 (16.1) | 43.3 (39.9-46.8) | 38.7 (35.5-42.1) | 17.9 (16.2-19.8) | 1.16 (1.03-1.31) | |
| Anxiety | ||||||
| No | 5831 (58.9) | 45.3 (43.6-46.9) | 40.6 (38.9-42.3) | 14.1 (13.3-15.0) | 1 | <0.01 |
| Yes | 4068 (41.1) | 43.5 (41.4-45.7) | 38.7 (36.6-40.9) | 17.7 (16.6-18.9) | 1.13 (1.03-1.23) | |
| BMI | ||||||
| Normal | 3063 (32.3) | 53.0 (50.9-55.0) | 35.2 (33.3-37.1) | 11.8 (10.8-12.9) | 1 | |
| Overweight | 3830 (40.4) | 39.5 (37.3-41.8) | 43.3 (41.1-45.6) | 17.1 (16.0-18.3) | 1.77 (1.60-1.96) | <0.001 |
| Obese | 2578 (27.3) | 31.7 (28.6-35.0) | 48.5 (45.2-51.9) | 19.8 (18.1-21.6) | 2.45 (2.15-2.79) | <0.001 |
| Physical activity | ||||||
| Low | 4740 (47.8) | 44.3 (42.4-46.2) | 39.8 (37.8-41.7) | 16.0 (15.0-17.0) | 1 | |
| Moderate | 3693 (37.3) | 43.6 (41.4-45.9) | 40.0 (37.8-42.2) | 16.4 (15.1-17.7) | 1.06 (0.93-1.21) | 0.39 |
| High | 1478 (14.9) | 45.5 (42.3-48.6) | 39.3 (35.8-42.8) | 15.3 (13.3-17.4) | 1.08 (0.95-1.23) | 0.24 |
| Hypertension | ||||||
| No | 7134 (72.1) | 46.1 (44.5-47.6) | 40.8 (39.2-42.4) | 13.2 (12.4-14) | 1 | <0.001 |
| Yes | 2759 (27.9) | 40.4 (33.8-47.3) | 38.4 (31.8-45.5) | 21.2 (19.2-23.4) | 1.28 (1.14-1.43) | |
| Diabetes | ||||||
| No | 6958 (70.2) | 47.1 (45.6-48.6) | 39.9 (38.3-41.4) | 13.0 (12.3-13.8) | 1 | <0.001 |
| Yes | 2953 (29.8) | 35.3 (31.9-38.9) | 42.9 (39.3-46.6) | 21.8 (19.8-23.9) | 1.62 (1.46-1.80) |
The odds ratios are adjusted based on gender, age, education, smoking status, and BMI. CI=Confidence interval; BMI=Body mass index; AOR=Adjusted odd ratio
Predictors of dyslipidemia
By adjusting baseline indicators as probable confounders [Table 3], sex, advanced age, overweight, obesity, diabetes, hypertension, and depression status could effectively predict DL in the study population. Females were 1.78 times more likely to be diagnosed with DL (adjusted odds ratio [AOR] =1.78; 95% CI: 1.61, 1.98). Increase in age was associated with higher odds of being diagnosed with DL. For example, OR for those aged above 55 years was more than two times higher than for those aged 15–24 years. Participants with depression and anxiety were 16% (AOR = 1.16; 95% CI: 1.03, 1.31) and 13% (AOR = 1.13; 95% CI: 1.03, 1.23) more likely to be diagnosed with DL, respectively. Other significant variables were BMI level, and history of hypertension and diabetes.
Chances of DL increased significantly in women (AOR = 1.78; P < 0.001), people >24 years of age (P < 0.001), individuals that were overweight and obese (AOR = 1.77 and 2.45, respectively P < 0.001), patients with diabetes (AOR = 1.62; P < 0.001) and hypertension (AOR = 1.28; P < 0.001), and individuals with a depression status [Table 3]. In this context, education level, smoking, opium use, and physical activity were not found as significant determinants of DL.
The overall prevalence of DL and UDL was lower in Phase 2 compared to Phase 1 of the study [Figure 2a and b]. Furthermore, in both instances, the prevalence was higher in females compared to males. On the other hand, the prevalence of DDL was higher in Phase 2 compared to Phase 1 and also in females compared to males [Figure 2c]. The prevalence of UDL in all age groups was lower in Phase 2 compared to Phase 1 [Figure 3a]; however, the prevalence of DDL was not different up to the age of 65 years where the prevalence in Phase 2 overtook that of Phase 1 [Figure 3b].
