Abstract
Background
Lenvatinib is a novel multiple receptor tyrosine kinase inhibitor used for hepatocellular carcinoma (HCC) treatment. Although its main function is to suppress VEGFR and FGFR pathway, its immunomodulatory activity in HCC is not elucidated. Thus, this study aimed to investigate the immunomodulatory capability of lenvatinib in HCC.
Material and methods
Totally 47 patients with HCC were enrolled in this study, and the immune cells and serum cytokine profiles before initiation of treatment and after 1 and 3 months were measured. The immune checkpoint receptors on the immune cells were also evaluated. Kaplan–Meier survival estimate and log rank tests were used to assess the prognostic value.
Result
The frequency of T helper (Th) cells and T regulatory (Treg) cells reduced after lenvatinib treatment, while cytotoxic T lymphocyte (CTL) cells increased significantly. The cytokine profiles showed IL‐2, IL‐5, IFN‐γ increased; other cytokines including IL‐6, IL‐10, TNF‐ α and TNF‐ β decreased with lenvatinib therapy. Furthermore, the PD‐1 and TIM‐3 expressed on CTL had greatly decreased; the expression of TIM‐3 and CTLA‐4 was reduced on Treg cells as well. Besides, the new index CTL/Treg ratio was created, and low ratio was associated with the unfavorable outcome of HCC patients.
Conclusion
Our results confirmed that lenvatinib is capable of improving patients’ immune status, saving the effector cells from exhaustion status and inhibiting the number and function of immunosuppressive cells. The novel index CTL/Treg ratio qualifies as a predictor for the outcome of patients with lenvatinib therapy.
Keywords: CTL/Treg ratio, HCC, immunomodulatory activity, lenvatinib
The immunomodulatory activity of lenvatinib saves the effector cells from exhaustion status and inhibiting the number and function of immunosuppressive cells.

1. INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors globally and remains as the fourth leading cause of cancer‐related death during the recent decade. 1 The most effective and preferred curative treatment is surgical removal of tumor. 2 , 3 However, due to the insidious progression of that fatal disease, most patients were already in advanced stage at the time of diagnosis, missing the chance to receive curative resection. 4 , 5 Fortunately, benefited from the development of genetics and genomic technology, the landscape of molecular alternation of HCC was initially established, among which some critical driver mutations could lead to druggable targets. 6 Lenvatinib is a such kind of molecule inhibitor of multiple receptor tyrosine kinases, which approved for the emerging first‐line therapy of advanced stage HCC in China. 7 , 8 In addition, it showed noninferiority in time to progression and overall survival (OS) compared with sorafenib in clinical trial. 9 As reported, lenvatinib extends its antitumor and antiangiogenic activities through suppressing VEGF‐VEGFR and FGF‐FGFR system, which were considered as the most important factors in tumorigenesis and disease progression. 10 On the other hand, immune modulatory effects also played a vital role in tumor development. Several research had already showed that when combined with immune‐checkpoint inhibitors, lenvatinib could greatly enhance its antitumor effect 11 , 12 ; other reports indicated lenvatinib itself has ability to alleviate the immune suppression 13 and further affects the immune cells 14 so as to improve the prognosis of patients with tumor burden. However, there is no relevant research explains how lenvatinib regulates the immune status of HCC patients.
So, in this study, our team conducted an retrospective research to evaluate the change of immune cells and relevant cytokines in patients with HCC after they received lenvatinib therapy.
2. MATERIAL AND METHODS
2.1. Enrollment and specimen collection
A total of 47 patients with unresectable HCC were enrolled in Zhongshan Hospital, Fudan University, from July 2020 to October 2020. The inclusion criteria were as follows: Patients were diagnosed with HCC according to the Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition) 15 and received only lenvatinib treatment (8 mg/day for weight <60 kg; 12 mg/day for weight ≥60 kg). If no dose‐limiting toxicity (DLT) occurred in the first phase, the second phase would be initiated using the recommended dose from the DLT phase, and regimens may be changed and even discontinued with the occurrence of unacceptable or serious Adverse events. The exclusion criteria were as follows: (1) patients with history of other tumors; (2) patients had received treatments before admission; and (3) patients had 50% or higher occupation of liver; (4) patients with major diseases of other system, such as severe cardiovascular, lung diseases and neurological diseases, or mental disease. This research had been approved by the research ethics committee of Zhongshan Hospital, Fudan University. The written informed consent was obtained from every patient in this study. The detailed clinical characteristics are shown in Table 1.
TABLE 1.
The clinicopathologic characteristics of patients with hepatocellular carcinoma enrolled in the study
| Clinical characteristic | Number of patients |
|---|---|
| Age | |
| ≤50 | 8 |
| >50 | 39 |
| Sex | |
| Male | 41 |
| Female | 6 |
| AFP | |
| ≤400 ng/ml | 10 |
| >400 ng/ml | 37 |
| ALT | |
| ≤75 U/L | 18 |
| >75 U/L | 29 |
| HbsAg | |
| Negative | 6 |
| Positive | 41 |
| ECOG | |
| 0 | 11 |
| 1 | 36 |
| Portal vessel invasion | |
| No | 28 |
| Yes | 19 |
| Child–Pugh score | |
| A | 14 |
| B | 33 |
| BCLC stage | |
| B | 15 |
| C | 32 |
Abbreviations: AFP, alpha fetoprotein; ALT, alanine aminotransferase.
