Abstract
Background
Acute lower respiratory infection (ALRI) is a major cause of morbidity and mortality worldwide in young children and is predominately caused by viral respiratory pathogens. This study aims to identify the viral etiologies of ALRI in hospitalized children in Jordan University Hospital and compare the clinical characteristics of influenza virus infection with other respiratory viruses.
Methods
A retrospective viral surveillance study that included 152 children below 15 years of age admitted with ALRI from December 2018 through April 2019 was conducted. We recorded results of real-time reverse transcriptasepolymerase chain reaction (RT-PCR) for common respiratory viruses. Clinical and demographic information of the study population was collected from patients' electronic medical records.
Results
152 patients were identified with a median age of 1 year (mean was 2.1 years). Ninety-five patients (62.5%) were males. One or more viral respiratory pathogens were detected in 145 (95.3%) children. Respiratory syncytial virus was the most detected virus in 68 patients (44.8%). Influenza virus was detected in 25 patients (16.4%). Children with influenza infection had more fever and lower leukocyte count compared to children infected with other viruses. The severity of the ALRI correlated significantly with several factors, including age less than six months and the presence of neuromuscular disease (p<0.05).
Conclusion
Viral detection was common among children admitted with ALRI. Viruses, including influenza, are recognized as significant contributors to the morbidity associated with ALRI. More attention is needed on strategies for the prevention and detection of viral ALRI in developing countries.
Key words: Influenza, Jordan, acute lower respiratory infections, pediatrics, RT-PCR
Introduction
Acute lower respiratory infections (ALRI) are among the leading causes of morbidity and mortality in pediatric patients worldwide, with a significant burden on health care facilities [1]. Deaths caused by ALRI account for approximately 15% of mortality in children below 5 years [2]. Viruses are responsible for the majority of ALRI in children [3,4]. Rhinoviruses, influenza viruses, parainfluenza viruses, respiratory syncytial virus, coronaviruses, and adenovirus are the leading causes of viral respiratory infections. Various reports worldwide have investigated the role of individual viruses and their impact on childhood health.
Respiratory syncytial virus (RSV) was reported to be the pathogen most commonly recovered from children with bronchiolitis as it was responsible for as many as 50-90% of children hospitalized with bronchiolitis during the winter [5]. In another study from Manitoba, (Canada), adenovirus infection was associated with significant respiratory morbidities, especially in young infants [6]. Calvo and coresearchers found that rhinovirus infection was detected in hospitalized infants with respiratory tract disease and was the second etiologic agent associated with recurrent wheezing in hospitalized children of the age of 2 years [7]. A study by Zohng et al. compared the clinical characteristics of different types of Human Parainfluenza virus (HPIV) and demonstrated that coinfection of HPIV with other viruses was more severe than single infection but was milder than coinfection with other viruses. Moreover, HPIV coinfection with atypical bacteria was more severe than HPIV single infection and coinfection with other viruses [8].
Seasonal influenza virus is one of the most significant pathogens that cause ALRI. It is caused by influenza A or B viruses, and rarely influenza C viruses. It usually occurs as annual outbreaks, but the timing of the onset, peak, and end of influenza activity varies among different areas worldwide [9].
In Jordan, like other countries in the Northern hemisphere, the epidemics of influenza typically occur during the fall and winter, with reported cases that tend to peak in October, but disease activity can extend as late as May. According to the latest WHO data published in 2018, influenza and ALRI deaths in Jordan reached 3.78% of total deaths with an adjusted death rate of 21.86 per 100,000 population, ranking at 110 in the world [10]. The World Health Organization estimates that the annual influenza epidemics worldwide result in about 3-5 million severe illness cases and about 250,000 to 500,000 deaths [11]. According to the CDC (Centre for Disease Control), seasonal influenza virus infection is so common that its incidence can only be estimated. The CDC maintains surveillance for several measures, such as the percentage of respiratory specimens submitted to clinical laboratories that are positive for influenza and the percentage of outpatient visits to sentinel physicians for influenza-like illness. The seasonal incidence of influenza is approximated as 5-20% [12] .
Although influenza infection is a commonly self-limited and uncomplicated disease in healthy children, it can be associated with severe morbidity and mortality. The highest rate of influenzarelated pediatric deaths has been reported in infants less than 6- months of age [13]. Pediatric influenza virus infections are responsible for significant health and social consequences; they are associated with increased frequency of emergency room visits, hospitalization, and antibiotic utilization, missed school days for the patient and patient’s siblings, and missed workdays for the parents [14,15]. The hospitalization rate with influenza was more significant in children <5 years than children aged 5 through 17 [14]. Interestingly, hospitalization of younger infants with influenza is often attributed to undifferentiated febrile illness, which may require an evaluation for severe bacterial infection because of the acute onset of fever and absence of localizing signs, including respiratory signs [15].
Seasonal influenza also represents a major economic burden with increased healthcare costs, workplace absences, and reduced productivity. The World Health Organization cites reports from developed countries that estimate the total annual cost of influenza infections between U$1 million to U$6 million per 100,000 [16]. Epidemiological studies on ALRI in general and pediatric influenza are scarce in the Middle East, including Jordan [17,18].
Routine surveillance for the total number of laboratory-confirmed influenza infections is not conducted. Therefore, the number of published studies that include such data is limited. A multicenter study of five East-Mediterranean countries, including Jordan, investigated viral etiology of hospitalized patients with severe ALRI over seven years; influenza was responsible for 11.8% of admitted patients [17]. Noteworthy, a workforce of an influenza surveillance program for the Middle East, North, East, and South Africa region published a report in 2018. This report concluded that influenza poses a significant threat, especially in high-risk groups (children under five, elderly, pregnant women, and immunosuppressed individuals). This finding called for additional funding and planning to address such a threat [18]. 6-year surveillance on influenza hospitalization with SARS was performed in Jordan by Abdullat et al., it concluded that influenza was associated with substantial morbidity and mortality in the country; with influenza detected in 9% of SARS cases and had a death rate of 3% [19].
Our study conducted a retrospective study that reviewed viral ALRI-related hospitalizations in Jordan University Hospital over the influenza season of 2019. This study’s objectives were to estimate the burden of influenza infection among other viruses in terms of frequency, severity, and length of hospitalization and investigate the risk factors of severe ALRI.
