Highlights
-
•
Combination of oncolytic viruses armed with ICI and cytokine gene provides an efficient way for cancer treatment.
-
•
Combined treatment showed a high mice survival rate (100%).
-
•
Combined treatment produced a vaccine-like response in re-challenged experiment.
Keywords: Oncolytic virus, HSV-1, IL-12, PD-1 antibody, Cancer immunotherapy
Abstract
Cancer immunotherapy is a new therapeutic strategy for cancer treatment that targets tumors by improving or restoring immune system function. Therapies targeting immune checkpoint molecules have exerted potent anti-tumor effects and prolonged the overall survival rate of patients. However, only a small number of patients benefit from the treatment. Oncolytic viruses exert anti-tumor effects by regulating the tumor microenvironment and affecting multiple steps of tumor immune circulation. In this study, we engineered two oncolytic viruses that express mouse anti-PD-1 antibody (VT1093M) or mouse IL-12 (VT1092M). We found that both oncolytic viruses showed significant anti-tumor effects in a murine CT26 colon adenocarcinoma model. Importantly, the intratumoral combined injection with VT1092M and VT1093M inhibited growth of the primary tumor, prevented growth of the contralateral untreated tumor, produced a vaccine-like response, activated antigen-specific T cell responses and prolonged the overall survival rate of mice. These results indicate that combination therapy with the engineered oncolytic virus may represent a potent immunotherapy strategy for cancer patients, especially those resistant to PD-1/PD-L1 blockade therapy.
Graphical abstract

Introduction
Immune escape is a key characteristic of cancer cells, which survive during all stages of the anti-tumor immune response. Immune escape of cancer cells is usually achieved by upregulation of the expression of immune checkpoint receptor ligands and the secretion of immunosuppressive cytokines [1]. Cancer immunotherapy is a strategy for targeting tumors by restarting and maintaining the tumor immune cycle and restoring the normal anti-tumor immune response [2]. To overcome the immune evasion mechanisms of tumors, immunotherapy involves the introduction of immunomodulatory molecules or genes at tumor sites. In recent years, immunotherapies, such as immune checkpoint inhibitors (ICIs) [3], [4], [5], have achieved significant clinical efficacy as anti-tumor therapies by enhancing the effect of the host immune system effect on tumor cells and are considered a new era of cancer treatment [6,7].
Cytokines play key functions in regulating the immune response and participate in the inflammatory response, wound healing, and tumor growth and decline [8]. Interleukin-12 (IL-12) is considered to have good anti-tumor activity and activates natural killer cells, T lymphocytes, and macrophages, promotes dendritic cell maturation, and inhibits vascular formation. IL-12 also induces the production of interferon-γ (IFN-γ) and other immune factors, thus exerting strong anti-tumor effects [9,10].
The programmed cell death protein 1 (PD-1) antibody blocks the interaction between programmed cell death-Ligand 1 (PD-L1) and programmed cell death-Ligand 2 (PD-L2) and their ligands and thus prevents PD-1 pathway–mediated immunosuppressive responses and anti-tumor immune responses [11]. However, most cancer patients are resistant or partially insensitive to PD-1/PD-L1 blockade because of low T cell infiltration [12,13].
Multiple ICIs have been approved by the Food and Drug Administration (FDA) for a variety of advanced malignancies and have significantly improved patient survival and quality of life [14], [15], [16], [17]. However, many patients do not respond after immunotherapy or do not show a durable response [18]. To overcome immunotherapy resistance, improved treatment methods in combination with ICIs should be explored to obtain long-term anti-tumor effects.
Oncolytic virotherapy has shown a broad application prospect [19], [20], [21]. Oncolytic viruses (OVs) selectively infect and lyse tumor cells, which then release progeny viruses to further infect and replicate in nearby cancer cells. OV infection also stimulates the host immune response, which leads to the production of an adaptive anti-tumor immune response [22]. In addition, studies have shown that OVs can be used as a cancer vaccine and acts as a therapeutic vaccine without recognizing tumor-specific neoantigens [23], [24], [25]. The OVs drug talimogene laherparepvec (T-VEC) was approved for the treatment of advanced unresectable melanoma.
In this study, we reported that the combined treatment of OVs encoding IL-12 (VT1092M) and anti-PD-1 antibody (VT1093M) inhibited the growth of the primary tumor, completely prevented the growth of the contralateral untreated tumor, and produced a vaccine-like response. In all assays, the combination of VT1092M and VT1093M showed a higher response rate than the single treatment groups.
Materials and methods
Cell lines
The colon cancer cell line CT26.WT and liver cancer cell line H22 were obtained from the National Collection of Authenticated Cell Cultures (Shanghai, China) and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% FBS in an incubator at 37 °C with 5% CO2.