Two millilitre of blood anticoagulated with EDTA‐k2 and 2 ml of serum were collected from each patient with HCC before initiation of the treatment and after 1 and 3 months of the treatment with lenvatinib.
2.2. The follow‐up of patients with hepatocellular carcinoma
The observation period began from the start of therapy with lenvatinib to patients’ death or the last visit. Every patient was monitored by serum testing including alpha fetoprotein (AFP), alanine aminotransferase (ALT), aspartate aminotransferase, etc. and received imaging test including CT or MRI scans 4–6 weeks after the initiation of therapy and thereafter every 2–3 months. The patients were further divided into four groups, including complete response (CR) group, partial response (PR) group, stable disease (SD) group, and progressive disease (PD) group based on the prognosis according to the Response Evaluation Criteria in Solid Tumors (RECIST)1.1, 16 and the RECIST result was the smallest sum, longest diameter recorded since the start of the treatment or the appearance of one or more new lesions. CR was defined as the disappearance of intratumoral arterial enhancement in any target lesions and no new lesion appearance; PR was defined as a ≥30% reduction in the sum of the diameters of target lesions; PD was defined as a ≥20% increase in the sum of the diameters of target lesions or appearance of new lesions; and SD was defined as any other case that did not qualify for either PR or PD. Progression‐free survival (PFS) was defined as the interval between the initiation of therapy and disease progression. OS was defined as the interval between the initiation of therapy and death. The treatment response rate for patients with HCC was shown in Table S1.
2.3. Flow cytometry
Peripheral blood mononuclear cells were isolated through Ficoll (GE Healthcare Bio‐sciences AB, Sweden) from whole blood of patients with HCC and resuspended in 0.5 ml of PBS. Then the immunofluorescent antibodies were deployed to further distinguish the different kinds of immune cells in peripheral blood. The immunofluorescent antibodies used in the study were shown as followed: CD3‐FITC, CD4‐APC, CD25‐PE‐Cy7, PD‐1‐BV421, Foxp3‐V450, CTLA‐4(CD152)‐PE, TIM‐3‐PerCP‐Cy5.5, CD8‐ APC‐Cy7, CD56‐PE, and CD19‐ PerCP‐Cy5.5 (BD Bioscience). The related isotype antibodies were used as negative control. The result was analyzed with Flowjo 10.6.2 (BD Bioscience).
2.4. Cytokines profiles
The cytokines including IL‐1β, IL‐2, IL‐4, IL‐5, IL‐6, IL‐8, IL‐10, IL‐12p, IL‐17a, IL‐17f, IL‐22, TNF‐ α, TNF‐β, and IFN‐γ were measured using CBA cytokine kit (QuantoBio). The measurement was followed by the manufacturer's protocol.
2.5. Statistical analysis
Statistical analyses were performed using SPSS 19.0 software (IBM). Experimental values were expressed in the form of mean ± SD. The difference between the groups was compared using Student t‐test, chi‐square test, or Fisher test, as appropriate. Kaplan–Meier survival curves were performed defining the survival rates, log‐rank test was used to access the different between the groups. p value <0.05 was considered as statistically significant.
3. RESULT
The change of immune cells in peripheral blood of patients with HCC after receiving lenvatinib treatment.
The level of various kinds of circulating immune cells in patients with HCC was measured and evaluated by flow cytometry prior to lenvatinib therapy as well as 1 month (approximately 29–32 days) and 3 months (approximately 88–93 days) after the treatment. The result showed that the T helper cells (CD4+ Th cells) had slightly decreased with the process of lenvatinib treatment from 36.38 ± 8.36% to 31.02 ± 9.34% (1 month) (p = 0.015) and 30.46 ± 10.79% (3 months) (p = 0.031), whereas cytotoxic T lymphocyte cells (CD8+ CTL cells) had dramatically increased from 23.31 ± 7.92% to 30.36 ± 8.83% (1 month) (p = 0.001) and 29.28 ± 10.34% (3 months) (p = 0.003). Statistically significant reduction was also observed in the frequency of T regular (Treg) cells after 1 month of lenvatinib treatment (9.48 ± 5.31%) (p = 0.004) compared with its baseline values (13.42 ± 5.94%), and it kept falling during 3 months (8.94 ± 5.11%) (p = 0.003). However, no obvious change of B cells or natural killer (NK) cells was observed during the experiments (Figure 1A). Next, we further evaluate the frequency of these three types immune cells in patients with HCC with different clinical response, and the result indicated that after 3‐month treatment, the frequency of CTL cells was significantly elevated in CR and PR groups (35.95 ± 8.92%) (totally 18 patients) compared with those in SD and PD groups (23.93 ± 8.51%) (totally 29 patients) (p < 0.001). On the contrary, the frequency of Th (CR/PR:26.37 ± 8.52%; SD/PD:30.00 ± 11.38%; p = 0.039) and Treg cells (CR/PR:5.79 ± 3.33%; SD/PD:10.39 ± 4.74%; p < 0.001) was decreased in those patients with better prognosis (Figure 1B). Interestingly, when we combined CTL and Treg cells to form a new index (the ratio of CTL/Treg) based on the third‐month result, patients in CR and PR groups had much higher CTL/Treg ratio value (p = 0.008). We then stratified patients with HCC as CTL high and CTL low, Th high and Th low, Treg high and Treg low, and CTL/Treg ratio high and CTL/Treg ratio low based on the median of these factors. The result showed patients with high CTL/Treg ratio value (p < 0.001), high CTL (p = 0.006), and low Treg (p = 0.003) achieved better outcome, and the CTL/Treg ratio had the best efficacy, suggesting the new index had great ability in discriminating the different outcome of patients with HCC with lenvatinib treatment (Figure 1C).