Methods
Study design and setting
This retrospective study included children acutely ill with respiratory symptoms admitted to the pediatric department or Pediatric intensive care unit (PICU) at Jordan University Hospital in Amman. Jordan University Hospital is a tertiary teaching hospital in Amman and the central area of Jordan with a bed capacity of about 500. We enrolled children between one month and 15 years old. The study was conducted from December 2018 through April 2019. Data of children enrolled in the study were obtained from laboratory records of all children admitted to the hospital tested with PCR (polymerase chain reaction)-viral detection test. We recorded PCR test results and clinical and demographic information of the study population collected from patients’ electronic medical records.
Children hospitalized with febrile illness (>38.0°C) or symptoms of ALRI were included. Children were diagnosed as ALRI if they presented with cough, difficulty breathing, and signs indicative of lower respiratory tract infections such as tachypnea, retraction, grunting, flaring, and auscultatory findings such as wheezing or crackles. Patients were classified as having severe ALRI if they had a cough or difficulty breathing in addition to one or more of the following: retractions, nasal flaring, grunting, oxygen saturation (SpO2) <90% at room air, intensive care unit (ICU) admission, or patient death [20].
Viral detection
Nasopharyngeal samples were collected using diagnostic swabs and transport medium (CITO -test transport system with sterile Floctil collected ultra-swab). Samples were stored at - 20°C.One hundred and thirteen (113) patients were tested with real-time reverse transcriptase-polymerase chain reaction (RTPCR) for viruses using a multiplex identification kit (Fast-Track Diagnostic, Esch-sur-Alzette, Luxembourg) [21]. The viruses identified were RSV, human Rhinovirus (HRV), human adenovirus (HAdV), human bocavirus (HBoV), influenza A, A/H1N1 and B, Metapneumovirus A and B, α coronaviruses 229E and NL63 (HCoV1), β coronaviruses OC43 and HKU1 (HCoV2), and human parainfluenza viruses (HPIV) 1-4. A group of 39 patients (25.6%) were tested with a rapid FLU test for influenza viruses only (influenza A, A/H1N1, and influenza B).
Nucleic acid extraction
Viral nucleic acid from samples was extracted using an EasyMAG (Biomeurex, Marcy l’Etoile, France) automated extractor according to the manufacturer’s instructions. Briefly, the extraction was done from a 400 μl homogenized sample, which was added to a 1500 μl lysis buffer and was incubated for 10 min off-board. The samples were loaded into the EasyMAG, and 100 μl of magnetic silica was added to each sample and mixed well. Finally, the nucleic acid was eluted in a volume of 110 μl, of which 50 μl was used for the FTD assay and 54 μl for the custom essay.
Multiplex real-time RT-PCR
The multiplex real-time PCR FTD assay was performed on an ABI 7500 Fast instrument (Life Technologies, Carlsbad, CA, USA) as per the manufacturer’s instructions using an AgPath-ID™ One-Step RT-PCR kit (Ambion Inc., Austin, TX, USA) with the FTD Respiratory pathogens kit (Fast Track Diagnosis) for the detection of 12 viruses using five tubes containing primer and probe mix for different viruses; tube-1 [influenza A (flu A), influenza A subtype H1N1 (pandemic H1N1), human rhinovirus (HRV), influenza B (flu B)], tube-2 [human coronaviruses NL63 (HCoV-NL63), 229E (HCoV 229E), OC43 (HCoV-OC43), and HKU1 (HCoV HKU1)], tube-3 [human parainfluenza viruses, 2, 3, and 4 (HPIV- 2, 3 and 4) & IC], tube-4 [human parainfluenza viruses-1, human bocavirus (HBoV), human metapneumovirus (HMPV A/B)] and tube-5 [respiratory syncytial virus (RSVA/B), human adenovirus (HAdV). The multiplex real-time RT-PCR thermal profile for the FTD kit was as follows; 50°C for 15 min, 95°C for 10 min, 40 cycles of 95°C for 8 s, 60°C for 34 s, whereas the thermal profile for the custom assay was set at 50°C for 30 min, 95°C for 10 min, 45 cycles of 95°C for 15 s, 55°C for 30 s.
Standardization of uniplex real-time PCR
Initial standardizations were done for uniplex real-time PCR using the FTD positive samples as controls. These positive samples included the following viruses: HRV, HAdV, flu A, pandemic H1N1, flu B, HPIV-1, HPIV-2, HPIV-3, HPIV-4, RSVA/B HMPV A/B, HCoV-OC43, HCoV-NL63, HCoV 229E, HCoV HKU1, (HBoV). AgPath (Ambion) one step RT-PCR master mix was used to amplify and detect viral nucleic acid. Briefly, each reaction was performed in a 20 μl volume which consisted of 12.5 μl of buffer, 1 μl of enzyme, 2 picomoles of each primer (corresponding to each virus), 2 picomoles of probe (corresponding to each virus) (synthesized by Life Technologies), 5 μl of the extracted nucleic acid of the positive control/sample and made to a final volume of 20 μl with nuclease-free water. The thermal cycling profile for the uniplex RT-PCR was 50°C for 30 min (1 cycle), 95°C for 10 min (1 cycle) followed by 90°C for 15 s and 55°C for 30 s (4 cycles). Specimens were considered positive when the Ct value was <35.