Viruses
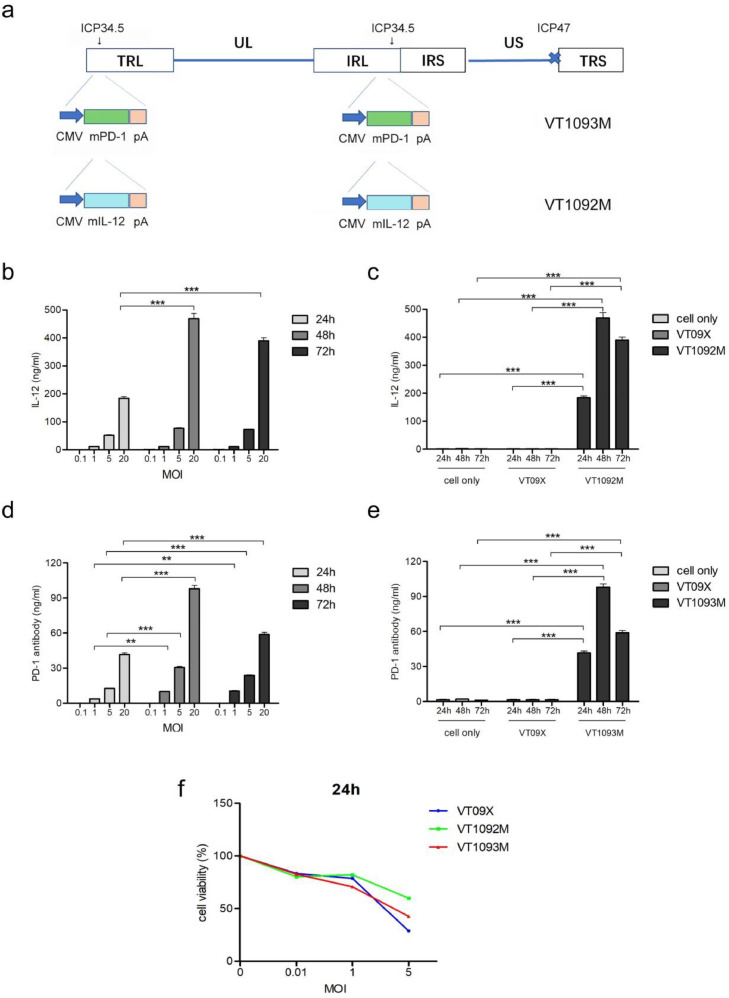
We used three viruses in the current study. VT09X was based on the wild-type herpes simplex virus type 1 (HSV-1) virus strain; the copies of the ɤ34.5 gene and the α47 gene in the genome were deleted by homologous recombination. Recombinant VT09X virus was obtained after purification and isolation. The VT1092M virus, which expresses the mouse-derived IL-12 gene, was constructed using the VT09X vector (Fig. 1a). We also used the VT09X vector to construct the VT1093M virus, which expresses the mouse-derived anti-PD-1 antibody gene (Fig. 1a).
Fig. 1.
Construction and infection of VT1092M and VT1093M. (a) The structure of VT1092M and VT1093M. (b-e) CT26 cells were plated in 24-well plates and infected with viruses at the indicated MOIs for the indicated times. The expression levels of IL-12 (b) and PD-1 antibody (d) were detected by ELISA. VT09X and CT26 cell supernatant (cell only) were used as negative controls (c, e). (f) Cells were plated in 96-well plates and treated with viruses at the indicated MOIs; the virus-mediated killing of CT26 cells was examined by CCK8 assay. Data are presented as the mean ± SD (n = 3). Statistical significance was calculated by one-way ANOVA. (*P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001).
VT09X and VT09X-GFP were obtained from WellGene and the copies of the ɤ34.5 gene and the α47 gene were deleted by homologous recombination. The gene encoding GFP was inserted into both ICP34.5 loci in VT09XGFP. To generate VT1093M and VT1092M, VT09XGFP was co-transfected with p-34.5PD1 or p-34.5IL12 into Vero cells. After several rounds of plaque purification, positive clones that did not express GFP were selected and confirmed by PCR and sequencing. All recombinant viruses were amplified in Vero cells and purified as described previously.
Reagents
Cyclophosphamide was purchased from Selleck (Beijing, China). The anti-mouse PD-1 antibody (m-PD-1 antibody) was purchased from Bio X Cell (Beijing, China).
Enzyme-linked immunosorbent assay (ELISA)
CT26 cells were seeded at 2 × 105 cells per well into a 24-well plate and cultured overnight. Cells were infected with virus at MOIs of 0.1, 1, 5 and 20 for 24, 48 or 72 h. VT09X and CT26 cell supernatant were used as negative controls. IL-12 and PD-1 antibody were measured using ELISA kits (Solarbio, Beijing, China).
In vitro cell cytotoxicity assay
CT26 cells were seeded at 5 × 103 cells per well into a 96-well plate and incubated overnight. Cells were infected with virus at MOIs of 0, 0.1, 1 and 5 for 24, 48 or 72 h. Proliferation was measured using the Cell Counting Kit-8 (CCK8) (Beyotime, Shanghai, China).