FIGURE 1.
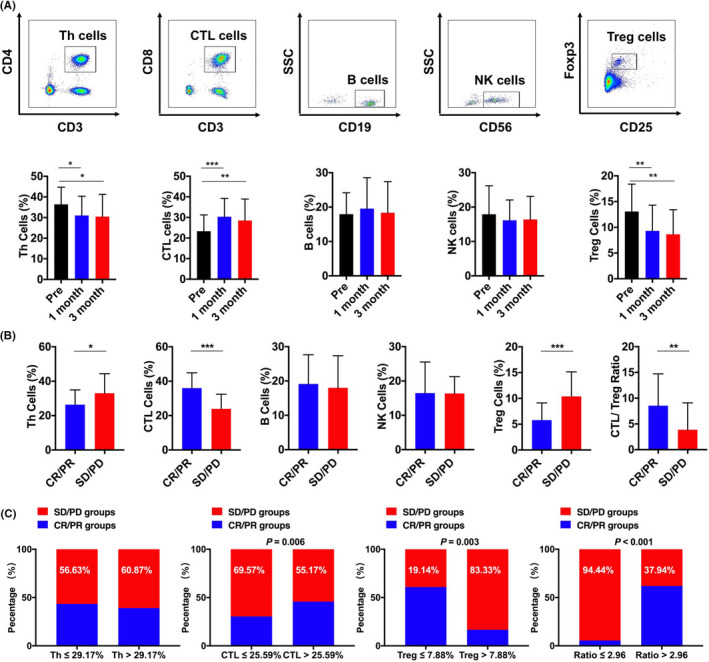
The change of immune cells from patients with HCC after lenvatinib therapy. (A) Frequency of CD4+ Th cells, CD8+CTL cells, Treg cells, B cells, and NK cells pretreatment and after 1 and 3 months. (B) The frequency of Th cells, CTL cells, B cells, NK cells, and CTL/Treg ratio in CR/ PR group and SD/ PD group. (C) The percentage of poor outcome in Th group, CTL group, Treg group, and CTL/Treg ratio group. * p < 0.05; ** p < 0.01; *** p < 0.001
3.1. Association of CTL/Treg ratio with clinicopathologic parameters and its clinical significance in patients with hepatocellular carcinoma
Next, we tried to seek the association between CTL/Treg ratio and clinicopathologic parameters in patients with HCC. The results showed that CTL/Treg ratio was related to AFP (p = 0.012), indicating patients with lower CTL/Treg ratio tended to have higher AFP. Meanwhile, patients in BCLC‐0‐A had little bit higher CTL/Treg ratio value than those in BCLC‐B‐D stage (p = 0.060), although it had not yet reached a statistical significance. Other clinical characteristics, including age, sex, ALT, HbsAg, portal vessel invasion, and Child–Pugh score had no significant difference between the high and low CTL/Treg ratio groups (Table 2).
TABLE 2.
Correlation between CTL/Treg ratio and clinicopathologic characteristics
| Clinical characteristic | Number of patients | CTL/Treg ratio ≤2.96 | CTL/Treg ratio >2.96 | p |
|---|---|---|---|---|
| Age | ||||
| ≤50 | 8 | 5 | 3 | 0.461 |
| >50 | 39 | 18 | 21 | |
| Sex | ||||
| Male | 41 | 21 | 20 | 0.666 |
| Female | 6 | 2 | 4 | |
| AFP | ||||
| ≤400 ng/ml | 10 | 1 | 9 | 0.012 |
| >400 ng/ml | 37 | 21 | 16 | |
| ALT | ||||
| ≤75 U/L | 18 | 8 | 10 | 0.763 |
| >75 U/L | 29 | 13 | 14 | |
| HbsAg | ||||
| Negative | 6 | 1 | 5 | 0.188 |
| Positive | 41 | 22 | 19 | |
| Portal vessel invasion | ||||
| No | 28 | 15 | 13 | 0.556 |
| Yes | 19 | 8 | 11 | |
| Child–Pugh score | ||||
| A | 14 | 5 | 9 | 0.341 |
| B | 33 | 18 | `15 | |
| BCLC stage | ||||
| 0‐A | 15 | 4 | 11 | 0.060 |
| B‐D | 32 | 19 | 13 | |
Abbreviations: AFP, alpha fetoprotein; ALT, alanine aminotransferase.