Incidence rate of dyslipidemia
The data of 2820 persons who participated in both phases were used for incidence rate calculation [Table 4]. The overall incidence rate was 2.58 per 100-person years. The incidence rate of DL increased more or less age dependently from 1.28 persons/100-person years in the 15–24-year-old group to 3.48 in the 55–64-year-old group. On the other hand, the highest incidence rate of DL was observed in people with diabetes (5.45 persons/100-person years) and in obese individuals (3.97 persons/100-person years) [Table 4].
Table 4.
Age-sex-specific incidence rate (persons/100 person-years) of dyslipidemia between 1st (2009-2011, n=5855) and 2nd (2014-2018, n=9911) phases of community-based cohort study - Kerman Coronary Artery Disease Risk Factors Study, Kerman, Iran (n=2820 match cases)
| Subgroups to round 1 | Number of normal people turned dyslipidemia | Person-years | Incidence rate (95% CI) | P |
|---|---|---|---|---|
| Overall | 94 | 2030.30 | 2.58 (2.09-3.15) | |
| Sex | ||||
| Male | 50 | 1268.69 | 2.20 (1.63-2.88) | 0.06 |
| Female | 44 | 761.61 | 3.24 (2.36-4.32) | |
| Age group (year) | ||||
| 15-24 | 8 | 355.40 | 1.28 (0.55-2.50) | <0.05 |
| 25-34 | 17 | 390.83 | 2.42 (1.42-3.85) | |
| 35-44 | 22 | 392.81 | 3.12 (1.97-4.69) | |
| 45-54 | 20 | 390.73 | 2.83 (1.74-4.34) | |
| 55-64 | 16 | 255.57 | 3.48 (2.0-5.59) | |
| 65-74 | 10 | 168.86 | 3.29 (1.59-5.97) | |
| +75 | 1 | 66.20 | 0.84 (0.02-4.59) | |
| Cigarette smoker | ||||
| No | 85 | 1802.20 | 2.63 (2.10-3.25) | 0.61 |
| Yes | 9 | 228.09 | 2.20 (1.01-4.15) | |
| Addiction | ||||
| No | 78 | 1721.44 | 3.82 (3.03-4.75) | 0.22 |
| Yes | 15 | 303.99 | 2.73 (1.53-4.46) | |
| Depression | ||||
| No | 60 | 1428.45 | 2.34 (1.79-3.0) | 0.16 |
| Yes | 34 | 601.85 | 3.16 (2.20-4.38) | |
| Anxiety | ||||
| No | 28 | 516.18 | 3.02 (2.02-4.34) | 0.33 |
| Yes | 66 | 1514.12 | 2.43 (1.89-3.10) | |
| Obesity | ||||
| No | 75 | 1727.14 | 2.42 (1.91-3.02) | 0.05 |
| Yes | 18 | 257.29 | 3.97 (2.37-6.19) | |
| Diabetes | ||||
| No | 84 | 1919.01 | 2.45 (1.95-3.02) | 0.03 |
| Yes | 10 | 111.29 | 4.97 (2.41-8.96) | |
| Hypertension | ||||
| No | 78 | 1768.34 | 2.46 (1.95-3.07) | 0.20 |
| Yes | 16 | 256.34 | 3.47 (2.0-5.57) | |
| Physical activity | ||||
| Low | 36 | 764.28 | 2.62 (1.84-3.61) | 0.92 |
| Other | 58 | 1266.01 | 2.56 (1.95-3.30) |
CI=Confidence interval
DISCUSSION
The main findings of the study were that 57% of the studied population had at least one abnormal serum lipid (DL), about three-quarters of whom were undiagnosed. Advanced age, obesity, hypertension, diabetes, anxiety, and depression were the main predictors of DL in the study population. The prevalence of DL was reduced by about 25% between the two phases of the study. Both UDL and DDL were more prevalent in women. The 5-year incidence rate of DL was 2/6 persons/100 person-years, and people with diabetes and obese individuals had the highest incidence rate of DL during the last 5 years.