Animal experiments
All animal experimental protocols were approved by the Ethics Committee of Yantai University (IACUC No. 2018-DA-12) and conducted according to the Care and Use of Laboratory Animals of the National Institutes of Health. Female BALB/c mice (5–6 weeks old) were purchased from Jinan Pengyue Laboratory Animal Breeding Co. Ltd. (Jinan, China). CT26 cells were resuspended in serum-free medium with Matrigel basement membrane matrix (BD Biosciences, San Jose, CA, USA) at a 1:1 ratio and then subcutaneously injected into the right flank of each animal (5 × 106 cells per mouse). After tumors grew to approximately 250–300 mm3 in size, mice were randomly divided into six groups (n = 22/group). Mice received a single intratumoral injection treatment of sodium chloride (NaCl), 1 × 107 PFU of VT09X, 1 × 107 PFU of VT1092M, or 1 × 107 PFU of VT1093M in a volume of 50 µL at days 1,2,3,6,9,12,15 and 18 or an intratumoral injection treatment of VT1092M (at days 1,3,9,15 in 50 µL) and VT1093M (at days 2,6,12,18 in 50 µL); a single intraperitoneal injection of m-PD-1 antibody at 5 mg/kg twice a week and a single intraperitoneal injection of cyclophosphamide at 100 mg/kg once a week .
Tumor dimensions and body weights were recorded every three days. For analysis of tumor-infiltrating immune cells, six animals from each group were sacrificed at day 20 for flow cytometry assay and three animals per group were sacrificed from each group for quantitative PCR assay. For survival analyses, six animals were used and tumors were monitored for 46 days as described above.
For the re-challenge experiment, the animals that survived in the survival study were subcutaneously injected with 5 × 106 CT26 cells in the upper left of the back and 2 × 106 H22 cells in the bottom left of the back on day 46. Eight untreated mice were injected with cells for the control group. The tumor volumes were monitored for 20 days as described above.
Flow cytometry
Tumors were harvested and digested, and single-cell suspensions were produced using the Tumor Dissociation Kit (Miltenyi Biotec, Gladbach, Germany) with gentleMACS™ Octo Dissociator with Heaters (Miltenyi Biotec) for surface labeling. Mouse blood was collected by removing eyeball and lysed with Red Blood Cell Lysis Buffer to exclude the red cells. Cells were incubated in 0.5 µg Fc Block (Invitrogen, San Jose, CA, USA) for 5 min at 4 °C, and Fixable Viability Stain 510 (Invitrogen) was used to distinguish live cells and dead cells. Cells were incubated with an antibody cocktail including Brilliant Violet 605-CD45, PE-CD11b, PerCP-CD3e, APC—H7-CD4 and FITC—CD8 (BioLegend, San Diego, CA, USA) in the dark for 30 min at room temperature. Cell surface molecule expressions were measured and analyzed on an BD FACSCelesta flow cytometer (BD Biosciences) equipped with 405, 488 and 640 nm excitation lasers.
Quantitative PCR assay
Total RNA was extracted from tumors using the RNA extraction kit (Qiagen, Germantown, MD, USA) and converted to cDNA using the cDNA reverse transcription kit (Qiagen) according to the manufacturer's instructions. qRT-PCR was performed using the Roche LightCycler 480 PCR System with the following reaction components: 0.8 µL cDNA sample, 10 µL of 2 × SuperReal PreMix (probe), and 0.4 µL TaqMan probes in a final volume of 20 µL. Gene expression levels were normalized to levels of the β-actin housekeeping gene.
Statistics
Data were analyzed by a ttest for comparison of two groups and one-way ANOVA for comparison of multiple groups using GraphPad Prism 5. P < 0.05 was considered statistically significant.
Results
Transgene expression in VT1092M- and VT1093M-infected CT26 cells
The mouse IL12 gene and anti-PD-1 antibody gene were cloned into two ICP34.5 regions of HSV-1 with deletion of ICP47 to obtain VT1092M and VT1093M, respectively, as described in the Materials and Methods (Fig. 1a). We analyzed the expression level of IL-12 and PD-1 antibody in CT26 cells infected with VT1092M or VT1093M compared with cells infected with VT09X or control cells using ELISA. Cells were infected with viruses at MOI of 0.1, 1, 5 and 20 for 24, 48, and 72. The expressions of IL-12 and PD-1 antibody at 48 h and 72 h were significantly higher than expression at 24 h (P < 0.001) (Fig. 1b, d). In the VT09X-infected cells and CT26 cell negative controls, no expressions of IL-12 and PD-1 antibody were detected (Fig. 1c,e). These results indicated that the transgenes were expressed from the recombinant HSV-1 virus.
Insertion of transgene to influence viral replication and oncolytic ability
We next analyzed the killing of CT26 cells after infection with VT1092M or VT1093M compared with VT09X by CCK8 assay. The results showed that both VT1092M and VT1093M efficiently lyse CT26 cells at the same level as VT09X (Fig. 1f).