3.2. The prognostic value of immune cells and new index in patients with hepatocellular carcinoma treated with lenvatinib
Because we have found that CTL, Th, Treg cells, and CTL/Treg ratio had great difference in CR/PR and SD/PD groups, the prognostic value of these immune cells and the novel index were further exploded using Kaplan–Meier survival curve. The log‐rank result indicated high CTL/Treg ratio was associated with longer PFS (p = 0.039) and OS (p = 0.030) of patients with HCC undergoing lenvatinib treatment. Although low Treg correlated with longer PFS (p = 0.056) and OS (p = 0.030). In addition, high CTL cells had slight connection with better OS (p = 0.062), but it showed no significant prognostic value in PFS (p = 0.524), whereas Th cells had no prognostic significance for both PFS (p = 0.553) and OS (p = 0.862) (Figure 2A,B).
FIGURE 2.
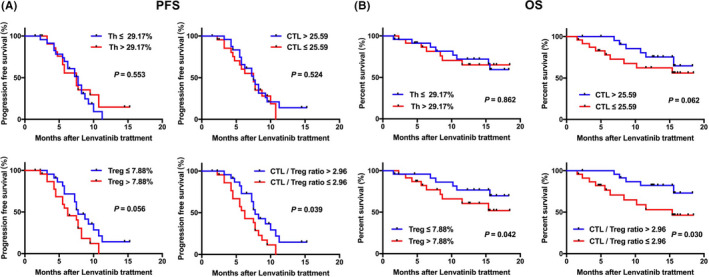
Kaplan–Meier survival analysis of patients with high‐ and low‐lymphocyte cells and the novel index in the two cohorts
3.3. Serum cytokine profiles
We next evaluated the expression levels of relevant cytokines of these 47 patients with HCC before and after the treatment with lenvatinib during 3 months. The result showed that IL‐2 (1 month: p = 0.008; 3 months: p = 0.014), IL‐5 (1 month: p = 0.042), IFN‐γ (1 month: p = 0.001; 3 months: p < 0.001) increased, whereas several cytokines including IL‐6 (3 months: p = 0.038), IL‐10 (1 month: p = 0.001; 3 months: p < 0.001), TNF‐ α (3 months: p = 0.004), and TNF‐ β (1 month: p = 0.013) decreased, the remaining cytokines had no significant change (Figure 3A). We then further evaluated the expression level of those cytokine of statistically significance in patients with HCC with different clinical response. IL‐10 (p < 0.001) and TNF‐ α (p = 0.004) were significantly evaluated in SD/PD groups. Besides, IL‐2 (p = 0.001) and IFN‐γ (p = 0.001) were decreased in SD/PD groups compared with CR/PR groups. However, IL‐5, IL‐6, and TNF‐ β showed no difference between CR/PR groups and SD/PD groups (Figure 3B).
FIGURE 3.
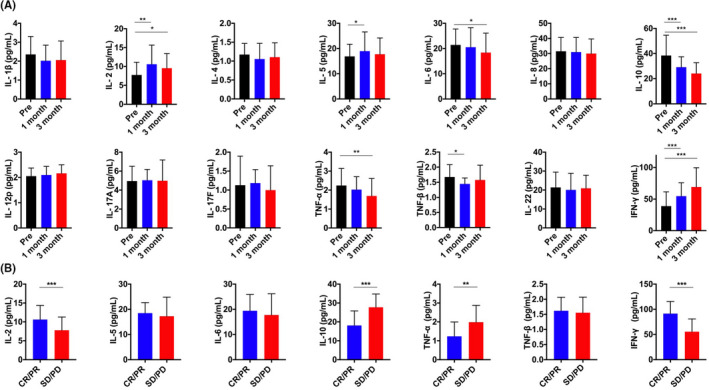
The change of 14 cytokines in the serum from patients with HCC after lenvatinib therapy. (A) The serum level of 14 cytokines pretreatment and after 1 and 3 months. (B) The serum level of 14 cytokines in CR/ PR group and SD/ PD group. * p < 0.05; ** p < 0.01; *** p < 0.001
3.4. The expression level of immune checkpoint receptors on T cells
viSNE based on flow cytometry was used to further analyzed the phenotypic characteristics of immune checkpoint receptor including programmed death 1 (PD‐1), cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4), and mucin domain‐containing protein 3 (TIM‐3) on T cells. As shown in Figure 4A, PD‐1 was expressed most on CTL and Th cells, CTLA‐4 was expressed on Treg and Th cells, and TIM‐3 was expressed on CTL cells. After lenvatinib treatment, the PD‐1 (p = 0.008) and TIM‐3 (p = 0.015) expressed on CTL had significantly decreased. The reduction of TIM‐3 (p = 0.013) and CTLA‐4 (p = 0.001) was also observed on Treg cells. However, there was no great change of CTLA‐4 on Th cells (Figure 4B,C). Next, we further analyzed the expression of these immune checkpoint receptors in patients with HCC with different clinical response. Patients in CR/PR groups had lower expression of TIM‐3 (p = 0.007) and PD‐1 (p = 0.022) on CTL cells, additionally, the expression of CTLA‐4 (p < 0.001) and TIM‐3 (p = 0.006) on Treg in CR/PR groups was much lower compared with DS/PD groups. (Figure 4D).
FIGURE 4.