Hyperlipidemia is considered the main risk factor for CAD and CVD, diseases that cause about 46% of mortality in Iran.[19] Almost 20% of the studied population had borderline cholesterol levels. These individuals are at risk of becoming hypercholesterolemic in near future, especially that this phenomenon was found to be age dependent. On the other hand, the prevalence of UDL was 2.5 times higher than that of DDL. These statistics show we need promotion in health education, screening, and controlling the lipid profile in the residents of this region of the country.
Our data showed that DL prevalence was declined after 5 years in Kerman. Meanwhile 70% of the individuals with DL are undiagnosed, while in Phase 1, this measure was 85%. This means that both overall and undiagnosed proportions of this abnormality have decreased in the last 5 years. The reason may be the provision of more treatment facilities by the government during the last 5 years.[20] In Iran, in recent years, the government has performed a health promotion plan that includes covering the treatment costs by providing more efficient health insurance to almost all Iranians. The tendency for replacement of saturated oil with unsaturated forms in cooking may also have played a role. Darroudi et al. reported the DL prevalence of 83.4% in adults in Mashhad city,[21] while in our study, the overall prevalence of DL was 56.8%, showing a lower prevalence of DL in Kerman. The prevalence of DL was much lower in China and Saudi Arabia than it was in the present study (31.3% and 33% vs. 57% here).[22,23] Regarding DL components, Hedayatnia et al. reported that the prevalence of low HDL-C, high LDL-C, hypertriglyceridemia, and hypercholesterolemia was 43.9%, 35.5%, 46%, and 41.6% in Mashhad (the largest city in the northeast of Iran), respectively,[11] while in the present study, they were 42.9%, 5.3%, 11.8%, and 6.4%, respectively. Except for low HDL-C, the other types of DL showed significantly higher prevalence in Mashhad compared to Kerman, indicating better health status and control of DL in Kerman.[2,11,21] In a study on a population aged 20–83 years, in Turkey in 2014, it was shown that more than three-quarters of people (79.6%) had DL,[24] which shows a higher overall prevalence in Turkey than we found in the present study. Furthermore, DL was not significantly different between men and women in that study (78.7% in men vs. 80.4% in women). However, abnormal DL component prevalence including HDL-C, LDL-C, and TG was 41.5%, 36.2%, and 35.7%, respectively, which is similar to the findings of the present study.
The second finding of the study was that the prevalence and incidence rate of DL was higher in women compared to men. Although these results are consistent with the findings of some published studies,[6,25] they are different from other studies.[19] After adjusting for the impact of other covariates, there was a significant relationship between sex and DL. The chance of DL in women was about 1.8 times that of men. The present findings seem to be consistent with other research that found gender is a contributing factor in DL.[6,25,26] This result may be explained by the fact that women are more overweight/obese, less physically active, and more anxious than men.[15,17,27] The prevalence of low HDL-C in women was higher than it was in men, although compared with 5 years earlier, the prevalence of low HDL-C has decreased. The same results were also reported by other studies.[1,6] Opoku et al. reported that DL and low HDL-C level were higher in women.[6] Low HDL-C was the most prevalent DL in the present study. This is in accord with the results of Garcez et al. in Sao Paulo, Brazil.[1] By contrast, a study on an adult population in Jordan showed that hypercholesterolemia was the most prevalent DL type, and interestingly, low HDL-C had the lowest prevalence compared with other forms of DL.[28] Liu et al. showed that the overall DL prevalence was 32.2% and the prevalence in men was higher than it was in women (42.8% vs. 26.2%).[26] They also found that gender (men), advanced age, overweight and obesity, type 2 diabetes mellitus, and hypertension were risk factors for DL. We found that hypercholesterolemia was not different between men and women, and that hypertriglyceridemia was more prevalent in men. The explanation for these discrepancies can be related to the year of the study, the nature of the geographical region, lifestyle, ethnicity, and the place of residence. For example, the sample of Liu et al. was from rural areas in China, but in the present study, the target population was from the urban region of southeastern Iran.[26]
None of the mentioned studies include opium use and mental status in their studies. There is a belief among most opium users that this substance will reduce the level of serum lipids. This study did not verify this unsafe belief. Anxiety and depression also increased the chance of having DL. These results point to an important health condition risk, as it was shown that the prevalence of these mental disorders is high in this population (77.1% and 34.7%, respectively).[27]
Even though, compared with Phase 1, DL and UDL prevalence has decreased, still UDL is more prevalent, and it is important that health authorities improve their screening programs in order to detect UDL and reduce its burden on health and the economy.