Clinical data collection
Data were collected from medical records of patients who have been admitted during the study period and had nasopharyngeal swabs taken for PCR testing. Data included patient age, gender, birth weight, gestational age. Prematurity is defined as gestational age less than 37 weeks (26-36 weeks), and low birth weight is defined as birth weight <2.5Kg [21]. Other data collected included NICU admission, number of siblings, sick contacts, breastfeeding history, smoking exposure, daycare attendance, antibiotics use before hospitalization, daycare attendance, and vaccination profile, including flu vaccine. Chronic diseases were also reported in study populations such as cystic fibrosis (CF), asthma, anatomical lung problems, congenital heart disease, neuromuscular, neurological, growth retardation, down syndrome, renal, and metabolic disease. Patients’ signs included pulse oximeter reading (SPO2) on admission, axillary-measured temperature, respiratory rate, presence of respiratory distress such as grunting, flaring of ala nasi, and retractions. Tachypnea was defined as respiratory rate >60/min for infants younger than 2 months, >50/min for infants 2 to 12 months old, >40/min for children, >20/min for children 1 to 5 years, and >20/min for children >5 years [20]. We collected laboratory and diagnostic investigations such as complete blood count and inflammatory markers, serum electrolytes and kidney function, blood cultures. Chest films performed for children during admission were also reviewed and interpreted by a radiologist blinded to patients’ clinical data and respiratory viral tests. We entered details on patients’ course during hospitalization like the length of stay (LoS), admission to PICU, oxygen supplementation, and respiratory support requirement. Medications used during admission were documented, including antibiotics and oseltamivir (tamiflu). Finally, discharge diagnosis (bronchiolitis, lobar pneumonia, bronchopneumonia, and exacerbation of asthma) and survival outcomes were entered.
Statistical analysis
Data are described using means and standard deviation (SD) for continuous variables and frequencies and percentages for categorical variables. The frequency of influenza and other viruses in the study population are presented as frequency distributions. The relevant demographic and clinical characteristics of influenza-positive patients were compared with those of influenza-negative patients using the chi-square test. We conducted a multivariate analysis of factors associated with longer hospital stay using the general linear model procedure. Clinical variables predicting the risk for severe ALRI presentation and mortality were analyzed with binary logistic regression analysis. A p of <0.05 was considered statistically significant. All analysis was performed using IBM SPSS 20 (SPSS Inc., Chicago, IL, USA).
Results
Study population
Clinical and demographic data
The study population’s clinical and demographic characteristics are shown in Table 1. One hundred and fifty-two (152) patients were identified for the study from December 2018 to April 2019. Their ages ranged between 1 month and 15 years, with a median age of 1 year (mean 2.1±3). Most children of the study population were males and were less than six months old (27.6%). Chronic illness was present in 54 (35.5%) of children, including chronic lung disease, congenital heart disease, neurological or neuromuscular disorders, and asthma. The most common primary diagnosis on admission was bronchopneumonia in 52 children (34%). Figure 1 displays the proportion of individual virus detection by admission diagnoses.
Laboratory investigations
Lymphopenia, which is defined as a peripheral blood lymphocyte count <1500/mm3 (or <2000 in children <6 years of age) [22], was noticed in 13 patients (8.5%) with a mean value (SD) of 965/mm3 (± 270). Blood cultures were obtained from 72 patients (47.3%) and were positive in 2 patients only (0.03%).
Length of Stay
The mean LoS (Lenght of Stay) for previously healthy patients admitted for ALRI (n=141) was measured to be 6.2±3.2 days. Patients who had an underlying chronic medical condition (n=11) required prolonged hospitalization to IMU (intermediate care unit); hence their mean LoS was calculated separately (58±46 days). Among our cohort (97 patients), 64% of the study population received oxygen therapy, (44 patients) 29% were admitted to the PICU. One patient died, a 3.5-year boy with neuromuscular disease who presented with severe ALRI and respiratory failure requiring prolonged mechanical ventilation (28 days).
Table 1.
Characteristics of patients with influenza-positive ALRI compared with those of patients with Influenza-negative ALRI.
| Variable | Total number | Influenza-positive patients | Influenza-negative patients | p | ||
|---|---|---|---|---|---|---|
| 152 | 16% | n=25 | 79% | n=120 | ||
| Gender | 0.76 | |||||
| Male | 95 | 64% | 16 | 61% | 73 | |
| Female | 57 | 36% | 9 | 39% | 47 | |
| Age | 0.18 | |||||
| ≤6 months | 52 | 25% | 5 | 39% | 47 | |
| 6–24 months | 59 | 44% | 11 | 38% | 46 | |
| 2–5 years | 26 | 20% | 5 | 15% | 18 | |
| >5 years | 15 | 16% | 4 | 8% | 9 | |
| Clinical risk factors | ||||||
| Prematurity | 45 | 24% | 6 | 32% | 38 | 0.44 |
| Chronic lung disease | 6 | 4% | 1 | 4% | 5 | 0.96 |
| Neuromuscular disease | 20 | 12% | 3 | 13% | 15 | 0.94 |
| Asthma | 21 | 20% | 5 | 13% | 16 | 0.38 |
| Clinical presentation | ||||||
| Fever | 114 | 92% | 23 | 71% | 85 | 0.027 |
| Tachypnea | 130 | 84% | 21 | 86% | 103 | 0.81 |
| Retractions | 56 | 20% | 5 | 40% | 48 | 0.05 |
| Apnea | 15 | 8% | 2 | 10% | 12 | 0.75 |
| Cyanosis | 26 | 4% | 1 | 19% | 23 | 0.06 |
| O2 requirement | 97 | 40% | 10 | 70% | 84 | 0.004 |
| Chest X-ray findings | ||||||
| Interstitial markings | 115 | 60% | 15 | 85% | 97 | 0.02 |
| Hyperinflation | ||||||
| Peribronchial | ||||||
| Thickening | ||||||
| Consolidation | 44 | 36% | 9 | 26% | 32 | 0.61 |
| Atelactasis | ||||||
| Normal | 33 | 40% | 10 | 17% | 20 | 0.008 |
| Markers of inflammation | ||||||
| Positive CRP | 93 | 68% | 17 | 62% | 74 | 0.55 |
| Neutrophilia | 21 | 8% | 2 | 16% | 19 | 0.31 |
| Lymphopenia | 9 | 24% | 6 | 3% | 3 | 0.001 |
| Viral detection by PCR | ||||||
| One virus | 90 | 68% | 17 | 61% | 73 | 0.5 |
| Multiple viruses | 55 | 32% | 8 | 39% | 47 | 0.5 |
| Blood gas | ||||||
| Normal | 3 | 8% | 2 | 15% | 18 | 0.35 |
| Acidosis | 15 | 4% | 1 | 12% | 14 | 0.25 |
| Clinical course | ||||||
| Length of stay in days | 6 ± 3.4 | 6.2 ± 3.2 | 0.88 | |||
| (mean ± SD) | ||||||
| Severe ALRI* | 92 | 40% | 10 | 66% | 79 | 0.015 |
| ICU admission | 44 | 28% | 7 | 29% | 35 | 0.9 |
ALRI, acute lower respiratory infection; ICU, intensive care unit; SD, standard deviation; PcO2: carbon dioxide level; CRP, C-reactive protein; WBC, white blood cells; *severe ALRI defines as respiratory symptoms with one or more of the following: retractions, nasal flaring, grunting, oxygen saturation (SpO2) <90% at room air, ICU admission, or patient death; the demographic and clinical characteristics of influenza-positive patients were compared with those of influenza-negative patients using the chi-square test; values in bold are statistically significant at p<0.05.