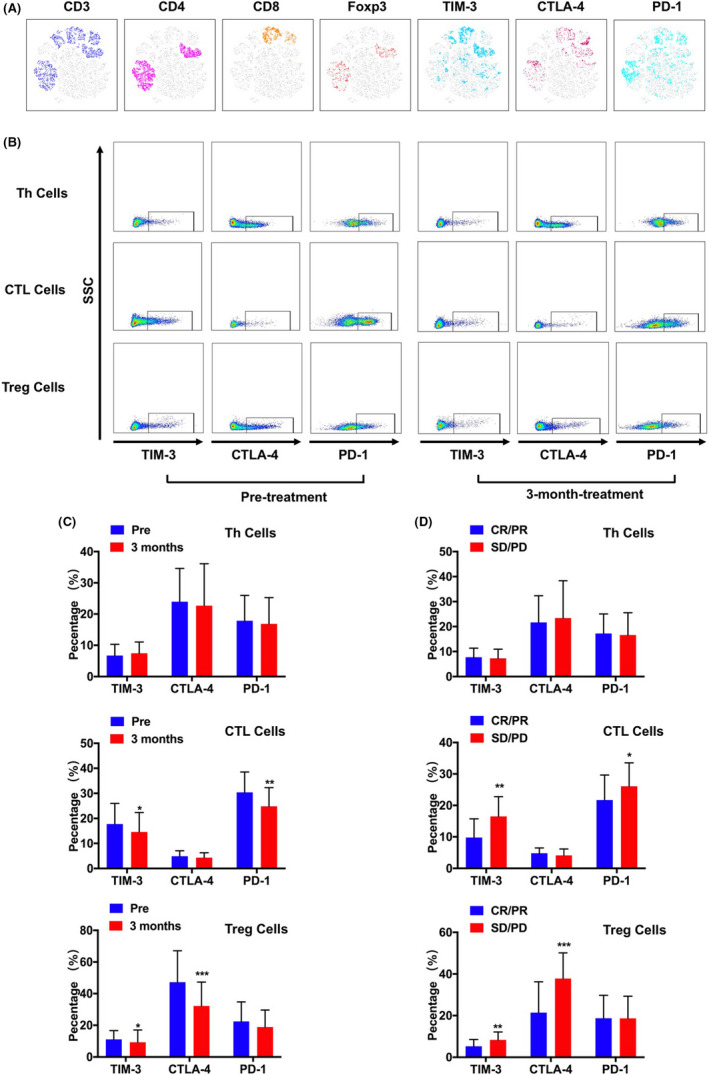
The expression of immune checkpoint receptors from immune cells of patients with HCC were measured by flow cytometry followed by viSNE analysis. (A) The expression of CD3, CD4, CD8, TIM‐3, CTLA‐4, and PD‐1 was indicated by the different color. (B) Representative flow cytometry result showing the expression level of TIM‐3, CTLA‐4, and PD‐1 on Th cells, CTLA cells and Tregs. (C) The change of immune checkpoint receptors from immune cells pretreatment and after 3 months. (D) The expression level of immune checkpoint receptors on Th cells, CTLA cells, and Tregs in CR/ PR group and SD/ PD group. * p < 0.05; ** p < 0.01; *** p < 0.001
4. DISCUSSION
HCC is one of the most common malignant tumors in the world, and its incidence and mortality rate are increasing annually, bringing heavy burden and waste to social medical resources. 17 For the prognosis of patients with HCC, early diagnosis and treatment are always the major issue. Currently, liver resection, tumor ablation, and liver transplantation are commonly used treatments. In addition, with developing advancements including living donor transplantation and laparoscopic liver resection, new therapies provide more options for different patients and influence their long‐term survival. 18 , 19 In the past decade, systematic treatment attracted a great attention. Sorafenib was considered as the most important and effective first‐line medicine in treating advanced and unresectable HCC. However, the choice of first‐line treatment still remained limited because few agents showed non‐inferiority or superiority to sorafenib. 20 , 21
Lenvatinib is a selective, oral, small‐molecule inhibitor of multiple‐receptor tyrosine kinases. With different dosages, it can exert different inhibitory effects on a variety of pathways related to pro‐angiogenesis and tumorigenesis, 22 , 23 , 24 among which VEGFR and FGFR are the main targets, which are overexpressed in tumor and have great connection with proliferation of cancer cells and angiogenesis. Several clinical trials demonstrated that lenvatinib could significantly prolong the median OS of patients with HCC, and it even showed better clinical efficacy than sorafenib. 8 , 25 , 26 Moreover, as reported, when combined with immune checkpoint therapy, the prognosis of patients with HCC had been greatly improved. 11 , 12 , 27 In addition, pervious research demonstrated that lenvatinib monotherapy could increase tumor‐infiltrating macrophages and CD8+ T cells through mice model in anaplastic thyroid cancer. 28 However, whether lenvatinib could regulate or affect the immune status of patients with HCC had not been fully clarified so far. In this study, we first found that after lenvatinib therapy, the frequency of CD8+ T cytotoxic cells in peripheral blood of patients with HCC had continuously and significantly increased; meanwhile, those patients with higher CTL cells appeared to achieve better clinical response and OS. Pervious study showed that the immune escape of tumor cells is one of major mechanisms of tumor progression and metastasis, resulting from the exhaustion of CTL cells, which predominantly exert antitumor efficacy. 29 A great number of studies also gave strong support that CTL cells, especially tumor‐infiltrating CTL cells, which have been associated with better prognosis and lower recurrence rates after proper treatment. 30 , 31 , 32 , 33 In detail, CTL cells have the capability to recognize respective tumor antigens in autologous HCC tissue, killing the target cells through cytotoxic effect. 34 Recently, Kimura et al. had reported that lenvatinib showed more effective antitumor activity than sorafenib in immune‐competent mice and weakened by CTL cell depletion, 35 suggesting lenvatinib's antitumor effect might be related to the number and viability of CTL cells, which interestingly was consistent with ours. Besides, we further explored the immune checkpoint receptors on CTL cells. As showed in Figure 4, PD‐1 and TIM‐3 was significantly upregulated on CTL cells before the initiation of treatment. Previous reports demonstrated that high expression level of PD‐1 on T cell was considered as a major marker of T cell progressive dysfunction. 36 In addition, high level of TIM‐3 was confirmed as a negative regulator of T cells function. 37 Apparently, CTL cells were in the status of exhaustion with high expression of TIM‐3 and PD‐1. 