Enhanced anti-tumor activities against primary tumors
We next examined the anti-tumor activity of the OVs in tumors in BALB/c mice. A schematic for the experiment is shown in Fig. 2a. Tumors were established in mice as described in the Materials and Methods, and mice were intratumorally injected with various viruses, NaCl and intraperitoneal injection with m-PD-1 antibody. The tumor volumes in the groups injected with VT09X, VT1092M, VT1093M, and the combination of VT1092M and VT1093M were remarkably smaller compared with the NaCl control group (Fig. 2b). Tumor weights in the VT1092M, VT1093M and the combination groups were significantly reduced compared with the VT09X group (P < 0.05); however, there was no significant difference among the VT1092M, VT1093M and the combination groups (Fig. 2c). The average tumor weight in the VT1093M group was significantly lower than the m-PD-1 antibody group (P < 0.001) (Fig. 2c). No changes in body weight were observed among groups during the experiment (Fig. 2d).
Fig. 2.
Anti-tumor activities of OVs against primary tumors. (a) CT26 cells were injected into the right flank of mice. After tumors grew to approximately 250–300 mm3 in size, mice were randomly divided into six groups: the NaCl group (e), m-PD-1 antibody group (f), VT09X group (g), VT1093M group (h), VT1092M group (i) and VT1092M+VT1093M group (j). Tumor volume (b), body weights (d) and relative tumor inhibition rate (k) were recorded every three days. Tumor weight was compared at day 20 after treatment (c). The blue dotted lines in panels (e) through (g) indicate that mice died that day. Data are presented as the mean ± SD (n = 21 mice). Statistical significance was calculated by one-way ANOVA. (*P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
All animals in the four virus-treated groups, except one in the VT09X group, were alive at the end of the experiment, and the tumor growth rate was much slower in the VT1092M group, VT1093M group and VT1092M + VT1093M group than the NaCl and m-PD-1 antibody groups (Fig. 2e–j). These findings indicate that the single VT1092M or VT1093M as well as the combined therapy effectively inhibited tumor growth, with a tumor growth inhibition rate of more than 80% (Fig. 2k). The combined therapy group showed the highest tumor growth inhibition (Fig. 2j).
Tumor-specific anti-tumor immune response from virus therapy
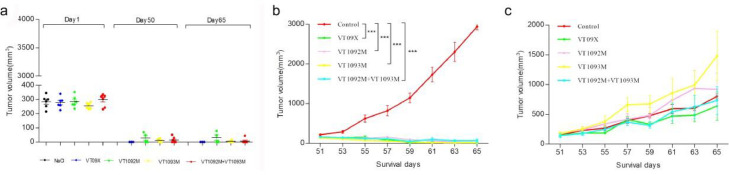
To determine whether intratumoral injection of the OV causes a systemic anti-tumor immune response, we performed a re-challenge experiment and inoculated two different tumor cell lines on the opposite side on the back of mice (upper left side, CT26 cells; lower left side, H22 cells) on day 46 (28 days after the termination of administration). The primary tumor volume (treatment side tumor) was smaller than 60 mm3, and most mice were tumor-free in the virus administration groups on day 50 (VT09X: 3/6; VT1092M: 2/6; VT1093M: 4/6; VT1092M+VT1093M: 6/6). There was no tumor growth on the right primary inoculation sites after the re-challenge experiment, until the end of the experiment (Fig. 3a). The volume of the re-challenged tumor (left side, CT26 cells) in the virus administration group was significantly decreased compared with the control group (P < 0.001) (Fig. 3b). There was no significant difference on H22 tumor growth between the re-challenge groups and control group (Fig. 3c). These data demonstrate that intratumoral injections with virus can provoke a tumor-specific anti-tumor immune response irrespective of the therapeutic gene.
Fig. 3.
Tumor-specific anti-tumor immune response of the virus. Mice were inoculated with CT26 cells on the right back at day 1. On day 46, the mice were inoculated with CT26 cells on the upper left and H22 cells on the lower left for the re-challenge experiment. Animals were sacrificed at day 65. (a) Right CT26 cell-derived tumor. (b) Left CT26 cell-derived tumor. (c) Left H22 cell-derived tumor. Data are presented as the mean ± SD (n = 6 mice). ⁎⁎P < 0.01 or ⁎⁎⁎P < 0.001 compared with the control group, determined by one-way ANOVA.
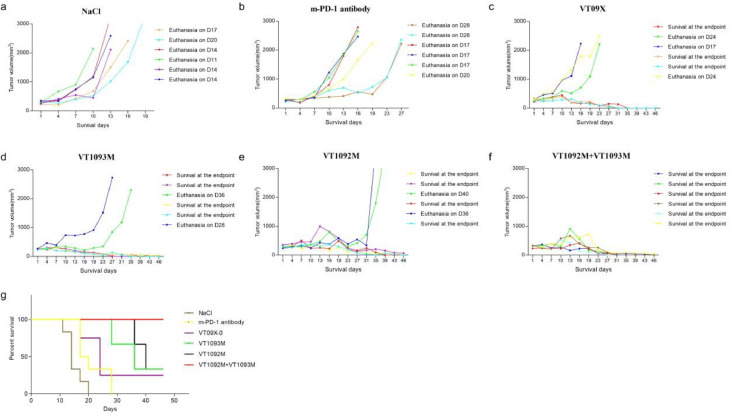
Prolonged survival by VT1092M in combination with VT1093M therapy
Six mice per group were used in the survival study. Animals were monitored for tumor burden and euthanized when tumors reached to 2000 mm3 according to the guidelines for animal ethics. By day 20, all animals in the NaCl group were euthanized. Two mice each in the VT1093M and VT1092M groups demonstrated tumor progression during the survival study, and all animal tumor volumes in the combined group for survival study were well controlled until the experiment endpoint (Fig. 4a–f). The results indicated that the combination treatment prolonged the survival time of tumor-bearing mice (Fig. 4g).