Viral detection
At least one virus was detected in 145 patients (95.3%) of the total children. RSV was the most detected virus in 68 patients (44.8%). Influenza A virus was detected in 25 (16.4%). The least detected viruses were influenza A/H1N1, coronavirus, and enterovirus. Viral co-detection was common; 55 patients (36%) had more than one virus detected (Figure 2).
The viral distribution among different age groups is shown in Figure 3. RSV is the most common virus among children belonging to three age groups. However, the proportion of all types of influenza added together (A and B and A/H1N1) constitutes a larger frequency of 25% (4 patients). Figure 1 demonstrates the proportion of individual virus detection by diagnosis on admission.
Characteristics of patients with influenza-positive ARI compared to those with influenza-negative ARI
Table 1 shows the relevant demographic and clinical characteristics of influenza -positive patients compared with influenza-negative patients. In comparison to influenza-negative patients, influenza-positive children presented with more fever (92% vs 70.8%; p=0.027) and were more likely to have lower leukocyte count (24% vs 2.5%; p≤0.001). Influenza-positive children were more likely to have normal chest X-rays on admission (40% vs 16.6%, p=0.008), while the non-influenza group was more likely to have hyperinflation, interstitial markings, and peribronchial thickening on their chest films (p=0.02). In terms of clinical presentation, the non -influenza group was more likely to present with retractions (40% vs 20%; p=0.05), and they require more oxygen supplementation (70% vs 40%; p=0.04). Moreover, markers of severity were more pronounced in non-influenza patients (64.8% vs 40%; p=0.015), influenza -negative children were more likely to have interstitial marking and hyperinflation on chest X-ray at presentation (85%vs 60%, p=0.02). The two study groups were not significantly different regarding PICU admission, death, and length of stay.
Co-infection with influenza
Coinfection with at least one virus was observed in 8 (32%) of the influenza -positive patients. Three (37.5%) were coinfected with RSV, 1 (12.5%) with MPVa and MPVb, 2 (25%) with parainfluenza, 1 (12.5%) with adenovirus and 1 (12.5%) with enterovirus. Patients coinfected with other viruses were compared with those infected with influenza alone; no statistically significant difference between those two groups was found regarding the risk of increased length of stay, the severity of ALRI, or PICU admission.
Treatment
Of the study population hospitalized as LRTI, 97 (64%) patients received oxygen therapy,139 (91.4%) received systemic antibiotics and 29 (19%) patients received oseltamivir.
Antiviral administration
Oseltamivir antiviral therapy was started in 29 patients. Oseltamivir was commenced in patients with suspected or confirmed influenza virus infection who were less than 24 months of age and presented with febrile ALRI; those with high risk for complications, especially patients with neuromuscular diseases and asthma, and in children who presented with severe ALRI requiring PICU admission [23]. Twelve patients were positive for influenza. Those patients were compared to influenza positive patients who did not receive the medication. Data analysis revealed that 46% of the patient without oseltamivir therapy had severe disease compared to 33% in the treated group; this, however, was not statistically significant (p=0.5). Concerning oseltamivir therapy and length of stay, the treated group had a shorter LoS with a mean (SD) of 5.9 vs 6.3 (4.9 vs 1.6), but it was also not statistically significant (p=0.7) (Table 2).
Figure 1.
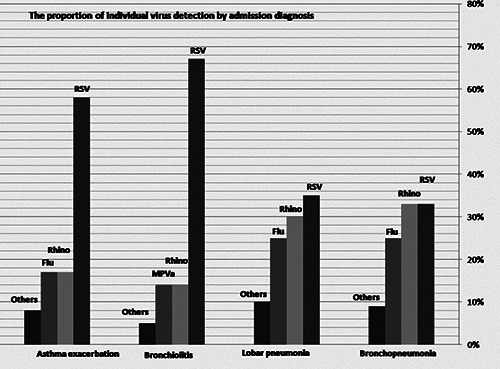
The proportion of individual virus detection by admission diagnoses.
Figure 2.
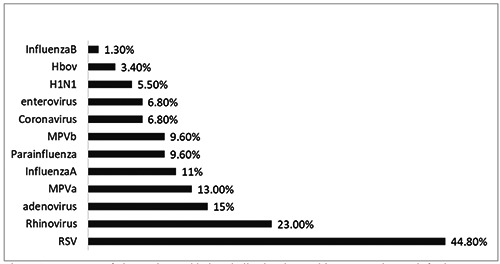
Frequency of viruses detected in hospitalized patients with acute respiratory infection.
Figure 3.

The proportion of viral pathogens detected by age group.
Factors associated with severe ALRI
Several clinical and demographic factors were investigated to confirm the association with the severity of ALRI. Table 2 shows the multivariate analysis of the factors associated with severe ALRI. More severe ALRI was confirmed among patients who presented with fever (OR: 0.57, p=0.02), patients with neuromuscular disease (OR: 0.00, p=0.021), and children who were exposed to smoking (OR: 4, p=0.007). Additionally, patients who required O2 support (OR: 0.1, p≤0.001) and those who stayed longer in the hospital (OR: 0.56, p=0.01) had more severe disease. RSV and MPVb were significantly associated with an increased risk of severe disease (OR: 0.8, p=0.004, OR: 4, p=0.04).
Table 3 demonstrates the severity of ALRI according to children age groups, patients who are less than 6 months were most likely to have severe disease (p≤0.001), with acute viral bronchiolitis being the most common diagnosis among this age group (48%). This is followed by patients who are more than five years of age who also had severe disease with a p of 0.04, with influenza viruses being most common among this age group (25%).