38 , 39 Sawada et al. thought that PD‐1+ Tim3+ CTL cells were a kind of immune cells that lost cytotoxic activity in cancer and associated with poor outcome. 40 In our study, we found after therapy, the expression of PD‐1 and TIM‐3 on CTL cells had dramatically reduced. Moreover, those patients who had complete and partial response had lower PD‐1 and TIM‐3 expression, suggesting the CTL cells might recover from exhaustion status and form a new immune balance with lenvatinib treatment processing. Coincidently, the cytokine profiles were in favor of our hypothesis, after 3‐month treatment, IL‐2 and IFN‐γ were elevated continuously. IL‐2 is mainly secreted by CD4+ cells 41 and considered as one of the strongest stimuli causing the proliferation and differentiation of naive CD8+ cells and formation of functional memory cells as well. 42 In patients with HCC, high level of IL‐2 further activated CD8+ cells, promoting them to secrete IFN‐γ to enhance the efficiency of killing cancer cells. This phenomenon was consistent with what we had observed in the study.
On the contrary, CD4+ T helper cells had slightly decreased, whereas the frequency of Treg in peripheral blood showed great difference before and after medication. Multiple research had already confirmed that Treg is one of the most vital immunosuppressive cells in the progression of tumor, standing out for its ability to inhibit various kinds of immune cells through contact‐dependent interaction and cytokines it secrets including TGF‐ β, IL‐10, and IL‐35. 43 , 44 , 45 , 46 In the study, we stratified the patients with HCC into two groups based on the median of Treg frequency, the result showed those patients with lower Treg tended to achieve better outcome and longer OS. To our surprise, two patients’ Treg dropped significantly from 41% to 2% and 38% to 1.8% during the lenvatinib therapy, suggesting lenvatinib itself might bring some influence on Treg generation. Moreover, the immune checkpoint receptors on Treg were measured as well. We had noticed that after treatment the expression level of CTLA‐4 and TIM‐3 all decreased greatly. As reported, CTLA‐4 is a key molecule expressed on the surface of Treg, whose main function is to downregulate the costimulatory molecules CD80/86 expression in antigen‐presenting cells, thereby inhibiting the activation of effector T cells. 47 Additionally, several studies demonstrated that TIM‐3 was identified as a novel marker of Treg cells that were highly effective at suppressing T cell proliferation despite of high expression of PD‐1. 48 , 49 Thus, with the reduction of CTLA‐4 and TIM‐3 expression, the function of Treg was clearly impaired, meanwhile the significant drop of IL‐10 in serum also gave strong support for it, suggesting lenvatinib might affect immune to some extent to improve the immune status of patients with HCC.
So, based on the result above, we constructed a novel index CTL/Treg ratio to evaluate the prognosis of patients with HCC after lenvatinib treatment, which could effectively reflect whether the patient's immune system was suppressed or not. The median of CTL/Treg ratio was used as a clinical cutoff value. We found patients with high CTL/Treg ratio (>2.96) had better clinical response. In addition, the Kaplan–Meier survival curve also indicated the CTL/Treg ratio had great discrimination ability as a prognosis predictor for PFS and OS. The reason might be that high CTL/Treg ratio suggesting the rebalance of the immune status with activation of CTL while suppression of Treg, which perform high antitumor capacity. On the other hand, we also found the correlation between CTL/Treg ratio and AFP, suggesting low CTL/Treg ratio might reflect the great damage of hepatocytes as well.
However, there were still remain some limitations in our study. First of all, the number of patients recruited in the research was small, the follow‐up period was short as well. Second, most patients in China have hepatitis B virus–positive background, which is quite different from the population in USA, Japan, Canada, and Europe. Therefore, the hypothesis we proposed needs to be further validated in larger and different patients from other geographic areas to explode the true relationship between immune cells and lenvatinib. Third, we found that lenvatinib could influence the number of immune cells and regulated the expression of immune checkpoint receptors on them, but the specific mechanism behind is still unclear, so further investigation into the underlying mechanism might provide a new sight of this medicine in treating HCC.