Fig. 4.
Prolonged survival of mice by the combination of VT1092M with VT1093M. (a–f) Six mice per group were used to evaluate the overall survival after treatment with the single agents or combined therapy. (g) Survival curves of the six groups of mice.
In the comparison of the survival rate between VT1093M and the m-PD-1 antibody groups, all animals in the m-PD-1 antibody group were euthanatized on day 28, while two mice in the VT1093M group demonstrated tumor progression and were euthanized on day 28 and day 36 (Fig. 4b and d). The remaining three mice survived and effective inhibition of tumor growth was observed (the tumors were cured and could not be measured).
Enhanced immune cell proportions in peripheral blood by the combined treatment compared with single treatments
We next analyzed the immune cell infiltration in peripheral blood and local tumor after intratumoral injections with the OVs or NaCl or intraperitoneal injection with m-PD-1 antibody. The VT1092M and VT1093M combination group showed markedly enhanced percentages of CD11b−, CD3+, CD4+ and CD8+T cells compared with the single VT1092M and VT1093M groups (Fig. 5a–d). All virus groups showed increased percentages of CD3+and CD4+T cells compared with the NaCl group (Fig. 5f–g). Except for the decrease of CD8+T cells in the VT1092M group (Fig. 5h), most other immune cells showed a small increase in the virus administration group compared with the NaCl group. These results suggest that the combined virus treatment showed superior potency in enhancing immune cell infiltration in peripheral blood compared with the single treatments.
Fig. 5.
Enhanced immune cell proportions in peripheral blood in the combined treatment group compared with single treatment. (a–d) Statistical analysis of the CD3+, CD4+ and CD8+ T cell proportions in peripheral blood from the indicated groups. (e–h) Statistical analysis of the CD11b−, CD3+, CD4+ and CD8+T cell infiltration in tumors from the indicated group. Data are presented as the means ± SD (n = 5 mice). Statistical significance was calculated by one-way ANOVA. (*P < 0.05, **P < 0.01, ***P < 0.001).
Modulation of the expression of genes related to T cell response
The above findings suggest that the combination therapy induced intratumoral immune cell infiltration. Next, we analyzed the expression level of several genes related to T cell response by qRT-PCR. Beta-2-microglobulin (β2 M) and major histocompatibility complex I (MHC I) gene expressions were upregulated in the combined group compared with NaCl group, although differences in β2 M gene expression were not significant (Fig. 6a,b). This result indicated that oncolytic therapy could affect the antigen-presentation pathway. We also observed a tendency of higher gene expression of IFN-γ and PD-L1 in the virus administration groups compared with the NaCl groups (Fig. 6c,d). IFN-γ and PD-L1 gene expressions were significantly enhanced in the VT1092M+VT1093M group compared with the NaCl group (P < 0.05).
Fig. 6.
Expression of genes related to the T cell response. qRT-PCR determination of expression of β2M (a), MHC I (b), IFN-γ (c) and PD-L1 (d) genes in tumor specimens from mice at day 20. Data are presented as the mean ± SD (n = 3). Statistical significance was calculated using the t-test. (*P < 0.05).
Enhanced T cell proportion in peripheral blood in the re-challenge experiment
We further collected mouse blood for flow analysis in the re-challenge mouse groups. The virus administration group showed enhanced CD3+/CD45+ and CD4+/CD45+ immune cell proportions compared with the control group, but without statistical significance (Fig. 7b,c). The groups administered VT09X, VT1092M and VT1093M showed significantly increased percentages of CD8+/CD45+cells compared with the control group (P < 0.05). The combined group showed significantly enhanced percentages of CD11b−/CD45+ (P < 0.05) and CD8+/CD45+ (P < 0.001) T cells compared with the control group (Fig. 7a,d). These data suggest that the combined treatment enhanced T cell proportions in peripheral blood in the re-challenge experiment.
Fig. 7.
Enhanced T cell proportions in peripheral blood in the re-challenge experiment. Mouse blood was collected for flow analysis in the re-challenge mouse groups. Statistical analyses of the proportions of CD11b−, CD3+, CD4+and CD8+T cells to CD45 cells. Data are presented as the mean ± SD (n = 5). Statistical significance was calculated by one-way ANOVA. (*P < 0.05, ⁎⁎P < 0.01 and ⁎⁎⁎P < 0.001).