Factors associated with Length of Stay
The presence of chronic lung disease, neuromuscular disease, elevated C-reactive protein, the diagnosis of lobar pneumonia with the presence of consolidation on chest X-ray was significantly associated with a longer mean LoS (Table 4). Patients infected with enterovirus had a longer mean LoS than those who were positive for other viruses (p=0.04). The majority of patients positive for enterovirus were below 12 months, and 80% of their PCR test results revealed multiple viruses. Influenza-positive ALRI patients’ analysis revealed that disease severity was the only significant predictive factor for increased LoS. The mean LoS was 7.8 days for influenza-positive patients with severe disease compared to 4.5 days for influenza-positive patients with non-severe disease (p=0.017).
Discussion
This study demonstrates the burden of different viruses among children admitted with ALRI at JUH during the 2019 flu season. Our study confirmed that respiratory viruses are identified among most children admitted with fever or respiratory symptoms. The most common virus among all age groups was RSV (44.8%), followed by HRV (23%). This is consistent with two other national studies, which confirmed that 81.5% and 95% of children hospitalized during winter months tested positive for a virus, with RSV and HRV being the most common viruses identified [24,25]. The first of the two studies was a three-year prospective surveillance cohort study conducted in Amman by Khouri et al., where over 3,000 children were tested for 11 major respiratory viruses. It showed that of the 3,168 studied subjects, 2,581 (82%) had at least one respiratory virus detected, with respiratory syncytial virus (RSV) being the most predominant pathogen isolated [26].
The second prospective surveillance study was performed in Irbid, Northern Jordan, during the winter of 2,016, showing a significant burden of ARI/ALRI-associated hospitalization caused by viruses (95%); RSV was identified as the most common viral cause of ARI [24]. This is in line with published data in neighboring countries in the Middle East [26-29]. RSV infection is responsible for most ALRI in children, as reported by various reports globally [30-33].
In the former study by Najwa and coworkers, influenza viruses were reported in 4% of the 3,168 children tested for 11 viruses by RT-PCR [25]. In a multicenter cohort in the East Mediterranean region, 11.8% of influenza detection rate was reported. The higher percentage of influenza virus detection in our study can be attributed, in part, to the fact that a group of patients was tested for influenza virus only without the rest of the viral panel; this was a cost-effective measure as influenza has a specific treatment with an antiviral medication unlike the other viruses in the multiplex identification kit. It has been shown that although real-time PCR testing improved detection rate, it did not reduce additional diagnostic procedures, antibiotic use, antibiotic costs, or duration of hospital stay [34].
In comparison with other viruses identified, patients with influenza had more fever, which is well-described about influenza in contrast to other common viruses [35,36]. In addition to that, influenza positive group had a lower leukocyte count. According to previous studies, lymphopenia is a common feature for seasonal influenza and influenza A (H1N1) infection, although this finding is prominent in adult patients and less common in the pediatric population [37,38]. In terms of clinical presentation, the noninfluenza group had more pronounced signs of respiratory distress with more cyanosis, retractions, and the need for oxygen supplementation. This is consistent with finding by Khouri et al., where children who were positive for non-influenza viruses, RSV being most prevalent, were belonging to the younger age group and were more likely to require oxygen, present with cough and shortness of breath, and less likely to present with fever [25]. This observation was also reported in studies outside Jordan [30,31]. Previous work identified several risk factors for severe ALRI in the pediatric age group, including male gender, history of prematurity, chronic lung disease, malnutrition, lack of breastfeeding, low socio-economic status, and living with siblings.
Table 2.
Factors associated with severe ALRI.
| Variables* | Odds ratio | p |
|---|---|---|
| Age | 1.24 | 0.109 |
| Fever | 0.57 | 0.021 |
| Smoking exposure | 4 | 0.007 |
| Neuromuscular disease | 0.00 | 0.021 |
| Asthma | 2.55 | 0.07 |
| O2 requirement | 0.1 | 0.001 |
| Other viruses | 0.36 | 0.215 |
| CXR consolidation | 0.185 | 0.23 |
| Length of stay | 0.56 | 0.01 |
| RSV | 0.8 | 0.004 |
| Multiple viruses | 2.6 | 0.1 |
| MPVb | 4 | 0.04 |
| Influenza | 0.1 | 0.8 |
*Risk factors of severe disease were analyzed with binary logistic regression; ALRI, acute lower respiratory infection; CXR, chest x-ray; RSV, respiratory syncytial virus; MPVb, metapneumovirus
Table 3.
Severity of ALRI according to patients’ age groups.
| Age group | Severity | p |
|---|---|---|
| ≤6 months | 39/42 | 0.001 |
| 6-24 months | 34/59 | 0.5 |
| 2-5 years | 13/26 | 0.11 |
| >5 years | 6/15 | 0.04 |
The frequency of severe cases among all age groups analyzed by the chi-square test; values in bold are statistically significant at p<0.05.
Table 4.
Multivariate analysis of factors associated with length of stay in the study population.
| Median length of hospitalization (in days) | p | |
|---|---|---|
| Prematurity | 7 | 0.203 |
| Chronic lung disease | 10.5 | 0.016 |
| Neuromuscular disease | 9 | 0.001 |
| Asthma | 5 | 0.111 |
| Elevated C-reactive protein | 6 | 0.007 |
| Diagnosis | ||
| Lobar pneumonia | 8.5 | 0.007 |
| Bronchiolitis | 5 | 0.5 |
| Consolidation on chest film | 7 | 0.035 |
| Multiple viruses on RT-PCR | 6 | 0.087 |
| Enterovirus on RT-PCR | 7 | 0.04 |
Multivariate analysis performed with general linear model (GLM); values in bold are statistically significant at p<0.05. bold are statistically significant at p<0.05.
In our study, several factors were identified as risk factors for severe ALRI. These factors included the following; age younger than six months, diagnosis of viral bronchiolitis, atelectasis on chest films, infection with certain viruses (influenza A, Coronavirus, and RSV), and neuromuscular presence disease. Fever on presentation, age group more than five years, and exposure to smoking were other factors associated with the severity of ALRI. As it would be expected, longer LoS and oxygen supplementation are associated with severe disease.