To our knowledge, this is the first research to demonstrate the immunomodulatory activity of lenvatinib in patients with HCC. Our results confirmed that lenvatinib is capable of improving patients’ immune status, saving the effector cells from exhaustion status and inhibiting the number and function of immunosuppressive cells. The novel index CTL/Treg ratio qualifies as a predictor for the outcome of patients with lenvatinib therapy, which is easy determination and low cost.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS APPROVAL
Ethics approval was obtained from the Research Ethics Committee of Zhongshan Hospital, Fudan University. Informed consent was obtained from each patient.
Supporting information
Table S1
ACKNOWLEDGMENT
The authors thank the patients with hepatocellular carcinoma who participated in this research for the source of clinical blood samples.
Zhu J, Fang P, Wang C, et al. The immunomodulatory activity of lenvatinib prompts the survival of patients with advanced hepatocellular carcinoma. Cancer Med. 2021;10:7977–7987. doi: 10.1002/cam4.4312
Jie Zhu and Peiqi Fang contributed equal to this manuscript
Funding information
Beili Wang was supported by The National Science Foundation of China (81902139); Specialized Fund for the clinical researches of Zhongshan Hospital affiliated Fudan University (2020ZSLC54); the key medical and health projects of Xiamen (YDZX20193502000002); Wei Guo was supported by the National Natural Science Foundation of China (81972000, 81772263), Specialized Fund for the clinical researches of Zhongshan Hospital affiliated Fudan University (2018ZSLC05); the constructing project of clinical key disciplines in Shanghai (shslczdzk03302); the key medical and health projects of Xiamen (YDZX20193502000002); Shanghai Medical Key Specialty (ZK2019B28); Xinrong Yang was supported by The National Key R&D Program of China (2019YFC1315800, 2019YFC1315802); The National Natural Science Foundation of China (81872355 and 82072715); The Shanghai Municipal Health Commission Collaborative Innovation Cluster Project (2019CXJQ02); Shanghai “Rising Stars of Medical Talent” Youth Development Program (Outstanding Youth Medical Talents); The Projects from the Shanghai Science and Technology Commission (19441905000, 21140900300); The Projects from Science Foundation of Zhongshan Hospital, Fudan University (2021ZSCX28, 2020ZSLC31).
Contributor Information
Xinrong Yang, Email: yang.xinrong@zs-hospital.sh.cn.
Beili Wang, Email: wang.beili1@zs-hospital.sh.cn.
DATA AVAILABILITY STATEMENT
The raw data will not be shared. All potential findings based on raw data analysis are presented in the manuscript or additional files.
REFERENCE
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Kanwal F, Singal AG. Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology. 2019;157(1):54‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gunasekaran G, Bekki Y, Lourdusamy V, et al. Surgical treatments of hepatobiliary cancers. Hepatology. 2021;73:128‐136. [DOI] [PubMed] [Google Scholar]
- 4. Bruix J, Reig M, Sherman M. Evidence‐based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835‐853. [DOI] [PubMed] [Google Scholar]
- 5. Chen S, Cao Q, Wen W, et al. Targeted therapy for hepatocellular carcinoma: challenges and opportunities. Cancer Lett. 2019;460:1‐9. [DOI] [PubMed] [Google Scholar]
- 6. Llovet JM, Zucman‐Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [DOI] [PubMed] [Google Scholar]
- 7. Al‐Salama ZT, Syed YY, Scott LJ. Lenvatinib: a review in hepatocellular carcinoma. Drugs. 2019;79(6):665‐674. [DOI] [PubMed] [Google Scholar]
- 8. Eisai Inc. Lenvima® (Lenvatinib mesylate) capsules: Chinese Product Specifications. 2018. Accessed 26 March 2019. http://202.96.26.102/index/detail/id/543
- 9. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus Sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. Lancet. 2018;391:1163‐1173. [DOI] [PubMed] [Google Scholar]
- 10. Spallanzani A, Orsi G, Andrikou K, et al. Lenvatinib as a therapy for unresectable hepatocellular carcinoma. Expert Rev Anticancer Ther. 2018;18(11):1069‐1076. [DOI] [PubMed] [Google Scholar]
- 11. Liu Z, Lin Y, Zhang J, et al. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Jiang M, Zhu J, et al. The safety and efficacy of Lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed Pharmacother. 2020;132:110797. [DOI] [PubMed] [Google Scholar]
- 13. Kawazoe A, Fukuoka S, Nakamura Y, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first‐line or second‐line setting (EPOC1706): an open‐label, single‐arm, phase 2 trial. Lancet Oncol. 2020;21(8):1057‐1065. [DOI] [PubMed] [Google Scholar]
- 14. Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38(26):2981‐2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 17. Lu C, Rong D, Zhang B, et al. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol Cancer. 2019;18:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guro H, Cho JY, Han H‐S, et al. Current status of laparoscopic liver resection for hepatocellular carcinoma. Clin Mol Hepatol. 2016;22(2):212‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoon YI, Lee SG. Living donor liver transplantation for hepatocellular carcinoma: an Asian perspective. Dig Dis Sci. 2019;64(4):993‐1000. [DOI] [PubMed] [Google Scholar]
- 20. European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182‐236. [DOI] [PubMed] [Google Scholar]