CONCLUSION
Overall the results showed that about 57% of the population under study suffer from DL, from whom 73.4% were undiagnosed. Although there were promising signs of reduction in DL and increase in DDL in the last 5 years, the current high prevalence of lipid abnormality, especially in the form of UDL, and its association with obesity, hypertension, diabetes, anxiety, and depression is a warning that the health-care management system should improve its strategies toward diagnosis and treatment of this metabolic abnormality and CAD risk factor.
Financial support and sponsorship
Authors are grateful from Vice Chancellor for Research and Technology at Kerman University of Medical Sciences for financial support of the project (grant No IR.KMU.REC.93/310).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Garcez MR, Pereira JL, Fontanelli Mde M, Marchioni DM, Fisberg RM. Prevalence of dyslipidemia according to the nutritional status in a representative sample of São Paulo. Arq Bras Cardiol. 2014;103:476–84. doi: 10.5935/abc.20140156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Najafipour H, Shokoohi M, Yousefzadeh G, Azimzadeh BS, Moshtaghi Kashanian G, Bagheri MM, et al. Prevalence of dyslipidemia and its association with other coronary artery disease risk factors among urban population in Southeast of Iran: Results of the Kerman Coronary Artery Disease Risk Factors Study (KERCADRS) J Diabetes Metab Disord. 2016;15:49. doi: 10.1186/s40200-016-0268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin HQ, Wu JY, Chen ML, Chen FQ, Liao YJ, Wu YT, et al. Prevalence of dyslipidemia and prediction of 10-year CVD risk among older adults living in southeast coastal regions in China: A cross-sectional study. Clin Interv Aging. 2019;14:1119–29. doi: 10.2147/CIA.S207665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akbartabar Toori M, Kiani F, Sayehmiri F, Sayehmiri K, Mohsenzadeh Y, Ostovar R, et al. Prevalence of hypercholesterolemia, high LDL, and Low HDL in Iran: A systematic review and meta-analysis. Iran J Med Sci. 2018;43:449–65. [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee EJ, Kim HC, Kim JH, Lee EY, Kim BJ, Kim EM, et al. Guidelines for the management of dyslipidemia. Korean J Intern Med. 2019;34:723–71. doi: 10.3904/kjim.2019.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opoku S, Gan Y, Fu W, Chen D, Addo-Yobo E, Trofimovitch D, et al. Prevalence and risk factors for dyslipidemia among adults in rural and urban China: Findings from the China National Stroke Screening and Prevention Project (CNSSPP) BMC Public Health. 2019;19:1500. doi: 10.1186/s12889-019-7827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seyedi SH, Mottaghi A, Mirmiran P, Hedayati M, Azizi F. The relationship between dietary patterns and lipoprotein-associated phospholipase A2 levels in adults with cardiovascular risk factors: Tehran Lipid and Glucose Study. J Res Med Sci. 2020;25:3. doi: 10.4103/jrms.JRMS_256_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sajjadieh H, Sajjadieh A, Koopaei ZK, Oveisgharan S. Correlation between vitamin D level and coronary artery calcification. J Res Med Sci. 2020;25:51. doi: 10.4103/jrms.JRMS_1080_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirdamadi A, Mirmohammadsadeghi M, Banazade Dardashty A, Arabi Z. The value of epicardial adipose tissue thickness for outcome prediction of patients undergoing coronary artery bypass grafting surgery. J Res Med Sci. 2019;24:93. doi: 10.4103/jrms.JRMS_1024_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Journal of Vascular Medicine. Volume 2017, Article ID 6061306, 5 pages. doi: 10.1155/2017/6061306. [Google Scholar]
- 11.Hedayatnia M, Asadi Z, Zare-Feyzabadi R, Yaghooti-Khorasani M, Ghazizadeh H, Ghaffarian-Zirak R, et al. Dyslipidemia and cardiovascular disease risk among the MASHAD study population. Lipids Health Dis. 2020;19:42. doi: 10.1186/s12944-020-01204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rinkūnienė E, Laucevičius A, Petrulionienė Z, Dženkevičiūtė V, Kutkienė S, Skujaitė A, et al. The prevalence of dislipidemia and its relation to other risk factors: A nationwide survey of Lithuania. Clin Lipidol. 2015;10:219–25. [Google Scholar]