Additionally, we identified several clinical and demographic factors that may predict longer LoS, chronic lung disease, neuromuscular disease, elevated C-reactive protein, and the diagnosis of lobar pneumonia with consolidation on chest films.
Influenza A virus has been reported to be associated with severe ALRI in children with a higher hospitalization rate, particularly those with a chronic medical disease, neurological or neuromuscular disease [39,40]. The relationship between RSV infection and severe viral bronchiolitis is well-established in the literature [25,41,42].
Chronic medical conditions and neuromuscular disorders were among risk factors for severe disease in infants admitted with viral ALRI in Italy’s large birth cohort study [43]. Our findings support what has been reported about CRP’s significant prognostic value in community-acquired pneumonia and its use as a marker of severity [44-46]. The diagnosis of lobar pneumonia with radiological consolidation is distinct from the rest of the study population and is associated with a more extended hospital stay [47,48]. A finding can be attributed to bacterial etiology and the need for antibiotic administration with or without oxygen supplementation. However, our study demonstrated that most patients admitted with ALRI (139 patients - 91.4%) were started on antibiotics reflecting overuse of antibiotics to treat pneumonia. A finding addressed in several previous studies that reported the overuse of antibiotics in children and that most radiographic pneumonia treated with antibiotics may not have a bacterial etiology [49,50].
Despite international guidelines which limit performing chest films in young children with ALRI to certain indications [51], all out of the 152 children of the study admitted with fever and respiratory symptoms had a chest firm performed on admission. Both groups’ radiological findings were interpreted most commonly as hyperinflation, followed by interstitial lung disease, peribronchial thickening, consolidation, and atelectasis. In a retrospective analysis of radiological findings in 210 confirmed viral pneumonia cases, the predominant findings were bilateral patchy areas of consolidation, followed by interstitial lung disease, diffuse areas of air space consolidation, and lobar consolidation [52]. Some variability of radiological findings reporting is noted among radiologists in different centers [52].
Abnormal chest films were reported in 80% and 60% of influenza positive and influenza negative groups. Influenza-viruspositive patients were more likely to have a normal chest X-ray, while the influenza-negative patients were more likely to have radiological changes consistent with viral pneumonia, including hyperinflation and peribronchial thickening and interstitial infiltrate. This is consistent with research from Malaysia, which reported normal chest radiographs among at least 50% of influenza-positive patients [53]. Consolidation and atelectasis on chest films were significantly associated with severe ALRI. This was supported by a prospective study of 213 children by Shaw et al. [54]. Consolidation was also associated with increased LoS, a finding that may reflect the effect of potential bacterial coinfection and the need to use antibiotics. Bacterial and viral coinfection is increasingly recognized as a cause for ALRI and is responsible for increasing the severity of the disease [55]. This is consistent with the study by Awad et al. where both atelectasis and consolidation were associated with increased LoS [24]. It is noteworthy that the mean LoS in our study population (6 days) was longer than that reported in the two other national studies by Khouri and Samah (5 and 3.5 days, respectively). It may be explained by the fact that almost all our patients received courses of antibiotics during their admission for ALRI, which lengthened their stay in hospital awaiting pending blood cultures. The wide use of antibiotics in our population may be attributed to several factors: i) empirical antibiotics for children with the clinical diagnosis of pneumonia (fever, respiratory symptoms, elevated inflammatory markers with or without radiological changes); ii) bacterial and viral coinfection may often exist in hospitalized children with community-acquired (CA) pneumonia [55,56]; iii) empirical antibiotics for febrile infants with rule-out sepsis diagnosis, and the iv) challenge which may exist with differentiation between viral and bacterial etiologies for CA pneumonia [57].
Therefore, healthcare workers in Jordan are advised by our results to limit their use to specific indications according to published guidelines to minimize the cost and duration of hospitalization [20].
RT-PCR for the common viruses was not performed in all patients. Rapid test for influenza viruses was ordered for a group of those children, and this may be explained by the following; children with LRTI are often hospitalized with more severe symptoms needing appropriate intervention and support, and the influenza virus has a specific treatment unlike most other viruses; therefore, testing for influenza was a cost-effective measure. Secondly, influenza test was mainly obtained for older children (children >24 months). At the same time, non-influenza viruses (mainly RSV virus) were shown to be responsible for most admissions in the younger age group [24,25,29,32] with their contribution to LRTIrelated admissions falling significantly in the older age group in favor of influenza virus [25,58].
This study confirmed what has been described in the literature about the effect of tobacco exposure on infants and young children in terms of increased risk of hospitalization with viral ALRI and increasing the severity of illness among hospitalized children [59,60]. However, the current study could not show a significant correlation between the severity of disease or LoS with gender, birth weight, gestational age, and bronchial asthma. Our study is the first viral surveillance study in the pediatric age group conducted in Jordan University Hospital. It is the first to focus on the burden on seasonal influenza among this age group.
This study has several limitations. First, a complete respiratory virus panel was not ordered in all study populations, and many of the children were tested for influenza virus only for cost and treatment planning consideration, hindering more accurate estimation of viral burden. Second, the surveillance was limited to one season in one center, potentially affecting the generalization of results to other seasons of the year in Jordan’s entire population. Third, bacterial testing in respiratory specimens was not performed, limiting the assessment of bacterial coinfection and its potential influence on the clinical presentation and outcome.
Conclusion
Our study confirmed that viral detection was common among children admitted with febrile or nonfebrile ALRI as most children had a positive PCR test. RSV was the most detected virus among the study population, followed by rhinovirus. Influenza virus came third and was detected in about 16% of children with a positive PCR test. Bronchopneumonia was the most common diagnosis among patients admitted with ALRI. Children with influenza had more fever, less leukocyte count than children with other viruses. Influenza virus was the most detected virus in children more than five years of age with severe ALRI. Overuse of systemic antibiotics and radiological chest imaging was reported in the majority of the study population. Our results advise healthcare workers in Jordan to limit their use to specific indications according to published guidelines to minimize the cost and duration of hospitalization.