Discussion
Although ICIs, especially anti-PD-1 antibody, have shown great success in tumor treatment in the past 10 years, some patients do not respond to treatment [11,13,26]. In the tumor microenvironment, anti-PD-1 antibody activates T cells to induce anti-tumor effects; however, tumors without T cell infiltration do not respond to anti-PD-1 treatment [12,27]. OVs function as a cancer vaccine that attracts T cell infiltration, making it an effective combination treatment with anti-PD-1 therapy [23]. Insertion of the anti-PD-1 antibody gene into an OV enables its expression in situ to block the PD-1/PD-L1 interaction. Indeed, some studies showed that OV encoding the anti-PD-1 antibody gene exerted strong anti-tumor effects [28,29]. To achieve better anti-tumor efficacy, we selected IL-12, which shows potent effects in T cell stimulation, for expression in OVs. In the combination treatment strategy using both recombinant OVs in this study, the OV and expressed anti-PD-1 antibody and IL-12 all induce anti-tumor effects by different mechanisms.
The abilities of ICIs and IL-12 to treat cancers through immune activation have been demonstrated [9,12,[30], [31], [32]]. Oncolytic virus infects tumor cells to release tumor antigen and induce killing of cytotoxic T cells, and IL-12 can stimulate proliferative and induces the production of interfero-γ of activated T cells, but the negative feedback regulation mechanism of PD-L1 will inhibit T cell activation. The addition of PD-1 antibody will break this inhibition, significantly enhance anti-tumor effect and increase the response rate of test mice. Two studies reported a synergetic anti-tumor efficacy of IL-12 in combination with anti-PD-1 antibody [33,34]. Yan et al. reported that the oncolytic herpes simplex virus T3855 expressing murine IL-12 and anti-PD-1 antibody was superior to systemic administration of IL-12 and PD-1 antibody [33]. The tumor growth inhibition rate of T3855 on A20, MC38, MFC and SCC7 tumors was approximately 60%. In this study, we evaluated the effects of our combination treatment of OV-driven IL-12 and PD-1 antibody expression on a CT26 cell-derived tumor model in BALB/c mice. Local injection of VT1092M and VT1093M resulted in a tumor inhibition rate of more than 90%. The difference between our results and the previous study (90% and 60%, respectively) may be from the different expression levels of IL-12 and PD-1 antibody; IL-12 and PD-1 antibody are expressed from VT1092M and VT1093M in a range of 30–400 ng/ml, while in the T3855, the two genes are expressed simultaneously in a range of 0–500 pg/ml. With the combination strategy, the levels of VT1092M and VT1093M can be modulated at different ratios for expressions of IL-12 and antibody to PD-1 for optimized anti-tumor efficacy, but the expression ratio of IL-12 and PD-1 from T3855 is not changeable. In this study, the anti-tumor effect of intratumoral injection of OV expressing PD-1 antibody was significantly enhanced compared with intraperitoneal injection of PD-1 antibody (P < 0.001), and approximately 86% of animals had an immune response to VT1093M. In addition, the infiltration of CD8+ and CD4+ T cells in the tumor and peripheral blood was also enhanced, suggesting the advantages of the combination of ICIs and oncolytic viruses. Our in vitro studies showed that at a high MOI, the killing ability of VT1092M and VT1093M towards tumor cells was weaker than that of VT09X. However, in vivo experimental results showed that the anti-tumor effects of VT1092M and VT1093M were significantly stronger than VT09X, indicating that the insertion of these genes enhanced the anti-tumor immune response. While there was no significant difference in tumor volume between the single treatment group and the combination group, we found that half of the mice in the single treatment group showed tumor growth remission after the end of the administration, while the combination group showed no remission. These results demonstrated that the combination of VT1092M and VT1093M exerted potent anti-tumor effects and significantly prolonged the survival rate of mice compared with single treatments.
Local injection of OV in the VT1092M+VT193M group increased immune cell infiltration, enhanced the expression of MHCI and β2M genes in antigen-presenting cells, upregulated a variety of immune-related pathways, and induced tumor regression without injection of distant tumors. The number of infiltrating immune cells was low, while the immune response of distant tumors lasted for a long time, indicating that tumor-specific lymphocytes may also migrate to secondary lymphatic organs and be maintained as memory T cells [35]. This will result in rejection of the introduced tumor which different with primary tumor in re-challenge experiment. In the re-challenge experiment, the CT26 cell-derived tumors in the virus-administered group undergoing tumor re-challenge continued to decrease, while the H22 cell-derived tumors inoculated at the same time continued to grow; the tumor volume was not much different from the control group at the end of the experiment, indicating that the OV caused an antigen-specific anti-tumor immune response after administration. However, we were unable to confirm that tumor-specific memory T cells infiltrated the CT26 cell-derived tumor in the re-challenge experiment, and this requires further analysis in subsequent experiments.