- 21. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358‐380. [DOI] [PubMed] [Google Scholar]
- 22. Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angio‐ genesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of Lenvatinib (E7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014:638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kudo M. Lenvatinib may drastically change the treatment land‐scape of hepatocellular carcinoma. Liver Cancer. 2018;7(1):1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim JJ, McFarlane T, Tully S, et al. Lenvatinib versus sorafenib as first‐line treatment of unresectable hepatocellular carcinoma: a cost‐utility analysis. Oncologist. 2020;25(3):e512‐e519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamashita T, Kudo M, Ikeda K, et al. REFLECT‐a phase 3 trial comparing efficacy and safety of Lenvatinib to Sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol. 2020;55(1):113‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin P‐T, Teng W, Jeng W‐J, et al. Combining immune checkpoint inhibitor with Lenvatinib prolongs survival than Lenvatinib alone in Sorafenib‐experienced hepatocellular carcinoma patients. Eur J Gastroenterol Hepatol. 2020; Publish Ahead of Print. doi: 10.1097/MEG.0000000000001956 [DOI] [PubMed] [Google Scholar]
- 28. Gunda V, Gigliotti B, Ashry T, et al. Anti‐PD‐1/PD‐L1 therapy augments Lenvatinib's efficacy by favorably altering the immune microenvironment of murine anaplastic thyroid cancer. Int J Cancer. 2019;144(9):2266‐2278. [DOI] [PubMed] [Google Scholar]
- 29. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565‐1570. [DOI] [PubMed] [Google Scholar]
- 30. Yao W, He J‐C, Yang Y, et al. The prognostic value of tumor‐infiltrating lymphocytes in hepatocellular carcinoma: a systematic review and meta‐analysis. Sci Rep. 2017;7:7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gabrielson A, Wu Y, Wang H, et al. Intratumoral cd3 and cd8 t‐cell densities associated with relapse‐free survival in hcc. Cancer Immunol Res. 2016;4:419‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao Q, Qiu S‐J, Fan J, et al. Intratumoral balance of regulatory and cytotoxic t cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586‐2593. [DOI] [PubMed] [Google Scholar]
- 33. Unitt E, Marshall A, Gelson W, et al. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol. 2006;45:246‐253. [DOI] [PubMed] [Google Scholar]
- 34. Hofmann M, Tauber C, Hensel N, et al. CD8+ T cell responses during HCV infection and HCC. J Clin Med. 2021;10(5):991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kimura T, Kato YU, Ozawa Y, et al. Immunomodulatory activity of Lenvatinib contributes to antitumor activity in the Hepa1‐6 hepatocellular carcinoma model. Cancer Sci. 2018;109(12):3993‐4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen DS, Mellman I. Oncology meets immunology: the cancer‐immunity cycle. Immunity. 2013;39:1‐10. [DOI] [PubMed] [Google Scholar]
- 37. Zhou Q, Munger ME, Veenstra RG, et al. Coexpression of Tim‐3 and PD‐1 identifies a CD8+ T‐cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117(17):4501‐4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thommen DS, Schumacher TN. T cell dysfunction in cancer. Cancer Cell. 2018;33(4):547‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sawada M, Goto K, Morimoto‐Okazawa A, et al. PD‐1+ Tim3+ tumor‐infiltrating CD8 T cells sustain the potential for IFN‐γ production, but lose cytotoxic activity in ovarian cancer. Int Immunol. 2020;32(6):397‐405. [DOI] [PubMed] [Google Scholar]
- 41. Leonard WJ. Cytokines and immunodeficiency diseases. Nat Rev Immunol. 2001;1(3):200‐208. [DOI] [PubMed] [Google Scholar]
- 42. Kamimura D, Bevan MJ. Naive CD8+ T cells differentiate into protective memory‐like cells after IL‐2–anti–IL‐2 complex treatment in vivo. J Exp Med. 2007;204(8):1803‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou S‐L, Zhou Z‐J, Hu Z‐Q, et al. Tumor‐associated neutrophils recruit macrophages and T‐regulatory cells to promote progression of hepatocellular carcinoma and resistance to Sorafenib. Gastroenterology. 2016;150(7):1646‐1658.e17. [DOI] [PubMed] [Google Scholar]
- 44. Langhans B, Nischalke HD, Krämer B, et al. Role of regulatory T cells and checkpoint inhibition in hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68(12):2055‐2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490‐500. [DOI] [PubMed] [Google Scholar]
- 46. Zhou Y, Wang B, Wu J, et al. Association of preoperative EpCAM Circulating Tumor Cells and peripheral Treg cell levels with early recurrence of hepatocellular carcinoma following radical hepatic resection. BMC Cancer. 2016;16:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wing K, Onishi Y, Prieto‐Martin P, et al. CTLA‐4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271‐275. [DOI] [PubMed] [Google Scholar]
- 48. Liu Z, McMichael EL, Shayan G, et al. Novel effector phenotype of Tim‐3+ regulatory T cells leads to enhanced suppressive function in head and neck cancer patients. Clin Cancer Res. 2018;24(18):4529‐4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dong Q, Cai C, Gao F, et al. Defective Treg response in acute kidney injury was caused by a reduction in TIM‐3+Treg cells. Immunol Invest. 2019;48(1):27‐38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The raw data will not be shared. All potential findings based on raw data analysis are presented in the manuscript or additional files.