Our study is the first surveillance study showing the significant burden of influenza virus in children admitted with ALRI during the flu season in Jordan University Hospital in Amman. Stakeholders in the health care system are encouraged by this study’s findings to prioritize health care interventions such as increasing influenza vaccination coverage, especially in limited resources settings. Future surveillance studies should be conducted annually on a national level to establish influenza’s burden in different risk groups within the country.
Acknowledgments
The authors wish to acknowledge Professor Najwa Khuri- Bulos and Dr. Faisal Al-Khatib for their contribution to the preparation of this manuscript.
References
- 1.Fischer Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013;381:1405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis 2002;2:25-32. [DOI] [PubMed] [Google Scholar]
- 3.Glezen P, Denny FW. Epidemiology of acute lower respiratory disease in children. N Engl J Med 1973;288:498-505. [DOI] [PubMed] [Google Scholar]
- 4.Pavia AT. Viral infections of the lower respiratory tract: Old viruses, new viruses, and the role of diagnosis. Clin Infect Dis 2011;52:S284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979-1997. J Infect Dis 2001;183:16-22. [DOI] [PubMed] [Google Scholar]
- 6.Alharbi S, Van Caeseele P, Consunji-Araneta R, Zoubeidi T, Fanella S, Souid AK, Alsuwaid AR. Epidemiology of severe pediatric adenovirus lower respiratory tract infections in Manitoba, Canada, 1991-2005. BMC Infect Dis 2012;12:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvo C, Garcia-Garcia ML, Blanco C, Pozo F, Casas Flecha I, Perez-Brena P. Role of rhinovirus in hospitalized infants with respiratory tract infections in Spain. Pediatr Infect Dis J 2007;26:904-8. [DOI] [PubMed] [Google Scholar]
- 8.Henrickson KJ, Kuhn SM, Savatski LL. Epidemiology and cost of infection with human parainfluenza virus types 1 and 2 in young children. Clin Infect Dis 1994;18:770-9. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Influenza transmission zones 2018. Available from: https://www.who.int/influenza/surveillance_monitoring/updates/Influenza_Transmission_Zones20180914.pdf [Google Scholar]
- 10.World Life Expectancy [Internet]. Influenza and pneumonia in Jordan. Available from: https://www.worldlifeexpectancy.com/jordan-influenza-pneumonia [Google Scholar]
- 11.World Health Organization. Influenza (Seasonal). Available from: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) [Google Scholar]
- 12.Centers for Disease Control and Prevention [Internet]. Weekly U.S. Influenza Surveillance Report. Available from: https://www.cdc.gov/flu/weekly/index.htm [Google Scholar]
- 13.Centers for Disease Control and Prevention [Internet]. Influenzaassociated pediatric mortality. Available from: https://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html [Google Scholar]
- 14.Bender JM, Ampofo K, Gesteland P, Sheng X, Korgenski K, Raines B, et al. Influenza virus infection in infants less than three months of age. Pediatr Infect Dis J 2010;29:6-9. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Influenza vaccines. Available from: https://www.who.int/wer/2005/wer8033.pdf?ua=1 [Google Scholar]
- 16.Garten R, Blanton L, Abd Elal AI, Alabi N, Barnes J, Biggerstaff M, et al. Update: Influenza activity in the United States during the 2016-17 season and composition of the 2017-18 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018;67:634-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horton KC, Dueger EL, Kandeel A, Abdallat M, El-Kholy A, Al-Awaidy S, et al. Viral etiology, seasonality and severity of hospitalized patients with severe acute respiratory infections in the Eastern Mediterranean Region, 2007-2014. PLoS One 2017;12:e0180954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abusrewil S, Algeer A, Aljifri A, Al Slail F, Andrew MK, Awad Tag Eldin M, et al. Influenza surveillance in Middle East, North, East and South Africa: Report of the 8th MENA Influenza Stakeholders Network. Influenza Other Respir Viruses 2019;13:298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Abdallat M, Dawson P, Jeries Haddadin A, El-Shoubary W, Dueger E, Al-Sanouri T, et al. Influenza hospitalization epidemiology from a severe acute respiratory infection surveillance system in Jordan, January 2008-February 2014. Influenza Other Respir Viruses 2016;10:91-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. Executive summary: The management of community- acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011;53:617-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Eleventh revision of the International Classification of Diseases. 2019. Available from: https://apps.who.int/gb/ebwha/pdf_files/WHA72/A72_29-en.pdf [Google Scholar]
- 22.Naeim F, Rao N, Song AX, Grody WW. Atlas of hematopathology: morphology, immunophenotype, cytogenetics, and molecular approaches. Amsterdam: Elsevier; 2013. [Google Scholar]
- 23.Uyeki TM, Bernstein HH, Bradley JS, Englund JA, File TM, Fry AM, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 Update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis 2019;68:e1-e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Awad S, Khader Y, Mansi M, Yusef D, Alawadin S, Qudah W, Khasawneh R. Viral surveillance of children with acute respiratory infection in two main hospitals in northern Jordan, Irbid, during winter of 2016. J Pediatr Infect Dis 2020;15:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khuri-Bulos N, Lawrence L, Piya B, Wang L, Fonnesbeck C, Faouri S, et al. Severe outcomes associated with respiratory viruses in newborns and infants: A prospective viral surveillance study in Jordan. BMJ Open 2018;8:e021898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janahi I, Abdulkayoum A, Almeshwesh F, Alkuwari M, Al hammadi A, Alameri M. Viral aetiology of bronchiolitis in hospitalised children in Qatar. BMC Infect Dis 2017;17:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrag MA, Hamed ME, Amer HM, Almajhdi FN. Epidemiology of respiratory viruses in Saudi Arabia: toward a complete picture, Arch Virol 2019;164:1981-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khamis FA, Al-Kobaisi MF, Al-Areimi WS, Al-Kindi H, Al-Zakwani I. Epidemiology of respiratory virus infections among infants and young children admitted to hospital in Oman. J Med Virol 2012;84:1323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Ayed MS, Asaad AM, Qureshi MA, Ameen MS. Viral etiology of respiratory infections in children in southwestern Saudi Arabia using multiplex reverse-transcriptase polymerase chain reaction Saudi Med J 2014;35:1348-53. [PMC free article] [PubMed] [Google Scholar]