- 13.Yarnell J, Yu S, McCrum E, Arveiler D, Hass B, Dallongeville J, et al. Education, socioeconomic and lifestyle factors, and risk of coronary heart disease: The PRIME Study. Int J Epidemiol. 2005;34:268–75. doi: 10.1093/ije/dyh267. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong T, Bull F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ) J Public Health. 2006;14:66–70. [Google Scholar]
- 15.Najafipour H, Kahnooji M, Baneshi MR, Yeganeh M, Ahmadi Gohari M, Shadkam Farokhi M, et al. The prevalence and 5-year incidence rate of low physical activity in an urban population of 10,000 in southeastern Iran: Relationship with other cardiovascular risk factors. J Phys Act Health. 2020;17:435–42. doi: 10.1123/jpah.2019-0426. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Steer RA, Garbin MG. Psychometric properties of the beck depression inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 17.Najafipour H, Sanjari M, Shokoohi M, Haghdoost AA, Afshari M, Shadkam M, et al. Epidemiology of diabetes mellitus, pre-diabetes, undiagnosed and uncontrolled diabetes and its predictors in general population aged 15 to 75 years: A community-based study (KERCADRS) in southeastern Iran. J Diabetes. 2015;7:613–21. doi: 10.1111/1753-0407.12195. [DOI] [PubMed] [Google Scholar]
- 18.Center for Disease Control and Prevention (CDC). Principles of Epidemiology in Public Health Practice; An Introduction to Applied Epidemiology and Biostatistics, 3rd Ed. U.S. Department of Health & Human Services. [Updated Nov 2011]. https://www.cdc.gov/csels/dsepd/ss1978/index.html.
- 19.Aghamohammadi S, Kazemi E, Khosravi A, Kazemeini H. Trend of ten leading causes of death in the Islamic Republic of Iran, 2006- 2011. IJE. 2017;12:1–11. [Google Scholar]
- 20.Olyaeemanesh A, Behzadifar M, Mousavinejhad N, Behzadifar M, Heydarvand S, Azari S, et al. Iran's health system transformation plan: A SWOT analysis. Med J Islam Repub Iran. 2018;32:39. doi: 10.14196/mjiri.32.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darroudi S, Saberi-Karimian M, Tayefi M, Arekhi S, Motamedzadeh Torghabeh A, Seyedzadeh Sani SM, et al. Prevalence of combined and noncombined dyslipidemia in an Iranian population. J Clin Lab Anal. 2018;32:e22579. doi: 10.1002/jcla.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi L, Hu J, Zhu K, Fu Y, Xia R, Hu X. Changes of prevalence of dyslipidemia among adults: A cross-sectional study with a 2-year follow-up in urban southeast China. Clin Lipidol. 2014;9:33–47. [Google Scholar]
- 23.Saad Alzahrani G, Aljehani SM, Al-Johani JJ. Risk factors of dyslipidemia among Saudi population, 2017. Egypt J Hosp Med. 2018;71:2262–5. [Google Scholar]
- 24.Bayram F, Kocer D, Gundogan K, Kaya A, Demir O, Coskun R, et al. Prevalence of dyslipidemia and associated risk factors in Turkish adults. J Clin Lipidol. 2014;8:206–16. doi: 10.1016/j.jacl.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Tabatabaei-Malazy O, Qorbani M, Samavat T, Sharifi F, Larijani B, Fakhrzadeh H. Prevalence of dyslipidemia in Iran: A systematic review and mete analysis study. Int J Prev Med. 2014;5:373–93. [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Yu S, Mao Z, Li Y, Zhang H, Yang K, et al. Dyslipidemia prevalence, awareness, treatment, control, and risk factors in Chinese rural population: The Henan rural cohort study. Lipids Health Dis. 2018;17:119. doi: 10.1186/s12944-018-0768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Najafipour H, Banivaheb G, Sabahi A, Naderi N, Nasirian M, Mirzazadeh A. Prevalence of anxiety and depression symptoms and their relationship with other coronary artery disease risk factors: A population-based study on 5900 residents in Southeast Iran. Asian J Psychiatry. 2016;20:55–60. doi: 10.1016/j.ajp.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Khader YS, Batieha A, El-Khateeb M, Al Omari M, Ajlouni K. Prevalence of dyslipidemia and its associated factors among Jordanian adults. J Clin Lipid. 2010;4:53–8. doi: 10.1016/j.jacl.2009.12.004. [DOI] [PubMed] [Google Scholar]