Previous studies showed that OVs were not detected in the contralateral or distal tumor after local intratumoral injection of OVs, indicating that OVs are not distributed throughout the body [36]. However, in this study, the CT26 and H22 cell–derived tumors on the non-administration side of the mouse completely disappeared in the re-challenge experiment. This observation suggests that the OV not only causes an antigen-specific anti-tumor immune response, but it may also induce a systemic antigen non-specific immune response. This possibility requires further investigation and should be examined in future studies.
In summary, our data shows that the OVs VT1092M and VT1093M have significant anti-tumor effects when used in combination, and the combination treatment significantly extended the survival time of tumor-bearing mice. These findings provide evidence for a novel combination therapy against colon cancer.
CRediT authorship contribution statement
Xin Xie: Investigation, Data curation, Formal analysis, Writing – original draft. Jingwen Lv: Investigation, Data curation, Formal analysis. Wei Zhu: Investigation, Investigation, Data curation, Writing – review & editing. Chao Tian: Investigation, Data curation. Jingfeng Li: Investigation, Investigation, Data curation, Writing – review & editing. Jiajia Liu: . Hua Zhou: Investigation, Data curation. Chunyang Sun: Investigation, Data curation. Zongfeng Hu: Investigation, Data curation. Xiaopeng Li: .
CRediT authorship contribution statement
Xin Xie: Investigation, Data curation, Formal analysis, Writing – original draft. Jingwen Lv: Investigation, Data curation, Formal analysis. Wei Zhu: Investigation, Investigation, Data curation, Writing – review & editing. Chao Tian: Investigation, Data curation. Jingfeng Li: Investigation, Investigation, Data curation, Writing – review & editing. Jiajia Liu: . Hua Zhou: Investigation, Data curation. Chunyang Sun: Investigation, Data curation. Zongfeng Hu: Investigation, Data curation. Xiaopeng Li: .
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The study was funded by the Top Talents Program for One Case One Discussion of Shandong Province and the National Natural Science Foundation of Shandong Province (ZR2019PH056). We thank Gabrielle White Wolf, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn/) for editing the English text of a draft of this manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101287.
Appendix. Supplementary materials
References
- 1.Bommareddy P.K., Shettigar M., Kaufman H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018;18:498–513. doi: 10.1038/s41577-018-0014-6. [DOI] [PubMed] [Google Scholar]
- 2.Lichty B.D., Breitbach C.J., Stojdl D.F., Bell J.C. Going viral with cancer immunotherapy. Nat. Rev. Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 3.Kong X., Lu P., Liu C., Guo Y., Meng J. A combination of PD1/PDL1 inhibitors: the prospect of overcoming the weakness of tumor immunotherapy (Review) Mol. Med. Rep. 2021;23:362. doi: 10.3892/mmr.2021.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sehgal A., Whiteside T.L., Boyiadzis M. Programmed death-1 checkpoint blockade in acute myeloid leukemia. Expert Opin. Biol. Ther. 2015;15:1191–1203. doi: 10.1517/14712598.2015.1051028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zs A., Bo L.B., Ch B., Wx A., Yi C.B., Ling C.B., Min L. An oncolytic vaccinia virus armed with anti-human-PD-1 antibody and anti-human-4-1BB antibody double genes for cancer-targeted therapy. Biochem. Biophy. Res. Commun. 2021;559:176–182. doi: 10.1016/j.bbrc.2021.04.078. [DOI] [PubMed] [Google Scholar]
- 6.de Almeida N.A.A., Ribeiro C.R.A., Raposo J.V., de Paula V.S. Immunotherapy and gene therapy for oncoviruses infections: a review. Viruses. 2021;13:822. doi: 10.3390/v13050822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gajewski T.F., Schreiber H., Fu Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S., Margolin K. Cytokines in cancer immunotherapy. Cancers. 2011;3:3856–3893. doi: 10.3390/cancers3043856. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valerio L., Andrea V., Valentina G., Julie R., Mara S., Catia B., Anna Z., Patrizia N., Pier-Luigi L., Costanza C., et al. A fully-virulent retargeted oncolytic HSV armed with IL-12 elicits local immunity and vaccine therapy towards distant tumors. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim K.J., Moon D., Kong S.J., Yu S.L., Choi K.J. Antitumor effects of IL-12 and GM-CSF co-expressed in an engineered oncolytic HSV-1. Gene. Ther. 2020;28:186–198. doi: 10.1038/s41434-020-00205-x. [DOI] [PubMed] [Google Scholar]