- 30.Pickles RJ, DeVincenzo JP. Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis. J Pathol 2015;235:266-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009;360:588-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith DK, Seales S, Budzik C. Respiratory syncytial virus bronchiolitis in children. Am Fam Physician 2017;95:94-9. [PubMed] [Google Scholar]
- 33.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010;375:1545-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oosterheert JJ, van Loon AM, Schuurman R, Hoepelman AIM, Hak E, Thijsen S, et al. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin Infect Dis 2005;41:1438-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention [Internet]. Cold versus flu. Available from: https://www.cdc.gov/flu/symptoms/coldflu.htm [Google Scholar]
- 36.Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Inter Med 2000;160:3243-47. [DOI] [PubMed] [Google Scholar]
- 37.Merekoulias G, Alexopoulos EC, Belezos T, Panagiotopoulou E, Jelastopulu E. Lymphocyte to monocyte ratio as a screening tool for influenza. PLoS Curr 2010;2:RRN1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cunha B, Pherez F, Schoch P. Diagnostic importance of relative lymphopenia as a marker of swine influenza (H1N1) in adults. Clin Infect Dis 2009;49:1454-6. [DOI] [PubMed] [Google Scholar]
- 39.Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000;342:232-9. [DOI] [PubMed] [Google Scholar]
- 40.Mistry RD, Fischer JB, Prasad PA, Coffin SE, Alpern ER. Severe complications in influenza-like illnesses. Pediatrics 2014;134:e684-90. [DOI] [PubMed] [Google Scholar]
- 41.Plesca DA, Cora F, Buzoianu E, Moiceanu M, Hurduc V. Risk factors for severe bronchiolitis - A retrospective study. Eur Respir J 2012;40:P4660. [Google Scholar]
- 42.Praznik A, Vinšek N, Prodan A, Erčulj V, Pokorn M, Mrvič T, Paro D, et al. Risk factors for bronchiolitis severity: A retrospective review of patients admitted to the university hospital from central region of Slovenia. Influenza Other Respir Viruses 2018;12:765-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanari M, Prinelli F, Adorni F, Di Santo S, Vandini S, Silvestri M, Musicco M. Risk factors for bronchiolitis hospitalization during the first year of life in a multicenter Italian birth cohort. Ital J Pediatr 2015;41:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chalmers JD, Singanayagam A, Hill AT. C-reactive protein is an independent predictor of severity in community-acquired pneumonia. Am J Med 2008;121: 219-25. [DOI] [PubMed] [Google Scholar]
- 45.Menendez R, Cremades MJ, Martinez-Moragon E, Soler JJ, Reyes S, Perpina M. Duration of length of stay in pneumonia: Influence of clinical factors and hospital type. Eur Respir J 2003;22:643-8. [DOI] [PubMed] [Google Scholar]
- 46.Youssef HA, Nasseh S, Hafiz HA, Gawesh A. Evaluation of diagnostic and prognostic value of high sensitivity C reactive protein (Hs-CRP) in community acquired pneumonia. Egypt J Chest Dis Tuberc 2013;6:301-4. [Google Scholar]
- 47.McClain L, Hall M, Shah SS, Tieder JS, Myers AL, Auger K, et al. Admission chest radiographs predict illness severity for children hospitalized with pneumonia. J Hosp Med 2014;9:559-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reynolds JH, McDonald G, Alton H, Gordon SB. Pneumonia in the immunocompetent patient. Br J Radiol 2010;83:998-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coon ER, Young PC, Quinonez RA, Morgan DJ, Dhruva SS, Schroeder AR. Update on pediatric overuse. Pediatrics 2017;139:e20162797. [DOI] [PubMed] [Google Scholar]
- 50.van Houten CB, Cohen A, Engelhard D, Hays JP, Karlsson R, Moore E. Antibiotic misuse in respiratory tract infections in children and adults-a prospective, multicentre study (TAILORED Treatment). Eur J Clin Microbiol Infect Dis 2019;38:505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 2011;66:ii1-23. [DOI] [PubMed] [Google Scholar]
- 52.Guo W, Wang J, Sheng M, Zhou M, Fang L. Radiological findings in 210 paediatric patients with viral pneumonia: a retrospective case study. Br J Radiol 2012;85:1385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bux S, Mohd Ramli N, Ahmad Sarji S, Kamarulzaman A. Chest imaging features of patients afflicted with influenza A (H1N1) in a Malaysian tertiary referral centre. Biomed Imaging Interv J 2010;6:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw KN, Bell LM, Sherman NH. Outpatient assessment of infants with bronchiolitis. Am J Dis Child 1991;145:151-5. [DOI] [PubMed] [Google Scholar]
- 55.Cawcutt K, Kalil AC. Pneumonia with bacterial and viral coinfection. Curr Opin Crit Care 2017;23:385-90. [DOI] [PubMed] [Google Scholar]
- 56.Burk M, El-Kersh K, Saad M, Wiemken T, Ramirez J, Cavallazzi R. Viral infection in community-acquired pneumonia: a systematic review and meta-analysis. Eur Respir Rev 2016;25:178-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Gageldonk-Lafeber AB, Wever BC, van der Lubben IM, de Jager CPC, Meijer A, de Vries MC, et al. The aetiology of community- acquired pneumonia and implications for patient management. Neth J Med 2013;71:418-25. [PubMed] [Google Scholar]
- 58.Tran DN, Trinh QD, Pham NTK, Vu MP, Ha MT, Nguyen TQN, et al. Clinical and epidemiological characteristics of acute respiratoryvirus infections in Vietnamese children. Epidemiol Infect 2016;144:527-36. [DOI] [PubMed] [Google Scholar]
- 59.DiFranza JR, Masaquel A, Barrett AM, Colosia AD, Mahadevia PJ. Systematic literature review assessing tobacco smoke exposure as a risk factor for serious respiratory syncytial virus disease among infants and young children. BMC Pediatr 2012;12:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aujard Y, Fauroux B. Risk factors for severe respiratory syncytial virus infection in infants. Respir Med 2002;96(Suppl. B):S9-14. [PubMed] [Google Scholar]