- 11.Pardoll M.D. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G., Kang X., Chen K.S., Jehng T., Chen S.Y. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses. Nat. Commun. 2020;11:1395. doi: 10.1038/s41467-020-15229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tumeh P.C., Harview C.L., Yearley J.H., Shintaku P.I., Taylor E.J.M., Robert L., Chmielowski B., Spasic M., Henry G., Ciobanu V., et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribas A., Hamid O., Daud A., Hodi F.S., Wolchok J.D., Kefford R., Joshua A.M., Patnaik A., Hwu W.J., Weber J.S., et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 16.Hodi F.S., O'Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., Schadendorf D., Dummer R., Smylie M., Rutkowski P., et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma, Reply. N. Engl. J. Med. 2015;273:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolgin E. Oncolytic viruses get a boost with first FDA-approval recommendation. Nat. Rev. Drug Discov. 2015;14:369–371. doi: 10.1038/nrd4643. [DOI] [PubMed] [Google Scholar]
- 20.Andtbacka R.H., Kaufman H.L., Collichio F., Amatru Da T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S., et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 21.Hu J., Coffin R.S., Davis C.J., Graham N.J., Coombes R.C. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 2006;12:6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 22.Giulia M., Anwen H., Lemoine N.R., Wang Y. Oncolytic viral therapy and the immune system: a double-edged sword against cancer. Front. Immunol. 2018;9:866. doi: 10.3389/fimmu.2018.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang H., Rivera-Molina Y., Clise-Dwyer K., Bover L., Vence L., Yuan Y., Lang F.F., Toniatti C., Hossain M.B., Gomez-Manzano C. Oncolytic adenovirus and tumor-targeting immune modulatory therapy improve autologous cancer vaccination. Cancer Res. 2017;77:3894–3907. doi: 10.1158/0008-5472.CAN-17-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartlett D.L., Liu Z., Sathaiah M., Ravindranathan R., Guo Z., He Y., Guo Z.S. Oncolytic viruses as therapeutic cancer vaccines. Mol. Cancer. 2013;12:1–16. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sivendran S., Pan M., Kaufman H.L., Saenger Y. Herpes simplex virus oncolytic vaccine therapy in melanoma. Expert Opin. Biol. Ther. 2010;10:1145–1153. doi: 10.1517/14712598.2010.495383. [DOI] [PubMed] [Google Scholar]
- 26.Arianna D., Aled C.C., Andrew F., Marco D. Acquired resistance to cancer immunotherapy. Semin Immunopathol. 2018;41:31–41. doi: 10.1007/s00281-018-0692-y. [DOI] [PubMed] [Google Scholar]
- 27.Kleinpeter P., Fend L., Thioudellet C., Geist M., Sfrontato N., Koerper V., Fahrner C., Schmitt D., Gantzer M., Remy-Ziller C., et al. Vectorization in an oncolytic vaccinia virus of an antibody, a Fab and a scFv against programmed cell death -1 (PD-1) allows their intratumoral delivery and an improved tumor-growth inhibition. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2016.1220467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z., Ravindranathan R., Kalinski P., Guo Z.S., Bartlett D.L. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat. Commun. 2017;8:14754. doi: 10.1038/ncomms14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C.Y., Hutzen B., Wedekind M.F., Cripe T.P. Oncolytic virus and PD-1/PD-L1 blockade combination therapy. Oncol. Virother. 2018;7:65–77. doi: 10.2147/OV.S145532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakao S., Arai Y., Tasaki M., Yamashita M., Murakami R., Kawase T., Amino N., Nakatake M., Kurosaki H., et al. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Sci. Transl. Med. 2020;12:7992. doi: 10.1126/scitranslmed.aax7992. eaax. [DOI] [PubMed] [Google Scholar]
- 31.Thomas E.D., Meza-Perez S., Bevis K.S., Randall T.D., Gillespie G.Y., Langford C., Alvarez R.D. IL-12 expressing oncolytic herpes simplex virus promotes anti-tumor activity and immunologic control of metastatic ovarian cancer in mice. J. Ovarian Res. 2016;9:70. doi: 10.1186/s13048-016-0282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Najmuddin S., Amin Z.M., Tan S.W., Yeap S.K., Alith Ee N N.B. Cytotoxicity study of the interleukin-12-expressing recombinant newcastle disease virus strain, rAF-IL12, towards CT26 colon cancer cells in vitro and in vivo. Cancer Cell Int. 2020;20:278. doi: 10.1186/s12935-020-01372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan R., Zhou X., Chen X.Q., Liu X.J., tang Y.x., ma j., wang l., liu z.w., zhang b.r., chen h., et al. enhancement of oncolytic activity of oHSV expressing IL-12 and Anti PD-1 antibody by concurrent administration of exosomes carrying CTLA-4 miRNA. Immunol. 2019;5:1–10. [Google Scholar]
- 34.Xu C., Zhang Y.P., Rolfe P.A., Hernández V.M., Guzman W., Kradjian G., Marelli B., Qin G., Qi J., Wang H., et al. Combination therapy with NHS-muIL12 and avelumab (anti-PD-L1) enhances antitumor efficacy in preclinical cancer models. Clin. Cancer Res. 2017;23:5869–5880. doi: 10.1158/1078-0432.CCR-17-0483. [DOI] [PubMed] [Google Scholar]
- 35.Garrido F., Aptsiauri N. Cancer immune escape: MHC expression in primary tumours versus metastases. Immunology. 2019;158:255–266. doi: 10.1111/imm.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamarin D., Holmgaard R.B., Subudhi S.K., Park J.S., Mansour M., Palese P., Merghoub T., Wolchok J.D., Allison J.P. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014;6:226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.